Abstract
Many mammalian viruses have properties that can be commandeered for the treatment of cancer. These characteristics include preferential infection and replication in tumor cells, the initiation of tumor cell lysis, and the induction of innate and adaptive anti-tumor immunity. Furthermore, viruses can be genetically engineered to reduce pathogenicity and increase immunogenicity resulting in minimally toxic therapeutic agents. Talimogene laherparepvec (T-VEC; Imlygic™), is a genetically modified herpes simplex virus, type 1, and is the first oncolytic virus therapy to be approved for the treatment of advanced melanoma by the US FDA. T-VEC is attenuated by the deletion of the herpes neurovirulence viral genes and enhanced for immunogenicity by the deletion of the viral ICP47 gene. Immunogenicity is further supported by expression of the human granulocyte-macrophage colony-stimulating factor (GM-CSF) gene, which helps promote the priming of T cell responses. T-VEC demonstrated significant improvement in durable response rate, objective response rate, and progression-free survival in a randomized phase III clinical trial for patients with advanced melanoma. This review will discuss the optimal selection of patients for such treatment and describe how therapy is optimally delivered. We will also review future directions for oncolytic virus immunotherapy, which will likely include combination T-VEC clinical trials, expansion of T-VEC to other types of non-melanoma skin cancers and renewed efforts at oncolytic virus drug development with other viruses.
1. Introduction
Melanoma is a malignant tumor of melanocytes with a slowly increasing incidence over the last 50 years. In 2016 there will be an estimated 76,380 cases of invasive melanoma in the United States[1]. The overall lifetime risk of developing melanoma is 1 in 33 for men and 1 in 52 for women[2]. Complete surgical excision is curative for in situ and minimally invasive lesions, and excision is the standard of care for early melanoma treatment[3]. Once tumors invade deeper than 0.75–1.0 mm into the dermis, metastatic spread through subdermal lymphatic vessels is possible. Melanoma typically spreads in an organized fashion, first to regional lymph nodes where the disease may be arrested through lymph node dissection. Sentinel lymph node biopsy is currently recommended in patients with intermediate thickness melanomas to identify the subset of such patients with regional nodal metastases and can help select appropriate patients for completion lymphadenectomy[3]. If the disease is not contained locally or within tumor-draining lymph nodes, systemic metastasis to almost any organ in the body is possible. Prior to 2011, metastatic melanoma had been resistant to systemic therapy except for a small subset of patients who responded to treatment with high-dose interleukin-2 (IL-2)[4]. Progress in the treatment of metastatic melanoma after 2011 has been significant with major advances in both targeted and tumor immunotherapy[5].
A better understanding of the molecular signaling pathways that drive melanoma progression and the ability to rapidly obtain genomic mutation data from individual tumor specimens led to the widespread application of BRAF and/or MEK inhibitors for the treatment of BRAF-mutant metastatic melanoma. While these agents have been associated with high initial response rates, drug resistance and disease recurrence have been major challenges with these agents[6]. Advances in tumor immunotherapy, especially the recognition that molecular “checkpoints”, as represented by the cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed cell death 1 specific immunity have revolutionized the treatment of metastatic melanoma[7]. Randomized clinical trials of CTLA-4 and PD-1 inhibiting monoclonal antibodies demonstrated significant improvement in overall survival and response rates for patients with metastatic melanoma, and resulted in FDA approval of three new T cell checkpoint inhibitors since 2011, one targeting CTLA-4 (ipilimumab) and two targeting PD-1 (pembrolizumab and nivolumab)[8–10]. Further, combination strategies with T cell checkpoint inhibitors, such as ipilimumab and nivolumab, have shown additive therapeutic activity leading to FDA approval of the first combination immunotherapy, although the adverse event rate is also higher with combination treatment[11].
The development of predictive biomarkers to aid in appropriate patient selection for treatment, is a high priority. To date, there has been some evidence that tumor expression of the PD-1 ligand (PD-L1) may serve as a predictor of patients more likely to respond to checkpoint inhibitor monotherapy but this has not been universally consistent[12–14]. The accumulation of tumor-infiltrating lymphocytes with the tumor microenvironment has also been suggested as a potential biomarker of response, yet why some tumors have such cells and some do not is not completely understood[15]. The availability of clinically validated biomarkers is important since many patients do not respond to these therapeutic approaches, eventual drug resistance with disease recurrence may occur with these agents and an improved understanding of why certain factors may serve as a predictive biomarker might inform further clinical investigation[16]. For example, agents that can increase PD-L1 expression and tumor infiltration in the tumor microenvironment would be of especially high importance.
There are several viruses that have been identified as oncolytic with many now in clinical trials against a variety of cancers. These include adenovirus, coxsackievirus, reovirus, Newcastle Disease virus, poliovirus, measles virus, vesicular stomatitis virus, vaccinia virus, and herpes simplex virus (HSV) [17]. Early phase clinical trials have generally demonstrated a tolerable safety profile and therapeutic responses have been reported [18, 19]. There has been considerable excitement in the field with the completion of the first randomized phase III clinical trial of an oncolytic virus in patients with advanced melanoma. This trial employed an oncolytic HSV-1 virus encoding human granulocyte-macrophage colony-stimulating factor (GM-CSF) and has been designated Talimogene laherparepvec (Imlygic®; or T-VEC). T-VEC demonstrated a significant improvement in durable response rate for patients with unresectable stage III and IV melanoma and was approved by the U.S. Food and Drug Administration for the treatment of patients with advanced melanoma in 2015 and for the treatment of Stage III and IV M1a melanoma by the European Medicines Agency [20]. T-VEC treatment has been associated with an increase in melanoma-specific CD8+ T cells and corresponding decrease in suppressive immune cells, such as CD4+FoxP3+ regulatory T cells and myeloid-derived suppressor cells within the tumor microenvironment [21]. In this review, we will discus the basic biology of T-VEC, describe the clinical trial results in more detail, provide practical tips for T-VEC administration and suggest future areas of high priority research with T-VEC.
2. Basic Biology of Oncolytic Viruses
2.1 General mechanisms of action
Oncolytic viruses represent a new approach to cancer treatment based on the ability of certain viruses to preferentially replicate in tumor cells and their ability to promote immune responses[17]. Of the nearly 300,000 mammalian viruses, many are known to infect cancer cells and induce potent T cell responses, and in most cases, humoral immunity as well. Oncolytic viruses may consist of native viral particles or be genetically manipulated for decreased pathogenicity or increased immunogenicity[17]. In general, all oncolytic viruses induce anti-tumor activity through both direct infection of tumor cells with resultant lysis of the cancer cell and secondarily, though induction of anti-tumor immunity. Oncolytic viruses may be especially effective because viral infection results in the local release of pro-inflammatory factors, including viral and cellular DNA, calreticulin, HMGB1 and other danger-associated molecular factors, which can promote host innate immunity[22–24]. Further, dying tumor cells may release soluble antigens or necrotic cells may be engulfed by local antigen-presenting cells in the context of a pro-inflammatory milieu associated with acute viral infection[25, 26]. Viral infection also induced host interferon responses, which can enhance PD-L1 expression and promote T cell infiltration into sites of active infection, such as the tumor microenvironment[27]. The death of virally infected tumor cells can foster cross presentation of tumor antigens and activation of adaptive, tumor-specific immune responses. The priming of tumor-specific T cells can then mediate tumor regression at sites of tumor growth without requiring active viral infection, resulting in a so-called abscopal effect allowing rejection of both injected and un-injected tumors [28].
2.2 Biology and development of oncolytic herpes viruses for cancer treatment
Herpes simplex virus type 1 (HSV-1) is a double-stranded DNA virus that has large segments of non-coding DNA allowing for genetic manipulation. HSV-1 enters cells through the herpes virus entry mediator (HVEM) or through a family of nectin proteins using an outer layer of glycoproteins in the herpes virus envelope. Following cell entry, HSV-1 particles traffic to the nucleus. The virus completes the replication cycle largely within the host cell nucleus, although insertional mutagenesis does not occur [19]. Following replication the viral capsid is assembled and mature virions accumulate outside the nucleus and eventually result in cell lysis. HSV-1 can enter a latent phase in some cells, such as neurons, and viral particles can be released at later times, such as during host stress [29]. HSV-1 is ubiquitous in the environment and by age 70, it is estimated that 90% of people have been exposed to the virus[30]. Several native strains of herpes virus, including HSV-1, have shown oncolytic effects in which tumor cells are infected and killed following infection.
First generation oncolytic HSV-1 viruses were engineered by creating mutations in single genes of interest. The single gene mutations were initially created to limit neuronal latency following viral infection and to limit the oncolytic activity to neoplastic cells. A complete list of up to date HSV-1 mutants, mutated genes and their functions are described in a review by Rabkin et al[31]. Knipe and colleagues engineered a thymidine kinase (tk) UL23 mutant HSV-1 Dlsptk by using HSV strain KOS. The sequences from the Bgl I site to the Pvu I site downstream of the tk gene were digested to obtain the tk deletion mutant [32]. Thymidine kinase expression is required for HSV-1 infection of neuronal ganglia and for viral reactivation in latent ganglia. Thymidine kinase catalyzes the conversion of deoxythymidine to deoxythymidine 5′-phosphate (dTMP) and thus helps in creating building blocks for viral DNA synthesis. The deletion of tk restricts viral replication in non-dividing cells and, thus enables selective targeting to rapidly dividing tumor cells. Dlsptk is the first genetically engineered oncolytic HSV-1 and while it originally was made to study the effects of tk deletion on viral replication in mouse ganglia, it was later studied in the context of oncolysis. In murine studies Dlsptk was able to selectively lyse U87 glioblastoma cells and resulted in prolonged survival of nude mice bearing U87 orthotopic gliomas, confirming the hypothesis that mutations in tk gene can be used for oncolytic activity [33].
