Keywords: epithelial Na+ channel, pendrin, potassium
Abstract
Pendrin is an intercalated cell Cl−/ exchanger thought to participate in K+-sparing NaCl absorption. However, its role in K+ homeostasis has not been clearly defined. We hypothesized that pendrin-null mice will develop hypokalemia with dietary K+ restriction. We further hypothesized that pendrin knockout (KO) mice mitigate urinary K+ loss by downregulating the epithelial Na+ channel (ENaC). Thus, we examined the role of ENaC in Na+ and K+ balance in pendrin KO and wild-type mice following dietary K+ restriction. To do so, we examined the relationship between Na+ and K+ balance and ENaC subunit abundance in K+-restricted pendrin-null and wild-type mice that were NaCl restricted or replete. Following a NaCl-replete, K+-restricted diet, K+ balance and serum K+ were similar in both groups. However, following a Na+, K+, and Cl−-deficient diet, pendrin KO mice developed hypokalemia from increased K+ excretion. The fall in serum K+ observed in K+-restricted pendrin KO mice was enhanced with ENaC stimulation but eliminated with ENaC inhibition. The fall in serum K+ observed in K+-restricted pendrin KO mice was enhanced with ENaC stimulation but eliminated with ENaC inhibition. However, reducing ENaC activity also reduced blood pressure and increased apparent intravascular volume contraction, since KO mice had lower serum Na+, higher blood urea nitrogen and hemoglobin, greater weight loss, greater metabolic alkalosis, and greater NaCl excretion. We conclude that dietary Na+ and K+ restriction induces hypokalemia in pendrin KO mice. Pendrin-null mice limit renal K+ loss by downregulating ENaC. However, this ENaC downregulation occurs at the expense of intravascular volume.
NEW & NOTEWORTHY Pendrin is an apical Cl−/ exchanger that provides renal K+-sparing NaCl absorption. The pendrin-null kidney has an inability to fully conserve K+ and limits renal K+ loss by downregulating the epithelial Na+ channel (ENaC). However, with Na+ restriction, the need to reduce ENaC for K+ balance conflicts with the need to stimulate ENaC for intravascular volume. Therefore, NaCl restriction stimulates ENaC less in pendrin-null mice than in wild-type mice, which mitigates their kaliuresis and hypokalemia but exacerbates volume contraction.
INTRODUCTION
During NaCl restriction, the cortical collecting duct (CCD) increases electroneutral NaCl absorption by stimulating pendrin-mediated Cl−/ exchange in tandem with Na+-dependent Cl−/ exchange [through the electroneutral Na+/ exchanger (NDCBE)], which are encoded by Slc26a4 and Slc4a8, respectively (1, 2). Because both NDCBE and pendrin are electroneutral exchangers, and because NDCBE gene ablation reduces NaCl absorption by the CCD without changing transepithelial K+ transport, the pendrin/NDCBE complex is thought to mediate K+-sparing NaCl absorption (2).
The effect of pendrin gene ablation on K+ homeostasis has varied among studies. In a mouse model of pseudohypoaldosteronism type II, Lopez-Cayuqueo et al. (3) observed that serum K+ is 0.6 mM lower in mice that were pendrin null relative to those with wild-type pendrin. Similarly, in aldosterone-treated mice, Xu et al. (4) observed serum K+ to be 0.3 mM lower in pendrin-null mice relative to wild-type mice, although we did not observe a difference in serum K+ between pendrin-null and wild-type mice under similar conditions (5). Soleimani et al. (6) also observed no difference in serum K+ between mice that were both NaCl cotransporter (NCC)- and pendrin-null relative to mice that were NCC null alone. Finally, some studies have observed higher serum K+ in pendrin-null mice relative to wild-type mice (7). Therefore, although pendrin is thought to mediate K+-sparing NaCl absorption, the treatment conditions that unmask a K+ phenotype in pendrin-null mice are unclear.
In the absence of a transporter that mediates electroneutral NaCl absorption, such as NCC, NDCBE, or pendrin, the maintenance of intravascular volume is thought to be increasingly epithelial Na+ channel (ENaC) dependent (1, 8, 9). In NaCl-restricted NCC- and NDCBE-null mice, Sinning et al. (9) observed that both pendrin and ENaC subunit abundance were increased, whereas serum K+ was reduced relative to mice that were NDCBE null alone. The authors concluded that ablation of genes encoding electroneutral mechanisms of NaCl absorption increases ENaC activity, which promotes K+ excretion and thus reduces serum K+.
Based on the Sinning et al. (9) model, ENaC should be upregulated in the absence of pendrin/NDCBE-mediated electroneutral NaCl absorption. Following NaCl restriction, pendrin-null mice are in negative NaCl balance and are therefore volume contracted (10). This volume contraction should increase angiotensin II and aldosterone, thereby stimulating ENaC-mediated Na+ absorption. Instead, we observed that ENaC subunit abundance, ENaC abundance in the most apical region of the cell, ENaC channel open probability, and ENaC-mediated Na+ absorption are all lower in pendrin-null mice relative to wild-type mice (11–13). Because NaCl-restricted pendrin-null mice are volume contracted and have lower blood pressure, how ENaC downregulation is of benefit to these mice has been puzzling. We hypothesized that in the absence of pendrin-mediated K+-sparing NaCl absorption, K+ excretion increases. The pendrin-null kidney attenuates this renal K+ loss by downregulating ENaC. We further hypothesized that during dietary NaCl restriction, the need for ENaC-mediated Na+ absorption to maintain intravascular volume limits the ability of the pendrin-null kidney to mitigate urinary K+ loss through ENaC downregulation.
The purpose of the present study was to determine if pendrin-null mice have an impaired ability to conserve K+ when dietary K+ is restricted, to explore the role of NaCl intake and ENaC in maintaining K+ homeostasis in the K+-restricted pendrin-null mouse, and to determine if there is a sex difference in this response.
METHODS
Animals
Most experiments studied Slc26a4−/− and Slc26a4+/+ littermates that were generated by breeding Slc26a4+/− mice on a C57Bl/6J background. In some experiments, we compared wild-type mice (ENaCWTWT; Slc26a4+/+), homozygous pendrin-null mice with wild-type ENaC (ENaCWTWT; Slc26a4−/−), homozygous Liddle’s mice with wild-type pendrin (ENaCLL; Slc26a4+/+), and homozygous Liddle’s mice that were homozygous pendrin null (ENaCLL; Slc26a4−/−). These mice were on a 129 SvEv background and have been previously described (13, 14).
Animal Conditioning
Treatment 1: Na+, K+, and Cl−-deficient synthetic diet.
For 3–8 days, mice were ration fed a balanced Na+, K+, and Cl−-deficient synthetic diet (TD 150434, Harlan Teklad) prepared as a gel (0.6% agar, 74.6% water, and 24.8% mouse chow), which provided mice with 0.03% Na+, 0.05% Cl−, and 0.1% K+/g of diet or 0.05 meq/day Na+, 0.05 meq/day Cl−, 0.04 meq/day K+, and 10 mL water/day.
Treatment 2: K+-deficient, NaCl-replete synthetic diet.
For 4 or 7 days, mice were ration fed the gelled diet as described for treatment 1, which was supplemented with NaCl to give mice 0.46% Na+, 0.1% K+, and 0.7% Cl− or 0.8 meq/day Na+, 0.04 meq/day K+, and 0.8 meq/day Cl−.
Treatment 3: Na+, K+, and Cl−-deficient synthetic diet supplemented with amiloride.
For 7 days, mice were ration fed the gelled diet as described for treatment 1, which was supplemented with amiloride to give 0.11 mg/day.
Treatment 4: NaCl-deficient, K+-replete, grain-based diet with normal or high water content.
Mice were ration fed a balanced NaCl-restricted, K+-replete diet (TD 90228) for 7 days, prepared as a gel, with either 75% water and 25% diet or with 53% water and 48% diet. By giving the former group 18 mL and the latter group 9.4 mL of the gelled diet each day, each group received 4.5 g of food each day, although daily water intake was 12 mL in the former group and 4.5 mL in the latter group, which compares with an expected water intake of 4 mL/day in a 25-g mouse eating a standard diet and drinking water ad libitum. Each group received 0.02% Na+, 0.8% K+, and 0.07% Cl−/g of powdered diet, giving each mouse 0.003 meq/day Na+, 0.9 meq/day K+, and 0.08 meq/day Cl−.
