Abstract
Background
Refractory epilepsy confers a considerable lifetime risk of sudden unexplained death (SUDEP). Mechanisms may overlap with sudden cardiac death (SCD), particularly regarding QTc prolongation. Guidelines in the US do not mandate the use of ECG in diagnostic evaluation of seizures or epilepsy.
Objective
We aimed to determine the frequency of ECG use, QT prolongation and whether it predicts mortality in patients with seizures.
Methods
We performed a retrospective cohort study including all patients seen at Mayo Clinic in Rochester, MN from January 1st 2000 to July 31st 2015 with index evaluation for seizure or epilepsy. Patients with an ECG were categorized by presence of a prolonged QT interval with a primary endpoint of all-cause mortality after the 15-year observation period.
Results
Optimal cut-off QT intervals most predictive of mortality were identified. Median age was 40.0 years. An ECG was obtained in 18,222 (57.4%) patients. After excluding patients with confounding ECG findings, primary prolonged QT intervals were seen in 223 (1.4%) cases, similar to general population. Kaplan-Meier analysis demonstrated a significant increase in mortality (Cox HR 1.90; 95%CI: 1.76, 2.05) for prolonged optimal cut-off QT, maintained after adjustments for age, Charlson Comorbidity Index and sex (HR 1.48; 95%CI: 1.37, 1.59).
Conclusions
Use of ECG in diagnostic workup of patients with seizures is poor. A prolonged optimal cut-off QTc interval predicts all-cause mortality in patients evaluated for seizure and those diagnosed with epilepsy. We advocate the routine use of a 12-lead ECG at index evaluation in patients with seizure or epilepsy.
Keywords: QT prolongation, ECG, seizure, epilepsy, SUDEP, sudden death
Graphical Abstract
1. Introduction
Epilepsy affects 1–2% (3–6 million) of Americans with significant morbidity and mortality 1–4. Patients with refractory epilepsy have a 35% cumulative lifetime risk of sudden unexpected death in epilepsy (SUDEP) likely due to an underlying (genetic or acquired) ion channelopathy and/or altered neural circulatory and respiratory control. Sudden cardiac arrest (SCA) claims over 350,000 lives in the US per annum; while the majority of cases in the general population are due to coronary artery disease, in those under the age of 40 years and with structurally normal hearts, ion channelopathies, including long QT syndrome (LQTS), Brugada syndrome (BrS), and catecholaminergic polymorphic ventricular tachycardia are significant contributors 5–7. LQTS can present with palpitations, syncope, SCA, and seizures 8. Similarly, BrS and CPVT are also associated with familial epilepsy, seizure-like episodes, as well as anoxic seizures secondary to arrhythmias 9, 10. European guidelines recommend a 12-lead electrocardiogram (ECG) at first presentation for all those with suspected or confirmed seizures or epilepsy 11, 12. However, no such guidelines exist in the US due in part to a lack of evidence, leading to clinician discretion with a wide variation in practice reflective of the referral pathways for assessment (e.g. patients can self-refer to a specialist without involving primary care). Furthermore, there are no data on the routine use of the ECG or the types of ECG abnormalities seen in patients with seizures from the US.
Prolonged QT intervals on surface ECGs are associated with increased risk of all-cause mortality in the general population and in those with established cardiovascular disease 13–15. Given ECGs are readily available, easy to perform, established, robust, reproducible and cost-effective, their use in persons with seizures and epilepsy may have benefit. Aside from being useful in the evaluation of patients with seizures to identify conduction system disease, cardiovascular disease, diagnosing LQTS and BrS, and monitoring toxic effects of medications16, the utility in predicting mortality in persons with seizures or epilepsy is unknown. Specifically, QT prolongation may be a useful biomarker of increased mortality in those evaluated for seizures and those with a diagnosis of epilepsy which may have implications for SUDEP risk.
The aims of this study were to determine the frequency of ECG use in patients undergoing index evaluation for first seizure or epilepsy seen at a tertiary care referral center, describe abnormalities on the ECG, determine the frequency of QT prolongation, and determine whether QT prolongation predicts mortality in patients with index evaluation for seizure or epilepsy.
2. Methods
Study population
This study complies with the Declaration of Helsinki, the research protocol has been approved by the Mayo Clinic Institutional Review Board and all patients have authorized the use of the data presented in this paper for research purposes. The data will be made available upon reasonable request to the corresponding author. The Mayo Clinic Epilepsy Registry (MCER) which includes all cases evaluated for seizures and/or epilepsy seen from January 1st, 1976 to present (n=59,499) was queried. In this retrospective analysis, we focused on all cases seen at Mayo Clinic in Rochester, MN with index evaluation for seizure or epilepsy between January 1st, 2000 and July 31st, 2015.
