Abstract
This retrospective traces the hypothesis of ion channels from an early statement in a 1970 essay in this journal (Hille, B., 1970, Prog. Biophys. Mol. Biol. 21, 1–32) to its realization today in biophysical, molecular, biochemical, and structural terms. The Na+ and K+ channels of the action potential have been isolated, reconstituted, cloned, mutated, and expressed. They are conformationally flexible, multi-pass glycosylated membrane proteins. Refined atomic structures of several conformational states are known. The discoveries over this half century history illustrate the growth of a field from initial ideas to a mature discipline of biology, physiology, and biomedical science.
Keywords: Na+ channel, K+ channel, Ca 2+ channel
1. Introduction
This is a retrospective on the essay, “Ionic channels in nerve membranes,” that I published more than 50 years ago in this journal (Hille, 1970). What were the context and the thesis then, and how have the conclusions matured in time?
Today’s explanation of membrane potentials and electrical excitability of axons is based on concepts developed by the Cambridge school of membrane biophysicists in the 1940s and 50s and refined over the next 70 years. It comprises demonstrations of the electrochemical ion activity differences set up by pumps across the plasma membrane, and the electrical consequences of opening and closing gated ion channels in the membrane. The channels catalyze nonequilibrium ionic current flows across the membrane that charge and discharge the membrane capacitance, accounting for the electrical signals of the nervous system. These biophysical ideas have now been transformed by modern biology from the original electrical language to a molecular and genetic understanding.
The subject of my 1970 essay was the newly evolving concept of ion channels as the molecular explanation for the electrical excitability of nerve. In 1952, Hodgkin and Huxley had published Nobel-Prize-winning measurements of ionic currents of the squid giant axon cell membrane using their new voltage clamp technique (Hodgkin et al., 1952; Hodgkin and Huxley, 1952a,b,c). In the final paper, “A quantitative description of membrane current and its application to conduction and excitation in nerve,” they dissected and described the currents by an empirical kinetic model of voltage-dependent ion permeability changes that could simulate propagated action potentials with remarkable fidelity (Hodgkin and Huxley, 1952d). Their paradigm-changing work was electrical and mathematical without any molecular commitments to tell us what a membrane conductance is. It came before we understood the molecular organization of lipids and proteins in cell membranes, and it came before fluxes due to what we now confidently call channels, pumps, and carriers had been fully distinguished. Indeed, at that time, some scientists still maintained that there was no membrane around cells, others felt that the electrical signals were surface epiphenomena reflecting propagating intracellular biochemical reactions, and others attributed propagation along axons to actions of acetylcholine. The closest that the Cambridge group came to structure is in the paper of Hodgkin and Keynes (1955) measuring the ratio of influx to efflux of radioactive K+ ions. There they concluded, in Hodgkin’s understated operational words: “The variation of [potassium] flux ratio with driving force is of the kind expected in a system in which the ions are constrained to move in single file.” Today this is often called “the long-pore effect.” Movements of Na+ did not suggest such a long-pore, single-file behavior.
Ten years later, Clay M. Armstrong and I sought a more molecular interpretation. We championed a view that Hodgkin and Huxley’s Na+ and K+ membrane conductances were due to proteinaceous, gated, and ion-selective pores that we would be calling Na+ channels and K+ channels consistently. Today, ideas we embraced so dogmatically would have been called a hypothesis. We began more than a decade of biophysical study with the voltage clamp to advance this thesis, which was originally regarded by many as radical, wrong, or at least untestable. Clay worked with squid giant axons and I worked with frog nodes of Ranvier. The key answers were the same. I have already reminisced on parts of this story in several recent essays (Hille, 2012; 2022). The “Ionic channels in nerve membranes” essay of 50 years ago (Hille, 1970) was the first attempted review of the channel ideas that Clay Armstrong and I were beginning to develop. As well, it was altogether my first review, and I am very grateful that Denis Noble invited me at the age of 27 to write it–and now at the age of 81 to write this retrospective. After reading the final draft of my essay, Kenneth S. Cole (Clay Armstrong’s former mentor who also introduced Alan Hodgkin to the squid giant axon) wrote me, “slightly worried that you may be pushing some of your channel arguments pretty far (K.S. Cole letter, June 2, 1969).
2. Two questions
The1970 essay (Hille, 1970) started with:
This review focuses on two questions:
Do the several permeant ions share a common pathway through the membrane or are the pathways for different ions different?
Is the ionic permeability a diffuse property of broad areas of membrane or is it localized in rare specializations of the membrane?
From today’s perspective, it seems remarkable that such basic questions had to be considered at all. In slightly more modern terms, they asked whether Na+ channels and K+ channels share the same permeation tunnel and whether “channel” phenomena are a broad emergent property of the way membranes are built or are due to rare specialized molecules imbedded in the membrane. The conclusion of the essay was that Na+ channels and K+ channels are separate, sparse, but as of 1970 still unidentified pore-forming membrane molecules and that in addition there were “leak” channels that had ion selectivity but no complex voltage dependence and no known pharmacology.
3. The arguments in 1970
The 1970 essay (Hille, 1970) presented four biophysical arguments compatible with separate pathways: (i) The Hodgkin and Huxley (1952d) model described the voltage-dependent kinetics of Na+ and K+ conductances by different equations as if they had no interdependence. The leak conductance had no voltage or time dependence. (ii) The experiments of Hodgkin and Keynes (1955) had suggested a long pore and single-file movements for K+ and no single filing for Na+. (iii) Ion selectivity experiments suggested that it was best to refer to two channel mechanisms with overlapping selectivity rather than to two mechanisms with exclusive Na+ or K+ selectivity or to one mechanism with temporally evolving selectivity. Thus, the permeability to NH4+ ions showed one kinetic component with Na+ channel kinetics and pharmacology and another with K+ channel kinetics and pharmacology (Binstock and Lecar, 1969). In addition, the Na+ channel exhibited a small permeability component to K+ (Chandler and Meves, 1965). And (iv) The pharmacology of the two current components suggested separate channels with a clean block of Na+ channels but not K+ channels by tetrodotoxin and saxitoxin, and a clean block of K+ channels but not Na+ channels by tetraethylammonium ions (Narahashi et al., 1964; Armstrong, 1966; Hille, 1966, 1967).
In addition, the essay (Hille, 1970) presented four biophysical arguments compatible with sparse pathways: (i) Physical principles can account for the observed membrane conductances if you postulate that channels are sparse aqueous tunnels in the membrane even if they had diameters small enough to distinguish among ions (Hille, 1968a). (ii) Hodgkin and Huxley (1952d) had postulated charged gating particles whose movements in the membrane electric field controlled each gate. Failed early attempts to observe this necessary charge movement suggested a very low density of channels. (iii) Tetrodotoxin blocked Na+ channels selectively (Narahashi et al., 1964; Hille, 1966). Failed early attempts to measure binding of radioactive tetrodotoxin to nerve membranes or depletion from the bathing medium suggested a very low density of Na+ channels (Moore et al., 1967). (iv) Kinetic interpretation of the rapid rate of entry of blocking tetraethylammonium ions into K+ channels in squid giant axons suggested (with remarkable accuracy) a single-channel conductance consistent with a low K+ channel density (Armstrong, 1966).