The Institutional Animal Care and Use Committee of Emory University approved all treatment protocols.
Measurement of Serum and Urine Chemistries, Osmolality, Arterial Blood Gases, Serum Aldosterone, and Blood Pressure
Blood was collected for serum chemistries and arterial blood gases through the abdominal aorta under isoflurane anesthesia. Urine Na+, K+, and Cl− concentrations were measured by Sound Diagnostic Lab (Seattle, WA) and using a ProLyte Electrolyte Analyzer (Diamond Diagnostics, Holliston, MA). Serum electrolytes and arterial blood gases were measured with an iSTAT Alinity V (Abbot Point of Care, Princeton, NJ). Serum K+ was also measured by IDEXX (North Grafton, MA). Serum aldosterone was measured by radioimmunoassay (Michigan State Veterinary Diagnostic Lab/IDEXX). Blood pressure was measured by tail-cuff with a Visitech Systems, BP-2000 Series II Blood Pressure analysis system (model BP-2000-CU with platform model BP-2000-MP-6), as we have previously described (15).
Urine and serum osmolality were measured using a vapor pressure osmometer (Wescor, Logan, UT). Using these values, free water clearance was calculated using the following relationship:
where U and P are urine and serum osmolality, respectively, and V is the urinary flow rate.
Electrolyte-free water clearance was calculated using the following relationship:
where UNa + K and PNa + K are urine and plasma concentrations of Na+ plus K+, respectively.
Primary Antibodies
We used ENaC antibodies, as previously described (16), that detect the β-subunit (Cat. No. SPC-404, StressMarq, 1:1,000 titer) and γ-subunit (Cat. No. SPC-405, StressMarq, 1:1,000 titer) of ENaC. To detect the 85- and 30-kDa fragments of the ENaC α-subunit, we used an α-ENaC antibody as previously described (17). The rabbit anti-rat aquaporin-2 (AQP2) antibody used has been previously described (18) and was a generous gift from Dr. Mark Knepper.
Immunohistochemistry
For standard immunohistochemistry, kidneys were fixed in situ and embedded in polyester wax [polyethylene glycol 400 distearate (Polysciences, Warrington, PA) and 10% 1-hexadecanol] (19). Two micrometer-thick sections were cut and mounted on gelatin-coated glass slides, as previously described (19).
Immunolocalization was done with standard immunoperoxidase procedures. Sections were dewaxed, rehydrated, and rinsed in distilled water. Endogenous peroxidase activity was blocked by incubating the sections in 3% H2O2 in distilled water for 45 min. Sections were then incubated at 4°C overnight with primary antibody diluted in Dako antibody diluent, washed in PBS, and incubated for 45 min in polymer-linked peroxidase-conjugated anti-mouse IgG diluted to 1:10 or 30 min in anti-rabbit IgG (Vector ImmPRESS, Vector Laboratories, Burlingame, CA), washed again with PBS, and exposed to diaminobenzidine (Vector DAB substrate kit) for 5 min. Sections were then washed in distilled water, dehydrated in graded ethanol and xylene, mounted, and observed by light microscopy.
Labeling was compared only between sections of the same thickness, which were from the same experiment done with identical reagents. Sections were examined using a Leica DM2000 microscope equipped with differential interference contrast (DIC) optics and a Leica DFC425 digital camera and Leica DFC Twain software and LAS application suite (Leica Microsystems, Buffalo Grove, IL).
Immunohistochemistry of tissue from both groups was performed simultaneously in the same experiment. Differences in wild-type and knockout (KO) label were evaluated by two blinded observers, who agreed on the overall differences in label distribution and intensity between the two groups.
Immunoblots
ENaC immunoblots were performed using previously reported methods (11, 20). Whole kidney lysates were isolated by harvesting mouse kidneys and placing them in ice-cooled buffer [0.3 M sucrose and 25 mM imidazole (pH 7.2) containing 1× Roche Complete Protease Inhibitor Cocktail]. Tissue was immediately homogenized using an Omni THQ Tissue Homogenizer and then centrifuged at 1,000 g for 15 min at 4°C. To prepare whole cell lysates, kidneys were homogenized in gentle lysis buffer (10 mM Tris·HCl, 10 mM NaCl, 2 mM EDTA, 0.5% Nonidet P-40, 1% glycerol, and Na3VO4 and freshly added 0.18 μg/mL Na3VO4, 10 μg/mL PMSF, 5 μg/mL aprotinin, and 1 μg/mL leupeptin). To enable equal protein loading in each lane, protein content in the soluble fraction of homogenates was measured using a RC-PC protein assay kit (DC Protein Assay Kit, Bio-Rad, Hercules, CA), and then dissolved in Laemmli buffer.
Aliquots containing equal amounts of protein from these lysates were separated by SDS-PAGE on 8.5% acrylamide gels and then electroblotted to PVDF membranes (Immobilon, Millipore, Bedford, MA). Blots were blocked with Odyssey blocking buffer (LI-COR Biosciences) following the manufacturer’s instructions and then incubated with primary antibody overnight at 4°C, followed by incubation for 2 h at room temperature with Alexa Fluor 680-linked anti-rabbit IgG (A-21109, Invitrogen, 1:5,000). To correct for possible differences between lanes in lysate protein loading, membranes were Coomassie stained as previously reported (21). Signals were visualized with an Odyssey Infrared Imaging System (LI-COR Biosciences). Immunoblot and Coomassie band densities were quantified using ImageJ (National Institutes of Health, available at http://rsb.info.nih.gov/). Immunoblot band density was normalized to the density of the Coomassie gel bands in the region similar mobility. To confirm protein loading, actin immunoreactivity was quantified in the same blots when possible using rabbit anti-actin antibody (A2066, Sigma-Aldrich, 1:1,000).
Statistics
Data are presented as means ± SE. Each n value used in the statistical analysis represents data from separate animals. To test for statistical significance between two groups, a paired or unpaired Student’s t test was used. To compare multiple groups, one-way ANOVA was used with Holm–Sidak or Tukey posttests, as appropriate (see figures). The criterion for statistical significance was P < 0.05.
