Abstract
Background
Tumor immune cell infiltration is important in the prognosis of patients with lung adenocarcinoma. The aim of this study was to develop a prognostic classification based on the tumor immunoscore.
Methods
Patients with KRAS‐mutant invasive non‐mucinous lung adenocarcinoma who underwent radical surgery were enrolled in the study. Histologic grading was assessed according to the recommendations of the International Association for the Study of Lung Cancer. Programmed death‐ligand 1 (PD‐L1) and CD8 expression was detected using immunohistochemistry. The number of CD8+ tumor‐infiltrating lymphocytes (TILs) per high‐power field was assessed. A classification based on histological grade and CD8+ TIL level was established (Grading‐Immunoscore type): low‐to‐medium grade with high or low infiltration (type A); high‐grade, high‐infiltration (type B); and high‐grade, low‐infiltration (type C).
Results
A total of 112 patients participated. In the multivariable analysis, histological grading and level of CD8+ TILs were independent prognostic factors for overall survival (OS) and progression‐free survival (PFS) (p < 0.001 and p = 0.007, respectively). Patients with type A tumors had the best OS and PFS, whereas those with type C tumors had the worst OS (89.6%, 65.0%, and 29.5% 5‐year OS for types A, B, and C, respectively). PD‐L1 positivity and high expression rate was highest in type B tumors (tumor proportion score [TPS] ≥ 1%: 29.4%, 73.1%, and 42.9%; TPS ≥50%: 7.8%, 42.3%, and 17.1%, for types A, B, and C, respectively).
Conclusions
The Grading‐Immunoscore classification refines the prognostic grouping of histological grading and might aid in the screening of potential candidates for immunotherapy in patients with KRAS‐mutant adenocarcinoma.
Keywords: CD8+ tumor‐infiltrating lymphocytes, KRAS mutation, lung adenocarcinoma, PD‐L1, prognosis
The Grading‐Immunoscore classification refines the prognostic grouping of histological grading and might aid in the screening of potential candidates for immunotherapy in patients with KRAS‐mutant adenocarcinoma.
INTRODUCTION
With the development of molecular profiling and targeted therapeutic drugs, marked changes have been made in the treatment of non–small cell lung cancer (NSCLC). EGFR, ALK, and KRAS are the most common driver genes in NSCLC. Targeted therapies such as tyrosine kinase inhibitors for EGFR and ALK have greatly benefitted patients. However, targeting KRAS mutations has proven extremely challenging, and there is no apparent benefit of chemotherapy in patients with KRAS mutations. 1 , 2 The KRAS G12C‐specific inhibitor, sotorasib, has recently been approved by the United States (US) Food and Drug Administration, but it only targets the KRAS‐G12C‐mutant site, which accounts for ~39% of KRAS‐mutant lung adenocarcinomas (LUADs). 3 In general, compared with KRAS wild‐type tumors, KRAS‐mutant lung cancer is associated with poor overall survival (OS), especially in those with advanced‐stage disease. 3 , 4 Therefore, attention is being paid to immunotherapy with checkpoint inhibitors in clinical applications.
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of NSCLC. Several studies have reported that using programmed death‐ligand 1 (PD‐1)/PD‐L1 inhibitors improved the OS compared to standard chemotherapy in KRAS‐mutant NSCLC patients. 5 , 6 , 7 , 8 , 9 Previous research by our team showed that KRAS mutation is significantly related to the high expression of PD‐L1. 10 Although ICIs have become a milestone in anticancer treatment, only ~20% of unselected patients with advanced NSCLC showed a durable response. 5 , 11 Therefore, a detailed understanding of key predictors is imperative to determine, which patients may benefit from ICI treatment. Importantly, accumulating evidence shows that tumor‐infiltrating lymphocytes (TILs) are prognostic indicators of response to ICI treatment. 12 , 13 CD8+ T‐cells, which are the principal cytotoxic cells, play a pivotal role in cell‐mediated antitumor immune responses. Tumeh et al. 14 found that tumor‐regression response to PD‐1 blockade therapy requires pre‐existing CD8+ T‐cells that are negatively regulated by PD‐1/PD‐L1‐mediated adaptive immune resistance. The evaluation of the immune infiltrate within tumors has been well studied in colon and breast cancer. It is called the “immunoscore” and provides prognostic information equal to or better than that obtained from the established tumor, nodes, and metastases (TNM) staging system. 15 , 16 A recent study showed that CD8+ TIL is the most promising candidate marker for immune cell score in NSCLC. 17 However, there are few reports on the immunoscore of NSCLC, and a standardized scoring system has not yet been established.
In this study, we aimed to explore the features of PD‐L1 expression and propose strategies for immunoscoring of CD8+ TILs. We are currently working on the implementation of a combined Grading‐Immunoscore type (G‐I type) for KRAS‐mutant adenocarcinoma. This stratification, as a potential indicator of responsiveness to immunotherapy, can indicate the prognosis of adenocarcinoma and provide pathological evidence for the selection of immunotherapy strategies.
