Abstract
Background:
The association between socioeconomic factors and development of peripheral artery disease (PAD) has not been as well characterized compared to other cardiovascular diseases (CVD). We sought to define how annual income, sex, race, and education level are associated with newly diagnosed PAD in a well-characterized, diverse set of adults with CKD.
Methods:
The Chronic Renal Insufficiency Cohort Study (CRIC) is a multicenter, prospective cohort study designed to examine risk factors for progression of CKD and cardiovascular disease. Demographic and clinical data including ankle brachial index (ABI) and interventions were collected at baseline, as well as yearly during follow-up visits. Annual income was categorized as: < $25,000, $25,000–50,000, $50,000–100,000, or above $100,000. We excluded those with pre-existing PAD, defined as enrollment ABI of <0.9 or >1.4, or missing income data. Cox proportional hazards regression was used to estimate the risk for incident PAD during CRIC enrollment, defined as a drop in ABI to < 0.90 or a confirmed PAD intervention, including revascularization or amputation.
Results:
A total of 3,313 patients met inclusion criteria, the mean age was 58.7 yrs, 56% were male, and 42% were Black. Over a median follow-up of 10.1 years, 639 participants (19%) were newly diagnosed with PAD. After adjusting for cardiovascular risk factors, all lower levels of annual household income were associated with increased incidence of PAD (income <$25,000 HR 1.7, 95% CI 1.1–2.4, p=0.008; income $25,000–50,000 HR 1.5, 95% CI 1.1–2.3, p=0.009; income $50,000–100,000 HR 1.6, 95% CI 1.2–2.4, p=0.004), relative to a baseline annual income of >$100,000 (overall p-value=0.02). In the multivariable model, there was no association between education level and PAD incidence (p=0.80). Black race (HR 1.2, 95% CI 1.0–1.5, p=0.023) and female sex (HR 1.7, 95% CI 1.4–2.0, p<0.001) were independently associated with PAD incidence. Multiple imputation analysis provided similar results.
Conclusions:
In the CRIC, a multi-center cohort of prospectively followed CKD patients undergoing yearly CVD surveillance, lower annual household income, female sex, and Black race were significantly associated with the PAD incidence. In contrast, level of education was not independently associated with incident PAD.
1.0. Introduction
Even with continued improvements in cardiovascular care in the US over the last several decades, substantial disparities remain in cardiovascular disease (CVD) incidence and outcomes, with many improvements in CVD care not experienced equally by all socioeconomic groups.1 Factors including patient income, educational level, race, and sex have consistently been demonstrated to be important contributors to overall health and CVD outcomes.2 Peripheral arterial disease (PAD) is a common disease in the US elderly population, with an estimated prevalence of approximately 8 million in the United States, and is a major source of morbidity and mortality, resulting in functional impairment, limb loss, as well as death. While low socioeconomic status (SES) has been linked with increased prevalence of coronary artery disease (CAD) and increased CAD mortality,4 there are few studies that have investigated the relationship between SES and PAD.5,6,7 Prior research has been cross sectional in design, only able to assess for associations between SES factors and PAD prevalence, which is susceptible to lead time bias. The onset of PAD has been poorly characterized, due to many studies being cross sectional as well as the time and resources required to periodically assess asymptomatic subjects. Further, some uncertainty surrounding PAD onset and progression likely stems from the fact that PAD surveillance is not conducted in any state or nation. Additionally, most studies examine risk factors such as tobacco use and diabetes and their impact on PAD progression, but the relationship between SES factors and PAD incidence has not been clearly defined. Patients who are at particularly high risk for PAD include those with chronic kidney disease (CKD). Data from the National Health and Nutrition Examination Survey (NHANES) indicated that 25% of patients with CKD stage 3 or higher had PAD as defined by an ankle brachial index (ABI) < 0.9, compared to 4% in those normal renal function.8 The National Kidney Foundation Task Force has previously issued a statement suggesting that patients with CKD be considered in the highest risk category for subsequent cardiovascular events such as myocardial infarction, stable and unstable angina, and cardiac death.9 The Chronic Renal Insufficiency Cohort (CRIC) is a multi-center, prospective cohort study of CKD participants which is well-balanced with regard to education level, race, sex, age, and collects data regarding arterial circulation of the lower extremities at standardized intervals.10,11 As such, it is an ideal population to assess for SES based differences in PAD incidence among CKD patients. The objective of this study was to assess the association of SES factors on PAD incidence in a well-established prospective cohort of patients with CKD that undergoes yearly PAD assessment.