International Classification of Diseases and International Classification for Diseases, version 9 (ICD-9) codes utilized to identify the appropriate patients Online Table 1. A new incident case was defined as ≥2 included diagnostic codes (in any combination) ≥30 days apart and within 1 year, as this strategy reduces false positives from ambiguous coding issues. Our cohort was defined using diagnosis codes intended to identify a ‘sensitive population worked-up for first seizures or suspected epilepsy (regardless of whether the final diagnosis was epilepsy) as this assesses the utility of ECG in the diagnostic evaluation. This group includes all those evaluated for possible seizures including those with atypical spells, referred to as Cohort 1. Two subsets were created and analysed: a more specific population with a final diagnosis of epilepsy confirmed by electroencephalographically (EEG) and meeting ILAE criteria, called Cohort 2; and a local population subset by Zip Code (to minimize effects of referral bias) called Cohort 3. In brief, Cohort 1 was based on ICD-9 criteria used in our area by the Rochester Epidemiology Project. Cohort 2, a specific population was refined from Cohort 1 by one of the study staff (REA) masked to codeset, and randomly checking 100 cases at a time to determine if they had epilepsy as International League of Epilepsy criteria.17 The initial specificity was <70% and we wanted a specificity of >90% to have epilepsy. The codeset was refined to remove unclear codes until a specificity of 92% epilepsy achieved Online Table 2. Cohort 3, was taken from Cohort 1, restricted to the local population by Zip code. See methods and results reported in the online supplemental material for details.
3. Results
Demographics and ECG use
Table 1 includes baseline characteristics for the study cohorts Online Table 3. Figure 1 illustrates the case selection process. A total of 31,732 patients were seen with index evaluation for seizure or epilepsy during the specified timeframe (Jan. 1st, 2000-Jul. 31st, 2015). Only 18,222 (57.4%) had a surface 12-lead ECG at index presentation. Median (25th, 75th) age was 40.0 (19.0, 57.9) years and was similar to those in the MCER. Patients receiving an ECG were significantly older, with a difference in median age by 26.8 years (p<0.0001). The median (25th, 75th) age of patients receiving an ECG was 51.1 (33.5, 65.9) years; 55.1% were female. The majority of participants were White. The median follow-up period was 1.2 years with a total person-years of follow-up of 50,375.
Table 1:
Baseline Characteristics of Cases in the Mayo Clinic Epilepsy Registry and the Study Cohorts.
| Variable | MCER (1976–2017) |
Cohort 1 (Sensitive) (Jan. 1, 2000-Sep. 30, 2015) |
Cohort 2 (Specific) (Jan. 1, 2000-Sep. 30, 2015) |
||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n=59,499 | All n=29,760 |
Without ECG n=13,510 |
With ECG n=16,250 |
All n=6,290 |
Without ECG n=3,514 |
With ECG n=2,776 |
|
| Age at index visit Median (25th, 75th centile) years | 40.4 (19.8, 60.1) | 40.0 (19.0, 57.9) | 24.3 (8.0, 43.4) | 51.1 (33.5, 65.9) | 28.6 (10.7, 50.2) | 16.5 (5.8, 33.9) | 47.7 (27.7, 63.3) |
| Male sex n (%) | 28,463 (47.8) | 13,684 (46 .0) | 6,394 (47. 3) | 7,290 (44.9) | 3,219 (51.2) | 1,815 (51. 7) | 1,404 (50.6) |
| Height mean ±SD cm | 160±30 | 160±30 | 140±40 | 170±20 | 150±40 | 140±40 | 160±30 |
| Weight mean ±SD kg | 70.6±29.7 | 68.1±30.8 | 56.3±33.8 | 76.6±25.2 | 61.3±32.6 | 50.7±33.1 | 73.6±27.2 |
| BMI Median (25th, 75th centile) kg/m2 | 26.4 (21.2, 30.4) | 25.82 (20.4, 30.0) | 22.3 (17.5, 28.0) | 26.6 (22.8, 31.1) | 23.8 (18.5, 28.8) | 20.8 (16.8, 26.8) | 26.0 (22.0, 30.5) |
| White ethnicity n (%) | 45,045 (75.7) | 24,693 (83.0) | 10,827 (80.1) | 13,866 (85.8) | 5,258 (83.6) | 2,901 (82.6) | 2,357 (84.9) |
| Smoking history available no. (%) | 30,177 (50.7) | 17,866 (60.0) | 6,627 (49.0) | 11,239 (69.1) | 4,146 (65.9) | 1,979 (56.3) | 2,167 (78.0) |
| Current smoker no. (%) | 7,896 (26.2) | 4,889 (27.4) | 1,660 (25.0) | 3,229 (28.7) | 1,011 (24.4) | 390 (19.7) | 621 (28.7) |
| Past smoker no. (%) | 6,088 (20.2) | 3,420 (19.1) | 869 (13.1) | 2,551 (22.7) | 649 (15.7) | 179 (9.0) | 470 (21.7) |
| Never smoker no. (%) | 16,193 (53.7) | 9,557 (53.5) | 4,098 (61.8) | 5,459 (48.6) | 2,486 (60.0) | 1,410 (71.2) | 1,076 (49.7) |
| CCI mean ±SD (range) | 1.0±2.0 (0.0–19.0) | 0.9±1.9 (0.0–18.0) | 0.4±1.1 (0.0–15.0) | 1.4±2.3 (0.0–18.0) | 0.9±2.0 (0.0–18.0) | 0.4±1.0 (0.0–10.0) | 1.6±2.6 (0.0–18.0) |
All data points were not available for the entire sample population
BMI, body mass index; CCI = Charlson Comorbidity Index; ECG, electrocardiogram; MCER, Mayo Clinic Epilepsy egistry; SD, standard deviation
Figure 1. Selection of the Study Cohorts.