Other first generation oncolytic viruses included the hR3 mutant, which was engineered by placing the E. coli LacZ gene into ICP6 (UL39), which encodes the large subunit of the viral ribonucleotide reductase and is necessary for formation of deoxyribonucleotides (dNMP) from ribonucleotides[34]. During replication HSV-1 needs an abundant supply of dNMPs to synthesize new viral genomes. Thus, the deletion of ICP6 results in preferential replication of HSV-1 in rapidly dividing cells. As more information on the molecular biology of HSV-1 became known, additional genetic mutants were developed to improve the oncolytic capability of the virus.
Following cell infection, HSV-1 can replicate and lyse the host cell, provided its anti-viral machinery does not eradicate the virus. A major part of the host defense against herpes viruses is the interaction with host cell protein kinase R (PKR), an RNA-dependent kinase that can be induced by interferon-α and activated by double-stranded RNA produced by HSV-1 replication. Once PKR is activated, it induces the phosphorylation of the eIF-2α translation factor resulting in the inactivation and disruption of viral protein synthesis. Many viruses have evolved mechanisms for inhibiting PKR-mediated inhibition of viral replication, and native HSV-1 contains two copies of the infected cell protein 34.5 (termed ICP 34.5, γ-34.5 or γ1-34.5 protein), which binds directly to cellular protein phosphatase 1α, which then dephosphorylates eIF-2α and re-induces viral protein synthesis[35]. HSV-1 vectors that lack the ICP34.5 genes are significantly compromised in their ability to replicate in normal cells and neurons that have activated PKR capability, but are able to replicate in tumor cells, which generally lack PKR activity. R3616 is a mutant HSV-1 engineered by the deletion of diploid γ34.5 genes present in the long repeat region of HSV-1 [36]. Deletion of the γ34.5 genes also limits HSV-1 replication in the brain and dorsal ganglia, reducing the pathogenicity of viral infection [36].
While the first generation of genetically engineered HSV-1 laid the foundation for improving the selective replication in tumor cells and addressed the issue of neuronal latency, additional safety features were realized in second-generation viral constructs. These modified HSV-1 vectors were developed with multiple gene deletions. The first such oncolytic HSV-1 strain was called G207, and was generated by deleting both ICP34.5 genes and a large portion of the ICP6 gene The G207 also encodes the β-galactosidase gene as a marker. In a pre-clinical study of U-87MG glioblastoma in BALB/c mice, G207 was able to significantly reduce tumor volumes compared to wild type HSV-1. In these studies, active replication of G207 within the tumor tissue was confirmed by immunohistochemistry [37].
HSV1716 was derived from the Glasgow HSV-1 17+ strain and is a replication-competent, tumor-selectively mutant HSV-1 in which both copies of the ICP34.5 genes have been deleted [38]. HSV1716 lyses human glioblastoma cells in vitro and has demonstrated therapeutic activity in murine models of glioblastoma without evidence of replication in normal tissues[39–42]. The HSV 1716 has been widely studies in early phase clinical trials after initial studied demonstrated an acceptable safety profile of direct intra-tumoral injection of doses up to 105 plaque forming units (pfu) in patients with recurrent high-grade glioma[43]. Similar findings were reported in patients with metastatic melanoma [44].
2.3 Biology of T-VEC
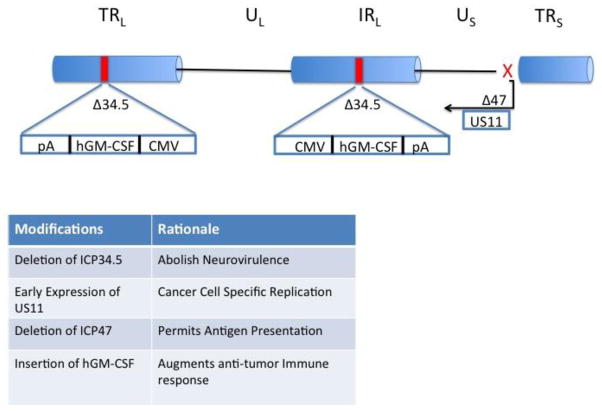
T-VEC is a genetically modified HSV-1 based on the JS1 strain, a minor human pathogen and the causative agent of fever blister disease. Deletion of specific viral genes have further enhanced T-VEC’s efficacy as an oncolytic therapy (see Fig. 1). HSV-1 has two copies of the infected cell protein (ICP) 34.5 gene, which encodes the neurovirulence factors. Deletion of both copies of the ICP34.5 gene prevents replication of the virus in neurons, but does not affect the replication of the virus in other cells [45, 46]. This deletion results in decreased pathogenicity and also promotes selective replication in tumor cells since ICP34.5 gene product interacts with host cell protein kinase R (PKR) to block viral protein synthesis since activated PKR can induce cellular apoptosis following viral infection. In addition, T-VEC contains a deletion of the herpes virus ICP47 gene, whose gene product blocks viral peptide entry into the antigen processing machinery as a method of avoiding immunologic detection during infection. In the absence of ICP47, antigenic viral and tumor-associated peptides gain access to MHC class I complexes, which promotes host immune responses and viral immunogenicity [47]. Another genetic alteration in T-VEC is placing the herpes unique short 11 (US11) gene under an early/immediate gene promoter, rather than the native late promoter. The US11 gene product promotes early inhibition of PKR phosphorylation, which prevents rapid viral clearance, prolongs the life cycle of the virus and results in more profound cytolytic effects. Finally, in place of the ICP34.5 genes T-VEC contains two copies of the human granulocyte-macrophage colony stimulating factor (GM-CSF) gene, which attracts local dendritic cells and promotes their maturation after sampling dying tumor cells and soluble antigen, thereby generating systemic T cell immune response against the virus and tumor cell. Recently, HSV-derived vectors have been shown to replicate more efficiently in host cells harboring gain-of-function oncogene mutations, such as in Ras mutated cancer cells; thus patients with mutations in the MAPK kinase pathway may be particularly susceptible to oncolytic therapy with T-VEC [48, 49].
Figure 1.
Schematic showing the engineering of Talimogene laherparepvec (T-VEC). The backbone is the JS17 strain of herpes Simplex virus, type 1 in which the two viral ICP34.5 genes have been deleted and replaced with copies of the human GM-CSF genes under control of a CMV promoter. In addition, the viral ICP47 gene is deleted transitioning the viral US11 gene to an immediate-early promoter. The implications of these genetic modifications are listed in the box. Abbreviations: CMV, cytomegalovirus; hGM-CSF, human granulocyte-macrophage colony-stimulating factor; ICP, infected cell protein; IRL, long inverted repeat region; pA, polyadenylation tail; TRL, long terminal repeat; TRS, short terminal repeat; UL, unique long region; US, unique short region; US11, unique short sequence 11.
T-VEC has a dual mechanism of action, first directly infecting and killing tumor cells (so-called oncolytic effect) and secondly, through induction of local and systemic immune responses (so-called immunotherapy effect). T-VEC is administered by local injection into cutaneous, subcutaneous, or nodal sites of melanoma. The cancer-selective properties, as described above, allow preferential T-VEC replication within cancer cells. Replication of the herpes virus leads to lysis of infected tumor cells, which then releases soluble tumor-associated antigens, danger signals and necrotic tumor cell fragments, all of which help initiate local immune responses. The local expression of GM-CSF further enhances dendritic cell migration and maturation. The dendritic cells then travel to regional lymph nodes where they present antigens to specific CD4+ T helper cells and CD8+ T effector cells, triggering a systemic T cell response. These tumor-specific T cells can then traffic to sites of distant metastases where immune-mediated regression occurs [29, 50, 51]. In addition, a “bystander effect” is also likely in which progeny viral particles that are released go on to infect neighboring tumor cells expanding the anti-tumor activity. The response rate in distant metastases is lower than the response rate in injected disease, and the reasons for this are not fully understood, but presumably, the tumor-specific T cell response is not able to expand sufficiently, or local suppressive factors in the distant metastases overwhelm the effector T cells. These limitations may be overcome by direct injection of visceral metastases and/or through other immunotherapy agents that can promote expansion of reactive T cell populations.
T-VEC has demonstrated tumor cell killing in several in vitro and in vivo models, against a wide range of cancer cell lines and murine tumors. In studies of the murine A20 tumor, direct injection of T-VEC with or without GM-CSF resulted in rejection of the injected tumor, but eradication of a contralateral flank tumor occurred only when GM-CSF was incorporated into the viral construct [45]. The viral vectors augmented with GM-CSF also demonstrated increased IFN-gamma production compared to non-GM-CSF containing virus, and this may help promote anti-tumor immunity by enhancing MHC class I expression on the tumor cells and mediating a Th1 shift fostering tumor immunity. In more recent studies, the cytopathic effects of HSV-1 were studied in murine squamous cell carcinoma cells. Infection was associated with release of several danger-associated molecular pattern (DAMP) factors, such as adenosine triphosphate (ATP) and high mobility group box 1 (HMGB1), and translocation of calreticulin to the cell membrane [46]. In this system, cell death was reduced by pan-caspase inhibitors, suggesting that activation of apoptotic and pyroptotic pathways may play a role in oncolytic virus cell death. Further research is needed to better understand how T-VEC kills tumor cells and induces anti-tumor immunity.