2.0. Methods:
The CRIC Study is a multi-center prospective study that examines risk factors for progression of CVD among patients with CKD. A total of 5,499 participants were recruited between 2003–2013 from 7 clinical centers across the US (Supplemental Table 1),11 and include a racially and ethnically diverse group of men and women who were aged 21 to 74 years old at the time of inclusion and have CKD based on age-based estimated GFR (eGFR) thresholds.10 Age-based GFR was defined as the following: for age 21 to 44, GFR 20 to 70 mL/min per 1.73 m2, age 45 to 64, GFR 20 to 60 mL/min per 1.73 m2; and age 65 to 74, GFR 20 to 50 mL/min per 1.73 m2. Participants were identified through searches of laboratory databases, medical records, and referrals from health care providers. The CRIC excluded those with cirrhosis, HIV infection, polycystic kidney disease, renal cell carcinoma, those on dialysis or recipients of a kidney transplant, and those taking immunosuppressant drugs. All study participants provided written informed consent, and the study protocol was approved by institutional review boards at each of the participating sites. All study data were collected by trained study staff during the CRIC clinical visits. Participants completed questionnaires at enrollment about socioeconomic, demographic, and medical history and returned for yearly visits where this information was updated. Annual household income was derived as a four-level categorical variable: below $25,000, $25,000–50,000, $50,000–100,000, or above $100,000. Highest level of educational attainment was also treated as a four-level categorical variable: some high school, high school graduate, some college, or college graduate. All data collection procedures and equipment were standardized across study sites. Baseline characteristics of participants were summarized as means (standard deviation, SD) for continuous variables and percentages for categorical variables by PAD status. Statistical significance was tested using either t-test or ANOVA for continuous variables and the χ2 test for categorical variables. Statistical analyses were performed using STATA version 15 (College Station, TX). All p-values were two-sided and statistical significance was defined as p<0.05.
2.1. PAD Assessment
The ankle brachial index (ABI) is the standard test utilized to diagnose PAD. After lying supine for 5 minutes, systolic blood pressure was measured in both arms and in the dorsalis pedis artery and posterior tibial artery using a Doppler probe. The ABI was calculated by dividing the systolic blood pressure for each sided pedal artery by the higher systolic blood pressure of the brachial artery. In participants with functioning arteriovenous fistula or grafts, the available contralateral brachial artery blood pressure was used. An ABI below 0.9 has been well established to be diagnostic for PAD with a sensitivity of 68% and a specificity of 99%.12,13 Patients were classified as having PAD at enrollment if they had a history of a PAD intervention prior to enrollment (history of PAD-related lower extremity amputation, angioplasty, or bypass) or an enrollment ABI of less than 0.9. Those with PAD at study enrollment were excluded from all analyses. Those with an enrollment ABI >1.4 were also excluded due to their uncertainty regarding PAD status in those with noncompressible vasculature. PAD symptoms were not assessed during yearly patient visits.
2.2. Incident PAD During the Study Period
Patients were identified to have incident PAD if they did not have PAD at enrollment, and experienced one of the following during the study period: (1) underwent lower extremity revascularization procedure (angioplasty or surgical bypass), (2) underwent major lower extremity amputation (below the knee or above the knee), or (3) had an ABI value of less than 0.9 in either lower extremity during an annual study visit. Subjects were censored at the time of death, study withdrawal, or at completion of the study. Associations with traditional cardiovascular and socioeconomic factors were explored with univariate Cox regression using the outcome of incident PAD. A multivariable Cox regression model was created containing selected variables and backwards elimination was used to create the final multivariable Cox regression model. Separate sensitivity analyses were performed with imputation of those with missing income data and inclusion of patients with noncompressible vasculature (enrollment ABI of >1.4). Imputation of missing income data was performed using multiple imputation with chained equation methods with final HR estimates combined using Rubin’s formula.14,15
3.0. Results:
There were 5,499 total CRIC participants enrolled, with a mean age of 59.5 years, 56.4% were male, and 44.3% that were Black. There were 621 patients with missing income information and 516 patients with enrollment ABI >1.4 that were excluded from initial analysis. There were 860 participants (20.2%) in the CRIC cohort found to have PAD at enrollment (see Methods: PAD Assessment), who were also excluded.
There were 3,313 participants from the initial 5,499 CRIC cohort that met inclusion criteria. The median length of follow up time was 10.1 years [IQR 3.8 – 12.6], mean age was 58.7 years, 56.1% were males, and 42.0% were Black. A total of 639 study participants (19%) developed PAD during the study, with 543 diagnosed by an ABI below 0.9 (85%) and 96 diagnosed by undergoing a PAD procedure (revascularization or lower extremity amputation). Patient demographic data and clinical characteristics by PAD outcome are shown in Table 1 It is notable that there were significant differences between the groups with regard to age, sex, race, SES factors, and comorbid conditions. Mean time to incident PAD diagnosis was 3.7 ± 3.0 years (median 2.8 years, IQR 1.2 – 5.0) with an incidence rate of 2.8 cases of PAD per 100 person-years.
Table 1.