METHODS
Patient cohort
A total of 112 tissue samples of surgically resected cases of KRAS‐mutant invasive non‐mucinous adenocarcinoma were collected from March 2013 to December 2017. The TNM stage was determined according to the American Joint Committee on Cancer's (AJCC) 8th edition of the TNM classification system. 18 Patients with tumor histology other than absent or failed genotyping, and patients without adequate follow‐up documentation or imaging, were excluded. Data were collected on all clinical characteristics (including age, sex, pathological TNM staging, postoperative adjuvant chemotherapy, and molecular alterations). OS was calculated as the date from pathological diagnosis to the time of death from any cause. Progression‐free survival (PFS) was calculated from the date of pathological diagnosis to the time of the last clinical evidence of recurrence, progression, or death. Informed consent was obtained from the patients and ethical approval was obtained from the Ethics Review Board of the Peking University Cancer Hospital and Institute, Beijing, China (Approval No. 2018YJZ19).
Histologic evaluation
Two experienced senior pathologists (K.W.C. and W.S.) reviewed all the pathological sections and checked the diagnosis according to the fifth edition of the World Health Organization classification criteria. 19 Histologic evaluation was performed according to the standards specified by the International Association for the Study of Lung Cancer (IASLC), 20 including IASLC grading, predominant pattern, lymphovascular invasion, pleural invasion, nuclear grade, mitotic grade, cytologic grade, necrosis, and spread through air spaces (STAS).
Immunohistochemistry
PD‐L1 immunohistochemistry was determined using the PD‐L1 22C3 pharmDx kit (SK006; Dako) on the Dako Link 48 platform. PD‐L1 expression was evaluated according to the tumor proportion score (TPS), which is defined as the percentage of tumor cells showing partial or complete membranous staining: TPS ≥1% (positive expression), and TPS ≥50% (high expression). Immunohistochemical staining for CD3 (Clone poly, Shanghai GeneTech) and CD8 (Clone SP16, Beijing Zhongshan Golden Bridge Biotechnology) was performed according to the standard EnVision procedure. CD3 and CD8 staining occurs in the membrane of lymphocytes. The evaluation of CD3+ TILs was based on a semiquantitative assessment performed by estimating the percentage of lymphocytes with positive membrane staining. A binary classification was used to grade the CD3+ TILs within the edge of tumor infiltration as follows: group 1, none to mild infiltration; or group 2, moderate to severe infiltration, as recommended by the International Immuno‐Oncology Biomarkers Working Group. 21
For CD8+ TIL scoring, each slide was screened for CD8+ TIL evaluation regions at 200× magnification. The highest‐grade pattern area was selected, using one high‐power field (HPF) at 400× magnification with a 0.55‐mm field diameter; five visual fields were randomly selected to calculate the count of CD8+ T cells, and the mean value of five HPFs was considered as the density of CD8+ TILs, similar to the counting method used in a previous study. 22 , 23 Both CD8+ TILs within the tumor epithelial component and CD8+ cells in the stroma or adjacent tumor were considered, disregarding CD8+ TILs beyond the tumor invasive margin. CD8+ TIL levels were assessed using a two‐tiered scoring system with the median as the cutoff value.
All sections were examined microscopically and evaluated independently by two pathologists (K.W.C. and W.S.) who were blinded to the clinical data pertaining to the subjects. In case of discrepancy, the final score was determined as that obtained on consensus by the pathologists.
Grading‐Immunoscore type
To stratify KRAS mutation cases, we introduced CD8+ TIL as an immune factor into the IASLC grading system and established a new classification, the G‐I type. According to the IASLC histologic grading (containing 20% or more high‐grade structure) and level of CD8+ TILs, we classified patients into the following four subgroups: low‐to‐medium grade, high infiltration (group 1); low‐to‐medium grade, low infiltration (group 2); high grade, high infiltration (group 3); and high grade, low infiltration (group 4). We, then, further combined the groups 1 and 2 into type A and classified group 3 as type B, and group 4 as type C.
Classic genomic aberrations
All resected lung adenocarcinoma cases underwent routine molecular testing using an amplification refractory mutation system‐based polymerase chain reaction (ARMS‐PCR) platform. Routine clinical data with complete data of common genomic driver genes, including EGFR, ALK, KRAS, and ROS1, were collected for KRAS mutation cases. KRAS‐frequent codon variants at exons 2 and 3, including codons 12, 13, and 61, were detected using the ACCB Gene Mutation Detection Kit (ACCB Biotech).
Statistical analyses
Pearson's χ2 and Fisher's exact tests were used to analyze correlations between CD8+ T‐cell density and PD‐L1 expression with clinicopathological variables. Bland–Altman plots and intraclass correlation coefficients were used to assess the consistency between observers. Survival analysis was performed using the Kaplan–Meier method. Correlations between clinicopathological variables and OS or PFS were performed using univariate Cox proportional hazards regression analysis. Multivariable Cox proportional hazards regression was performed to evaluate variables with p‐values of <0.05 in the univariate analysis. All analyses were performed using IBM SPSS Statistics 20.0 (IBM Corporation). Statistical significance was defined as a two‐sided p‐value <0.05.