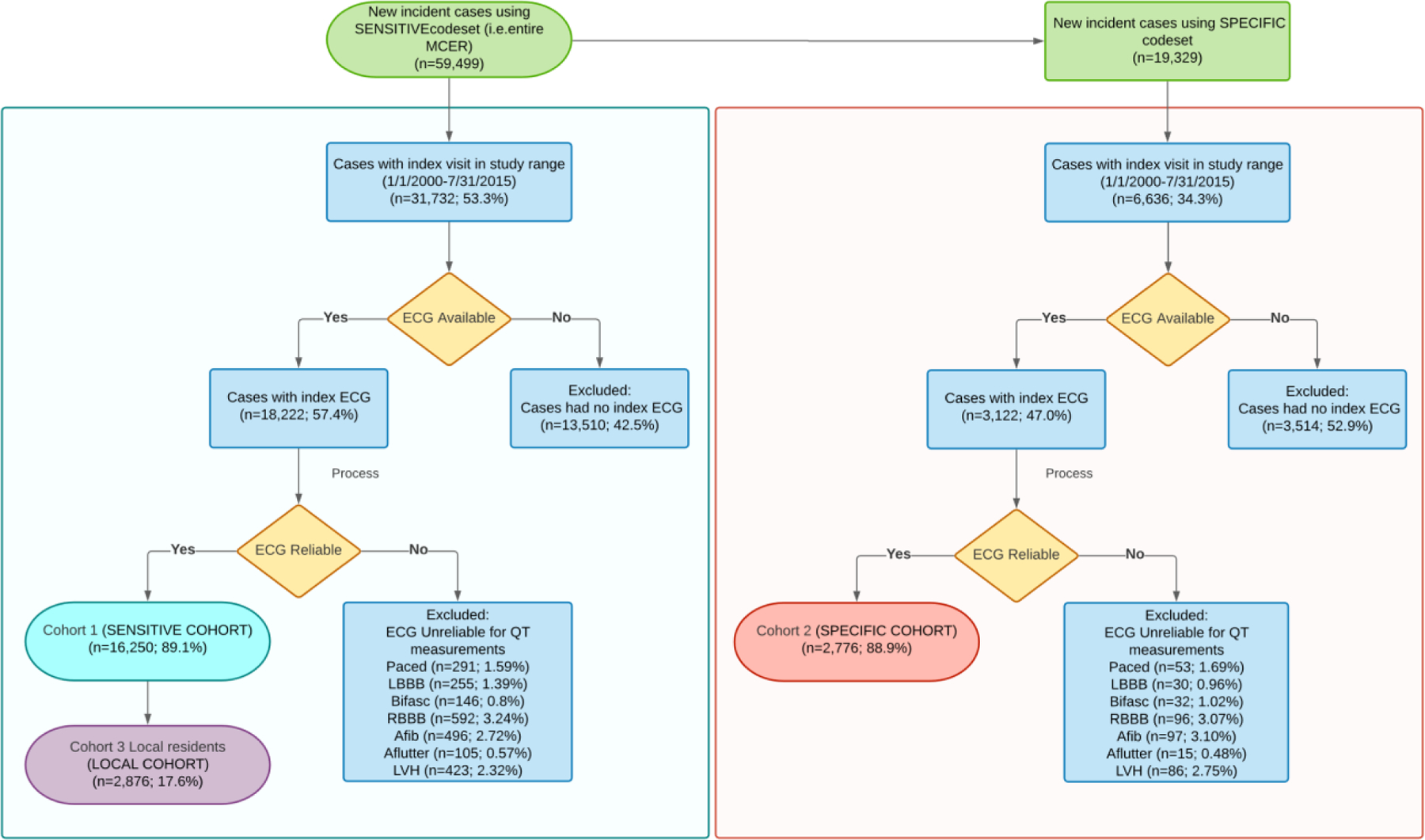
AFib = atrial fibrillation; Aflutter = atrial flutter; bifasc = bifascicular block; LBBB = left bundle branch block; LVH = left ventricular hypertrophy; Paced = ventricular paced rhythm; RBBB = complete right bundle branch block. Note: some patients had multiple exclusion criteria.
