Abstract
Finding reliable parameters to identify patients with heart failure (HF) that will respond to cardiac resynchronization therapy (CRT) represents a major challenge. We and others have observed post-translational modifications of Ryanodine Receptor (RyR) in several tissues (including skeletal muscle and circulating lymphocytes) of patients with advanced HF. We designed a prospective study to test the hypothesis that RyR1 glycation in circulating lymphocytes could predict CRT responsiveness in patients with non-ischemic HF. We enrolled 94 patients who underwent CRT and 30 individuals without HF, examining RyR1 glycation in peripheral lymphocytes at enrollment and after 1 year. We found that baseline RyR1 glycation independently predicts CRT response at 1 year after adjusting for age, diabetes, QRS duration and morphology, echocardiographic dyssynchrony, and hypertension. Moreover, RyR1 glycation in circulating lymphocytes significantly correlated with pathologic intracellular calcium leak. Taken together, our data show for the first time that RyR1 glycation in circulating lymphocytes represents a novel biomarker to predict CRT responsiveness.
Keywords: CRT, glycosylation, heart failure, post-translational modifications, RyR
Cardiac resynchronization therapy (CRT) is an effective treatment for patients with heart failure (HF).1-3 HF patients have been shown to exhibit a pathologic post-translational remodeling of Ryanodine Receptor calcium release channels (RyRs) in different organs and cell types, including skeletal muscle and circulating lymphocytes, mirroring the detrimental remodeling of cardiac RyRs.4
Recent evidence suggests that RyR glycation could lead to cardiac mitochondrial damage.5 However, the actual significance of RyR glycation in clinical practice has been poorly investigated.
We designed a prospective cohort study to test the hypothesis that glycation of type 1 RyR (RyR1) in circulating lymphocytes could predict the response to CRT in patients with non-ischemic HF. We prospectively enrolled 94 patients with non-ischemic HF who underwent CRT (all patients fulfilled the CRT guidelines criteria); 30 individuals without HF were characterized as well. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments and approved by the Ethical Committee; a written informed consent was obtained from each participant.
We examined RyR1 glycation in peripheral lymphocytes at enrollment and after 1 year using established assays: briefly, peripheral lymphocytes were isolated as described,4 RyR glycation was measured via a sandwich ELISA (Hycult),5 and intracellular calcium leak was determined as we described.6,7 Non-responders to CRT were defined after 1 year as patients that did not improve their clinical composite score.3
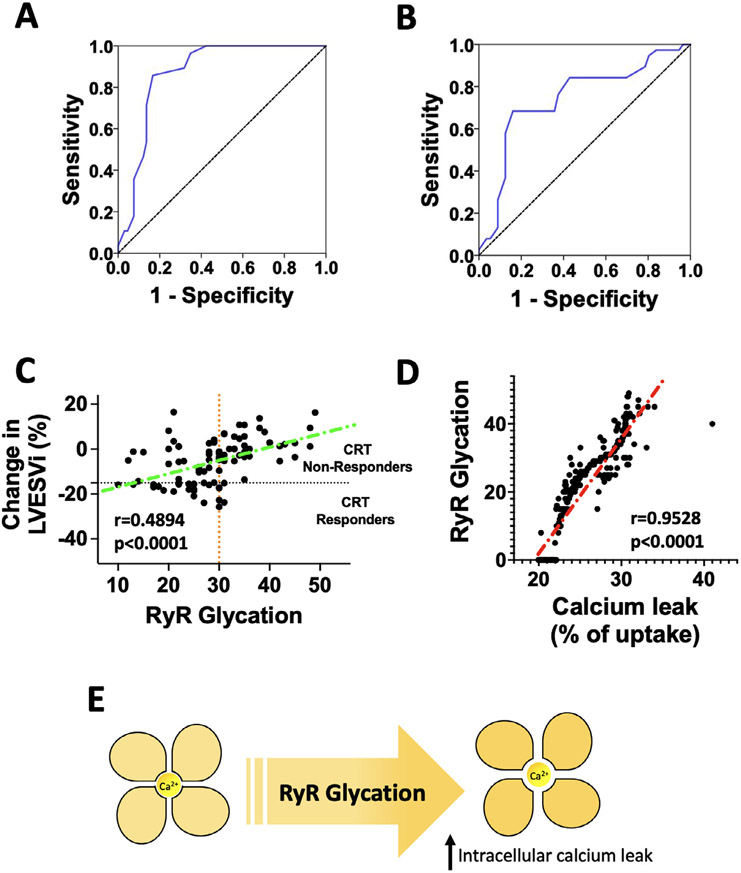