RESULTS
Clinicopathological characteristics
Among 2098 NSCLC patients who underwent KRAS genomic analysis at our institution between 2013 and 2017, 170 (8.1%) had tumors harboring KRAS mutations. Fifty‐eight patients were excluded because of invasive mucinous adenocarcinoma subtypes and incomplete baseline data. Finally, 112 patients with invasive non‐mucinous adenocarcinoma were included in the analysis. All had EGFR wild‐type tumors, and three had combined ALK gene mutations. The median age at diagnosis was 61 years (range: 35–79 years). Three‐quarters of the cohort (75%) were male, and the majority of patients (74.1%) had a history of smoking. The average follow‐up period was 47.99 months (median: 45.95 months, range: 4.13–94.77 months). The distribution of patients according to the TNM staging system was as follows: I (n = 67, 59.8%), II (n = 25, 22.3%), and III (n = 20, 17.9%). The detailed clinicopathological variables are shown in Table 1, and representative hematoxylin and eosin staining and immunohistochemical staining images are shown in Figure 1.
TABLE 1.
Clinicopathologic characteristics of patients and the correlations between Grading‐Immunoscore type and clinicopathological variables
| All n (%) | Classification a | p Value | |||
|---|---|---|---|---|---|
| Type An (%) | Type Bn (%) | Type Cn (%) | |||
| All | 112 | 51 (45.4) | 26 (23.2) | 35 (31.2) | |
| Age (y) | 0.964 | ||||
| ≤60 | 50 (44.6) | 23 (45.1) | 12 (46.2) | 15 (42.9) | |
| >60 | 62 (55.4) | 28 (54.9) | 14 (53.8) | 20 (57.1) | |
| Sex | 0.028 | ||||
| Female | 28 (25.0) | 18 (35.3) | 2 (7.7) | 8 (22.9) | |
| Male | 84 (75.0) | 33 (64.7) | 24 (92.3) | 27 (77.1) | |
| Smoking | 0.007 | ||||
| Yes | 83 (74.1) | 31 (60.8) | 24 (92.3) | 28 (80.0) | |
| No | 29 (25.9) | 20 (39.2) | 2 (7.7) | 7 (20.0) | |
| IASLC histological grading | |||||
| Grade 1 | 9 (8.0) | 9 (17.6) | 0 | 0 | |
| Grade 2 | 42 (37.5) | 42 (82.4) | 0 | 0 | |
| Grade 3 | 61 (54.5) | 0 (0.0) | 26 (100.0) | 35 (100.0) | |
| CD8+ TILs | |||||
| Low density | 56 (50.0) | 21 (41.2) | 0 | 35 (100.0) | |
| High density | 56 (50.0) | 30 (58.8) | 26 (100.0) | 0 | |
| STAS | 0.005 | ||||
| Present | 13 (11.6) | 1 (2.0) | 4 (15.4) | 8 (22.9) | |
| Absent | 99 (88.4) | 50 (98.0) | 22 (84.6) | 27 (77.1) | |
| Lymphovascular invasion | <0.001 | ||||
| Present | 27 (24.1) | 3 (5.9) | 8 (30.8) | 16 (45.7) | |
| Absent | 85 (75.9) | 48 (94.1) | 18 (69.2) | 19 (54.3) | |
| PD‐L1 | 0.001 | ||||
| TPS <1% | 63 (56.2) | 36 (70.6) | 7 (26.9) | 20 (57.1) | |
| TPS ≥1% | 49 (43.8) | 15 (29.4) | 19 (73.1) | 15 (42.9) | |
| PD‐L1 | 0.002 | ||||
| TPS <50% | 91 (81.2) | 47 (92.2) | 15 (57.7) | 29 (82.9) | |
| TPS ≥50% | 21 (18.8) | 4 (7.8) | 11 (42.3) | 6 (17.1) | |
| pStage | 0.062 | ||||
| I | 67 (59.8) | 37 (72.5) | 13 (50.0) | 17 (48.6) | |
| II | 25 (22.3) | 8 (15.7) | 5 (19.2) | 12 (34.3) | |
| III | 20 (17.9) | 6 (11.8) | 8 (30.8) | 6 (17.1) | |
| pT | 0.377 | ||||
| 1 | 46 (41.1) | 24 (47.1) | 9 (34.6) | 13 (37.1) | |
| 2 | 53 (47.3) | 21 (41.2) | 12 (46.2) | 20 (57.1) | |
| 3 | 13 (11.6) | 6 (11.8) | 5 (19.2) | 2 (5.7) | |
| pN | 0.053 | ||||
| 1 | 76 (67.9) | 40 (78.4) | 17 (65.4) | 19 (54.3) | |
| 2 | 36 (32.1) | 11 (21.6) | 9 (34.6) | 16 (45.7) | |
| Postoperative adjuvant chemotherapy | 0.010 | ||||
| Yes | 41 (36.6) | 11 (21.6) | 12 (46.2) | 18 (51.4) | |
| No | 71 (63.4) | 40 (78.4) | 14 (53.8) | 17 (48.6) | |
| Molecular alteration | 0.688 | ||||
| KRAS G12C | 44 (39.3) | 21 (41.2) | 7 (26.9) | 16 (45.7) | |
| KRAS G12V | 24 (21.4) | 11 (21.6) | 7 (26.9) | 6 (17.1) | |
| KRAS G12A+G12D+G12S | 34 (30.4) | 16 (31.4) | 8 (30.8) | 10 (28.6) | |
| KRAS Codon 13+61 | 10 (8.9) | 3 (5.9) | 4 (15.4) | 3 (8.6) | |
Abbreviations: IASLC, International Association for the Study of Lung Cancer; TIL, tumor infiltrating lymphocyte; TPS, tumor proportion score.