Summary statistics for those who did not enter the CRIC study with pre-existing PAD, stratified by development of PAD during the study period.
| No PAD (n = 2674) | Incident PAD (n = 639) | p-value | ||
|---|---|---|---|---|
| Age | < 45 years | 328 (12.3%) | 53 (8.3%) | 0.002 |
| 45 to less than 65 years | 1507 (56.4%) | 350 (54.8%) | ||
| ≥ 65 years | 839 (31.4%) | 236 (36.9%) | ||
| Annual Household Income | Below $25,000 | 768 (28.7%) | 255 (39.9%) | <0.001 |
| $25,000–$50,000 | 768 (28.7%) | 190 (29.7%) | ||
| $50,000-$100,000 | 675 (25.2%) | 149 (23.3%) | ||
| Above $100,000 | 463 (17.3%) | 45 (7.0%) | ||
| Education Level | Some High School | 389 (14.5%) | 127 (19.9%) | <0.001 |
| High School Grad | 439 (16.4%) | 125 (19.6%) | ||
| Some College | 784 (29.3%) | 213 (33.3%) | ||
| College Grad | 1062 (39.7%) | 174 (27.2%) | ||
| Male Gender | 1588 (59.4%) | 281 (44.0%) | <0.001 | |
| Black Race | 1058 (39.6%) | 333 (52.1%) | <0.001 | |
| eGFR (mL/min per 1.73 m2) | Below 30 | 296 (11.1%) | 108 (16.9%) | <0.001 |
| 30–60 | 1618 (60.5%) | 436 (68.2%) | ||
| Above 60 | 760 (28.4%) | 95 (14.9%) | ||
| History of CVD | 623 (23.3%) | 204 (31.9%) | <0.001 | |
| History of DM | 1120 (41.9%) | 336 (52.6%) | <0.001 | |
| History of Hypertension | 2198 (82.2%) | 587 (91.9%) | <0.001 | |
| History of High Cholesterol | 1997 (74.7%) | 527 (82.5%) | <0.001 | |
| Tobacco Use | 251 (9.4%) | 129 (20.2%) | <0.001 | |
| Alcohol Use | 686 (25.7%) | 115 (18.0%) | <0.001 | |
| Medication Use | Statin Use | 1389 (52.3%) | 379 (59.9%) | <0.001 |
| Antiplatelet Use | 1144 (43.1%) | 303 (47.4%) | 0.028 | |
| Anticoagulant Use | 166 (6.2%) | 36 (5.6%) | 0.59 | |
| Body Mass Index | Below 25 | 407 (15.3%) | 87 (13.6%) | <0.001 |
| 25–30 | 835 (31.3%) | 161 (25.2%) | ||
| Above 30 | 1424 (53.4%) | 391 (61.2%) | ||
| Urine Sodium Concentration, mmol/L (SD) | 83.0 (35.5) | 82.2 (34.1) | 0.62 | |
| Urine Protein/Creatinine Ratio (SD) | 0.68 (1.9) | 0.86 (2.0) | 0.035 | |
| Cause of Renal Disease | Diabetes | 518 (19.4%) | 167 (26.1%) | <0.001 |
| Hypertension | 418 (15.6%) | 94 (14.7%) | ||
| Other | 432 (16.2%) | 73 (11.4%) | ||
| Unknown | 1306 (48.8%) | 305 (47.4%) | ||
| CRIC Site | University of Pennsylvania | 334 (12.5%) | 93 (14.6%) | <0.001 |
| The Johns Hopkins University | 357 (13.4%) | 98 (15.3%) | ||
| Case Western Reserve University | 331 (12.4%) | 149 (23.3%) | ||
| University of Michigan | 409 (15.3%) | 46 (7.2%) | ||
| University of Illinois at Chicago | 442 (16.5%) | 128 (20.0%) | ||
| Tulane University Health Science Center | 305 (11.4%) | 51(8.0%) | ||
| Kaiser Permanente of Norther California | 496 (18.5%) | 74 (11.6%) |
PAD: Peripheral Artery Disease, CVD: Cardiovascular Disease, DM: Diabetes Mellitus
Notable associations from the univariate and multivariable Cox regression model are shown in Table 2. In the adjusted multivariable Cox regression model, which included demographic and clinical factors relevant from the univariate analysis, Black race (HR 1.2, 95% CI 1.0–1.5, p = 0.0023), female sex (HR 1.7, 95% CI 1.4–2.0, p < 0.001), and lower level of annual household income (p = 0.02) remained independently associated with development of PAD during the study. When compared to a baseline annual income of >$100,000, all lower levels of annual household income were associated with increased incidence of PAD (income < $25,000 HR 1.7, 95% CI 1.1–2.4, p = 0.008; income $25,000–50,000 HR 1.6, 95% CI 1.1–2.3, p = 0.009; income $50,000–100,000 HR 1.7, 95% CI 1.2–2.4, p = 0.002). In contrast, level of educational attainment was not associated with the development of PAD in the adjusted analysis (p = 0.80). Inclusion of statin, antiplatelet, or anticoagulant use did not significantly alter the association between SES factors and incident PAD in the adjusted model. No significant interaction was found between age, race, or sex with either annual household income or level of educational attained. The full multivariable model is shown in supplement Table 2.
Table 2.
