Abstract
Redox homeostasis is elemental for the normal physiology of all cell types. Cells use multiple mechanisms to tightly regulate the redox balance. The onset and progression of many metabolic and aging-associated diseases occur due to the dysregulation of redox homeostasis. Thus, it is critical to identify and therapeutically target mechanisms that precipitate abnormalities in redox balance. Reactive oxygen species (ROS) produced within the immune cells regulate homeostasis, hyperimmune and hypoimmune cell responsiveness, apoptosis, immune response to pathogens, and tumor immunity. Immune cells have both cytosolic and organelle-specific redox regulatory systems to maintain appropriate levels of ROS. Nicotinamide nucleotide transhydrogenase (NNT) is an essential mitochondrial redox regulatory protein. Dysregulation of NNT function prevents immune cells from mounting an adequate immune response to pathogens, promotes a chronic inflammatory state associated with aging and metabolic diseases, and initiates conditions related to a dysregulated immune system such as autoimmunity. Although many studies have reported on NNT in different cell types, including cancer cells, relatively few studies have explored NNT in immune cells. This review provides an overview of NNT and focuses on the current knowledge of NNT in the immune cells.
Keywords: immune cells, inflammation, NNT, oxidative stress, ROS
INTRODUCTION
Nicotinamide nucleotide transhydrogenase (NNT) is a nuclear-encoded inner mitochondrial membrane protein that functions as a redox-driven proton pump. NNT catalyzes the transhydrogenation between NADH and nicotinamide adenine dinucleotide phosphate (NADP+), utilizing the proton gradient to bring about H+ movement down the electrochemical potential across the inner mitochondrial membrane. The forward reaction of NNT results in a reduced nicotinamide adenine dinucleotide phosphate (NADPH)-to-NADP+ ratio much higher than the NADH-to-nicotinamide adenine dinucleotide (NAD+) ratio in the mitochondrial matrix (1), thus making NNT a prominent redox regulatory protein. NNT is an efficient and vital generator of NADPH under physiological conditions in the mitochondria (2). In addition to NNT, enzymes such as mitochondrial NAD(P)+-dependent malic enzyme (ME2), glutamate dehydrogenase (GDH), NADP+-dependent isocitrate dehydrogenase (IDH2), mitochondrial NAD kinase (NADK2), and mitochondrial folate cycle can generate NADPH (1). Reviews and research articles on NNT and its role in cell metabolism, health, and disease have shed some light on the protein. However, the cellular functions of NNT remain an intriguing enigma. NNT, a significant regulator of mitochondrial redox balance, can influence immune cell function and organismal health. Thus, it is essential to investigate the role of NNT in the immune system. This review summarizes the recent findings on NNT, its function, and what needs further investigation regarding this protein in the context of the immune system and inflammation.
CELLULAR ANTIOXIDANT MECHANISMS
Cells, including immune cells, have various mechanisms to maintain redox homeostasis. The primary sources of reactive oxygen species (ROS) are the mitochondrial respiratory chain and enzyme-catalyzed reactions carried out by NADPH oxidase (NOX), nitric oxide (NO) synthase, xanthine oxidase, arachidonic oxide, lipoxygenase, cyclooxygenase, and cytochrome P450 enzymes. To maintain ROS homeostasis, cells have many exogenous and endogenous mechanisms. Exogenous dietary antioxidants, vitamins (A, C, and E), endogenous antioxidant molecules glutathione, ferritin, bilirubin, coenzyme Q, and endogenous enzymes manganese superoxide dismutase (MnSOD), copper-zinc superoxide dismutase (CuSOD), catalase, glutathione peroxidase, glutathione reductase, thioredoxin (Trx), and peroxiredoxins (Prxs) maintain ROS at appropriate levels to promote cellular redox homeostasis (3).
Evaluation of animal models that experimentally lack the antioxidant enzymes reveals the importance of redox homeostasis. For example, Sod1−/− deficient mice had oxidative damages in organs and the onset of several age-related pathologies, which were attenuated by treatment with antioxidants (4; reviewed in Refs. 5–8). Cellular systems also rely on a prevalent low molecular weight thiol, glutathione, for redox homeostasis. The recycling of oxidized glutathione (GSSG) to reduced glutathione (GSH) depends on the availability of NADPH. In addition, glutathione metabolism is essential for inducing trained immunity, evidenced by glutathione regulating proinflammatory cytokine production (9). Recent studies highlight the importance of NNT in maintaining mitochondrial NADPH and thiol-dependent systems (10).
NNT AND REDOX REGULATION AN OVERVIEW
NNT is an integral mitochondrial membrane protein expressed in eukaryotic cells, including immune cells (11, 12). NNT is highly expressed in the heart muscle, gall bladder, cerebral cortex, lung, stomach, and testes. Tissues with low expression are the bone marrow, lymph nodes, esophagus, smooth muscles, and thyroid. NNT is one of the primary regulators of cellular redox balance due to its ability to supply the mitochondria with NADPH. Recent work shows that NNT can operate in reverse under certain circumstances (discussed later), disrupting the antioxidant defense (13). NNT may have a pro or antioxidative role depending on the metabolic status of the cell and may be dictated by the proton motive force. It is important to note that high NADPH levels may slow down the forward reaction of NNT and may not necessarily reverse the reaction. Although definitive experimental evidence is lacking, some studies have attempted to evaluate NNT’s direction of action (13, 14).
NNT always exists as a homodimeric protein, and each monomer is composed of NAD(H) binding domain I (dI), membrane domain II (dII), and NADP(H) binding domain III (dIII; 15). Proton translocation through the membrane-intercalated domain is coupled to hydride transfer between NAD(H) and NADP(H) bound to extramembranous domains. The domains I and III are present in the matrix side of the mitochondria in eukaryotes (16). Finer details of the molecular structure of mammalian NNT revealed by cryo-electron microscopy offer further insight into the functioning of the protein (17). The NADP(H) binding domain of NNT opens the proton channel to the opposite sides of the membrane (mitochondrial matrix and cytoplasmic sides). The proton channel exists in two conformations depending on the binding of the dIII domain and has a degree of conformational flexibility necessary for proton translocation. Based on this model, the investigators propose a robust mechanism that explains the effects of NADP+ and NADPH on the conformation of the protein, the pH dependencies of the reverse and cyclic reactions, and the reversibility of NNT.