The classification is based on the Grading‐Immunoscore type, which combines histological grade and CD8+ TIL level as follow: type A, low‐ to medium‐grade with high or low infiltration; type B, high‐grade, high‐infiltration; and type C, high‐grade, low‐infiltration.
FIGURE 1.
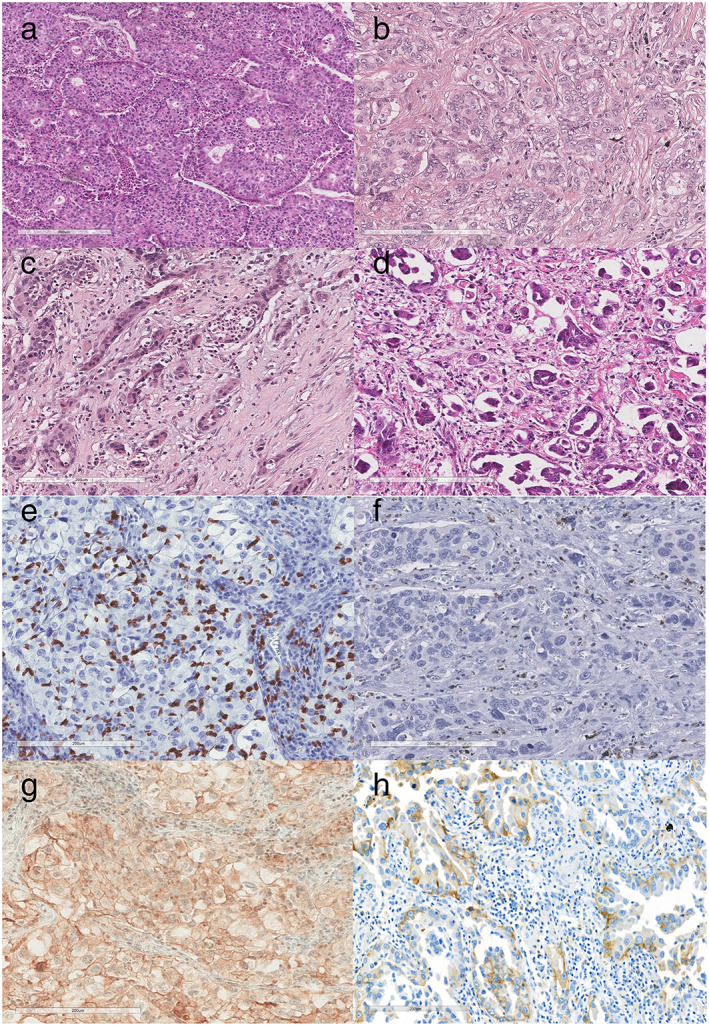
(a)–(d) High‐grade patterns in KRAS‐mutant LUAD. (a) Cribriform pattern; (b) fused glands in a continuous spectrum between solid and acinar patterns; (c) poorly formed and irregular glands in desmoplastic stroma; (d) small cell clusters, and single cells infiltrating desmoplastic stroma. (e)–(h) Representative images of immunohistochemical staining for density of CD8+ TILs, PD‐L1 expression. (e) High density of CD8+ TILs (count 45 per high power field); (f) low density of CD8+ TILs (count 1 per high power field); (g) PD‐L1 22C3 expression (TPS = 90%); (h) PD‐L1 22C3 expression (TPS = 30%). Abbreviations: LUAD, lung adenocarcinoma; TIL, tumor‐infiltrating lymphocyte; TPS, tumor proportion score
Associations between Grading‐Immunoscore type and clinicopathological variables
The results of the χ2 tests showed that the G‐I type was correlated with sex (p = 0.028), history of smoking (p = 0.007), lymphovascular invasion (p < 0.001), postoperative adjuvant chemotherapy (p = 0.010), PD‐L1 expression (p = 0.001), and PD‐L1 high expression (p = 0.002). However, there was no significant association with other clinicopathological factors such as age (p = 0.964), pStage (p = 0.062), and molecular alterations (p = 0.688) (Table 1). Moreover, type B exhibited a higher positive rate of PD‐L1 expression than types A and C (TPS ≥1%: 29.4%, 73.1%, and 42.9%; TPS ≥50%: 7.8%, 42.3%, and 17.1% for types A, B, and C, respectively).
Reproducibility assessment of CD8 + tumor‐infiltrating lymphocyte scoring
The assessments by the pathologists had a high degree of interobserver reliability for CD8+ TIL count scoring (intraclass correlation: 0.918, 95% confidence interval [CI], 0.882–0.943). The Bland–Altman plot is shown in Figure S1. For the binary result of CD8+ TILs, there was agreement in 90% of the cases (101 of 112) among observers. The difference in count in the disagreements ranged from 5 to 20. In 63.6% of the inconsistent cases (7 of 11), the difference was >10. The discordant results for large differences were mainly owing to the observed differences in the location of the high‐grade patterns.
Based on the substantial repeatability of CD8+ TIL scoring between the pairs of observers, we used the mean value of counts evaluated by the two pathologists as the final score. The median of CD8+ TIL scoring was 12.75, so that CD8+ TIL levels were assessed using a two‐tiered scoring system; 0–13 was classified as low‐density infiltration, and >13 was classified as high‐density infiltration.
