Abstract
Background
Interstitial lung abnormality (ILA) is closely related to lung cancer. This study aimed to assess whether the presence of ILA is associated with the clinicoradiological features of elderly patients (≥70 years) with early‐stage non‐small cell lung cancer (NSCLC).
Methods
Elderly patients who underwent surgical resection for stage I or II NSCLC with preserved lung function between 2012 and 2019 were retrospectively identified. ILA was evaluated using a three‐point scale. Univariate analyses were performed for clinicoradiological features based on the presence of ILA. Logistic and linear regression analyses were performed for cancer staging and tumor size, respectively.
Results
A total of 254 patients were evaluated. The presence of ILA (score = 2) was significantly associated with male sex, current or former smoker status, higher pack‐years of smoking, low forced expiratory volume in one second/forced vital capacity ratios and diffusing capacity of the lung for carbon monoxide, and presence of emphysema (≥5%). Tumor characteristics, such as lower lobe and outer one‐third location, squamous cell carcinoma, and higher cancer stage (stage II) were significantly associated with ILA. The presence of ILA independently predicted a higher cancer stage (adjusted odds ratio, 1.81; 95% confidence interval, 1.10–2.96; p = 0.02) and a larger tumor size in linear regression analysis (p = 0.04).
Conclusions
Patients with ILA showed clinicoradiological features similar to those of idiopathic pulmonary fibrosis in elderly patients with early‐stage NSCLC. Identifying the clinical implications of ILA in early‐stage lung cancer will guide clinicians in providing appropriate management for these patients.
Keywords: early‐stage non‐small cell lung cancer, elderly patients, idiopathic pulmonary fibrosis, interstitial lung abnormality
This study revealed that the presence of ILA was significantly associated with higher cancer staging and larger tumor size. Also, ILA showed clinicoradiological features that are similar to those of IPF among elderly patients with early‐stage NSCLC.
INTRODUCTION
Lung cancer is the leading cause of cancer‐related deaths 1 and is mainly diagnosed in elderly patients (median age of diagnosis: 71 years). Furthermore, 86% of all lung cancer cases involve non‐small cell lung cancer (NSCLC). 2 , 3 Given that lung cancer screening with low‐dose computed tomography (LDCT) has become part of evidence‐based medicine for mortality reduction, this method can now be broadly applied worldwide. 4 Lung cancer screening has yielded interesting results. First, a study indicated that the incidence rates of early‐stage lung cancer (stages I and II) and late‐stage lung cancer (stages III and IV) are increasing and decreasing, respectively. 5 Another study showed that only 15% of lung cancers detected in routine care were stage I NSCLC, whereas the ratio was reported to be up to 70% when NSCLC was detected via lung cancer screening. 6 , 7 Second, an increase in LDCT scans leads to an increase in nodule detection and in other incidental but important radiological abnormalities, particularly interstitial lung abnormalities (ILAs) or emphysema. Older age is related to the probability of discovering incidental findings. 5 Considering that incidental findings are more prevalent in older patients and that lung cancer is mainly found in elderly patients, the appropriate management of incidental findings will become more important in the future.
ILA refers to incidental radiological findings without significant clinical symptoms or functional decline. Old age and smoking are well‐known risk factors for ILA. 8 ILA is closely related to lung cancer. The National Lung Cancer Trial showed that ILA is an independent risk factor for lung cancer and is also related to cancer‐specific mortality. 9 Evaluating ILA using preoperative computed tomography (CT) is beneficial for elderly patients with early‐stage NSCLC because pretreatment ILA can serve as a predictive factor for postoperative pulmonary complications due to early‐stage NSCLC in elderly patients after curative resection. 10 , 11 Therefore, it is essential to determine how the clinicoradiologic features of lung cancer patients differ based on the presence of ILA because this information can lead to the establishment of an appropriate treatment strategy.
This study aimed to assess whether the presence of ILA is related to the clinicoradiological features of elderly patients (≥70 years) with early‐stage NSCLC.
METHODS
This study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (approval no. CNUHH‐2021‐242). The requirement for informed consent was waived owing to the retrospective study design.
Patients
This study included patients aged ≥70 years who underwent surgical resection at Chonnam National University Hwasun Hospital. The inclusion criteria were (i) NSCLC with clinical or pathologic stage I or II and (ii) preserved lung function with normal spirometry results. Considering that ILA is an incidental radiological abnormality without clinical symptoms or functional decline, we included “normal spirometry results” as an inclusion criterion. A normal spirometry result was defined as a prebronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio of >0.70 and as FVC ≥80% of the predicted value.
Data collection
Clinical data for all patients were obtained by reviewing their electronic medical records. These data included age, sex, smoking status, pack‐years of smoking, body mass index (BMI; kg/m2), diabetes mellitus, hypertension, cardiovascular disease, presence of double primary cancer, and history of cancer. The results of preoperative pulmonary function tests involved prebronchodilator FEV1/FVC ratio, percentage of predicted FEV1, FVC, and diffusing capacity of the lung for carbon monoxide (DLCO). Clinical or pathologic staging was re‐evaluated according to the eighth tumor–node–metastasis (TNM) staging system. The detailed histology of the resected pathological specimens and tumor size were also investigated. Tumor size was defined as the maximal diameter of the pathologic specimen.
