Abstract
Whilst vaccination for the SARS-CoV-2 virus has been successful in reducing the severity and burden of the COVID-19 pandemic, there have been recent reports of mRNA vaccines triggering autoimmune hepatitis in the native liver. There have been no descriptions thus far of recurrent ‘autoimmune hepatitis’ after liver transplantation in the context of SARS-CoV-2 vaccination. We describe a patient transplanted for autoimmune hepatitis who was stable for many years until they had immune-mediated flares coinciding with Pfizer-BioNTech mRNA vaccination. Intravenous steroid treatment was required to suppress histologically evident interface hepatitis. We firmly believe that mRNA vaccination was responsible for this ‘recurrence’ and that clinicians should be vigilant for this reaction in patients transplanted for autoimmune hepatitis.
Keywords: SARS-CoV2, mRNA vaccine, Autoimmune hepatitis, Liver transplantation
Dear Editor
Whilst vaccination for the SARS-CoV-2 virus has been successful in reducing the severity and burden of the COVID-19 pandemic, there have been recent reports of mRNA vaccines triggering autoimmune hepatitis in the native liver [1]. There have been no descriptions thus far of recurrent ‘autoimmune hepatitis’ after liver transplantation in the context of SARS-CoV-2 vaccination. We describe a patient transplanted for autoimmune hepatitis who subsequently had immune-mediated flares coinciding with Pfizer-BioNTech mRNA vaccination.
A 32 year old woman of European origin underwent elective orthotopic liver transplantation for autoimmune hepatitis in 2014 with a donor following brainstem death. She was being maintained on conventional immunosuppression in the form of tacrolimus (Prograf 4 mg mane, 3 mg nocte), azathioprine 50 mg daily and prednisolone 5 mg daily. In addition, she was prescribed aspirin 75 mg daily, vitamin D supplementation and atenolol 50 mg daily for hypertension. From 2015 onwards her ALT remained broadly in the normal range apart from during successful pregnancy in 2020 when it rose during the second trimester, peaked at 95 U/L and settled back to normal post-partum where it remained on subsequent testing.
On 17 December 2021 she underwent routine outpatient review. Blood tests in primary care 4 days earlier showed an unexpected ALT elevation at 288 U/L with ALP 115 U/L and bilirubin 29 μmol/L. She reported no recent medication changes or new supplement intake and full compliance with her immunosuppression. She felt entirely well with no specific symptoms. Tacrolimus trough levels were all in the range 8–12 μg/L during 2021 with her latest level at 11.1 μg/L. She was admitted for further assessment including unremarkable examination and liver ultrasonography which showed normal parenchyma, biliary tree and vasculature. Epstein-Barr virus DNA, cytomegalovirus DNA and acute serology for hepatitis A to E were negative and her immunoglobulin G level was normal. Prior liver autoantibody testing in 2021 was positive only for soluble liver antigen/liver pancreas antibody.