4. Going forward
From 1970 to 2021, this nascent field grew enormously from just a handful of papers and a handful of investigators to at least 300,000 papers listed on the Pubmed data base authored by thousands of investigators. Ion channels are taught to all students of biology even at the undergraduate level and to all health professionals. They are the subject of several journals, numerous books (Ashcroft, 1999; Hille, 2001; Zheng and Trudeau, 2015), many awards, and three Nobel Prizes. The existence of ion channels and of membranes is no longer in question, and our understanding expands every day. The original concepts have been modified very little, but the details, scope, and impact have expanded well beyond what could have been anticipated more than 50 years ago. At least 300 genes of the human genome code for protein subunits of ion channels. Although classic biophysical measurements with voltage clamp of axons were foundational for the ion channel concept, many subsequent advances in the last five decades resulted from new techniques including the patch clamp with single-channel recording (Hamill et al., 1981) and measuring fluorescence changes with fluorescently tagged channels; new preparations including muscles, neuron cell bodies, and dendrites; protein isolation and sequencing; cloning, gene sequencing, and genomics; reconstitution and transfection; site-directed and spontaneous mutation; linkage to diseases; targets of therapeutic agents; imaging; atomic structures; molecular dynamic simulation; and use of online computers for data collection, analysis, and modeling. The discussion below touches on the classical ideas with their modern evolution, and it highlights just a few interesting references without presenting a scholarly or balanced analysis of the field today (Hille, 2001; Zheng and Trudeau, 2015). The thesis is that the 1970 review was a baby step towards the later spectacular growth of a burgeoning field of science.
5. Channels are locally clustered
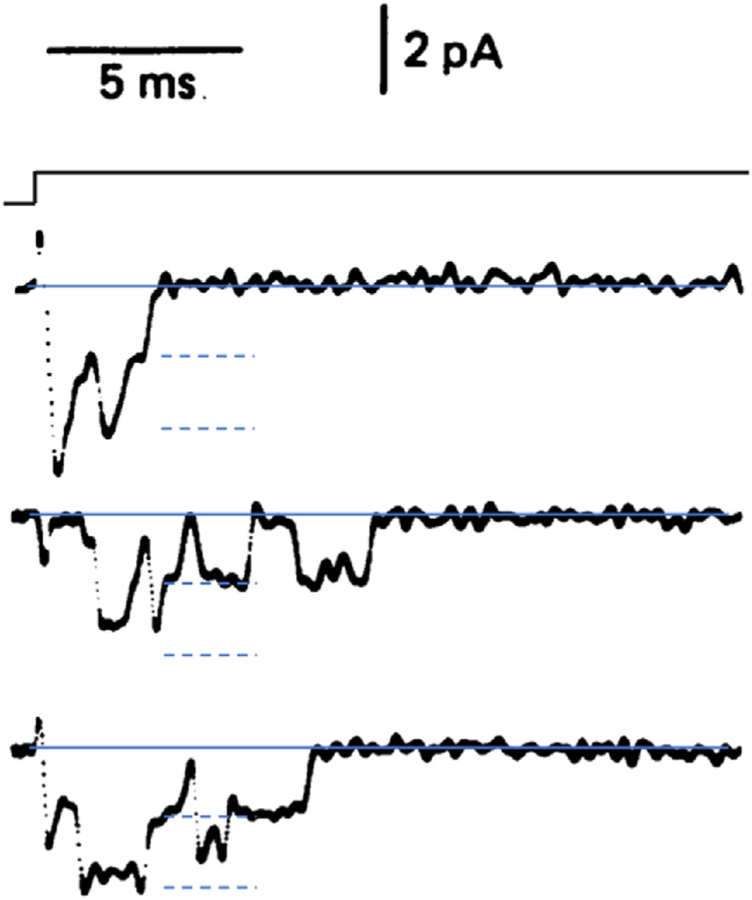
Let us start with the second question of Hille (1970) using modern numbers: Is the ionic permeability a diffuse property of broad areas of membrane or is it localized in rare specializations of the membrane? The concept of sparseness has held up well in the sense that the number of copies of ion channels in the plasma membrane of an excitable cell is minute compared to the number of copies of all membrane molecules. We know the number of channel copies approximately in many different examples from measuring abundances with antibody or toxin binding, or from counting with electron and super-resolution microscopy, or from observing gating charge movement with increasing refinement during voltage steps (Armstrong and Bezanilla, 1973), or most simply from calculations comparing total membrane conductance with the conductances of single channels obtained by patch recording or fluctuation analysis. Single-channel conductance can be seen directly in the amplitudes of current steps as the gates of channels open and close stochastically in an all-or-nothing manner in patch recording. (Fig. 1). The −1.5 pA (inward) unitary-current step sizes in that figure correspond to a single-channel conductance of ~15 pS for those Na+ channels. A modern channel-density calculation might then compare the maximum Na+ and K+ conductance of e.g. the squid giant axon model (ḡNa = 120 mS/cm2; ḡK = 36 mS/cm2; Hodgkin and Huxley, 1952d) to the approximate 2–20 pS single-channel conductance of various Na+ and K+ channels (Hille, 2001) (conductance values 10-fold lower than the 1970 essay (Hille, 1970) supposed). This would correspond to 60 to 600 Na+ channels/μm2 and a smaller density of K+ channels. Considering that the same area of membrane bilayer might have 2 × 106 phospholipid and cholesterol molecules, on average each Na+ channel protein is outnumbered ~3600- to 36,000-fold by lipid molecules. Thus, on average, channel proteins are sparse even in squid giant axons. Using current densities reported in the frog node of Ranvier (Dodge and Frankenhaeuser, 1959), the Na+ channel density there might be three times higher.
Fig. 1.
Early patch clamp recording of Na+ currents in voltage-gated Na+ channels. The outside-out patch from a bovine chromaffin cell is depolarized in a voltage step from −80 mV to −50 mV. Each channel opening is seen as a downward deflection as Na+ ions flow briefly (open time ~1 ms) in from the extracellular solution when one or two Na+ channels are opened stochastically. (Fenwick et al. (1982).