Imaging acquisition and analysis
All CT scans, including high‐resolution CT images, were obtained using the following multidetector CT scanners: LightSpeed 16 (GE Healthcare), LightSpeed VCT (GE Healthcare), Somatom Definition Flash (Siemens Healthineers, Erlangen, Germany), and Revolution (GE Healthcare). The slice thickness used in our hospital was 2.0–3.0 mm.
The presence of ILA and emphysema, and the location of the tumors were evaluated by reviewing the preoperative CT scans. We evaluated ILA using a three‐point scale: 0 = no ILA, 1 = equivocal for ILA, and 2 = presence of ILA. The presence of ILA (score = 2) was defined as an incidentally detected CT abnormality comprising reticulation, ground‐glass abnormality, traction bronchiectasis, nonemphysematous cysts, and honeycombing that exceeds 5% of the entire lung. “Equivocal for ILA” (score = 1) was defined as focal or unilateral ground‐glass abnormality, focal or unilateral reticulation, and ground‐glass abnormality involving less than 5% of the entire lung 12 (Figure 1).
FIGURE 1.
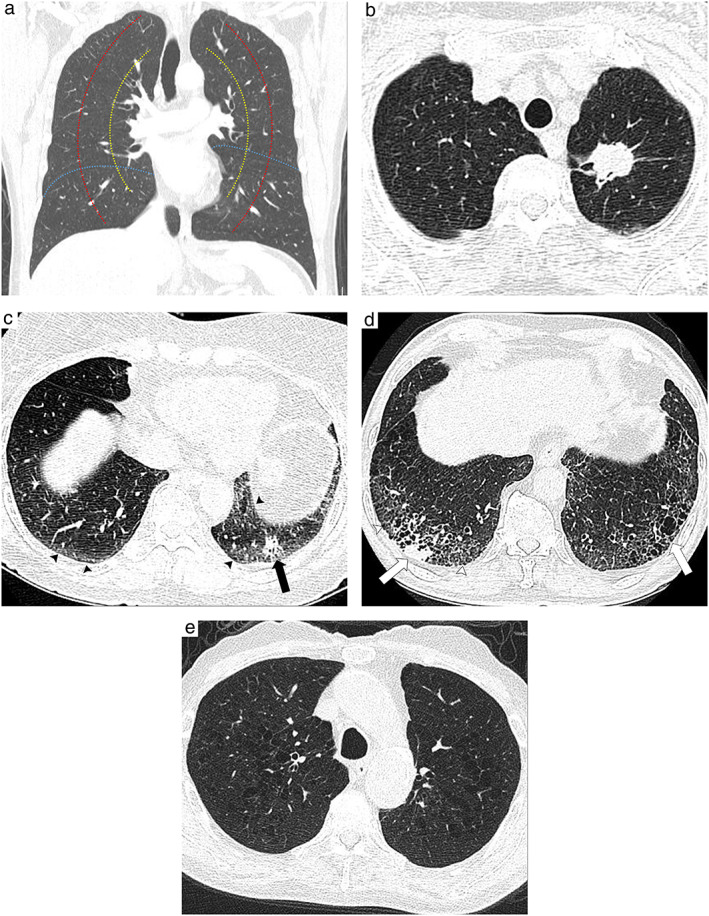
Definitions of tumor location and laterality and interstitial lung abnormality. (a) Boundaries separating laterality and location. The red and yellow lines indicate the boundaries of the inner, middle, and outer one‐third from the pulmonary hilum. The blue line indicates the boundary between the lower lobe and nonlower lobe. (b) Axial high resolution computed tomography (CT) scan shows an approximately 3.1‐cm mass in the left upper lobe. The mass is located in the inner one‐third of the pulmonary hilum. (c) Axial high resolution CT scan shows an approximately 1.5‐cm nodule in the left lower lobe (black arrow). The nodule is located in the outer one‐third of the pulmonary hilum. Ground‐glass abnormalities and mildly dilated bronchi involving less than 5% of the entire lung area are visible in the subpleural areas of both lower lobes (black arrowheads), thus indicating equivocal results for ILA (score = 1). (d) Axial high‐resolution CT scan shows an approximately 2‐cm nodule in the right lower lobe (white arrow). The nodule is located in the outer one‐third of the pulmonary hilum. Reticulation (white arrowheads) and traction bronchiectasis (dotted arrow) are visible in the subpleural areas of both lower lobes, thus indicating ILA (score = 2). (e) Axial high‐resolution CT scan shows centrilobular lucency exceeding 5% of the lung parenchyma, thus indicating centrilobular emphysema
The presence of emphysema was defined as involvement of more than 5% of the entire lung in accordance with the Fleischner Society guidelines. 13 The location of the tumors was assessed by two factors: lower lobe location and laterality. First, the location of the tumor was divided into the lower and nonlower lobes. The right middle lobe was considered as the nonlower lobe. Second, the laterality of the tumor was evaluated as the distance from the epicenter of the tumor to the pulmonary hilum. We used concentric lines arising from the hilum to define the inner one‐third, middle one‐third, and outer one‐third in the definition of central lung cancer. 14 Tumor size was evaluated using the eighth TNM staging system (Figure 1).