Univariate and multivariable cox regression model results for association between development of incident PAD during CRIC study period and socioeconomic factors
| Univariate Cox HR | Univariat e p-value | Multivariab le Cox HR | Multivariab le p-value | ||
|---|---|---|---|---|---|
| Annual Household Income | Below $25,000 | 3.4 (2.5–4.7) | <0.001 | 1.7 (1.1–2.4) | 0.023 |
| $25,000–$50,000 | 2.5 (1.8–3.4) | 1.6 (1.1–2.3) | |||
| $50,000–$100,000 | 2.1 (1.5–3.0) | 1.7 (1.2–2.4) | |||
| Above $100,000 | Ref | Ref | |||
| Education Level | Some High School Education | 2.0 (1.6–2.5) | <0.001 | 1.0 (0.8–1.4) | 0.800 |
| High School Graduate | 1.8 (1.4–2.2) | 1.0 (0.8–1.3) | |||
| Some College Education | 1.6 (1.3–2.0) | 1.1 (0.9–1.4) | |||
| College Graduate | Ref | Ref | |||
| Black Race | 1.7 (1.5–2.0) | <0.001 | 1.2 (1.0–1.5) | 0.023 | |
| Female Gender | 1.7 (1.5–2.0) | <0.001 | 1.7 (1.4–2.0) | <0.001 |
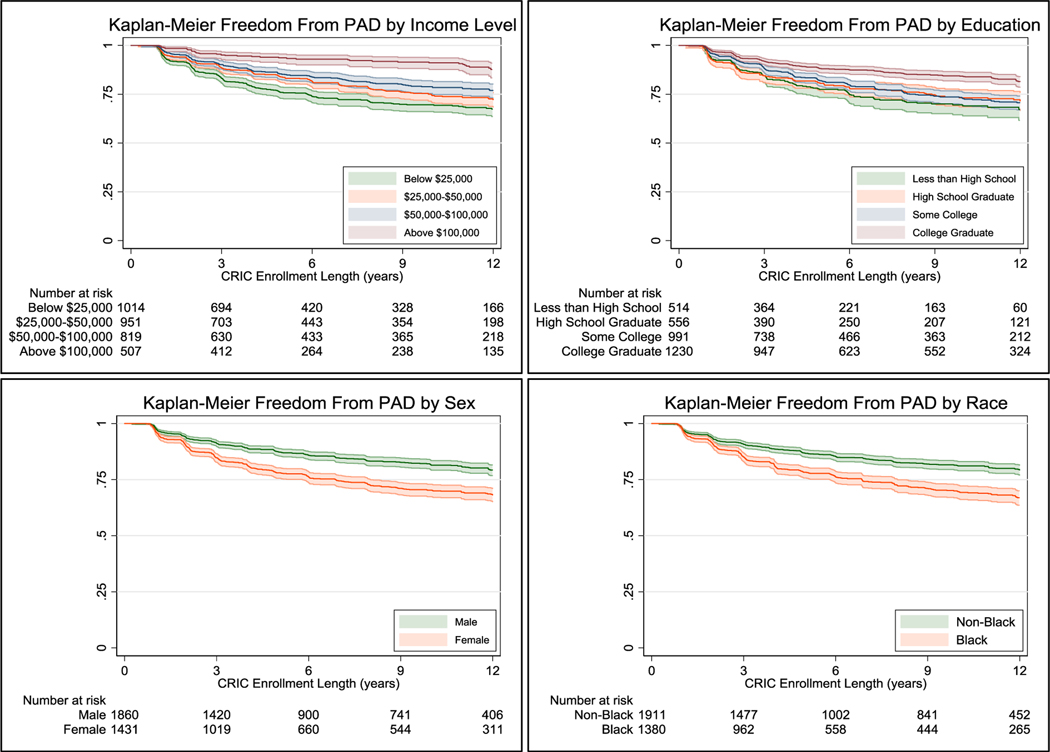
Kaplan–Meier curves are shown in Figure 1. Overall, low income (log-rank test, p < 0.001), lower education level (log-rank test, p < 0.001), female sex (log-rank test, p < 0.001), and Black race (log-rank test, p < 0.001) were associated with lower rates of freedom from PAD.
Figure 1.
Kaplan-Meier curves denoting freedom from PAD across SES factors
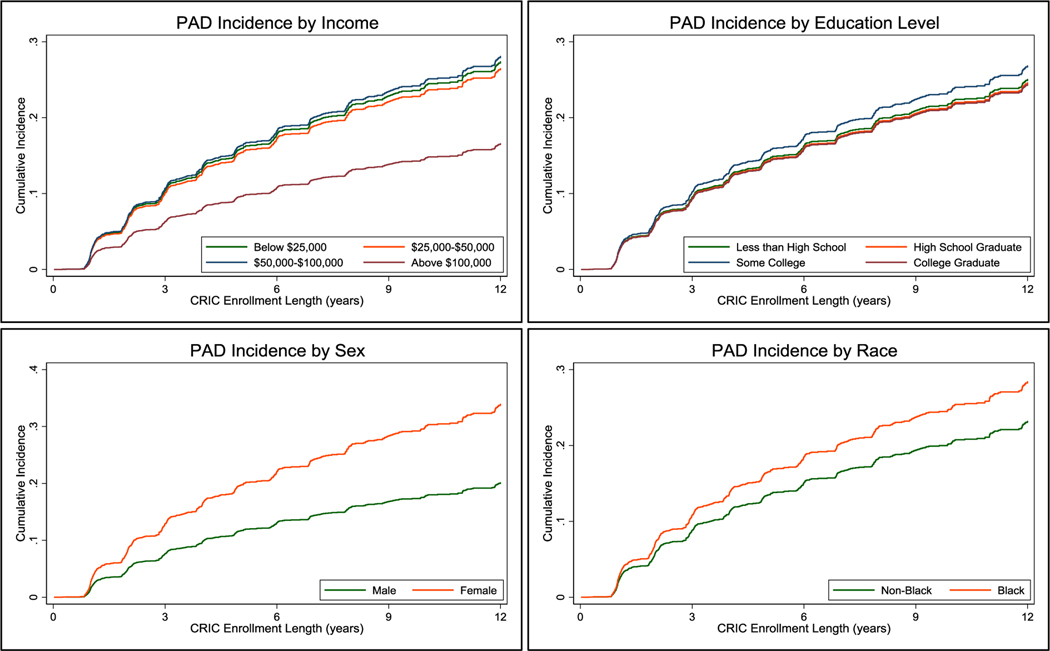
Cumulative PAD incidence curves from the adjusted analysis are shown in Figure 2, and reflect that low annual household income, Black race and female sex remained significantly associated with an increased rate of PAD.