RESULTS
Following Dietary Restriction of Na+, K+, and Cl−, Pendrin-Null Mice Excrete More NaCl Than Wild-Type Mice, Which Produces Hyponatremia
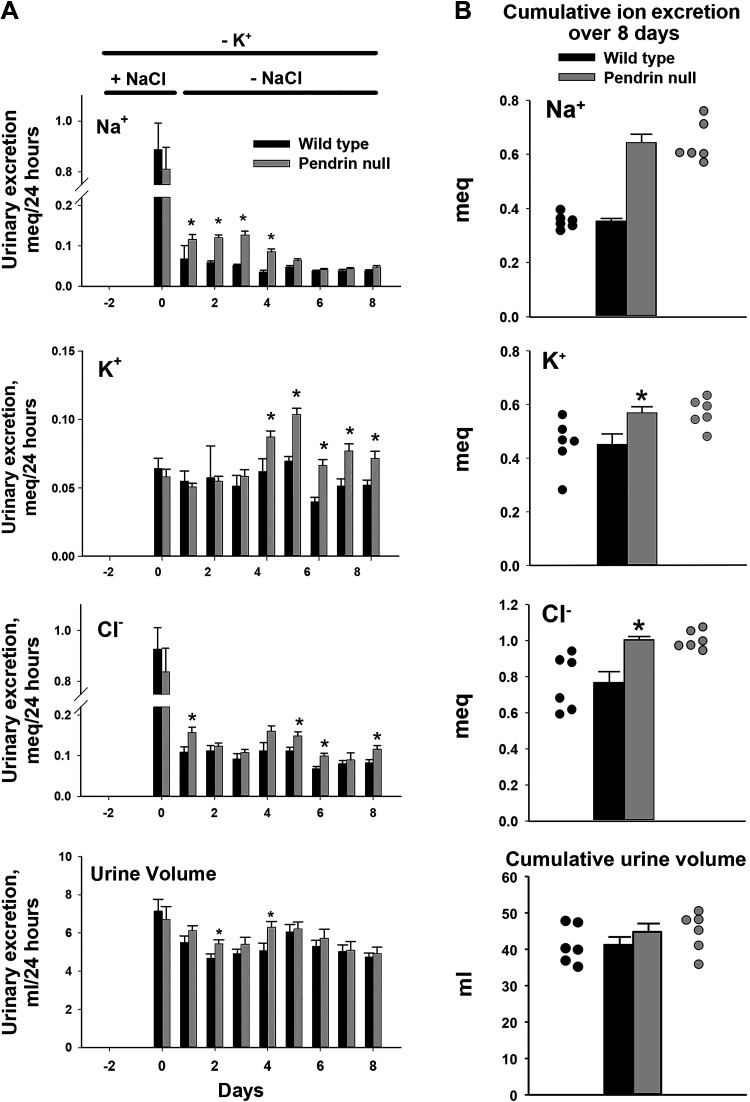
Dietary NaCl restriction greatly increases apical plasma membrane pendrin abundance, which augments renal NaCl absorption (10). Therefore, in the absence of pendrin-mediated NaCl absorption, natriuresis is observed (11). Since dietary K+ restriction reduces aldosterone (5) and may downregulate pendrin (22), we asked if the difference in NaCl excretion observed between NaCl-restricted wild-type and pendrin-null mice is blunted or eliminated when K+ is also restricted. To answer this question, we established a protocol for dietary K+ and Na+ restriction. Male mice were first conditioned with a K+-restricted diet containing control quantities of Na+ and Cl− (treatment 2) and then given a Na+, K+, and Cl−-restricted diet (treatment 1) for an additional 3 or 8 days. Following the NaCl-replete, K+-restricted diet, urinary Na+, K+, and Cl− excretion was similar in both groups (day 0; Fig. 1). However, with the introduction of the Na+, K+, and Cl−-restricted diet (treatment 1), Na+ excretion was greater in pendrin-null mice than in wild-type mice over the ensuing 3 or 4 days (Fig. 1), despite a higher serum aldosterone concentration in the mutant mice, which should increase Na+ absorption (Table 1). Over the ensuing 5 days of Na+, K+, and Cl− restriction (i.e., days 4 and 8), daily Na+ excretion was similar in both groups (Fig. 1). Figure 1 also shows that cumulative Na+ excretion over these 8 days of Na+, K+, and Cl− restriction was 82% higher in pendrin-null mice than in their wild-type littermates (P = 4.4 × 10−6). We conclude that pendrin-null mice excrete more Na+ than wild-type mice following NaCl restriction (23), whether or not K+ intake is also limited. Moreover, the greater Na+ excretion seen in pendrin-null mice cannot be explained by changes in serum aldosterone concentration (Table 1).
Figure 1.
Effect of pendrin gene ablation on urinary Na+, K+, and Cl− excretion following dietary Na+, K+, and Cl− restriction. Male pendrin-null and wild-type mice on a C57Bl/6 background were given 4 days of the NaCl-replete, K+-restricted diet (treatment 2), and then 8 days of the Na+, K+, and Cl−-restricted diet (treatment 1). Daily 24-h urinary volume as well as Na+, K+, and Cl− excretion were measured in samples that were collected continuously over the last 9 days of the protocol (A). B: cumulative urinary volume and urinary Na+, K+, and Cl− excretion over the 8 days of dietary Na+, K+, and Cl− restriction. Solid black circles indicate individual data points for wild-type mice; solid gray circles are data points for pendrin-null mice. *P < 0.05 (Student’s t test).
Table 1.
Effect of pendrin gene ablation on serum aldosterone and electrolytes in mice fed a NaCl-replete, K+-deficient diet for 4 days, and then either 3 or 8 days of a Na+, K+, and Cl−-deficient diet
| Strain | n | Na+, meq | K+, meq | Cl−, meq | Aldosterone Treatment |
|
|---|---|---|---|---|---|---|
| n | nM | |||||
| Four days of a NaCl-replete, K+-deficient diet (treatment 2) and then 3 days of a Na+, K+, and Cl−-deficient diet (treatment 1) | ||||||
| Males | ||||||
| Wild type | 9 | 141 ± 1 | 3.5 ± 0.1 | 110 ± 1 | 5 | 1.72 ± 0.19 |
| Pendrin KO | 8 | 136 ± 0.3* | 3.1 ± 0.1* | 100 ± 1* | 5 | 2.98 ± 0.38* |
| Females | ||||||
| Wild type | 7 | 142 ± 1 | 3.9 ± 0.1 | 109 ± 1 | NM | |
| Pendrin KO | 7 | 137 ± 1* | 3.2 ± 0.2* | 98 ± 2 | NM | |
| Four days of a NaCl-replete, K+-deficient diet (treatment 2) and then 8 days of a Na+, K+, and Cl−-deficient diet (treatment 1) | ||||||
| Males | ||||||
| Wild type | 14 | 145 ± 1 | 4.1 ± 0.1 | 113 ± 1 | 5 | 1.28 ± 0.13 |
| Pendrin KO | 13 | 138 ± 1* | 3.0 ± 0.1* | 97 ± 1* | 5 | 1.57 ± 0.21 |
| Females | ||||||
| Wild type | 11 | 142 ± 1 | 4.0 ± 0.1 | 112 ± 1 | 5 | 1.53 ± 0.06 |
| Pendrin KO | 12 | 137 ± 1* | 3.0 ± 0.1* | 99 ± 1* | 5 | 2.65 ± 0.22* |
Values are means ± SE; n, number of mice studied. Mice were on a C57Bl/6 background. An unpaired Student’s t test was used to compare wild-type and knockout (KO) mice of the same sex. *P < 0.05. NM, not measured.
Table 1 shows that serum Na+ is lower in pendrin-null mice than in their wild-type littermates of both sexes following either 3 or 8 days of the Na+, K+, and Cl−-restricted diet. This hyponatremia does not occur from greater AQP2 water channel abundance in pendrin-null mice than in wild-type mice (Supplemental Fig. S1; all Supplemental Material is available at https://doi.org/10.6084/m9.figshare.16803613). Although Na+ excretion was greater in the mutant mice than in wild-type mice, urinary volume, electrolyte-free water excretion, and free water clearance were similar in male pendrin-null mice and wild-type mice (Fig. 1 and Supplemental Table S1). Thus, the fall in serum Na+ seen in pendrin-null mice compared with wild-type mice may occur, at least in part, from greater excretion of Na+ than water.
During Dietary Na+ and K+ Restriction, Pendrin-Null Mice Excrete More K+ Than Wild-Type Mice, Which Contributes to Their Hypokalemia
The fall in ENaC-mediated Na+ absorption and ENaC subunit abundance seen in mice lacking pendrin (11, 13) reduces the lumen-negative transepithelial voltage (11), thereby reducing the driving force for K+ secretion, which should reduce the excretion of K+. Therefore, we asked if serum K+ is higher in NaCl-restricted pendrin-null mice than in wild-type mice (7) and if this serum K+ difference occurs through changes in K+ excretion. We thus compared serum K+ and urinary K+ excretion in male wild-type and pendrin-null mice following 4 days of the NaCl-replete, K+-restricted diet (treatment 2), and then 3 or 8 days of the Na+, K+, and Cl−-restricted diet (treatment 1). Table 1 shows that with 3 days of the Na+, K+, and Cl−-deficient diet, serum K+ was 0.4 mM lower in the mutant mice than in wild-type mice of both sexes.
To determine if this fall in serum K+ occurs from urinary K+ wasting, we examined K+ urinary excretion in male pendrin-null mice and wild-type mice over the treatment period. Because urinary K+ excretion should fall with hypokalemia, we expected less K+ excretion by pendrin-null mice than their wild-type littermates (Table 1). Since urinary K+ excretion was instead similar in both groups with 3 days of dietary Na+, K+, and Cl− restriction, the pendrin-null kidney has an impaired ability to conserve K+.