We found that baseline RyR1 glycation was significantly lower in circulating lymphocytes isolated from CRT responders compared to non-responders (Table 1); equally important, RyR1 glycation in lymphocytes of CRT responders at follow-up was significantly reduced compared to pre-CRT levels (Table 1). No major differences in terms of pharmacologic treatment were noted between groups. By analyzing receiver operating characteristic (ROC) curves, RyR1 glycation > 30% was identified as the optimum cut-off (maximum Youden’s index) to predict a negative response to CRT (AUC: 0.859), as shown in Figure 1A. In a multivariate logistic regression analysis, we observed that baseline RyR1 glycation in peripheral lymphocytes independently predicts CRT response at 1 year [Odds ratio (OR): 1.197, 95% C.I.: 1.096-1.306; p < .001] after adjusting for age, diabetes, hypertension, left ventricular ejection fraction, QRS duration and morphology, presence of septal flash (an established marker of dyssynchrony2), New York Heart Association class, and N-terminal pro hormone B-type natriuretic peptide.
Table 1.
Main Characteristics of the Study Population
| Non-HF | Baseline |
Follow-up |
|||
|---|---|---|---|---|---|
| CRT Responders | CRT Non-Responders | CRT Responders | CRT Non-Responders | ||
| n | 30 | 65 | 29 | 65 | 29 |
| Age (years) | 60.5±17.8 | 59.2±9.7 | 60.76±5.7 | 60.2±9.7 | 61.76±5.7 |
| Sex (Female, %) | 40.0 | 43.1 | 34.5 | 43.1 | 34.5 |
| BMI (kg/m2) | 24.6±3.2 | 24.6±2.61 | 23.78±2.2 | 24.8±2.3 | 23.6±2.0 |
| HR (bpm) | 75.4±8.2 | 76.4±8.67 | 77.31±8.2 | 76.5±8.5 | 78.2±8.5 |
| QRS (ms) | 93.1±6.5 | 166.6±9.9 | 165.56±12.9 | 154.3±9.2b | 155.7±12.1b |
| Septal flash (%) | - | 76.6 | 42.9a | - | - |
| Apical rocking (%) | - | 85.5 | 45.8a | - | - |
| MWD ≥860 mmHg·(%) | - | 81.8 | 26.1a | - | - |
| NYHA (I-IV) | N/A | 2.88±0.7 | 2.82±0.6 | 2.66±0.67b | 2.93±0.52a,b |
| LVEF (%) | 61.1±7.2 | 27.1±2.8 | 27.3±3.3 | 33.0±4.0b | 27.8±4.3 |
| LVEDVi (ml/m2) | 28.3±2.7 | 132.8±14.9 | 128.03±17.3 | 119.3±21.8b | 130.1±14.1 |
| LVESVi (ml/m2) | 17.5±1.7 | 80.7±8.2 | 77.4±8.2 | 73.7±10.7 | 79.0±10.9 |
| LAVi (ml/m2) | 21.5±2.1 | 32.2±4.1 | 32.9655±5.1 | 27.1±5.4b | 34.2±6.1a |
| Mitral Regurgitation (1-4) | 0.43±0.5 | 2.1±0.8 | 2.3±1.2 | 1.7±0.9 | 2.3±1.0 |
| Diabetes (%) | 13.3 | 32.3 | 31.0 | 32.3 | 31.0 |
| Hypertension (%) | 30.0 | 56.9 | 48.3 | 55.4 | 48.3 |
| Hyperlipidemia (%) | 40.0 | 55.4 | 55.2 | 55.4 | 55.2 |
| Kidney failure (%) | 3.33 | 15.4 | 13.8 | 15.4 | 17.2a,b |
| LV lead position (n, %) | |||||
| -Postero-latero-basal | N/A | N/A | N/A | 38 (58.5) | 17 (58.6) |
| -Postero-latero-apical | N/A | N/A | N/A | 4 (6.15) | 2 (6.8) |
| -Antero-latero-basal | N/A | N/A | N/A | 22 (33.8) | 10 (34.5) |
| -Antero-latero-apical | N/A | N/A | N/A | 1 (1.54) | 0 (0) |
| Biventricular pacing | N/A | N/A | N/A | 92.7±4.7 | 90.9±5.4 |
| β Blockers (%) | 6.7 | 90.8 | 93.1 | 87.7 | 96.6a |
| ACEi/ARBs (%) | 16.7 | 92.3 | 96.6 | 89.2 | 96.6 |
| Diuretics (%) | 10.0 | 86.2 | 86.2 | 76.9b | 89.7a |
| QoL | N/A | 39.2±7.17 | 38.76±9.3 | 34.5±6.6b | 43.3±10.5a,b |
| 6-min walk (m) | N/A | 301.81±25.8 | 301.38±25.02 | 335.5±42.9b | 295.3±22.7a |
| NT-proBNP (pg/ml) | 77.9±29.2 | 1500.1±497 | 1656.9±443 | 1166.1±387b | 1766.9±534 |
| RyR Glycation (%) | 3.46±6.5 | 26.05±7.7 | 35.03±5.54a | 23.3±6.94b | 38.0±4.81a,b |