ECG characteristics/abnormalities
Table 2 describes the ECG characteristics and abnormalities identified with Online Table 4 for the rest of the cohorts. The mean heart rate, PR interval, and QRS duration were 76.3, 157, and 89.4 ms. Primary prolonged QT intervals were seen in 223 (1.4%) cases.
Table 2:
ECG Findings
| Variable | Cohort 1 (Sensitive) n=16,250 |
|---|---|
| ECG Findings | |
| Heart rate mean ±SD bpm | 76.3±19.6 |
| PR interval mean ±SD ms | 157±32 |
| QRS duration mean ±SD ms | 89.4±11.9 |
| QTc mean ±SD ms | 428.5±27.7 |
| Primary Prolonged QT, n (%) | 223 (1.4) |
| ECG Abnormalities* | |
| Paced rhythm, n (%) | 291 (1.6) |
| LBBB, n (%) | 255 (1.4) |
| Bifascicular block, n (%) | 146 (0.8) |
| RBBB, n (%) | 592 (3.2) |
| Atrial fibrillation, n (%) | 496 (2.7) |
| Atrial flutter, n (%) | 105 (0.6) |
| LVH, n (%) | 423 (2.3) |
ECG Abnormalities were considered exclusion criteria; therefore, these patients were not included in QTc analysis.
LBBB, left bundle branch block; LVH, left ventricular hypertrophy; RBBB, complete right bundle branch block
Prolonged QT interval at index evaluation
Table 3 describes the summative results of Kaplan-Meier analysis based on presence of a prolonged QT interval with Online Table 5 for all cohorts. This analysis revealed a significant difference in all-cause mortality with an unadjusted hazard ratio (HR) of 1.75 (95% CI 1.39, 2.21). Statistical analysis revealed the optimal QTc cutpoint to be ≥448 ms with an associated all-cause mortality HR of 1.90 (95% CI 1.76, 2.05) (Table 3). Figure 2 depicts the Kaplan-Meier survival plot for both the primary prolonged QT definition (>500 ms) and the prolonged optimal cut-point QT analysis with Online Figure 1 and Online Figure 2 for the other cohorts
Table 3:
All-Cause Mortality Stratified by Prolonged QT Interval
| n | Deaths | Median survival in years | Unadjusted HR (95% CI) | p-value | Adjusted HR (95% CI)* | p-value | |
|---|---|---|---|---|---|---|---|
| Primary Definition | |||||||
| Not Prolonged | 16,027 | 3,115 | 11.7 | ||||
| Prolonged | 223 | 73 | 7.9 | 1.75 (1.39, 2.21) | <0.0001 | 1.61 (1.27, 2.03) | <0.0001 |
| Optimal Cutpoint | |||||||
| QTc <448 ms | 12,834 | 2,194 | 12.9 | ||||
| QTc ≥448 ms | 3,416 | 994 | 7.6 | 1.90 (1.76, 2.05) | <0.0001 | 1.48 (1.37, 1.59) | <0.0001 |
Adjusted HR for age at index visit, sex and Charlson Comorbidity Index
HR, hazard ratio
Figure 2. Kaplan-Meier Survival Plots of Mortality as Predicted by a Prolonged QT Interval for Patients with index evaluation for seizure or epilepsy (Cohort 1; ‘sensitive’).
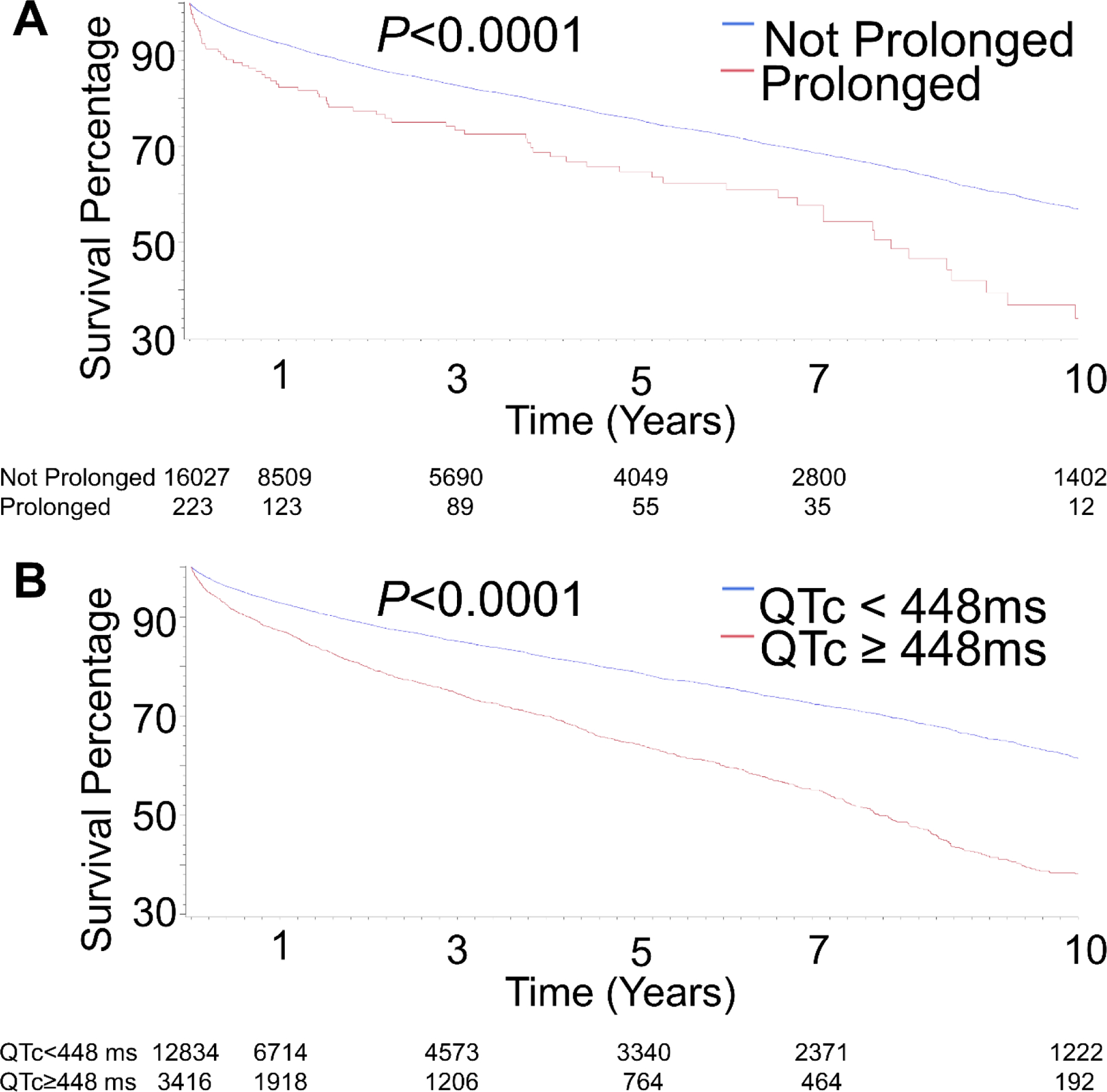
A. comparing QT≥500 vs QT<500 ms. B. comparing patients based on the optimal cutpoint assessment.
