Abstract
Pseudoxanthoma elasticum (PXE; OMIM 264800) is a rare heritable multisystem disorder, characterized by ectopic mineralization affecting elastic fibers in the skin, eyes, and the cardiovascular system. Skin findings often lead to early diagnosis of PXE, but currently no specific treatment exists to counteract the progression of symptoms.
PXE belongs to a group of Mendelian calcification disorders linked to pyrophosphate metabolism, which also includes generalized arterial calcification of infancy (GACI), and arterial calcification due to CD73 deficiency (ACDC). Inactivating mutations in ABCC6, ENPP1 and NT5E are the genetic cause of these diseases, respectively, and all of them result in reduced inorganic pyrophosphate (PPi) concentration in the circulation. Although PPi is a strong inhibitor of ectopic calcification, oral supplementation therapy was initially not considered because of its low bioavailability. Our earlier work however demonstrated that orally administered pyrophosphate inhibits ectopic calcification in the animal models of PXE and GACI, and that orally given Na4P2O7 is absorbed in humans.
Here we report that gelatin encapsulated Na2H2P2O7 has similar absorption properties in healthy volunteers and people affected by PXE. The sodium-free K2H2P2O7 form resulted in similar uptake in healthy volunteers, and inhibited calcification in Abcc6−/− mice as effectively as its sodium counterpart. Novel pyrophosphate compounds showing higher bioavailability in mice were also identified. Our results provide an important step toward testing oral PPi in clinical trials in PXE, or potentially any condition accompanied by ectopic calcification including diabetes, chronic kidney disease or ageing.
Keywords: pseudoxanthoma elasticum, ectopic mineralization, pathologic calcification, pyrophosphate, investigational therapy
INTRODUCTION
Pyrophosphate (PPi) is an endogenous metabolite which not only has a crucial role in bone and teeth formation, but is also among the strongest inhibitors of ectopic calcification (pathological hydroxyapatite deposition). Two major mechanisms responsible for extracellular or systemic presence of PPi have been identified. First, it was found that release of ATP from the liver is the main source of PPi in the circulation1. ABCC6, a transporter expressed in the basolateral membrane of hepatocytes facilitates ATP release to sinusoid capillaries, and ENPP1, a membrane-bound ectonucleotide pyrophosphatase/phosphodiesterase, effectively hydrolyzes ATP to produce PPi that appears in the circulation. Mutations in the ABCC6 gene are associated with pseudoxanthoma elasticum (PXE, OMIM 177850), a late onset, slowly progressing monogenic mineralization disease2–4. In PXE, calcification of the elastic fibers in the skin, clinically manifesting as skin folds and yellowish papules, represent the first sign of the disease. Mineralization of the Bruch’s membrane of the eyes is accompanied with peau d’orange, angioid streaks, and sometimes choroidal neovascularization and retinal bleeding resulting in macular atrophy. Arterial calcification affecting the media layer of arteries leads to peripheral arterial disease manifesting with intermittent claudication, hypertension and rarely myocardial infarcts. Mutations in the ENPP1 gene are responsible for generalized arterial calcification of infancy type 1 (GACI1, OMIM 2080005), a more severe disorder characterized by prenatal calcification of the internal elastic lamina of large- and medium-sized arteries and the joins. Both pathological conditions are characterized by low plasma PPi concentration: ~40% of the control in PXE and virtually zero in GACI, and this is the common causal metabolic alteration in the two diseases. There are overlapping phenotypic features between the two disorders, and in a few cases mutations in the ABCC6 gene are also causative in GACI type 2 (OMIM 614473)6,7.
Secondly, another mechanism regulating PPi metabolism involves ANKH, which was identified earlier as a pyrophosphate transporter releasing the metabolite from the cytosol to the extracellular space8. A recent study, however, clearly proved that ANKH is an ATP transporter and its conversion to PPi is due to ENPP1 hydrolytic activity9. Mutations of ANKH cause craniometaphyseal dysplasia, a bone dysplasia characterized by overgrowth of craniofacial bones and abnormal metaphyses of long bones10. Other proteins, such as tissue non-specific alkaline phosphatase (TNAP) and CD73, are also involved in the regulation of local or systemic PPi levels [for reviews, see: 11 and 12]. Similar calcification as that observed in PXE is present in patients with diabetes13, chronic kidney disease14 and can also occur as a result of ageing15. Thus, PXE can be considered a model to study age-associated medial arterial calcification and cardiovascular disease15.
The role of PPi as an inhibitor of ectopic mineralization was established in the 1960’s16. The inhibitory effect of PPi is based on blocking crystal growth centers on forming hydroxyapatite17. It was demonstrated in various animal studies that supplementation treatment by injecting PPi effectively counteracts ectopic calcification: uremic vascular calcification was prevented in rats18 and the PXE-like spontaneous calcification was inhibited in Abcc6−/− mice19. Daily transient increase of PPi in the circulation was sufficient to inhibit mineralization in these animal models consistent with its proposed mechanism of action.
It was claimed that orally administrated PPi is not effective in inhibiting ectopic calcification20, and it was suggested that PPi cannot appear in the circulation because of the hydrolytic pyrophosphatase activities in the gut21. On the other hand, oral administration would be preferred to treat patients lifelong in diseases such as PXE.
