Abstract
Across diverse research and application areas, dynamic functionality—such as programmable changes in biochemical property, in mechanical property, or in microscopic or macroscopic architecture—is an increasingly common biomaterials design criterion, joining long-studied criteria such as cytocompatibility and biocompatibility, drug release kinetics, and controlled degradability or long-term stability in vivo. Despite tremendous effort, achieving dynamic functionality while simultaneously maintaining other desired design criteria remains a significant challenge. Reversible dynamic functionality, rather than one-time or one-way dynamic functionality, is of particular interest but has proven especially challenging. Such reversible functionality could enable studies that address the current gap between the dynamic nature of in vivo biological and biomechanical processes, such as cell traction, cell-extracellular matrix (ECM) interactions, and cell-mediated ECM remodeling, and the static nature of the substrates and ECM constructs used to study the processes. This review assesses dynamic materials that have traditionally been used to control cell activity and static biomaterial constructs, experimental and computational techniques, with features that may inform continued advances in reversible dynamic materials. Taken together, this review presents a perspective on combining the reversibility of smart materials and the in-depth dynamic cell behavior probed by static polymers to design smart bi-directional ECM platforms that can reversibly and repeatedly communicate with cells.
Keywords: Smart materials, dynamic, reversible, cell activity, extracellular matrix, computational
Introduction
In vivo, many extracellular matrices (ECMs) undergo remodeling during development, homeostasis, disease, and wound healing, yet synthetic mimics of the ECM used in basic and applied biomedical science generally are limited to materials with fixed properties that are unable to recreate the dynamic in vivo microenvironment. This review broadly aims to provide a perspective on (I) the need for “smart” ECM mimics, (II) the potential for better understanding of cell/ECM interactions to advance fundamental and applied biomedical science, and (III) the emerging opportunities to bridge the gap between existing static ECM materials used for studying cell activity and more dynamic, biomimetic, bioinspired smart ECM materials.
I. The need for a smart extracellular matrix (ECM)
Most existing research in the area of cell-biomaterial interactions focuses on biomaterial-based platforms that mimic a static ECM or an irreversible change of ECM properties, and it is widely recognized that efforts to date have not succeeded in faithfully recreating the dynamic, ever-changing nature of many ECMs.[1] On the macro scale, most biological processes such as healing of wounds, development of organs, and the various stages of illnesses are dynamic and demonstrate a time-based progression.[2–4] Similarly, in tissue regeneration at the cellular level, the timeframe for cells to adhere to the ECM and migrate, divide, or differentiate within the ECM is predetermined and well-orchestrated.[5] Biomaterial systems with reversible and/or repeatable control over ECM properties have the potential to provide the ability to closely emulate the intricacies of feedback mechanisms between the ECM and the cells, continuously process those signals, and remodel the cells’ microenvironment.[6]
A leading strategy to mimic the native ECM is by employing smart materials. Since their conception in the 1950s, the definition of a smart material has evolved into one that can respond to stimuli such as multiple pH values, light, electric current, magnetic field, mechanical stresses, and enzyme levels, and be applied to carry out a certain function. [7, 8] This definition remained largely unchanged for several decades. However, due to the continuous advancements in the field, smart materials found applications in areas such as sensor design, diagnostic imaging, and drug delivery.[9] More recently, smart biomaterials have been used in novel ways to trigger cells by designing the biomaterial platforms to respond to external stimulus. Their applications include tissue regeneration, smart scaffolds, and accelerated wound healing. [10]
To address recent progress on the use of smart materials as substrates responsive to cell activity and possibly serve as model platforms to study disease development, [11, 12] in this review, we have analyzed smart materials that offer repeated, reversible modification of their properties — thus offering a more biologically-relevant platform to study and control cell behavior. Based on the field’s progress, we anticipate and discuss the potential for the definition of smart materials to be expanded to include materials that are engineered with bi-directional responsiveness; these materials will not only be used to modulate cell activity, but also to receive feedback from cells and remodel. Such systems would mimic the existing physiological environment of the cells and serve as more accurate platforms to study the progression of diseases such as fibrosis and cancer.
Despite advances in the design of smart biomaterials that can reversibly control cell activity, these smart materials do not allow the bi-directional transmission of signals between cells and substrate. Thus, some limitations such as unresponsiveness to changes in the ECM due to cell activity remain to be addressed. It is well known that the reversible control over cell activity can be influenced by various input stimuli such as changes in pH,[13–16] glucose concentration,[14, 15] light,[17–25] temperature,[15, 20, 25–30] tensile forces,[31] and voltage.[32–35] Smart materials have also been designed to respond to biologically relevant signals such as ligand-receptor,[36] antigen,[26, 30, 34] and MMPs,[11] while physical aspects (e.g., stiffness) of other synthetic constructs are programmed to simulate cellular activity in native biophysical systems.[28, 37–39] These smart polymer systems, however, do not account for changes in the ECM as a result of the cell activity. Due to the deep interdependence between the polymer properties, parameters utilized for triggering, and cell-specific responses, the ability to fabricate such smart polymer constructs that can reversibly control and be controlled by the activity of cells incorporated in them present a challenge to material scientists and biologists alike.
II. The interplay between cells and their extracellular environment
The remodeling of the ECM architecture is of biological and biomedical importance due to the critical mechanobiological interdependence between cellular behavior and the ECM. The dynamic in vivo microenvironment to which cells are exposed has been described to a great extent in existing literature.[40–42] In addition to sensing biological stimuli, like enzyme levels, cell adhesion peptides, calcium ion (Ca2+) concentrations, and biochemical signals, such as pH levels, temperatures, and electric potential differences, it is well known that the cells are subjected to continuous mechanical forces — for instance — shear, compression, and tension, while also responding to morphological and stiffness-related cues.[43–46] These stimuli collectively result in specific biological responses, including cell division, differentiation, migration, proliferation, and tumorigenesis. These responses, in turn, are transmitted via feedback pathways that impact the cytoskeletal response, including adhesion, spreading, and division of the cells. Moreover, the biological responses also provide a significant feedback signal for the remodeling of the ECM, which subsequently affects the cells residing within it.[6, 47] Due to active contractions caused in them, the cells continue exerting tension on the surrounding ECM. The growth of tissues and organs is highly dependent on the dynamic interactions between cells and their local ECM. For example, the abnormal growth of tissue into a tumor is accelerated by the cells’ ability to degrade the native ECM proteins and aggressively deposit their own ECM components, which generally results in the stiffening of the surrounding tissue. This stiffened tissue further disrupts cell-ECM communication and eventually leads to tumor metastasis.[48, 49] Mechanobiological feedback thus plays a critical role in the time-dependent, dynamic, and constant evolution of many ECM structures and, consequently, cellular behaviors.
III. Advances in smart ECM to control cell fate and static ECM used for studying cell activity
Several research labs have only characterized the changes in properties of cells seeded on smart ECMs. The ECMs used in these studies assume that the cells and ECM behave as perfectly elastic materials. However, possible related phenomena, for example, viscoelasticity and stress relaxation of the ECM constructs were not analyzed. Moreover, as discussed above, the properties of cells in vivo change continuously in response to biological processes and signal transmissions.[54] While being subjected to such stimuli, the output from the cells offers a feedback signal, which directly affects cell-cell communication and cell-ECM interaction, which in turn impacts remodeling of the ECM. [55, 56]
With respect to probing the activity of cells resulting in ECM remodeling, several physiologically relevant interactions between the cell and ECM have been studied systematically. Most of these studies involve a combination of experimental, theoretical, and modeling strategies to characterize the effect of cell mobility on ECM remodeling. [57, 58] Progress in this area led to several research labs utilizing a 3D collagenous matrix, a combination of collagen and Matrigel, or a purely synthetic platform in their studies.[31, 59–64] Furthermore, biological factors, such as anisotropic stress generated by cells, strain-stiffening behavior of the ECM, and RGD-functionalization, have been monitored. Moreover, some reports have incorporated nanopatterned structures modified with collagen to study the morphology and migratory behavior of cells. Most studies in the past two decades have focused on an irreversible or a one-way change in the ECM to provide signaling to the cells, and they have been reviewed in great detail elsewhere.[1]
A systematic review from both the ECM and cells’ points of view
In this review, we highlight reports from recent years that have adopted and developed smart materials-based mechanisms which allow reversible application of a stimulus with the potential to regulate cell fate. Specifically, we categorize the studies based on the applied stimulus causing (A) changes in biochemical property or (B) changes in mechanical property. Wherever applicable, those reports are further classified according to utilization of (I) exogenous and (II) endogenous cues for designing smart polymers. As such, studying cell behavior in response to these stimuli is crucial towards the understanding of how the chemical and structural cues presented by the polymer matrix affect cell fate (Scheme 1). Lastly, we review and reconcile an underexplored area of research that targets data-driven quantification of cell-ECM interaction and changes in the static ECM microstructure due to cell activity by amalgamating experimental, theoretical, and computational biology approaches. In our concluding remarks, we discuss current shortcomings and disparities between these ‘dynamic’ and ‘static’ areas of cell-ECM research by looking at different scientific disciplines and proposing promising strategies to bridge this gap in the years to come. We also define a new class of smart materials and our vision on how these materials can be developed in the near future.
Scheme 1.