2.4 Preclinical studies
There have been several pre-clinical studies demonstrating that HSV-1 vectors, including the forerunner of T-VEC, could infect and lyse tumor cells in vitro and can mediate anti-tumor activity in vivo[52]. In early studies to evaluate whether the deletion of the ICP34.5 genes allowing preferential replication and lysis of tumor cells would allow enough time for transgene expression, an ICP34.5-deleted HSV-1 vector was engineered to express the marker gene β-galactosidase [53]. This vector was able to replicate in tissue culture and exhibited little toxicity following intracranial or foot-pad delivery to mice. Strong expression of the β-galactosidase transgene was observed in both the brain and dorsal root ganglia, suggesting that the deletion of ICP34.5 did not block transgene expression in HSV-1 and provided a strategy for HSV-1 oncolytic virus development with potent gene expression while avoiding neurotoxicity.
In an effort to improve the lytic activity of HSV-1, an ICP47 gene-deleted JS1 strain was selected based on enhanced lytic activity in vitro [45]. This had two important implications for therapeutic action of the virus. First, ICP47 normally functions to block antigen processing in HSV-infected cells limiting the host anti-viral immune responses. In the absence of ICP47, presumably a stronger immune response could be generated and this was hypothesized to include response against tumor-associated antigens. The second major change was that the deletion of ICP47 transitioned the US11 gene under an immediate-early gene promoter further enhancing viral replication. The ICP34.5−/ICP47− HSV-1 virus resulted in significant killing of directly injected established xenograft tumors in mice [45]. This attenuated HSV-1 vector was also able to preferentially eradicate breast tumor cell lines while not lysing hematopoetic cells in mixed culture studies. In this study, bone marrow aspirates from patients with breast cancer were also exposed to the attenuated HSV-1 virus ex vivo and preferential killing of metastatic breast cancer cells was reported[54]. To further augment the anti-tumor activity, T-VEC encodes the genes for GM-CSF, which resulted in the rejection of contralateral, uninjected A20 tumors in a mouse model. T-VEC treatment was associated with the generation of A20-specific cytotoxic T lymphocytes and re-challenge experiments demonstrated that mice who had rejected tumors with the HSV-1 construct had developed long-term memory to A20 but not other tumor cells[45].
The clearance of peripheral HSV-1 infections requires adaptive immunity, which is dependent on the function of dendritic cells (DCs). Since HSV-1 has been shown to both infect DCs and block their maturation, studies have been conducted to evaluate how DCs respond to HSV-1 infection. In one study, HSV-1 infection was found to induce a more mature phenotype in both directly infected human DCs and in uninfected bystander DCs[55]. This report demonstrated that type I interferons produced by the infected DCs were able to mature nearby uninfected DCs and induce the expression of IL-12. The investigators went on to show that this effect was dependent on HSV glycoprotein D binding to receptors on the surface of DCs and was mediated NF-kB and p38 pathway activation[55]. The authors concluded that while HSV-1 might inhibit DC maturation following infection, the release of type I interferons could counterbalance this effect and result in DC maturation, subsequently priming T cell immune responses.
There have been fewer studies of how HSV-1 oncolytic viruses induce immune responses in the setting of established cancers. In one study using an ICP34.5-deleted HSV-1 vector in a murine model of melanoma intra-cranial metastases, an increase in total CD4+ T cells and macrophages was seen upon the treatment [55]. The authors also reported the induction of melanoma-specific cytotoxic and proliferative T cell responses without evidence of an increase in neutralizing viral or tumor-specific antibody titers. This data is consistent with data in which human melanoma patients treated with T-VEC demonstrated the accumulation of MART-1-specific CD8+ T cells within the tumor microenvironment of injected lesions [21]. In this report, injected lesions were also associated with a decrease in CD4+FoxP3+ regulatory T cells and CD14+ myeloid-derived suppressor cells (MDSC). Collectively, these studies support the ability of attenuated HSV-1 vectors, such as T-VEC, to mediate anti-tumor activity and promote tumor-specific T cell immunity.
2.5 Barriers to oncolytic virus delivery and strategies to overcome them
Although oncolytic viruses are undergoing intense evaluation in melanoma and several other types of cancer, several barriers can limit the therapeutic effectiveness of these agents. For example, the presence of pre-existing neutralizing antibodies or various complement factors can inactivate potential virions prior to infecting tumor cells. The complexity of the tumor microenvironment, including hypoxia, decreased tissue vascularization, tumor necrosis, elevated interstitial fluid pressure, metabolic acidosis and dense extracellular matrix composition, impose significant physical obstacles that can block viral infection. Another unresolved issue is how various routes of administration influence viral infection of tumor cells [56–59]. While intra-tumoral delivery may bypass some of the physical and peripheral blood barriers, intra-venous delivery allows a more effective route for widespread viral dissemination and access to visceral and clinically hidden tumor tissue. The recognition of these challenges has led to several interesting strategies to overcome them and, subsequently, improved the therapeutic activity of oncolytic viruses. We will explore some of these strategies with a focus on those most relevant to clinical development.
The inactivation of oncolytic viruses by neutralizing antibodies depends on the type of virus used, the local distribution of the virus, and the incidence and prevalence of human exposure and efficiency of priming a humoral response to the native virus. The type of virus is important as some strains, such as HSV-1, are highly prevalent in the human population and pre-existing antiviral antibody titers are frequently found in cancer patients – who generally tend to be older. To date, the presence of neutralizing anti-HSV antibody titers has not correlated with clinical responses to T-VEC, and this may relate to the ability of these viruses to evade the detection by the immune system and the local delivery route used in current clinical trials[51]. In contrast to HSV-1, some viruses are not endemic and human exposure is limited, such as for rhabdovirus and some strains of coxsackievirus. Other viruses, such as adenovirus, measles virus, and polio virus, where vaccination or previous exposure and antibody titers are common, viral clearance may be reduced by serotype switching, polymer coating, pegylation or covalent conjugation of viral particles that block antibody binding[60–62]. In addition to modifying the viral particle, an alternative approach being explored is to suppress host anti-viral immunity by pre-treating patients with immunosuppressive agents, such as cyclophosphamide, which has demonstrated enhanced efficacy when given just before oncolytic HSV-1 treatment[63].
One of the roles of the complement system is opsonization and targeting of pathogenic microorganisms for destruction by phagocytosis. Components of the complement system can bind to some viruses, including HSV-2 and vaccinia virus, thus targeting the viruses for inactivation by the immune system [64–66]. Furthermore, the induction of anti-viral neutralizing antibodies may further enhance complement activation and amplify viral inactivation, especially when the viruses are administered through an intravenous route. In an interesting pre-clinical model, the use of a C3-specific complement inhibitor was shown to improve anti-tumor activity with an oncolytic virus, and this effect was seen with both intravenous and intra-tumoral virus delivery[66]. This general strategy is currently entering clinical trials.
Another barrier to oncolytic virus therapy is based on the extensive heterogeneity encountered across different tumor cells within an individual host. The anti-viral machinery that function to eradicate viral infection in normal cells is generally dysfunctional in malignant cells providing oncolytic viruses with a preferential replication advantage in cancer cells. Due to genomic heterogeneity, however, the anti-viral response largely mediated by type I interferons and interferon-responsive elements, may be partially restored limiting viral replication[67]. The application of small molecule viral sensitizers can simulate deleted viral virulence gene products and block interferon responses within cells to enhance oncolytic virus replication and lysis[67, 68]. The exact mechanisms through which these sensitizers restore viral replication are not fully understood.
In addition to the genomic heterogeneity in tumor cells, the tumor microenvironment is a complicated assortment of cells and soluble factors that may exhibit immune suppressive effects that can block anti-tumor immunity and potentially inhibit the therapeutic activity of oncolytic viruses. Cancer-associated fibroblasts (CAFs), for example, are altered by tumor cell-derived transforming growth factor β (TGF-β), which confers increased susceptibility to oncolytic virus infection compared to normal fibroblasts. Infected CAF secrete higher levels of fibroblast growth factor 2 (FGF2), which inhibits retinoic acid-inducible gene 1 (RIG-I) in tumor cells blocking viral detection [69]. Thus, reciprocal cross talk between cells within the tumor microenvironment can influence oncolytic virus replication and therapeutic effectiveness although further research is needed to more fully understand these complex interactions.
Other strategies to improve the therapeutic responses of oncolytic viruses include combination regimens in which viral therapy is combined with other approaches that target non-overlapping anti-tumor pathways. Such approaches have included immunotherapy, most notably with T cell checkpoint inhibitors (see below), targeted therapy, radiation therapy, adoptive T cell therapy, tumor vaccines, cytotoxic chemotherapy, and surgical intervention [70–72]. Another interesting strategy under consideration is the use of heterologous prime-boosting, in which different oncolytic viruses are used in tandem [73]. This approach may avoid the impact of neutralizing antibodies against one virus. Further investigations will likely focus on optimizing the best viral constructs to use and the best sequencing in which to deliver them. Finally, oncolytic virus therapy may be improved by alternate delivery methods, such as nanocarriers, cell carriers, and convection-enhanced delivery techniques, which attempt to shield the viral particles from the systemic circulation but allow efficient delivery directly into the tumor cell or tumor microenvironment[74–77].
