Abstract
Heterotopic pancreas is a rare congenital abnormality that occurs during the growth and development process. It can be found in any part of the digestive tract, but the most common sites are the stomach, duodenum, and jejunum. Malignant transformation especially in the esophagus is rare. Here, we aim to report an unusual case of mid‐esophageal adenocarcinoma that originated from a heterotopic pancreas.
Keywords: esophagus, heterotopic pancreas, malignant transformation
INTRODUCTION
Heterotopic pancreas refers to pancreatic tissue found outside its normal location which has no anatomical or vascular relationship with the normal pancreas. 1 It is a congenital malformation that can occur in any part of the digestive tract with the most common sites being the stomach, duodenum, and jejunum. 2 , 3 It is typically asymptomatic and the lesion is normally discovered incidentally during an unrelated surgery, imaging, or even at autopsy. A few patients may present with nonspecific symptoms such as abdominal pain, dyspepsia, obstruction, bleeding, and inflammation depending on the site of the lesion. Malignant transformations especially those involving the esophagus are rare. 4 Here, we report an unusual case of mid‐esophageal adenocarcinoma that originated from a heterotopic pancreas.
Case report
A 60‐year‐old gentleman presented to our hospital with a history of epigastric discomfort for 1 month, with no symptoms of dysphagia, heartburn, acid reflux, abdominal distention, diarrhea, or weight loss. Physical examination did not reveal any abnormalities. Routine hematological, biochemical, and tumor marker (CEA, CA 19–9, CA 72.4, CA 125) tests were within the normal range. Esophagogastroduodenoscopy demonstrated a submucosal cauliflower‐like mass with a diameter of 3 cm at the middle esophagus causing luminal stenosis. Biopsy revealed a poorly differentiated adenocarcinoma. Contrast‐enhanced computed tomography (CT) demonstrated a 3.3 × 3.0 cm tumor at the right‐side wall of the mid‐esophagus without any adjacent pleural invasion. Positron emission tomography (PET) revealed a mid‐esophageal soft tissue mass with high fluorodeoxyglucose (FDG) uptake without regional lymph node involvement or distant metastases. The patient was diagnosed with mid‐esophageal adenocarcinoma that was clinically staged as cT3N0M0 according to the eighth TNM staging system (Figure 1). A minimal McKeown esophagectomy was performed and a mid‐esophageal tumor measuring 3.0 × 3.0 cm that did not infiltrate through the adventitia was identified. A 4.0 × 2.0 cm diverticulum was found adjacent to the tumor. The postoperative recovery of the patient was unremarkable. Histopathological examination showed characteristic moderately differentiated adenocarcinoma with cribriform structure, heterotopic pancreas composed of lobular acini, and hyperplastic ductal glands scattered in the submucosa and mucosa muscularis of the esophagus (Heinrich's type I). Lymph‐vascular invasion, or neural infiltration was not identified and all resected lymph nodes were negative. Immunohistochemistry tests were as follows: BRCA (+<25%), C‐met (2+), EGFR (3+), ERCC‐1 (+50–75%), HER2 (0), Ki‐67 (+50–75%), CK7 (partly +), CK20 (+), CDX‐2 (+), TP63 (−), CA 19–9 (partly +), CEA (+), TP53 (−), P16 (−), and CyclinD1 (−) (Figure 2). The patient did not receive adjuvant therapy postoperatively, but had regular follow‐up at the outpatient department. Unfortunately, he had mediastinal lymph node recurrence and lung metastasis 37 months after the operation and received adjuvant cisplatin‐based chemotherapy for recurrence. The patient did not respond well to chemotherapy and the tumor subsequently slowly progressed. The patient died 20 months after recurrence.
FIGURE 1.