Since its discovery in 1953, the regulation of many cellular functions has been attributed to NNT, most notably its role in mitochondrial NADPH production (14). Mitochondria have several defense mechanisms against ROS as they are the hub of cellular respiration, which is a significant generator of ROS. However, it is essential to know that mitochondria also serve as “ROS sinks” because of the potent ROS defense system (RDS) within the organelle. RDS is specialized for removing the different species of ROS and almost all of the enzymes of RDS rely on NADPH as the source of reducing equivalent for their activity. Because of the large pools of NADPH and GSH in the mitochondria, short bursts of ROS would not affect the function of mitochondria. However, a prolonged insult from ROS would require adequate NADPH and GSH to maintain the RDS. A unique feature of mitochondrial RDS is that the organelle acts as a sink and deals with intramitochondrial ROS and can detoxify exogenous ROS species capable of crossing the inner mitochondrial membrane (1, 18, 19). Evidence suggests that the mitochondrial NADPH shuttle allows for NADPH flux between the cytosol and the mitochondria. Classic studies using cytosolic and mitochondria-targeted redox-sensitive GFP (roGFP) show the existence of redox cross-talk between mitochondria and the cytosol (20). Reducing power from the cytoplasm can be transmitted to the mitochondria by direct transport of 1) GSH to the mitochondrial matrix (21), and 2) mitochondrial NADPH shuttles that transfer NADPH reducing equivalents (22). Thus, the dysregulation of mitochondrial redox balance can promote impaired overall cellular redox balance, which is implicated in the onset and progression of many metabolic and inflammatory diseases (23–28). The role of ROS in the development of various pathologies was recently reviewed (29).
NNT AND MITOCHONDRIAL BIOENERGETICS
An intricate relationship between NNT and mitochondrial bioenergetics exists, which was demonstrated experimentally in some cell types. Silencing NNT in PC12 cells decreased oxidative phosphorylation (oxphos) with no effect in anaerobic glycolysis (30). In addition, the alteration of the redox status preceded the impairment of mitochondrial bioenergetics. The authors conclude that the disruption of the electron flux from fuel substrate to redox components induced redox imbalance and mitochondrial dysfunction, which are interdependent events regulated by NNT. Lopert and Patel (31) demonstrated the mechanistic link between NNT, mitochondrial respiration, and the catabolism of hydrogen peroxide in brain mitochondria. The brain mitochondria removed hydrogen peroxide when respiration substrates malate and glutamate feed NADH into enzyme complex I or to a lesser extent when succinate feeds FADH2 into complex II. In the brain, NNT activity linked the ability of mitochondria to remove hydrogen peroxide in a substrate and respiration-dependent manner to the thioredoxin/peroxiredoxin (Trx/Prx) system. NNT inhibition resulted in decreased mitochondrial hydrogen peroxide removal rates, reduced cellular NADPH levels, thioredoxin reductase (TrxR), and thioredoxin (TrX) activity in isolated mitochondria, and increased mitochondrial peroxiredoxin (PrX) oxidation and oxidative cell death.
NNT-deficient SK-Hep1 cells showed increased dependence on oxidative phosphorylation but had reduced ability to maintain NAD+ and NADPH levels (32). More recently, to understand the link between mitochondrial redox circuits and bioenergetics, the energy supply was manipulated by varying the flux rate through β-oxidation in muscle mitochondria in the presence of pharmacological and genetic inhibition of redox buffering circuits (33). Accelerating the flux through β-oxidation resulted in a corresponding increase in the rate of H2O2 production, and ∼80% of H2O2 was reduced to H2O by electrons drawn from redox buffering circuits supplied by NADPH. Most importantly, the electron flux through the redox buffering circuits was directly linked to the changes in oxygen consumption mediated by NNT.
Collectively, these findings show the intricate mechanistic link between NNT and mitochondrial bioenergetics. The results will have implications in understanding, managing, and treating mitochondrial diseases in general and those promoted by dysfunctional or deficient NNT, such as familial glucocorticosteroid deficiency (FGD).
NADPH-DEPENDENT CELLULAR REDOX SYSTEMS
The mitochondrial antioxidant systems have an innate ability to clear mitochondrial and cellular ROS, leading to the idea that mitochondria serve as “ROS stabilizing devices or ROS sinks” that buffer cellular H2O2 (1). Glutathione (GSH) and thioredoxin (Trx2) are the main H2O2-detoxifying pathways in the mitochondria. The thioredoxin system comprises thioredoxin and the enzyme thioredoxin reductase. The glutathione system includes glutathione and glutathione reductase. The GSH and Trx2 systems rely on the provision of NADPH to eliminate ROS (1).These two antioxidant systems act in concert or serve as a backup to maintain the redox balance.
The mitochondrial free radical theory of aging (MFRTA) is a prominent theory that hypothesizes that mitochondrial free radicals cause oxidative damage. The dysregulation of the mitochondrial RDS results in oxidative damage and is one of the prominent driving forces of aging. Attempts to either restore antioxidants by overexpression of antioxidant enzymes or by supplementation with antioxidants do not show positive improvements in prolonging the life span in general. Hence, it is acknowledged that antioxidants might need NADPH to recycle them to their active reduced forms, and supplements would not be beneficial if the levels of NADPH are deficient, as observed during aging (20). Since redox regulators such as TrX and GSH rely on NADPH, it underscores the importance of NNT and NADPH in aging (34, 35). Increasing the expression of mitochondrial thioredoxin had shown promising results in mice and primate studies, where the protein levels and activities in fibroblasts positively correlated with longevity (36). Interestingly, the study showed that overexpression of mitochondrial thioredoxin reductase but not the cytoplasmic or the partial mitochondrial form resulted in life span extension during normoxia (36).
NNT IN IMMUNE CELL FUNCTION
Loss of Nnt, whether in C57BL/6J mice or β-cell lines, is shown to reduce glucose-stimulated insulin secretion (GSIS) significantly (37). C57BL/6J mice that carry Nnt mutation are more prone to develop obesity and metabolic disease on a high-fat diet than mice without Nnt mutation. The studies by Ripoll et al. (12), showed the mechanistic link between NNT and macrophage immune response. This study was one of the first to show that the immune cells richly expressed NNT, a finding confirmed later by others and our group (11). NNT expression is also considerable in immune organs, such as the spleen and the bone marrow. Resting macrophages expressed Nnt mRNA; however, activation in response to lipopolysaccharides resulted in its downregulation. Interestingly, priming macrophages overnight with IFNγ also resulted in further downregulation of NNT mRNA and protein.