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows (version 28.0; IBM Corp.). Data are presented as numbers and percentages for categorical variables and as median values with interquartile ranges for continuous variables. The independent t‐test was used for continuous variables, while the chi‐squared test and Fisher's exact test were used for categorical variables. Logistic regression analysis was performed to evaluate the association between cancer staging and relevant clinicopathological factors. Linear regression analysis was performed to evaluate the association between the maximal tumor diameter and relevant clinicoradiological factors. Two experienced thoracic radiologists (W.G.J., 11 years of experience; J.E.L., 8 years of experience) independently reviewed all CT images. They evaluated the presence of ILA using a three‐point scale, the presence of emphysema exceeding 5% of the entire lung, and the laterality of the tumor. Interobserver agreements for ILA scoring, presence of emphysema, and laterality of the tumor were assessed using the kappa value of agreement with 95% confidence intervals (CIs). After all CT scans were reviewed by two readers, discrepant cases underwent a consensus‐building process. Statistical significance was set at p < 0.05.
RESULTS
Among the 550 patients who underwent lung cancer surgery because of early NSCLC between January 2012 and December 2019, 273 patients were excluded because they had abnormal spirometry results. Patients who had multiple primary lung cancers (n = 4) and a history of lung or esophageal cancer (n = 19) were also excluded. A total of 254 patients were finally included (Figure 2). The presence of ILA (score = 2) was observed in 17.7% (45/254) of the patients, and 32 patients (12.6%) showed equivocal results for ILA (score = 1) on preoperative CT scans. The remaining 177 patients (69.7%) did not show ILA (score = 0) (Figure 2).
FIGURE 2.
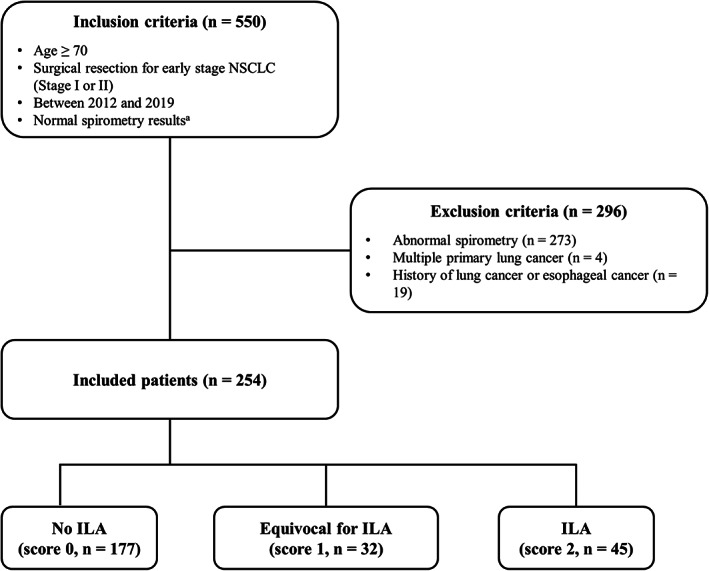
Flowchart of the selection and exclusion of the study population. NSCLC, non‐small cell lung cancer; ILA: interstitial lung abnormality. a Normal spirometry result was defined as a prebronchodilator forced expiratory volume in one second (FEV1) to forced vital capacity (FVC) ratio of >70 or FVC ≥80% of the predicted value
The median (interquartile range) age of all patients was 73 (71–76) years. There was no statistical difference between patients with ILA scores of two and those with ILA scores of zero or one. Among the 254 patients, 130 were men (51.2%), and the presence of ILA (score = 2) was significantly associated with male sex, compared with “no ILA” (score = 0) or “equivocal for ILA” (score = 1) (percentage: 42.6% vs. 91.1%, p < 0.001). A total of 114 patients were former or current smokers (44.9%). The presence of ILA was significantly associated with former or current smokers (p < 0.001) and higher pack‐years of smoking (median pack‐years of smoking: 0 vs. 40, p < 0.001). The median BMI was 23.6 kg/m2. Hypertension was observed in approximately half of the patients (141 patients, 55.5%). BMI and history of lung or esophageal cancer did not show statistical differences between the two groups. Patients with ILA (score = 2) showed a significantly lower FEV1/FVC ratio (median percentage: 76 vs. 73, p = 0.01) and lower percentage predicted DLCO (median percentage: 105 vs. 80, p < 0.001) than those with no ILA (score = 0) or “equivocal for ILA” (score = 1). Emphysema (≥5%) was observed in 23 patients (9.1%). The presence of ILA (score = 2) was significantly associated with the presence of emphysema (≥5%) compared with “no ILA” (score = 0) or “equivocal for ILA” (score = 1) (Table 1).
TABLE 1.