Cox proportional hazards modelling and all-cause mortality
Univariable Cox regression modelling demonstrated age, male sex and CCI score were strongly predictive. When adjusted for age, sex and CCI, a primary prolonged QT interval maintained its predictive significance with an adjusted HR of 1.61 (95% CI 1.27, 2.03) (Table 3, Online Table 6).
Subset with final diagnosis epilepsy and a local population subset
The same methods of analysis were used for the subset with a final diagnosis of epilepsy and the local population. Unadjusted HR for the “specific” cohort 2 and “local population” cohort 3 were 1.2 (95% CI 0.70, 1.94) unsignificant and 2.06 (95% CI 1.47, 2.89) for the primary prolonged QT. Statistical analysis revealed an optimal cutpoint at QTc ≥445 ms for the “specific” cohort 2 and “local population” cohort 3 in predicting all-cause mortality, with HR of 1.99 ( 95% CI 1.71, 2.324) and 2.13 (95% CI 1.86, 2.44). (see online supplemental section).
4. Discussion
To our knowledge, this is the first study to report the frequency and utility of 12-lead ECG during index evaluation in persons with index evaluation for first seizure and/or subsequently diagnosed epilepsy in a US population and has identified important findings. First, only 57.4% of suspected seizure cases evaluated received a 12-lead ECG at index evaluation. Second, in those who do obtain ECG the most frequent abnormality is AF with a frequency of at least 2.7% similar to the local prevalence. Third, the frequency of primary QT prolongation at index evaluation is 1.4%. Fourth, a prolonged optimal cut-off QTc interval in this population is a powerful predictor of all-cause mortality, with a cut-off of ≥448 ms being associated with a HR of 1.90 (p<0.0001). Considered together, these findings have important clinical implications for all-cause mortality.
Our findings demonstrate a 12-lead ECG was performed on only 57.4% of patients most likely reflective of a lack of guideline-mandated ECG acquisition and referral pathways in the US. Patients who develop seizures may be evaluated directly by non-cardiologists. Most patients with a suspected first seizure are not likely to see a cardiologist until neurologic contributors have been ruled out. Similarly, atypical seizures, atonic drop attacks, and absence seizures could have instead been evaluated by a cardiologist initially and would be much more likely to receive a 12-lead ECG. Older patients were more likely to have had an ECG, probably reflecting provider concern over cardiovascular pathology. However, while age was an independent risk factor for subsequent mortality, those in younger age groups also remain at higher risk for mortality and should be considered for ECG screening.