To further understand the physiological significance of NNT in macrophage immune response, the researchers overexpressed murine full-length Nnt complementary deoxyribonucleic acid (CDNA) in macrophage-like cell lines and made a series of observations. First, Nnt overexpression and the ability of macrophages to produce ROS and NO in response to activation were evaluated. Baseline ROS and ROS upon stimulation with phorbol 12-myristate 13-acetate (PMA) and TNFα/zymosan were lower in Nnt overexpressing cells and independent of NADPH oxidases (NOX). Second, Nnt overexpression resulted in a lower generation of nitric oxide (NO). Third, the cells had a higher GSH/GSSG ratio at basal levels and after 8 h of lipopolysaccharides (LPS) stimulation.
Most importantly, overexpression resulted in reduced secretion of cytokines IL-6, TNFα, and IL-1β, reduced activation of the mitogen-activated protein kinase (MAPK) pathway, but no change was observed in NF-kB activation (12). However, Nnt overexpressing cells’ inability to produce robust ROS had negative consequences in pathogen clearing as the cells were inefficient in clearing intracellular Escherichia coli. Corroborating this data, mice that lacked Nnt were more resistant to pulmonary infection by Streptococcus pneumoniae, whereas the Nnt gene rescued mice were susceptible to the infection. Thus, the inability of Nnt overexpressing cells to mount a robust immune response reveals a new role of NNT in regulating macrophages function.
An interesting association was observed in lymphocytes from patients affected with familial glucocorticosteroid deficiency (FGD) carrying a novel homozygous NNT F215S mutation (38). FDG is an autosomal recessive disorder, and Nnt was identified as the causative gene for FDG. Lymphocytes from FDG patients had 70% loss of function of NNT, deficits in oxphos capacity, lower mitochondrial mass, increased protein nitration, and mitochondrial DNA deletions. Parameters such as mtDNA deletion, increased protein nitration, and decreased oxphos capacity were affected only at <30% NNT activity. The patient’s parents were both heterozygous for the mutation and remained clinically healthy. The difference in the effects of the mutation between homozygous and heterozygous carriers shows that there might be an NADPH threshold that affects the antioxidant capacity or mtDNA repair and replication pathway. The authors also speculate that NNT might be the primary provider of NADPH for the thymidylate synthesis, and defects in dTTP could affect the maintenance of mtDNA integrity. Loss of mitochondrial mass increased mtDNA deletions, and lower complex I, IV, and V activity were also observed in C57BL/6J mice with a spontaneous Nnt mutation. These data collectively show that NNT has a significant role in maintaining mtDNA integrity, in addition to its well-known functions of providing the mitochondria with NADPH and alleviating oxidative stress.
NNT IN T CELLS
Redox balance is critical for all cell types, and T cells are not an exception. T cells provide antigen-specific adaptive immunity and they rapidly proliferate into effector cells upon encountering the cognate antigen. Their metabolic programs characterize the different functional phenotypes of T cells. The naïve T cells, T regulatory cells (Treg), and memory T cells rely on fatty acid oxidation and oxidative metabolism as their energy requirements are relatively low. On the other hand, robust and rapid glycolysis, pentose phosphate pathway, and glutaminolysis are required to support the proliferation, clonal expansion, and effector T cell function (39). Mitochondrial ROS and cellular NADPH oxidases derived ROS regulate T cell differentiation, proliferation, and metabolism (40). Yamamoto et al. (41) showed that NNT plays a vital role in regulating tumor environment, apoptosis, and ROS signaling in human T cell lines continuously exposed to asbestos, a known carcinogen. Human lymphotropic virus 1 immortalized human T cells (HTLV-1) continuously exposed to a low dose followed by exposure to transient but high doses of minerals found in asbestos were assessed for alterations in ROS signaling, antioxidant mechanisms, and apoptotic pathways. Excess production of IL-10, transforming growth factor β (TGF-β), and decreased production of IFNγ, TNFα, along with reduced cell surface expression of CXCR3 was observed in cells exposed to asbestos.
In addition, a higher expression of NNT enhanced Treg function, BCL-2 phosphorylation, and cell cycle progression, reduced proapoptotic factors induction, and decreased cell death signals were observed, collectively indicating that the cells exposed to asbestos lacked antitumor activity. The cells might upregulate NNT as an adaptation to the presence of continuous stressors. Nnt knockdown did not affect the cell proliferation or resistance to asbestos-induced apoptosis but showed recovery of ROS production. Thus, modulating NNT might be therapeutic by initiating ROS-mediated cytotoxicity and antitumor activity. Seminal findings by Ripoll et al. (12) also confirmed that NNT overexpressing macrophages could not clear bacterial infection due to low ROS, and the same hypothesis is valid regarding antitumor immunity.
Our recent findings in CD4+ T cells from lean, normoglycemic older subjects (average age 62 yr) showed that the older subjects’ T cells had higher expression of NNT, but produced higher levels of ROS and proinflammatory diabetogenic Th17 cytokines (42). We could not point definitively to mitochondrial ROS and NNT as the specific drivers of the Th17 inflammatory phenotype based on our data. However, uncontrolled cellular ROS is an inducer of Th17 cytokines by activating the transcription factor signal transducer and activator of transcription 3 (STAT3). As mentioned earlier, cells continuously exposed to stressors might upregulate NNT activity as an adaptation. The heightened activity and expression of the protein but without the alleviation of ROS may be explained by NNT operating in reverse mode, as demonstrated by Nickel et al. (13). The work elegantly revealed the unexpected Yin-Yang role of NNT, showing that NNT can function in a reverse mode. A pathological increase in cardiac workload reverses the direction in which NNT operates. Instead of generating NADPH by hydride transfer, NNT supports NADH and ATP production resulting in NADPH oxidation. Thus, NNT switches to a pro-oxidative reverse mode during pressure overload in the heart, which increases mitochondrial ROS emission. C57BL/6J mice lacking functional NNT were protected from ROS generated by pressure overload (13). The status of NNT direction and function in aging T cells needs experimental validation.