Figure 2.
Cumulative incidence of peripheral artery disease by SES factors. Using Cox Regression model at the mean age of the cohort (57 years) as well as the mean for other variables in the model, adjusted for diabetes mellitus, race, gender, income level, educational level, smoking, body mass index, glomerular filtration rate, coronary artery disease, hypertension, and geographic location.
3.1. Sensitivity Analyses
3.1.1. PAD incidence with imputation of missing income information
Imputation of missing income data was performed using multiple imputation with chained equation methods.14 Using 10 chained equations, imputation of 410 missing income levels (66%) was successful for a total sample size of 3,723 patients. Based on prior simulations in chained model imputation, our model imputation performed as expected given our sample and the number of imputations.14 In the multivariable Cox regression model with imputation, those with an annual income above $100,000 had a lower risk of incident PAD during the study period compared to those with an income < $25,000 (HR 1.6, 95% CI 1.1–2.3, p = 0.008), $25,000–50,000 (HR 1.5, 95% CI 1.1–2.2, p = 0.012), and $50,000–100,000 (HR 1.6, 95% CI 1.1–2.2, p = 0.006) with an overall p-value of 0.040. There was no association between education level and risk of incident PAD (p = 0.83) in the adjusted imputed model with imputation. Black race (HR 1.3, 95% CI 1.1–1.5, p = 0.007) and female sex (HR 1.7, 95% CI 1.4–2.0, p < 0.001) remained independently associated with development of incident PAD in the adjusted model with imputation.
3.1.2. PAD incidence with inclusion of those with baseline ABI>1.4
Inclusion of the 516 patients initially excluded from analyses due to enrollment ABI>1.4 resulted in a total of 3,829 patients included in the Cox regression analysis for risk of incident PAD during the CRIC study period. A total of 735 developed incident PAD in this expanded cohort (19%) compared to 639 patients on the initial analysis, with an additional 50 patients diagnosed with PAD who required a PAD intervention and 46 patients diagnosed by progression of ABI to less than 0.9. In the final multivariable Cox regression model, Black race (HR 1.3, 95% CI 1.1–1.5, p = 0.002), female sex (HR 1.6, 95% CI 1.4–1.9, p < 0.001), and lower level of annual household income remained independently associated with development of PAD. Those with an annual income above $100,000 had a lower risk of incident PAD during the study period compared to those with an income < $25,000 (HR 1.6, 95% CI 1.1–2.3, p = 0.006), $25,000–50,000 (HR 1.6, 95% CI 1.1–2.2, p = 0.008), and $50,000–100,000 (HR 1.6, 95% CI 1.2–2.2, p = 0.003) with an overall p-value of 0.022. Level of educational attainment was not associated with development of PAD with inclusion of those with noncompressible baseline ABI (p = 0.71).
4.0. Discussion:
In this study, we demonstrated the associations of household income, education level, sex, and race, with development of PAD in a well-established prospective longitudinal cohort of patients at high risk for PAD development. Even with multivariable adjustment, annual household income, female sex, and Black race remain strongly associated with incident PAD. As seen in prior literature assessing PAD prevalence, the association between educational level and development of PAD appeared to be largely driven by demographic and cardiovascular risk factors, as there was an association between education level and risk of PAD development on univariate cox regression model, but no association on multivariable model.16 The relationship between annual household income and risk of incident PAD persisted after adjusting for multiple key covariates, with lower levels of annual income associated with a similar risk of PAD development when compared to annual income above $100,000. Further, we found no interaction between annual income and other SES factors with regard to PAD incidence. The association between income and CVD has been well documented for other disease entities with similar results to ours.5,17 The etiology of the association between low income level and CVD is likely multifactorial. Possible contributing factors include: the documented association between low annual income and chronic low-grade inflammation, the association between lower SES with fewer social supports and decreased likelihood of engaging in healthy lifestyle choices, those with lower income level experiencing increased difficulty in gaining access to community resources and/or exercise accessories due to employment and financial constraints, and increased difficulty in attaining access to preventive care or screening initiaives.5, 17–20 Our results support the need for innovative solutions to address the increased PAD incidence observed among these disadvantaged patients. This may entail expansion of existing cardiovascular health outreach services as well as enactment of new health and social policies to mitigate disparities associated with lower income. Those with higher income, especially annual household income above $100,000, may also be experiencing an associated protective effect from PAD development. Given the similar HRs noted for all income categories below $100,000, it appears that all of these cohorts are equally susceptible the previously noted health risks associated with lower income, and a dose-response relationship was not observed.