Patient demographics according to the interstitial lung abnormality scoring
| Characteristics | ILA score | |||
|---|---|---|---|---|
| 0 or 1 (n = 209) | 2 (n = 45) | Total (n = 254) | p‐value | |
| Age (years) | 73 (71,76) | 73 (71.5, 75.5) | 73 (71,76) | 0.45 |
| Male | 89 (42.6) | 41 (91.1) | 130 (51.2) | <0.001 |
| Smoking status | <0.001 | |||
| Never | 137 (65.6) | 3 (6.7) | 140 (55.1) | |
| Former/current | 72 (34.4) | 42 (94.4) | 114 (44.9) | |
| Pack years of smoking | 0 (0, 21.3) | 40 (27, 50) | 0 (0, 30) | <0.001 |
| BMI, kg/m2 | 23.5 (21.6, 25.7) | 24.0 (21.5, 25.6) | 23.6 (21.6, 25.6) | 0.15 |
| DM | 61 (29.2) | 18 (40) | 79 (31.1) | 0.11 |
| HTN | 120 (57.4) | 21 (46.7) | 141 (55.5) | 0.13 |
| Cardiovascular disease | 20 (9.6) | 4 (8.9) | 24 (9.4) | 0.58 |
| Double primary cancer | 15 (7.2) | 2 (3.0) | 17 (6.7) | 0.39 |
| Previous cancer history | 38 (18.2) | 8 (17.8) | 46 (18.1%) | 0.57 |
| PFT | ||||
| FVC (% pred) | 90 (83, 99) | 86 (82, 95.5) | 89 (83,99) | 0.17 |
| FEV1 (% pred) | 99 (91, 108) | 94 (88.8, 109.5) | 99 (90, 108) | 0.28 |
| FEV1/FVC | 76.0 (73.0, 79.7) | 73.0 (70.7, 77.5) | 75.6 (72.5, 79.3) | 0.01 |
| DLCO (% pred) | 105 (90.8, 118.6) | 80 (67.0, 93.5) | 101.5 (86.0, 117.6) | <0.001 |
| Emphysema (≥5%) | 10 (4.8) | 13 (28.9) | 23 (9.1) | <0.001 |
Note: Continuous variables are presented as median values (interquartile range). Values for the number of patients are presented as numbers and percentages (in parenthesis). Bold values indicate that p < 0.05.
Abbreviations: BMI, body mass index; DLCO, diffusing capacity of the lung for CO; DM: diabetes mellitus; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; HTN, hypertension; ILA, interstitial lung abnormality.
Among 254 tumors, 99 and 102 tumors were located in the lower lobe (39%) and outer one‐third (40.2%), respectively. The presence of ILA (score = 2) was significantly associated with the lower lobe (p = 0.02) and outer one‐third (p < 0.001) of the tumors. Approximately half of the tumors were at stage T1 (≤30 mm) (140/254, 55.1%). A total of 61 (24%) and 39 (15.4%) patients had stage T2a tumors (>30 mm and ≤ 40 mm) and stage T2b tumors (>40 mm and ≤ 50 mm), respectively. Although T staging did not show a statistical difference, the maximal tumor diameters of the two groups were significantly different (median diameter: 25 mm vs. 30 mm) (Figure 3). Twenty patients showed positive nodal metastases (N1, 7.9%). N staging did not show a statistically significant difference. Fifty tumors were squamous cell carcinomas (19.7%). The presence of ILA (score = 2) was significantly associated with squamous cell carcinoma (p < 0.001) compared with “no ILA” (score = 0) or “equivocal for ILA” (score = 1). A total of 53 patients had stage II lung cancer (20.9%). Patients with ILA (score = 2) showed significantly higher cancer staging (p < 0.001) than those with no ILA (score = 0) or “equivocal for ILA” (score = 1) (Figure 4, Table 2). Multivariate regression analysis revealed that among several relevant risk factors, including ILA (score = 2), age, male sex, smoking status, and emphysema (≥5%), the presence of ILA (score = 2) independently predicted higher cancer staging (adjusted odds ratio, 1.81; 95% CI: 1.1–2.96; p = 0.02) (Table 3). The presence of ILA (score = 2) also predicted a larger tumor size in the linear regression analysis (p = 0.04) (Table 4).
FIGURE 3.
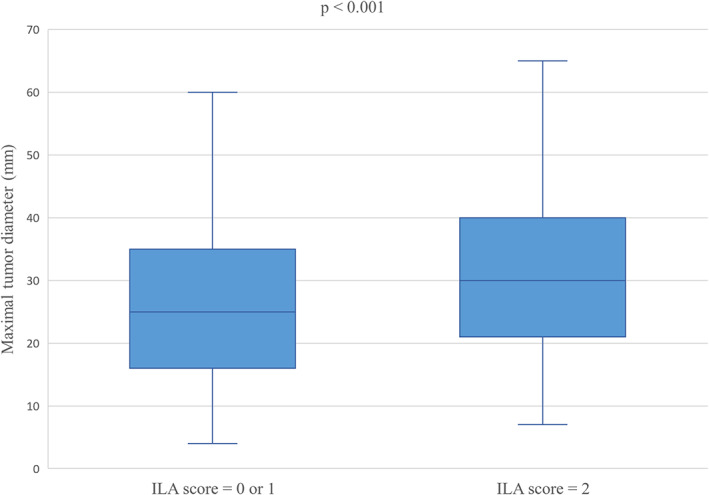
Box plot for maximal tumor diameter according to interstitial lung abnormality scoring. Abbreviations: ILA, interstitial lung abnormality
FIGURE 4.