ACEi, Angiotensin converting enzyme inhibitors; ARBs, Angiotensin II receptor blockers; BMI, Body mass index; CRT, Cardiac resynchronization therapy; HR, heart rate; LAVi, Left atrium volume indexed by body surface; LV, Left ventricle; LVEDVi, Left ventricular end-diastolic volume indexed by body surface; LVEF, Left ventricular ejection fraction; LVESVi, Left ventricular end-systolic volume indexed by body surface; MWD, Myocardial work difference, lateral-to-septal (missing in 16 patients); NT-proBNP, N-terminal pro hormone B-type natriuretic peptide; NYHA, New York Heart Association (both NYHA and mitral regurgitation outcomes were also confirmed when considered as categorical variables); QoL, Quality of life (Minnesota living with HF);. QRS configuration was not significantly different between responders and non-responders at baseline (Left bundle branch block, LBBB, 76.9% vs 62.1%; Intraventricular conduction delay, IVCD, 18.5% vs 27.6%; RBBB, Right bundle branch block, 4.6% vs 10.3%). The presence or absence of septal flash was missing in 2 patients; the presence or absence of apical rocking was not assessed in 15 patients.
Data are means ± SD or percent.
p < .05 vs CRT-Responders (defined according to the clinical composite score)
p < .05 vs Baseline; Fisher exact test for dichotomous variables, Student's t test or Mann-Whitney U test for continuous variables (the normal distribution was verified using the Shapiro-Wilk test).
Figure 1.
(A) Determination of the optimal cut off value of RyR glycation to determine CRT response using receiver operating characteristic (ROC) curve defining CRT response according to the clinical composite score (Panel A, AUC: 0.859; 95% C.I.: 0.784-0.933; Youden’s index at 30: 0.539; Sensitivity for glycation >30: 0.831; 95% C.I.: 0.784-0.933; Specificity for glycation >30: 0.827; 95% C.I.: 0.78-0.874) or volumetric parameters (Panel B, AUC: 0.743; 95% C.I.: 0.636-0.850; Youden’s index at 30: 0.523; Sensitivity for glycation >30: 0.839; 95% C.I.: 0.806-0.872; Specificity for glycation >30: 0.684; 95% C.I.: 0.633-0.735). (C) Correlation between RyR glycation and left ventricular reverse remodeling (the black dotted line represents 15% reduction in left ventricular end-systolic volume index (LVESi), while the orange dotted line represents the proposed cut-off value for RyR glycation (95% C.I.: 0.3129 to 0.6333); (D) Correlation between RyR glycation and intracellular calcium leak (95% C.I.: 0.9393 to 0.9633). E) Schematic drawing representing the tetrameric structure of RyR and how its glycation increases intracellular calcium (Ca2+) leak through the channel pore.
These findings were confirmed when CRT response was defined according to volumetric parameters (i.e., decrease in left ventricular end-systolic volume ≥ 15% and/or absolute increase of 5% in left ventricular ejection fraction8), both in terms of ROC curve (Figure 1B) and multivariate logistic regression analysis [OR: 1.103, 95% C.I.: 1.035-1.175; p = .002]; strikingly, we also found that RyR glycation significantly correlated with echocardiographic reverse remodeling following CRT (Figure 1C).