In both univariate and multivariable analysis, Black race was associated with increased PAD incidence. The independent association between Black race and CVD, even in this well followed prospective cohort with adjustment of baseline comorbid conditions and SES, has been seen in other areas of cardiovascular health.17,21 This observation has been attributed to more severe comorbid conditions in the Black population (including increased levels of baseline blood pressure in those with hypertension, higher blood glucose in those with diabetes, and increased frequency of central obesity in those with elevated BMI) and increased baseline levels of psychological distress experienced by this population.17,21 Additionally, the Black population has been noted to have poorer access to medical care, even with adjustment of other socioeconomic factors, which may contribute to our findings.17,22 Further, female sex was associated with incident PAD on univariate and multivariable cox regression analysis. Prior reports have shown conflicting results in regard to the association between sex and PAD. There have been prior studies demonstrating an association between female sex and PAD prevalence, but in these studies this was attributed to increased risk of comorbid cardiovascular conditions and decreased cardiovascular medication use in women.23,24 More recent cohort studies have demonstrated that women may present with more severe PAD and have lower rates of limb salvage compared to men.25–28 Additionally, prior literature has suggested that younger women diagnosed with PAD have more rapid decline in function compared to men, which may justify earlier and more aggressive screening measures in women in compared to men.27,28 Our results support the association between female sex and increased PAD incidence, which may differ from historical studies due this study investigating associations with PAD incidence or due to the detailed tracking of cardiovascular conditions that is done in the CRIC cohort allowing for appropriate adjustment of comorbid factors.25,26
This study did not show significant differences in cardiovascular medication regimen by SES factors as a possible explanation for our findings. Income level, education level, and race were not associated with differing rates of prescriptions for antiplatelet agents, anticoagulants, or statins, although medication compliance was not assessed. Additionally, these medications did not have a significant association with PAD incidence in multivariable models.
The disparities highlighted in this study demonstrate the need for improved approaches to raise PAD awareness, as well as focused research endeavors and treatment strategies that target low SES individuals, as they are the most at risk for developing PAD. Previous studies demonstrated that patients with both PAD and low SES are more likely to undergo amputations compared to those with higher SES, who are more likely to undergo revascularization or debridement.29,30 Continued efforts to identify and implement programs in these subpopulations would reduce morbidity caused by delayed diagnosis. Notably, in patients who survived more than five years, over 20% developed PAD during the study period. Given the high prevalence of PAD in those with CKD, as well as the often asymptomatic nature of PAD, earlier ABI screening in this subpopulation may be warranted. In this study, 85% of patients diagnosed with PAD were identified by ABI screening, which could lead to earlier disease treatment in these individuals. There are important limitations to our study. Annual income and education level are self-reported and only collected upon participant enrollment into the CRIC; thus, misclassification is possible. However, prior studies have shown minimal discrepancy between self-reported income and true wage or salary.31 In addition, annual household income may not always approximate total wealth and varies by family size, especially in those who are retired. However, analyses stratified by age < or ≥65 years yielded similar relationships in all models. Patients with CKD and/or diabetes can have calcified, noncompressible vessels, which can lead to an overestimation of the ABI and misclassification. Our primary analyses excluded those with noncompressible vessels, though we observed similar relationships between SES factors and development of PAD in sensitivity analyses in which they were reincorporated into the cohort.. Additionally, the CRIC is a longitudinal study designed for those with CKD, which may limit generalizability to the broader US population. Further, patients able to comply with the yearly visits for CRIC participation may not be generalizable to those in the US who are at most risk for PAD development. While we were able to control for presence or absence of traditional cardiovascular risk factors, we were unable to control for severity of these comorbidities (i.e. A1C level, higher level of LDL, etc). Thus, there may have been residual confounding by disease severity. Lastly, we were able to assess for PAD by both ABI criteria and need for intervention, but otherwise PAD symptoms were not tracked in this prospective cohort. Nonetheless, this is a rich dataset which provides important insights into a highly relevant population which is commonly affected by PAD.
Additionally, since this is a prospectively followed cohort, we were able to assess socioeconomic associations with incident PAD, eliminating the lead time bias present in prior studies.