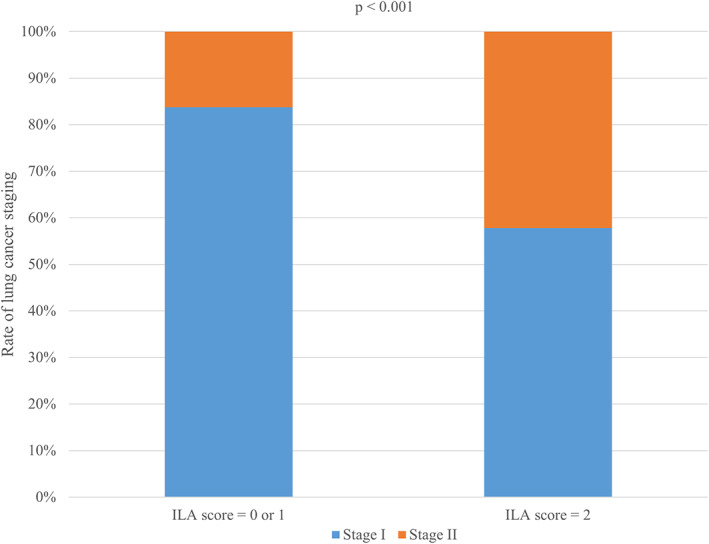
Rate of lung cancer staging according to interstitial lung abnormality scoring. ILA, interstitial lung abnormality. Patients with ILA score 2 had a significantly higher rate of stage II lung cancer
TABLE 2.
Tumor characteristics according to interstitial lung abnormality scoring
| Characteristics | ILA score | |||
|---|---|---|---|---|
| 0 or 1 (n = 209) | 2 (n = 45) | Total (n = 254) | p‐value | |
| Location | 0.02 | |||
| Nonlower lobe | 134 (64.1) | 21 (46.7) | 155 (61.0) | |
| Lower lobe | 75 (35.9) | 24 (53.3) | 99 (39.0) | |
| Location (laterality) | <0.001 | |||
| Inner one‐third | 36 (17.2) | 6 (13.3) | 42 (16.5) | |
| Middle one‐third | 100 (47.8) | 10 (22.2) | 110 (43.3) | |
| Outer one‐third a | 73 (34.9) | 29 (64.4) | 102 (40.2) | |
| T stage b | 0.08 | |||
| 1 (≤30 mm) | 122 (58.4) | 18 (40.0) | 140 (55.1) | |
| 2a (>30 mm, ≤40 mm) | 48 (22.9) | 13 (28.9) | 61 (24.0) | |
| 2b (>40 mm, ≤50 mm) | 30 (14.4) | 9 (20.0) | 39 (15.4) | |
| 3 (>50 mm, ≤70 mm) | 9 (4.3) | 5 (11.1) | 14 (5.5) | |
| Tumor size (mm) b | 25.0 (16.0, 35.0) | 30.0 (21.0, 40.5) | 25.5 (17.8, 35.0) | <0.001 |
| N stage | 0.37 | |||
| 0 | 194 (92.8) | 40 (88.9) | 234 (92.1) | |
| 1 | 15 (7.2) | 5 (11.1) | 20 (7.9) | |
| Histology | <0.001 | |||
| Non‐SQC | 178 (85.2) | 26 (57.8) | 204 (80.3) | |
| SQC | 31 (14.8) | 19 (42.2) | 50 (19.7) | |
| Lung cancer stage c | <0.001 | |||
| Stage I | 175 (83.7) | 26 (57.8) | 201 (79.1) | |
| Stage II | 34 (16.3) | 19 (42.2) | 53 (20.9) | |
Note: Continuous variables are presented as median values (interquartile range). Values for the number of patients are presented as numbers and percentages (in parenthesis). Bold values indicate that p < 0.05.
Abbreviations: ILA, interstitial lung abnormality; SQC, squamous cell carcinoma.
Significant differences considering Bonferroni's correction for post hoc analysis.
By the eighth TNM staging.
Maximal tumor diameter.
TABLE 3.
Logistic regression analysis for cancer staging
| Characteristics | OR | 95% CI | p‐value |
|---|---|---|---|
| ILA (score 2) a | 1.81 | 1.10–2.96 | 0.02 |
| Age | 1.05 | 0.95–1.15 | 0.35 |
| Male sex | 1.37 | 0.38–4.86 | 0.63 |
| Former/current smoker b | 1.23 | 0.32–4.75 | 0.77 |
| Emphysema (≥5%) | 1.01 | 0.35–2.90 | 0.98 |
| Histology (SQC) c | 1.92 | 0.85–4.30 | 0.12 |
Note: Bold values indicate that p < 0.05.
Abbreviations: CI, confidence interval; ILA, interstitial lung abnormality; OR, odds radio; SQC, squamous cell carcinoma.
Comparison of ILA score 2 with ILA score 0 or 1.