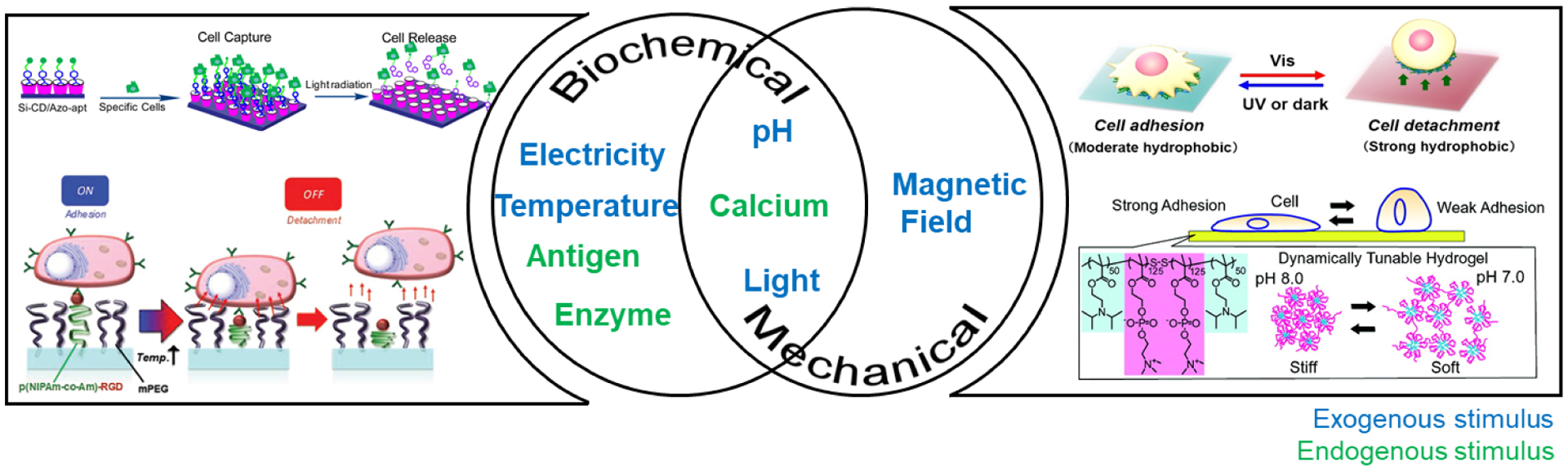
Pictorial representation of the exogenous and endogenous parameters reversibly influencing biochemical and mechanical properties of smart materials. Figure permissions - (top left): Reproduced with permission from reference [17], Copyright (2016) American Chemical Society, (bottom left): Reproduced with permission from reference [65] Copyright 2020 John Wiley and Sons, (top right): Reproduced with permission from reference [66], Copyright (2016) Creative Commons Attribution License 4.0 International, (bottom right): Reproduced from reference[67], Copyright (2011) with permission from American Chemical Society.
A. Direct changes in biochemical properties
I. Applying exogenous stimulus changes biochemical property
Synthetic smart ECMs that respond to exogenous stimuli were originally engineered to study cell behavior due to their ease of tailored responsiveness to appropriate stimuli as well as flexibility in terms of incorporation of the desired cell-binding sites. Most of the early work on dynamically manipulating properties of a synthetic smart ECM interface was performed using exogenous stimuli due to the high level of spatial, temporal, or spatiotemporal control, and the ability to target specialized cell-ECM interaction scenarios through biomimicry. To this end, here we focus on studies over the past few years which have been dedicated to the design of smart biomaterials that utilize non-biological stimuli such as pH, temperature, and light to reversibly control biochemical properties such as coordination complex, electronic configuration, and surface wetness.
During the last decade, pH was one of the widely used inputs to control polymer architecture and examine consequent cell activity. Taking advantage of this, Zhan et al. developed a gold surface coated with phenylboronic acid (PBA) and cyclodextrin (CD), where the CD was modified with different bioactive ligands (Figure 1I.).[13] The PBA-cis-diol complex was pH-sensitive and reversible, the PBA surface could be recovered by decreasing the pH to dissociate the PBA-fructose complex.
Figure 1.
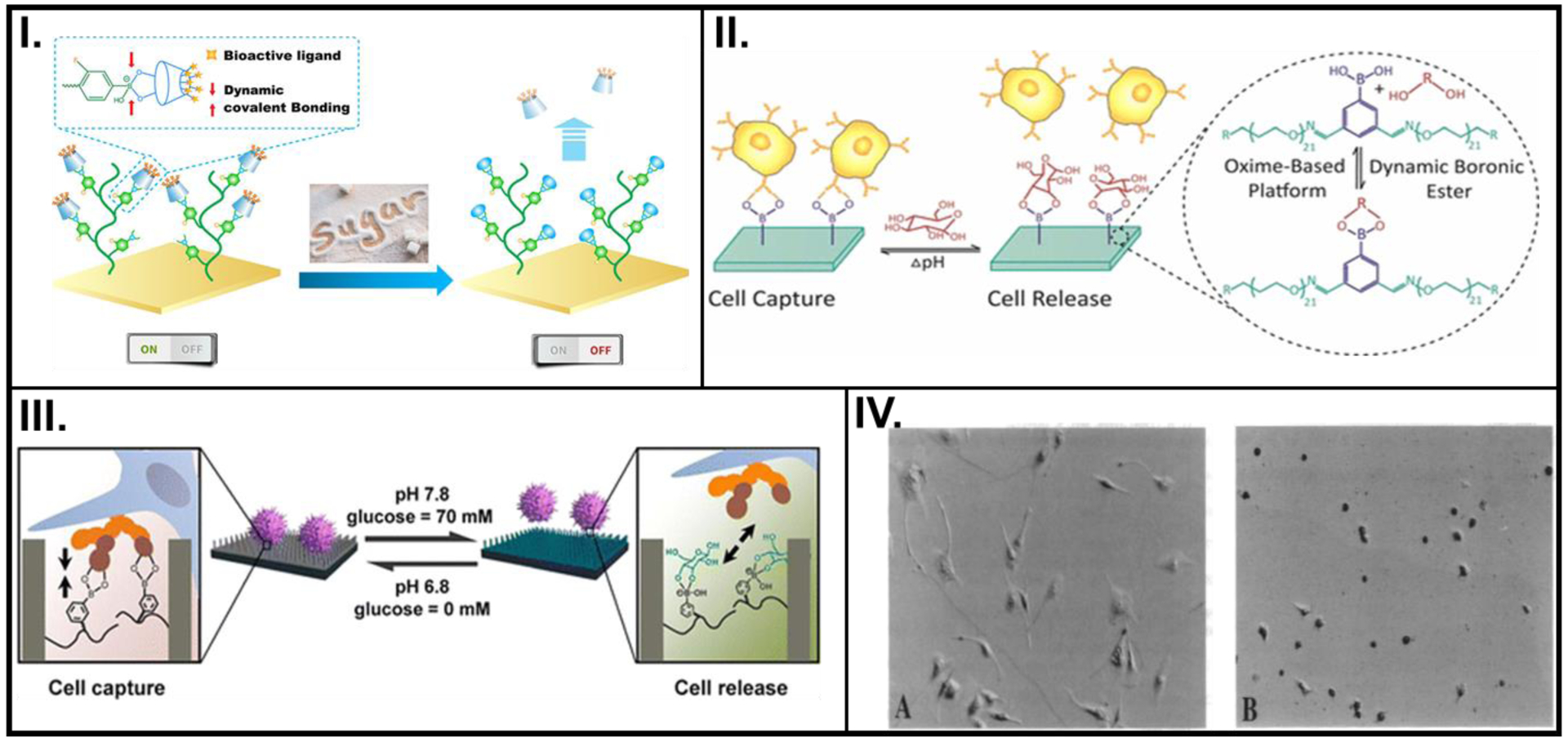
pH- or voltage- controlled changes in the biochemical property of smart polymers used to control cell activity. (I.) PBA-based polymeric brush with surface immobilized CD-X (left) reversibly detects fructose (right). (II.) On-demand, dual-responsiveness to pH and glucose, and associated cell capture and release achieved by PEG hydrogels built on an oxime platform. (III.) pH and glucose-responsive, reversible attachment and detachment of cells afforded by a SiNW substrate with pendant PBA groups. (IV.) Light microscopy images showing shapes of endothelial cells before (left) and after (right) application of – 0.5 V, cultured on FN-PP substrates. A: Reprinted with permission from reference [13], Copyright (2018) American Chemical Society, B: Reprinted with permission from reference [14], Copyright (2017) American Chemical Society, C: Reprinted with permission from reference [15], Copyright (2013) American Chemical Society, D: Reproduced from reference [32], Copyright (1994) with permission from National Academy of Sciences, U.S.A.
Another example of reversible pH sensitivity was exhibited by an oxime-based polyethylene polymer where udiMCF-7 cells were captured by the PBA surface due to the formation of the PBA-carbohydrate complex with a surrounding pH of 6.8 and no glucose (Figure 1II.).[14] The reversibility of this boronate-ester formation enabled multiple cell captures and releases (four cycles), with small loss of potency in cell adhesion or release amounts.