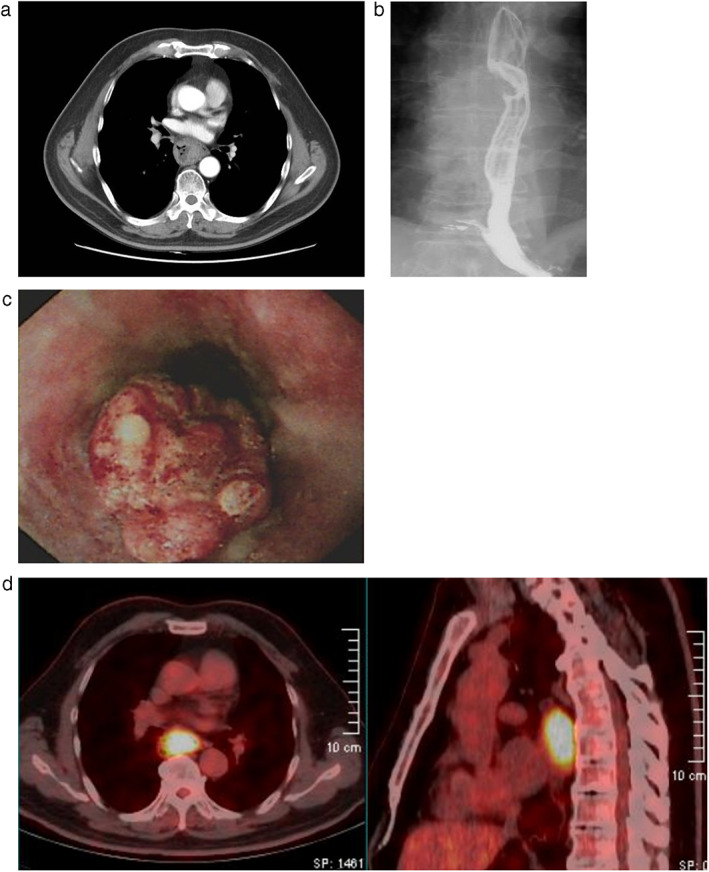
(a) Contrast‐enhanced chest computed tomography demonstrates thickening of the middle esophageal wall. (b) Barium swallow shows a filling defect of the middle esophagus. (c) Endoscopy reveals a 4.0 cm mid‐esophageal mass. (d) PET/CT image shows a mid‐esophageal soft tissue mass with high FDG uptake without lymph node involvement
FIGURE 2.
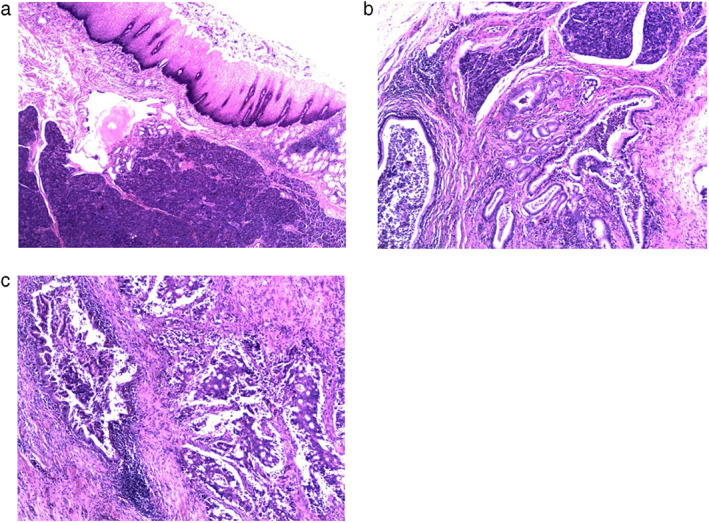
(a) Ectopic pancreas with lobular structure highlighted in the submucosa and mucosa muscularis of the esophagus (×40 magnification). (b) Ectopic pancreas with lobular acini and hyperplastic ductal glands (×100 magnification). (c) Right‐sided moderately differentiated adenocarcinoma with cribriform structure. On the left side, a ductal gland was identified with pancreatic intraepithelial neoplasia 1 (PanIN‐1) changes next to the carcinomatous glands, which indicates that this adenocarcinoma originated from the pancreatic duct (×100 magnification)
DISCUSSION
Heterotopic pancreas, also known as an aberrant or ectopic pancreas (EP), is described as pancreatic tissue in an abnormal location without any anatomic, vascular, or neural continuity with the normal pancreas. It is more predominant in men than women and typically is not discovered until the fifth to sixth decade of life. Only a few cases are diagnosed in children. 5 There are several theories regarding the development of heterotopic pancreas: (i) the dislocation theory states that during foregut rotation, the original pancreatic components are separated, deposited, and develop ectopically, thus gradually forming mature pancreatic tissue. (ii) The metaplasia theory posits that heterotopic pancreas originates from the endoderm of the pancreatic metaplasia region and then migrates to the submucosa during embryogenesis. (iii) In the totipotent cell theory, the totipotent endodermal cells lining the gut differentiate into pancreatic tissue. 6
Patients with a heterotopic pancreas are usually asymptomatic and it is only occasionally found during routine imaging or endoscopy, after surgery, or even at autopsy. Therefore, the true incidence is hard to estimate. The reported incidence ranges from 0.55% to 13.7% on autopsy and 0.2% during upper abdominal surgery. 2 , 7 A few patients may present with various nonspecific symptoms such as abdominal pain, nausea, dysphagia, dyspepsia, bleeding, and obstruction depending on the site of the lesion.