Univariate survival analysis
Univariate analysis was used to analyze 22 clinicopathological factors in patients with KRAS‐mutant LUAD. The results showed that the IASLC histologic grading (p < 0.001), pStage (p = 0.031), T stage (p = 0.024), N stage (p = 0.047), pleural invasion (p = 0.041), and postoperative adjuvant chemotherapy (p = 0.004) were associated with lower OS, whereas CD3+ TILs (p = 0.014) and CD8+ TILs (p = 0.002) were associated with higher OS. The IASLC histological grading (p < 0.001), pStage (p = 0.020), T stage (p = 0.006), N stage (p = 0.012), lymphovascular invasion (p = 0.001), STAS (p = 0.034), cytologic grade (p = 0.028), and postoperative adjuvant chemotherapy (p = 0.001) were associated with lower PFS, whereas high‐density CD8+ TILs (p = 0.006) was associated with higher PFS. The survival analysis curves of the OS and PFS are shown in Figure 2, and the detailed results of the univariate analysis are shown in Table S1.
FIGURE 2.
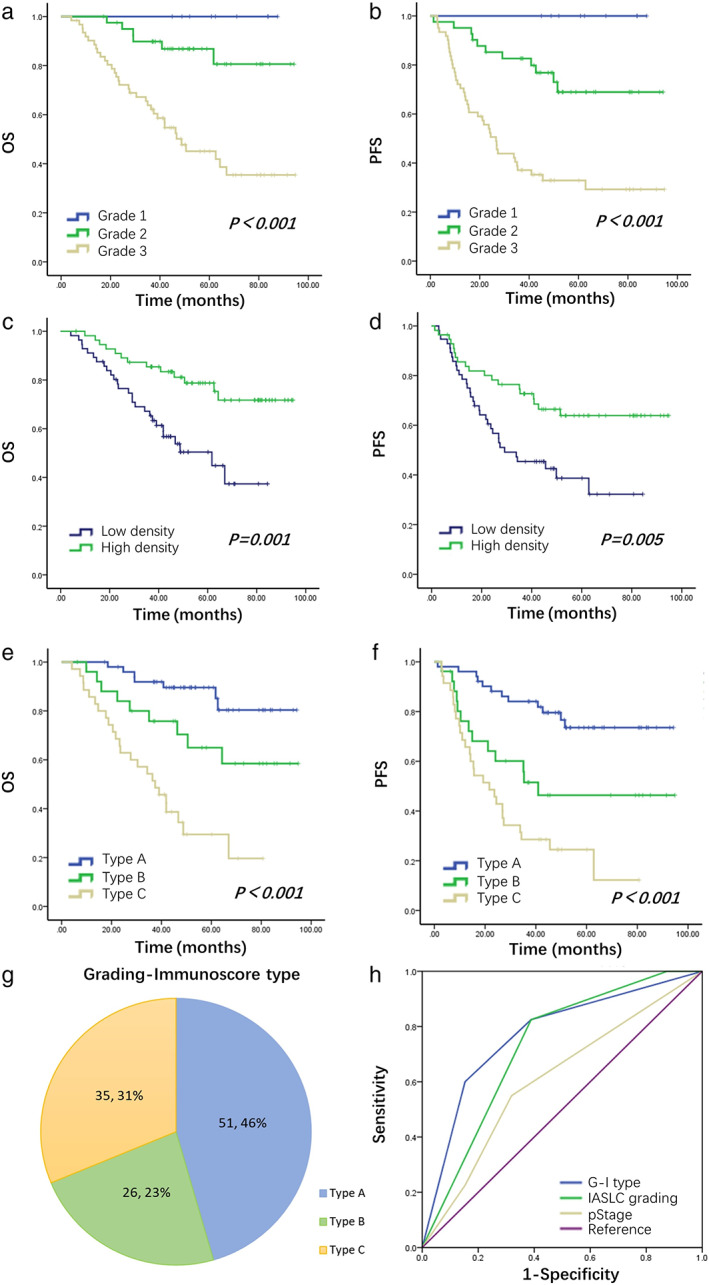
Survival curves per the IASLC histologic grading ((a) for OS and (b) for PFS), CD8+ TIL level ((c) for OS and (d) For PFS) and Grading‐Immunoscore type ((e) for OS and (f) for PFS). (g) Pie chart of the distribution of G‐I type. (h) Death ROC curve of G‐I type, IASLC histologic grading and pStage in patients with KRAS‐mutant LUAD. Abbreviations: G‐I type, Grading‐Immunoscore type; IASLC, International Association for the Study of Lung Cancer; LUAD, lung adenocarcinoma; OS, overall survival; PFS, progression‐free survival; ROC, receiver‐operating characteristic; TIL, tumor‐infiltrating lymphocyte
Multivariable Cox regression analysis
The significant risk factors (IASLC histologic grading, CD8+ TILs, postoperative adjuvant chemotherapy, and pStage) were included in the multivariable analysis. The results showed that IASLC histologic grading (HR, 4.177, 95% CI, 1.811–9.633; p = 0.001) and CD8+ TILs (HR, 0.392, 95% CI, 0.198–0.777; p = 0.007) were associated with lower OS and higher OS respectively, independent of the pStage and postoperative adjuvant chemotherapy, and the same results were obtained in PFS analysis (Table 2).