The versatility of using pH levels as the stimulus for cell capture and release was demonstrated by Liu et al., where a smart platform was developed by coating PBA on silicon nanowires (SiNWs).[15] At higher pH values, PBA preferentially attached with glucose, while breaking the complex formed with the oversecreted sialic acid residues secreted by MCF-7 cancer cells. Moreover, the captured cells were released and PBA, which has higher affinity toward glucose, switched its complexed state (Figure 1III.). A key highlight of this study was their use of high-resolution scanning electron microscopy (SEM) images and quartz crystal microbalance (QCM) for their analysis needs.
Recent studies suggest that light-responsive model polymers systems which can capture complex reversible cell-ECM interactions could be used to evaluate the effect of ECM stiffness on cells. [68, 69] Smart materials that respond to light-based triggers have been extensively studied as photo-modulated platforms that can be used as tools to study cell-ECM interactions.[70] To specifically isolate and capture breast cancer cells and trigger their release using ultraviolet irradiation (UV), Bian et al. designed an azobenzene-based platform.[17] Their mechanism focused on host-guest interactions to capture MCF-7 breast cancer cells with some chemical modifications and additions. Interestingly, fluorescence microscopy images indicated that the reversible adhesion and release was cell-specific and did not capture other cancer cells present. This implies that their approach has potential for the identification of targeted tumorigenic cells for diagnostic purposes.
A facile method for utilizing a thermoresponsive polymer to control the attachment and desorption of cells using biologically safe irradiation wavelengths (UV-A and visible light) was first reported by Edahiro et al.[18] Although the authors suggested that cell attachment to their photo responsive cell culture surface could be achieved repeatedly, the repeatability of the cell-polymer interactions was unclear. In a different strategy implemented by Li W. et al, they aimed to utilize the upconversion capabilities of core-shell nanoparticles (UCNPs) synthesized from the elements of the lanthanide series.[19] The attachment/detachment of cells based on the light-triggered spiropyran-merocyanine isomerism could be repeated for more than 10 cycles, making this technique a remarkable advance in manipulating cell-supporting matrix interactions.
The drastic change in physical properties of poly n-isopropylacrylamide (pNIPAM) as it transitions between its hydrophobic and hydrophilic states in response to changes in temperature has garnered significant interest in the cell biomechanics community.[71] In a study by Cui et al., a thermoresponsive polymer, pNIPAM, was coated on SiNWs surfaces, forming a conduit between photo and thermally-activated platforms.[20] While switching the NIR-irradiation ‘on’ rendered the pNIPAM surface hydrophobic and cell adhesive, turning it ‘off’ led to hydrophilic characteristics at ~26°C, resulting in the detachment of >95 % of the cells from the hydrophilic substrate. The surface modifications remained stable, and the cell capture and release phenomenon could be repeated for 20 cycles.
To control the temperature-responsive, enzyme-free capture and release of cells. another such dynamic material based on glycine-arginine-glycine-aspartate-serine (GRGDS)- modified pNIPAM platform was recently developed by Brunato et al.[65] Their synthetic smart material platform with tethered biorecognition sites captured cells at temperatures below the LCST of the polymer, while elevating the temperature above the LCST rendered the GRGDS sites unavailable for cell adhesion and consequent detachment. When compared to traditional NIPAM-based materials that rely solely on the hydrophilic to hydrophobic transition to control cell activity, this new approach which offers additional control via bioactive pendant groups has the potential to provide greater control over dynamic bio-tagging and cell-biomaterial interaction.
While continuing to use UV and visible light to integrate cell imaging with simultaneous substrate triggering, a self-assembled monolayer (SAM) platform comprised of GRGDS peptides as the biofunctionalized moiety along with azobenzene was fabricated.[21] The ability to reversibly control the molecular configuration of azobenzene by employing UV and visible light opened a wide range of possibilities for designing devices that can reversibly capture and release cells by simply using wavelengths of light produced by the mercury lamp of a fluorescence microscope.
In contrast to the traditional enzyme-based methods used to detach confluent cells from tissue culture polystyrene, Cicotte et al. fabricated a thermoresponsive material obtained by electrospinning pNIPAM. MC3T3-E1 (3T3) and EMT6 (cancerous tumor) cells formed confluent sheets on the electrospun pNIPAM with the substrate maintained at 31°C.[72] Upon cooling, 80 % of the cells could be detached from the surface within 5 min. Furthermore, reversible retrieval and adhesion of cells was demonstrated by employing polyvinylidene fluoride (PVDF) substrate as a secondary platform.
Stimuli such as pH and temperature are generally used due to their ease of incorporation because of the involvement of cell culture media. A different approach was used by Wong and coworkers to control cell activity where polypyrrole (PP) was electrodeposited on an optically transparent Indium Tin Oxide (ITO) electrode in the presence of tetramethyl ammonium p-toluenesulfonate as the electrolyte containing the counter anions (SO3−).[32] Bovine aortic endothelial cells (BAECs) cultured atop fibronectin (FN)-coated PP (in its natural, oxidized form) showed an appreciable level of cell spreading and elongated morphology. Upon the application of an electric potential, the cells rounded up, and negligible spreading was observed (Figure 1IV.) Since this novel application of PP as candidates for studying cell-substrate interactions was still in its nascent stages, several hypotheses that could potentially govern the cellular behavior were proposed. Despite the absence of reversible control tests to observe cell morphology, the inherent ability of such polymers to switch between their oxidized, reduced, or neutral oxidation states bears immense potential for the future of smart conductive polymers as a non-invasive platform for studying cellular activities.
Conductive polymers combine desired features, such as the high conductivity of metals as well as the convenience of facile synthesis and refining of polymers. Due to their minimal latency in response and their ability to switch states under relatively mild conditions, there has recently been a growing interest in conductive polymers for studying cellular activity.[73, 74] In their widely cited study, Ng et al. exploited two different chemistry combinations to study a switchable surface for cell adhesion. Specifically, they fabricated SAMs that could either expose or conceal RGP peptides, which would render the surfaces cell-adhesive or cell-repulsive, respectively.[33] Even though their switching experiments needed 45 min for stabilization of parameters and regeneration of incubation conditions, such a reversibly switching platform provides a critical tool for precisely controlling the cell capture and release zones, with the added possibility of introducing selective capture by decorating specific antibodies or cell-adhesion peptides.
While smart ECMs that respond to exogenous stimuli used to reversibly control cell activity has been the oft-used combination for several years, these strategies often face limited biological applicability because of issues such as discrepancy between synthetic biomolecules-based signaling expected to intercept sophisticated cellular signaling, inconsistencies in pH between experimental in vitro simulations and targeted in vivo conditions, and the continued narrow focus on concepts such as cell attachment and detachment.[75] Next, we discuss endogenous triggers that could potentially help alleviate some of these issues.
II. Applying endogenous cues regulates biochemical changes
Smart biomaterial systems can also be fabricated to respond to factors such as the presence or concentration of certain biological factors including enzymes, antigens, or antibodies by demonstrating a conformational change in their properties (e.g., swelling/deswelling, degradation, and crosslinking). As presented in the previous section, many existing reports discuss the use of external factors such as pH, temperature, and electric potential to modulate changes in a smart polymer to observe dynamic changes in cell behavior. While such stimuli can be precisely controlled spatially or temporally, these strategies rely on the transduction of these signals into one that can be meaningful to direct cellular biological output. Endogenous cues such as antigen or enzyme levels, on the other hand, could bypass some of these challenges by utilizing already existing signaling pathways within the cell.
Hydrogels based on highly specific antibody-antigen binding were among the first smart materials which responded to a biological stimulus. A strategy for the synthesis of an antigen-responsive hydrogel was first described by Miyata and coworkers.[26] The facile strategy involved fabricating a semi-interpenetrating network composed of polymer chains containing vinyl-functionalized antibodies polymerized with vinyl-functionalized antigens In addition to detecting a specific antigen from a mixture of antigens, the ability of this biopolymer to repeatedly detect the presence and absence of antigen was characterized by recording the swelling and deswelling behaviors, respectively (Figure 2I.).[76] Moreover, the stimulation by antigen also allowed the permeation of hemoglobin, thus indicating the potential use of the biopolymer for antigen-specific delivery of a drug payload.
Figure 2.