We have demonstrated in our previous work that in spite of the consideration cited above, PPi given orally to Abcc6−/− and Enpp1−/− mice does inhibit ectopic calcification22. The two animal models recapitulate the calcification syndromes of the corresponding human diseases. We have also shown that orally given PPi is absorbed in humans to an extent that it can elevate plasma PPi level from pathologically low to above normal. Importantly, it was also shown that progression of ectopic calcification in PXE patients can be halted by treatment with a non-hydrolysable pyrophosphate analog, etidronate23,24, and early administration of etidronate to Abcc6−/− mice completely prevents the ectopic mineralization25. Collectively, these results argue for the potential effectiveness of oral pyrophosphate therapy.
In our previous experiments we used the Na4P2O7 form of PPi and delivered it dissolved in drinking water. In spite of its clear inhibitory effect, this form has disadvantages: high ion load which results in gastro-intestinal (GI) discomfort and unpleasant bitter taste. In addition, it results in an unwanted excess of sodium intake. In the present study our aim was to overcome the above downside of administration, and to find the optimal chemical form and delivery method of orally given PPi and to reduce or completely avoid the presence of sodium. We also asked the question if oral absorption of PPi in patients with PXE is similar to that in normal volunteers.
METHODS
Approval of the human studies:
Oral uptake study involving healthy human volunteers (female and male) was approved by the National Review Board of the Ministry of Health, Hungary (ETT TUKEB). The permit based on the above approval has been issued by National Public Health and Medical Officer Service (NNK; authorization number: 16412–5/2021). Informed consent was obtained from each volunteer prior to the study and experiments conformed to the principles of Declaration of Helsinki what is indicated in the above document. All patient samples were handled in an anonymized form also approved by the above document.
Oral uptake study involving PXE patients was approved by the Genetic Alliance Review Board, and mutation analysis confirming the diagnosis in PXE patients was approved by the Institutional Review Board of Thomas Jefferson University. The diagnosis of PXE was based on characteristic skin findings, supported by characteristic histopathology, and ocular and vascular involvement.
Animals and animal studies:
The animal studies have been approved by the Ethical Committee of Animal Experiments, Governmental Office of Pest County, Hungary; permit No. PE/EA/748–2/2021 and XVI-I-001/707–4/2012, and were conducted according to the national guidelines.
All animals were housed in approved animal facilities at the Research Centre for Natural Sciences. Mice were kept under routine laboratory conditions with a 12-hour light-dark cycle and ad libitum access to water and chow. Anesthesia was carried out by intraperitoneal injection of the mixture of Zoletil (30 mg/kg, Virbac, France), Xylazine (12.5 mg/kg, Produlab Pharma, The Netherlands) and Butorphanol (3 mg/kg, Richterpharma, Austria) during all procedures.
Cryo-injury was performed as described previously26,22, with 10 mM PPi treatment provided as Na2H2P2O7 or K2H2P2O7 or no PPi added (control) to the drinking water of female Abcc6−/− mice27.
For PPi uptake studies in mice, three-month-old C57/BL6 female mice were fasted for 4 hours prior to PPi administration. PPi solution was given under anesthesia via gastric gavage (the dose equivalent to 39 mg/kg PPi) in a volume of 5 μl/g. Blood was taken by cardiac puncture prior and after 10, 30 and 60 minutes (n≥3 mice at each timepoint) of administration, and collected into tubes containing 50 μl CTAD (BD, Franklin Lakes, NJ). Following filtration through Centrisart I 300,000 MWCO filters (Sartorius, Frankfurt, Germany), plasma PPi was assayed as described1.
Synthesis of pyrophosphate compounds
Detailed description of the synthesis of K2H2P2O7, (NH4)2P2O7, monoarginine-H2P2O7, monolysine-H2P2O7 and bisethanolamine-H2P2O7 is given in the Supplementary Material.
Human uptake and plasma pyrophosphate assay
Na4P2O7 (anhydrous, Code 118) and Na2H2P2O7 (both food grade) were purchased from ICL Food Specialist (St. Louis, MO, USA) and from Fosfa (Breclav, Czech Republic). The K2H2P2O7 form in Good Manufacturing Practice (GMP) quality was from ERAS Labo (Grenoble, France). Gelatin capsules were from Molar Chemicals (Halásztelek, Hungary), acid resistant HPMCP (hydroxypropylmethyl cellulose phthalate) type capsules were from Capsuline (Davie, FL, USA). Capsules were filled manually with the pyrophosphate salts to be tested the day before the uptake experiment, and were stored in a sealed container at room temperature until use.
Volunteers and PXE patients (after overnight fasting) were involved in the studies. The duration of ingestion was less than one minute. Blood samples were collected from the vena cubiti before ingestion (0 min) and after 30, 60, 120 and 240 min into CTAD anticoagulated tubes (BD, Franklin Lakes, NJ, USA), and filtered through Centrisart I 300,000 MWCO filters (Sartorius, Frankfurt, Germany). Plasma PPi was assayed as described1.