OBESITY AND INFLAMMATION
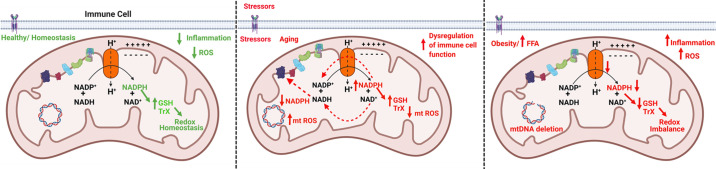
T cells play essential roles in obesity-associated insulin resistance and inflammation by producing cytokines that induce glucose intolerance and insulin resistance in many cell types (43). Th17 cells, a subtype of CD4+ T cells were recently identified as one of the major contributors to inflammation and hyperglycemia and have been implicated in age-associated chronic inflammatory diseases (42–44), and autoimmune diseases like type 1 diabetes (37, 43, 45–49). C57BL/6J mice carry two spontaneous mutations in Nnt gene resulting in a truncated nonfunctional protein. Transgenic expression of the functional Nnt gene in mice with a C57BL/6J background promoted improvements in glucose tolerance compared with nontransgenic mice following a glucose challenge (37). Decreased Nnt expression correlated with reduced insulin secretion, which was significantly improved in the transgenic mice (37). Conversely, in inbred mouse strains of diabetes susceptible DBA/2 mice, a positive correlation between insulin secretion and NNT activity was observed, and only the mouse strains with high NNT activity exhibited severe diabetes after becoming obese. Thus, we can concur that overactive NNT can result in insulin hypersecretion and may predispose DBA/2 mice to β-cell failure (50). These studies collectively show that modulation of NNT can have therapeutic significance. In this regard, we sought to determine if NNT has a mechanistic link to inflammation in the context of obesity (11). Peripheral blood mononuclear cells (PBMCs) from lean normoglycemic young adults (average age 33 yr) were activated with T cells specific αCD3/αCD28 stimulus and were treated with physiologically relevant doses of dietary saturated (palmitate) and monounsaturated (oleate) fatty acids, respectively. Cellular ROS production was higher in palmitate-treated cells at 20 and 40 h posttreatment. At 40 h posttreatment, higher ROS production coincided with quantifiable proinflammatory Th17 and Th17 supportive cytokine production. Palmitate treatment resulted in blunted NNT expression and low NADPH and GSH. PBMCs from normoglycemic age-matched subjects with obesity with higher plasma free fatty acids recapitulated the redox imbalance observed in palmitate-treated cells and produced higher amounts of Th17 cytokines, IL-17A, IL-17F, and IL-23 along with lower NNT expression. Genetic inhibition of NNT in cells from lean subjects recapitulated redox and inflammatory phenotype of cells from subjects with obesity and palmitate-treated cells. Our data show a direct link between the NNT function and Th17 cytokine production. The redox imbalance and the subsequent alterations in broader cell signaling mechanisms propagated due to the reduced function of NNT are most likely to initiate the inflammatory response. Mitochondrial ROS scavenger mitoTempo did not have an impact on Th17 cytokines, which may indicate mtROS independence of Th17 cytokines. However, we were unable to conclusively exclude mtROS as a perpetrator of signaling events. The link between mitochondrial ROS and induction of Th17 cytokines is reported (51; Fig. 1).
Figure 1.
Model figure. Regulation of mitochondrial redox balance by NNT. Mitochondrial redox balance influences immune cell function in the context of physiological aging and pathological situations such as obesity, infection, and autoimmunity. Healthy mitochondria maintain redox homeostasis with NNT functioning at its optimal levels (solid green arrows far left). Homeostatic conditions exists within the mitochondria with adequate levels of NADPH to sustain NAPDH-dependent ROS defense systems (RDSs) such as GSH and thioredoxin. In this scenario, considering the function of mitochondria as “ROS sinks,” which helps to regulate oxidative stress, the levels of inflammation induced by oxidative stress would be low within the cellular milieu, thus promoting health. Redox imbalance can occur in situations such as a pathological increase in cardiac workload, which can reverse the direction of NNT and favor the generation of NADH to support oxidative phosphorylation and ATP production at the expense of NADPH (dotted red arrows, middle). In such situations, NADPH oxidation occurs resulting in low levels of NADPH, failure of NADPH-dependent RDS, leading to higher oxidative stress and increase in mitochondrial ROS. Redox imbalance (reductive stress) can also occur by hyperactivation/overexpression of NNT, which can result in high levels of mitochondrial NAPDH, GSH, and low ROS. Reduced ROS prevents immune cells from mounting an adequate immune response toward invading pathogens or tumorigenesis and results in dysregulated immune cell function (solid red arrows, middle). Upregulation of expression of NNT is reported in aging immune cells and peripheral blood of older male MS patients. Downregulation of NNT can occurs in immune cells during obesity or exposure to high levels of dietary free fatty acids (solid red arrows, far right). Downregulation of NNT perpetuates redox imbalance resulting in low NADPH and low activity of NADPH-dependent RDS, high ROS leading to loss of mitochondrial DNA (mtDNA) integrity, mtDNA deletions, exaggerated ROS-induced production of proinflammatory cytokines and obesity-associated Inflammation, leading to the onset of metabolic diseases. The figure was created using Biorender.com with permission. GSH, reduced glutathione; MS, multiple sclerosis; NADPH, reduced nicotinamide adenine dinucleotide phosphate; NNT, nicotinamide nucleotide transhydrogenase; ROS, reactive oxygen species.
CARDIOVASCULAR DISEASES AND AUTOIMMUNITY
Macrophage oxidative stress and onset of atherosclerosis were reported in mice with Nnt deficiency (52). A gradient increase in oxidant production was observed with the highest oxidant production in the Nnt double mutant mice, followed by single mutant, and the least was produced in the wild-type mouse liver mitochondria and macrophages. The single and double Nnt mutants also had parallel increases in mitochondrial biogenesis and inflammatory markers. Increased lipid accumulation and foam cell formation in macrophages in response to low-density lipoprotein (LDL) treatment were attributed to the upregulation of CD36 and downregulation of the cholesterol efflux regulatory ATP-binding cassette transporters (ABCA1s) in the Nnt mutants. Thus, this study shows the direct link between Nnt, inflammatory responses, and the development of atherosclerosis. NNT involvement in the regulation of endothelial cell function was also reported (10). NNT expression and activity were higher in human aortic endothelial cells upon treatment with angiotensin II. Knockdown of NNT resulted in exaggerated ROS, impaired glutathione peroxidase and glutathione reductase activities, and lower NADPH-to-NADP+ ratio. It is important to note that NNT dysregulation in the endothelial cells, coupled with dysregulation of the NNT in the circulatory immune cells, as seen during obesity (11), can quickly accelerate an inflammatory milieu resulting in vascular dysfunction and the onset of microvascular and macrovascular diseases.