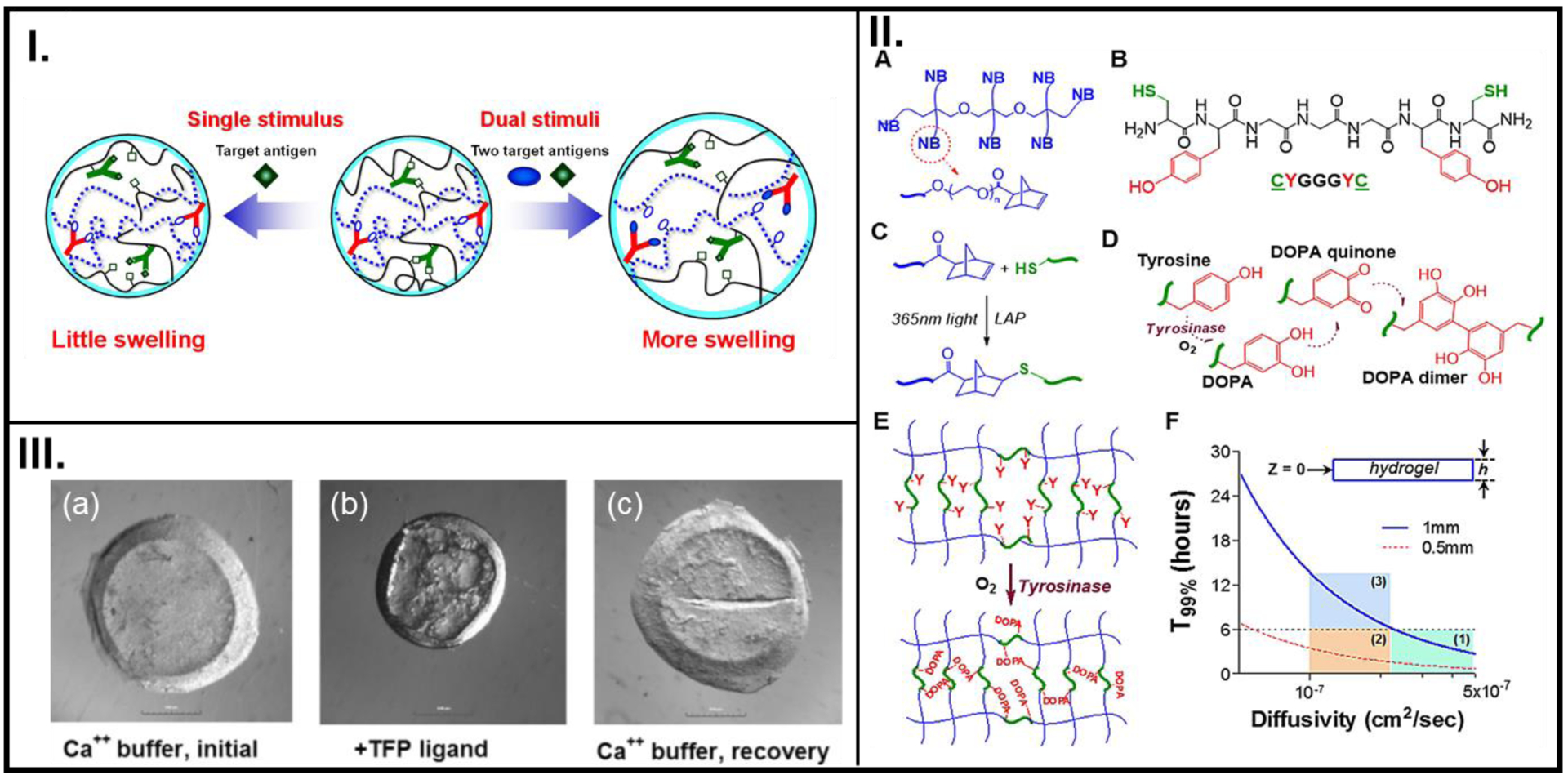
Reversible utilization of biologically relevant stimuli used in the design of smart polymer systems to dictate cell fate. (I.) The swelling-deswelling mechanism of an acrylamide-based antigen-responsive hydrogel in response to detecting competitive binding with free antigen. (II.) Chemical structure of an 8-arm NB-PEG (A), A model bis-cysteine crosslinker (B), Photo-click chemistry between-SH and NB to create NB-PEG-peptide hydrogel (C), Tyrosinase enzyme-mediated change in the oxidation state of tyrosine (D), the related DOPA intra-molecular crosslinking that leads to elevated crosslinking (E), Representation of different cases of hydrogel thickness (h) required for sufficient infiltration during a 6 hr incubation in tyrosinase. Regions 1 and 2 meet the diffusivity criteria (T99%), while region 3 does not (F). (III.) Light microscope images showing the swelling-deswelling phenomenon in CaM-based hydrogels in their virgin condition in a Ca2+ buffer (a), upon interaction with a TFP ligand (b), and recovering their virgin state when re-introduced into a Ca2+ buffer (c). A: Reproduced with permission from reference [76] Copyright (2016) under Creative Commons Attribution License 4.0 International, B: Reproduced with permission from reference [11], Copyright (2017) Acta Materialia Inc. Published by Elsevier Ltd., C: Reproduced from reference [36], Copyright (2007), with permission John Wiley and Sons.
In a more recent study, in addition to using a specific antibody-conjugated molecule, Kim and group fabricated smart polymer beads with a carboxylated polystyrene core and decorated it further with a temperature-responsive, -NH2 functionalized p(NIPAAM) polymer.[34] At an elevated temperature, these beads were able to selectively capture EpCAM positive human prostate cancer (PC3) cells; when cooled, these beads released approximately 70 % of cells. This allowed for a straightforward design for the development of biopolymer strategies involving reversible temperature and antigen-specific response, as well as isolation by electric stimulus.
It is known that the stiffness of biological tissues increases during pathological events such as fibrosis and cancer.[11] To recreate this elevated stiffening via crosslinking of peptides, a precursor solution containing norbornene (NB)-modified polyethylene glycol (PEG), a bis-cysteine-bis-tyrosine peptide crosslinker, and pancreatic stellate cells (PSCs) was first polymerized into a 3D structure under UV (365 nm) light.[11] Additional crosslinking was facilitated by further treating the polymer with tyrosinase (Figure 2II.). The enzyme-mediated degradation by collagenase demonstrated that the peptide crosslinker was susceptible to proteolytic degradation by MMP activity. Moreover, the time-dependent deferred degradation mechanisms of the stiff polymers could potentially be matched with the timeline of stiffening during tumor tissue formation. As such, this strategy can provide a robust means for utilizing dynamic stiffening of biopolymers to emulate and study cellular fate during tumorigenesis.
A dynamic synthetic ECM was constructed with embedded biological components to study protein-ligand interaction by Sui et al.[36] Instead of the usual threonine residues, the authors appended the peptide with cysteine residues (-SH groups). A reversible and repeatable deswelling-swelling phenomenon was observed across multiple cycles as the gels recovered their expanded dumbbell formation and swollen state upon introduction in Ca2+ buffer (Figure 2III.). This dynamic system for reversible binding between the cell protein and adhesive ligand presented by the drug can serve as a platform for examining other specific adhesions between cell proteins and ECM ligands.
When engineering a 3D ECM that can mimic properties and conditions offered by the native environment, one strategy is to include enzyme-cleavable peptides in the composition. Existing data in the domain of encapsulated cells in a 3D culture setting suggest that cell viability remains relatively high in a soft-to-low stiffness network (with modulus generally ranging from hundreds of Pa to less than 5 kPa) for up to 14 days in culture.[77] However, even though the goal of 3D cultures is often to eventually engineer complex structures including tissues and organs, the spreading and proliferation rate of cells, a crucial element for creating functional structures, is often not included in the scope of such studies. To emulate the dynamic conditions by presenting cues that can be identified by cells, researchers have included MMP-cleavable peptides as crosslinkers into synthetic 3D ECMs, thus engineering constructs catering to accommodate transient cell behavior. Within these ECM designs, factors such as covalent crosslinking of a calculated mole fraction of MMP-cleavable sequence into the polymer to match the desired ECM degradation and the rate of nascent protein deposition by cells, along with tailoring the MMP-cleavable sequence selection to the specific cell type, will have significant implications toward realizing a 3D ECM that mimics native conditions.
B. Direct changes in mechanical properties
Similar to the outcomes observed by means of conformational changes in biochemical properties of smart ECM constructs due to applied triggers, exogenous and endogenous stimuli also have the potential to change mechanical properties of such constructs. It has been increasingly documented that cells have the ability to sense the changes to mechanical attributes of their microenvironment and translate those inputs via their complex signaling pathways to exhibit changes in their biological output.
I. Applying exogenous cues to change mechanical properties
To evaluate and control cell response to dynamic stimulation of the smart ECM, exogenous stimuli such as pH, magnetic field, and light in different wavelengths have been used to dynamically change mechanical properties (such as stiffness and viscoelasticity) of the smart ECM.
To achieve this via a probe-free technique, Yoshikawa et al. synthesized a pH-sensitive triblock ABA copolymer.[16] At pH 8, the polymer was stiff and showed strong adhesion of C2C12 mouse fibroblast cells; decreasing the pH to 7 caused a decrease in stiffness, to which cells did not adhere (Figure 3I.). This reversible change in elasticity could be repeated 40 times without appreciable loss of polymer resilience.
Figure 3.