Plasma inorganic phosphate assay
Plasma inorganic phosphate was measured from ultrafiltered plasma used for PPi assay since about 85 to 90% of serum phosphate is free and is ultrafilterable28. We have tested that the anticoagulant mixture in the CTAD blood collection tubes does not interfere with the assay. Plasma inorganic phosphate was measured by ammonium molybdate method: 10 μl of plasma sample or calibration standard was added to a mixture of 0.3 ml reagent containing 2.5 M H2S04, 1% ammonium molybdate, and 0.014% antimony potassium tartrate and 0.7 ml 20% acetic acid. For the reduction of the complex 0.15 ml of 1% ascorbic acid (freshly prepared) was added. Optical density was determined after 15 minutes at 880 nm.
RESULTS and DISCUSSION
To reduce the ion load and the amount of sodium in PPi, we first turned to the Na2H2P2O7 salt form which contains half as much sodium as the previously utilized tetrasodium (Na4P2O7) variant22. A case report has been published describing oral pyrophosphate treatment of a PXE patient utilizing Na2H2P2O7 dissolved in water29. To avoid the unpleasant taste we used capsulated PPi salts instead of water-based solution as the form of delivery. Two different capsules were tested: gelatin and HPMCP-type cellulose capsules, which can resist dissolution at the acidic pH of gastric fluid30.
The human absorption experiments were performed and plasma PPi levels were monitored as described in the Methods Section. We first recorded the absorption curves of three different delivery methods: Na4P2O7 dissolved in drinking water (40 mg pyrophosphate/kg), and Na2H2P2O7 loaded into gelatin or HPMCP capsules (39 mg pyrophosphate/kg each), as shown in Figure1. While there are individual differences in the extent of absorption among the healthy volunteers in the case of each formulation, similar to our previous observations22, the absorption from the two different types of capsules was very different (compare panels B, C and D in Figure1), with gelatin capsules being more effective than HPMCP. Furthermore, both capsule-Na2H2P2O7 combinations were superior when compared to the Na4P2O7-water solution (compare panels a and d). The major outcome of this experiment was that the introduction of gelatin capsule-Na2H2P2O7 formulation not only provided a solution for the problem of the unpleasant taste, but proved to be the most effective delivery method resulting in the highest absorption. In addition, the GI discomfort experienced by volunteers earlier with tetrasodium salt was reduced to the minimum. The two types of capsules release their cargo in different compartments of the GI system: gelatin capsules dissolve within minutes of reaching the stomach31, in contrast, HPMCP capsules can withstand the acidic pH of the gastric fluid, and release their cargo at a higher pH in the small intestine30. We hypothesize that the significantly more effective absorption from gelatin capsule is, at least partly, due to the acidic environment of the stomach. At low pH the pyrophosphate anion carries less negative charge which makes passive diffusion through the organ barrier of the GI system more probable.