3 Clinical Development of Oncolytic Viruses
The regulatory approval of T-VEC has generated significant interest in the development of oncolytic viruses as both monotherapy and as part of combination immunotherapy regimens for the treatment of a variety of human cancers. Table 1 lists a representative sample of current cancer clinical trials in progress. In this section we will focus on the development of T-VEC since this was the first oncolytic virus to achieve approval and describe basic patient management guidelines that have evolved from the clinical experience in caring for these patients. We will also briefly discuss some of the other oncolytic viruses in the clinic.
Table 1.
Representative Oncolytic Virus Clinical Trials in Progress (as of November 2016
| PRIMARY OUTCOME | DRUGS | PI | CONDITION |
|---|---|---|---|
| Best overall response rate (BORR) [Time Frame: at 24 weeks] [Designated as safety issue: No] | Replication-competent HSV-1 Oncolytic Virus, and Ipilimumab | Robert Andtbacka | Stage IIIB, Stage IIIC, or Stage IV Unresectable or Metastatic Malignant Melanoma |
| Maximally-tolerated dose (MTD) and/or maximum-feasible dose (MFD) of JX-594 administered by intravenous (IV) infusion [Time Frame: 4 weeks] [Designated as safety issue: Yes] | Recombinant Vaccinia GM-CSF; RAC VAC GM-CSF (JX-594) | David Kim, MD | Melanoma Lung Cancer Renal Cell Carcinoma |
| Safety and tolerability of two doses of Coxsackievirus A21 administered intratumourally. [Time Frame: Days 1, 3, 6, 8,10, 13, 17, 24, 38, 52, 87] [Designated as safety issue: Yes] | Coxsackievirus A21 | Mark Smithers Damien Thomson |
Stage IV Melanoma |
| Number of participants with treatment-related adverse events as defined by CTCAE v4.03. [Time Frame: 2.5 years] [Designated as safety issue: Yes] |
Biological: GL-ONC1 Biological: Eculizumab |
Kaitlyn Kelly | Solid Organ Cancers |
| Response rate for injected tumor(s) [Time Frame: Initial response assessment at 6 weeks] [Designated as safety issue: No] |
Biological: JX-594 | James Burke | Melanoma |
| Safety and tolerability (CTCAE version 4.0). [Time Frame: up to Week 16] [Designated as safety issue: Yes] Adverse events will be evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE version 4.0). |
Biological: TBI-1401(HF10) | Naoya Yamazaki | Solid Tumor |
| Phase I: Maximum Tolerated Dose (MTD)/Recommended Phase II Dose (RP2D) [Time Frame: Up to 6 months] [Designated as safety issue: Yes] MTD/RP2D of talimogene laherparepvec administered with neoadjuvant paclitaxel-doxorubicin/cyclophosphamide | Biological: Talimogene laherparepvec Drug: Paclitaxel |
Hatem Soliman | Breast Cancer Ductal Carcinoma Invasive Breast Carcinoma Invasive Ductal Breast Carcinoma |
| To determine whether intratumoral injection or intravenous infusions of HSV1716 is safe in adolescents and young adults with non-CNS solid tumors. [Time Frame: Dose limiting toxicities will be assessed at 28 days after injection of HSV1716.] [Designated as safety issue: | Biological: HSV1716 | Timothy Cripe | Rhabdomyosarcoma Osteosarcoma Ewing Sarcoma Soft Tissue Sarcoma |
| The incidence of dose-limiting toxicities (DLT) of intravenous pembrolizumab in combination with intratumoral CAVATAK will be assessed using CTCAE v. 4.0. [Time Frame: Up to 2 years] [Designated as safety issue: Yes] | Biological: CAVATAK Drug: Pembrolizumab |
Howard L Kaufman | Melanoma |
3.1 Clinical Development of T-VEC
The first human study of T-VEC was conducted in a phase I study of 30 patients with either refractory cutaneous or subcutaneous metastases from breast, gastrointestinal adenocarcinoma, malignant melanoma, or epithelial cancer of the head and neck [78]. Thirteen patients received a single-dose of virus at 106, 107 or 108 plaque-forming units (pfu)/mL. The virus was delivered by local intralesional injection instead of systemic delivery since it was expected that intratumoral injections would be better tolerated and would have increased tumor cell infectivity. In this study, T-VEC was well tolerated, and the most common adverse events were local inflammation, erythema, and flu-like symptoms. The local reactions were dose limiting at 107 pfu/ml in HSV-seronegative patients. Therefore, in the multi-dose phase of the study, seronegative patients were given 106 pfu/mL as the initial dose to allow seroconversion, followed three weeks later by multiple doses of up to 108 pfu/mL given every two weeks. This regimen with a low-dose priming at 106 pfu/mL followed by high-dose maintenance at 108 pfu was well tolerated. Although no objective responses were seen in this trial, evidence of biological activity was documented, including viral replication, GM-CSF expression and HSV antigen-associated necrosis in patients with melanoma, breast cancer, and head and neck cancer. Areas of necrosis with tumor cells were strongly associated with positive staining for HSV particles. In comparison, non-tumor tissue rarely stained positive for HSV proteins and no evidence of necrosis was found outside the tumor microenvironment [78]. Three patients were noted to have stable disease; six patients had tumors flattening at injected lesions and/or nearby uninjected lesions, and four patients had systemic immune responses. Of note, baseline HSV serology status did not shown any effect on response to T-VEC. Therefore, the dosing regimen established was an initial dose of 106 pfu/mL regardless of baseline serology, followed three weeks later by multiple doses of 108 pfu/mL every two weeks until confirmed disease progression or unacceptable toxicity.
A phase II multi-institutional trial was conducted in patients with unresectable or metastatic melanoma with stage IIIc or IV disease [55]. Fifty patients were enrolled in the study with stages IIIc (n=10), IVM1a (n=16), IVM1b (n=4), IVM1c (n=20). Most of the population was previously treated as 74% of the population had received prior therapy for advanced disease. Patients received initial intratumoral injections of 106 pfu/mL of T-VEC. The injected volume administered was up to 4 mL, depending on the size of the lesions using bidirectional clinical measurements. The initial injection was followed 3 weeks later with higher doses of up to 4 mL of 108 pfu/mL every 2 weeks for up to 24 treatments. Treatment was well tolerated with adverse effects limited primarily to transient flu-like symptoms, fever, chills, nausea and local injection site reactions. The primary endpoint was objective response rate assesse by the Response Evaluation Criteria in Solid Tumors (RECIST) criteria and was 26%, which included in overall disease burden responses (i.e. injected and uninjected lesions). Eight patients had a complete response and 5 patients had a partial response. Regression of local and distant tumors was observed. Of note, the duration of response was 16–40+ months from first T-VEC dose and the one-year overall survival rate was 58% and 24-month overall survival rate was 52% for all patients.
Based on the encouraging landmark survival analyses, durability of responses, and low toxicity from the phase I and II studies, a phase III trial testing T-VEC (formerly known OncovexGM-CSF) in melanoma was designed. This trial, known as the OncovexGM-CSF Pivotal Trial in Melanoma (OPTiM) was an open-label study in which 436 patients with unresected stage IIIB to IV melanoma were randomized in a 2:1 fashion to intralesional T-VEC therapy or subcutaneous GM-CSF. The T-VEC group received initial dose of 106 pfu/mL, followed by a 108-pfu/mL booster at 3 weeks and then 108 pfu/mL every 2 weeks for 24 total doses. In this clinical trial, all melanoma lesions could be injected including any new lesions that appeared after regression of established disease. The control group received subcutaneous recombinant GM-CSF at 125 μg/m2 with 14 day on and 14 days off for 1 year. This control was selected based on retrospective data supporting a clinical benefit with GM-CSF in stage III and IV resected melanoma, the immunotherapy mechanism of action for both agents and the inclusion of GM-CSF in both treatment cohorts. The primary endpoint measured was durable response rate (DRR) defined as an objective response (partial or complete) based on modified WHO criteria lasting continuously for ≥ 6 months and beginning within the first 12 months of treatment. Significant secondary end points included were overall survival (OS) and objective response rate (ORR). Primary end point of DRR was achieved in 16.3 % (95% CI of 12.1 to 20.5%) in the T-VEC group compared to 2.1% (95% CI, 0% to 4.5%) in GM-CSF group, with an odds ratio of 8.9 (p<0.001). In the T-VEC group, a higher ORR of 26.4 % compared to 5.7% in CM-CSF group was reported. Median overall survival was 23.3 months (95% CI, 19.5 to 29.6 months) with T-VEC and 18.9 months (95% CI, 16.0 to 23.7 months) with GM-CSF (hazard ratio [HR]=0.79; 95%CI = 0.62–1.00; P = 0.051). Eleven percent of patients had complete response in the T-VEC group compared to <1% in GM-CSF group. The most common adverse events with T-VEC were fatigue, chills, pyrexia, nausea and local injection site reactions. Cellulitis occurred in 2.1% of patients, and this was the only grade 3 or 4 AE that occurred in ≥2% of T-VEC treated patients. There were no fatal treatment-related events reported [79].
