Abstract
Heparan sulfate (HS) is a linear polysaccharide attached to a core protein, forming heparan sulfate proteoglycans (HSPGs) that are ubiquitously expressed on the surface of almost all mammalian cells and the extracellular matrix. HS orchestrates the binding of various signal molecules to their receptors, thus regulating many biological processes, including homeostasis, metabolism, and various pathological processes. Due to its wide distribution and negatively charged properties, HS is exploited by many viruses as a cofactor to attach to host cells. Therefore, inhibition of the interaction between virus and HS is proposed as a promising approach to mitigate viral infection, including SARS-CoV-2. In this review, we summarize the interaction manners of HS with viruses with focus on significant pathogenic RNA viruses, including alphaviruses, flaviviruses, and coronaviruses. We also provide an overview of the challenges we may face when using HS mimetics as antivirals for clinical treatment. More studies are needed to provide a further understanding of the interplay between HS and viruses both in vitro and in vivo, which will favor the development of specific antiviral inhibitors.
Keywords: alphavirus, coronavirus, flavivirus, heparan sulfate, virus, SARS-CoV-2
STRUCTURE, FUNCTION, AND BIOGENESIS OF HEPARAN SULFATE
Heparan sulfate (HS) was first identified as a distinct molecule “heparin monosulfuric acid” in 1948 (1). Accumulating studies point out that HS is an important component of vertebrate and invertebrate tissues and plays vital functions in various biological processes. HS belongs to the group of polysaccharides called glycosaminoglycans (GAGs), which are unbranched chains of disaccharide repeat units consisting of amino and acidic sugars (2). Hexosamine, the amino sugar of GAGs, can be either N-acetylated or N/O-sulfated. In HS, the amino sugar is N-acetylated glucosamine (GlcNAc) that undergoes deacetylation and N/O-sulfation after chain polymerization. The acidic sugar of the GAGs disaccharide unit is glucuronic acid (GlcA) that can be epimerized to iduronic acid (IdoA) at the polymer level. An HS polymer chain of 10–100 disaccharides repeats (3) consisting of the alternating GlcNAc and GlcA units is variably sulfated and modified at different positions (Fig. 1). Together with core protein, one or multiple HS chains form heparan sulfate proteoglycans (HSPGs).
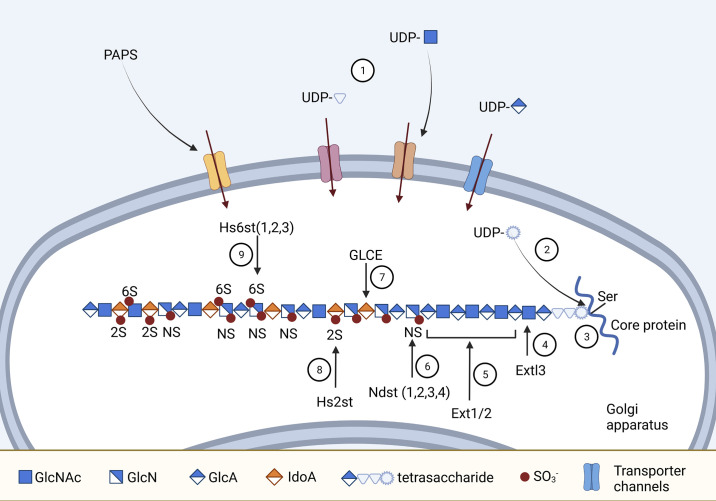
Figure 1.
Structure and biogenesis of heparan sulfate proteoglycans (HSPGs). 1: UDP-sugars and 3′-phosphoadenosine 5′-phosphosulfate (PAPS) enter the Golgi bodies via special channels in the membrane. 2: UDP-Xylose is formed from UDP-glucuronic acid (GlcA) in Golgi. 3: Glucosyl-transferases sequentially transfer the sugars from UDP to the serine residue of the protein core, making the tetrasaccharide linker (Xyl-Gal-Gal-GlcA). 4: Extl3 incorporates the first N-acetylated glucosamine (GlcNAc) into the heparin sulfate (HS) chain. 5: Ext1 and Ext2 alternatively insert GlcA and GlcNAc to the growing HS chain. 6: Isoforms of Ndst (1/2/3/4) remove N-acetyl from GlcNAc and replace it with sulfate group . 7: Deacetylation and N-sulfation of GlcNAc is followed by other modifications; for instance, GLCE catalyzes the conversion of GlcA to iduronic acid (IdoA) at certain positions. 8: Hs2st drives 2-O-sulfation of IdoA unit. 9: Hs6st transfers sulfate to the 6-O position of N-sulfated or N-acetylated GlcN. Figure created with Biorender.com.
HS biosynthesis process, which takes places in the Golgi network, is highly regulated by multiple enzymes. Compared with heparin exclusively expressed in mast cells, HS synthesis occurs in almost all mammalian cells. The sources of GlcNAc and GlcA for HS synthesis are UDP-N-acetyl glucosamine (UDP-GlcNAc) and UDP-glucuronic acid (UDP-GlcA), respectively. The UDP-sugars are synthesized in the cytoplasm and translocated to the Golgi apparatus via specialized nucleotide sugar transporters (4). The initiation of HS chain formation requires the synthesis of a linkage tetrasaccharide in the serine residue of the core protein. This tetrasaccharide is composed of xylose-galactose-galactose-glucuronic acid that is assembled sequentially by glycosyltransferases. Once the tetrasaccharide linker has organized on the protein core, different enzymes come into play to extend and modify the glycan chain, as illustrated in Fig. 1. The sulfate groups for the modification come from sulfate donors of 3′-phosphoadenosine 5′-phosphosulfate (PAPS) that are generated in the cytosol and transported into the Golgi lumen by PAPS transporters PAPST1 and PAPST2 (5).
After synthesis, HSPGs are transported to the cell surface and extracellular matrix (ECM) where the HS chains can be further modified by HS degradation enzyme heparanase (Hpse) and glucosaminyl 6-O-sulfatasess (Sulfs) (6). The core protein of HSPG on the cell surface can also be cleaved by sheddase, releasing HSPG fragments. Owing to the diversity in composition, sulfation pattern, and type of core protein, HSPGs perform a wide range of functions in mammalian physiology. Cell membrane-bound HSPGs often act as coreceptors for growth factors and modulate the receptor-ligand complex formation. Hence, one of the most elucidated functions of HSPG is to mediate the activities of signaling molecules and cellular growth. HSPGs also facilitate endocytosis (7). In addition, pathogens, including parasites, bacteria, and viruses, exploit cell-surface HSPGs for adhesion and entry to the host cell (8).