Despite these consistent results, newer work suggests that we should not assume that channels are spread out evenly across the cell surface. In a classical example of channel clustering at fast chemical synapses, both the presynaptic and the postsynaptic channels are focally organized. This occurs presynaptically for both the voltage-gated Ca2+ channels (CaV) and the calcium-gated K+ channels (KCa) in the active zone, which form a stereotyped microarray with a roughly 10-nm lattice spacing (Roberts et al., 1990; Harlow et al., 2001) and postsynaptically for neurotransmitter-gated channels densely packed in the postsynaptic density (Salpeter and Loring, 1985). Similarly, the CaV channels of skeletal muscle T-tubules and the ryanodine receptor channels of the adjacent sarcoplasmic reticulum are organized in complementary near-regular lattices forming the couplons of excitation-contraction coupling (Ríos et al., 2019). Recent examples suggest that such tight lateral organization may be wide spread. Thus, some CaV channels in heart form clusters of at least 8 channels that interact both at the molecular level and through their local calcium signals and therefore gate cooperatively in multi-unit steps (Moreno et al., 2016). Further, some CaV and KCa channels of neuron somata aggregate tightly in clusters of 10–100 channels in super-resolution microscopy, possibly like an intramembrane phase condensate (Vivas et al., 2017). More generically, ion channels, like other signaling proteins, enter into macromolecular complexes with many other cellular and membrane proteins at membrane-membrane junctions (Johnson et al., 2018), and some channels localize in “liquid ordered,” membrane lipid rafts of the plasma membrane where they can interact more directly with other signaling molecules (Myeong et al., 2021). In summary, from the work of Hodgkin and Huxley, we had presumed that voltage-gated ion channels communicated with each other only through their electrical signals spread by “cable properties.” This presumption is not always correct. It is evident that Ca2+-permeable channels communicate with themselves, their neighbors, and downstream effectors through local intracellular calcium signals, and many channels communicate through physical interactions formed when the channels and effectors become organized as multi-molecular signaling complexes. Clustering of ion channels confers new mechanical and chemical interdependence.
6. NaV and KV channels are seperate proteins
We return to the first question of the 1970 essay (Hille, 1970): Do the several permeant ions share a common pathway through the membrane, or are the pathways for different ions physically distinct? As anticipated then, Na+ and K+ do use separate pathways formed by separate channel proteins and coded by separate genes, but, as not even speculated then, the Na+ and K+ channel proteins and those of CaV channels nevertheless share important structural similarities and are evolutionary homologues. Through many gene duplications, they are members of an even larger superfamily of related channels. The existence of many members of this superfamily would not have been suspected from electrophysiology in 1970 or even a decade later.
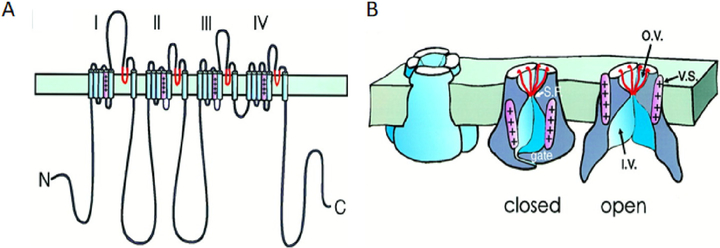
The first hint that voltage-gated ion channels are proteins came from the simple finding that intracellular perfusion of the protease pronase removes the inactivation gating of squid Na+ channels (Armstrong et al., 1973) as if the gate could be compromised by cleaving peptide bonds. There followed a couple of decades’ work in biochemistry and molecular biology laboratories to fully identify the molecules. Tetrodotoxin served as the marker for purification, reconstitution, sequencing, and cloning of the first NaV channel and its auxiliary subunits (Noda et al., 1984; Catterall, 1984), and dihydropyridine blockers served for the first CaV channel and its subunits (Tanabe et al., 1987; Takahashi et al., 1987). Grossly, the Na+ and CaV channels were remarkably similar. Their long polypeptide chain crossed the membrane 24 times and contained 4 internal repeats (Fig. 2A) potentially folding as a pseudo-tetramer with a hole in the middle (Fig. 2B). The transmembrane segments of each repeat domain were numbered S1-S6. The pore-forming principal subunit had four copies of the voltage sensor, bearing many positive charges (pink and ++++) in S4, and four membrane-reentrant loops (red, between S5 and S6) bearing a net negative charge that came together to form the outer vestibule and selectivity filter. The outer vestibule and selectivity filter would include the receptor for tetrodotoxin (Terlau et al., 1991; Shen et al., 2018), and the inner vestibule and selectivity filter would form the receptor for local anesthetics and CaV channel blockers (Zhao et al., 2019). At the time, there was no high-affinity label for KV channels so they were first cloned by “chromosome walking” to a gene locus that coded for a Drosophila KV channel (Papazian et al., 1987). The KV channel polypeptide chain was a quarter as long with only 6 transmembrane segments, and contained only one voltage sensor domain S4 and one membrane reentrant loop, so the KV channel macromolecule assembles as a tetramer of four similar or identical subunits. The cloned NaV, CaV, and KV channel sequences were clear evolutionary homologues that must have derived by tandem (side-by-side) duplications and accumulated divergent mutations from a single-repeat voltage-gated ancestor such as a KV channel subunit.
Fig. 2.
Cartoons from the 1990s of a four-fold pseudosymmetric voltage-gated cation channel in the plasma membrane. Peptide chain folding (A) and functional components (B) are shown diagrammatically with selectivity filter regions in red and the voltage sensor S4 segment in pink. The drawing in B was made before structural work showed where the voltage sensors lie. They are drawn too close to the pore itself and should be shifted more laterally virtually into the lipid membrane (see Fig. 4). The folding diagram is for an Na+ channel with four homologous repeat domains labeled I-IV. Ca2+ channels would be similar, and K+ channels are homo- or hetero-tetramers of subunits similar to a single Na+ channel domain. Abbreviations: S.F., selectivity filter; v.s., voltage sensor; o.v., outer vestibule; and i.v., inner vestibule. (A) After Catterall (1992) and (B) from Armstrong and Hille (1998).
7. Ion channel diversity
Within a decade of this first cloning, entire genome sequences of eukaryotes began to appear (budding yeast, 1996; C. elegans, 1998; human, 2001–2004), and it gradually became possible to try to annotate all ion channel principal subunits related to the pseudo-tetrameric/tetrameric voltage-gated cation channels. Although electrophysiology had begun to reveal numerous channel types in the same cell (McAllister et al., 1975; Tsien et al., 1988), genomes revealed many more than any physiological studies had anticipated. A 2004 analysis identified a cation-channel superfamily of 143 relatives in the human genome, including many channels that were totally unsuspected in 1970 (Yu and Catterall, 2004). The expanding list, appending also newer entries from the 2021 IUPHAR/BPS Guide to Pharmacology (https://www.guidetopharmacology.org), includes (the number in parentheses indicates how many genes for channel principal subunits are listed in each group): NaV (9), Navi (1), CaV (11), KV (40), K inward rectifier (15), K2p (15), KCa (6), KNa (2), HV (1), TRP (28), TPC (2), cyclic nucleotide gated (6), HCN (4), RyR (3), IP3R (3), and Catsper (4). We can anticipate that this 2021 list will grow with recognition of more distant tetrameric cation channels like perhaps synaptic glutamate receptor channels. The voltage-gated cation channel superfamily list includes 28 members of the unsuspected TRP family, a most versatile multimodal group that contains channels sensitive to heat, cold, acid, chili peppers, oregano, garlic, wasabe, menthol, or mechanical stimuli (Ramsey et al., 2006).