Currently, there is one study where NNT expression was evaluated in the context of multiple sclerosis (MS). Eftekharian et al. (53) assessed NNT and NNT antisense 1 in the peripheral blood of patients with MS. NNT-AS1 is a long noncoding RNA (lncRNA) and has recently been shown to exert antiproliferative effects (54).
In addition to the low sample size in this study, the expression analysis was performed on total RNA extracted from the peripheral blood, and researchers did not assess gene or protein expression of NNT in specific immune cells types in peripheral blood (53).
The presence of macrophages in demyelinating lesions show the immune cells take part in the pathogenesis of this debilitating illness (55). The changes in the nervous tissues during the onset of MS result in the infiltration of the macrophages, which bring about the destruction of myelin (56). Along with the macrophages, both CD4+ and CD8+ T lymphocytes are also known to infiltrate early on during the onset of the disease but begin to dwindle as the disease progresses (57). Studies conducted in animal MS models such as experimental autoimmune encephalitis (EAE) show that both Th1 and Th17 subsets of CD4+ T cells, as well as CD8+ T cells, participate in the etiology of MS.
NNT expression was higher in male subjects with MS and aged over 50 yr compared with healthy control subjects without neurological or inflammatory disorders. No significant differences in Nnt mRNA expression were observed in female subjects, which shows that NNT might play a sex-specific role in the pathogenesis of MS. Macrophages functions are known to be influenced by the presence and concentrations of sex hormones and are known to display sex-specific cytokine release upon activation by T lymphocytes (58). Therefore, the researchers hypothesize that the observed upregulation of NNT in older male patients with MS is indicative of a distinct role of NNT in this subgroup.
NNT AND TUMORIGENESIS
Although not much is known regarding NNT in the context of immune cells and tumorigenesis, it is important to mention that NNT is known to have a unique role in tumorigenesis of many other cell types. Since the immune system is intricately involved in the onset and progression of malignancy, there must exist a close cross-talk between the immune and malignant cells. The factors that influence the malignant cell may also influence the immune cells and vice versa. It is also critical to note that the role of NNT in tumorigenesis might be cell type and context specific. NNT function is studied in many cancer cell types, and the possibility of modulating NNT as a therapeutic intervention for various cancers is explored much. Transient knockdown of NNT in adrenocortical carcinoma cells resulted in increased intracellular levels of oxidative stress, inhibition of cell proliferation, but promoted apoptosis and susceptibility to chemically induced oxidative stress (59). Since NNT function is inhibited for a reasonable amount of time, it is likely the cells experience a drop in NADPH levels resulting in decreased recycling of GSH and increased oxidative stress due to an accumulation of GSSG and the impairment of other NADPH dependent RDS. It is reasonable to speculate that transient knockdown of NNT can be therapeutically exploited to prevent proliferation and promote apoptosis of cancer cells. Targeted therapies, such as intratumoral knockdown of NNT can be explored. Interestingly, a stable knockdown of NNT and prolonged suppression resulted in metabolic adaptation of the cells, leading to the restoration of redox balance and resilience to oxidative stress. However, the cells did not regain their ability to proliferate (60). Overexpression of NNT correlated with shorter survival of patients with gastric cancer (61). The decreased ability of NNT overexpressing cells to produce ROS, as seen in the context of infection, might result in an accelerated and unchecked proliferation of the malignant cells. Intratumoral injection of NNT small interfering RNA (siRNA) significantly suppressed gastric tumor growth in patient-derived xenograft models.
A long noncoding RNA, NNT-AS1, is also overexpressed in hepatocellular carcinoma (HCC), which prevents the CD4+ T cells from infiltrating the tumor (62). The NNT-AS1 regulated activation of TGF-β pathway prevented the infiltration of T cells into the tumor, thus causing tumor immune evasion. The role of TGF-β in chemotaxis of immune cells is well known, and NNT-AS1-mediated alterations in the TGF-β pathway would impact immune cell infiltration. Similarly, higher NNT-AS1 in patients correlated with shorter survival. The levels of NNT-AS1 were also positively associated with transforming growth factor β receptor 1 (TGFBR1) and SMAD family member 5 (SMAD5) levels. Although the levels of TGF-β, TGFBR1, and SMAD5 were higher, the infiltrating CD4+ and CD8+ cells were lower in HCC. When NNT-AS1 was knocked down in HepG2 cells, the levels of TGF-β, TGFBR1, and SMAD5 were significantly decreased showing the regulatory influence of NNT-AS1 on TGF-β signaling and tumor immune response.
NNT expression significantly enhances the formation and aggressiveness of tumors in murine models of lung cancer (63). To reiterate and pinpoint the importance of ROS signaling in immune response, the aggressiveness of the tumors could be due to the inability of cells to produce appropriate oxidative stress response and curb tumor progression. sh-RNA-mediated loss of NNT in in vitro cell culture models resulted in a significant reduction in the mitochondrial respiratory capacity, a diminished function of mitochondrial Fe-S proteins, and promoted metabolic defect. Loss of NNT-promoted mitochondrial dysfunction independent of an increase in oxidative stress. It is essential to mention that research is ongoing to understand the role of NNT in oncogenesis (59, 61). Although the importance of NNT function in malignant transformation of many cell types is evaluated, the understanding of the role of NNT in immune cell tumor immunity is still at the nascent stages.