THE INTERPLAY OF VIRUSES AND HEPARAN SULFATE
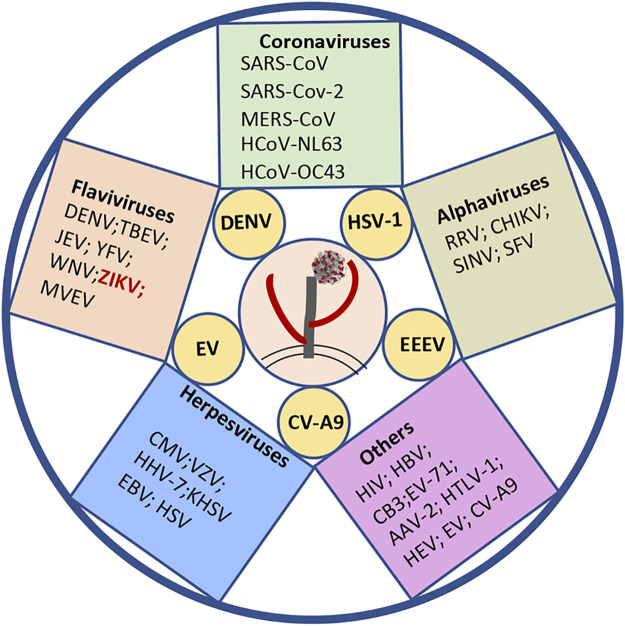
The initial step for virus infection is attachment to the target cell receptors. These receptors are usually surrounded by wide-distributed HSPGs on the cell surface. It is not surprising that viruses have evolved with different strategies to deal with HSPGs in favor of viral cell entry. HS is involved in cell attachment for various species of viruses, such as coronaviruses (SARS-CoV, SARS-CoV-2, and MERS-CoV), flaviviruses (DENV, TBEV, and JEV), retroviruses (HIV), and herpesviruses (EBV, KSHV, HSV) (Fig. 2). Among the viruses associated with human disease, only some natural virus isolates have been investigated for their interaction with HS, such as DENV, HSV-1, EEEV, Echovirus (EV), and Coxsackievirus A9 (CV-A9). While for most other virus species, HS only exhibits a high-affinity interaction with their cell-adapted strains or laboratory strains (Fig. 2).
Figure 2.
The human pathogenic viruses utilize heparan sulfate (HS) as an attachment factor directly, or indirectly, or under debate. The natural isolates of viruses labeled in yellow circles are able to bind to HS. The others can also interact with HS, either from cell-adapted or lab strains (in the squares). ZIKV marked in red indicates its interaction with HS, which is under debate. Coronaviruses: SARS-CoV, severe acute respiratory syndrome coronavirus (9); SARS-CoV-2, severe acute respiratory syndrome coronavirus 2 (10–14); HCoV-NL63, human coronavirus NL6 (15); HCoV-OC43, human coronavirus (16); and MERS-CoV, Middle East respiratory syndrome coronavirus (11). Alphaviruses: RRV, Ross river virus (17); EEEV, Eastern equine encephalitis virus (18, 19); SINV, Sindbis virus (20, 21); CHIKV, Chikungunya virus (22–24); SFV, Semliki forest viruses (25); Flaviviruses: DENV, Dengue virus (26–29); TBEV, Tick-borne encephalitis virus (30, 31); JEV, Japanese encephalitis virus (32, 33); YFV, Yellow fever virus (34); WNV, West Nile viruses (35); and Murray Valley encephalitis virus (MVEV) (33). Herpesviruses: CMV, cytomegalovirus (36, 37); VZV, Varicella zoster virus (38–40); HHV-7, human herpesvirus 7 (41, 42); KSHV, Kaposi’s sarcoma-associated herpesvirus (43–46); EBV, Epstein Barr-virus (47); and HSV, herpes simplex virus (48–51). Others (reviewed from Ref. 52): HIV, human immunodeficiency virus; HBV, hepatitis B virus; CB3, Coxsackievirus B3; EV-71, Enterovirus 71; AAV-2, adeno-associated virus 2; HTLV-1, human T cell leukemia virus type-1; HEV, hepatitis E virus; EV, Echovirus 5/6; and CV-A9, Coxsackievirus A9.
In this review, we summarize the findings of the interplay between HS and important RNA viruses (single stranded and positive senses) from the genera of alphavirus, flavivirus, and coronavirus. Based on what we know so far, we also discuss the challenges we face in creating effective therapeutics for RNA viruses-associated diseases by targeting HS-virus interactions.
Interaction of Alphavirus and Heparan Sulfate
Alphaviruses are enveloped, single-stranded, positive-sense RNA viruses with a genome around 12 kb in length, encoding nonstructural proteins and structural proteins from two open reading frames (53). The viral surface’s E1/E2 glycoprotein spikes are engaged in receptor binding and entry (54). Though distinct host factors have been identified as receptors for different alphavirus, HS on the cell surface is found to be involved in the attachment to cells for most species of the virus family, including Sindbis virus (SINV) (20, 21), Chikungunya virus (CHIKV) (22–24, 55), Ross River virus (RRV) (17, 56), Semliki Forest virus (SFV), Venezuelan equine encephalitis virus (VEEV) (19), and Eastern equine encephalitis virus (EEEV) (18, 57, 58).
A wild-type CHIKV strain has been demonstrated to enter cells in an HS-independent way (59); however, emerging evidence showed that cell-culture adapted CHIKV (through serial passages in vitro) enhances viral ability to attach to HS by increasing positively charged amino acids in the E2 envelope glycoprotein (24). One interesting finding is that the increased HS-binding capacity usually leads to attenuated virulence in vivo. By serial passages of wild-type CHIKV-LR on Chinese hamster ovary K1 fibroblasts and C6/36 Aedes albopictus mosquito cells, several E2 mutants were produced and their enhanced dependence on HS was paralleled with less virulence in a murine model (60). Moreover, it was found that the infectivity of the live-attenuated vaccine strain (CHIKV-181/25) was highly dependent on HS (59).
Bernard and colleagues identified five cell-adapted VEEV mutants that conferred the ability to interact with HS on the cell surface, and the interaction was correlated with low morbidity of the mice (19). Due to the ubiquitous expression of HS on the cell surface, they hypothesized that binding to HS would lead to a rapid clearance of the virus from the circulation. The results from Bernard and coworker’s study corroborated their hypothesis by the evidence that all five HS-binding VEEV mutants were cleared more rapidly from the blood of mice than those mutants incapable of binding to HS (19).
A similar scenario was found concerning interaction of SINV with HS (61–64). The SINV strain AR339 exhibits HS independence in cell attachment, while the cell-adapted mutants with the accumulation of positively charged amino acids from arginine at positions 1 and 114 of E2 protein significantly boosted viral competency to interact with HS (62). Selective acquirement of mutations in E2 of SINV, in favor of HS attachment, could be achieved by a few passages (65), suggesting that enhancement of HS binding may represent one of the adaptive mechanisms utilized by alphavirus to propagate effectively in vitro. Different from the SINV laboratory strain with a relatively high affinity to HS, the SINV mutants with decreased binding to HS could be generated by infecting mice. Those mouse-adapted SINV mutants are likely to form large plaques, resulting in higher-titer viremia and often causing higher mortality when injected subcutaneously into neonatal mice (61).
Natural EEEV is capable of binding to HS probably through the positive charge in 71–77 aa region of E2. Interaction of EEEV and HS is associated with viral pathogenesis. Binding to HS promotes the neurovirulence of EEEV but attenuates the viral replication in lymphoid tissue (18).
Interaction of Flavivirus and Heparan Sulfate
Flaviviruses, mainly transmitted by arthropods (mosquitoes and ticks), can cause a broad spectrum of diseases ranging from nonsymptomatic or mild infections to severe encephalitis, acute flaccid paralysis, congenital abnormalities, and even fetal death. Flaviviruses are enveloped, single-stranded, positive sense-RNA viruses, encoding three structural proteins and seven nonstructural proteins (66). Among the structural proteins, the envelope protein (E) is primarily responsible for attachment to cellular factors and mediates cell fusion (67). A large number of cellular factors have been suggested as attachment factors for flaviviruses entry. One of the best-characterized attachment factors is HS (67). HS has been demonstrated to link the cell entry of many flaviviruses directly or indirectly, including Dengue virus (DENV) (26–29), Zika virus (ZIKV) (68, 69), Yellow fever virus (YFV) (34), Tick-borne encephalitis virus (TBEV) (31), West Nile virus (WNV) (35), and Japanese encephalitis virus (JEV) (32, 33).