Corroborating these results, we show that RyR1 glycation in circulating lymphocytes significantly correlates with intracellular calcium leak (R: 0.9508, 95% C.I.: 0.9356 to 0.9624, p < .0001, Figure 1D).
To the best of our knowledge, this is the first study showing that CRT response in HF patients can be predicted by the level of baseline RyR1 glycation in peripheral lymphocytes, introducing a novel independent and reliable biomarker of CRT responsiveness. We acknowledge that the complexity of the technique to quantify RyR glycation, compared with other biomarkers, might represent a limitation for its wide implementation in clinical practice. Our findings also indicate that CRT responders exhibit a significantly reduced RyR1 glycation in lymphocytes compared to baseline and to CRT non-responders. Consistent with our results, previous preclinical research had shown that CRT rescued subcellular structural alterations and enhanced myofilament phosphorylation of components of the Z-disk and M-band.9 Albeit the exact mechanisms by which CRT delivers its benefit are not clearly established and the exact physiopathology of RyR glycation in HF requires further investigation, several potential mechanisms could be speculated, including a regulation of the left ventricular coordination and a modulation of myocardial remodeling and cardiac hemodynamics. Furthermore, since RyRs are responsible for efficient electromechanical coupling,4,5,10 post-translational modifications of RyR, including its glycation, could be linked to an altered synchrony of calcium release, thereby impairing myocardial contractility, worsening diastolic function, and/or triggering life-threatening arrhythmias.
Acknowledgments
We thank Dr. X. Wang for helpful discussion and technical assistance.
The Santulli’s Lab is supported in part by the National Institutes of Health (NIH: R01-HL146691, R01-DK033823, R01-HL159062, R01-DK123259, R56-AG066431, T32-HL144456, and R00-DK107895, to G.S.), by the Diabetes Action Research and Education Foundation (to G.S.), by the Irma T. Hirschl and Monique Weill-Caulier Trusts (to G.S.), and by the American Heart Association (AHA-20POST35211151 to J.G. and AHA-21POST836407 to S.S.J.).
Footnotes
Disclosure statement
The authors declare that they have no conflict of interest.
References
- 1.Aalen JM, Donal E, Larsen CK, et al. Imaging predictors of response to cardiac resynchronization therapy: left ventricular work asymmetry by echocardiography and septal viability by cardiac magnetic resonance. Eur Heart J 2020;41:3813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calle S, Delens C, Kamoen V, De Pooter J, Timmermans F. Septal flash: at the heart of cardiac dyssynchrony. Trends Cardiovasc Med 2020;30:115–22. [DOI] [PubMed] [Google Scholar]
- 3.Varma N, Boehmer J, Bhargava K, et al. Evaluation, management, and outcomes of patients poorly responsive to cardiac resynchronization device therapy. J Am Coll Cardiol 2019;74:2588–603. [DOI] [PubMed] [Google Scholar]
- 4.Kushnir A, Santulli G, Reiken SR, et al. Ryanodine receptor calcium leak in circulating B-lymphocytes as a biomarker in heart failure. Circulation 2018;138:1144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruiz-Meana M, Minguet M, Bou-Teen D, et al. Ryanodine receptor glycation favors mitochondrial damage in the senescent heart. Circulation 2019;139:949–64. [DOI] [PubMed] [Google Scholar]
- 6.Santulli G, Pagano G, Sardu C, et al. Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J Clin Invest 2015;125:1968–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lombardi A, Trimarco B, Iaccarino G, Santulli G. Impaired mitochondrial calcium uptake caused by tacrolimus underlies beta-cell failure. Cell Commun Signal 2017;15:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park MY, Altman RK, Orencole M, et al. Characteristics of responders to cardiac resynchronization therapy: the impact of echocardiographic left ventricular volume. Clin Cardiol 2012;35:777–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirk JA, Holewinski RJ, Kooij V, et al. Cardiac resynchronization sensitizes the sarcomere to calcium by reactivating GSK-3beta. J Clin Invest 2014;124:129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kansakar U, Varzideh F, Jankauskas SS, Gambardella J, Trimarco B, Santulli G. Advances in the understanding of excitation-contraction coupling: the pulsing quest for drugs against heart failure and arrhythmias. Eur Heart J Cardiovasc Pharmacother 2021;7:e91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]