There are several good reasons to advise 12-lead ECG should be considered mandatory in all cases of suspected first seizures at index evaluation. Cardiovascular diseases, of which the most important are channelopathies of LQTS and BrS, can have dual seizure and arrhythmia presentations, secondary arrhythmic seizures, or may be misdiagnosed due to seizure-like episodes 18. These conditions should be considered as “no-miss” cases because they can lead to sudden death; LQTS in particular is manageable lowering the risk of sudden death. Other comorbid cardiovascular disorders may also be identified as well as cardiac conduction system disease which can lead to brady-arrhythmia and SCD. Furthermore, ECGs can be helpful in identifying and ameliorating secondary causes of QT prolongation, such as electrolyte abnormalities and drug therapy, further supporting the case for initial and serial ECG monitoring in patients with suspected seizures and epilepsy 19.
The frequency of AF observed is similar to the general population, and is consistent with the age-dependent incidence and prevalence of AF 20. Comorbid AF is associated with significant morbidity and mortality and has recently been shown to have an association with SCD. This risk may be additive and directly applicable to understanding the causative factors of SUDEP, particularly in cases with post-infarct epilepsy that are also subject to significant SUDEP risk.3, 21
A prolonged optimal cut-off QTc has been shown to predict all-cause mortality in multiple populations, reflecting potential utility as a biomarker 13–15, 22–24. This is the first study of our knowledge to report utility in a population evaluated for seizures and a subset with a final diagnosis of epilepsy. Interestingly, the optimal cutoff of ≥448 ms our data identified is similar to that reported by Schwartz et al 40 years ago24. Similarly, a population-based study of 5,888 individuals ≥ 65-years-old who were participants in Cardiovascular Health Study, were followed for a mean 6.7 ±2.3 years.13 A QTc >450 ms on ECG at enrolment in both males and females, whether with known CVD or negative for CVD, had all-cause mortality relative risk of 2.3 (95%CI 1.6–3.3). This is analogous to troponin utilization as a biomarker in predicting mortality in populations with or without cardiovascular disease. Prolongation of the QT interval can be transient and most cases do not reflect genetic LQTS. Our data show a prolonged optimal cutoff QTc on ECG in patients with initial evaluation for seizure and/or epilepsy independently predicts risk of all-cause mortality. Among patients who received an index ECG, prevalence of a primary prolonged QT interval is higher than the expected frequency of 1% seen in the general population but lower than that in cohorts studies: 7.4% as seen in the Cardiovascular Health Study13 and 2% seen in the third National Health and Nutrition Examination Survey (NHANES)14. This is similar to the 1.2% frequency observed in a study from our institution which focused on ECGs performed in the Emergency Department 25. This high frequency of primary QT prolongation persisted in those who were found to have a final diagnosis of epilepsy (see “specific” cohort in the online supplement). Many AEDs and drugs used to treat depression, a frequent finding in persons with epilepsy, may also act synergistically to prolong the QT interval.
One challenge with using our definition for prolonged QT interval is that while this threshold is more specific to an underlying rhythm disorder, it may contribute to an elevated false negative rate. The QT interval varies with age and sex as well as baseline neural circulatory control. To be considered prolonged, the corrected values must be >99th centile for age and sex 26. When using time-dependent analysis, a QTc of ≥448 ms yielded an adjusted HR of 1.90, suggesting that even modest QTc prolongations are predictive of mortality. Additionally, we excluded patients with known electrocardiographic abnormalities and used a more stringent QT cutoff for patients with a prolonged QRS duration to prevent the contribution of conduction delay on QT prolongation and to allow more direct comparison to the aforementioned emergency department study. However, this strategy may have underestimated the mortality risk associated with a prolonged QT interval.
Arrhythmias using Implantable Loop Recorders
A recent study by Serdyuk .et al27 reported using implantable loop recorders to detect ictal rhythm abnormalities and found no significant malignant arrhythmias in patients who died on follow up. Of note only 5 patients had SUDEP and only one of them had a borderline prolonged QT interval of 453 ms, and borderline prolonged QT was noted in 16 patients overall out of 193 patients with drug resistant epilepsy. A prolonged QT interval may be a biomarker of advanced disease rather than directly leading to Torsade’s de pointes and death. It is our belief this is analogous to cardiac troponins and mortality, irrespective of an acute coronary syndrome. Even in genetic long QT syndrome, the event rate of torsades de points and death from it is low.
Relevance to SUDEP
While speculative, this data may have implications for SUDEP. The MCER data only contain survival status; therefore, specifics on the cause(s) of death are not available. Thus, we are unable to determine which patients died of SUDEP. However, our data do support predicting all-cause mortality and it is highly likely that some of these deaths were SUDEP given it is the leading cause of epilepsy-related death in persons with epilepsy. Cohort 2 a specific cohort with confirmed epilepsy diagnosis accounted to 17% of our study population. These findings may be directly applicable to investigating and preventing SUDEP. Identifying potential risk stratification and prevention tools for SUDEP is a priority in epilepsy management. Routine ECG at index presentation may be a cost-effective strategy to identify those who may be at higher risk of death and enable interventions to reduce risk. Our data support a change in guidelines in the US to align with those from Europe.