Natural circulating isolates of DENV were found to use HS as an attachment factor. Recombinant subviral particles, which encoded prM-gPE sequences of two low-passage clinical isolates, were capable of binding to heparin (a clinical used anticoagulant having similar structure as HS) specifically (70). In addition, heparin exhibits inhibitory effects against infection of four serotypes of dengue viruses to BHK-21 cells (71). However, the study from Lin et al. (72) claimed that in contrast to DENV-2, the clinical isolates of DENV-1, DENV-3, and DENV-4 were less effectively inhibited by heparin. The possible reasons for these seemingly contradictory observations could be due to the difference in DENV strains used in the studies (with distinct passage histories) and the assays applied. Similar to alphaviruses, serial passages of DENV in cells could produce high HS-binding mutated strains. By passaging neurovirulent prototype low-passage (NGC) strain PUO-218 in BHK-21 and SW13 cells, Lee and coworkers (73) identified four mutations on the E2 protein of DENV-2. The mutants are associated with an increase in HS-binding affinity due to the gain of net positive charges on the viral surface. Similar to the characteristics of alphaviruses, high HS-binding DENV strains are correlated with reduced virulence in animals. In addition, by assessing the relationship of HS affinity and virulence in mice deficient in alpha/beta/gamma infection responses, Lee and colleagues (73) found there is a strong correlation between high GAG affinity and low neuroinvasiveness.
A similar phenomenon regarding HS dependence and viral virulence in animals was also observed in TBEV, JEV, and WNV (33, 35, 74). Adapting JEV and WNV to grow in human adenocarcinoma (SW13) cells resulted in substitutions of E2 (residues 49, 138, 306, or 389/390) with positively charged amino acids, which increased the viral affinity for HS. Cell culture-adapted TBEV with a single amino acid mutation in the envelope protein led to an enhanced affinity for HS. These cell-adapted mutants appeared to show reduced virulence or neuroinvasiveness in mouse models as compared with their parental strains. One of the mechanisms underlying the attenuated virulence for those high HS-binding flavivirus mutants is linked to the reduction of virus spread and secondary infection by virtue of their more rapid clearance from the circulation. Those collective observations point to a common feature for flaviviruses (DENV, TEBV, JEV, and WNV); e.g., serial passages in cell culture are prone to select for mutants with increased affinity for HS, whereas these cell-adapted HS-dependent mutants are often related to reduced virulence in vivo (31, 33, 74).
For another important member of the flaviviruses, ZIKV, its relationship with HS is still under debate. A couple of studies showed a potential dependence of ZIKV on HS for cell entry (68, 69). By a surface plasmon resonance binding assay, recombinant ZIKV envelope protein has been demonstrated to interact with several glycosaminoglycans, including HS (68). A recent study indicated that HS acted as a key factor to mediate ZIKV infection on Vero cells (69).
However, some other studies supported that HS is dispensable for the attachment of ZIKV to cells. In a cell model, knocking out the HS biosynthesis enzymes beta-1,3-glucoronlytransferase (B3GAT3) or beta 1,4-galactosyltransferase (B4GALT7), or the PAPS transporter (SLC35B2), significantly reduced HS expression, which led to a dramatic reduction of DENV-2 attachment to the cells, but no effect on ZIKV entry (75). Knockdown of HS biosynthesis or removal of cellular HS by enzymes did not impact ZIKV infection, further indicating that ZIKV did not utilize HS as its attachment factor to infect cells (76). As heparin is often used as an HS mimetic, several studies evaluated the effect of heparin on ZIKV infection of cells. The study by Ghezzi and colleagues (77) found that heparin failed to inhibit ZIKV replication in human neural progenitor cells. However, a significant inhibitory activity of heparin on ZIKV infection on Vero cells was reported by Tan et al. (76). In contrast, another study showed a promoting effect of heparin on ZIKV infection of Vero cells (78). The conflicting findings regarding to the role of HS in ZIKV infection could be ascribed to the different cell types and experimental methods used, and they could also be associated with the complexity of ZIKV and HS interaction. Though heparin has usually been used as a substituent to evaluate the role of HS in viral attachment, it probably has other functions in virus infection instead of acting as the antagonist of HS-virus interaction at the entry step.
Interaction of Coronavirus and Heparan Sulfate
Coronaviruses (CoVs), with wide-spectrum hosts, are enveloped viruses with a positive-sense, single-stranded RNA genome. The genome of CoVs is around 30 kb, the largest among all RNA viruses, mainly encoding four structural proteins (nucleocapsid protein-N, spike protein-S, membrane protein-M, and envelope protein-E) along with several nonstructural proteins and accessory proteins (79). Among many species of CoVs, seven types of CoVs have been associated with human diseases. Four of them (HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1) usually only cause nonsymptomatic or mild infections of the upper respiratory tract. In contrast, the other three CoVs, including the Middle East respiratory syndrome coronavirus (MERS-CoV), the severe acute respiratory syndrome coronavirus (SARS-CoV), and SARS-CoV-2, can lead to severe symptoms and even death (80). In general, CoVs utilize the spike protein to attach to the specific receptors on the cell surface, which was well highlighted during the outbreaks of SARS-CoV and SARS-CoV-2. Except for the functional receptors, these viruses often hijack other cellular factors to concentrate on the cell surface, e.g., HS. It has been reported that cell surface HS acts as an attachment factor for animal and human infective CoVs, including HCoV-NL63, SARS-CoV, and SARS-CoV-2 (9–15, 81). Instead of using spike protein, HCoV-NL63 exploited its M protein to orchestrate the entry by interacting with HS on the cell surface (82). In response to the COVID-19 pandemic caused by SARS-CoV-2, extensive studies have been focused on elucidating the roles of HS on SARS-CoV-2 entry (Table 1).
Table 1.