Because there are no specific clinical manifestations or imaging signs, accurate diagnosis is usually difficult. In many cases, the correct diagnosis cannot be determined until the resected specimen is examined by histopathology. Most lesions are solitary, with a diameter of less than 3 cm. Typical endoscopic findings show a submucosal mass covered by normal mucosa with an intraluminal growth pattern. In some endoscopic cases, central umbilication can be found, but the exact diagnosis is not easy without a biopsy. These lesions are frequently misdiagnosed as gastrointestinal stromal tumors (GIST) or leiomyomas before a complete resection is performed. Endoscopic ultrasonography may exhibit an intermediate echogram between the echo dense submucosa and hyperechoic muscularis propria layer. The lesions appear to be hypoechoic to the submucosa and isoechoic to the muscularis propria. 1 CT images usually show oval submucosal masses with unclear boundaries and lobular morphology. Lesions exhibiting enhancement greater than or the same as those of the orthotopic pancreas are dominated by acini, whereas lesions with less enhancement are dominated by ducts and hypertrophied muscle. 7
The heterotopic pancreas is classified into three Heinrich types. The first and most common type is composed of all the elements of normal pancreatic tissue, including acini, ducts, and islet cells. The second and third histological types are dominated by either acini or ducts. In 1973, Gaspar‐Fuentes modified this classification system and added a fourth histological type which is composed of only endocrine islet cells without exocrine pancreatic tissue (Table 1). 8 Malignant transformations of the heterotopic pancreas are rare events, with a reported incidence rate of 0.7% to 1.8%. It is even more rare for lesions located at the esophagus. Only three cases of malignant transformations of the heterotopic pancreas at the esophagus have been reported in the literature (Table 2). 3 , 5 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23
TABLE 1.
Heinrich's classification of heterotopic pancreas
| Type | Histopathological characteristics |
|---|---|
| Type I | Typical pancreatic tissue with ducts, acini, and islet cells |
| Type II | Numerous acini, few ducts, and no islet cells |
| Type III | Numerous ducts, few to no acini, and no islet cells |
| Type IV | Endocrine islets without exocrine pancreatic tissue |
TABLE 2.