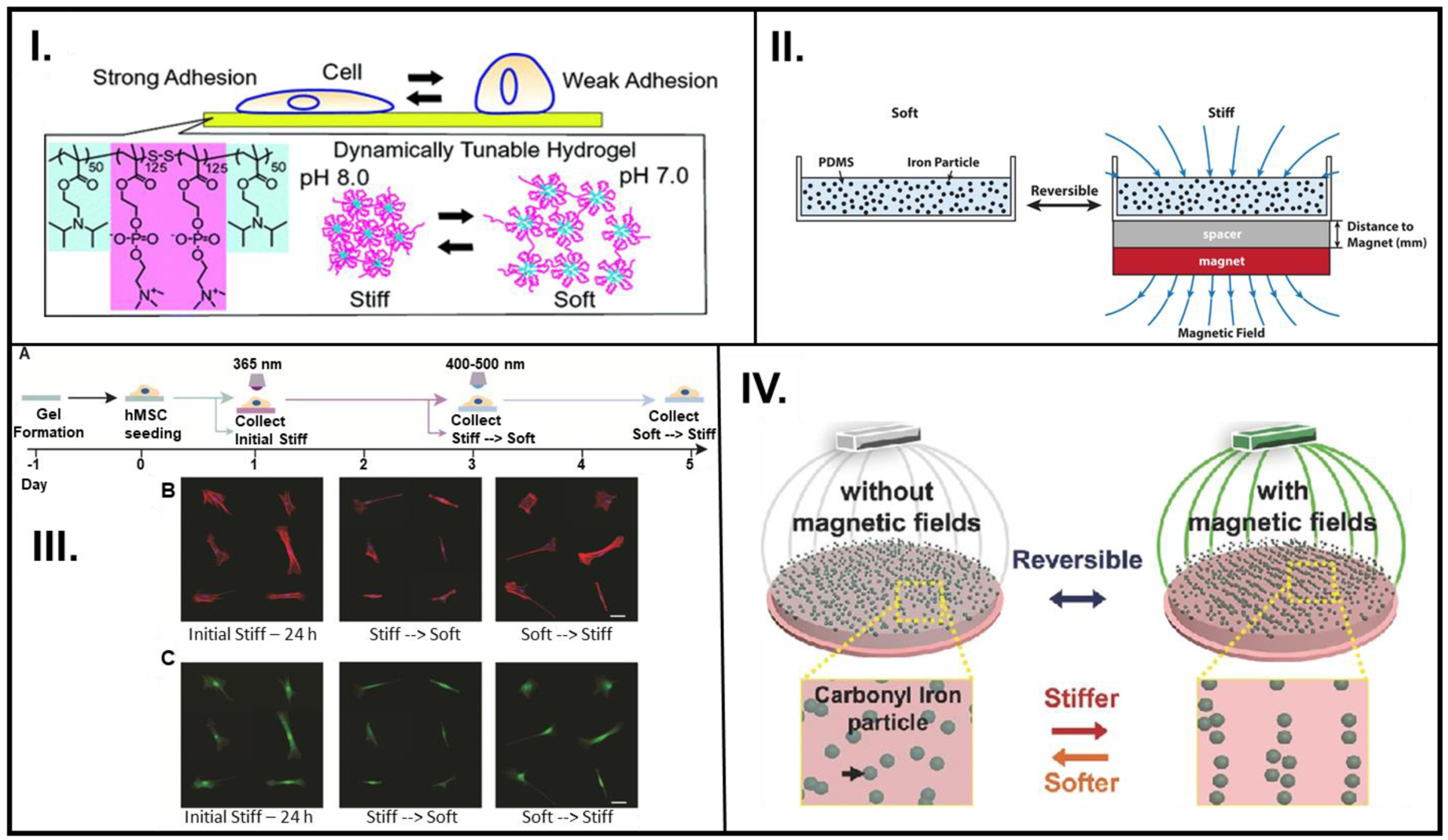
pH, magnetic field, or light employed in a reversible/repeatable manner to study cell activity. (I.) A reversibly pH-responsive PDPA-PMPC-PDPA copolymer that is favorable for cell adhesion (pH 8) and cell averse (pH 7). (II.) Employing magnetic field to reversibly control the arrangement of iron particles in polymeric material composed of PDMS with embedded iron particles. (III.) (left to right) Hydrogel formation, 400–500 nm irradiation causes crosslinking of MA groups (A), Representative images of f-actin stained (red) and nuclei (blue) (B), and YAP/TAZ (green) stained cells (C) as they go through the stiff-soft-stiff phases. (IV.) A pAAm gel was synthesized using free radical chemistry, where the pAAm-based polymer containing CI particles can reversibly transition between stiff and soft phases based on the alignment of CI particles. A: Reprinted with permission from reference [16] Copyright (2011), American Chemical Society, B: Reprinted with permission from reference [51] Copyright 2019, American Chemical Society, C: Reproduced from reference [22] Copyright 2017, with permission from Elsevier, D: Reproduced from reference [50], Copyright 2016, with permission John Wiley and Sons.
Smart materials with magnetically responsive particles have recently emerged as a popular choice for cell stimulation because they allow non-invasive actuation.[78] Additionally, the application of magnetic fields has also been shown to influence the activity of stem cells.[79] Generally, cells used in smart materials-related cell culture experiments are actively treated to not undergo differentiation. In a specialized instance, however, Corbin et al. probed the effect of dynamic instantaneous magnetic-field induced stiffening of PDMS substrates with embedded carbonyl ion (CI) on human-derived induced pluripotent stem cell cardiomyocytes (iPSC-CMs).[51] A relatively facile technique was employed to control the polymer stiffness by adjusting the magnitude of the magnetic field produced by a nearby magnet (Figure 3II.). A dynamic stepwise increase in the stiffness resulted in an increase in cell spreading coverage area while the opposite behavior was observed for the ramp down regime. Although not explicitly demonstrated, this experimental design had the potential to achieve reversible stiffness (first ramp up and then ramp down stiffness of the same sample), which could allow for the assessment of other biological systems that are affected by temporal stiffening. This design also plausibly constitutes the basis for the study of other mechanoresponsive cellular systems (e.g., osteogenesis, adipogenesis).
Abdeen and coworkers also explored the possibility of utilizing CI particles embedded in a viscoelastic polyacrylamide (pAAm) material as a platform for reversibly controlling the activity of mesenchymal stem cells (MSCs) seeded on them (Figure 3IV.).[50] Cyclic modulation of the applied field demonstrated that the stiffness values could fluctuate between two orders of magnitude showing higher stiffness with the field on versus significantly lower values with field off, with negligible hysteresis for up to 10 cycles. A significant decrease was observed in cell coverage area of MSCs when the stiffness of the underlying substrate was decreased. By examining the expression of RUNX2, a transcription factor, with the MSCs seeded on the magnetoelastic gels, it was concluded that early reception of mechanical stimulus was essential for MSC osteogenesis. Such a remote, non-invasive control over the mechanical properties of the ECM opens the doors to many more cellular biology experiments in which the ECM morphology could be modulated in alignment with changes in tissue stiffness during the progression of a particular type of disease.
Smart materials with the ability to provide on-demand temporal control over cues to modulate the materials’ stiffness and direct cell fate have been adopted by polymer scientists. In one instance, Homma et al. recently demonstrated a 2-D smart polymer platform for cell culture that could be stiffened by light irradiation at specific time points to potentially serve as a phantom that recreates transient changes in the cellular microenvironment during disease advancement.[80] Another 2-D cell culture platform was pioneered to emulate changes in the stiffness of tissues during abnormal biological conditions such as tumor progression and fibrosis [81] Their findings suggested that a modular smart material designed to undergo multi-step crosslinking could be used to trigger a change in substrate stiffness on discrete days, which in turn manipulated stem cell differentiation. Several systems that allow light-based dynamic stiffening and softening of the cell culture platform have been used to study cell processes.[47] To modulate reversible control over the properties of the polymer matrix, Rosales et al. designed a tailored network consisting of a biomimetic backbone, UV-degradable component, photo-initiated crosslinker, and cell adhesion peptide (Figure 3III.).[22] The o-nitrobenzene acrylate could be cleaved to decrease the stiffness, leading to a decrease in cell coverage of human mesenchymal stem cells (hMSCs) on these substrates. Furthermore, the methacrylate that was already present in the polymer matrix could be crosslinked in the presence of visible light and a photoinitiator, increasing the modulus as well as the cell coverage. The elastic modulus of the substrates in this study made it a promising candidate for emulating in vivo moduli of tissues as well as disease progression.
Other emerging approaches include the creation of smart fibrillar hydrogel constructs that can better emulate the properties of the in vivo ECM have already been reviewed in detail elsewhere [82] These constructs can be used for experiments involving development of organoids to provide dynamic conditions to mimic natural development and the fabrication of highly anisotropic complex structures that recreate the alignment of fibers in the physiological environment.
II. Applying endogenous cues to regulate mechanical changes
Strikingly, few studies have explored the use of biological stimuli to tune mechanical properties of smart ECMs, which would, in turn, direct cell activity. Using Ca2+ ions to form interpenetrating networks has emerged as a popular choice for biologically-inspired cues in the design of smart material systems that can regulate cell fate, especially in 3D models.[83, 84] While this stimulus does not offer spatiotemporal control like some other non-biological counterparts, its relevance to in vivo applicability due to better integration with cell-ECM signal transduction pathways makes it a promising target strategy for controlling the cell behavior.[85]
3D biopolymer cell culture substrates with temporally tunable stiffness can serve as a crucial tool to study the evolution of the phenotype of cells embedded in it. Stowers et al. sought to fabricate 3D ECM comprised mainly of alginate as the polymer backbone along with liposome constructs.[23] NIR-light assisted heating of a liposome construct encapsulating gold nanorods and Ca2+ beyond its transition temperature resulted in increased crosslinking and stiffening of the alginate polymer. Conversely, cleavage of ionic crosslinks between Ca2+ and alginate could reversibly soften the polymer. Spatial control was achieved by shining the NIR-laser only in certain regions of the polymer constructs to selectively initiate ionic crosslinking, creating stiff and soft zones (Figure 4I.). NIH 3T3 fibroblasts embedded in alginate – Ca2+ loaded liposomes and Matrigel composites showed an elongated morphology due to an initial increase in stiffness, which later transitioned to be more rounded as the stiffness increased. From the results of another cell culture strategy whereby RGD-modified alginate was progressively photopolymerized, it can be deduced that stiffness of the 3D polymer construct was the key to determining cell morphology.