Interaction of heparan sulfate and SARS-CoV-2
| References | Role of Heparan Sulfate | The Types of Virus Stains/Protein | Note |
|---|---|---|---|
| Clausen et al. (14) | Attachment factor | 1) SARS-CoV-2 live virus (USA-WA1/2020)2) pp-SARS2 | Binding to HS changes the conformation of the spike protein |
| Kim et al. (83) | Interaction with recombinant S | Recombinant SARS-CoV-2 spike protein | Recombinant spike protein interacts with the GAG-binding motif at the S1/S2 site and [453-459 (YRLFRKS)] |
| Mycroft-West et al. (12) | Heparin inhibits the SARS-CoV-2 entry | Italy/UniSR1/2020 isolate (GISAID accession ID: EPI_ISL_413489) | Binding to the RBD is more likely dependent on the structures of 2-O- or 6-O-sulfate groups |
| Schuurs et al. (84) | Interaction with spike protein | Bioinformatics and molecular dynamics simulations | The heparan sulfate binds the furin cleavage site and S247R; heparin binds the furin cleavage site and surrounding glycosylation structures, but not S247R |
| Chu et al. (10) | Attachment factor | 1) SARS-CoV-2 HKU-001a (GenBank accession number MT230904)2) SARS-CoV-2 HKU-001a S1/S2mut (GenBank accession number MT621560)3) pp-SARS2 | Both human lung epithelial cells and ex vivo human lung tissues were used in assessing the role of heparan sulfate |
| Tandon et al. (85) | Sulfated polysaccharides exhibit potent anti-SARS-CoV-2 activity | pp-SARS2 | |
| Yan et al. (86) | Binding to the spike protein | pp-SARS2 | SARS-CoV-2 spike protein binds stronger to human lung heparan sulfate than bat lung heparan sulfate |
| Partridge et al. (87) | Unfractionated heparin inhibits spike protein interaction | Recombinant SARS-CoV-2 spike protein | Chondroitin sulfate did not inhibit the binding of the spike protein |
| Yue et al. (88) | Facilitates spike protein-mediated SARS-CoV-2 cell entry | pp-SARS2 | Overexpression of Ext1 in 293 T-ACE2 cells increased pp-SARS2 infection |
| Liu et al. (89) | Attachment factor | Recombinant protein | HS Functions as initial attachment factor, which facilitates the binding of spike protein to ACE2 |
| Hao et al. (11) | Binding to the spike protein | Recombinant protein | S proteins are able to bind to HS in a sulfation-dependent manner specifically |
| Gupta et al. (90) | Heparin inhibits SARS-CoV-2 infection | SARS-COV2/MT020880.1 | |
| Shiliaev et al. (91) | Heparin can effectively inhibit Cell adapted SARS-CoV-2 but not natural isolates | 1) SARS-CoV-2 strain 2019nCoV/USA_WA1/20202) CoV-2/UAB | Serial passages of natural SARS-CoV-2 isolates in Vero-E6 increases the affinity to HS |
| Bermejo-Jambrina et al. (92) | Attachment receptor | 1) SARS-Related Coronavirus 2, Isolate Italy-INMI1, NR-52284; 2) PP-SARS2 | Heparan sulfate proteoglycans Syndecan 1 and 4 are important for SARS-CoV-2 binding |
| Chittum et al. (93) | Interacts with SARS-CoV-2 spike | HS microarray/SARS-CoV-2 protein | Equivalent importance of IdoA2S−GlcNS6S and GlcNS3S on binging to RBD |
| Zheng et al. (94) | Heparin binding to SARS-CoV-2 spike protein | mFc-tagged S1 protein | Spike protein directly promotes blood coagulation and thrombosis in the zebrafish model |
HS, heparin sulfate; pp-SARS2: pseudotyped SARS-CoV-2.
Clausen and colleagues (14) have pointed out that HS plays a vital role in the interaction of SARS-CoV-2 spike protein and the functional receptor angiotensin-converting enzyme 2 (ACE2) based on evidence obtained by multiple experimental settings. By computational analysis, HS was predicted to strongly interact with the positively charged Arg346, Arg355, Lys444, and Arg466 residues of SARS-CoV-2 spike protein receptor-binding domain (RBD). The RBD-HS binding amino acids on the spike protein are adjacent to the residues responsible for binding to ACE2 (95), which provides the basis for RBD to bind to HS and ACE2 in a cooperative manner. Besides, Clausen and coworkers (14) also corroborated that the entry of SARS-CoV-2 is HS dependent using pseudotyped and authentic SARS-CoV-2 through knocking down of HS synthesis enzymes or eliminating HS, or by addition of heparin as an inhibitor for RBD-HS interaction. Several reported and ongoing studies using different approaches show that HS is an important attachment factor for SARS-CoV-2 entry (see Table 1), suggesting that it is a reasonable and promising tactic to block SARS-CoV-2 infection by HS mimetics.
However, regarding the HS dependence of SARS-CoV-2, there is an updated study indicating a different view. Shiliaev and coworkers (91) found that SARS-CoV-2 natural isolates had a low affinity to HS, which was evident by the weak binding of the virus to heparin-agarose beads and viral resistance to heparin inhibition for cell entry. Interestingly, their study showed that the HS binding ability was dramatically escalated by gaining net positive charges in the NH2-terminal domain and RBD domains of the spike protein through serial passages in Vero-E6 cells. The cell-adaptive SARS-CoV-2 mutants with high HS affinity are not surprising since similar features were discovered in many other RNA viruses, especially among the alphaviruses and flaviviruses (as discussed above). This finding raises a lot of questions about the roles of HS on SARS-CoV-2 infection. Is low affinity to HS a common feature for natural isolates/clinical isolates? If the circulating SARS-CoV-2 mutants in the human population have different affinities to HS? Whether there is a common adaptation mechanism for CoVs in their interaction with HS? It would be highly interesting to compare the current SARS-CoV-2 variants (both clinical isolates and cell-adapted strains), with regard to their dependence on cell surface HS for virus entry and virulence.
PERSPECTIVE AND CHALLENGE FOR MITIGATING SARS-COV-2/RNA VIRUS INFECTION BY TARGETING HEPARAN SULFATE
RNA virus-associated diseases have been emerging as one of the most serious global health problems, such as the ongoing COVID-19 pandemic. The urgent need for the development of effective prevention and treatments draws researchers’ attention to one of the critical host factors, HS, due to its role as an attachment factor for a wide-range of species of RNA viruses (see Fig. 2). Based on the roles of HS acting as a “virus collector,” disturbing the interaction between HS and viruses by HS mimetics is believed to be a promising approach to reduce viral infection. However, many questions remain to be clarified regarding the application of HS mimetics as anti-RNA virus inhibitors, especially for SARS-CoV-2.
First, HS is ubiquitously expressed on the cell surface, serving as a coreceptor for many protein ligands essential in biological processes. To selectively inhibit virus infection, the specific structure of HS required for virus attachment should be elucidated in detail. So far, more attention in the studies from alphaviruses, flaviviruses, and coronaviruses has been paid to the key amino acids on viral proteins for binding to HS, while the knowledge about HS-specific structures that are essential for interacting with viruses is largely lacking, although few potential key structures on HS for binding to SARS-CoV-2 spike protein have been reported (12, 93). Thus biological experiments using live SARS-CoV-2 and the cells that express different HS structures (e.g., eliminating or overexpressing HS modification enzymes) are needed.
Second, in terms of interaction of virus and HS, cell-adapted viral strains were used by most studies; hence, the role of HS on clinical stains infection is not fully understood. While dozens of viruses proved to be capable of binding to HS with high affinity after adapting to cell culture, only a few reports indicate that clinical viral strains are HS dependent (as listed in Fig. 2). Therefore, whether the clinical strains of viruses have a similar binding affinity to HS as the laboratory strains and how viruses balance their HS dependence and transmissibility in vivo remain to be investigated. Regarding SARS-CoV-2, the binding affinity of HS with clinical strains should be investigated to further corroborate if the phenomenon discovered in the study by Shiliaev and coworkers (91) is common for all the SARS-CoV-2 circulating in the human population. Our own study found that heparin was not able to block cell entry of several SARS-CoV-2 clinical isolates (low passages) even at high concertation (800 μg/ml) (unpublished data), suggesting that clinical SARS-CoV-2 strains may behave differently from the cell-adapted strains in the affinity to HS. It is also of interest to assess the interplay between HS and SARS-CoV-2 variants, especially for the variants of concern, which have higher transmissibility or the increased receptor binding affinity.
Third, the interaction of HS and viral envelope proteins mediated by electronic is usually very weak. As a competitive inhibitor, the HS mimetics expected to block SARS-CoV-2 spike protein binding to ACE2 should display a much higher affinity to the spike protein. A recent report pointed out that heparin cannot block SARS-CoV-2 spike protein binding to ACE2 in vitro assay (89). It is worth investigating if there are other mechanisms underlying HS mimetic-mediated antiviral effects.