Figure 1.: Oral uptake of pyrophosphate in humans delivering different forms and formulations.
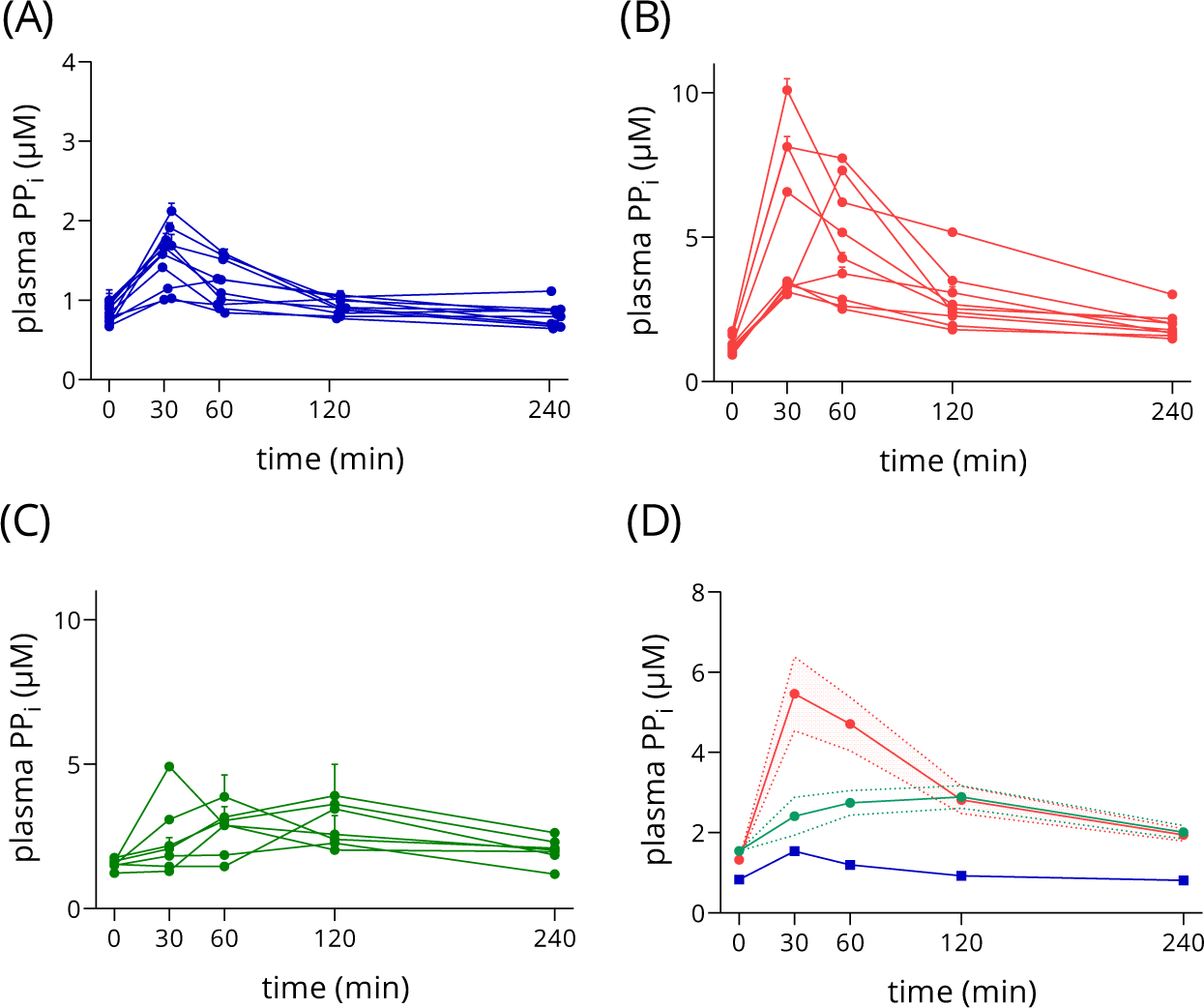
Plasma PPi levels were determined by the luminescent method1. Error bars represent SD on panels (A), (B) and (C), while mean and SEM are displayed on (D).
Panel A: oral delivery of Na4P2O7-water solution, 40 mg/kg pyrophosphate, n=10.
Panel B: oral delivery of Na2H2P2O7 in gelatin capsule formula, 39 mg/kg pyrophosphate, n=9.
Panel C: oral delivery of Na2H2P2O7 in HPMCP capsule formula, 39 mg/kg pyrophosphate, n=7.
Panel D: comparison of uptake curves presented on Panels a, b and c, n= 10, 9 and 7, respectively.
After establishing the Na2H2P2O7-gelatin capsule as a more effective delivery method we asked the question: How is PPi absorbed in individuals affected by PXE compared to its absorption in non-PXE volunteers? We tested the exact same experimental setup in the case of PXE patients using the same batch of Na2H2P2O7 and the same batch of gelatin capsules. Figure 2A displays PPi absorption in PXE patients, showing individual differences, while on panel b the compared absorption curves of patients versus healthy volunteers confirm very similar uptake in the two groups. The peak PPi concentrations are 5.5 ± 0.9 μM in the non-PXE group and 5.7±1.4 μM in the PXE group. Baseline concentrations, as expected, are different, 1.3±0.1 and 0.5±0.1 μM in the non-PXE and PXE group, respectively (Figure 2C). Gastro-intestinal discomfort was not reported.
Figure 2.: Absorption of orally delivered pyrophosphate in PXE patients.

Plasma PPi levels were determined by the luminescent method 1. Error bars represent SD on panel A and C, and SEM on panel B.
Panel A: oral uptake of Na2H2P2O7 in gelatin capsule formula by PXE patients, 39 mg/kg pyrophosphate, n=9.
Panel B: comparison of uptake curves of healthy volunteers and PXE patients taking 39 mg/kg Na2H2P2O7 in gelatin capsules, n=9.
Panel C: baseline plasma pyrophosphate levels of healthy volunteers and PXE patients involved in the study (n= 9, p-value calculated by Mann-Whitney U test).
Next, we have embarked upon the development of sodium-free pyrophosphate forms to avoid excessive delivery of sodium. Figure 3A shows the chemical structures of compounds synthesized for this study (K2H2P2O7, (NH4)2P2O7, monoarginine-H2 P2O7, monolysine-H2P2O7 and bisethanolamine-H2P2O7) along with Na2H2P2O7. The synthesis and purification of the above compounds are detailed in Supplementary Materials.
Figure 3.: Absorption of sodium-free pyrophosphate compounds and Na2H2P2O7 in mice.

Panel A: the chemical structure of the pyrophosphate compounds studied.
Panel B: Plasma PPi levels of mice after dosing with various pyrophosphate compounds via gastric gavage. Dose was 39 mg/kg pyrophosphate in each case. Mean ± SEM are displayed, nγ3 at each data point.