Comparison of former/current smoker with never smoker.
Comparison of squamous cell carcinoma with nonsquamous cell carcinoma.
TABLE 4.
Linear regression analysis for maximal tumor diameter
| Characteristics | Standardized coefficients beta | t | p‐value |
|---|---|---|---|
| ILA (score = 2) a | 0.15 | 2.05 | 0.04 |
| Age | 0.46 | 0.75 | 0.46 |
| Male sex | −0.01 | −0.12 | 0.90 |
| Former/current smoker b | 0.04 | 0.31 | 0.76 |
| Emphysema (≥5%) | 0.02 | 0.32 | 0.75 |
| Histology (SQC) c | 0.13 | 0.84 | 0.07 |
Note: Bold values indicate that p < 0.05.
Abbreviations: CI, confidence interval; ILA, interstitial lung abnormality; OR, odds radio; SQC, squamous cell carcinoma.
Comparison of ILA score 2 with ILA score 0 or 1.
Comparison of former/current smoker with never smoker.
Comparison of squamous cell carcinoma with nonsquamous cell carcinoma.
The two radiologists showed substantial interobserver agreements for ILA scoring (κ = 0.61; 95% CI: 0.51–0.71; p < 0.001), presence of emphysema (κ = 0.78; 95% CI: 0.65–0.90; p < 0.001), and tumor laterality (κ = 0.70; 95% CI: 0.62–0.77; p < 0.001).
DISCUSSION
Patients with combined lung cancer and idiopathic pulmonary fibrosis (IPF) are known to have several characteristic clinicoradiological features, such as a preponderance in male smokers, lower lobe and peripheral location of tumors, and a higher prevalence of squamous cell carcinoma. 15 Our study revealed that the clinicoradiological features in elderly patients presenting with ILA and early‐stage NSCLC resemble those in patients with combined lung cancer and IPF. Considering that ILA is a subclinical stage of interstitial lung disease (ILD) and that fibrotic ILA, a subcategory of ILA, is an important early precursor of IPF, the aforementioned characteristics are sufficiently explainable. Currently, ILA and ILD are considered to lie in the spectra of pulmonary fibrosis, and not in separate diseases. 16 ILA and ILD are independent risk factors for lung cancer development. 9 , 17 Regarding ILD, particularly IPF, several studies have reported that the pathomechanism of IPF overlaps with that of lung cancer. 18 The abnormality of the epithelial layer in IPF is closely related to carcinogenesis, which subsequently results in the transition to invasive cancer. 19 Fibrosis in IPF causes cellular metaplasia and repeated tissue damage and repair, thus increasing vulnerability to lung cancer. 20 A study in Japan reported that lung cancer risk is 3.5 times higher in patients with fibrotic lesions regardless of smoking status. 21 Two other studies reported that patients with IPF have 4.99 and 7.3 times higher risk of developing lung cancer. 22 , 23 The close relationship between fibrosis and carcinogenesis also affects the tumor location. Lung cancers in IPF patients tend to be located in the interface between normal lung tissue and fibrosis or within fibrosis. 24 Lower lobe and peripheral predilection are also well‐known features of lung cancer in IPF patients.
This study revealed that the presence of ILA was significantly associated with higher cancer staging (stage I vs. stage II) and larger maximal tumor diameter among several relevant clinical and radiological factors. Jang et al. 25 reported that the severity of IPF is associated with higher cancer staging because widespread areas of fibrosis affect tumor development. Assuming that ILA is a precursor to IPF, fibrosis inherent in ILA may have influenced tumor arousal, thus resulting in higher cancer staging and larger tumor size. Squamous cell carcinoma is associated with an adverse prognosis compared with adenocarcinoma. 26 Tumor staging reflects patient outcomes, that is, a higher staging is associated with a worse prognosis. The current study revealed that the presence of ILA is significantly associated with squamous cell carcinoma and higher cancer staging. These results have two important clinical implications. First, risk factor modification, such as smoking cessation, must be recommended for patients throughout their lives to prevent ILA development. Second, when ILA is observed on thoracic CT, including lung cancer screening, close follow‐up or thorough evaluation may be necessary for the early detection of lung cancer.
The characteristics of our study that show similarities to IPF are also supported by pathological evidence. Although ILA is a subclinical stage of pulmonary fibrosis, histopathological findings of ILA are similar to those of pulmonary fibrosis and usual interstitial pneumonia (UIP). Several authors have determined the histopathological features of ILA. Miller et al. 27 reported that subpleural fibrosis and UIP findings are strongly associated with subpleural ILA. Chae et al. 28 also reported that definite or probable UIP patterns were observed on histopathological examinations in majority of subpleural fibrotic ILA subjects.
Lung cancer treatment consists of various components, such as surgery, chemotherapy, and radiation therapy. In addition, personalized medicine is actively developed and adapted owing to its intractability and higher cancer‐related mortality. Pretreatment risk stratification is especially emphasized in lung cancer patients with IPF, as the lung is vulnerable to cancer‐related therapies in this group, thus unexpectedly leading to a disastrous event. 29 Although previous studies already demonstrated that pretreatment ILA is a risk factor for pulmonary complication following cancer‐related therapies, 8 our study goes one step further and raises the need to put patients with pretreatment ILA on the same line as IPF and establish different treatment strategies.