Figure 4.
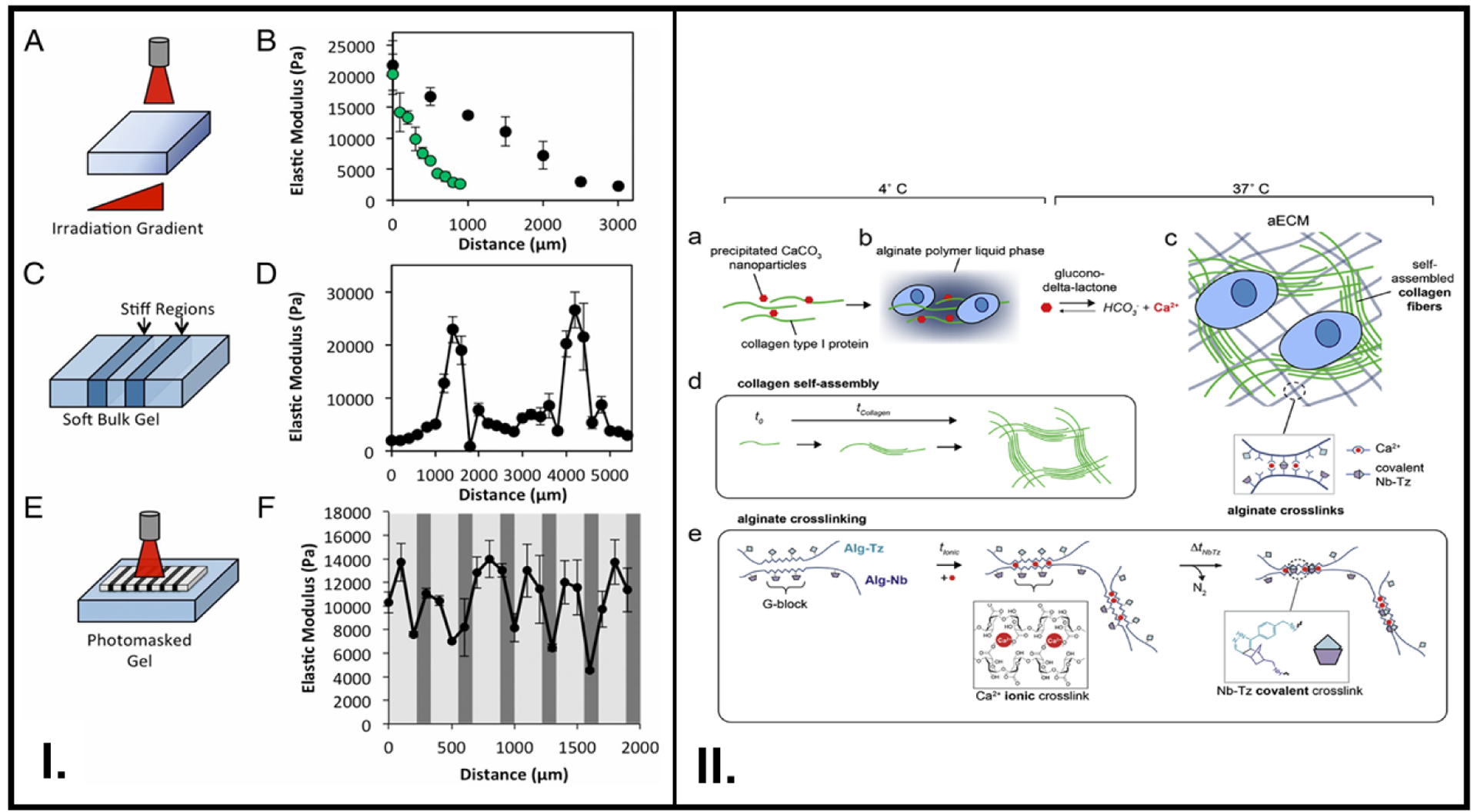
Dynamic tuning of smart polymer stiffness with physiologically-relevant phenomena such as change in Ca2+ concentration and critical stress offered by the ECM. (I.) Modulation of stiffness via NIR radiation (A-B), , irradiation of specific regions (C-D), use of a photomask to produce stiff regions in a controlled region (E-F). (II.) A hybrid ECM consisting of covalent and ionic crosslinks was synthesized, where collagen type I (green) and CaCO3 (red) (a), were mixed with an alginate solution (b), The addition of glucono-delta-lactone and incubation at 37°C resulted in hydrogel formation first due to ionic crosslinks between Ca2+ and alginate backbone as and then due to Nb-Tz covalent crosslinking between Nb (purple)- and Tz (light blue)- functionalized alginate chains(c), Collagen self-assembles and gets increasingly crosslinked with time (d), Detailed reaction chemistry of the ionic and covalent crosslinks (e). A: Reproduced from reference [23] Copyright (2015) with permission from National Academy of Sciences, B: Reproduced from reference [52], Copyright (2019) with permission from Elsevier
Previous work on engineering the structure of dynamic materials that possess the characteristics of the native ECM, thus allowing easy adhesion and encapsulation of cells, have already been discussed in detail elsewhere.[86] Cytocompatible hydrogels whose modular covalent bond chemistry can potentially adapt to the stiffness and stress relaxation of several tissues of the body offer an interesting dynamic approach to account for the transient nature of the complex structure of the physiological environment. Other such studies with detailed reports of tunable hydrogels to match target organ/tissue moduli have also been reviewed previously.[87]
One method of designing a smart polymer with reversible viscoelastic properties to control cell behavior is by creating an interpenetrating network of biopolymers.[88] For their MSC-based experiments, Vining et al. prepared alginate polymers containing ionic crosslinks mediated by calcium (Ca2+) ions, as well as covalent bonds due to the pendant groups such as norbornene (Nb) or tetrazene (Tz) functionalized on the glucuronic acid block (G-block) (Figure 4II.).[52] Their findings revealed that tuning the viscoelastic properties of a biopolymer could be used to invoke immune-system mediated expression of signaling factors in MSCs, which bears significant potential in specific cell therapy-oriented approaches in cancer treatment.
Transitioning from a one-way to a bi-directional communication between cells and ECM
All the discussions above have been focused on the design of smart materials to control cell activity without considering the interactions of cells with the smart materials at the microscale. The possible remodeling of the ECM due to cell behavior also has not been discussed. Current research at the intersection of polymer chemistry and cell biology has focused mainly on materials whose properties are static and cannot be changed on demand. These studies, however, provide in-depth analyses related to the interactions between the cell and its neighboring ECM on the microscale. Although we know that the biological microenvironment is a dynamic system which can seldom be represented by homogeneous parameters alone, static polymer platforms have offered valuable explanations to help us comprehend the native tissue microenvironment to a certain extent. Some examples include the effect of stiffness on cell lines,[89] control over cell phenotype in a 2D environment,[39, 90] and the utility of polymeric constructs, especially smart hydrogels that can match biologically-relevant timescales,[91, 92] and potentially facilitate the eventual development of vascularized artificial tissues. Thus, for the purposes of this review, to include bi-directional functionality, these smart materials can be defined as materials that can not only regulate cell behavior but can also accept feedback from the same cells, and dynamically remodel their architecture in synergy with the cells. To achieve this, we discuss the various experimental, theoretical, and computational tools used in the studies on static cell culture as well as the interactions between the cells and their environment. The objective of the following section is to highlight the continual development of assays and techniques used for studying cell mechanobiology in the arena of static cell culture constructs and propose the adoption and application of some of these concepts to produce smart materials that can continually sense signals from cells and intervene autonomously should an anomaly arise.
Experimental, Theoretical, and Computational Biology Tools Used in Static Cell Culture
Biomaterials with fixed static properties have been used for over a decade as constructs to examine cell-biomaterial interaction. Due to their inherent limitations, current biomaterials with fixed properties are unable to recapitulate the complexity of the physiological environment.[2, 93–95] Smart biomaterials, on the other hand, allow for the manipulation of their properties upon the application of an external trigger and are highly sought after in the fields of bioengineering, biomedicine and polymer chemistry.[96, 97] Most of these existing materials, however, provide only a one-way or irreversible control over their responsive elements.[98–102] Additionally, these materials mainly act as a platform for measuring static cellular adhesion-based phenomena and are therefore limited in their ability to provide cues to the cells to observe dynamic processes such as attachment-detachment, migration, division, and differentiation. Therefore, a current challenge is focusing on smart material platforms that recapitulate the interplay between cells and their extracellular matrix with greater accuracy.
Many researchers have described experimental and theoretical techniques used when studying the interdependence between cells and their surrounding ECM. Guvendiren et al. developed hydrogels with tunable stiffness to study the responses of hepatic stellate cells during differentiation.[103] Aung et al. developed a reference-free quantitative assay for measuring 3D traction stressed generated during cell invasion into a Matrigel based ECM (Figure 5I).[60] Beningo et al. prepared polyacrylamide substrates for mouse embryonic fibroblasts and used microbeads to aid in their traction stress measurements and demonstrated that the mechanical properties of the substrate play an important role in defining fibroblast morphology (Figure 5III.).[57]
Figure 5.