TABLE 2.
Multivariable Cox regression analysis of CD8+ tumor‐infiltrating lymphocytes and clinicopathological variables on overall survival and progression‐free survival
| Overall survival | Progression‐free survival | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p‐Value | HR | 95% CI | p‐Value | |
| CD8+ TILs | ||||||
| High density versus low density | 0.392 | 0.198–0.777 | 0.007 | 0.534 | 0.299–0.953 | 0.034 |
| IASLC histological grading | ||||||
| Grade 3 versus grade 1 + grade 2 | 4.177 | 1.811–9.633 | 0.001 | 3.380 | 1.722–6.635 | <0.001 |
| Postoperative adjuvant chemotherapy | ||||||
| Yes versus no | 1.728 | 0.866–3.449 | 0.121 | 1.671 | 0.915–3.051 | 0.095 |
| pStage | ||||||
| Stage II + III versus I | 1.313 | 0.663–2.601 | 0.435 | 1.289 | 0.712–2.335 | 0.401 |
| Grading‐Immunoscore type | ||||||
| Type B versus type A | 2.416 | 0.891–6.552 | 0.083 | 2.554 | 1.150–5.670 | 0.021 |
| Type C versus type A | 7.242 | 3.036–17.274 | <0.001 | 4.842 | 2.382–9.844 | <0.001 |
| Type B versus type C | 0.334 | 0.152–0.734 | 0.006 | 0.527 | 0.270–1.030 | 0.061 |
| pStage | ||||||
| Stage II + III versus I | 1.281 | 0.826–2.955 | 0.479 | 1.262 | 0.698–2.282 | 0.441 |
| Postoperative adjuvant chemotherapy | ||||||
| Yes versus no | 1.745 | 0.871–3.493 | 0.116 | 1.665 | 0.911–3.044 | 0.098 |
Abbreviations: CI, confidence interval; HR, hazard ratio; IASLC, International Association for the Study of Lung Cancer; OS, overall survival; PFS, progression‐free survival; TIL, tumor‐infiltrating lymphocyte.
Prognostic significance of the Grading‐Immunoscore type
The Kaplan–Meier analysis showed that the low‐to‐medium grade, high‐infiltration group and the low‐to‐medium grade, low‐infiltration group had similar survival rates, which were not statistically significant. The above two groups were grouped together for analysis as type A (Figure S2 and Table S2). Furthermore, type C were associated with a shorter OS and PFS compared with types A and B (5‐year OS, 89.6%, 65.0%, and 29.5% for types A, B, and C, respectively; p < 0.001; 3‐year PFS, 84.1%, 51.5%, and 28.6% for types A, B, and C, respectively; p < 0.001) (Figure 2(e) and (f)). The stratification of survival was more evident with the G‐I type than with the IASLC grading system (Figure 2(a) and (b)). The significant risk factors (G‐I type, pStage, postoperative adjuvant chemotherapy) were included in the Cox regression model again for multivariable analysis. The results revealed that the G‐I type was an independent prognostic factor for OS and PFS in the cohort (Table 2).
The prognostic distinguishing ability of the pStage was represented by an area under the receiver‐operating characteristic (ROC) curve (AUC) of 0.609 for death and 0.617 for recurrence. The application of the IASLC histologic grading resulted in an AUC of 0.729 and 0.727 for death and recurrence, respectively. The introduction of the G‐I type improved the prognostic stratification of KRAS‐mutant adenocarcinoma with an AUC of 0.772 and 0.749 for death and recurrence, respectively (Figure 2(h)).
DISCUSSION
The IASLC grading system based on the predominant and high‐grade patterns is a strong prognostic classifier of invasive non‐mucinous adenocarcinoma, considered as a complement to the current classification of lung adenocarcinoma. 20 Our study validated that the IASLC grading system and density of CD8+ TILs were independent prognostic factors of OS and PFS in patients with KRAS‐mutant adenocarcinoma. However, the pathological stage was not an independent prognostic factor, and the difference in prognosis between pStage II and pStage III was not significant. As reported in a recent study, 24 EGFR mutations are associated with IASLC moderate grade, whereas KRAS mutations and ALK fusions are significantly more prevalent in IASLC high‐grade tumors. In our study, almost 54.5% of participants had poorly differentiated LUAD in the IASLC grading system. Therefore, to stratify the poorly differentiated KRAS mutation cases, we introduced CD8+ TILs as immune factors into the IASLC grading and established a new classification, the G‐I type. This classification provides a good manifestation of the heterogeneity of KRAS‐mutant LUAD and shows higher diagnostic and prognostic value than the IASLC histologic grading and pStage (AUC for death: 0.772, 0.729, and 0.609, respectively).