Generalizability
Mayo Clinic Rochester is a large tertiary care center. Our patient population may not represent that of a typical community center. However, Mayo Clinic Rochester also serves as the primary hospital and primary care center for a wide region. The “local population” cohort included in the online supplement was analysed to help address generalizability. This subset analysis showed our findings with this cohort persisted. The local population is predominantly white and generalizability to the non-white US population may be limited.
Strengths and limitations
This is the first and largest series assessing ECG use, ECG abnormalities, and the prognostic role of QT prolongation in those with index evaluation for seizure or epilepsy. Limitations include inherent bias associated with a retrospective cohort study. None of the patients received a formal diagnosis of genetic LQTS (none were referred for specialist). The age of our population, particularly who had an ECG, is older than the prototypical population presenting with first onset of seizures. This may represent a provider preference for ECG in an older patient who likely has other cardiac comorbidities, a delay in diagnosis of seizures or epilepsy, or other confounders contributing to selection bias skewing the decision to obtain an ECG. While we performed adjusted analyses to minimize the effect of confounders, variation in types of medications and serum electrolyte levels at the time of ECG as well as unknown factors may have contributed to QT prolongation. Survival status was based on the EMR and state-wide death information, which may underestimate the number of deaths. Furthermore, we do not have details on the cause of death. Lastly, misclassification of exposure may have occurred as the QTc was a single measure at index evaluation measured before the event and may have changed over time.
5. Conclusions
Routine ECG use at first presentation in patients with seizures and/or epilepsy identifies important co-existing ECG abnormalities. There is a distinct lack of ECG recordings in our population, AF is the most frequent arrhythmia, a higher frequency of primary QT prolongation (1.4% vs 1% in the general population), and a prolonged optimal cut-off QTc interval predicts mortality in patients evaluated for seizure and those eventually diagnosed with epilepsy. We advocate the routine use of a 12-lead ECG at index presentation in all those with a suspected first seizure or epilepsy. This cost-effective strategy may identify important abnormalities, provide impetus for corrective intervention, select those who should be co-reviewed by a cardiologist, help personalize risk stratification of those at greatest risk of subsequent mortality, and conceivably enable identification of a preventable death. There may be implications for SUDEP in the future.
Supplementary Material
Acknowledgements
Secretarial support from Ms. Lea Dacy, Mayo Clinic Department of Neurology, and assistance of Mrs. Alison B. Schultz, Division of Biomedical Statistics and Informatics, Department of Health Sciences Research, Mayo Clinic.
Funding
Supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences at the National Institutes of Health [grant numbers UL1 TR000135, UL1 TR002377 RR024150–01]; National Institutes of Health [grant numbers NIH HL65176 to C.A.A.C. and V.K.S., NIH HL134885 to C.A.A.C. and V.K.S.]; the American Heart Association [grant number 17POST33400211 to C.A.A.C.; Paul and Ruby Tsai Foundation; Student Scholarship in Cardiovascular Disease to J.A.G.]; and the Mayo Clinic Windland Smith Rice Comprehensive Sudden Death Program [to M.J.A.]. VKS is also supported by the Alice Sheets Marriott Endowed Professorship. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
EKSL research support from the Mayo Clinic Center for Translational Science Activities (CCaTS), supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health [1 UL1 RR024150–01].
MJA consultant for Audentes Therapeutics, Boston Scientific, Gilead Sciences, Invitae, Medtronic, MyoKardia, and St. Jude Medical. MJA and Mayo Clinic have an equity/royalty relationship with AliveCor, Blue Ox Health Corporation, and StemoniX. However, none of these entities were involved in this study in any way.
VK Somers consultant for Respicardia, Bayer, Baker Tilly, Sleep Number and Jazz Pharmaceuticals. He works with Mayo Health Solutions and their industry partners on intellectual property related to sleep and to obesity.
The rest of the authors had no conflict to disclose.