Last, other roles of HS in addition to mediating virus binding to its receptor remain to be addressed. Using HS mimetics to disturb virus infection is mainly based on the role of HS as an attachment molecule. However, HS on the cell surface is tightly related to the cellular biological processes and some of those signaling pathways might be utilized for SARS-CoV-2 or other virus infections. Whether HS is also engaged in SARS-CoV-2 propagation in the cells and release from the cells is still blank on the map. Interestingly, a recent study showed that HS bound with furin (96), a crucial cellular enzyme, facilitating SARS-CoV-2 cell entry. Moreover, as mentioned above, heparin could prevent ZIKV-induced cell death independent of blocking virus entry (77). These observations pointed out that HS may have other important pathophysiological roles in virus infection.
Altogether, HS is involved in the infection of a broad-spectrum viruses directly or indirectly, making it an attractive molecule as one of the potential antiviral therapeutical targets. A deep and comprehensive understanding of HS’s roles in RNA virus infection will advance the development of HS mimetics as antiviral inhibitors. While in exploring this strategy, one should bear in mind about the adaptive mutations of these viruses, which seems changing the properties of the viruses with regard to interaction with HS and virulence. Thus it is highly recommended to test the HS mimetics on clinical strains in several different cell lines.
GRANTS
This work was funded by Swedish Research Council (to J.-P.L.; 2020-05759) and partially supported by European Union’s Horizon 2020 Research Innovation Program Grant 874735 (VEO) and the SciLifeLab, Pandemic Laboratory Preparedness (LPP1-007) and the Swedish Cancer Foundation (to J-.P.L.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
This article is part of the special collection “Deciphering the Role of Proteoglycans and Glycosaminoglycans in Health and Disease.” Liliana Schaefer, MD, served as Guest Editor of this collection.
AUTHOR CONTRIBUTIONS
A.K. prepared figures; J. Ling, J. Li, and A.K. drafted manuscript; J. Ling, J.Li, A.L., and J.-P.L. edited and revised manuscript; J. Ling, J.Li, A.K., A.L. and J.-P.L. approved final version of manuscript.
REFERENCES
- 1.Jorpes JE, Gardell S. On heparin monosulfuric acid. J Biol Chem 176: 267–276, 1948. doi: 10.1016/S0021-9258(18)51026-7. [DOI] [PubMed] [Google Scholar]
- 2.Lindahl U, Couchman J, Kimata K, Esko JD. Proteoglycans and sulfated glycosaminoglycans. In: Essentials of Glycobiology, edited by Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH.. New York: Cold Spring Harbor, 2015, p. 207–221. [Google Scholar]
- 3.Sasisekharan R, Shriver Z, Venkataraman G, Narayanasami U. Roles of heparan-sulphate glycosaminoglycans in cancer. Nat Rev Cancer 2: 521–528, 2002. doi: 10.1038/nrc842. [DOI] [PubMed] [Google Scholar]
- 4.Blume A, Angulo J, Biet T, Peters H, Benie AJ, Palcic M, Peters T. Fragment-based screening of the donor substrate specificity of human blood group B galactosyltransferase using saturation transfer difference NMR. J Biol Chem 281: 32728–32740, 2006. doi: 10.1074/jbc.M600424200. [DOI] [PubMed] [Google Scholar]
- 5.Kamiyama S, Suda T, Ueda R, Suzuki M, Okubo R, Kikuchi N, Chiba Y, Goto S, Toyoda H, Saigo K, Watanabe M, Narimatsu H, Jigami Y, Nishihara S. Molecular cloning and identification of 3′-phosphoadenosine 5′-phosphosulfate transporter. J Biol Chem 278: 25958–25963, 2003. doi: 10.1074/jbc.M302439200. [DOI] [PubMed] [Google Scholar]
- 6.Vives RR, Seffouh A, Lortat-Jacob H. Post-synthetic regulation of HS structure: the yin and yang of the sulfs in cancer. Front Oncol 3: 331, 2014. doi: 10.3389/fonc.2013.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christianson HC, Belting M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol 35: 51–55, 2014. doi: 10.1016/j.matbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Dinglasan RR, Jacobs-Lorena M. Insight into a conserved lifestyle: protein-carbohydrate adhesion strategies of vector-borne pathogens. Infect Immun 73: 7797–7807, 2005. doi: 10.1128/IAI.73.12.7797-7807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang J, Yang N, Deng J, Liu K, Yang P, Zhang G, Jiang C. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS One 6: e23710, 2011. doi: 10.1371/journal.pone.0023710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu H, Hu B, Huang X, Chai Y, Zhou D, Wang Y, Shuai H, Yang D, Hou Y, Zhang X, Yuen TT, Cai JP, Zhang AJ, Zhou J, Yuan S, To KK, Chan IH, Sit KY, Foo DC, Wong IY, Ng AT, Cheung TT, Law SY, Au WK, Brindley MA, Chen Z, Kok KH, Chan JF, Yuen KY. Host and viral determinants for efficient SARS-CoV-2 infection of the human lung. Nat Commun 12: 134, 2021. doi: 10.1038/s41467-020-20457-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao W, Ma B, Li Z, Wang X, Gao X, Li Y, Qin B, Shang S, Cui S, Tan Z. Binding of the SARS-CoV-2 spike protein to glycans. Sci Bull (Beijing) 66: 1205–1214, 2021. doi: 10.1016/j.scib.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mycroft-West CJ, Su D, Pagani I, Rudd TR, Elli S, Gandhi NS, Guimond SE, Miller GJ, Meneghetti MC, Nader HB, Li Y, Nunes QM, Procter P, Mancini N, Clementi M, Bisio A, Forsyth NR, Ferro V, Turnbull JE, Guerrini M, Fernig DG, Vicenzi E, Yates EA, Lima MA, Skidmore MA. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the spike S1 receptor-binding domain with heparin. Thromb Haemost 120: 1700–1715, 2020. doi: 10.1055/s-0040-1721319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, Chen CZ, Swaroop M, Xu M, Wang L, Lee J, Wang AQ, Pradhan M, Hagen N, Chen L, Shen M, Luo Z, Xu X, Xu Y, Huang W, Zheng W, Ye Y. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discov 6: 80, 2020. doi: 10.1038/s41421-020-00222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clausen TM, Sandoval DR, Spliid CB, Pihl J, Perrett HR, Painter CD, et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell 183: 1043–1057, 2020. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J, Pyrc K. Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. J Virol 88: 13221–13230, 2014. doi: 10.1128/JVI.02078-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Haan CA, Haijema BJ, Schellen P, Wichgers Schreur P, Te Lintelo E, Vennema H, Rottier PJ. Cleavage of group 1 coronavirus spike proteins: how furin cleavage is traded off against heparan sulfate binding upon cell culture adaptation. J Virol 82: 6078–6083, 2008. doi: 10.1128/JVI.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W, Heil M, Kuhn RJ, Baker TS. Heparin binding sites on Ross River virus revealed by electron cryo-microscopy. Virology 332: 511–518, 2005. doi: 10.1016/j.virol.2004.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner CL, Ebel GD, Ryman KD, Klimstra WB. Heparan sulfate binding by natural eastern equine encephalitis viruses promotes neurovirulence. Proc Natl Acad Sci U S A 108: 16026–16031, 2011. doi: 10.1073/pnas.1110617108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernard KA, Klimstra WB, Johnston RE. Mutations in the E2 glycoprotein of Venezuelan equine encephalitis virus confer heparan sulfate interaction, low morbidity, and rapid clearance from blood of mice. Virology 276: 93–103, 2000. doi: 10.1006/viro.2000.0546. [DOI] [PubMed] [Google Scholar]