Because there was no significant difference in the survival rate between the low‐grade, high‐infiltration group and the low‐grade, low‐infiltration group, we grouped the two together for analysis as type A. Type B had a high‐grade histological pattern and a high density of CD8+ TILs, suggesting that it is an adaptive immune tolerance type. The high PD‐L1 expression rate of type B was 42.3%, whereas that of type A and type C was 7.8% and 17.1%, respectively, indicating that type B is most likely to benefit from immunotherapy. These results suggest that the G‐I type may be related to the KRAS molecular subtype (KP subgroup and KL subgroup). 25 , 26 The molecular data of the RT‐qPCR were collected retrospectively, and most of the tissue samples were collected 5 years before the analysis, making it difficult to conduct further genetic tests. In future studies gene‐panel next‐generation sequencing should be performed and analyzed according to the G‐I type, to confirm this hypothesis.
High levels of CD8+ T‐cell infiltration have been shown to have positive effects on the prognosis of patients with NSCLC. 27 , 28 , 29 , 30 We validated these observations and developed an accessible scoring system for clinical application. At present, there are only limited data addressing CD8+ TILs in lung cancer, based on manual quantitative analysis. Compared with the percentage evaluation method, the CD8+ TILs counting method that we evaluated eased the scoring and improved its consistency. Moreover, the presence of TIL is not a simple dichotomous variable, and both the density and location of TILs and their interaction with the PD‐L1 positive tumor microenvironment should be considered. 14 In this study, the evaluation region we chose was close to a high‐grade pattern tumor, which better reflects the response of the immune system to high‐grade tumors. In view of its robust prognostic effect, relative ease of measurement, and high interobserver consistency (percent agreement: 90%, intraclass correlation: 0.918), the quantitative evaluation of CD8+ TILs in high‐grade lung cancer could be considered for clinical application.
This study had certain limitations. First, the sample size used to classify the G‐I type was relatively small in resectable KRAS‐mutant LUAD. Second, although multiple groups have verified the impact of CD8+ TILs on clinical outcomes, a standardized scoring system has not yet been established. This study emphasizes a new approach of CD8+ TIL evaluation in KRAS‐mutant LUAD, which needs to be validated by other research teams in various clinical settings. The G‐I type proposed in our study is based on the KRAS‐mutant LUAD, a special molecular subtype; whether it is applicable to patients without driver gene changes needs further attention in follow‐up research. Third, tissue specimens used in this study were not recently obtained, which could affect the PD‐L1 expression and may lead to an underestimation of the TPS of PD‐L1 expression. Finally, there are no clinical data on the effectiveness of PD‐L1 checkpoint inhibitors in this study to support the clinical significance of the pathological classification, but similar clinical studies have been reported successively, initially showing its clinical application value. 31 , 32 , 33
CONCLUSION
In summary, these results highlight the importance of the tumor immune microenvironment in prognosis and promote the establishment of an immunoscore for LUAD. The strength of this study is that it innovatively proposes a new classification of KRAS‐mutant LUAD, which is more accurate than the IASLC grading at distinguishing the prognosis, which may be useful in identifying patients who can be included in a future trial for perioperative immunotherapy. The results should be validated for distinct clinical conditions and further be extended to clinical application in advanced lung cancers.
CONFLICT OF INTEREST
The authors declare no conflicts of interests.
ETHICS APPROVAL
Ethical approval was obtained from the Ethics Committee Board of the Peking University Cancer Hospital and Institute, Beijing, China (Approval No. 2018YJZ19).
PATIENT CONSENT
All patients provided informed consent.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81871860 and 82003155), Capital Funds for Health Improvement and Research (2020‐2‐1025).
Chi K, Sun W, Yang X, Wu J, Wang H, Liu X, et al. A prognostic classification based on the International Association for the Study of Lung Cancer histologic grading and immunoscore in KRAS ‐mutant invasive non‐mucinous adenocarcinoma. Thorac Cancer. 2022;13:1050–1058. 10.1111/1759-7714.14360
Funding information Capital Funds for Health Improvement and Research, Grant/Award Number: 2020‐2‐1025; National Natural Science Foundation of China, Grant/Award Numbers: 82003155, 81871860
DATA AVAILABILITY
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Shepherd FA, Domerg C, Hainaut P, Jänne PA, Pignon JP, Graziano S, et al. Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early‐stage resected non‐small‐cell lung cancer in four trials of adjuvant chemotherapy. J Clin Oncol. 2013;31(17):2173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Macerelli M, Caramella C, Faivre L, Besse B, Planchard D, Polo V, et al. Does KRAS mutational status predict chemoresistance in advanced non‐small cell lung cancer (NSCLC)? Lung Cancer. 2014;83(3):383–8. [DOI] [PubMed] [Google Scholar]
- 3. El Osta B, Behera M, Kim S, Berry LD, Sica G, Pillai RN, et al. Characteristics and outcomes of patients with metastatic KRAS‐mutant lung adenocarcinomas: the lung cancer mutation consortium experience. J Thorac Oncol. 2019;14(5):876–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mascaux C, Iannino N, Martin B, Paesmans M, Berghmans T, Dusart M, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta‐analysis. Br J Cancer. 2005;92(1):131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borghaei H, Paz‐Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373(17):1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): a multicentre, open‐label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–46. [DOI] [PubMed] [Google Scholar]