We have tested the absorption of the novel PPi salts in mice. Oral delivery for the absorption experiments was carried out as follows: three-month-old female C57/BL6 mice were fasted for 4 hours prior to PPi administration. PPi solution was given under anesthesia via gastric gavage (the dose equivalent to 39 mg/kg PPi). Blood was taken by cardiac puncture before and after 10, 30 and 60 minutes of delivery (n≥3 mice at each timepoint), and plasma PPi was assayed as described in the Methods section. We have observed PPi absorption in the case of each form, however the two amino acid derivatives, the monoarginine-H2P2O7 and the monolysine-H2P2O7 resulted in the highest concentration at the maximum of the absorption curve (15.6±1.5 and 13.5±1.3 μM, respectively). The potassium and sodium forms showed approximately same maximum (9.4±0.9 and 10.4±2.7 μM, respectively), while administration of the bisethanolamine and the (NH4)2PPi derivatives yielded only 5.3±0.5 and 3.4±0.6 μM peak plasma concentrations, respectively.
Currently, only the K2H2P2O7 salt is available in GMP quality (see Material and Methods), and therefore, following our protocol utilized in the human studies (oral administration of a 39 mg PPi/kg dose loaded in gelatin capsules) we investigated the properties of the potassium salt, K2H2P2O7. We designed this experiment with the same healthy volunteers in the Na2H2P2O7 and K2H2P2O7 treatment groups, as we wanted to establish a clear comparison of the two salt forms.
Figure 4A summarizes the uptake curves of all volunteers involved in the study while the individual absorption plots of six healthy volunteers are presented on Figure 4C. Figure 4A and C panels again indicate large individual differences similar to those observed in the case of the sodium forms (see above). The individual curves demonstrate better PPi uptake in two cases when K2H2P2O7 was taken and approximately the same extent of absorption as with Na2H2P2O7 in the case of the other four volunteers.
Figure 4.: The potassium pyrophosphate K2H2P2O7 shows similar absorption characteristics in humans as Na2H2P2O7, and plasma inorganic phosphate remains in the normal range. K2H2P2O7 and Na2H2P2O7 inhibit dystrophic cardiac calcification of Abcc6−/− mice when given orally.
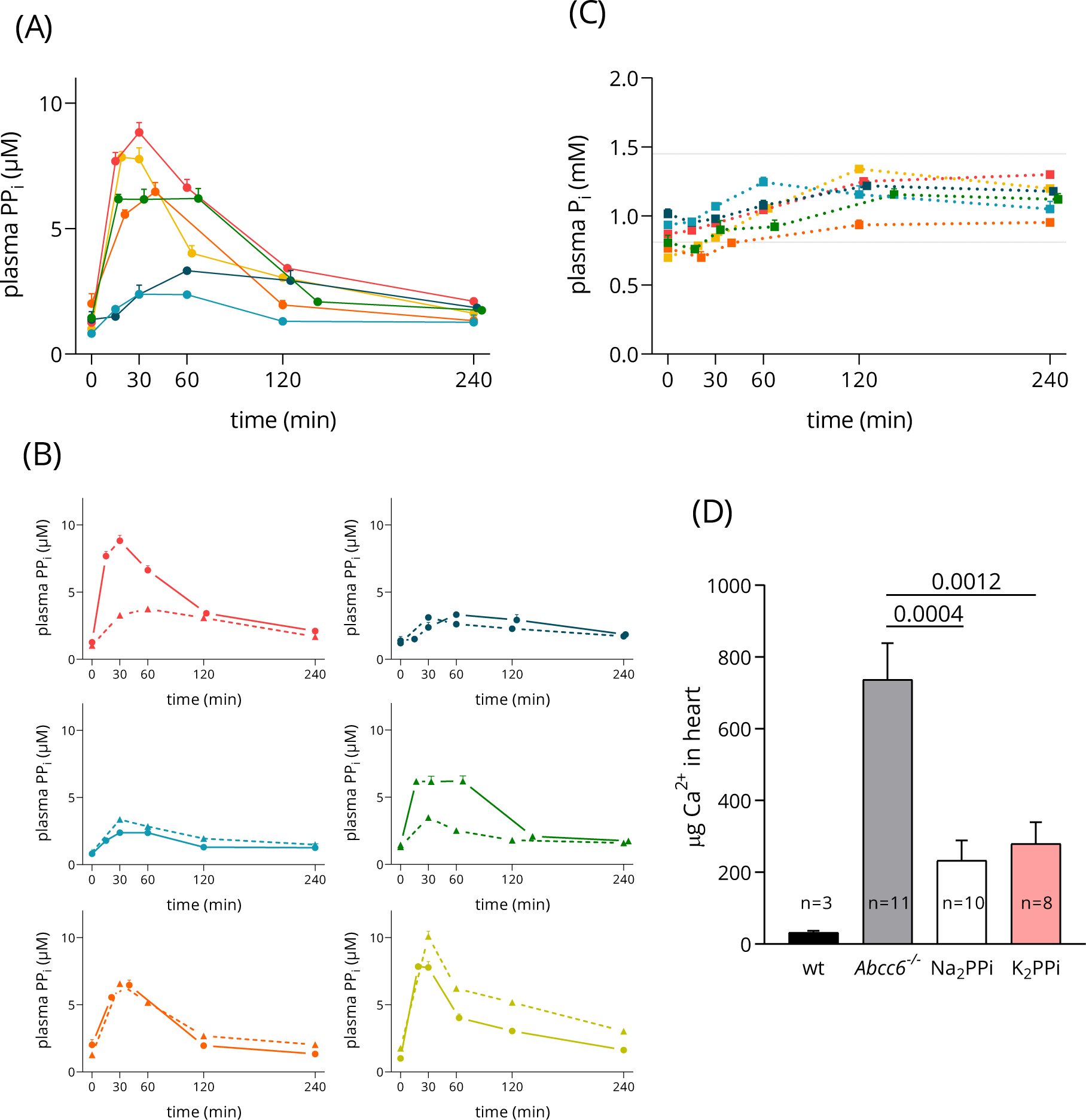
Panel A: oral uptake of K2H2P2O7 in gelatin capsule formula by healthy volunteers, 39 mg/kg pyrophosphate. Mean ± SD values are displayed, n=6.