- 20.Byrnes AP, Griffin DE. Binding of Sindbis virus to cell surface heparan sulfate. J Virol 72: 7349–7356, 1998. doi: 10.1128/JVI.72.9.7349-7356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu W, Wang L, Yang Y, Jia J, Fu S, Feng Y, He Y, Li JP, Liang G. Interaction of E2 glycoprotein with heparan sulfate is crucial for cellular infection of Sindbis virus. PLoS One 5: e9656, 2010. doi: 10.1371/journal.pone.0009656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAllister N, Liu Y, Silva LM, Lentscher AJ, Chai W, Wu N, Griswold KA, Raghunathan K, Vang L, Alexander J, Warfield KL, Diamond MS, Feizi T, Silva LA, Dermody TS. Chikungunya virus strains from each genetic clade bind sulfated glycosaminoglycans as attachment factors. J Virol 94: e01500-20, 2020. doi: 10.1128/JVI.01500-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka A, Tumkosit U, Nakamura S, Motooka D, Kishishita N, Priengprom T, Sa-Ngasang A, Kinoshita T, Takeda N, Maeda Y. Genome-wide screening uncovers the significance of n-sulfation of heparan sulfate as a host cell factor for chikungunya virus infection. J Virol 91: e00432-17, 2017. doi: 10.1128/JVI.00432-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva LA, Khomandiak S, Ashbrook AW, Weller R, Heise MT, Morrison TE, Dermody TS. A single-amino-acid polymorphism in Chikungunya virus E2 glycoprotein influences glycosaminoglycan utilization. J Virol 88: 2385–2397, 2014. doi: 10.1128/JVI.03116-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smit JM, Waarts BL, Kimata K, Klimstra WB, Bittman R, Wilschut J. Adaptation of alphaviruses to heparan sulfate: interaction of Sindbis and Semliki forest viruses with liposomes containing lipid-conjugated heparin. J Virol 76: 10128–10137, 2002. doi: 10.1128/jvi.76.20.10128-10137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, Linhardt RJ, Marks RM. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med 3: 866–871, 1997. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 27.Germi R, Crance JM, Garin D, Guimet J, Lortat-Jacob H, Ruigrok RW, Zarski JP, Drouet E. Heparan sulfate-mediated binding of infectious dengue virus type 2 and yellow fever virus. Virology 292: 162–168, 2002. doi: 10.1006/viro.2001.1232. [DOI] [PubMed] [Google Scholar]
- 28.Hilgard P, Stockert R. Heparan sulfate proteoglycans initiate dengue virus infection of hepatocytes. Hepatology 32: 1069–1077, 2000. doi: 10.1053/jhep.2000.18713. [DOI] [PubMed] [Google Scholar]
- 29.Dalrymple N, Mackow ER. Productive dengue virus infection of human endothelial cells is directed by heparan sulfate-containing proteoglycan receptors. J Virol 85: 9478–9485, 2011. doi: 10.1128/JVI.05008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroschewski H, Allison SL, Heinz FX, Mandl CW. Role of heparan sulfate for attachment and entry of tick-borne encephalitis virus. Virology 308: 92–100, 2003. doi: 10.1016/s0042-6822(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 31.Mandl CW, Kroschewski H, Allison SL, Kofler R, Holzmann H, Meixner T, Heinz FX. Adaptation of tick-borne encephalitis virus to BHK-21 cells results in the formation of multiple heparan sulfate binding sites in the envelope protein and attenuation in vivo. J Virol 75: 5627–5637, 2001. doi: 10.1128/JVI.75.12.5627-5637.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su CM, Liao CL, Lee YL, Lin YL. Highly sulfated forms of heparin sulfate are involved in japanese encephalitis virus infection. Virology 286: 206–215, 2001. doi: 10.1006/viro.2001.0986. [DOI] [PubMed] [Google Scholar]
- 33.Lee E, Lobigs M. Mechanism of virulence attenuation of glycosaminoglycan-binding variants of Japanese encephalitis virus and Murray Valley encephalitis virus. J Virol 76: 4901–4911, 2002. doi: 10.1128/jvi.76.10.4901-4911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee E, Lobigs M. E protein domain III determinants of yellow fever virus 17D vaccine strain enhance binding to glycosaminoglycans, impede virus spread, and attenuate virulence. J Virol 82: 6024–6033, 2008. doi: 10.1128/JVI.02509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee E, Hall RA, Lobigs M. Common E protein determinants for attenuation of glycosaminoglycan-binding variants of Japanese encephalitis and West Nile viruses. J Virol 78: 8271–8280, 2004. doi: 10.1128/JVI.78.15.8271-8280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Compton T, Nowlin DM, Cooper NR. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 193: 834–841, 1993. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- 37.Boyle KA, Compton T. Receptor-binding properties of a soluble form of human cytomegalovirus glycoprotein B. J Virol 72: 1826–1833, 1998. doi: 10.1128/JVI.72.3.1826-1833.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen JI, Seidel KE. Absence of varicella-zoster virus (VZV) glycoprotein V does not alter growth of VZV in vitro or sensitivity to heparin. J Gen Virol 75: 3087–3093, 1994. doi: 10.1099/0022-1317-75-11-3087. [DOI] [PubMed] [Google Scholar]
- 39.Jacquet A, Haumont M, Chellun D, Massaer M, Tufaro F, Bollen A, Jacobs P. The varicella zoster virus glycoprotein B (gB) plays a role in virus binding to cell surface heparan sulfate proteoglycans. Virus Res 53: 197–207, 1998. doi: 10.1016/S0168-1702(97)00149-4. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Z, Gershon MD, Ambron R, Gabel C, Gershon AA. Infection of cells by varicella zoster virus: inhibition of viral entry by mannose 6-phosphate and heparin. Proc Natl Acad Sci U S A 92: 3546–3550, 1995. doi: 10.1073/pnas.92.8.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Secchiero P, Sun D, De Vico AL, Crowley RW, Reitz MS, Jr, Zauli G, Lusso P, Gallo RC. Role of the extracellular domain of human herpesvirus 7 glycoprotein B in virus binding to cell surface heparan sulfate proteoglycans. J Virol 71: 4571–4580, 1997. doi: 10.1128/JVI.71.6.4571-4580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skrincosky D, Hocknell P, Whetter L, Secchiero P, Chandran B, Dewhurst S. Identification and analysis of a novel heparin-binding glycoprotein encoded by human herpesvirus 7. J Virol 74: 4530–4540, 2000. doi: 10.1128/jvi.74.10.4530-4540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birkmann A, Mahr K, Ensser A, Yağuboğlu S, Titgemeyer F, Fleckenstein B, Neipel F. Cell surface heparan sulfate is a receptor for human herpesvirus 8 and interacts with envelope glycoprotein K8.1. J Virol 75: 11583–11593, 2001. doi: 10.1128/JVI.75.23.11583-11593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang FZ, Akula SM, Pramod NP, Zeng L, Chandran B. Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparan sulfate. J Virol 75: 7517–7527, 2001. doi: 10.1128/JVI.75.16.7517-7527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akula SM, Pramod NP, Wang FZ, Chandran B. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284: 235–249, 2001. doi: 10.1006/viro.2001.0921. [DOI] [PubMed] [Google Scholar]