Further analysis at 3 years from last randomization with a median follow-up of 49 months (range, 37–63 months) showed similar results to the primary analysis with only one 1 additional event in the T-VEC group. Five-year survival in the T-VEC arm was 33.4% (95% CI, 27.7–39.2). Based on exploratory analyses, it was suggested that T-VEC effect had an enhanced effect in patients with stage IIIB/C/IVM1a melanoma and in patients with treatment-naïve disease [21]. Based on these results, T-VEC became the first oncolytic virus therapy to demonstrated significant clinical benefit in a phase III prospective, randomized clinical trial. These results led to T-VEC approval as monotherapy for the treatment of melanoma in patients with unresectable cutaneous, subcutaneous or nodal lesions after initial surgery by the U.S. Food and Drug Administration and the Australian Therapeutic Goods Administration in 2015. T-VEC was also approved for the treatment of stage III and IV M1a melanoma by the European Medicines Agency.
3.2 Clinical management of patients on T-VEC
T-VEC is the first-in-class oncolytic virus approved for the treatment of cancer. Therapy is associated with several important management considerations, including the need for appropriate storage and handling of the agent as T-VEC is a live, replicating virus, and concerns related to virus administration, biosafety control and prevention of household contact transmission. The virus needs to be stored at −70°C or colder freezer and prepared in a sterile biosafety cabinet. The virus comes in vials with either a 106 pfu/ml dose (yellow) or 108 pfu/ml dose (blue). Patients need to be seen in the clinic prior to dosing at each visit so that accessible tumor can be measured and the volume of virus determined (see Fig. 2). The dosing schedule is shown in Table 1. Lesions for injection are chosen based on size in longest diameter (generally injecting the largest lesion first), temporal appearance of lesions (generally injecting the most recent lesions first) and location (avoiding lesions located near mucosal surfaces or large blood vessels). For example, if a patient presents with a 3 centimeter left leg cutaneous melanoma, a 1.5 centimeter abdominal wall soft tissue melanoma and a 5 millimeter right carotid neck lymph node mass, the patients would receive T-VEC at 2 mL to the left leg lesion and 0.5 mL to the abdominal wall lesion (total volume 2.5 mL). The neck lesion may be left alone because of the proximity to the carotid artery and jugular vein but an ultrasound can also be used to isolate deeper lesions and monitor needle injection.
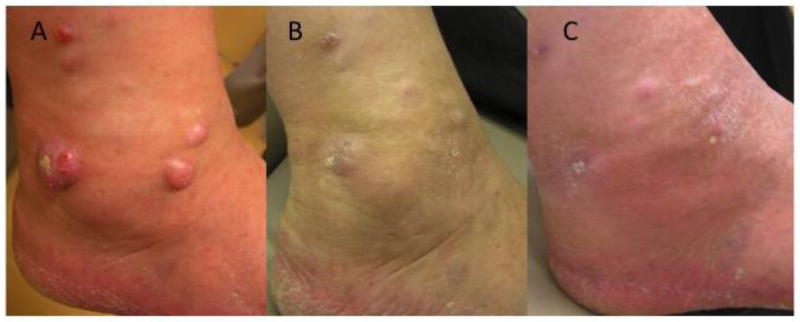
Figure 2.
Photograph of a melanoma patient (A) before; (B) 6 months after starting treatment with Talimogene laherparepvec (T-VEC); and (C) 9 months after starting treatment. The patient had multiple cutaneous and soft tissue metastases following amputation of a left great toe melanoma and failing systemic chemotherapy. A biopsy at 9 months confirmed the absence of viable melanoma cells.
The clinical management of patients is improved with standard operating procedures that can be used to instruct ambulatory staff and patients. At our center, we utilize a single room for all T-VEC injections on a given day. Patients are initially measured and then treated in the same room. We utilize universal precautions requiring healthcare providers administering the virus to have appropriate education and wear gloves, gowns and eye protection. The virus is delivered in individual syringes for injection from the pharmacy in a double enclosure container. Healthcare providers are instructed to wash their hands before and after handling the virus. After verifying the patient name, date, virus dose and volume, the injection site is wiped with alcohol. Although local anesthetic can be used, this is often not necessary. The virus is injected in a four quadrant fan-like manner to ensure wide distribution of the virus. Following the injection, the site is massaged gently for several seconds with a 2 x 2 or 4 x 4 dry gauze to promote viral dispersal and then covered with dry gauze and Tegaderm™ dressing. Patients are given extra dressings and a plastic biohazard bag for soiled dressings to take home with them. They are also instructed to wash hands prior to and after touching the dressings. They can bring any waste back to the clinic for disposal in the biohazard bags.
To date, there have been no cases of documented household contact transmission with T-VEC. It is important to remember that native HSV-1 is ubiquitous in the environment and early reports suggests that nearly 90% of patients have been exposed to HSV-1 by the time they are 70 years old[30]. T-VEC, like other HSV viruses, is susceptible to most anti-viral agents, such as acyclovir and famciclovir. Healthcare workers should receive general education about herpes virus handling when working with T-VEC. There have been three healthcare worker exposures in two individuals, all related to accidental needle stick injury. In one case, a herpetic whitlow developed at the injection site but was cleared by acyclovir treatment. Nonetheless, it is important to train workers in proper preparation, transportation and handling techniques and needle lock systems should be used whenever possible to avoid needle stick injuries. Anti-virals should be started immediately in the event of an accidental exposure. Although spills are unlikely with the small volumes of virus being used, the clinic rooms and any accidental spills should be cleaned with a 10% bleach solution. Physicians using T-VEC may also want to notify their institutional biosafety and/or hospital infection committees to ensure familiarity with the proper handling of the agent.
3.3 Other oncolytic viruses in clinical development
Although there are a large number of oncolytic viruses currently in clinical development (see Table 1), we will highlight several that have received more clinical attention. Pelareorep (Reolysin®) is a formulation consisting of a live replication-competent naturally occurring reovirus, type 3 Dearing strain (RT3D). Reovirus is ubiquitous and is found in almost all parts of the world. The genome of reovirus is present in the form of a linear double stranded RNA (dsRNA) with 10 segmental repeats[80]. Several clinical and preclinical studies have confirmed the ability of reovirus to selectively lyse tumor cells. In a preclinical study Coffey et al. have shown the ability of reovirus to selectively target tumors with activated RAS pathway, thus the tumor selectivity of reovirus can be attributed to altered cell signaling mechanisms[81]. Several clinical trials have shown promising results using reovirus alone or in combination to treat a variety of tumors including non-small cell lung cancer, pancreatic cancer, and extra-cranial solid tumors in children[82–84]. Reovirus binds to the cell by interacting with the sialic acid receptors present on the cell surface[85]. The interaction of reovirus with sialic acid residues might not be sufficient for the virus to internalize and it is now becoming clear that the epidermal growth factor receptor (EGFR) plays a prominent role in reovirus infection[86]. Cells that are susceptible to reovirus allow the synthesis of viral proteins and thereby enhance viral replication and infectivity. Cells that are not susceptible to infection will halt viral translation, and the inactivation of viral protein synthesis is dependent on the activation of PKR, thus promoting abortive replication in normal cells[87]. Reovirus has a biphasic mechanism of action. Tumor cells infected with virus may undergo a direct form of cell death via viral oncolysis. In addition, reoviruses have the ability to activate dendritic cells and trigger pro-inflammatory cytokine secretion, which can further attract NK and T cells to the tumor microenvironment[88].
Coxsackievirus A21 (CAV21; Cavatak™) is an enterovirus that is currently in clinical development based on the oncolytic properties of the native virus. CAV21 is a naturally occurring single-stranded RNA virus that enters cells via intercellular adhesion molecule (ICAM-1) present on the cell surface. A secondary protein named decay-accelerating factor (DAF) is responsible for facilitating viral attachment, internalization, and subsequent tumor cell lysis. Thus, the tumor cell selective infectivity of CAV21 is attributed to the up-regulation of ICAM-1 and the presence of decay-accelerating factor (DAF). Several malignant tumors including melanoma show high expression of these two proteins[89–91]. After the preliminary infection of tumor cells with CAV21, the virus replicates rapidly and produces progeny that can infect other metastatic lesions. Preliminary results from a multi-institutional phase II study in patients with accessible stage IIIC-IV melanoma demonstrated encouraging response rates and immune-related progression-free survival at 6 months in 22 of 57 patients (38.5%). The adverse events included low-grade constitutional and local injection site inflammation[92].
Pexastimogene devacirepvec (JX-594) is an immunotherapeutic replication-competent vaccinia virus encoding GM-CSF. JX-594 is currently being studied in clinical trials for its oncolytic ability to destroy cancer cells. JX-594 can destroy cancer cells via viral lysis and it can induce immune responses augmented by GM-CSF expression. JX-594 has been further genetically modified to augment anti-tumor responses by the deletion of the viral thymidine kinase gene, which enables selective replication in tumor cells[93]. A study by Bell et al. confirmed the ability of JX-594 to lyse cancer cells derived from the NCI60 panel [93]. In this study, the investigators also confirmed the replicative ability of JX-594 in primary tumor biopsy specimens. Altered cell signaling pathways may promote JX-594 selective replication in tumor cells[94]. A phase 1b study of JX-594 was conducted in 15 patients with treatment-refractory colorectal cancer[94]. JX-594 was administered by intravenous delivery every 2 weeks at dose levels of 1 × 106, 1 × 107, or 3 × 107 plaque-forming units (pfu)/kilogram. All patients injected with JX-594 were evaluated for safety and efficacy. Most patients experienced mild pyrexia and/or chills after infusion and no patients were discontinued from this study due to adverse side effects. Ten patients (67%) had radiographic stable disease and the authors concluded that further studies of JX-594 alone or in combination with cytotoxic chemotherapy were warranted[94].