Esophageal ectopic pancreas cases in the literature
| Case | Sex | Age | Presentation | Location | Treatment | Pathology | Heinrich's type | Follow‐up results |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 43 | Hematemesis | Distal | Thoracotomy | EP | I | Asymptomatic |
| 2 | M | 25 | Vomiting | Middle | Ivor Lewis esophagectomy | EP | N/A | The intramural esophageal cyst was enucleated by right thoracotomy |
| 3 | M | 60 | Dysphagia, epigastric pain | EGJ | Tumor resection by left thoracotomy and proximal stomach resection | Adenocarcinoma | N/A | Initially asymptomatic, but died 3 months postoperatively |
| 4 | F | 47 | Epigastric pain | EGJ | Ivor Lewis esophagectomy | EP | I | N/A |
| 5 | M | 45 | Dysphagia | EGJ | Sweet esophagectomy | Anaplastic carcinoma | N/A | Survived over 3 years with no recurrence |
| 6 | F | 24 | Nausea and vomiting, fever, and chills | EGJ | Esophageal enucleation | EP | I | Asymptomatic at 1 year |
| 7 | M | 52 | Episodic dysphagia | EGJ | Dietary adjustment | EP | N/A | Asymptomatic |
| 8 | F | 41 | Dysphagia, epigastric pain | EGJ | Gastrectomy with Roux‐en‐Y esophagojejunostomy | EP | I | N/A |
| 9 | F | 26 | Epigastric pain, nausea | EGJ | Laparoscopic excision | EP | I | Asymptomatic at 2 months |
| 10 | M | 63 | Asymptomatic | Middle | Conservative management | EP | II | Asymptomatic for 5 years |
| 11 | F | 14 | Periumbilical abdominal pain | Distal | Conservative management | EP | II | N/A |
| 12 | F | 58 | Dysphagia | EGJ | Ivor Lewis esophagectomy | Intraductal papillary mucinous neoplasm | N/A | Asymptomatic at 3 months |
| 13 | M | 25 | Epigastric pain | EGJ | VATS resection | EP | N/A | Asymptomatic at 2 months |
| 14 | F | 73 | Epigastric pain, heartburn, nausea, vomiting | EGJ | Endoscopic resection | EP | I | N/A |
| 15 | M | 34 | Dysphagia | Distal | Left thoracotomy | EP | I | Asymptomatic at 3 months |
| 16 | M | 70 | Heartburn, nausea, abdominal bloating | Distal | Endoscopic resection | EP | I | N/A |
There is no standardized guideline for the management of heterotopic pancreas, but treatment depends on the patient's clinical symptoms and the location of the lesion. For asymptomatic patients or those with small lesions, regular observation, or medication is appropriate. For symptomatic patients, or those with the possibility of a malignant transformation, endoscopic or surgical resection should be carried out. Resection is the gold standard of management for a definitive diagnosis.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Yang Y‐B, Liu Y‐Q, Dai L, Yan W‐P, Liang Z, Chen K‐N. Malignant transformation of heterotopic pancreas as middle esophagus adenocarcinoma—A rare case report and comprehensive literature review. Thorac Cancer. 2022;13:1083–1087. 10.1111/1759-7714.14344
Funding information National Key R&D Program of China, Grant/Award Number: 2017YFC0907504; the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support, Grant/Award Number: ZYLX201509; the Digestive Medical Coordinated Development Center of Beijing Municipal Administration of Hospitals, Grant/Award Number: XXT18; the National High Technology Research and Development Program of China, Grant/Award Number: 2015AA020403; the Organization Department of Beijing Municipal Party Committee, Grant/Award Number: 2018000021469G259
REFERENCES
- 1. Rezvani M, Menias C, Sandrasegaran K, Olpin JD, Elsayes KM, Shaaban AM. Heterotopic pancreas: histopathologic features, imaging findings, and complications. Radiographics. 2017;37(2):484–99. [DOI] [PubMed] [Google Scholar]
- 2. Kung JW, Brown A, Kruskal JB, Goldsmith JD, Pedrosa I. Heterotopic pancreas: typical and atypical imaging findings. Clin Radiol. 2010;65(5):403–7. [DOI] [PubMed] [Google Scholar]
- 3. Mack T, Lowry D, Carbone P, Barbick B, Kindelan J, Marks R. Multimodality imaging evaluation of an uncommon entity: esophageal heterotopic pancreas. Case Rep Radiol. 2014;2014:614347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cazacu IM, Luzuriaga Chavez AA, Nogueras Gonzalez GM, Saftoiu A, Bhutani MS. Malignant transformation of ectopic pancreas. Dig Dis Sci. 2019;64(3):655–68. [DOI] [PubMed] [Google Scholar]