At approximately day 4 of the ion-deficient diet, pendrin-null mice began to excrete more K+ than wild-type mice. Therefore, cumulative K+ excretion over 8 days of Na+, K+, and Cl− restriction was greater in KO mice than in wild-type mice (Fig. 1). This increase in K+ excretion contributed to the greater fall in serum K+ observed in pendrin-null mice seen with these additional 5 days of Na+, K+, and Cl− restriction (Table 1). Moreover, the mutant mice excreted more Cl− relative to wild-type mice, first as NaCl and later as KCl (Fig. 1).
The Fall in Serum K+ Concentration Seen With Pendrin Gene Ablation Is ENaC Dependent
We explored treatment conditions that unmask differences in serum K+ between pendrin-null and wild-type mice. Consistent with the results shown in Table 1, serum K+ was ∼0.9 meq lower in both male and female pendrin KO mice than in sex-matched wild-type mice following 7 days of the Na+, K+, and Cl−-deficient diet (treatment 1; Table 2). Because both Na+ absorption and K+ secretion increase with luminal flow (24), we asked if urinary flow influences pendrin-dependent change in serum K+. To answer this question, daily Na+, K+, and Cl− intake were held constant, whereas the water content of the gelled diet was varied, giving urine volumes of ∼1 and ∼9 mL/day (treatment 4) (25). Table 3 shows, however, that the hypokalemic phenotype seen in pendrin-null mice did not change appreciably when urinary flow was varied over this wide range.
Table 2.
Effect of dietary ion intake on serum K+ in WT and pendrin-null mice
| Ions Added to the Diet |
Serum K+ Concentration, meq |
||||||
|---|---|---|---|---|---|---|---|
| Na+ | K+ | Cl− | OtherTreatments | Strain | Male | Female | |
| Treatment 1 | − | − | − | WT | 3.9 ± 0.1 (n = 11) | 3.8 ± 0.2 (n = 3) | |
| KO | 3.1 ± 0.1 (n = 11)* | 3.0 ± 0.3 (n = 4) | |||||
| Treatment 2 | + | − | + | WT | 4.0 ± 0.3 (n = 4) | 4.4 ± 0.1 (n = 6) | |
| KO | 4.2 ± 0.3 (n = 3) | 4.1 ± 0.2 (n = 5) | |||||
| Treatment 3 | − | − | − | Amiloride | WT | 5.5 ± 0.3 (n = 9) | 4.3 ± 0.1 (n = 7) |
| KO | 5.7 ± 0.3 (n = 10) | 4.5 ± 0.1 (n = 6) | |||||
Data are means ± SE; n, number of mice studied. Mice were on a C57 Bl/6 background. *P < 0.05. KO, knockout; WT, wild type.
Table 3.
Effect of pendrin gene ablation on serum K+ in male and female mice when water intake is normal or high (treatment 4)
| n | Serum K+, meq | |
|---|---|---|
| Normal H2O intake (4.5 mL/day) | ||
| Wild type | 5 | 3.9 ± 0.2 |
| Pendrin null | 5 | 3.3 ± 0.2 |
| High H2O intake (12 mL/day) | ||
| Wild type | 5 | 3.9 ± 0.2 |
| Pendrin null | 5 | 3.4 ± 0.2 |
Data are means ± SE; n, number of mice studied. Mice were on a C57 Bl/6 background.
Because ENaC modulates renal K+ excretion, we asked if the fall in serum K+ seen in pendrin KO mice is ENaC dependent. To answer this question, renal ENaC activity was reduced by supplementing the Na+, K+, and Cl−-deficient diet with either NaCl (treatment 2) or amiloride (treatment 3). Table 2 shows that in both males and females serum K+ differences were blunted or eliminated with either NaCl or amiloride supplementation.
We asked if constitutively upregulating ENaC exacerbates the hypokalemia seen in pendrin-null mice. To do so, we used mice homozygous for wild-type ENaC or homozygous Liddle’s, a condition in which ENaC is constitutively upregulated (14). Serum K+ was first measured following 7 days of a NaCl-replete, K+-restricted diet (treatment 2) in ENaC wild-type mice that harbored either wild-type pendrin (ENaCWTWT; Slc26a4+/+) or were pendrin null (ENaCWTWT; Slc26a4−/−). Following such a diet, ENaC activity is very low in wild-type mice. As such, Table 4 shows that in mice homozygous for wild-type ENaC, serum K+ was similar in those with wild-type pendrin (ENaCWT/WT; Slc26a4+/+) and those that were pendrin null (ENaCWTWT Slc26a4−/−). However, in mice homozygous for Liddle’s mutation, serum K+ was lower in those that were pendrin null (ENaCLL; Slc26a4−/−) than in those with wild-type pendrin (ENaCLL; Slc26a4+/+). We conclude that reducing ENaC activity mitigates, whereas increasing ENaC activity enhances, the fall in serum K+ seen in pendrin-null mice. Therefore, pendrin-dependent changes in serum K+ are ENaC dependent.
Table 4.
Serum K+ and arterial blood gases in pendrin-null or wild-type pendrin mice harboring either wild-type ENaC or Liddle’s mutation given a NaCl-replete, K+-deficient diet (treatment 2)
| Strain | K+, meq |
|
|---|---|---|
| Male | Female | |
| Wild type(ENaCWTWT; Slc26a4+/+) | 3.9 ± 0.2 (n = 8) | 4.0 ± 0.1 (n = 3) |
| Pendrin null(ENaCWTWT; Slc26a4−/−) | 3.8 ± 0.4 (n = 4) | 3.8 ± 0.1 (n = 3) |
| Liddle’s(ENaCLL; Slc26a4+/+) | 3.5 ± 0.1 (n = 7) | 3.3 ± 0.2 (n = 4) |
| Liddle’s/pendrin null(ENaCLL; Slc26a4-/-) | 2.9 ± 0.2 (n = 6)* | 2.9 ± 0.1 (n = 5)† |
Data are means ± SE; n, number of mice studied. Mice were on a 129 SvEv Tac background. Sex-matched wild-type and pendrin-null littermates were compared with an unpaired t test. *P < 0.05; †P = 0.06.
Downregulation of ENaC Helps Maintain Serum K+ but at the Expense of Na+ Balance
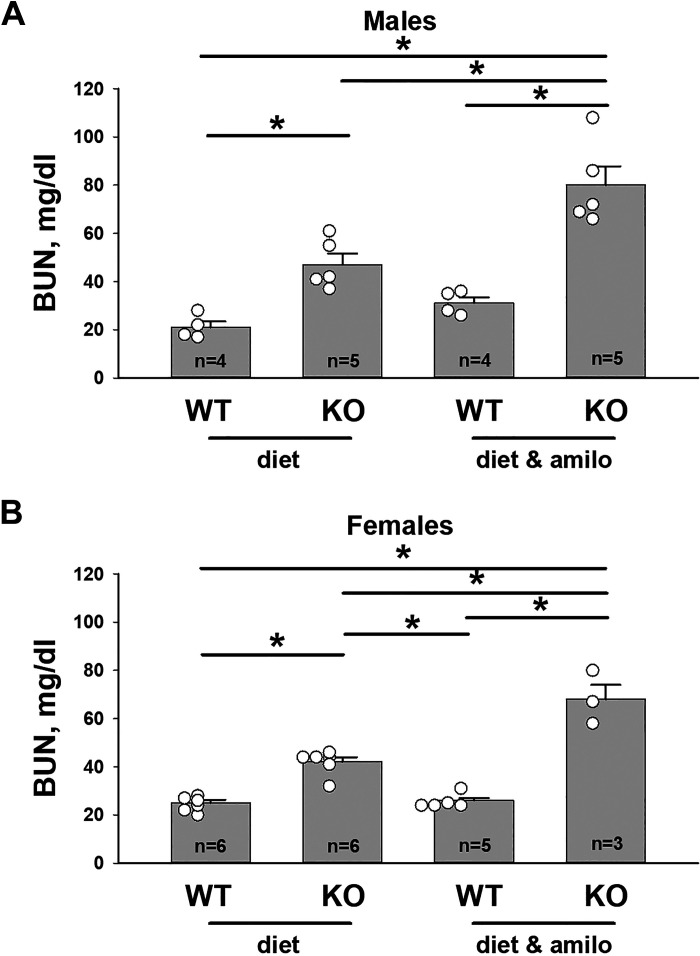
While ENaC blockade eliminates the serum K+ difference between pendrin-null mice and wild-type mice, we hypothesized that it will exacerbate differences in apparent intravascular volume. To test this hypothesis, we compared blood urea nitrogen (BUN) as an indicator of intravascular volume in male and female wild-type and pendrin-null mice following 7 days of the Na+, K+, and Cl−-deficient diet or diet plus the ENaC inhibitor amiloride. As shown in Fig. 2, A and B, BUN was higher in Na+, K+, and Cl−-restricted pendrin-null mice than in wild-type mice of both sexes (treatment 1). However, the difference in BUN between KO and wild-type mice was greater when they were also given amiloride (treatment 3). Amiloride also exacerbated the fall in serum Na+ observed in pendrin-null mice (Supplemental Fig. S2). We conclude that although ENaC inhibition eliminates serum K+ differences between wild-type and pendrin-null mice, it does so at the expense of intravascular volume.