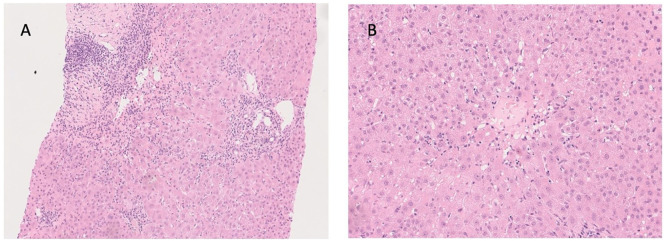
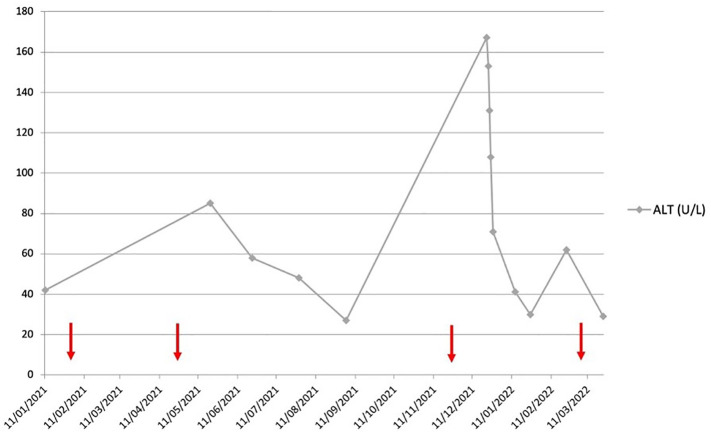
A percutaneous liver biopsy was undertaken which showed significant lymphocytic inflammation including plasma cells and with interface activity consistent with an immune-mediated hepatitis (Fig. 1 ). She received pulsed intravenous methylprednisolone (500 mg daily for 3 days) with subsequent higher dose oral prednisolone (20 mg daily). Her liver biochemistry normalised and her ALT was 30 U/L on 15 mg prednisolone at subsequent outpatient review end of January 2022. The prednisolone dose was reduced to 10 mg daily at that visit with subsequent mild rise in ALT to 62 U/L following which the azathioprine dose was increased to 75 mg daily and the ALT was seen to normalise again (Fig. 2 ). On further questioning, the patient reported having her third Pfizer-BioNTech vaccine on 20 November 2021, approximately 3 weeks before her initial ALT rise. Retrospectively, she was noted to have had an ALT spike of 85 U/L on 20 May 2021 having had her second Pfizer-BioNTech vaccination on 24 April 2021. Her initial Pfizer-BioNTech vaccine was on 30 January 2021 and she received a recent ‘booster’ dose of Pfizer-BioNTech vaccine on 5 March 2022.
Fig. 1.
A) x100 hematoxylin and eosin stain showing portal inflammatory infiltrate composed predominantly of lymphocytes, also with plasma cells and rare eosinophils. There is interface activity with focal rosetting and emperipolesis. There is also adjacent lobular inflammation with scattered acidophilic bodies. Portal vein endothelialitis is mild and focal. B) x200 hematoxylin and eosin stain showing terminal hepatic vein lymphohistiocytic inflammation with a rare acidophilic body but little endothelialitis.
Fig. 2.
Graph showing ALT course over 2021 into 2022. The arrows (red) show the time points for each administration of Pfizer-BioNTech SARS-CoV-2 vaccine. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In summary, we report hitherto undescribed post-transplant ‘recurrent autoimmune’ hepatitis associated temporally with mRNA vaccination for the SARS-CoV-2 virus. It is suggested that mRNA vaccines can result in molecular mimicry leading to autoimmune phenomena, via upregulation of specific immunological pathways [2]. The lack of alternative explanation as well as the ALT spike observed after her second vaccination are supportive. ALT values are lacking during the aftermath of her first dose so it is unknown whether any ALT flare occurred then. The lack of ALT rise after her fourth dose may be explained by additional immunosuppression and lower dose volume of a ‘booster’ rather than full vaccine. We suggest that transplant clinicians monitor for the possibility of ALT flares in ‘primed’ liver transplant recipients after SARS-CoV-2 mRNA vaccination; early recognition and prompt intervention may reduce morbidity from this potential side-effect.
Financial statement
No competing interests (all authors).
Author contributions
AM - manuscript preparation and data acquisition.
AD - data acquisition, preparation of figs.
WG - manuscript editing, project management.
Consent
Obtained from the patient in question and recorded in the clinical notes.
Declaration of Competing Interest
None to declare (all authors). No funding applicable.
References
- 1.Shwe Zin Tun G., Gleeson D., Al-Joudeh A., Dube A. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J. Hepatol. 2022;76:747–749. doi: 10.1016/j.jhep.2021.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talotta R. Do COVID-19 RNA vaccines put at risk of immune-mediated diseases? In reply to ‘potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases’. Clin. Immunol. 2021;224:108665. doi: 10.1016/j.clim.2021.108665. [DOI] [PMC free article] [PubMed] [Google Scholar]