4. Future Directions for Oncolytic Virus Development
4.1 T-VEC clinical development
T-VEC is the first oncolytic virus to be approved for the treatment of cancer, specifically advanced melanoma. Further studies of T-VEC will include combination clinical trials in melanoma, timing of T-VEC delivery in melanoma (e.g. neoadjuvant delivery), extension of T-VEC to other types of cancer and a focus on identifying predictive biomarkers of response. Given the tolerable safety profile of T-VEC, it is an interesting agent to use in combination approaches. The ability of viral infection to induce an interferon response, promote necrotic cell death and release of DAMPs, T-VEC is appealing as a combination agent with other forms of tumor immunotherapy. Studies combining T-VEC with immune T cell checkpoint inhibitors targeting cytotoxic T lymphocyte antigen 4 (CTLA-4) targeted and programmed cell death 1 (PD-1) are already underway[95]. Other therapeutic combinations of interest might also include radiation therapy, MAPK signaling pathway targeted therapy and adoptive T cell therapy[96]. To date, the combination trials with T cell checkpoints are the most advanced.
The combination of T-VEC with ipilimumab is being evaluated in an open-label, multi-institutional phase Ib/II study for safety and efficacy in patients with previously untreated, unresected stage IIIb-IV melanoma. In phase Ib, patients were primed with T-VEC by receiving an initial T-VEC dose of 106 PFU/mL, followed by T-VEC 108 PFU/mL on week 4 and then every two weeks [70]. Patients received ipilimumab starting on week 6 at 3 mg/kg every 3 weeks for total of 4 infusions. The primary endpoint of the phase Ib portion of the trial was safety with secondary endpoint of objective response rate. The results were reported after a median follow-up of 20 months and included 19 patients in the safety analysis. There was no dose-limiting toxicity reported while grade 3–4 treatment-related adverse events were documented in 26.3% of the participating subjects. These included 15.8% attributed to previously reported T-VEC adverse events and 21.1% attributed to typical ipilimumab-related adverse events. The objective response rate was 50% with 44% achieving durable responses lasting 6 months or greater. The median duration of T-VEC treatment was 13.3 weeks and the 18-month overall survival was 67%. The phase II portion of the trial randomized 200 melanoma patients to receive the combination or ipilimumab alone and results are anticipated soon[97].
T-VEC is also being studied in combination with pembrolizumab, a humanized anti-PD-1 monoclonal antibody. In a Phase 1b study designed to evaluate the safety, efficacy and tolerability of T-VEC and pembrolizumab, 21 melanoma patients were enrolled. The primary objective was to assess dose-limiting toxicities of the combination. In 16 evaluable patients, an objective response rate of 56.3% was seen and a disease control rate of 68.8% was reported[98]. Based on these encouraging initial results, a larger randomized phase III study was initiated to assess combination T-VEC and pembrolizumab versus pembrolizumab alone. The primary outcome measures of the phase III study will be progression-free and overall survival with up to 24 months of treatment allowed [98].
The timing of T-VEC delivery is also being evaluated in a randomized clinical trial exploring the role of neoadjuvant administration. A multicenter, randomized, open-label phase II trial of neoadjuvant T-VEC in patients who have completely resectable stage IIIB, IIIC, or IVM1a melanoma is now underway. In this study, patients will be randomized 1:1 to either immediate resection or 6 doses of neoadjuvant T-VEC followed by resection. The primary endpoint of the study will be recurrence-free survival [99]. This trial should allow a more comprehensive exploration of changes in the tumor microenvironment following T-VEC treatment and may identify the extent of immune system activation with T-VEC. The issue of whether T-VEC induces local or systemic anti-tumor immunity is also an unresolved issue. While more locally injected lesions regressed, there was evidence for regression of visceral melanoma in the OPTiM trial. Can T-VEC be delivered to visceral disease and will this improve systemic responses? These questions will be answered in the first visceral injection trial in which T-VEC will be delivered to primary and metastatic hepatic tumors through interventional radiology guided injection. This trial has recently started accrual.
Another important area for further investigation will be the expansion of T-VEC to other types of cancer. High priorities might be other tumors that are easily accessible for injection, such as non-melanoma skin cancer, soft tissue sarcoma, head and neck cancers and chest wall breast cancers. Such clinical trials of T-VEC monotherapy, or as part of a logical combination approach, are being actively pursued. Recent data suggesting that local radiation exposure can stimulate a systemic immune response leading to eradication of tumors through a so-called abscopal effect[100]. Thus, a study of localized radiation therapy and T-VEC is being tested in patients with advanced soft tissue sarcoma[101]. T-VEC currently is also being studied in other types of skin cancer, head and neck cancer, pancreatic cancer, rectal cancer, and as mentioned primary hepatocellular carcinoma and solid tumor liver metastases.
4.2 Future priorities for other oncolytic viruses in clinical development
Finally, the identification of predictive biomarkers would greatly accelerate progress and allow for better subject selection in high-priority clinical trials. To date, no biomarkers have been validated for T-VEC or any other oncolytic virus strategies. Interestingly, many of the putative biomarkers under study in the field may be appropriate for T-VEC. PD-L1 expression within the tumor microenvironment, the presence of tumor-infiltrating lymphocytes - especially at the invasive tumor margin, and interferon-gamma gene signature profiles are likely induced by T-VEC. The notion that some tumors may be “hot” as evidenced by T cell infiltrates and local interferon or PD-L1 expression has been advanced although why some tumors may be “hot” in the first place is not known. Perhaps T-VEC can be used to induce a “hot” tumor phenotype and then additional immunotherapy agents could be added to the treatment regimen. Other biomarkers may exist within the tumor cells since dysrgeulation of the mitogen activated protein kinase (MAPK) pathway as well as the interferon signaling pathway have been implicated in regulating oncolytic virus infection, replication and cell lysis. Further investigation is needed to better define these and other potential biomarkers before they can be recommended in clinical practice for targeted therapy in melanoma, expansion to other types of accessible cancers, visceral injections, and neoadjuvant delivery approaches. The identification of predictive biomarkers for therapeutic responses to oncolytic viruses will also be a high priority and may allow better patient selection and clinical management.
While the promise of oncolytic viruses has provided a new therapeutic option for patients with melanoma, there continue to be several challenges, including an incomplete knowledge of the barriers to viral replication within the tumor microenvironment, the optimal mechanism for avoiding premature immunologic viral clearance and a lack of data on how oncolytic viruses mediate systemic anti-tumor immune responses. In addition, the clinical implementation of oncolytic viruses requires special attention to storage, handling and administration of live, replication competent viruses. The adoption of local standard operating procedures for virus handling and spill management, staff education and training, and the use of anti-viral medications in case of inadvertent exposure, can be useful for the safe management of patients and healthcare workers. The safety profile and ease of administration makes oncolytic viruses good treatment options for patients with advanced locoregional disease, older patients with significant co-morbid conditions and patients unable to tolerate other forms of systemic therapy. Further studies of the basic mechanisms underlying the therapeutic activity, identification of biomarkers and combination clinical trials are high priorities for the field and will help expand the application of oncolytic viruses for patients with melanoma and, perhaps, other cancers as well.
Table 2.
Table of T-VEC injection volume determined by the longest diameter of the lesion(s) with up to a maximum of 4 mL of virus allowed for deliver at each visit.
| Tumor Size (longest dimension) | Maximum Injection Volume |
|---|---|
| > 5.0 cm | 4.0 mL |
| > 2.5 cm to 5.0 cm | 2.0 mL |
| > 1.5 cm to 2.5 cm | 1.0 mL |
| > 0.5 cm to 1.5 cm | 0.5 mL |
| ≤ 0.5 cm | 0.1 mL |
Key Points.
Oncolytic viruses are a new class of agents that likely work through two distinct mechanisms that include the direct killing of tumor cells and the induction of an immune response against injected tumors.
The first oncolytic virus, Talimogene laherparepvec (T-VEC) is a modified herpes virus that was recently approved for the treatment of melanoma by regulatory agencies in the United States, Australia and Europe.
Oncolytic viruses have an excellent safety profile and can be genetically manipulated to prevent toxicity, enhance immune responses, and promote tumor rejection.
Oncolytic viruses can be combined with immunotherapy, chemotherapy, targeted therapy and radiation therapy to improve the efficacy of both drugs.
Local and systemic barriers to oncolytic virus therapeutic activity are incompletely understood and predictive biomarkers to identify susceptible patents are high priorities for future research.
Acknowledgments
Funding
This work was supported, in part, by grant UM1 CA 186716-01 from the National Cancer Institute to HLK.