CONCLUDING REMARKS
Although the role of NNT in immune cell function had not been investigated extensively, there is renewed interest due to NNT’s role in autoimmunity, tumorigenesis, and the onset of metabolic and aging-associated diseases. Many studies point to mitochondrial dysregulation as a significant contributor to inflammation. Thus, inflammation could be the common denominator that promotes many pathological conditions. NNT, as a dominant regulator of mitochondrial redox environment, has the potential to drive the course of inflammation and thus can play a significant role in health and disease. A recent study in brain mitochondria shows the importance of NNT as an antioxidant and how NNT is specifically required to mitigate redox imbalance during hampered energy metabolism (64). Of the many interesting discoveries about NNT, the fascinating finding that NNT can function in reverse mode during pathological conditions is of particular interest in understanding the protein’s function in different contexts. However, many unanswered questions remain that need to be addressed, and it is also important to evaluate the contradictory findings. This review summarizes the recent findings on NNT to encourage research in understanding the regulatory role of NNT in the immune system.
GRANTS
This work was supported by National Institute on Aging Grant R15AG068957 (to L.P.B.). This work was also supported by the Pasini Fellowship (to L.P.B.), College of Health Sciences Faculty Development Grant (FDG), and Sakowich Center for Undergraduate Research and Creative Activities Grant (SCURCA), Merrimack College (to L.P.B.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.P.B. prepared figures; T.R., R.C., and L.P.B. drafted manuscript; T.R. and L.P.B. edited and revised manuscript; T.R., R.C., and L.P.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Mathew Switliski for editing the manuscript.
REFERENCES
- 1.Mailloux RJ. Mitochondrial antioxidants and the maintenance of cellular hydrogen peroxide levels. Oxid Med Cell Longev 2018: 7857251, 2018. doi: 10.1155/2018/7857251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ronchi JA, Francisco A, Passos LAC, Figueira TR, Castilho RF. The Contribution of nicotinamide nucleotide transhydrogenase to peroxide detoxification is dependent on the respiratory state and counterbalanced by other sources of NADPH in liver mitochondria. J Biol Chem 291: 20173–20187, 2016. doi: 10.1074/jbc.M116.730473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marengo B, Nitti M, Furfaro AL, Colla R, Ciucis CD, Marinari UM, Pronzato MA, Traverso N, Domenicotti C. Redox homeostasis and cellular antioxidant systems: crucial players in cancer growth and therapy. Oxid Med Cell Longev 2016: 6235641, 2016. doi: 10.1155/2016/6235641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet 11: 376–381, 1995. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe K, Shibuya S, Ozawa Y, Nojiri H, Izuo N, Yokote K, Shimizu T. Superoxide dismutase 1 loss disturbs intracellular redox signaling, resulting in global age-related pathological changes. Biomed Res Int 2014: 140165, 2014. doi: 10.1155/2014/140165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doskey CM, Buranasudja V, Wagner BA, Wilkes JG, Du J, Cullen JJ, Buettner GR. Tumor cells have decreased ability to metabolize H2O2: implications for pharmacological ascorbate in cancer therapy. Redox Biol 10: 274–284, 2016. doi: 10.1016/j.redox.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beno I, Staruchová M, Volkovová K, Bátovský M. Increased antioxidant enzyme activities in the colorectal adenoma and carcinoma. Neoplasma 42: 265–269, 1995. [PubMed] [Google Scholar]
- 8.Won H, Lim S, Jang M, Kim Y, Rashid MA, Jyothi KR, Dashdorj A, Kang I, Ha J, Kim SS. Peroxiredoxin-2 upregulated by NF-κB attenuates oxidative stress during the differentiation of muscle-derived C2C12 cells. Antioxid Redox Signal 16: 245–261, 2012. doi: 10.1089/ars.2011.3952. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira AV, Koeken VACM, Matzaraki V, Kostidis S, Alarcon-Barrera JC, de Bree LCJ, Moorlag SJCFM, Mourits VP, Novakovic B, Giera MA, Netea MG, Domínguez-Andrés J. Glutathione metabolism contributes to the induction of trained immunity. Cells 10: 971,.2021. doi: 10.3390/cells10050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao KNS, Shen X, Pardue S, Krzywanski DM. Nicotinamide nucleotide transhydrogenase (NNT) regulates mitochondrial ROS and endothelial dysfunction in response to angiotensin II. Redox Biol 36: 101650, 2020. doi: 10.1016/j.redox.2020.101650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCambridge G, Agrawal M, Keady A, Kern PA, Hasturk H, Nikolajczyk B, Bharath L. Saturated fatty acid activates t cell inflammation through a nicotinamide nucleotide transhydrogenase (Nnt)-dependent mechanism. Biomolecules 9: 79, 2019. doi: 10.3390/biom9020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ripoll VM, Meadows NA, Bangert M, Lee AW, Kadioglu A, Cox RD. Nicotinamide nucleotide transhydrogenase (NNT) acts as a novel modulator of macrophage inflammatory responses. FASEB J 26: 3550–3562, 2012. doi: 10.1096/fj.11-199935. [DOI] [PubMed] [Google Scholar]
- 13.Nickel AG, von Hardenberg A, Hohl M, Löffler JR, Kohlhaas M, Becker J, Reil J-C, Kazakov A, Bonnekoh J, Stadelmaier M, Puhl S-L, Wagner M, Bogeski I, Cortassa S, Kappl R, Pasieka B, Lafontaine M, Lancaster CRD, Blacker TS, Hall AR, Duchen MR, Kästner L, Lipp P, Zeller T, Müller C, Knopp A, Laufs U, Böhm M, Hoth M, Maack C. Reversal of mitochondrial transhydrogenase causes oxidative stress in heart failure. Cell Metab 22: 472–484, 2015. doi: 10.1016/j.cmet.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Favia M, Atlante A. Cellular redox state acts as switch to determine the direction of nnt-catalyzed reaction in cystic fibrosis cells. Int J Mol Sci 22: 967, 2021. doi: 10.3390/ijms22020967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen A, Karlsson GB, Rydström J. Proton-translocating transhydrogenase: an update of unsolved and controversial issues. J Bioenerg Biomembr 40: 463–473, 2008. doi: 10.1007/s10863-008-9170-x. [DOI] [PubMed] [Google Scholar]