Figure 2.
Epithelial Na+ channel inhibitors exacerbate the apparent intravascular volume contraction observed in pendrin-null mice. Blood urea nitrogen (BUN) was measured in male (A) and female (B) wild-type (WT) and pendrin-null [knockout (KO)] mice on a C57Bl/6 background following 7 days of the Na+, K+, and Cl−-restricted diet (treatment 1) or with diet and amiloride (treatment 4). Open circles represent the individual data points for mice in each group. *P < 0.05 (one-way ANOVA with a Holm–Sidak posttest).
Following Dietary Na+ and K+ Restriction, Changes in Na+ Excretion Correlate With Changes in ENaC Subunit Abundance
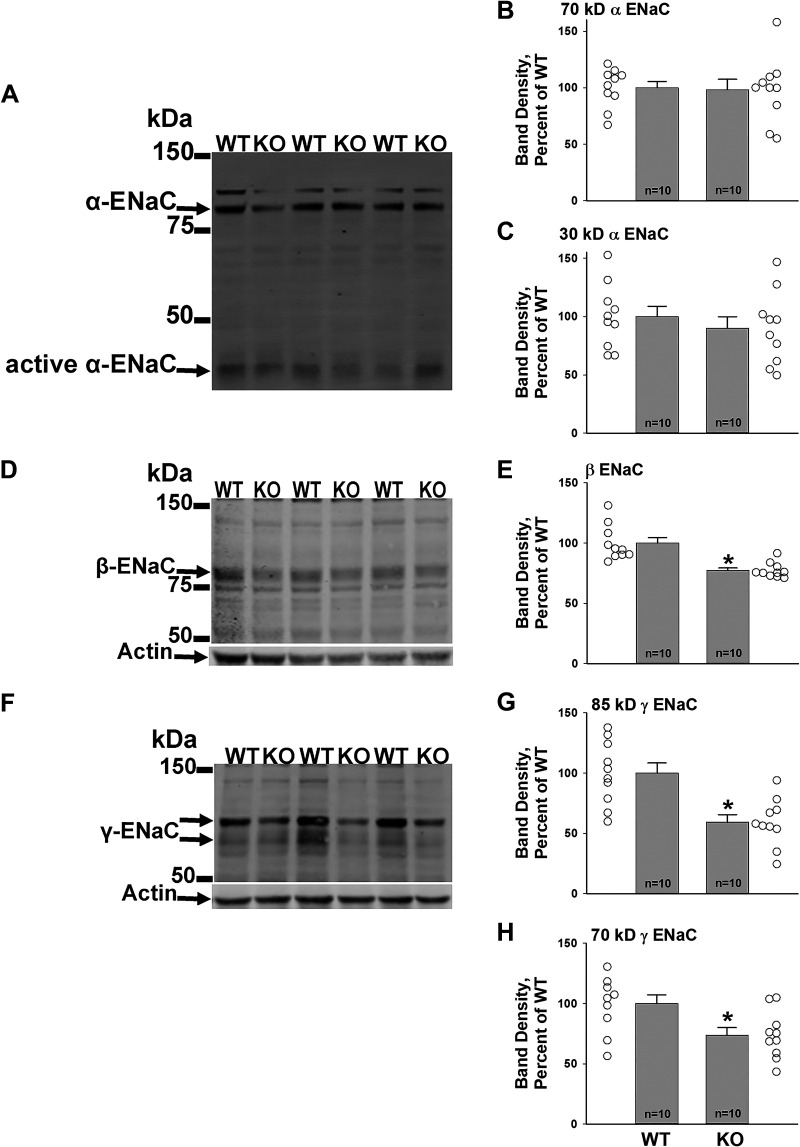
We hypothesized that with 3 days of dietary Na+, K+, and Cl− restriction, pendrin-null mice develop natriuresis because ENaC is downregulated. To test this hypothesis, ENaC subunit abundance was measured in male wild-type and pendrin-null mice that received 4 days of the NaCl-replete, K+-deficient diet (treatment 2), and then either 3 or 8 days of the Na+, K+, and Cl−-restricted diet (treatment 1). As shown in Fig. 3, after 3 days of the Na+, K+, and Cl−-restricted diet, α-subunit abundance was similar in both groups, although the abundance of the 70- and 85-kDa γ-ENaC fragments as well as the β-ENaC subunit were lower in male pendrin-null mice relative to wild-type mice, consistent with our previous study (11). Similar observations were made in females (Supplemental Fig. S3). However, in both males and females, smaller differences in ENaC subunit abundance were detected in KO and wild-type mice with 8 days of the Na+, K+, and Cl−-deficient diet (Fig. 4). These data are consistent with the similar rates of Na+ excretion seen in KO and wild-type mice at this time point. We conclude that when a Na+, K+, and Cl−-restricted diet is introduced, pendrin-null mice initially downregulate ENaC relative to that of wild-type mice, which increases Na+ excretion. Thereafter, Na+ excretion and ENaC abundance are similar in both groups.
Figure 3.
With 3 days of Na+, K+, and Cl− restriction, β- and γ-epithelial Na+ channel (ENaC) subunit abundance is lower in male pendrin-null [knockout (KO)] mice than in wild-type (WT) mice. Pendrin-null and WT mice were given 4 days of the NaCl-replete, K+-restricted diet (treatment 2), and then 3 days of the Na+, K+, and Cl−-restricted diet (treatment 1). As shown, although α-ENaC subunit abundance was similar in both groups (A–C), the abundance of both β-ENaC (D and E) and the 70- and 85-kDa fragments of γ-ENaC (F–H) were lower in kidneys from pendrin-null than from WT mice. Open circles represent individual data points for WT mice (left) and pendrin-null (right) mice. *P < 0.05 (unpaired Student’s t test).