Footnotes
Compliance with Ethical Standards
Conflicts of Interest
Dr. Kaufman has served on advisory boards for Amgen, Celldex, EMD Serono, Merck, Prometheus and Sanofi. He is a member of the Merck Speaker’s Bureau but does not received direct compensation for this activity. Mr. Bommareddy, Dr. Patel and Dr. Hossain have no conflicts to declare.
References
- 1.Brunssen A, et al. Impact of skin cancer screening and secondary prevention campaigns on skin cancer incidence and mortality: A systematic review. J Am Acad Dermatol. 2016 doi: 10.1016/j.jaad.2016.07.045. [DOI] [PubMed] [Google Scholar]
- 2.Feng Z, Zhang Z, Wu XC. Lifetime risks of cutaneous melanoma by histological subtype and race/ethnicity in the United States. J La State Med Soc. 2013;165(4):201–8. [PubMed] [Google Scholar]
- 3.Coit DG, et al. NCCN Guidelines Insights: Melanoma, Version 3.2016. J Natl Compr Canc Netw. 2016;14(8):945–58. doi: 10.6004/jnccn.2016.0101. [DOI] [PubMed] [Google Scholar]
- 4.Radny P, et al. Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases. Br J Cancer. 2003;89(9):1620–6. doi: 10.1038/sj.bjc.6601320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lau PK, Ascierto PA, McArthur G. Melanoma: the intersection of molecular targeted therapy and immune checkpoint inhibition. Curr Opin Immunol. 2016;39:30–8. doi: 10.1016/j.coi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Redmond KL, et al. Overcoming Resistance to Targeted Therapies in Cancer. Semin Oncol. 2015;42(6):896–908. doi: 10.1053/j.seminoncol.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19(19):5300–9. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robert C, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 10.Robert C, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 11.Larkin J, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kluger HM, et al. Characterization of PD-L1 Expression and Associated T-cell Infiltrates in Metastatic Melanoma Samples from Variable Anatomic Sites. Clin Cancer Res. 2015;21(13):3052–60. doi: 10.1158/1078-0432.CCR-14-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taube JM, et al. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin Cancer Res. 2015;21(17):3969–76. doi: 10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue H, et al. Intratumoral expression levels of PD-L1, GZMA, and HLA-A along with oligoclonal T cell expansion associate with response to nivolumab in metastatic melanoma. Oncoimmunology. 2016;5(9):e1204507. doi: 10.1080/2162402X.2016.1204507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gajewski TF, et al. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Curr Opin Immunol. 2011;23(2):286–92. doi: 10.1016/j.coi.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaretsky JM, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016;375(9):819–29. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14(9):642–62. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douglas JT, et al. Efficient oncolysis by a replicating adenovirus (ad) in vivo is critically dependent on tumor expression of primary ad receptors. Cancer Res. 2001;61(3):813–7. [PubMed] [Google Scholar]
- 19.Goldufsky J, et al. Oncolytic virus therapy for cancer. Oncolytic Virother. 2013;2:31–46. doi: 10.2147/OV.S38901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andtbacka RHCF, Amatruda T, Senzer N, Chesney J, Delman K, et al. Final planned overall survival (OS) from OPTiM, a randomized phase III trial of talimogene laherparepvec (T-VEC) versus GM-CSF for the treatment of unresected stage IIIB/C/IV melanoma. J Immunother Cancer. 2014;(suppl3):P263. [Google Scholar]
- 21.Kaufman HL, et al. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17(3):718–30. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 22.Huang B, et al. Synergistic anti-tumor effects between oncolytic vaccinia virus and paclitaxel are mediated by the IFN response and HMGB1. Gene Ther. 2011;18(2):164–72. doi: 10.1038/gt.2010.121. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto S, et al. Coxsackievirus B3 is an oncolytic virus with immunostimulatory properties that is active against lung adenocarcinoma. Cancer Res. 2012;72(10):2609–21. doi: 10.1158/0008-5472.CAN-11-3185. [DOI] [PubMed] [Google Scholar]
- 24.Liikanen I, et al. Serum HMGB1 is a predictive and prognostic biomarker for oncolytic immunotherapy. Oncoimmunology. 2015;4(3):e989771. doi: 10.4161/2162402X.2014.989771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koks CA, et al. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int J Cancer. 2015;136(5):E313–25. doi: 10.1002/ijc.29202. [DOI] [PubMed] [Google Scholar]
- 26.Takasu A, et al. Immunogenic cell death by oncolytic herpes simplex virus type 1 in squamous cell carcinoma cells. Cancer Gene Ther. 2016;23(4):107–13. doi: 10.1038/cgt.2016.8. [DOI] [PubMed] [Google Scholar]
- 27.West EE, et al. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest. 2013;123(6):2604–15. doi: 10.1172/JCI67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–70. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu F, et al. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988–1994. J Infect Dis. 2002;185(8):1019–24. doi: 10.1086/340041. [DOI] [PubMed] [Google Scholar]
- 31.Peters C, Rabkin SD. Designing Herpes Viruses as Oncolytics. Mol Ther Oncolytics. 2015:2. doi: 10.1038/mto.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coen DM, et al. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci U S A. 1989;86(12):4736–40. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martuza RL, et al. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252(5007):854–6. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein DJ, Weller SK. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988;62(1):196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A. 1997;94(3):843–8. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chou J, et al. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990;250(4985):1262–6. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 37.Mineta T, et al. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1(9):938–43. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 38.MacLean AR, et al. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the ‘a’ sequence. J Gen Virol. 1991;72(Pt 3):631–9. doi: 10.1099/0022-1317-72-3-631. [DOI] [PubMed] [Google Scholar]
- 39.McKie EA, et al. Selective in vitro replication of herpes simplex virus type 1 (HSV-1) ICP34.5 null mutants in primary human CNS tumours--evaluation of a potentially effective clinical therapy. Br J Cancer. 1996;74(5):745–52. doi: 10.1038/bjc.1996.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kesari S, et al. Therapy of experimental human brain tumors using a neuroattenuated herpes simplex virus mutant. Lab Invest. 1995;73(5):636–48. [PubMed] [Google Scholar]
- 41.Randazzo BP, et al. Treatment of experimental intracranial murine melanoma with a neuroattenuated herpes simplex virus 1 mutant. Virology. 1995;211(1):94–101. doi: 10.1006/viro.1995.1382. [DOI] [PubMed] [Google Scholar]
- 42.McKie EA, et al. Histopathological responses in the CNS following inoculation with a non-neurovirulent mutant (1716) of herpes simplex virus type 1 (HSV 1): relevance for gene and cancer therapy. Neuropathol Appl Neurobiol. 1998;24(5):367–72. doi: 10.1046/j.1365-2990.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- 43.Rampling R, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7(10):859–66. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 44.MacKie RM, Stewart B, Brown SM. Intralesional injection of herpes simplex virus 1716 in metastatic melanoma. Lancet. 2001;357(9255):525–6. doi: 10.1016/S0140-6736(00)04048-4. [DOI] [PubMed] [Google Scholar]
- 45.Liu BL, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10(4):292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 46.Cassady KA, Gross M, Roizman B. The herpes simplex virus US11 protein effectively compensates for the gamma1(34.5) gene if present before activation of protein kinase R by precluding its phosphorylation and that of the alpha subunit of eukaryotic translation initiation factor 2. J Virol. 1998;72(11):8620–6. doi: 10.1128/jvi.72.11.8620-8626.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farassati F, Yang AD, Lee PW. Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat Cell Biol. 2001;3(8):745–50. doi: 10.1038/35087061. [DOI] [PubMed] [Google Scholar]
- 48.Pan W, et al. Utilizing ras signaling pathway to direct selective replication of herpes simplex virus-1. PLoS One. 2009;4(8):e6514. doi: 10.1371/journal.pone.0006514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim F, et al. Biosafety of gene therapy vectors derived from herpes simplex virus type 1. Curr Gene Ther. 2013;13(6):478–91. doi: 10.2174/156652321306140103224550. [DOI] [PubMed] [Google Scholar]
- 50.Atherton MJ, Lichty BD. Evolution of oncolytic viruses: novel strategies for cancer treatment. Immunotherapy. 2013;5(11):1191–206. doi: 10.2217/imt.13.123. [DOI] [PubMed] [Google Scholar]
- 51.Hu JC, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12(22):6737–47. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 52.Shen Y, Nemunaitis J. Herpes simplex virus 1 (HSV-1) for cancer treatment. Cancer Gene Ther. 2006;13(11):975–92. doi: 10.1038/sj.cgt.7700946. [DOI] [PubMed] [Google Scholar]
- 53.Coffin RS, et al. Gene delivery to the central and peripheral nervous systems of mice using HSV1 ICP34.5 deletion mutant vectors. Gene Ther. 1996;3(10):886–91. [PubMed] [Google Scholar]
- 54.Hu JC, et al. A novel HSV-1 virus, JS1/34.5-/47-, purges contaminating breast cancer cells from bone marrow. Clin Cancer Res. 2006;12(22):6853–62. doi: 10.1158/1078-0432.CCR-06-1228. [DOI] [PubMed] [Google Scholar]