- 16.Sundaresan V, Chartron J, Yamaguchi M, Stout CD. Conformational diversity in NAD(H) and interacting transhydrogenase nicotinamide nucleotide binding domains. J Mol Biol 346: 617–629, 2005. doi: 10.1016/j.jmb.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 17.Kampjut D, Sazanov LA. Structure and mechanism of mitochondrial proton-translocating transhydrogenase. Nature 573: 291–295, 2019. doi: 10.1038/s41586-019-1519-2. [DOI] [PubMed] [Google Scholar]
- 18.Starkov AA. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci 1147: 37–52, 2008. doi: 10.1196/annals.1427.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94: 909–950, 2014. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradshaw PC. Cytoplasmic and mitochondrial NADPH-coupled redox systems in the regulation of aging. Nutrients 11: 504, 2019. doi: 10.3390/nu11030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wadey AL, Muyderman H, Kwek PT, Sims NR. Mitochondrial glutathione uptake: characterization in isolated brain mitochondria and astrocytes in culture. J Neurochem 109: 101–108, 2009. doi: 10.1111/j.1471-4159.2009.05936.x. [DOI] [PubMed] [Google Scholar]
- 22.Blacker TS, Duchen MR. Investigating mitochondrial redox state using NADH and NADPH autofluorescence. Free Radic Biol Med 100: 53–65, 2016. doi: 10.1016/j.freeradbiomed.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai D-F, Chiao Y, Marcinek DJ, Szeto HH, Rabinovitch PS. Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan 3: 6, 2014. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen KS, Smith C. Ageing-associated oxidative stress and inflammation are alleviated by products from grapes. Oxid Med Cell Longev 2016: 6236309, 2016. doi: 10.1155/2016/6236309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P. Oxidative stress, aging, and diseases. Clin Interv Aging 13: 757–772, 2018. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han CY. Roles of reactive oxygen species on insulin resistance in adipose tissue. Diabetes Metab J 40: 272–279, 2016. doi: 10.4093/dmj.2016.40.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Zhou Z, Mitochondria MW. Mitochondria, oxidative stress and innate immunity. Front Physiol 9: 1487, 2018. doi: 10.3389/fphys.2018.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res 122: 877–902, 2018. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S, Lian G. ROS and diseases: role in metabolism and energy supply. Mol Cell Biochem 467: 1–12, 2020. doi: 10.1007/s11010-019-03667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin F, Sancheti H, Cadenas E. Silencing of nicotinamide nucleotide transhydrogenase impairs cellular redox homeostasis and energy metabolism in PC12 cells. Biochim Biophys Acta 1817: 401–409, 2012. doi: 10.1016/j.bbabio.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopert P, Patel M. Nicotinamide nucleotide transhydrogenase (Nnt) links the substrate requirement in brain mitochondria for hydrogen peroxide removal to the thioredoxin/peroxiredoxin (Trx/Prx) system. J Biol Chem 289: 15611–15620, 2014. doi: 10.1074/jbc.M113.533653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho HY, Lin YT, Lin G, Wu PR, Cheng ML. Nicotinamide nucleotide transhydrogenase (NNT) deficiency dysregulates mitochondrial retrograde signaling and impedes proliferation. Redox Biol 12: 916–928, 2017. doi: 10.1016/j.redox.2017.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith CD, Schmidt CA, Lin C, Fisher-Wellman KH, Darrell Neufer P. Flux through mitochondrial redox circuits linked to nicotinamide nucleotide transhydrogenase generates counterbalance changes in energy expenditure. J Biol Chem 295: 16207–16216, 2020. doi: 10.1074/jbc.RA120.013899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh D, Levault KR, Brewer GJ. Relative importance of redox buffers GSH and NAD(P)H in age-related neurodegeneration and Alzheimer disease-like mouse neurons. Aging Cell 13: 631–640, 2014. doi: 10.1111/acel.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Go YM, Jones DP. Redox theory of aging: implications for health and disease. Clin Sci (Lond) 131: 1669–1688, 2017. doi: 10.1042/CS20160897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pickering AM, Lehr M, Gendron CM, Pletcher SD, Miller RA. Mitochondrial thioredoxin reductase 2 is elevated in long-lived primate as well as rodent species and extends fly mean lifespan. Aging Cell 16: 683–692, 2017. doi: 10.1111/acel.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes 55: 2153–2156, 2006. [Erratum in Diabetes 63: 815, 2014] doi: 10.2337/db06-0358. [DOI] [PubMed] [Google Scholar]
- 38.Fujisawa Y, Napoli E, Wong S, Song G, Yamaguchi R, Matsui T, Nagasaki K, Ogata T, Giulivi C. Impact of a novel homozygous mutation in nicotinamide nucleotide transhydrogenase on mitochondrial DNA integrity in a case of familial glucocorticoid deficiency. BBA Clin 3: 70–78, 2015. doi: 10.1016/j.bbacli.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapman NM, Boothby MR, Chi H. Metabolic coordination of T cell quiescence and activation. Nat Rev Immunol 20: 55–70, 2020. doi: 10.1038/s41577-019-0203-y. [DOI] [PubMed] [Google Scholar]
- 40.Desdín-Micó G, Soto-Heredero G, Mittelbrunn M. Mitochondrial activity in T cells. Mitochondrion 41: 51–57, 2018. doi: 10.1016/j.mito.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto S, Lee S, Matsuzaki H, Kumagai-Takei N, Yoshitome K, Sada N, Shimizu Y, Ito T, Nishimura Y, Otsuki T. Enhanced expression of nicotinamide nucleotide transhydrogenase (NNT) and its role in a human T cell line continuously exposed to asbestos. Environ Int 138: 105654, 2020. doi: 10.1016/j.envint.2020.105654. [DOI] [PubMed] [Google Scholar]