Panel B: Plasma inorganic phosphate (Pi) levels of volunteers after dosing with K2H2P2O7 remain within the normal range of 0.81–1.45 mmol/L, indicated by shaded area. Mean ± SD values are plotted, n=6.
Panel C: Individual plasma PPi (pyrophosphate) curves to compare the absorption in the same healthy volunteers from K2H2P2O7 (solid line) versus Na2H2P2O7 (dashed line). Mean ± SD values are displayed.
Panel D: total Ca2+-content of the heart tissue was measured; mean ± SEM are graphed, p-values indicated were calculated by Mann-Whitney U test, animal numbers in each treatment group (n) are displayed on the figure.
Pyrophosphate is hydrolyzed in the gut to inorganic phosphate (Pi) and excreted via urine and feces as indicated by a study using rats32. We have determined Pi concentration in the blood of volunteers during the time course of K2H2P2O7 uptake (Figure 4B).
An increase of plasma Pi was detected from 0.96 to 1.34 mmol/L and with much smaller individual differences than in case of PPi in plasma. These values are within the normal range of 0.81–1.45 mmol/L33.
Next, the capacity of the K2H2P2O7 salt in inhibiting ectopic calcification in Abcc6−/− mice was tested by the cryo-injury based DCC-method as described in our earlier papers19,22. In this experiment we induce calcification in the myocardium and five days after we determine the Ca2+-content of the heart. As it is demonstrated in Figure 4D, this treatment does not induce mineralization in wild type mice but a massive calcification develops in the Abcc6−/− animals. Both Na2H2P2O7 and K2H2P2O7, when given orally (10 mM in drinking water), reduce calcification to the same extent, indicating that the newly synthesized potassium salt is as effective as the sodium form. Earlier we have demonstrated a similar inhibitory effect using the Na4P2O7 form22.
In summary, we have shown that a sodium-free PPi derivative, K2H2P2O7, is absorbed in humans when given orally, similar to Na2H2P2O7. This overcomes the problem of the excess sodium intake.
Importantly, we demonstrated that K2H2P2O7 effectively inhibits calcification in Abcc6−/− mice. Our results prove that formulation in gelatin capsules provides the highest uptake. These findings suggest that the K2H2P2O7-gelatin capsule formulation is a suitable candidate for therapeutic trials. Furthermore, we have identified two novel pyrophosphate forms (monolysine- and monoarginine derivatives), showing high absorption in animal experiments when given orally.
Large individual differences in uptake of pyrophosphate both in the group of healthy volunteers and in the group of PXE patients were observed. The dose we have applied was chosen to provide at least 2.5 fold elevation of plasma pyrophosphate concentration at the peak level even in the case of the lowest absorption efficacy (see Figure 4). It is notable that a daily transient increase of PPi in the circulation was sufficient to inhibit mineralization in Abcc6−/− mice19. The observed large individual variation in absorption may indicate a necessity of personalized dosing established prior therapy or a clinical trial. Gelatin capsules represent a suitable formulation for oral administration of PPi in double-blinded clinical trials, as both the therapeutic compound and the placebo can be delivered by the same type of vehicle, masking the characteristic salty-bitter taste of pyrophosphate salts.
Medial arterial calcification observed in PXE is also present in patients with diabetes13, chronic kidney disease14 and as a result of ageing15. PXE can be considered as a model to study medial arterial calcification in cardiovascular disease, and research can also lead to novel therapeutic interventions which would be relevant not only in PXE but could also reduce calcification and related cardiovascular risk in the above mentioned populations34. The intervention based on oral administration of pyrophosphate is one of the promising candidate approaches. Our study, in order to find the best chemical form and administration method of pyrophosphate, is an important step toward this direction. Indeed, a clinical trial in PXE based on the oral administration of pyrophosphate35 has already been registered.
Supplementary Material
Acknowledgments
The technical work of Gyorgyi Demeter and Gabriella Szotak is greatly appreciated. We thank Koen van de Wetering (Thomas Jefferson University, Philadelphia, USA) for critical comments on the manuscript and Carol Kelly for assistance in manuscript preparation. Most of all we are grateful for the individuals affected by pseudoxanthoma elasticum (PXE), and all healthy volunteers who participated in this study.
This work was supported by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR072695; JU, QL and AV); the National Research, Development and Innovation Office of Hungary grants: OTKA 127957,127933 (AV); PD- OTKA 128003 (VP). A financial support of CellPharma Ltd (Budapest, Hungary) is also acknowledged.