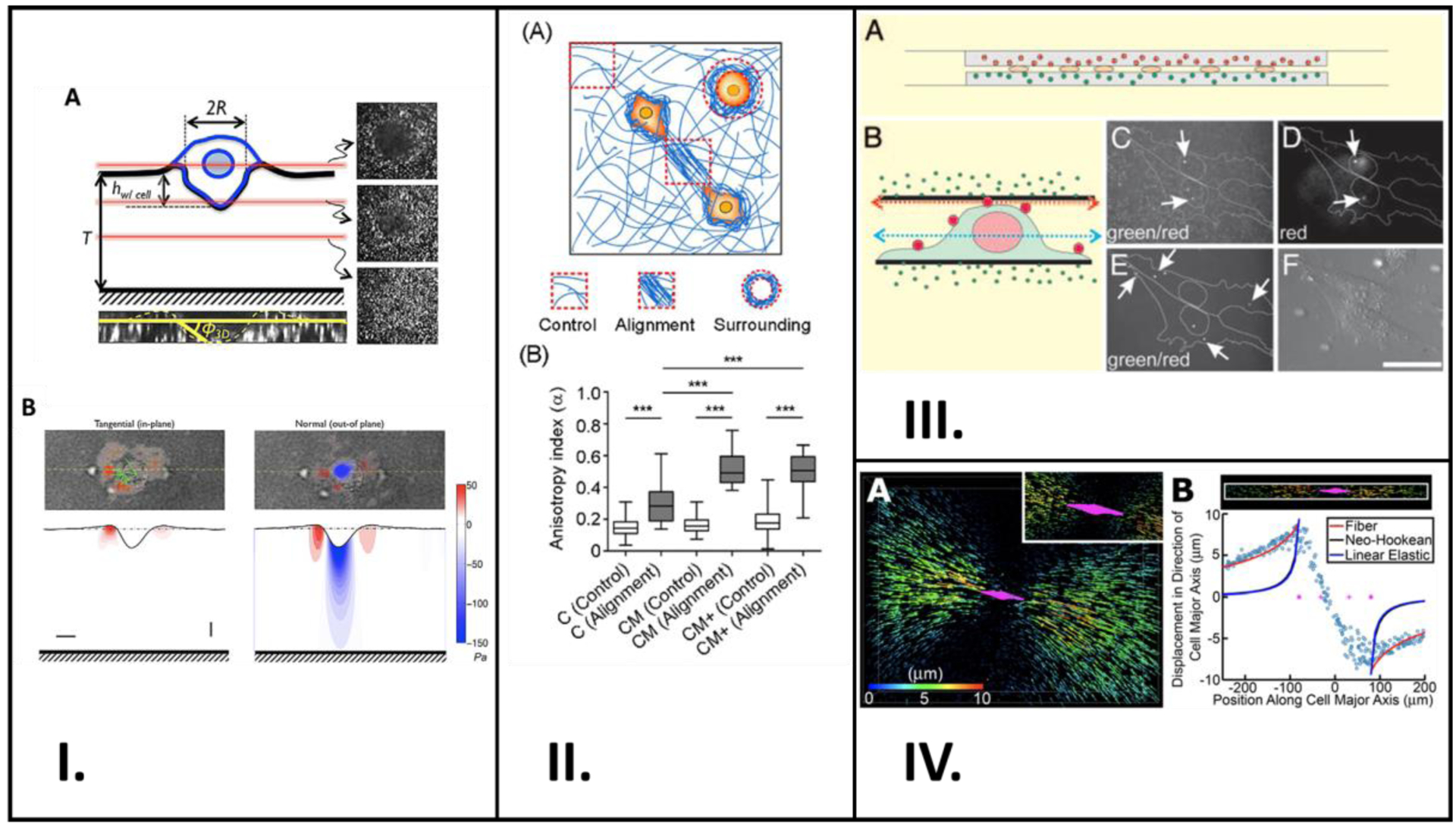
Studies involving application of experimental and theoretical techniques to study cell mechanotransduction. (I.) Schematic representation of a cancer cell invading Matrigel embedded with fluorescent beads are shown in (A). Confocal z-stacks shown are sectioned at the red lines. (B) shows two plots: tangential and normal traction stresses from deforming the Matrigel. (II.) (A) Schematic showing cells in orange and collagen fibers in blue, and the areas used in quantification. Measurements of control and alignment anisotropy in each hydrogel type are plotted in (B). (III.) (A) is a schematic showing the cultured NIH 3T3 cells between two sheets of polyacrylamide, and (B) shows the surface-labeling of the cells with polychromatic beads. (C) – (F) are all confocal microscopy images taken in the green channel focused at the red arow in (B), in the red channel focused at the red arrow in (B), in the green channel focused at the blue arrow in (B), and regularly, respectively. (IV.) Rendition of cell and displacement of fluorescent beads is shown in (A), with the cell in magenta and the displacement as arrows. Plot of displacements within a set radius along the cell’s major axis is shown in (B). A: Reproduced from reference [60], Copyright (2004) with permission Biophysical Society, B: Reproduced from reference [104], Copyright (2020) with permission from Creative Commons Attribution License 4.0 International, C: Reproduced from reference [57], Copyright (2004) with permission from National Academy of Sciences, U.S.A, D: Reproduced from reference [61], Copyright (2015) with permission from Creative Commons Attribution License 4.0 International.
Anguiano et al. used different hydrogel formulations with varying collagen and Matrigel content to study the alignment and remodeling of the ECM surrounding cancer cells.[104] Their study revealed that Matrigel promotes fiber alignment surrounding cancer cells which further encourages cells to migrate and continue aligning the fibers in their vicinity (Figure 5II.). This bi-directional relationship between cells and their surrounding environment was also shown by Kim et al. In their study, they discovered that HUVEC’s filopodia have the ability to reorganize their local ECM and the ECM can also control the filopodia’s actin fiber orientation; they employed computational methods to confirm those findings.[61] These findings of cell-ECM interdependence are also shared by Hall et al. in their research.[63] That group used fluorescent marker beads to study the relationship between breast cancer cells and their surrounding ECM, composed mainly of collagen (Figure 5IV.).
I. Experimental Techniques
Traction Force Microscopy (TFM)
Capabilities
TFM coupled with fluorescent beads has been increasingly implemented to probe forces exerted by cells on their underlying substrate (Figures 6I. and 6II.) [105–107] This technique excels at detecting microscopic traction forces between cells and their substrate with minimal adverse effects on the cells themselves.
Figure 6.
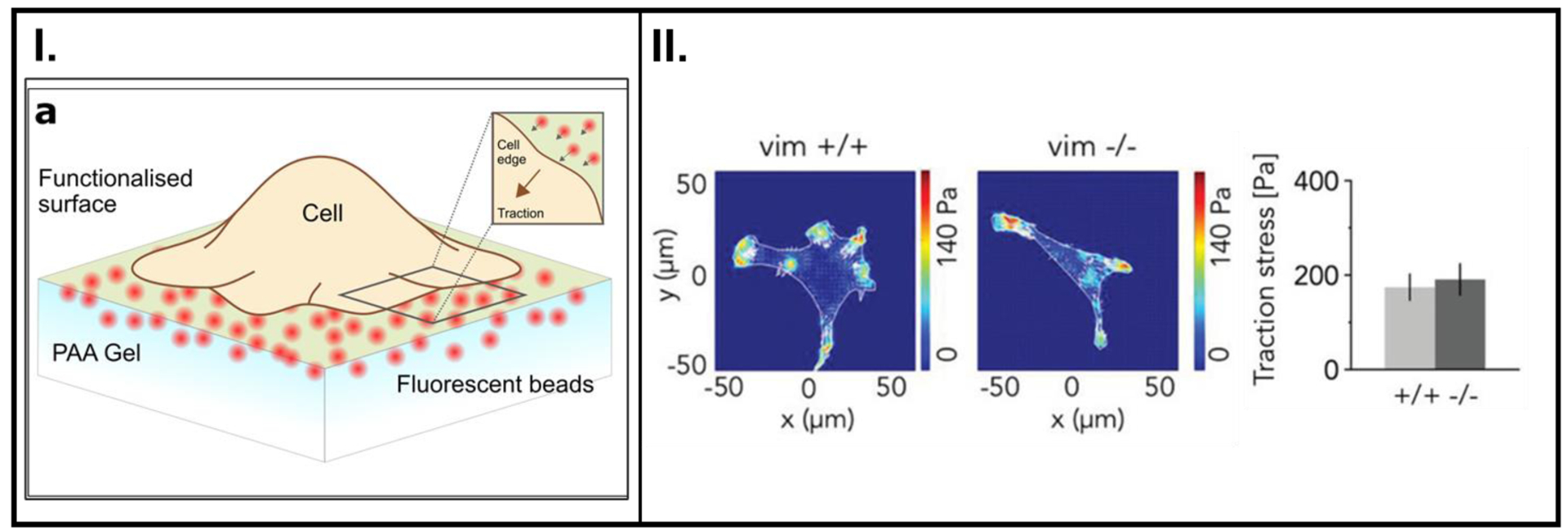
(I.) Schematic of a cell anchored onto a polymer substrate. Actomyosin chemistry inside the cells is the origin of contractions within the cell, and this tension is relayed to the substrate through focal adhesion (FA) complexes. This tension is known as cell traction force. FA proteins (integrins) form a connection to transmit forces between actin fibers from the cytoskeleton and the substrate. Among other processes, the quantification of cell traction forces is key to determining cell migratory behavior. (II.) Contractile forces within a cell quantified using TFM. The force charts (on the left) depict traction forces on synthetic polymer substrates. The graph on the right shows the traction forces generated during cell movement associated with the loss of a cytoskeletal filament protein, vimentin. A: Reproduced with permission from reference [105], Copyright (2018) Elsevier, B: Reproduced with permission from reference [106], Copyright (2019) John Wiley and Sons.