- 42.Bharath LP, Agrawal M, McCambridge G, Nicholas DA, Hasturk H, Liu J, Jiang K, Liu R, Guo Z, Deeney J, Apovian CM, Snyder-Cappione J, Hawk GS, Fleeman RM, Pihl RMF, Thompson K, Belkina AC, Cui L, Proctor EA, Kern PA, Nikolajczyk BS. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab 32: 44–55.e6, 2020. doi: 10.1016/j.cmet.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ip B, Cilfone NA, Belkina AC, DeFuria J, Jagannathan-Bogdan M, Zhu M, Kuchibhatla R, McDonnell ME, Xiao Q, Kepler TB, Apovian CM, Lauffenburger DA, Nikolajczyk BS. Th17 cytokines differentiate obesity from obesity-associated type 2 diabetes and promote TNFα production. Obesity (Silver Spring) 24: 102–112, 2016. doi: 10.1002/oby.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmitt V, Rink L, Uciechowski P. The Th17/Treg balance is disturbed during aging. Exp Gerontol 48: 1379–1386, 2013. doi: 10.1016/j.exger.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, Shapiro AMJ. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes 58: 1302–1311, 2009. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jagannathan-Bogdan M, McDonnell ME, Shin H, Rehman Q, Hasturk H, Apovian CM, Nikolajczyk BS. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol 186: 1162–1172, 2011. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sumarac-Dumanovic M, Stevanovic D, Ljubic A, Jorga J, Simic M, Stamenkovic-Pejkovic D, Starcevic V, Trajkovic V, Micic D. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes (Lond) 33: 151–156, 2009. doi: 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]
- 48.Arababadi MK, Nosratabadi R, Hassanshahi G, Yaghini N, Pooladvand V, Shamsizadeh A, Hakimi H, Derakhshan R. Nephropathic complication of type-2 diabetes is following pattern of autoimmune diseases? Diabetes Res Clin Pract 87: 33–37, 2010. doi: 10.1016/j.diabres.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 49.Nicholas DA, Proctor EA, Agrawal M, Belkina AC, Van Nostrand SC, Panneerseelan-Bharath L, Jones AR 4th, Raval F, Ip BC, Zhu M, Cacicedo JM, Habib C, Sainz-Rueda N, Persky L, Sullivan PG, Corkey BE, Apovian CM, Kern PA, Lauffenburger DA, Nikolajczyk BS. Fatty acid metabolites combine with reduced β oxidation to activate Th17 inflammation in human type 2 diabetes. Cell Metab 30: 447–461.e5, 2019. doi: 10.1016/j.cmet.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aston-Mourney K, Wong N, Kebede M, Zraika S, Balmer L, McMahon JM, Fam BC, Favaloro J, Proietto J, Morahan G, Andrikopoulos S. Increased nicotinamide nucleotide transhydrogenase levels predispose to insulin hypersecretion in a mouse strain susceptible to diabetes. Diabetologia 50: 2476–2485, 2007. doi: 10.1007/s00125-007-0814-x. [DOI] [PubMed] [Google Scholar]
- 51.Kaufmann U, Kahlfuss S, Yang J, Ivanova E, Koralov SB, Feske S. Calcium signaling controls pathogenic Th17 cell-mediated inflammation by regulating mitochondrial function. Cell Metab 29: 1104–1118.e6, 2019. doi: 10.1016/j.cmet.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salerno AG, Rentz T, Dorighello GG, Marques AC, Lorza-Gil E, Wanschel ACBA, de Moraes A, Vercesi AE, Oliveira HCF. Lack of mitochondrial NADP(H)-transhydrogenase expression in macrophages exacerbates atherosclerosis in hypercholesterolemic mice. Biochem J 476: 3769–3789, 2019. doi: 10.1042/BCJ20190543. [DOI] [PubMed] [Google Scholar]
- 53.Eftekharian MM, Taheri M, Arsang-Jang S, Komaki A, Ghafouri-Fard S. Nicotinamide nucleotide transhydrogenase expression analysis in multiple sclerosis patients. Int J Neurosci 129: 1256–1260, 2019. doi: 10.1080/00207454.2019.1660655. [DOI] [PubMed] [Google Scholar]
- 54.Huang Y, Shi J, Xu Y. Long non-coding RNA NNT-AS1 contributes to cell proliferation, metastasis and apoptosis in human ovarian cancer. Oncol Lett 15: 9264–9270, 2018. doi: 10.3892/ol.2018.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Valk P, De Groot CJA. Staging of multiple sclerosis (MS) lesions: pathology of the time frame of MS. Neuropathol Appl Neurobiol 26: 2–10, 2000. doi: 10.1046/j.1365-2990.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 56.Vogel DY, Vereyken EJ, Glim JE, Heijnen PD, Moeton M, van der Valk P, Amor S, Teunissen CE, van Horssen J, Dijkstra CD. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation 10: 35, 2013. doi: 10.1186/1742-2094-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Legroux L, Arbour N. Multiple sclerosis and t lymphocytes: an entangled story. J Neuroimmune Pharmacol 10: 528–546, 2015. doi: 10.1007/s11481-015-9614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller L, Hunt JS. Sex steroid hormones and macrophage function. Life Sci 59: 1–14, 1996. doi: 10.1016/0024-3205(96)00122-1. [DOI] [PubMed] [Google Scholar]
- 59.Ciccarese F, Ciminale V. Escaping death: mitochondrial redox homeostasis in cancer cells. Front Oncol 7: 117, 2017. doi: 10.3389/fonc.2017.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chortis V, Taylor AE, Doig CL, Walsh MD, Meimaridou E, Jenkinson C, Rodriguez-Blanco G, Ronchi CL, Jafri A, Metherell LA, Hebenstreit D, Dunn WB, Arlt W, Foster PA. Nicotinamide nucleotide transhydrogenase as a novel treatment target in adrenocortical carcinoma. Endocrinology 159: 2836–2849, 2018. doi: 10.1210/en.2018-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li S, Zhuang Z, Wu T, Lin J-C, Liu Z-X, Zhou L-F, Dai T, Lu L, Ju H-Q. Nicotinamide nucleotide transhydrogenase-mediated redox homeostasis promotes tumor growth and metastasis in gastric cancer. Redox Biol 18: 246–255, 2018. doi: 10.1016/j.redox.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Yang L, Dong X, Yang X, Zhang X, Liu Z, Zhao X, Wen T. Overexpression of NNT-AS1 activates TGF-β signaling to decrease tumor CD4 lymphocyte infiltration in hepatocellular carcinoma. Biomed Res Int 2020: 8216541, 2020. doi: 10.1155/2020/8216541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ward NP, Kang YP, Falzone A, Boyle TA, DeNicola GM. Nicotinamide nucleotide transhydrogenase regulates mitochondrial metabolism in NSCLC through maintenance of Fe-S protein function. J Exp Med 217: e20191689, 2020. doi: 10.1084/jem.20191689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Francisco A, Ronchi JA, Navarro CDC, Figueira TR, Castilho RF. Nicotinamide nucleotide transhydrogenase is required for brain mitochondrial redox balance under hampered energy substrate metabolism and high-fat diet. J Neurochem 147: 663–677, 2018. doi: 10.1111/jnc.14602. [DOI] [PubMed] [Google Scholar]