Abbreviations
- GACI
generalized arterial calcification of infancy
- HPMCP
hydroxypropylmethyl cellulose phthalate
- PXE
pseudoxanthoma elasticum
- PPi
inorganic pyrophosphate
Footnotes
Conflict of Interest Statement
AV: is co-inventor on a patent related to the use of pyrophosphates for therapeutic applications (NL20117471), entitled ‘Oral Pyrophosphate For Use In Reducing Tissue Calcification’, which is continued as US 16/333,856 and EP3512530. Two subsequent patent applications have been filed for further pyrophosphate salts which improve tolerability and bioavailability (NL2023491 and US 63/091,467) by C.B, X.Z., V.P and AV. The other co-authors have no conflict of interest to declare.
Author Contributions Statement (CRediT-compliant)
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE): AV, KF, EK, NT and PV contributed to conception and study design; KF, EK, NT, NR, QL, ST and JU contributed to data collection and data analysis. CB and XZ synthesized pyrophosphate compounds. AV prepared the manuscript, EK prepared the figures. All authors maintained full editorial control over the content of the manuscript and were responsible for all final decisions on the manuscript content, for final approval of the version for submission and publication.
Data Availability Statement
No datasets were generated or analyzed during the current study.
REFERENCES
- 1.Jansen RS, Duijst S, Mahakena S, et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler Thromb Vasc Biol. 2014;34(9):1985–1989. doi: 10.1161/ATVBAHA.114.304017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergen AA, Plomp AS, Schuurman EJ, et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet. 2000;25(2):228–231. doi: 10.1038/76109 [DOI] [PubMed] [Google Scholar]
- 3.Le Saux O, Urban Z, Tschuch C, et al. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat Genet. 2000;25(2):223–227. doi: 10.1038/76102 [DOI] [PubMed] [Google Scholar]
- 4.Ringpfeil F, Lebwohl MG, Christiano AM, Uitto J. Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci U S A. 2000;97(11):6001–6006. doi: 10.1073/pnas.100041297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutsch F, Ruf N, Vaingankar S, et al. Mutations in ENPP1 are associated with “idiopathic” infantile arterial calcification. Nat Genet. 2003;34(4):379–381. doi: 10.1038/ng1221 [DOI] [PubMed] [Google Scholar]
- 6.Li Q, Brodsky JL, Conlin LK, et al. Mutations in the ABCC6 gene as a cause of generalized arterial calcification of infancy: genotypic overlap with pseudoxanthoma elasticum. J Invest Dermatol. 2014;134(3):658–665. doi: 10.1038/jid.2013.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitschke Y, Baujat G, Botschen U, et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet. 2012;90(1):25–39. doi: 10.1016/j.ajhg.2011.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289(5477):265–270. doi: 10.1126/science.289.5477.265 [DOI] [PubMed] [Google Scholar]
- 9.Szeri F, Lundkvist S, Donnelly S, et al. The membrane protein ANKH is crucial for bone mechanical performance by mediating cellular export of citrate and ATP. PLoS Genet. 2020;16(7). doi: 10.1371/journal.pgen.1008884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nürnberg P, Thiele H, Chandler D, et al. Heterozygous mutations in ANKH, the human ortholog of the mouse progressive ankylosis gene, result in craniometaphyseal dysplasia. Nat Genet. 2001;28(1):37–41. doi: 10.1038/ng0501-37 [DOI] [PubMed] [Google Scholar]
- 11.Li Q, van de Wetering K, Uitto J. Pseudoxanthoma Elasticum as a Paradigm of Heritable Ectopic Mineralization Disorders: Pathomechanisms and Treatment Development. Am J Pathol. 2019;189(2):216–225. doi: 10.1016/j.ajpath.2018.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borst P, Varadi A, van de Wetering K. PXE, a mysterious inborn error clarified. Trends Biochem Sci. 2019;44(2):125–140. doi: 10.1016/j.tibs.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huesa C, Zhu D, Glover JD, et al. Deficiency of the bone mineralization inhibitor NPP1 protects mice against obesity and diabetes. Dis Model Mech. 2014;7(12):1341–1350. doi: 10.1242/dmm.017905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Marco L, Lima-Martínez M, Karohl C, Chacín M, Bermúdez V. Pseudoxanthoma Elasticum: An Interesting Model to Evaluate Chronic Kidney Disease-Like Vascular Damage without Renal Disease. Kidney Dis Basel Switz. 2020;6(2):92–97. doi: 10.1159/000505026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartstra JW, de Jong PA, Spiering W. Accelerated peripheral vascular aging in pseudoxanthoma elasticum - proof of concept for arterial calcification-induced cardiovascular disease. Aging. 2019;11(3):1062–1064. doi: 10.18632/aging.101821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleisch H, Bisaz S. Mechanism of Calcification: Inhibitory Role of Pyrophosphate. Nature. 1962;195(4844):911–911. doi: 10.1038/195911a0 [DOI] [PubMed] [Google Scholar]