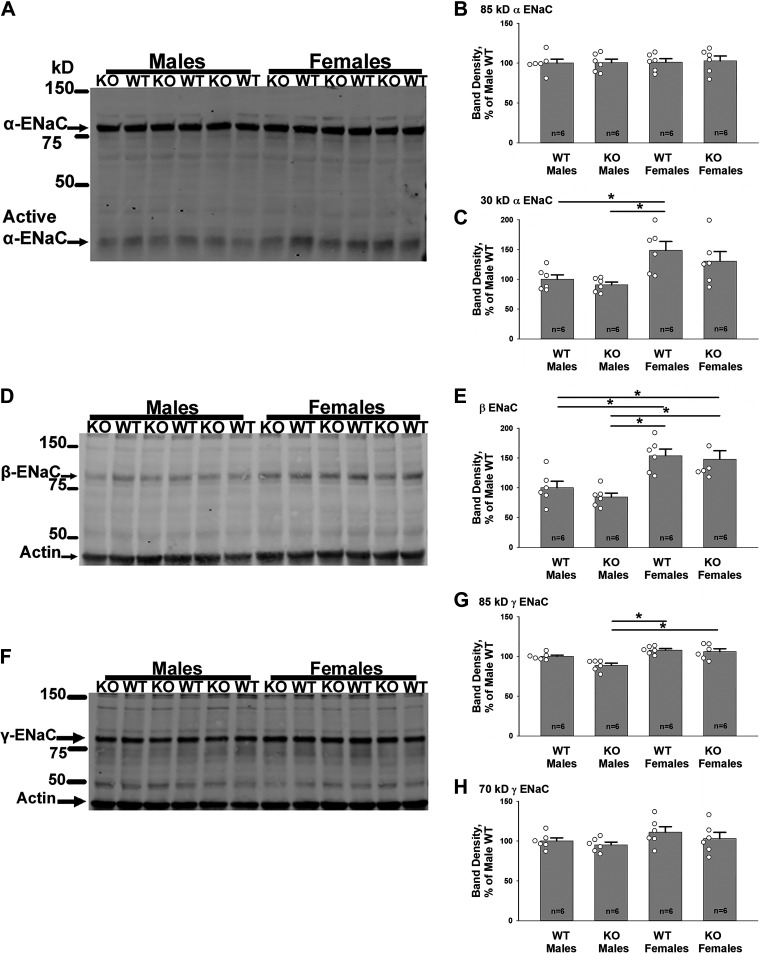
Figure 4.
With 8 days of the Na+, K+, and Cl−-restricted diet, epithelial Na+ channel (ENaC) subunit abundance is similar in pendrin-null [knockout (KO)] and wild-type (WT) kidneys, although subunit abundance is greater in kidneys from female mice than from male mice. Shown are representative α-, β-, and γ-ENaC subunit immunoblots of kidney lysates from male and female WT and pendrin-null mice following 4 days of the NaCl-replete, K+-deficient diet (treatment 2), and then 8 days of the Na+, K+, and Cl−-deficient diet (treatment 1; A, D, and F) and their respective band densities (B, C, E, G, and H). As shown, the abundance of both the β-ENaC subunit and the 85-kDa fragment of γ-ENaC were higher in female than in male pendrin-null mice, although the abundance of the other ENaC subunits was similar. However, in both sexes, ENaC subunit abundance was similar in KO and WT mice. Open circles represent individual data points of mice from each group. Groups were compared with one-way ANOVA with a Holm–Sidak posttest (30-kDa α-ENaC and β-ENaC) and a Tukey posttest (85-kDa γ-ENaC). *P < 0.05.
Following a Na+, K+, and Cl−-Restricted Diet, ENaC Subunit Abundance and Apparent Intravascular Volume Are Lower in Male Than in Female Pendrin-Null Mice
Because ENaC subunit abundance is higher in female rodents than in male rodents (11), we hypothesized that ENaC subunit abundance is also higher in female pendrin-null mice than in male pendrin-null mice. To test this hypothesis, we compared ENaC subunit abundance in male and female pendrin-null mice and their wild-type littermates following 4 days of the NaCl-replete, K+-deficient diet (treatment 2), and then 8 days of the diet deficient in Na+ and K+ (treatment 1). Figure 4 shows that ENaC subunit abundance was the same or higher in female compared with male pendrin-null mice.
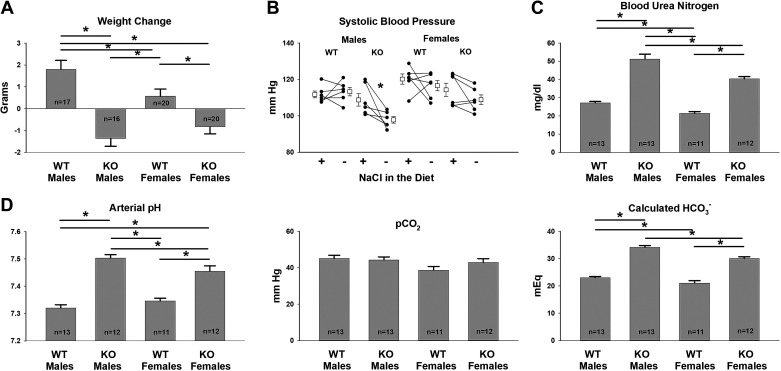
Because ENaC subunit abundance is lower in male than in female pendrin-null mice, we hypothesized that apparent intravascular volume is also lower in males. To test this hypothesis, we compared the weight change in male and female wild-type and pendrin-null mice seen with the treatment protocol shown in Fig. 1. Although both male and female pendrin KO mice lost weight over the 12 days of treatment (Fig. 5A), weight loss was greater in males than in females. Moreover, when transitioned from a NaCl-replete diet to a NaCl-deficient diet, blood pressure fell more in male than female pendrin-null mice (Fig. 5B).
Figure 5.
Following dietary Na+, K+, and Cl− restriction, apparent intravascular volume depletion is greater in male than in female pendrin-null [knockout (KO)] mice. Male and female wild-type (WT) and pendrin-null littermates were given a NaCl-replete, K+-deficient diet (treatment 2) for 4 days, and then 7 or 8 days of the Na+, K+, and Cl−-deficient diet (treatment 1). A: weight change over the 12-day treatment period in mice from these four groups. Blood pressure was compared in the same mice after the NaCl-replete, K+-deficient diet, and then the Na+, K+, and Cl−-deficient diet using a paired Student’s t test (B). Blood urea nitrogen (C) and arterial blood gases (D) were measured at the end of the 12-day treatment period in mice from each group. Multiple comparisons were made with one-way ANOVA and a Holm–Sidak posttest (A and D). Values in C were compared using Kruskal–Wallis one-way analysis of variance on ranks. *P < 0.05.
To assess apparent intravascular volume further, we compared arterial blood gases and BUN in male and female wild-type and pendrin-null mice following this 12-day treatment protocol. Both male and female Na+, K+, and Cl−-restricted pendrin-null mice developed metabolic alkalosis, whereas their wild-type littermates did not (Fig. 5B). However, both arterial pH and calculated were greater in male than female pendrin-null mice (Fig. 5B). Similarly, although BUN was similar in male and female wild-type mice, it was higher in male than female pendrin-null mice (Fig. 5C). As such, the metabolic alkalosis and the rise in BUN observed in pendrin KO mice following a Na+- and K+-deficient diet are more severe in males than in females, which is consistent with their greater apparent vascular volume contraction.
DISCUSSION
The present study shows that pendrin-null mice develop severe hypokalemia and renal K+ loss when dietary NaCl and K+ are restricted. Consistent with our observations in rodents, case reports have described severe hypokalemia in people with Pendred syndrome under basal conditions (26, 27) and following thiazide administration (26), ethanol, or vomiting (28, 29).
In people and in mice, inactivation sequence variants in the gene encoding pendrin, SLC26A/Slc26a4, produce an impaired ability to conserve NaCl, which lowers blood pressure and generates severe metabolic alkalosis during NaCl restriction (5, 10–12, 30, 31). This fall in intravascular volume seen in pendrin-null mice occurs in large part from reduced ENaC abundance and function, particularly following high aldosterone states, such as following dietary NaCl restriction or with the administration of aldosterone. Because of ENaC’s critical role in maintaining intravascular volume (11), why ENaC is downregulated in these volume contracted, Na+-restricted mutant mice has been puzzling (11–13). The present study demonstrates that the pendrin-null kidney has an inability to fully conserve K+. The kidney limits this renal K+ loss by downregulating ENaC, which helps maintain K+ homeostasis. However, if both Na+ and K+ are restricted, the need to downregulate ENaC for K+ homeostasis is at odds with the need to stimulate ENaC to maintain intravascular volume and blood pressure. As such, the ability of the pendrin-null kidney to downregulate ENaC is limited, which results in kaliuresis and hypokalemia. However, with a NaCl-replete diet, hypokalemia and renal K+ loss are not seen even when dietary K+ is restricted, because ENaC abundance and function are low and therefore ENaC-dependent K+ secretion is also low.