Presumably all of these related cation channels evolved by gene duplications that offered adaptive advantages for specialized localization, regulation, gating, and cell function. Genome studies revealed another striking fact: the genomes of many prokaryotes (eubacteria and archaebacteria!) also contained, for example, families of homologous KV channels, NaV channels (Koishi et al., 2004), and relatives of synaptic glutamate receptors. We have to conclude that, much as all of cellular life needs a cell membrane, it also needs ion channels. Evidently, the basic outlines of the channels now in our nervous systems were achieved several billion years ago before the divergence of eukaryotes, eubacteria, and archaebacteria. Voltage sensitivity and electrical excitability would have been possible even then. It will be fascinating to learn what physiological roles these prokaryotic ion channels served originally and today. In conclusion, the voltage-gated channel super family is large, ancient, and essential for life as we know it.
Thus far we have focused on the radiation and diversity of relatives of the voltage-gated channels of excitable cells, the subject of my 1970 essay. The diversity of all ion channels is much larger (Zheng and Trudeau, 2015). Many other families of membrane channels likely evolved independently of the tetrameric cation channels we discussed. Most numerous are 47 genes for ligand-gated pentameric channels of our fast chemical synapses. They include synaptic channels responding to acetylcholine (17), serotonin (5), GABA (19), glycine (5), and zinc (1) (IUPHAR/BPS Guide to Pharmacology https://www.guidetopharmacology.org). Again, an impressive list enabling the sophisticated specialization of brain and nerve-muscle synapses. The five ligand-binding channel protomers are arranged to form a cylinder that encloses a single ligand-gated ion pathway down its central axis. Other known channel families include ASIC, ENaC, P2X, various mechano-transduction channels, CLC chloride channels and transporters, several other families of anion channels, Orai, and aquaporins, which require in total at least 41 more genes so far. Most of these channels were recognized only after 1970, and several of them also have been traced back to prokaryotic origins. Some are dimers, some trimers, and Orai channels are hexamers. Some form one channel pore per complex, and others, two. Some are on the plasma membrane, and quite a number that we understand less are on intracellular membranes. We can single out the 7 homologous CLC chloride channel family members, all related in structure, some of which are Cl− channels and some Cl−-H+ exchangers, some of which are on the plasma membrane and others on internal membranes, and all of which are dimers forming either two pores or two transport pathways (Miller, 1982; Jentsch and Pusch, 2018). Tantalizingly, they illustrate how pores and transporters can be closely related. We also single out the two mechanosensitive piezo channel family members (Coste et al., 2010; Kefauver et al., 2020). They are trimers with as many as 38 transmembrane segments per protomer, perhaps suggesting that occupying lots of real estate aids mechanosensitivity. This enumeration neglects the non-pore-forming auxiliary subunits of ion channels, additional gene products that are obligate or optional modulators of channel activity.
Our discussion of diversity should make clear that the computational models for simulating electrical signaling in an excitable cell perhaps could include six to 20 active ion channel types rather than the two or three of the original Hodgkin-Huxley (Hodgkin and Huxley, 1952d) model for axons. Even better than speaking of a particular organism or cell, we should distinguish channel specialization in subdomains of the cell, e.g., distal dendrite, proximal dendrite, soma, axon hillock, axon, nerve terminal, and synapse.
All of these ion channels are proteins coded by genes, and we have learned that they are subjected to all regulatory processes known for other proteins. Channel DNA can be methylated and mutated, the RNA can be spliced, edited, trafficked, and degraded, and the proteins can be phosphorylated, glycosylated, sulfated, lipidated, ubiquitinated, sumoylated, ADP-ribosylated, and proteolytically clipped. Channels have signals for translocation, targeting, degradation, and for binding in complexes with other proteins including, for example, modulatory Gβγ subunits of heterotrimeric G proteins (Logothetis et al., 1987) and kinase-scaffolding AKAPs. These many interactions and modifications of ion channels mean that unlike what one might have supposed from the original Hodgkin-Huxley model (Hodgkin and Huxley, 1952d), the computational powers of nerves and muscles and the ion transport processes in all cells are continually modulated by ongoing dynamic intracellular, neurotransmitter, and hormonal signals.
Channel mutations underlie many hereditary diseases, called channelopathies (Ashcroft, 1999). For example, mutations of the anion channel CFTR underlie cystic fibrosis (Veit et al., 2016), mutations in cardiac KCNQ1 and its auxiliary subunit KCNE1 increase susceptibility to cardiac long-QT syndrome (Tristani-Firouzi and Sanguinetti, 2003), and mutations of pancreatic KATP channels and their SUR subunits can increase susceptibility to neonatal diabetes (Pipatpolkai et al., 2020). The availability of gene sequencing has meant that we can identify such mutations precisely. They are remarkably comprehensive. Thus, more than 2000 different mutations have been identified so far in the gene coding the 1480 amino acid residues of human CFTR (Veit et al., 2016). As for many other proteins in the human genome, mutations in most of the several hundred ion channels we have listed, are associated with one or more hereditary channelopathies and human diseases. Identifying these mutations is now routine in genetic medicine. We should anticipate that every coding and non-coding base pair is repeatedly being tested by spontaneous mutation and natural selection in the human population.
8. Ion channel structures
After the cloning and sequencing of many individual ion channels, it still has taken years to determine their 3-dimensional structure. But now we have some beautiful structures and elegant functional conclusions (Doyle et al., 1998; Shen et al., 2018; Zhao et al., 2019; Catterall et al., 2020; Pipatpolkai et al., 2020; van Goor et al., 2020; Zhang et al., 2021). Membrane proteins denature unless they are in a membrane-like environment, so forming crystals for x-ray crystallography required both the ability to make quantities of very pure protein as well as finding compatible lipids or detergents that allowed crystallization. The pioneering success with KcsA, a bacterial K+ channel with no voltage sensitivity (Doyle et al., 1998), was followed slowly by a broader range of other especially K+ channels. Fortunately, another less demanding technique, cryogenic electron microscopy (cryo-EM), has been gaining ground and yielding a broader range of ion channel structures. New generations of higher-sensitivity, direct electron-recording cameras and new software approaches correcting for beam-induced image motion made this possible. The sample is flash frozen in vitreous ice, and there is no need to make a crystal. Each channel particle is resolved as an image and sorted into different poses and even different conformations. Thousands of similar particle images are then averaged and combined. The results often reach comparable resolution to those from x-ray crystallography, and they are often sufficient to model the protein backbone, side chains, and bound ligands. Since ion channels are flexible macromolecules, at the very least having multiple gating states, a challenge for the structural biologist has been to deduce which physiological state each structure represents. Progress in trapping specific functional states of ion channels and determining their structures by cryo-EM promises in the near future to yield a full complement of structural models for the main functional states of ion channels and their transitions.