5.0. Conclusions:
In summary, in this prospectively followed CKD cohort, we found that lower annual household income, female sex, and Black race were significantly associated with development of PAD. In contrast, level of education was not independently associated with incident PAD after adjusting for demographic and cardiovascular conditions. Acknowledging the limitations of this study, the fact that these factors are strongly associated with PAD development should lead us to better consider our advocacy and education efforts around PAD. Without question, subpopulations exist US that remain at substantially higher risk of developing PAD. Improved public awareness efforts that are targeted toward these populations will hopefully lower the adverse impact of PAD that these vulnerable patients experience. Additionally, given the high incidence of PAD in this cohort, the role of periodic ABI screening for PAD in high risk populations should be considered.
Supplementary Material
Highlights.
We found that lower annual household income, female gender, and Black race were independently associated with development of PAD even with strict multivariable adjustment in this prospectively followed CKD cohort
Level of educational attainment was not independently associated with incident PAD, but educational level was a possible surrogate for annual income if missing
Given the high incidence of PAD in this cohort, with 19% developing PAD over a median of 10 years, the role of periodic PAD screening by ABI in high risk subpopulations should be considered
Acknowledgments
Sources of Funding
Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, U01DK060902 and U24DK060990). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131, Department of Internal Medicine, University of New Mexico School of Medicine Albuquerque, NM R01DK119199
Appendix:
The CRIC Study Investigators include Lawrence J. Appel, MD, MPH: Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Medical Institutions, Baltimore, MD; Alan S. Go, MD: Departments of Epidemiology, Biostatistics, and Medicine, University of California, San Francisco, CA; Jiang He, MD, PhD: Department of Epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, LA; John W. Kusek, PhD: National Institutes of Health, Bethesda, MD; James P. Lash, MD: Department of Medicine, University of Illinois College of Medicine, Chicago, IL; Mahboob Rahman, MD: Department of Medicine, Case Western Reserve University, Cleveland, OH.
Footnotes
Conception and design
JS, JBC, GW
Analysis and interpretation
JS, JBC, NB, JCC, RT, DX, HF, GW
Data collection
Not applicable
Writing the article
JS, GW
Statistical analysis
JS, DX, GW
Obtained funding
Not applicable
Overall responsibility
JS, GW
Final approval of the article
JS, JBC, NB, JCC, RT, DX, HF, GW
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of Disparities in Cardiovascular Health in the United States. Circulation. 2005;111:1233–1241. [DOI] [PubMed] [Google Scholar]
- [2].Alter DA, Franklin B, Ko DT, Austin PC, Lee DS, Oh PI, Stukel TA, Tu JV. Socioeconomic Status, Functional Recovery, and Long-Term Mortality among Patients Surviving Acute Myocardial Infarction. PLoS One. 2013;8:e65130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001. Sep 19;286(11):1317–24. [DOI] [PubMed] [Google Scholar]
- [4].Kaplan GA, Keil JE. Socioeconomic Factors and Cardiovascular Disease: A Review of the Literature. Circulation. 1993;88:1973–1998. [DOI] [PubMed] [Google Scholar]
- [5].Pande RL, Creager MA. Socioeconomic inequality and peripheral artery disease prevalence in US adults. Circ Cardiovasc Qual Outcomes. 2014;7:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kröger K, Dragano N, Stang A, Moebus S, Möhlenkamp S, Mann K, Siegrist J, Jöckel KH, Erbel R; Heinz Nixdorf Recall Study Investigator Group. An unequal social distribution of peripheral arterial disease and the possible explanations: results from a population-based study. Vasc Med. 2009;14:289–296. [DOI] [PubMed] [Google Scholar]
- [7].Kaplan GA, Keil JE. Socioeconomic Factors and Cardiovascular Disease: A Review of the Literature. Circulation. 1993;88:1973–1998. [DOI] [PubMed] [Google Scholar]
- [8].O’Hare Ann M, Glidden David V, Fox Caroline S, Hsu Chi-yuan. High Prevalence of Peripheral Arterial Disease in Persons With Renal Insufficiency. Circulation. 2004;109(3):320–323. doi: 10.1161/01.CIR.0000114519.75433.DD [DOI] [PubMed] [Google Scholar]
- [9].Sarnak Mark J, Levey Andrew S, Schoolwerth Anton C, Coresh Josef, Culleton Bruce, Lee Hamm L., McCullough Peter A, Kasiske Bertram L, Kelepouris Ellie, Klag Michael J, Parfrey Patrick, Pfeffer Marc, Raij Leopoldo, Spinosa David J, Wilson Peter W. Kidney Disease as a Risk Factor for Development of Cardiovascular Disease. Hypertension. 2003;42:1050–1065. [DOI] [PubMed] [Google Scholar]
- [10].Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline Characteristics and Associations with Kidney Function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. JASN. 2003;14(suppl 2):S148–S153. doi: 10.1097/01.ASN.0000070149.78399.CE [DOI] [PubMed] [Google Scholar]