- 17.Fleisch H, Russell R, Straumann F. Effect of pyrophosphate on hydroxyapatite and its implications in calcium homeostasis. Nature. 1966;212(5065):901–903. [DOI] [PubMed] [Google Scholar]
- 18.O’Neill WC, Lomashvili KA, Malluche HH, Faugere M-C, Riser BL. Treatment with pyrophosphate inhibits uremic vascular calcification. Kidney Int. 2011;79(5):512–517. doi: 10.1038/ki.2010.461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pomozi V, Brampton C, van de Wetering K, et al. Pyrophosphate Supplementation Prevents Chronic and Acute Calcification in ABCC6-Deficient Mice. Am J Pathol. 2017;187(6):1258–1272. doi: 10.1016/j.ajpath.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francis MD, Russell RG, Fleisch H. Diphosphonates inhibit formation of calcium phosphate crystals in vitro and pathological calcification in vivo. Science. 1969;165(3899):1264–1266. doi: 10.1126/science.165.3899.1264 [DOI] [PubMed] [Google Scholar]
- 21.Ferguson A, Watson WC, Maxwell JD, Fell GS. Alkaline phosphatase levels in normal and diseased small bowel. Gut. 1968;9(1):96–98. doi: 10.1136/gut.9.1.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dedinszki D, Szeri F, Kozák E, et al. Oral administration of pyrophosphate inhibits connective tissue calcification. EMBO Mol Med. 2017;9(11):1463–1470. doi: 10.15252/emmm.201707532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kranenburg G, de Jong PA, Bartstra JW, et al. Etidronate for Prevention of Ectopic Mineralization in Patients With Pseudoxanthoma Elasticum. J Am Coll Cardiol. 2018;71(10):1117–1126. doi: 10.1016/j.jacc.2017.12.062 [DOI] [PubMed] [Google Scholar]
- 24.Bartstra JW, de Jong PA, Kranenburg G, et al. Etidronate halts systemic arterial calcification in pseudoxanthoma elasticum. Atherosclerosis. 2020;292:37–41. doi: 10.1016/j.atherosclerosis.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Sundberg JP, Levine MA, Terry SF, Uitto J. The effects of bisphosphonates on ectopic soft tissue mineralization caused by mutations in the ABCC6 gene. Cell Cycle. 2015;14(7):1082–1089. doi: 10.1080/15384101.2015.1007809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pomozi V, Brampton C, Szeri F, et al. Functional Rescue of ABCC6 Deficiency by 4-Phenylbutyrate Therapy Reduces Dystrophic Calcification in Abcc6−/− Mice. J Invest Dermatol. 2017;137(3):595–602. doi: 10.1016/j.jid.2016.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorgels TGMF, Hu X, Scheffer GL, et al. Disruption of Abcc6 in the mouse: novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum Mol Genet. 2005;14(13):1763–1773. doi: 10.1093/hmg/ddi183 [DOI] [PubMed] [Google Scholar]
- 28.Walker HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Butterworths; 1990. Accessed June 10, 2021. http://www.ncbi.nlm.nih.gov/books/NBK201/ [PubMed] [Google Scholar]
- 29.Väärämäki S, Uusitalo H, Tőkési N, Pelttari S, Váradi A, Nevalainen PI. Pyrophosphate Treatment in Pseudoxanthoma Elasticum (PXE)-Preventing ReOcclusion After Surgery for Critical Limb Ischaemia. Surg Case Rep. 2019;2019(4):1–3. doi: 10.31487/j.SCR.2019.04.02 [DOI] [Google Scholar]
- 30.Fu M, Blechar JA, Sauer A, Al-Gousous J, Langguth P. In Vitro Evaluation of Enteric-Coated HPMC Capsules—Effect of Formulation Factors on Product Performance. Pharmaceutics. 2020;12(8):696. doi: 10.3390/pharmaceutics12080696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiwele I, Jones BE, Podczeck F. The shell dissolution of various empty hard capsules. Chem Pharm Bull (Tokyo). 2000;48(7):951–956. doi: 10.1248/cpb.48.951 [DOI] [PubMed] [Google Scholar]
- 32.Datta PK, Frazer AC, Sharratt M, Sammons HG. Biological effects of food additives. II.—sodium pyrophosphate. J Sci Food Agric. 1962;13(11):556–566. doi: 10.1002/jsfa.2740131102 [DOI] [Google Scholar]
- 33.Burtis C, Bruns D. Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics - 7th Edition. 7th ed. Elsevier; 2014. Accessed April 16, 2021. https://www.elsevier.com/books/tietz-fundamentals-of-clinical-chemistry-and-molecular-diagnostics/burtis/978-1-4557-4165-6 [Google Scholar]
- 34.Luo H, Li Q, Cao Y, Uitto J. Therapeutics Development for Pseudoxanthoma Elasticum and Related Ectopic Mineralization Disorders: Update 2020. J Clin Med. 2020;10(1). doi: 10.3390/jcm10010114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefthériotis G. PyROphosPHate Supplementation to Fight ECtopIc Calcification in PseudoXanthoma Elasticum: PROPHECI Study. clinicaltrials.gov; 2021. Accessed June 7, 2021. https://clinicaltrials.gov/ct2/show/NCT04868578 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during the current study.