Although serum K+ was lower in the Na+, K+, and Cl−-restricted mutant mice than in wild-type mice, serum K+ was similar following aldosterone administration and a Na+, K+, and Cl−-replete diet (5). Although the reason for this different response is unknown, it may reflect the ability of a K+-rich diet to offset the kaliuresis of KO mice. Alternatively, it may reflect the extent to which the animal can downregulate ENaC to maintain K+ homeostasis, while still maintaining adequate intravascular volume. Whereas aldosterone-treated wild-type mice consuming a NaCl-rich diet are slightly volume expanded, NaCl-restricted mice are somewhat volume contracted. Therefore, pendrin-null mice can probably downregulate ENaC to a greater extent in the former than in the latter model. Although ENaC subunit abundance was not compared directly in these two treatment protocols, the present and previous studies have shown that channel subunit abundance is probably reduced more in pendrin-null mice relative to wild-type mice following a Na+, K+, and Cl−-replete diet, and aldosterone administration than following the Na+, K+, and Cl−-deficient diet used in this study (11, 13).
In the present study, we observed that constitutively upregulating ENaC with the Liddle’s mutation enhances the fall in serum K+ seen in pendrin-null mice. One could speculate that with sufficient ENaC downregulation, serum K+ should be similar in these Liddle’s mice whether they have wild-type pendrin or are pendrin null. In a previous study, we examined the effect of pendrin gene ablation on ENaC activity in aldosterone-treated mice that were homozygous for Liddle’s mutation, and in mice with wild-type ENaC by measuring total and benzamil-sensitive transepithelial voltage (VT) in CCDs perfused in vitro from mice in each of these groups (13). Total and benzamil-sensitive VT were greater in CCDs from Liddle’s than ENaC wild-type mice, indicating greater ENaC activity in the former than in the latter. Although pendrin gene ablation reduced VT more in Liddle’s mice than in mice with wild-type ENaC, the lumen-negative VT remained higher in pendrin-null mice that were homozygous for Liddle’s mutation (ENaCLL/Slc26a4−/−) than in KO mice with wild-type ENaC (ENaCWTWT/Slc26a4−/−). Therefore, although pendrin gene ablation reduces ENaC abundance and activity more in mice with Liddle’s mutation than in mice with wild-type ENaC, ENaC abundance and activity remained higher in pendrin-null mice with Liddle’s mutation than in mice with wild-type ENaC, thereby providing the former with a greater driving force for renal K+ secretion.
The present study shows that Na+, K+, and Cl−-restricted pendrin-null mice develop severe metabolic alkalosis. We have previously observed that secretion is lower in CCDs perfused in vitro that were taken from aldosterone-treated pendrin-null mice relative to wild-type mice. Therefore, the alkalosis observed in pendrin-null mice occurs in part from the absence of pendrin-mediated secretion. Because pendrin provides the chief transport pathway for the excretion of , pendrin KO mice develop alkalosis when challenged with an alkaline-rich diet or excessive metabolic bicarbonate generation (5, 31, 32). However, the present study suggests two other potential mechanisms for the alkalosis. First, the hypokalemia we observed in pendrin-null mice should exacerbate the alkalosis. When serum K+ falls, proximal tubule ammonium production increases (33, 34), as does ammonium transfer from the medullary interstitium to the collecting duct lumen (35). Thus, the hypokalemia seen in pendrin-null mice following Na+, K+, and Cl− restriction increases renal ammonium excretion, which increases net acid excretion, thereby exacerbating the alkalosis. Second, we observed profound intravascular volume contraction in Na+, K+, and Cl−-restricted pendrin-null mice relative to wild-type mice, which increases aldosterone and angiotensin II production, which should stimulate other renal mechanisms of H+ secretion, such as Na+/H+ exchanger isoform 3 and H+-ATPase (36, 37).
Perspectives and Significance
The pendrin-null kidney has an impaired ability to conserve K+. It attenuates this renal K+ loss by downregulating ENaC. However, when Na+ is restricted, the need to downregulate ENaC to maintain K+ homeostasis is at odds with the need to stimulate ENaC to maintain intravascular volume. As such, following dietary Na+, K+, and Cl− restriction, ENaC is stimulated less in KO mice than in wild-type mice, which attenuates the kaliuresis and fall in serum K+, but at the expense of intravascular volume and blood pressure. Pendrin-null mice also develop severe metabolic alkalosis due to the loss of pendrin-mediated secretion and most probably from their hypokalemia and volume contraction.
SUPPLEMENTAL DATA
Supplemental Table S1 and Supplemental Figs. S1–S3: https://doi.org/10.6084/m9.figshare.16803613.
GRANTS
This work was funded by DK110375 [to S.M.W. (Principal Investigator) and to P.A.W. (Program Director)].
DISCLOSURES
Dr. S. M. Wall owns common stock in Johnson & Johnson, Merck, Abbott, Beam, Becton Dickinson, Linde, and ThermoFisher. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
T.D.P. and S.M.W. conceived and designed research; T.D.P., A.J.E., J.W.V., L.A.-Q, C.C., D.C.A., S.A.K., and J.L. performed experiments; T.D.P., A.J.E., J.W.V., L.A.-Q, C.C., D.C.A., S.A.K., J.L., P.A.W., and S.M.W. analyzed data; T.D.P., A.J.E., J.W.V., L.A.-Q, C.C., J.L., P.A.W., and S.M.W. interpreted results of experiments; T.D.P., A.J.E., J.W.V. L.A.-Q., and S.M.W. prepared figures; T.D.P. and S.M.W. drafted manuscript; T.D.P., A.J.E., J.W.V., L.A.-Q, C.C., P.A.W., and S.M.W. edited and revised manuscript; T.D.P., A.J.E., J.W.V., L.A.-Q, C.C., D.C.A., S.A.K., J.L., P.A.W., and S.M.W. approved final version of manuscript.