- 46.Akula SM, Wang FZ, Vieira J, Chandran B. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282: 245–255, 2001. doi: 10.1006/viro.2000.0851. [DOI] [PubMed] [Google Scholar]
- 47.Chesnokova LS, Valencia SM, Hutt-Fletcher LM. The BDLF3 gene product of Epstein-Barr virus, gp150, mediates non-productive binding to heparan sulfate on epithelial cells and only the binding domain of CD21 is required for infection. Virology 494: 23–28, 2016. doi: 10.1016/j.virol.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Donnell CD, Kovacs M, Akhtar J, Valyi-Nagy T, Shukla D. Expanding the role of 3-O sulfated heparan sulfate in herpes simplex virus type-1 entry. Virology 397: 389–398, 2010. doi: 10.1016/j.virol.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.WuDunn D, Spear PG. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol 63: 52–58, 1989. doi: 10.1128/JVI.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trybala E, Liljeqvist JA, Svennerholm B, Bergstrom T. Herpes simplex virus types 1 and 2 differ in their interaction with heparan sulfate. J Virol 74: 9106–9114, 2000. doi: 10.1128/jvi.74.19.9106-9114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99: 13–22, 1999. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 52.Cagno V, Tseligka ED, Jones ST, Tapparel C. Heparan sulfate proteoglycans and viral attachment: true receptors or adaptation bias? Viruses 11: 596, 2019. doi: 10.3390/v11070596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen R, Mukhopadhyay S, Merits A, Bolling B, Nasar F, Coffey LL, Powers A, Weaver SC, and Ictv Report Consortium. ICTV virus taxonomy profile: togaviridae. J Gen Virol 99: 761–762, 2018. doi: 10.1099/jgv.0.001072. [DOI] [PubMed] [Google Scholar]
- 54.Basore K, Kim AS, Nelson CA, Zhang R, Smith BK, Uranga C, Vang L, Cheng M, Gross ML, Smith J, Diamond MS, Fremont DH. Cryo-EM structure of chikungunya virus in complex with the Mxra8 receptor. Cell 177: 1725–1737, 2019. doi: 10.1016/j.cell.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Supramaniam A, Liu X, Ferro V, Herrero LJ. Prophylactic antiheparanase activity by PG545 is antiviral in vitro and protects against ross river virus disease in mice. Antimicrob Agents Chemother 62: e01959-17, 2018. doi: 10.1128/AAC.01959-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heil ML, Albee A, Strauss JH, Kuhn RJ. An amino acid substitution in the coding region of the E2 glycoprotein adapts Ross River virus to utilize heparan sulfate as an attachment moiety. J Virol 75: 6303–6309, 2001. doi: 10.1128/JVI.75.14.6303-6309.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen CL, Hasan SS, Klose T, Sun Y, Buda G, Sun C, Klimstra WB, Rossmann MG. Cryo-EM structure of eastern equine encephalitis virus in complex with heparan sulfate analogues. Proc Natl Acad Sci U S A 117: 8890–8899, 2020. doi: 10.1073/pnas.1910670117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gardner CL, Choi-Nurvitadhi J, Sun C, Bayer A, Hritz J, Ryman KD, Klimstra WB. Natural variation in the heparan sulfate binding domain of the eastern equine encephalitis virus E2 glycoprotein alters interactions with cell surfaces and virulence in mice. J Virol 87: 8582–8590, 2013. doi: 10.1128/JVI.00937-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gardner CL, Burke CW, Higgs ST, Klimstra WB, Ryman KD. Interferon-alpha/beta deficiency greatly exacerbates arthritogenic disease in mice infected with wild-type chikungunya virus but not with the cell culture-adapted live-attenuated 181/25 vaccine candidate. Virology 425: 103–112, 2012. doi: 10.1016/j.virol.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gardner CL, Hritz J, Sun C, Vanlandingham DL, Song TY, Ghedin E, Higgs S, Klimstra WB, Ryman KD. Deliberate attenuation of chikungunya virus by adaptation to heparan sulfate-dependent infectivity: a model for rational arboviral vaccine design. PLoS Negl Trop Dis 8: e2719, 2014. doi: 10.1371/journal.pntd.0002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Byrnes AP, Griffin DE. Large-plaque mutants of Sindbis virus show reduced binding to heparan sulfate, heightened viremia, and slower clearance from the circulation. J Virol 74: 644–651, 2000. doi: 10.1128/jvi.74.2.644-651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klimstra WB, Ryman KD, Johnston RE. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J Virol 72: 7357–7366, 1998. doi: 10.1128/JVI.72.9.7357-7366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klimstra WB, Heidner HW, Johnston RE. The furin protease cleavage recognition sequence of Sindbis virus PE2 can mediate virion attachment to cell surface heparan sulfate. J Virol 73: 6299–6306, 1999. doi: 10.1128/JVI.73.8.6299-6306.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu W, Fu S, He Y, Li J, Liang G. Amino acid substitutions in the E2 glycoprotein of Sindbis-like virus XJ-160 confer the ability to undergo heparan sulfate-dependent infection of mouse embryonic fibroblasts. Virol J 7: 225, 2010. doi: 10.1186/1743-422X-7-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McKnight KL, Simpson DA, Lin SC, Knott TA, Polo JM, Pence DF, Johannsen DB, Heidner HW, Davis NL, Johnston RE. Deduced consensus sequence of Sindbis virus strain AR339: mutations contained in laboratory strains which affect cell culture and in vivo phenotypes. J Virol 70: 1981–1989, 1996. doi: 10.1128/JVI.70.3.1981-1989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roby JA, Setoh YX, Hall RA, Khromykh AA. Post-translational regulation and modifications of flavivirus structural proteins. J Gen Virol 96: 1551–1569, 2015. doi: 10.1099/vir.0.000097. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y, Maguire T, Marks RM. Demonstration of binding of dengue virus envelope protein to target cells. J Virol 70: 8765–8772, 1996. doi: 10.1128/JVI.70.12.8765-8772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim SY, Zhao J, Liu X, Fraser K, Lin L, Zhang X, Zhang F, Dordick JS, Linhardt RJ. Interaction of zika virus envelope protein with glycosaminoglycans. Biochemistry 56: 1151–1162, 2017. doi: 10.1021/acs.biochem.6b01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Varese A, Dantas E, Paletta A, Fitzgerald W, Di Diego Garcia F, Cabrerizo G, Erra Diaz F, Defelipe LA, Pallares H, Dodes Traian M, Gamarnik A, Geffner J, Remes Lenicov F, Margolis L, Ceballos A. Extracellular acidosis enhances Zika virus infection both in human cells and ex-vivo tissue cultures from female reproductive tract. Emerg Microbes Infect 10: 1169–1179, 2021. doi: 10.1080/22221751.2021.1932606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Artpradit C, Robinson LN, Gavrilov BK, Rurak TT, Ruchirawat M, Sasisekharan R. Recognition of heparan sulfate by clinical strains of dengue virus serotype 1 using recombinant subviral particles. Virus Res 176: 69–77, 2013. doi: 10.1016/j.virusres.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kato D, Era S, Watanabe I, Arihara M, Sugiura N, Kimata K, Suzuki Y, Morita K, Hidari KI, Suzuki T. Antiviral activity of chondroitin sulphate E targeting dengue virus envelope protein. Antiviral Res 88: 236–243, 2010. doi: 10.1016/j.antiviral.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Lin YL, Lei HY, Lin YS, Yeh TM, Chen SH, Liu HS. Heparin inhibits dengue-2 virus infection of five human liver cell lines. Antiviral Res 56: 93–96, 2002. doi: 10.1016/S0166-3542(02)00095-5. [DOI] [PubMed] [Google Scholar]