- 5. Shamoon D, Sostre V, Patel V, Volfson A. A case of heterotopic pancreatic tissue discovered in the distal esophagus. Case Rep Gastrointest Med. 2020;4695184:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lai EC, Tompkins RK. Heterotopic pancreas. Review of a 26 year experience. Am J Surg. 1986;151(6):697–700. [DOI] [PubMed] [Google Scholar]
- 7. Wei R, Wang QB, Chen QH, Liu JS, Zhang B. Upper gastrointestinal tract heterotopic pancreas: findings from ct and endoscopic imaging with histopathologic correlation. Clin Imaging. 2011;35(5):353–9. [DOI] [PubMed] [Google Scholar]
- 8. Gaspar Fuentes A, Campos Tarrech JM, Fernandez Burgui JL, et al. Pancreatic ectopias. Rev Esp Enferm Apar Dig. 1973;39(3):255–68. [PubMed] [Google Scholar]
- 9. Razi MD. Ectopic pancreatic tissue of esophagus with massive upper gastrointestinal bleeding. Arch Surg. 1966;92(1):101–4. [DOI] [PubMed] [Google Scholar]
- 10. Salo JA, Dlouhy M, Virtanen I. Congenital cyst and heterotopic pancreatic tissue in the oesophagus. Ann Chir Gynaecol. 1993;82(4):263–5. [PubMed] [Google Scholar]
- 11. Guillou L, Nordback P, Gerber C, Schneider RP. Ductal adenocarcinoma arising in a heterotopic pancreas situated in a hiatal hernia. Arch Pathol Lab Med. 1994;118(5):568–71. [PubMed] [Google Scholar]
- 12. Noffsinger AE, Hyams DM, Fenoglio‐Preiser CM. Esophageal heterotopic pancreas presenting as an inflammatory mass. Dig Dis Sci. 1995;40(11):2373–9. [DOI] [PubMed] [Google Scholar]
- 13. Roshe J, Del Buono E, Domenico D, Colturi TJ. Anaplastic carcinoma arising in ectopic pancreas located in the distal esophagus. J Clin Gastroenterol. 1996;22(3):242–4. [DOI] [PubMed] [Google Scholar]
- 14. Temes RT, Menen MJ, Davis MS, Pett SB Jr, Wernly JA. Heterotopic pancreas of the esophagus masquerading as Boerhaave's syndrome. Ann Thorac Surg. 2000;69(1):259–61. [DOI] [PubMed] [Google Scholar]
- 15. Shalaby M, Kochman ML, Lichtenstein GR. Heterotopic pancreas presenting as dysphagia. Am J Gastroenterol. 2002;97(4):1046–9. [DOI] [PubMed] [Google Scholar]
- 16. Rodriguez FJ, Abraham SC, Allen MS, Sebo TJ. Fine‐needle aspiration cytology findings from a case of pancreatic heterotopia at the gastroesophageal junction. Diagn Cytopathol. 2004;31(3):175–9. [DOI] [PubMed] [Google Scholar]
- 17. Gananadha S, Hunt DR. A unique case of pancreatitis and retention cyst in esophageal heterotopic pancreas. Surg Laparosc Endosc Percutan Tech. 2005;15(6):345–7. [DOI] [PubMed] [Google Scholar]
- 18. Goto J, Ohashi S, Okamura S, Urano F, Hosoi T, Ishikawa H, et al. Heterotopic pancreas in the esophagus diagnosed by EUS‐guided FNA. Gastrointest Endosc. 2005;62(5):812–4. [DOI] [PubMed] [Google Scholar]
- 19. Qualia CM, Rossi TM, Ullah A. Heterotopic pancreatic tissue found in the esophagus of a 14‐year‐old girl. Gastroenterol Hepatol (N Y). 2007;3(12):939–40. [PMC free article] [PubMed] [Google Scholar]
- 20. Crighton E, Botha A. Intraductal papillary mucinous neoplasm of the oesophagus: an unusual case of dysphagia. Ann R Coll Surg Engl. 2012;94(2):e92–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lowry DM, Mack TE, Partridge BJ, Barbick BC, Marks RM, Kindelan JT. Thorascopic resection of esophageal heterotopic pancreas. Ann Thorac Surg. 2013;96(5):1850–1. [DOI] [PubMed] [Google Scholar]
- 22. Filip R, Walczak E, Huk J, Radzki RP, Bienko M. Heterotopic pancreatic tissue in the gastric cardia: a case report and literature review. World J Gastroenterol. 2014;20(44):16779–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ulrych J, Fryba V, Skalova H, Krska Z, Krechler T, Zogala D. Premalignant and malignant lesions of the heterotopic pancreas in the esophagus: a case report and review of the literature. J Gastrointestin Liver Dis. 2015;24(2):235–9. [DOI] [PubMed] [Google Scholar]