9. Functional properties of Na+ and K+ channels: ion selectivity
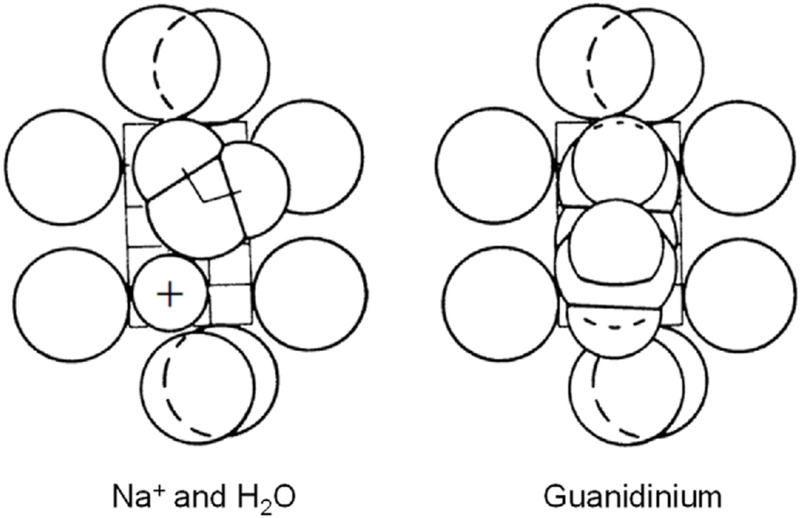
We consider several classical properties of the channels and then the ongoing story of their modern structural explanation. Key properties of the Na+ and K+ conductances of the Hodgkin and Huxley (1952d) model were ionic selectivity and voltage-dependent gating. Fifty years before, Bernstein (1902, 1912) had proposed that excitable cells are surrounded by a membrane that is selectively permeable to K+, a proposal that was quite familiar to physiologists, but the idea of a selective Na+ conductance was novel. So too was the idea of conductances regulated by voltage. A better understanding of selectivity and permeation was obtained in the early 1970s by measuring which small cations could pass through Na+ channels and which could not. Eleven cations passed through the Na+ channel (Chandler and Meves, 1965; Binstock and Lecar, 1969; Hille, 1971, 1972). The largest permeant ion was aminoguanidinium, a totally planar organic cation, and the smallest impermeant ion was methyl ammonium whose methyl hydrogens are not coplanar. Thus, it was possible to postulate that the Na+ channel selectivity filter forms a slit 3.1 Å wide and 5.1 Å high lined by oxygen dipoles that could hydrogen bond with permeating organic actions and with water (Hille, 1971) (Fig. 3). Two of the oxygens would be a negative carboxylic acid group whose protonation below pH 5.2 would account for block of Na+ channels by acid solutions (Hille, 1968b). Similar experiments showed that K+ channels pass only 4 small cation species and are limited to a small circular pore diameter of 3.2–3.3 Å (Bezanilla and Armstrong, 1972; Hille, 1973). Again, the pore would be lined with oxygen dipoles, but in this case no high-field-strength negative charge.
Fig. 3.
1971 hypothesis for the selectivity filter of a NaV channel viewed face on from the outside. Eight oxygen atoms outline a rectangular constricted pore just large enough to accept guanidinium and aminoguanidinium whose nitrogen atoms can form hydrogen bonds. A Na+ ion fits in with a lateral water molecule, which also hydrogen bonds. This hypothesis predates any sequence or structural work. The calibration grid shows Ångström dimensions (Hille, 1971).
In these original mechanical models, the selectivity filter acted as a sieve with a fixed pore size. However, that still did not explain why Na+ passes more easily through Na+ channels than K+. That required going back to energetic ideas of Gilbert Ling (1962) and George Eisenman (1962). Their concept was that aqueous ions are surrounded by water molecules in a strong dielectric interaction called hydration, and for an ion to enter a binding site (channel), some of these waters of hydration are removed and replaced by oxygen dipoles, negative charges, and other wall-lining atoms. Selectivity then requires comparing the dehydration and channel-entry energetics for different ions, differing in the case of Na+ versus K+, only in the ionic radius. A strong negative charge (carboxylic acid) in the Na+ channel and the possibility of retaining some tightly bound water molecules was invoked to account for its selectivity (Hille, 1971). Weaker negative charge and a greater stripping of more weakly bound water molecules was invoked to account for the K+ channel selectivity (Bezanilla and Armstrong, 1972; Hille, 1973).
Amazingly, amino acid sequence and structural studies have shown that the selectivity filter of virtually all K+ channels, eukaryote and prokaryote, is lined with four parallel copies of the amino acid sequence TTV/IGY/FG (from inside to outside) standing like columns. This “signature sequence” was so successful that it has not been further refined in several billion years. The uncharged, long-pore tunnel, lined with polar carbonyl oxygens, provides a single-file series of 4 or more K+ binding sites rigidly buttressed outside its walls to retain the critical internal dimensions (Doyle et al., 1998; Lockless et al., 2007) postulated 25 years before. As anticipated, the more recently resolved Na+ channel selectivity filter structures present a pseudo-fourfold but much shorter asymmetric pore with oxygen dipoles, more negative charge (carboxylic acids) than positive charge, and more dependence on polar and charged residue side chains than in K+ channels (Payandeh et al., 2011; Shen et al., 2018; Jiang et al., 2021). Again, the dimensions are compatible with the earlier biophysical deductions, although the presence of more charges and expected side-chain flexibility add new concepts to understand. The backbone of the Na+ channel filter may be as rigid as the K+ channel filter but its side chains that interact directly with the ions probably are not. At another extreme, the selectivity filter of TRPV1 channels seems highly adaptive, narrow for small alkali metal ions and becoming wider for larger somewhat hydrophobic organic cations (Zhang et al., 2021).
Now that atomic structures are available, we could anticipate that a complete understanding of ion selectivity might be reached by computer simulation using these structures (Callahan and Roux, 2018). However, the task remains daunting for several reasons: The energy differences are small and for a 10:1 selectivity ratio would be only 2.3 times thermal energy, a 0.1 Å error in structure could lead to a large energy error, yet the resolution of structures is currently 2–3 Å and many atoms have thermal motions and bond rotations. The energy models for essential water molecules are still improving, and the calculations should be done with fluctuating protein structures in fluctuating water and lipid environments. Ion permeation requires at least several microseconds of simulation in molecular dynamics calculations, which is difficult to obtain with the computer access available to most investigators. And to discuss selectivity would require comparing a large number of ion passages. Much effort is being devoted to solving each of these computational problems.
10. Voltage dependence
What about voltage dependence? Hodgkin and Huxley (1952d) recognized that the membrane electric field had to act on some regulatory charged or dipolar particles to do work on the gating mechanism and change the probability of what we now call channel opening. Movement of these gating particles in the electric field would necessarily produce a tiny transient transmembrane current, the gating current. Such small charge movements were first detected for CaV channels of muscle transverse tubules (Schneider and Chandler, 1973) and for NaV channels of squid giant axons (Armstrong and Bezanilla, 1973). The key to detection was the ability to record and average repeated noisy trials with a digital computer. Extensive electrical recordings of the gating charge movements in many types of channels have revealed many kinetic details of the microscopic steps of voltage sensing and gating including early on that the steps of activation gating and those of inactivation gating are more coupled than had been originally thought (Bezanilla, 2018).