- [12].Khan TH, Farooqui FA, Niazi K. Critical Review of the Ankle Brachial Index. Curr Cardiol Rev. 2008;4(2):101–106. doi: 10.2174/157340308784245810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schröder F, Diehm N, Kareem S, et al. A modified calculation of ankle-brachial pressure index is far more sensitive in the detection of peripheral arterial disease. J Vasc Surg. 2006;44(3):531–536. doi: 10.1016/j.jvs.2006.05.016 [DOI] [PubMed] [Google Scholar]
- [14].Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8(3):206–213. doi: 10.1007/s11121-007-0070-9 [DOI] [PubMed] [Google Scholar]
- [15].Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, Inc; 1987. doi: 10.1002/9780470316696 [DOI] [Google Scholar]
- [16].Pande RL, Creager MA. Socioeconomic Inequality and Peripheral Artery Disease Prevalence in US Adults. Circulation: Cardiovascular Quality and Outcomes. 2014;7:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Karlamangla AS, Merkin SS, Crimmins EM, Seeman TE. Socioeconomic and Ethnic Disparities in Cardiovascular Risk In the United States, 2001–2006. Annals of Epidemiology. 2010;20(8):617–628. doi: 10.1016/j.annepidem.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cohen B, Vittinghoff E, Whooley M. Association of Socioeconomic Status and Exercise Capacity in Adults With Coronary Heart Disease (from the Heart and Soul Study). The American Journal of Cardiology. 2008;101:462–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shishehbor MH, Gordon-Larsen P, Kiefe CI, Litaker D. Association of neighborhood socioeconomic status with physical fitness in healthy young adults: The Coronary Artery Risk Development in Young Adults (CARDIA) study. American Heart Journal. 2008;155:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Winkleby MA, Cubbin C, Ahn DK, Kraemer HC. Pathways by Which SES and Ethnicity Influence Cardiovascular Disease Risk Factors. Annals of the New York Academy of Sciences. 1999;896:191–209. [DOI] [PubMed] [Google Scholar]
- [21].Rooks RN, Simonsick EM, Miles T, Newman A, Kritchevsky SB, Schulz R, Harris T. The Association of Race and Socioeconomic Status With Cardiovascular Disease Indicators Among Older Adults in the Health, Aging, and Body Composition Study. J Gerontol B Psychol Sci Soc Sci. 2002;57:S247–S256. [DOI] [PubMed] [Google Scholar]
- [22].Matthews KA, Sowers MF, Derby CA, Stein E, Miracle-McMahill H, Crawford SL, Pasternak RC. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women’s Health Across the Nation (SWAN). American Heart Journal. 2005;149:1066–1073. [DOI] [PubMed] [Google Scholar]
- [23].Jelani Q, Petrov M, Martinez SC, Holmvang L, Al-Shaibi K, Alasnag M. Peripheral Arterial Disease in Women: an Overview of Risk Factor Profile, Clinical Features, and Outcomes. Curr Atheroscler Rep [Internet]. 2018. [cited 2020 Nov 17];20. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5984648/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sadrzadeh Rafie AH, Stefanick ML, Sims ST, Phan T, Higgins M, Gabriel A, Assimes T, Narasimhan B, Nead KT, Myers J, Olin J, Cooke JP. Sex differences in the prevalence of peripheral artery disease in patients undergoing coronary catheterization. Vasc Med. 2010;15:443–450. [DOI] [PubMed] [Google Scholar]
- [25].Diehm C, Schuster A, Allenberg JR, Darius H, Haberl R, Lange S, Pittrow D, von Stritzky B, Tepohl G, Trampisch H-J. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004;172:95–105. [DOI] [PubMed] [Google Scholar]
- [26].Sigvant B, Wiberg-Hedman K, Bergqvist D, Rolandsson O, Andersson B, Persson E, Wahlberg E. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. Journal of Vascular Surgery. 2007;45:1185–1191. [DOI] [PubMed] [Google Scholar]
- [27].Wang Grace J, Shaw Pamela A, Townsend Raymond R, Anderson Amanda H, Xie Dawei, Wang Xue, Nessel Lisa C, Mohler Emile R, Sozio Stephen M, Jaar Bernard G, Chen Jing, Wright Jackson, Taliercio Jonathan J, Ojo Akinlolu, Ricardo Ana C, Lustigova Eva, Fairman Ronald M, Feldman Harold I, Ky Bonnie Sex Differences in the Incidence of Peripheral Artery Disease in the Chronic Renal Insufficiency Cohort. Circulation: Cardiovascular Quality and Outcomes. 2016;9:S86–S93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McDermott Mary M, Ferrucci Luigi, Liu Kiang, Guralnik Jack M, Tian Lu, Kibbe Melina, Liao Yihua, Tao Huimin, Criqui Michael H. Women With Peripheral Arterial Disease Experience Faster Functional Decline Than Men With Peripheral Arterial Disease. Journal of the American College of Cardiology. 2011;57:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ferguson HJM, Nightingale P, Pathak R, Jayatunga AP. The Influence of Socio-economic Deprivation on Rates of Major Lower Limb Amputation Secondary to Peripheral Arterial Disease. European Journal of Vascular and Endovascular Surgery. 2010;40:76–80. [DOI] [PubMed] [Google Scholar]
- [30].Holman KH, Henke PK, Dimick JB, Birkmeyer JD. Racial disparities in the use of revascularization before leg amputation in Medicare patients. Journal of Vascular Surgery. 2011;54:420–426.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.