- 55.Andtbacka RHIKH, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;2014.58:3377. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 56.Shen BH, Hermiston TW. Effect of hypoxia on Ad5 infection, transgene expression and replication. Gene Ther. 2005;12(11):902–10. doi: 10.1038/sj.gt.3302448. [DOI] [PubMed] [Google Scholar]
- 57.Shen BH, Bauzon M, Hermiston TW. The effect of hypoxia on the uptake, replication and lytic potential of group B adenovirus type 3 (Ad3) and type 11p (Ad11p) Gene Ther. 2006;13(12):986–90. doi: 10.1038/sj.gt.3302736. [DOI] [PubMed] [Google Scholar]
- 58.Mok W, Boucher Y, Jain RK. Matrix metalloproteinases-1 and -8 improve the distribution and efficacy of an oncolytic virus. Cancer Res. 2007;67(22):10664–8. doi: 10.1158/0008-5472.CAN-07-3107. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen A, Ho L, Wan Y. Chemotherapy and Oncolytic Virotherapy: Advanced Tactics in the War against Cancer. Front Oncol. 2014;4:145. doi: 10.3389/fonc.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tesfay MZ, et al. PEGylation of vesicular stomatitis virus extends virus persistence in blood circulation of passively immunized mice. J Virol. 2013;87(7):3752–9. doi: 10.1128/JVI.02832-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morrison J, et al. Virotherapy of ovarian cancer with polymer-cloaked adenovirus retargeted to the epidermal growth factor receptor. Mol Ther. 2008;16(2):244–51. doi: 10.1038/sj.mt.6300363. [DOI] [PubMed] [Google Scholar]
- 62.O’Riordan CR, et al. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum Gene Ther. 1999;10(8):1349–58. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- 63.Fulci G, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci U S A. 2006;103(34):12873–8. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wakimoto H, et al. The complement response against an oncolytic virus is species-specific in its activation pathways. Mol Ther. 2002;5(3):275–82. doi: 10.1006/mthe.2002.0547. [DOI] [PubMed] [Google Scholar]
- 65.Ikeda K, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5(8):881–7. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 66.Evgin L, et al. Complement inhibition prevents oncolytic vaccinia virus neutralization in immune humans and cynomolgus macaques. Mol Ther. 2015;23(6):1066–76. doi: 10.1038/mt.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diallo JS, et al. A high-throughput pharmacoviral approach identifies novel oncolytic virus sensitizers. Mol Ther. 2010;18(6):1123–9. doi: 10.1038/mt.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berghauser Pont LM, et al. In vitro screening of clinical drugs identifies sensitizers of oncolytic viral therapy in glioblastoma stem-like cells. Gene Ther. 2015;22(12):947–59. doi: 10.1038/gt.2015.72. [DOI] [PubMed] [Google Scholar]
- 69.Ilkow CS, et al. Reciprocal cellular cross-talk within the tumor microenvironment promotes oncolytic virus activity. Nat Med. 2015;21(5):530–6. doi: 10.1038/nm.3848. [DOI] [PubMed] [Google Scholar]
- 70.Puzanov I, et al. Talimogene Laherparepvec in Combination With Ipilimumab in Previously Untreated, Unresectable Stage IIIB-IV Melanoma. J Clin Oncol. 2016;34(22):2619–26. doi: 10.1200/JCO.2016.67.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rehman H, et al. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J Immunother Cancer. 2016;4:53. doi: 10.1186/s40425-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rajani K, et al. Combination Therapy With Reovirus and Anti-PD-1 Blockade Controls Tumor Growth Through Innate and Adaptive Immune Responses. Mol Ther. 2016;24(1):166–74. doi: 10.1038/mt.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ilett E, et al. Prime-boost using separate oncolytic viruses in combination with checkpoint blockade improves anti-tumor therapy. Gene Ther. 2016 doi: 10.1038/gt.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hart SL. Multifunctional nanocomplexes for gene transfer and gene therapy. Cell Biol Toxicol. 2010;26(1):69–81. doi: 10.1007/s10565-009-9141-y. [DOI] [PubMed] [Google Scholar]
- 75.Mader EK, et al. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin Cancer Res. 2009;15(23):7246–55. doi: 10.1158/1078-0432.CCR-09-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muthana M, et al. Use of macrophages to target therapeutic adenovirus to human prostate tumors. Cancer Res. 2011;71(5):1805–15. doi: 10.1158/0008-5472.CAN-10-2349. [DOI] [PubMed] [Google Scholar]
- 77.Debinski W, Tatter SB. Convection-enhanced delivery to achieve widespread distribution of viral vectors: Predicting clinical implementation. Curr Opin Mol Ther. 2010;12(6):647–53. [PubMed] [Google Scholar]
- 78.Senzer NN, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27(34):5763–71. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 79.Aris M, Barrio MM. Combining immunotherapy with oncogene-targeted therapy: a new road for melanoma treatment. Front Immunol. 2015;6:46. doi: 10.3389/fimmu.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stanley NF. Reovirus--a ubiquitous orphan. Med J Aust. 1961;48(2):815–8. [PubMed] [Google Scholar]
- 81.Coffey MC, et al. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282(5392):1332–4. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 82.Villalona-Calero MA, et al. Oncolytic reovirus in combination with chemotherapy in metastatic or recurrent non-small cell lung cancer patients with KRAS-activated tumors. Cancer. 2016;122(6):875–83. doi: 10.1002/cncr.29856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mahalingam D, et al. The combination of intravenous Reolysin and gemcitabine induces reovirus replication and endoplasmic reticular stress in a patient with KRAS-activated pancreatic cancer. BMC Cancer. 2015;15:513. doi: 10.1186/s12885-015-1518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kolb EA, et al. A phase I trial and viral clearance study of reovirus (Reolysin) in children with relapsed or refractory extra-cranial solid tumors: a Children’s Oncology Group Phase I Consortium report. Pediatr Blood Cancer. 2015;62(5):751–8. doi: 10.1002/pbc.25464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Danthi P, et al. Reovirus receptors, cell entry, and proapoptotic signaling. Adv Exp Med Biol. 2013;790:42–71. doi: 10.1007/978-1-4614-7651-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Strong JE, Lee PW. The v-erbB oncogene confers enhanced cellular susceptibility to reovirus infection. J Virol. 1996;70(1):612–6. doi: 10.1128/jvi.70.1.612-616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strong JE, et al. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17(12):3351–62. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prestwich RJ, et al. Reciprocal human dendritic cell-natural killer cell interactions induce antitumor activity following tumor cell infection by oncolytic reovirus. J Immunol. 2009;183(7):4312–21. doi: 10.4049/jimmunol.0901074. [DOI] [PubMed] [Google Scholar]
- 89.Au GG, et al. Oncolysis of vascular malignant human melanoma tumors by Coxsackievirus A21. Int J Oncol. 2005;26(6):1471–6. doi: 10.3892/ijo.26.6.1471. [DOI] [PubMed] [Google Scholar]
- 90.Skelding KA, Barry RD, Shafren DR. Systemic targeting of metastatic human breast tumor xenografts by Coxsackievirus A21. Breast Cancer Res Treat. 2009;113(1):21–30. doi: 10.1007/s10549-008-9899-2. [DOI] [PubMed] [Google Scholar]
- 91.Berry LJ, et al. Potent oncolytic activity of human enteroviruses against human prostate cancer. Prostate. 2008;68(6):577–87. doi: 10.1002/pros.20741. [DOI] [PubMed] [Google Scholar]
- 92.Andtbacka RH, et al. A phase II study of Coxsackievirus A21 (CVA21) oncolytic virus immunotherapy in patients with advanced melanoma. ASCO May 2015, Journal Clinical Oncology. :9030. [Google Scholar]
- 93.Parato KA, et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol Ther. 2012;20(4):749–58. doi: 10.1038/mt.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park SH, et al. Phase 1b Trial of Biweekly Intravenous Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus in Colorectal Cancer. Mol Ther. 2015;23(9):1532–40. doi: 10.1038/mt.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zamarin D, Postow MA. Immune checkpoint modulation: rational design of combination strategies. Pharmacol Ther. 2015;150:23–32. doi: 10.1016/j.pharmthera.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 96.Hu-Lieskovan S, et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Transl Med. 2015;7(279):279ra41. doi: 10.1126/scitranslmed.aaa4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.AR, et al. A multicenter, open-label trial of talimogene laherparepvec (T-VEC) plus pembrolizumab vs pembrolizumab monotherapy in previously untreated, unresected, stage IIIB-IV melanoma. J Clin Oncol. 2015;33(suppl) abstr TPS9081. [Google Scholar]
- 98.Andtbacka R, et al. Phase 2, multicenter, randomized, open-label trial assessing efficacy and safety of talimogene laherparepvec (T-VEC) neoadjuvant treatment (tx) plus surgery vs surgery for resectable stage IIIB/C and IVM1a melanoma (MEL) J Clin Oncol. 2015;33(suppl) abstr TPS9094. [Google Scholar]
- 99.Zeng J, et al. Immune modulation and stereotactic radiation: improving local and abscopal responses. Biomed Res Int. 2013;2013:658126. doi: 10.1155/2013/658126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Milhem M. TVEC and Preop Radiation for Sarcoma. [accessed February 11, 2016]. https://clinicaltrials.gov/ct2/show/NCT02453191 .
- 101.H, et al. A Phase I, multicenter, open-label trial to evaluate the safety of talimogene laherparepvec (T-VEC) injected into liver tumors. Journal for ImmunoTherapy of Cancer. 2015;3(Suppl 2):P180. [Google Scholar]