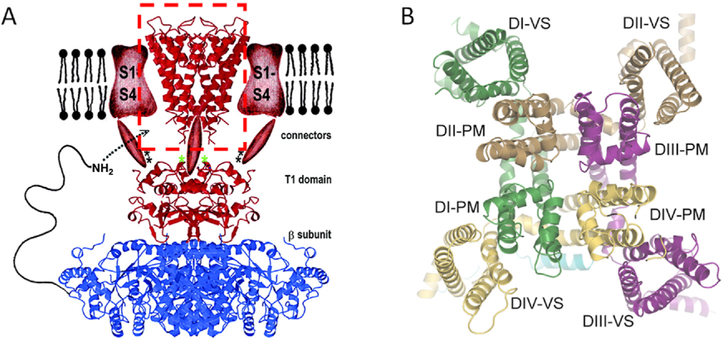
As soon as the first amino acid sequence of an NaV channel was published (Noda et al., 1984), a high density of positive charge in the S4 transmembrane segment was evident. Every third residue was an arginine or a lysine. For different voltage-gated channels, each S4 segment carries between 3 and 6 positive charges. These charges were Hodgkin and Huxley (1952d) the moving gating particles as was eventually proven by mutation, structure, and fluorescence monitoring. Especially valuable for tracing movements of each residue in S4 was the ability to add fluorescent labels at specific positions in specific repeat domains and to observe optical changes of the fluorophores at the same time as the electrical signals (Cha et al., 1999). In structures, segments S1-S4 (the voltage-sensor domain) extend laterally from the central pore domain out into the lipid membrane (Fig. 4). Of the four transmembrane segments in each voltage sensor, S4 lies closest to the pore domain (S5 & S6) where it can communicate mechanically to the gates via the S4–5 linker. During voltage sensing, the helical S4 segment slides though a short enveloping hourglass collar formed by S1-S3, neatly moving several gating charges from one side of the membrane to the other in a relatively short motion that exerts a torque on the S4–5 linker thus coupling mechanically to the gates (Catterall et al., 2017; Bezanilla, 2018). Although details still remain to work out, the broad strokes of electromechanical coupling in voltage sensors seem firmly in place.
Fig. 4.
Structures of the voltage-gated channel family. A. An early mockup cartoon representing a hypothetical KV channel in side view. The 2000 cartoon is a composite of several structures filled in with some artistic sketching. At the top center in a red dashed box is the crystal structure of the original KcsA channel (red), shown as a ribbon diagram of the inverted teepee. On either side, in the membrane, are sketched the expected positions of red voltage sensor domains (S1-S4). Below the membrane are sketched red cytoplasmic connectors joining to another crystal structure showing a cytoplasmic tetramerization domain (T1) of KV1.1 (red) complexed with a KV channel β2 subunit (blue), again as ribbon structures (Gulbis et al., 2000). This composite, anticipates the finding that channels may have extensive extracellular and intracellular components that make them much more than a simple conducting donut. B. A 2021 cryo-EM structure of NaV1.5 in face view from the cytoplasmic side. The pseudotetramer is formed from a single principal subunit with four repeat domains (DI-DIV) as in Fig. 2A. Each repeat has a different color. Around the outside are the four voltage-sensor domains (VS), and forming the pore in the middle are the four pore modules (PM). To obtain this structure, an intracellular component of the inactivation process was mutated to keep the channel open. After (Jiang et al., 2021).
11. Gating
Finally, what are the gates that regulate ion flow? Early experiments with cytoplasmic blockers of K+ and Na+ channels had suggested that the channel fast activation gate(s) lay at the cytoplasmic end of the pore (Armstrong, 1966; Hille, 1977), guarding a larger inner vestibule where the blocking drugs could bind only after the gates had opened as in Fig. 2B. Indeed, in the first K+ channel structure, KcsA, there was an inner vestibule just inside from the selectivity filter that was guarded on the cytoplasmic side by convergence of a bundle of four S6 transmembrane helices (one from each monomer) at the “bundle crossing” (Doyle et al., 1998). Many subsequent structural studies with various K+ and Na+ channels suggested that a widening of this bundle convergence would correspond to the opening of the channel (Black et al., 2021) and investigated how the movement of voltage sensors would couple to the widening of this activation gate. The S6 transmembrane helices were likened to the poles of a tepee oriented upside down (see the KcsA structure in the red dashed box as one component of the composite drawing in Fig. 4A), and the splaying out of the cytoplasmic ends of these poles during opening of the gate was envisioned as forming a “smoke hole” for the tepee where the ions could pass.
The 1998, pre-structure sketch in Fig. 2B represented the gates as hermetic flaps tightly closing off access to the inner vestibule, like heart valves. Now we recognize that gates can be more subtle. To close, they need to increase the energy needed for the ion to advance along the pore axis, which could be accomplished by structural shifts of only a fraction of an Ångström or by electrostatic changes. Furthermore, all components of gating in one channel do not need to use the same physical parts and strategies. For example, the gates of KV channels have been much debated and remain under discussion (Black et al., 2021); one widely held view is that the fast activation gate is the inner bundle crossing just where the classical experiments had suggested, but the fast (N-type) inactivation gate is an N-terminal chain that swings from the cytoplasm into the inner mouth of the channel pore to plug it (Hoshi et al., 1990), and slower (C-type) inactivation is a distortion of the selectivity filter (Choi et al., 1991; perhaps sometimes even an enlargement, Tan et al., 2021) that prevents K+ ion passage there. Recent structural studies of an NaV channel have captured its open state and revealed that subtle bending and rotation of the four S6 segments opens the activation gate (Jiang et al., 2021). Moreover, the results indicate that closure of the fast inactivation gate prevents the opening movements of the activation gate. The TRP family of channels has a similar teepee structure and there both the smoke hole and the selectivity filter can participate in gating (Zhang et al., 2021).
12. Conclusion
The field of ion channels has grown from tentative hypotheses to vigorous mainstream science in its first half century. Clay Armstrong and I happened to be there at the inception and were very fortunate to enjoy the fun of exciting science. When I was a beginning scientist, I had felt tinges of jealousy that Hodgkin and Huxley had come into electrophysiology when there was something fundamental and beautiful to be discovered about action potentials. However, now I realize that with luck, each of us may stand at the birth and participate in new conceptual developments that can expand into new fields. The awarding of Nobel Prizes and other scientific awards every year is testimony to the cornucopia of exciting questions. Each discovery reveals new questions and more opportunities for discovery.
Acknowledgements
I thank William A. Catterall for comments on the manuscript and all the past members of my laboratory and biophysical colleagues all over the world for their deep contributions to this field and for their friendship.