- 73.Lee E, Wright PJ, Davidson A, Lobigs M. Virulence attenuation of Dengue virus due to augmented glycosaminoglycan-binding affinity and restriction in extraneural dissemination. J Gen Virol 87: 2791–2801, 2006. doi: 10.1099/vir.0.82164-0. [DOI] [PubMed] [Google Scholar]
- 74.Goto A, Hayasaka D, Yoshii K, Mizutani T, Kariwa H, Takashima I. A BHK-21 cell culture-adapted tick-borne encephalitis virus mutant is attenuated for neuroinvasiveness. Vaccine 21: 4043–4051, 2003. doi: 10.1016/s0264-410x(03)00269-x. [DOI] [PubMed] [Google Scholar]
- 75.Gao H, Lin Y, He J, Zhou S, Liang M, Huang C, Li X, Liu C, Zhang P. Role of heparan sulfate in the Zika virus entry, replication, and cell death. Virology 529: 91–100, 2019. doi: 10.1016/j.virol.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 76.Tan CW, Sam IC, Chong WL, Lee VS, Chan YF. Polysulfonate suramin inhibits Zika virus infection. Antiviral Res 143: 186–194, 2017. doi: 10.1016/j.antiviral.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 77.Ghezzi S, Cooper L, Rubio A, Pagani I, Capobianchi MR, Ippolito G, Pelletier J, Meneghetti MC, Lima MA, Skidmore MA, Broccoli V, Yates EA, Vicenzi E. Heparin prevents Zika virus induced-cytopathic effects in human neural progenitor cells. Antiviral Res 140: 13–17, 2017. doi: 10.1016/j.antiviral.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim SY, Koetzner CA, Payne AF, Nierode GJ, Yu Y, Wang R, Barr E, Dordick JS, Kramer LD, Zhang F, Linhardt RJ. Glycosaminoglycan compositional analysis of relevant tissues in zika virus pathogenesis and in vitro evaluation of heparin as an antiviral against zika virus infection. Biochemistry 58: 1155–1166, 2019. doi: 10.1021/acs.biochem.8b01267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lai MM, Cavanagh D. The molecular biology of coronaviruses. Adv Virus Res 48: 1–100, 1997. doi: 10.1016/s0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meo SA, Alhowikan AM, Al-Khlaiwi T, Meo IM, Halepoto DM, Iqbal M, Usmani AM, Hajjar W, Ahmed N. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci 24: 2012–2019, 2020. doi: 10.26355/eurrev_202002_20379. [DOI] [PubMed] [Google Scholar]
- 81.Watanabe R, Sawicki SG, Taguchi F. Heparan sulfate is a binding molecule but not a receptor for CEACAM1-independent infection of murine coronavirus. Virology 366: 16–22, 2007. doi: 10.1016/j.virol.2007.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Naskalska A, Dabrowska A, Szczepanski A, Milewska A, Jasik KP, Pyrc K. Membrane protein of human coronavirus NL63 is responsible for interaction with the adhesion receptor. J Virol 93: e00355-19, 2019. doi: 10.1128/JVI.00355-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim SY, Jin W, Sood A, Montgomery DW, Grant OC, Fuster MM, Fu L, Dordick JS, Woods RJ, Zhang F, Linhardt RJ. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral Res 181: 104873, 2020. doi: 10.1016/j.antiviral.2020.104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schuurs ZP, Hammond E, Elli S, Rudd TR, Mycroft-West CJ, Lima MA, Skidmore MA, Karlsson R, Chen YH, Bagdonaite I, Yang Z, Ahmed YA, Richard DJ, Turnbull J, Ferro V, Coombe DR, Gandhi NS. Evidence of a putative glycosaminoglycan binding site on the glycosylated SARS-CoV-2 spike protein N-terminal domain. Comput Struct Biotechnol J 19: 2806–2818, 2021. doi: 10.1016/j.csbj.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tandon R, Sharp JS, Zhang F, Pomin VH, Ashpole NM, Mitra D, McCandless MG, Jin W, Liu H, Sharma P, Linhardt RJ. Effective inhibition of SARS-CoV-2 entry by heparin and enoxaparin derivatives. J Virol 95: e01987-20, 2021. doi: 10.1128/JVI.01987-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yan L, Song Y, Xia K, He P, Zhang F, Chen S, Pouliot R, Weiss DJ, Tandon R, Bates JT, Ederer DR, Mitra D, Sharma P, Davis A, Linhardt RJ. Heparan sulfates from bat and human lung and their binding to the spike protein of SARS-CoV-2 virus. Carbohydr Polym 260: 117797, 2021. doi: 10.1016/j.carbpol.2021.117797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Partridge LJ, Urwin L, Nicklin MJ, James DC, Green LR, Monk PN. ACE2-independent interaction of SARS-CoV-2 spike protein with human epithelial cells is inhibited by unfractionated heparin. Cells 10: 1419, 2021. doi: 10.3390/cells10061419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yue J, Jin W, Yang H, Faulkner J, Song X, Qiu H, Teng M, Azadi P, Zhang F, Linhardt RJ, Wang L. Heparan sulfate facilitates spike protein-mediated SARS-CoV-2 host cell invasion and contributes to increased infection of SARS-CoV-2 G614 mutant and in lung cancer. Front Mol Biosci 8: 649575, 2021. doi: 10.3389/fmolb.2021.649575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu L, Chopra P, Li X, Bouwman KM, Tompkins SM, Wolfert MA, de Vries RP, Boons GJ. Heparan sulfate proteoglycans as attachment factor for SARS-CoV-2. ACS Cent Sci 7: 1009–1018, 2021. doi: 10.1021/acscentsci.1c00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gupta Y, Maciorowski D, Zak SE, Kulkarni CV, Herbert AS, Durvasula R, Fareed J, Dye JM, Kempaiah P. Heparin: a simplistic repurposing to prevent SARS-CoV-2 transmission in light of its in-vitro nanomolar efficacy. Int J Biol Macromol 183: 203–212, 2021. doi: 10.1016/j.ijbiomac.2021.04.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shiliaev N, Lukash T, Palchevska O, Crossman DK, Green TJ, Crowley MR, Frolova EI, Frolov I. Natural and recombinant SARS-CoV-2 isolates rapidly evolve in vitro to higher infectivity through more efficient binding to heparan sulfate and reduced S1/S2 cleavage. J Virol 95: e0135721, 2021. doi: 10.1128/JVI.01357-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bermejo-Jambrina M, Eder J, Kaptein TM, van Hamme JL, Helgers LC, Vlaming KE, Brouwer PJ, van Nuenen AC, Spaargaren M, de Bree GJ, Nijmeijer BM, Kootstra NA, van Gils MJ, Sanders RW, Geijtenbeek TB. Infection and transmission of SARS-CoV-2 depend on heparan sulfate proteoglycans. EMBO J 40: e106765, 2021. doi: 10.15252/embj.2020106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chittum JE, Sankaranarayanan NV, O’Hara CP, Desai UR. On the selectivity of heparan sulfate recognition by SARS-CoV-2 spike glycoprotein. ACS Med Chem Lett 12: 1710–1717, 2021. doi: 10.1021/acsmedchemlett.1c00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zheng Y, Zhao J, Li J, Guo Z, Sheng J, Ye X, Jin G, Wang C, Chai W, Yan J, Liu D, Liang X. SARS-CoV-2 spike protein causes blood coagulation and thrombosis by competitive binding to heparan sulfate. Int J Biol Macromol 193: 1124–1129, 2021. doi: 10.1016/j.ijbiomac.2021.10.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581: 215–220, 2020. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 96.Zeng J, Meng Y, Chen SY, Zhao G, Wang L, Zhang EX, Qiu H. Structural characteristics of Heparan sulfate required for the binding with the virus processing Enzyme Furin. Glycoconj J 26: 1–11, 2021. doi: 10.1007/s10719-021-10018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]