REFERENCES
- 1.Zack MM KR. National and State Estimates of the Numbers of Adults and Children with Active Epilepsy — United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66:821–825.DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease C, Prevention. Epilepsy in adults and access to care--United States, 2010. MMWR Morb Mortal Wkly Rep Nov 16 2012;61:909–913.DOI. [PubMed] [Google Scholar]
- 3.Desai R, Rupareliya C, Patel U, et al. Burden of Arrhythmias in Epilepsy Patients: A Nationwide Inpatient Analysis of 1.4 Million Hospitalizations in the United States. Cureus Aug 8 2017;9:e1550.DOI: 10.7759/cureus.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. The Lancet Neurology Oct 2013;12:966–977.DOI: 10.1016/s1474-4422(13)70214-x. [DOI] [PubMed] [Google Scholar]
- 5.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med Nov 15 2001;345:1473–1482.DOI: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 6.Adabag AS, Peterson G, Apple FS, et al. Etiology of sudden death in the community: results of anatomical, metabolic, and genetic evaluation. Am Heart J Jan 2010;159:33–39.DOI: 10.1016/j.ahj.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semsarian C, Ingles J. Molecular autopsy in victims of inherited arrhythmias. J Arrhythm Oct 2016;32:359–365.DOI: 10.1016/j.joa.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacCormick JM, McAlister H, Crawford J, et al. Misdiagnosis of long QT syndrome as epilepsy at first presentation. Ann Emerg Med Jul 2009;54:26–32.DOI: 10.1016/j.annemergmed.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Gigli L, Bertero G, Vidal MC, et al. Juvenile myoclonic epilepsy and Brugada type 1 ECG pattern associated with (a novel) plakophillin 2 mutation. Journal of neurology Apr 2017;264:792–795.DOI: 10.1007/s00415-017-8414-2. [DOI] [PubMed] [Google Scholar]
- 10.Lahat H, Eldar M, Levy-Nissenbaum E, et al. Autosomal recessive catecholamine- or exercise-induced polymorphic ventricular tachycardia: clinical features and assignment of the disease gene to chromosome 1p13–21. Circulation Jun 12 2001;103:2822–2827.DOI: 10.1161/01.cir.103.23.2822. [DOI] [PubMed] [Google Scholar]
- 11.Nunes VD, Sawyer L, Neilson J, Sarri G, Cross JH. Diagnosis and management of the epilepsies in adults and children: summary of updated NICE guidance. BMJ Jan 26 2012;344:e281.DOI: 10.1136/bmj.e281. [DOI] [PubMed] [Google Scholar]
- 12.SIGN. Diagnosis and management of epilepsy in adults: A national Clinical Guideline2015 [Google Scholar]
- 13.Robbins J, Nelson JC, Rautaharju PM, Gottdiener JS. The association between the length of the QT interval and mortality in the Cardiovascular Health Study. The American journal of medicine Dec 15 2003;115:689–694.DOI: 10.1016/j.amjmed.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Post WS, Dalal D, et al. QT-interval duration and mortality rate: results from the Third National Health and Nutrition Examination Survey. Archives of internal medicine Oct 24 2011;171:1727–1733.DOI: 10.1001/archinternmed.2011.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Post WS, Blasco-Colmenares E, et al. Electrocardiographic QT interval and mortality: a meta-analysis. Epidemiology (Cambridge, Mass) Sep 2011;22:660–670.DOI: 10.1097/EDE.0b013e318225768b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French JA, Perucca E, Sander JW, et al. FDA Safety Warning on the Cardiac Effects of Lamotrigine: An Advisory From the Ad Hoc ILAE/AES Task Force. Epilepsy currents Feb 28 20211535759721996344.DOI: 10.1177/1535759721996344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia Apr 2017;58:512–521.DOI: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moss AJ, Schwartz PJ, Crampton RS, et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation Sep 1991;84:1136–1144.DOI: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 19.de Sousa JM, Fialho GL, Wolf P, Walz R, Lin K. Determining factors of electrocardiographic abnormalities in patients with epilepsy: A case-control study. Epilepsy research Jan 2017;129:106–116.DOI: 10.1016/j.eplepsyres.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation Jul 11 2006;114:119–125.DOI: 10.1161/circulationaha.105.595140. [DOI] [PubMed] [Google Scholar]
- 21.Devinsky O, Spruill T, Thurman D, Friedman D. Recognizing and preventing epilepsyrelated mortality: A call for action. Neurology Feb 23 2016;86:779–786.DOI: 10.1212/wnl.0000000000002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haugaa KH, Bos JM, Tarrell RF, et al. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clinic proceedings Apr 2013;88:315–325.DOI: 10.1016/j.mayocp.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Vrtovec B, Delgado R, Zewail A, et al. Prolonged QTc interval and high B-type natriuretic peptide levels together predict mortality in patients with advanced heart failure. Circulation Apr 8 2003;107:1764–1769.DOI: 10.1161/01.cir.0000057980.84624.95. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz PJ, Wolf S. QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation Jun 1978;57:1074–1077.DOI: 10.1161/01.cir.57.6.1074. [DOI] [PubMed] [Google Scholar]
- 25.Anderson HN, Bos JM, Haugaa KH, et al. Prevalence and Outcome of High-Risk QT Prolongation Recorded in the Emergency Department from an Institution-Wide QT Alert System. The Journal of emergency medicine Jan 2018;54:8–15.DOI: 10.1016/j.jemermed.2017.08.073. [DOI] [PubMed] [Google Scholar]
- 26.Rautaharju PM, Surawicz B, Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation Mar 17 2009;119:e241–250.DOI: 10.1161/circulationaha.108.191096. [DOI] [PubMed] [Google Scholar]
- 27.Serdyuk S, Davtyan K, Burd S, et al. Cardiac arrhythmias and sudden unexpected death in epilepsy: Results of long-term monitoring. Heart Rhythm Feb 2021;18:221–228.DOI: 10.1016/j.hrthm.2020.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