Funding
The author’s ion channel work was generously supported by the National Institutes of Health, United States, grant R3508174, for 53 years and by the McKnight Foundation, United States, and the Wayne E. Crill Endowed Professorship.
Footnotes
Declaration of competing interest
The author declares no competing interests.
References
- Armstrong CM, 1966. Time course of TEA+-induced anomalous rectification in squid giant axons. J. Gen. Physiol. 50, 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, Bezanilla F, 1973. Currents related to movement of the gating particles of the sodium channels. Nature 242, 459–461. [DOI] [PubMed] [Google Scholar]
- Armstrong CM, Bezanilla F, Rojas E, 1973. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J. Gen. Physiol. 62, 375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, Hille B, 1998. Voltage-gated ion channels and electrical excitability. Neuron 20, 371–380. [DOI] [PubMed] [Google Scholar]
- Ashcroft F, 1999. Ion Channels and Disease. Academic Press. [Google Scholar]
- Bernstein J, Armstrong CM, 1972. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J. Gen. Physiol. 60, 588–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein J, 1902. Untersuchungen zur Thermodynamik der bioelektrischen Ströme. Erster Theil. Pflügers Arch. 92, 521–562. [Google Scholar]
- Bernstein J, 1912. Elektrobiologie. Vieweg, Braunschweig, p. 215. [Google Scholar]
- Bezanilla F, Armstrong CM, 1972. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J. Gen. Physiol 60, 588–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F, 2018. Gating currents. J. Gen. Physiol. 150, 911–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binstock L, Lecar H, 1969. Ammonium ion currents in the squid giant axon. J. Gen. Physiol. 53, 342–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black KA, Jin R, He S, Gulbis JM, 2021. Changing perspectives on how the permeation pathway through potassium channels is regulated. J. Physiol. 599, 1961–1976. [DOI] [PubMed] [Google Scholar]
- Callahan KM, Roux B, 2018. Molecular dynamics of ion conduction through the selectivity filter of the NaVAb sodium channel. J. Phys. Chem. B 122, 10126–10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, 1984. The molecular basis of neuronal excitability. Science 223, 653–661. [DOI] [PubMed] [Google Scholar]
- Catterall WA, 1992. Cellular and molecular biology of voltage-gated sodium channels. Physiol. Rev. 72 (4 Suppl. l), S15–S48. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Wisedchaisri G, Zheng N, 2017. The chemical basis for electrical signaling. Nat. Chem. Biol. 13, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Lenaeus MJ, Gamal El-Din TM, 2020. Structure and pharmacology of voltage-gated sodium and calcium channels. Annu. Rev. Pharmacol. Toxicol. 60, 133–154. [DOI] [PubMed] [Google Scholar]
- Cha A, Ruben PC, George AL Jr., Fujimoto E, Bezanilla F, 1999. Voltage sensors in domains III and IV, but not I and II, are immobilized by Na+ channel fast inactivation. Neuron 22, 73–87. [DOI] [PubMed] [Google Scholar]
- Chandler WK, Meves H, 1965. Voltage clamp experiments on internally perfused giant axons. J. Physiol. 180, 788–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KL, Aldrich RW, Yellen G, 1991. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc. Natl. Acad. Sci. U.S.A. 88, 5092–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A, 2010. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge FA, Frankenhaeuser B, 1959. Sodium currents in the myelinated nerve fibre of Xenopus laevis investigated with the voltage clamp technique. J. Physiol. 148, 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R, 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77. [DOI] [PubMed] [Google Scholar]
- Eisenman G, 1962. Cation selective glass electrodes and their mode of operation. Biophys. J. 2 (2 Pt 2), 259–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick EM, Marty A, Neher E, 1982. Sodium and calcium channels in bovine chromaffin cells. J. Physiol. 331, 599–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbis JM, Zhou M, Mann S, MacKinnon R, 2000. Structure of the cytoplasmic β subunit-T1 assembly of voltage-dependent K+ channels. Science 289, 123–127. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ, 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv 391, 85–100. [DOI] [PubMed] [Google Scholar]
- Harlow ML, Ress D, Stoschek A, Marshall RM, McMahan UJ, 2001. The architecture of active zone material at the frog’s neuromuscular junction. Nature 409, 479–484. [DOI] [PubMed] [Google Scholar]
- Hille B, 1966. Common mode of action of three agents that decrease the transient change in sodium permeability in nerves. Nature 210, 1220–1222. [DOI] [PubMed] [Google Scholar]
- Hille B, 1967. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J. Gen. Physiol. 50, 1287–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B, 1968a. Pharmacological modifications of the sodium channels of frog nerve. J. Gen. Physiol. 51, 199–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B, 1968b. Charges and potentials at the nerve surface. Divalent ions and pH. J. Gen. Physiol. 51, 221–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B, 1970. Ionic channels in nerve membranes. Prog. Biophys. Mol. Biol. 21, 1–32. [DOI] [PubMed] [Google Scholar]
- Hille B, 1971. The permeability of the sodium channel to organic cations in myelinated nerve. J. Gen. Physiol. 58, 599–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B, 1972. The permeability of the sodium channel to metal cations in myelinated nerve. J. Gen. Physiol. 59, 637–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B, 1973. Potassium channels in myelinated nerve. Selective permeability to small cations. J. Gen. Physiol. 61, 669–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B, 1977. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J. Gen. Physiol. 69, 497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B, 2001. Ion Channels of Excitable Membranes. Sinauer Associates, Sunderland. [Google Scholar]
- Hille B, 2012. Bertil Hille. Chapter 4. In: Squire LR (Ed.), The History of Neuroscience in Autobiography, Vol., 7. Oxford Univ. Press, New York, pp. 140–187. [Google Scholar]
- Hille B, 2022. A Life of Biophysics. Annu. Rev. Biophys 51, 1–16. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF, 1952a. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J. Physiol. 116, 449–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF, 1952b. The components of membrane conductance in the giant axon of Loligo. J. Physiol. 116, 473–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF, 1952c. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J. Physiol. 116, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF, 1952d. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF, Katz B, 1952. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J. Physiol. 116, 424–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Keynes RD, 1955. The potassium permeability of a giant nerve fibre. J. Physiol. 128, 61–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW, 1990. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 250, 533–538. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Pusch M, 2018. CLC chloride channels and transporters: structure, function, physiology, and disease. Physiol. Rev. 98, 1493–1590. [DOI] [PubMed] [Google Scholar]