- 7. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): a phase 3, open‐label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non‐small cell lung carcinoma: a systematic review and meta‐analysis. JAMA Oncol. 2018;4(2):210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang Q, Zhang H, Hai J, Socinski MA, Lim E, Chen H, et al. Impact of PD‐L1 expression, driver mutations and clinical characteristics on survival after anti‐PD‐1/PD‐L1 immunotherapy versus chemotherapy in non‐small‐cell lung cancer: a meta‐analysis of randomized trials. Onco Targets Ther. 2018;7(12):e1396403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu J, Sun W, Wang H, Huang X, Wang X, Jiang W, et al. The correlation and overlaps between PD‐L1 expression and classical genomic aberrations in Chinese lung adenocarcinoma patients: a single center case series. Cancer Biol Med. 2019;16(4):811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med. 2015;373(2):123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism‐driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fridman WH, Pagès F, Sautès‐Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. [DOI] [PubMed] [Google Scholar]
- 14. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, et al. PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, et al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128–39. [DOI] [PubMed] [Google Scholar]
- 16. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor‐infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kilvaer TK, Paulsen EE, Andersen S, Rakaee M, Bremnes RM, Busund LTR, et al. Digitally quantified CD8+ cells: the best candidate marker for an immune cell score in non‐small cell lung cancer? Carcinogenesis. 2020;41(12):1671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldstraw P, Chansky K, Crowley J, Rami‐Porta R, Asamura H, Eberhardt WEE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. [DOI] [PubMed] [Google Scholar]
- 19. WHO Classification of Tumours Editorial Board . Thoracic tumours. WHO Classification of Tumours. Vol 5. 5th ed. Lyon(France): IARC Press; 2021. [Google Scholar]
- 20. Moreira AL, Ocampo PSS, Xia Y, Zhong H, Russell PA, Minami Y, et al. A grading system for invasive pulmonary adenocarcinoma: a proposal from the International Association for the Study of Lung Cancer pathology committee. J Thorac Oncol. 2020;15(10):1599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, et al. Assessing tumor‐infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international Immuno‐oncology biomarkers working group: part 2: TILs in melanoma, gastrointestinal tract carcinomas, non‐small cell lung carcinoma and mesothelioma, endometrial and ovarian carcinomas, squamous cell carcinoma of the head and neck, genitourinary carcinomas, and primary brain tumors. Adv Anat Pathol. 2017;24(6):311–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ovarian Tumor Tissue Analysis (OTTA) Consortium , Goode EL, Block MS, et al. Dose‐response association of CD8+ tumor‐infiltrating lymphocytes and survival time in high‐grade serous ovarian cancer. JAMA Oncol. 2017;3(12):e173290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang L, Conejo‐Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–13. [DOI] [PubMed] [Google Scholar]
- 24. Deng C, Zheng Q, Zhang Y, Jin Y, Shen X, Nie X, et al. Validation of the novel International Association for the Study of Lung Cancer grading system for invasive pulmonary adenocarcinoma and association with common driver mutations. J Thorac Oncol. 2021;16(10):1684–93. [DOI] [PubMed] [Google Scholar]
- 25. Skoulidis F, Byers LA, Diao L, Papadimitrakopoulou VA, Tong P, Izzo J, et al. Co‐occurring genomic alterations define major subsets of KRAS‐mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5(8):860–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, et al. STK11/LKB1 mutations and PD‐1 inhibitor resistance in KRAS‐mutant lung adenocarcinoma. Cancer Discov. 2018;8(7):822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim A, Lee SJ, Ahn J, Park WY, Shin DH, Lee CH, et al. The prognostic significance of tumor‐infiltrating lymphocytes assessment with hematoxylin and eosin sections in resected primary lung adenocarcinoma. PLoS One. 2019;14(11):e0224430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Donnem T, Hald SM, Paulsen EE, Richardsen E, al‐Saad S, Kilvaer TK, et al. Stromal CD8+ T‐cell density—a promising supplement to TNM staging in non‐small cell lung cancer. Clin Cancer Res. 2015;21(11):2635–43. [DOI] [PubMed] [Google Scholar]
- 29. Al‐Shibli KI, Donnem T, Al‐Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non‐small cell lung cancer. Clin Cancer Res. 2008;14(16):5220–7. [DOI] [PubMed] [Google Scholar]
- 30. Goc J, Germain C, Vo‐Bourgais TK, Lupo A, Klein C, Knockaert S, et al. Dendritic cells in tumor‐associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014;74(3):705–15. [DOI] [PubMed] [Google Scholar]
- 31. Yang JJ, Huang C, Fan Y, Pan H, Feng J, Jiang L, et al. Camrelizumab in different PD‐L1 expression cohorts of pre‐treated advanced or metastatic non‐small cell lung cancer: a phase II study. Cancer Immunol Immunother. 2021. 10.1007/s00262-021-03091-3. [online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Doroshow DB, Wei W, Gupta S, Zugazagoitia J, Robbins C, Adamson B, et al. Programmed death‐ligand 1 tumor proportion score and overall survival from first‐line Pembrolizumab in patients with nonsquamous versus squamous NSCLC. J Thorac Oncol. 2021;16(12):2139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Herbst RS, Garon EB, Kim DW, Cho BC, Gervais R, Perez‐Gracia JL, et al. Five‐year survival update from KEYNOTE‐010: Pembrolizumab versus Docetaxel for previously treated, programmed death‐ligand 1‐positive advanced NSCLC. J Thorac Oncol. 2021;16(10):1718–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