Several studies have reported that ILA presents a less obstructive pattern on pulmonary function test, 30 but the current study found that the FEV1/FVC ratio was significantly lower in patients with ILA. The large number of smokers and the older age of our study population may have caused the opposite result. Emphysema (≥5%) was significantly associated with the presence of ILA. The above results can be explained by the fact that smoking is a representative risk factor for ILA and emphysema. 8 Impaired gas exchange, which appears as low DLCO in the ILA population, has also been proven. 31
This study has several limitations. First, this was a retrospective, single‐center study. Second, this study only considered elderly patients with early‐stage NSCLC. Given that age is one of the representative risk factors for ILA, different results might be obtained when the study is performed without age limitations. Third, there was a risk of selection bias due to the exclusion of patients with abnormal spirometry results from our study. Considering the definition and current guidelines of ILA, patients with functional impairment should be managed as preclinical or early ILD rather than ILA. 8 ILA cannot be simply distinguished from preclinical or early ILD by spirometry results as they are in the same spectrum of ILD development and both disease entities imply asymptomatic patients and normal pulmonary function. 32 Nonetheless, we tried to exclude possible ILD cases by adapting spirometry results to eliminate confounding factors as much as possible. Pulmonary function tests are helpful in diagnosing and characterizing ILD. 33 FEV1/FVC ratio and percentage predicted FVC are two major determinants of pulmonary disease on the pulmonary function test. FEV1/FVC ratio over 0.7 or percentage predicted FVC under 80% indicate the possibility of mixed pattern and restrictive pattern pulmonary disease. 34 Fourth, we did not use the subclassification of ILAs based on the Fleischner Society classification system. The subclassification of ILAs may be more appropriate for risk stratification. Fifth, this study only considered fragmentary aspects of clinical features based on the presence of ILA. Survival analysis, such as overall survival or progression‐free survival, may be more meaningful in the application of these results in clinical practice. Finally, the quantification of ILD has been widely applied in many studies. Considering that the extent or degree of ILA varies among patients, further studies with quantification are needed.
In conclusion, patients with ILA showed clinicoradiological features that are similar to those of IPF in elderly patients with early‐stage NSCLC. Identifying the clinical implications of ILA in early‐stage lung cancer will guide clinicians in providing appropriate management for patients.
CONFLICT OF INTEREST
The authors of this work have nothing to disclose.
Cho SW, Jeong WG, Lee JE, Oh I‐J, Song SY, Park HM, et al. Clinical implication of interstitial lung abnormality in elderly patients with early‐stage non‐small cell lung cancer. Thorac Cancer. 2022;13:977–985. 10.1111/1759-7714.14341
REFERENCES
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. [DOI] [PubMed] [Google Scholar]
- 2. Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. SEER cancer statistics review, 1975–2017. Bethesda, MD: National Cancer Institute; 2020. [Google Scholar]
- 3. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non‐small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oudkerk M, Liu S, Heuvelmans MA, Walter JE, Field JK. Lung cancer LDCT screening and mortality reduction ‐ evidence, pitfalls and future perspectives. Nat Rev Clin Oncol. 2021;18:135–51. [DOI] [PubMed] [Google Scholar]
- 5. Jonas DE, Reuland DS, Reddy SM, Nagle M, Clark SD, Weber RP, et al. Screening for lung cancer with low‐dose computed tomography: updated evidence report and systematic review for the US preventive services task force. JAMA. 2021;325:971–87. [DOI] [PubMed] [Google Scholar]
- 6. Chansky K, Sculier JP, Crowley JJ, Giroux D, van Meerbeeck J, Goldstraw P. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non‐small cell lung cancer. J Thorac Oncol. 2009;4:792–801. [DOI] [PubMed] [Google Scholar]
- 7. Birim O, Kappetein AP, Takkenberg JJ, van Klaveren RJ, Bogers AJ. Survival after pathological stage IA nonsmall cell lung cancer: tumor size matters. Ann Thorac Surg. 2005;79:1137–41. [DOI] [PubMed] [Google Scholar]
- 8. Hatabu H, Hunninghake GM, Richeldi L, Brown KK, Wells AU, Remy‐Jardin M, et al. Interstitial lung abnormalities detected incidentally on CT: a position paper from the Fleischner society. Lancet Respir Med. 2020;8:726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whittaker Brown SA, Padilla M, Mhango G, Powell C, Salvatore M, Henschke C, et al. Interstitial lung abnormalities and lung cancer risk in the National Lung Screening Trial. Chest. 2019;156:1195–203. [DOI] [PubMed] [Google Scholar]