- Jiang D, Banh R, Gamal El-Din TM, Tonggu L, Lenaeus MJ, Pomés R, Zheng N, Catterall WA, 2021. Open-state structure and pore gating mechanism of the cardiac sodium channel. Cell 184, 5151–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B, Leek AN, Solé L, Maverick EE, Levine TP, Tamkun MM, 2018. KV2 potassium channels form endoplasmic reticulum/plasma membrane junctions via interaction with VAPA and VAPB. Proc. Natl. Acad. Sci. U.S.A. 115, E7331–E7340. 10.1073/pnas.1805757115, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefauver JM, Ward AB, Patapoutian A, 2020. Discoveries in structure and physiology of mechanically activated ion channels. Nature 587, 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koishi R, Xu H, Ren D, Navarro B, Spiller BW, Shi Q, Clapham DE, 2004. A superfamily of voltage-gated sodium channels in bacteria. J. Biol. Chem. 279, 9532–9538. [DOI] [PubMed] [Google Scholar]
- Ling GN, 1962. A Physical Theory of the Living State: the Association-Induction Hypothesis; with Considerations of the Mechanics Involved in Ionic Specificity. Blaisdell Publishing, New York. [Google Scholar]
- Lockless SW, Zhou M, MacKinnon R, 2007. Structural and thermodynamic properties of selective ion binding in a K+ channel. PLoS Biol. 5, e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE, 1987. The βγ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325, 321–326. [DOI] [PubMed] [Google Scholar]
- McAllister RE, Noble D, Tsien RW, 1975. Reconstruction of the electrical activity of cardiac Purkinje fibres. J. Physiol. 251, 1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C, 1982. Open-state substructure of single chloride channels from Torpedo electroplax. Philos. Trans. R. Soc. Lond. B Biol. Sci. 299, 401–411. [DOI] [PubMed] [Google Scholar]
- Moore JW, Narahashi T, Shaw TI, 1967. An upper limit to the number of sodium channels in nerve membrane? J. Physiol. 188, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno CM, Dixon RE, Tajada S, Yuan C, Opitz-Araya X, Binder MD, Santana LF, 2016. Ca2+ entry into neurons is facilitated by cooperative gating of clustered CaV1.3 channels. Elife 5, e15744. 10.7554/eLife.15744, 2016 May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myeong J, Park CG, Suh BC, Hille B, 2021. Compartmentalization of phosphatidylinositol 4,5-bisphosphate metabolism into plasma membrane liquidordered/raft domains. Proc. Natl. Acad. Sci. U.S.A. 118, e202534311. 10.1073/pnas.2025343118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T, Moore JW, Scott WR, 1964. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. J. Gen. Physiol. 47, 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Shimizu S, Tanabe T, Taka i T., Kayano T, Ikeda T, Takahashi H, Nakayama H, Kanaoka Y, Minamino N, Kangawa K, Matsuo H, Raftery MA, Hirose T, Inayama S, Hayashida H, Miyata T, Numa S, 1984. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature 312, 121–127. [DOI] [PubMed] [Google Scholar]
- Papazian DM, Schwarz TL, Tempel BL, Jan YN, Jan LY, 1987. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science 237, 749–753. [DOI] [PubMed] [Google Scholar]
- Payandeh J, Scheuer T, Zheng N, Catterall WA, 2011. The crystal structure of a voltage-gated sodium channel. Nature 475, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipatpolkai T, Usher S, Stansfeld PJ, Ashcroft FM, 2020. New insights into KATP channel gene mutations and neonatal diabetes mellitus. Nat. Rev. Endocrinol. 16, 378–393. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE, 2006. An introduction to TRP channels. Annu. Rev. Physiol. 68, 619–647. [DOI] [PubMed] [Google Scholar]
- Ríos E, Gillespie D, Franzini-Armstrong C, 2019. The binding interactions that maintain excitation-contraction coupling junctions in skeletal muscle. J. Gen. Physiol. 151, 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WM, Jacobs RA, Hudspeth AJ, 1990. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J. Neurosci. 10, 3664–3684. 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter MM, Loring RH, 1985. Nicotinic acetylcholine receptors in vertebrate muscle: properties, distribution and neural control. Prog. Neurobiol. 25, 297–325. 10.1016/0301-0082(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Schneider MF, Chandler WK, 1973. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature 242, 244–246. [DOI] [PubMed] [Google Scholar]
- Shen H, Li Z, Jiang Y, Pan X, Wu J, Cristofori-Armstrong B, Smith JJ, Chin YKY, Lei J, Zhou Q, King GF, Yan N, 2018. Structural basis for the modulation of voltage-gated sodium channels by animal toxins. Science 362, eaau2596. 10.1126/science.aau2596. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Seagar MJ, Jones JF, Reber BF, Catterall WA, 1987. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc. Natl. Acad. Sci. U. S. A. 84, 5478–5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X-F, Bae C, Stix R, Fernandez AI, Huffer K, Chang T-H, Jiang J, Faraldo-Gómez JD, Swartz KJ, 2021. Structure of the Shaker Kv channel and mechanism of slow C-type inactivation. bioRxiv. 10.1101/2021.09.21.461258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S, 1987. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature 328, 313–318. [DOI] [PubMed] [Google Scholar]
- Terlau H, Heinemann SH, Stühmer W, Pusch M, Conti F, Imoto K, Numa S, 1991. Mapping the site of block by tetrodotoxin and saxitoxin of sodium channel II. FEBS Lett. 293, 93–96. [DOI] [PubMed] [Google Scholar]
- Tristani-Firouzi M, Sanguinetti MC, 2003. Structural determinants and biophysical properties of HERG and KCNQ1 channel gating. J. Mol. Cell. Cardiol. 35, 27–35. [DOI] [PubMed] [Google Scholar]
- Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP, 1988. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 11, 431–438. [DOI] [PubMed] [Google Scholar]
- van Goor MK, de Jager L, Cheng Y, van der Wijst J, 2020. High-resolution structures of transient receptor potential vanilloid channels: unveiling a functionally diverse group of ion channels. Protein Sci. 29, 1569–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit G, Avramescu RG, Chiang AN, Houck SA, Cai Z, Peters KW, Hong JS, Pollard HB, Guggino WB, Balch WE, Skach WR, Cutting GR, Frizzell RA, Sheppard DN, Cyr DM, Sorscher EJ, Brodsky JL, Lukacs GL, 2016. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol. Biol. Cell 27, 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivas O, Moreno CM, Santana LF, Hille B, 2017. Proximal clustering between BK and CaV1.3 channels promotes functional coupling and BK channel activation at low voltage. Elife 30 (6), e28029. 10.7554/eLife.28029, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Catterall WA, 2004. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis, 2004 Oct 5 Sci. STKE 2004 (253). 10.1126/stke.2532004re15. [DOI] [PubMed] [Google Scholar]
- Zhang K, Julius D, Cheng Y, 2021. Structural snapshots of TRPV1 reveal mechanism of polymodal functionality. Cell 184, 5138–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Huang G, Wu J, Wu Q, Gao S, Yan Z, Lei J, Yan N, 2019. Molecular basis for ligand modulation of a mammalian voltage-gated Ca2+ channel. Cell 177, 1495–1506 e12. [DOI] [PubMed] [Google Scholar]
- Zheng J, Trudeau MC, 2015. Handbook of Ion Channels. CRC Press. [Google Scholar]