REFERENCES
- 1.Eladari D, Chambrey R, Picard N, Hadchouel J. Electroneutral absorption of NaCl by the aldosterone-sensitive distal nephron: implication for normal electrolytes homeostasis and blood pressure regulation. Cell Mol Life Sci 71: 2879–2895, 2014. doi: 10.1007/s00018-014-1585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leviel F, Hübner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Hatim H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010. [Erratum in J Clin Invest 121: 1668, 2011]. doi: 10.1172/JCI40145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.López-Cayuqueo KI, Chavez-Canales M, Pillot A, Houillier P, Jayat M, Baraka-Vidot J, Trepiccione F, Baudrie V, Büsst C, Soukaseum C, Kumai Y, Jeunemaître X, Hadchouel J, Eladari D, Chambrey R. A mouse model of pseudohypoaldosteronism type II reveals a novel mechanism of renal tubular acidosis. Kidney Int 94: 514–523, 2018. doi: 10.1016/j.kint.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Xu N, Hirohama D, Ishizawa K, Chang WX, Shimosawa T, Fujita T, Uchida S, Shibata S. Hypokalemia and pendrin induction by aldosterone. Hypertension 69: 855–862, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08519. [DOI] [PubMed] [Google Scholar]
- 5.Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM. Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension 42: 356–362, 2003. doi: 10.1161/01.HYP.0000088321.67254.B7. [DOI] [PubMed] [Google Scholar]
- 6.Soleimani M, Barone S, Xu J, Shull GE, Siddiqui F, Zahedi K, Amlal H. Double knockout of pendrin and Na-Cl cotransporter (NCC) causes severe salt wasting, volume depletion, and renal failure. Proc Natl Acad Sci USA 109: 13368–13373, 2012. doi: 10.1073/pnas.1202671109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazo-Fernandez Y, Aguilera G, Pham TD, Park AY, Beierwaltes WH, Sutliff RL, Verlander JW, Pacak K, Osunkoya AO, Ellis CL, Kim YH, Shipley GL, Wynne BM, Hoover RS, Sen SK, Plotsky PM, Wall SM. Pendrin localizes to the adrenal medulla and modulates catecholamine release. Am J Physiol Endocrinol Physiol 309: E534–E545, 2015. [Erratum in Am J Physiol Endocrinol Metab 30: E885, 2015]. doi: 10.1152/ajpendo.00035.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Cornière N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R. Renal β-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest 123: 4219–4231, 2013. doi: 10.1172/JCI63492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinning A, Radionov N, Trepiccione F, López-Cayuqueo KI, Jayat M, Baron S, Cornière N, Alexander RT, Hadchouel J, Eladari D, Hübner CA, Chambrey R. Double knockout of the Na+-driven Cl−/HCO3− exchanger and Na+/Cl− cotransporter induces hypokalemia and volume depletion. J Am Soc Nephrol 28: 130–139, 2017. doi: 10.1681/ASN.2015070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, Verlander JW. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl− conservation. Hypertension 44: 982–987, 2004. doi: 10.1161/01.HYP.0000145863.96091.89. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y-H, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, Shin WK, Verlander JW, Sutliff RL, Wall SM. Reduced ENaC protein abundance contributes to the lower blood pressure observed in pendrin-null mice. Am J Physiol Renal Physiol 293: F1314–F1324, 2007. doi: 10.1152/ajprenal.00155.2007. [DOI] [PubMed] [Google Scholar]
- 12.Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, Walp E, Kim Y-H, Sutliff RL, Bao H-F, Eaton DC, Wall SM. Pendrin modulates ENaC function by changing luminal HCO3. J Am Soc Nephrol 21: 1928–1941, 2010. doi: 10.1681/ASN.2009121257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pech V, Wall SM, Nanami M, Bao HF, Kim YH, Lazo-Fernandez Y, Yue Q, Pham TD, Eaton DC, Verlander JW. Pendrin gene ablation alters ENaC subcellular distribution and open probability. Am J Physiol Renal Physiol 309: F154–F163, 2015. doi: 10.1152/ajprenal.00564.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pradervand S, Wang Q, Burnier M, Beermann F, Horisberger JD, Hummler E, Rossier BC. A mouse model for Liddle's syndrome. J Am Soc Nephrol 10: 2527–2533, 1999. doi: 10.1681/ASN.V10122527. [DOI] [PubMed] [Google Scholar]
- 15.Verlander JW, Hong S, Pech V, Bailey JL, Agazatian D, Matthews SW, Coffman TM, Le T, Inagami T, Whitehill FM, Weiner ID, Farley DB, Kim YH, Wall SM. Angiotensin II acts through the angiotensin 1a receptor to upregulate pendrin. Am J Physiol Renal Physiol 301: F1314–F1325, 2011. doi: 10.1152/ajprenal.00114.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masilamani S, Kim G-H, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC α, β and γ subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 18.DiGiovanni SR, Nielsen S, Christensen EI, Knepper MA. Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad Sci USA 91: 8984–8988, 1994. doi: 10.1073/pnas.91.19.8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nanami M, Pham TD, Kim YH, Yang B, Sutliff RL, Staub O, Klein JD, Lopez-Cayuqueo KI, Chambrey R, Park AY, Wang X, Pech V, Verlander JW, Wall SM. The role of intercalated cell Nedd4-2 in BP regulation, ion transport, and transporter expression. J Am Soc Nephrol 29: 1706–1719, 2018. doi: 10.1681/ASN.2017080826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein JD, Martin CF, Kent KJ, Sands JM. Protein kinase C-α mediates hypertonicity-stimulated increase in urea transporter phosphorylation in the inner medullary collecting duct. Am J Physiol Renal Physiol 302: F1098–F1103, 2012. doi: 10.1152/ajprenal.00664.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welinder C, Ekblad L. Coomassie staining as loading control in Western blot analysis. J Proteome Res 10: 1416–1419, 2011. doi: 10.1021/pr1011476. [DOI] [PubMed] [Google Scholar]
- 22.Procino G, Milano S, Tamma G, Dossena S, Barbieri C, Nicoletti MC, Ranieri M, Di Mise A, Nofziger C, Svelto M, Paulmichl M, Valenti G. Co-regulated pendrin and aquaporin 5 expression and trafficking in type-B intercalated cells under potassium depletion. Cell Physiol Biochem 32: 184–199, 2013. doi: 10.1159/000356638. [DOI] [PubMed] [Google Scholar]
- 23.Ahn KY, Park KY, Kim KK, Kone BC. Chronic hypokalemia enhances expression of the H+-K+-ATPase alpha 2-subunit gene in renal medulla. Am J Physiol Renal Physiol 271: F314–F321, 1996. doi: 10.1152/ajprenal.1996.271.2.F314. [DOI] [PubMed] [Google Scholar]
- 24.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y-H, Pham TD, Zheng W, Hong S, Baylis C, Pech V, Beierwaltes WH, Farley DB, Braverman LE, Verlander JW, Wall SM. Role of pendrin in iodide balance: going with the flow. Am J Physiol Renal Physiol 297: F1069–F1079, 2009. doi: 10.1152/ajprenal.90581.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemoine S, Eladari D, Juillard L, Bonnefond A, Froguel P, Dubourg L. The Case | Hypokalemia and severe renal loss of sodium. Kidney Int 97: 1305–1306, 2020. doi: 10.1016/j.kint.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Trepiccione F, Suzumoto Y, Perna A, Capasso G. Pure Gitelman-like syndrome secondary to SLC26A4 (pendrin) mutation. Kidney Int 100: 947–948, 2021. doi: 10.1016/j.kint.2021.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Kandasamy N, Fugazzola L, Evans M, Chatterjee K, Karet F. Life-threatening metabolic alkalosis in Pendred syndrome. Eur J Endocrinol 165: 167–170, 2011. doi: 10.1530/EJE-11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanei-Moghaddam A, Wilson T, Kumar S, Gray R. An unfortunate case of Pendred syndrome. J Laryngol Otol 125: 965–967, 2011. doi: 10.1017/S0022215111001630. [DOI] [PubMed] [Google Scholar]
- 30.Kim BG, Yoo TH, Yoo JE, Seo YJ, Jung J, Choi JY. Resistance to hypertension and high Cl− excretion in humans with SLC26A4 mutations. Clin Genet 91: 448–452, 2017. doi: 10.1111/cge.12789. [DOI] [PubMed] [Google Scholar]
- 31.Verlander JW, Kim YH, Shin W, Pham TD, Hassell KA, Beierwaltes WH, Green ED, Everett L, Matthews SW, Wall SM. Dietary Cl− restriction upregulates pendrin expression within the apical plasma membrane of type B intercalated cells. Am J Physiol Renal Physiol 291: F833–F839, 2006. doi: 10.1152/ajprenal.00474.2005. [DOI] [PubMed] [Google Scholar]
- 32.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98: 4221–4226, 2001. doi: 10.1073/pnas.071516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abu Hossain S, Chaudhry FA, Zahedi K, Siddiqui F, Amlal H. Cellular and molecular basis of increased ammoniagenesis in potassium deprivation. Am J Physiol Renal Physiol 301: F969–F978, 2011. doi: 10.1152/ajprenal.00010.2011. [DOI] [PubMed] [Google Scholar]
- 34.Tannen RL, McGill J. Influence of potassium on renal ammonia production. Am J Physiol 231: 1178–1184, 1976. doi: 10.1152/ajplegacy.1976.231.4.1178. [DOI] [PubMed] [Google Scholar]
- 35.Good DW, Caflisch CR, DuBose TD Jr.. Transepithelial ammonia concentration gradients in inner medulla of the rat. Am J Physiol Renal Physiol 252: F491–F500, 1987. doi: 10.1152/ajprenal.1987.252.3.F491. [DOI] [PubMed] [Google Scholar]
- 36.Du Z, Weinbaum S, Weinstein AM, Wang T. Regulation of glomerulotubular balance. III. Implication of cytosolic calcium in flow-dependent proximal tubule transport. Am J Physiol Renal Physiol 308: F839–F847, 2015. doi: 10.1152/ajprenal.00601.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pech V, Zheng W, Pham TD, Verlander JW, Wall SM. Angiotensin II activates H+-ATPase in type A intercalated cells. J Am Soc Nephrol 19: 84–91, 2008. doi: 10.1681/ASN.2007030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1 and Supplemental Figs. S1–S3: https://doi.org/10.6084/m9.figshare.16803613.