- 10. Im Y, Park HY, Shin S, Shin SH, Lee H, Ahn JH, et al. Prevalence of and risk factors for pulmonary complications after curative resection in otherwise healthy elderly patients with early stage lung cancer. Respir Res. 2019;20:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeong WG, Kim YH, Lee JE, Oh IJ, Song SY, Chae KJ, et al. Predictive value of interstitial lung abnormalities for postoperative pulmonary complications in elderly patients with early‐stage lung cancer. Cancer Res Treat. 2021. 10.4143/crt.2021.772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hida T, Hata A, Lu J, Valtchinov VI, Hino T, Nishino M, et al. Interstitial lung abnormalities in patients with stage I non‐small cell lung cancer are associated with shorter overall survival: the Boston lung cancer study. Cancer Imaging. 2021;21:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lynch DA, Austin JH, Hogg JC, Grenier PA, Kauczor H‐U, Bankier AA, et al. CT‐definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner Society. Radiology. 2015;277:192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi H, Kim H, Park CM, Kim YT, Goo JM. Central tumor location at chest CT is an adverse prognostic factor for disease‐free survival of node‐negative early‐stage lung adenocarcinomas. Radiology. 2021;299:438–47. [DOI] [PubMed] [Google Scholar]
- 15. Kim HC, Lee S, Song JW. Impact of idiopathic pulmonary fibrosis on clinical outcomes of lung cancer patients. Sci Rep. 2021;11:8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hino T, Lee KS, Han J, Hata A, Ishigami K, Hatabu H. Spectrum of pulmonary fibrosis from interstitial lung abnormality to usual interstitial pneumonia: importance of identification and quantification of traction bronchiectasis in patient management. Korean J Radiol. 2021;22:811–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naccache JM, Gibiot Q, Monnet I, Antoine M, Wislez M, Chouaid C, et al. Lung cancer and interstitial lung disease: a literature review. J Thorac Dis. 2018;10:3829–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karampitsakos T, Tzilas V, Tringidou R, Steiropoulos P, Aidinis V, Papiris SA, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. 2017;45:1–10. [DOI] [PubMed] [Google Scholar]
- 19. Kawasaki H, Ogura T, Yokose T, Nagai K, Nishiwaki Y, Esumi H. p53 gene alteration in atypical epithelial lesions and carcinoma in patients with idiopathic pulmonary fibrosis. Hum Pathol. 2001;32:1043–9. [DOI] [PubMed] [Google Scholar]
- 20. Vancheri C. Common pathways in idiopathic pulmonary fibrosis and cancer. Eur Respir Rev. 2013;22:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mizuno S, Takiguchi Y, Fujikawa A, Motoori K, Tada Y, Kurosu K, et al. Chronic obstructive pulmonary disease and interstitial lung disease in patients with lung cancer. Respirology. 2009;14:377–83. [DOI] [PubMed] [Google Scholar]
- 22. Le Jeune I, Gribbin J, West J, Smith C, Cullinan P, Hubbard R. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir Med. 2007;101:2534–40. [DOI] [PubMed] [Google Scholar]
- 23. Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population‐based cohort study. Am J Respir Crit Care Med. 2000;161:5–8. [DOI] [PubMed] [Google Scholar]
- 24. Khan KA, Kennedy MP, Moore E, Crush L, Prendeville S, Maher MM, et al. Radiological characteristics, histological features and clinical outcomes of lung cancer patients with coexistent idiopathic pulmonary fibrosis. Lung. 2015;193:71–7. [DOI] [PubMed] [Google Scholar]
- 25. Jang HJ, Park MS, Kim YS, Chang J, Lee JH, Lee CT, et al. The relationship between the severity of pulmonary fibrosis and the lung cancer stage. J Cancer. 2021;12:2807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen S, Gao C, Du Q, Tang L, You H, Dong Y. A prognostic model for elderly patients with squamous non‐small cell lung cancer: a population‐based study. J Transl Med. 2020;18:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller ER, Putman RK, Vivero M, Hung Y, Araki T, Nishino M, et al. Histopathology of interstitial lung abnormalities in the context of lung nodule resections. Am J Respir Crit Care Med. 2018;197:955–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chae KJ, Chung MJ, Jin GY, Song YJ, An AR, Choi H, et al. Radiologic‐pathologic correlation of interstitial lung abnormalities and predictors for progression and survival. Eur Radiol. 2022. 10.1007/s00330-021-08378-8 [DOI] [PubMed] [Google Scholar]
- 29. Galioto F, Palmucci S, Astuti GM, Vancheri A, Distefano G, Tiralongo F, et al. Complications in idiopathic pulmonary fibrosis: focus on their clinical and radiological features. Diagnostics (Basel). 2020;10:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doyle TJ, Hunninghake GM, Rosas IO. Subclinical interstitial lung disease: why you should care. Am J Respir Crit Care Med. 2012;185:1147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368:2192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Podolanczuk AJ, Putman RK. Clinical relevance and management of "pre‐interstitial lung disease". Clin Chest Med. 2021;42:241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pugashetti JV, Kitich A, Alqalyoobi S, Maynard‐Paquette AC, Pritchard D, Graham J, et al. Derivation and validation of a diagnostic prediction tool for interstitial lung disease. Chest. 2020;158:620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tseng HJ, Henry TS, Veeraraghavan S, Mittal PK, Little BP. Pulmonary function tests for the radiologist. Radiographics. 2017;37:1037–58. [DOI] [PubMed] [Google Scholar]