Limitations
Despite significant advances and the widespread adoption of this technology, measuring these forces in 2-D has an inherent disadvantage since it is not an accurate representation of the native environment. While many research laboratories can track cell traction forces in 2-D, only a few multi-disciplinary research groups have now moved on to recapitulating in vivo conditions by exploiting three-dimensional (3-D) cell tracking of traction forces.[60, 108, 109]
In addition, although this technology enables the incorporation of several physiological stresses and biologically relevant parameters such as pH into the experimental design, complex deconvolution software is needed to interpret the displacement of the fluorescent beads and correlate it with stressors.
Fӧrster Resonance Energy Transfer (FRET)
Capabilities
FRET is a molecular-level probing technique that can detect tension between a FRET fluorophore pair (donor-acceptor) separated by a finite distance by photon-mediated coupling.[60, 107, 108] Since FRET phenomenon occurs at distances of ≤ 10 nm, deformations in the proteins as a result of mechanotransduction via the cytoskeletal elements (actin filament, ribosomes, Golgi apparatus, lysosomes, etc.) can be quantified by changes in emission spectra as a result of perturbations in the separation distance, allowing detection of forces at the single focal-adhesion scale (Figure 7).
Figure 7.
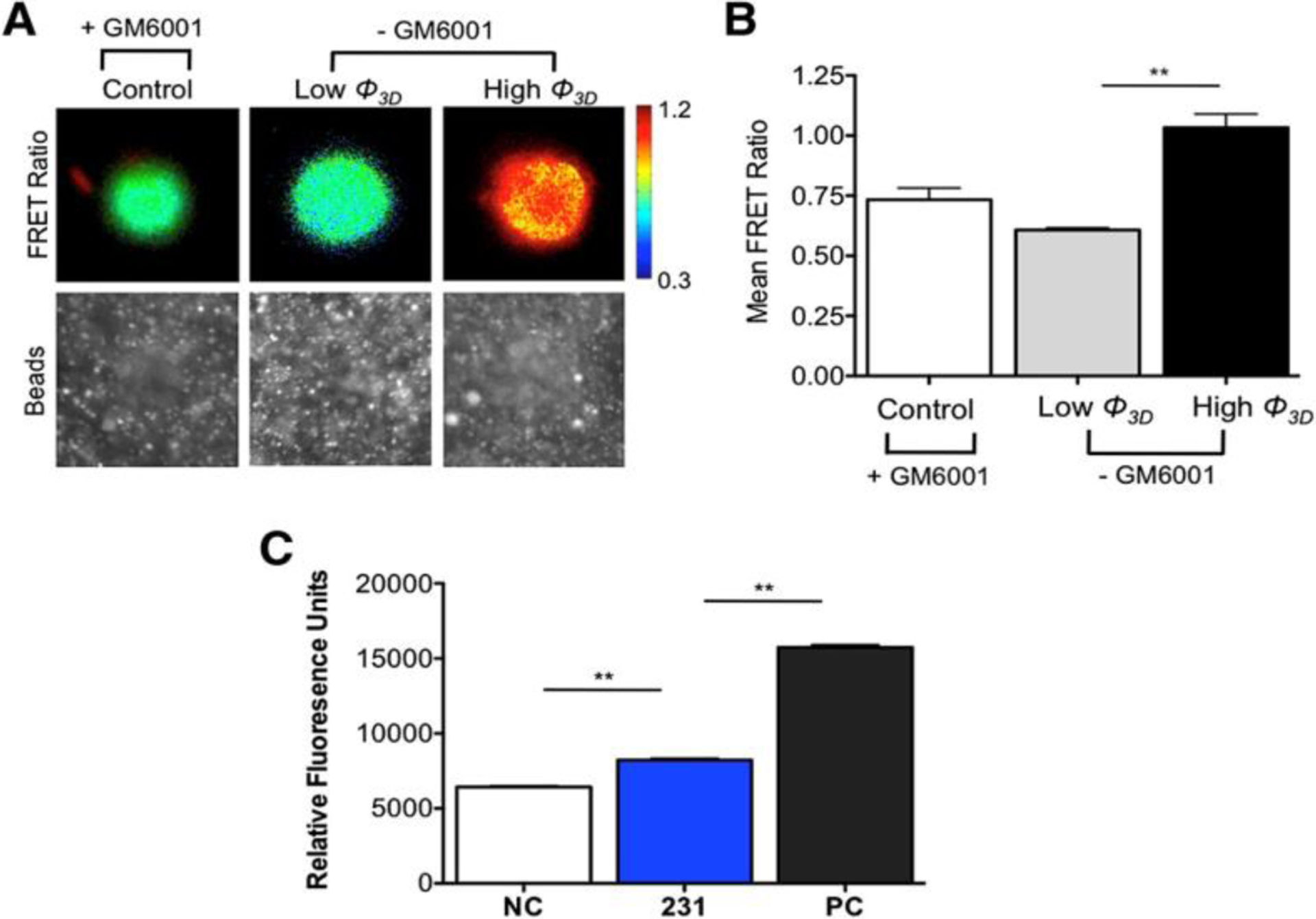
Cells transfected with the MT1-MMP FRET biosensor showed the MMP activity of cells infiltrating a Matrigel ECM at low (<10°) and high (>20°) angles of indentation (Φ) when cells invade and deform a 3D ECM; MMP activity was higher for the higher angle of indentation as seen by the heat map in (A) and mean FRET ratios presented in (B), The degradation of the Matrigel substrate correlated with the activity of MMP enzymes by analyzing the growth media via zymography (fluorogenic peptide assay) showed activity of MMPs for negative controls (NC), MDA-MB-231 cells (231), and positive controls (PC) (C). A: Reproduced with permission from reference [60], Copyright (2014) Elsevier.
Limitations
Despite appreciable advances, FRET-based tracking of cell forces is dependent on the chromophore-based tagging of native proteins, which might, in turn, disturb the original spacing, resulting in non-uniform adoption by different research groups. Additionally, FRET emission data can only provide the value of the force and not the direction vector. Therefore, using FRET to quantify 3D ECM systems might pose a challenge for the instrumentation and processing capabilities many laboratories.
FRET, and fluorescent marker beads are just some of the experimental techniques currently used; there is great potential for more robust experimental techniques. In addition, as theoretical and computational tools mature, more researchers will use those techniques to complement and augment their experimental procedures. In the ensuing sections, we will provide a review of some popular cell – ECM analysis techniques, which have been used extensively in cell biology but still relatively foreign to materials research.
The above techniques such as TFM, FRET, and fluorescent beads, along with other seldom used novel methods like QCM,[15] that are used to track ECM-cell interactions often demand interdisciplinary efforts that can only be achieved by the integration of specialized scientists from a wide array of expertise. At the very minimum, collaboration between domains such as materials science, polymer chemistry, chemistry, and theoretical biology is necessary. In addition, understanding the proliferation of cells seeded in 3D ECM constructs involves 3D cell tracking using molecular beads. This requires knowledge of computational biology and chemistry to effectively translate the relative motions of the beads into meaningful stressors to obtain magnitude and displacement information. While one angle to look at such experiments is from the ECM point of view (i.e., determining how the changes in the ECM dictate cell fate), another angle serves to understand the role of embedded cells in reorganizing their surrounding ECM to direct their own fate. Although both perspectives are essential for a well-rounded study, each requires training for specialized technicians, purchase of costly instrumentation, and maintenance, as has been previously noted.[110] Notwithstanding recent progress made in equipment and machinery that can be used for characterizing the aforementioned interdependence between cells and their surrounding ECM their widespread adoption still remains a challenge. Therefore, there is a need for data processing techniques that can be used to not only supplement experimental evidence, but to also extrapolate underlying mathematical equations to obtain predictive data.
II. Theoretical and Computational Biology Techniques
The growing interest in the introduction of mathematical modeling to cell-ECM experiments led to a handful of research groups adopting the use of algorithms in the late 1990s-early 2000s.[111–113] Since the inception of this concept, major advances have been enumerated recently by several studies. One of these reports employed CompuCell3D v3.6.0 (http://www.compucell3d.org) for agent-based modeling of the development of new blood vessels linked to the growth of multiple cell lines and ECM remodeling.[114]. Reinhardt et al.[62] also developed an agent-based model using NetLogo 4.1.1 with supporting algorithms. That study captured dynamic cell migration as well as remodeling of a fibrous ECM network by cells. NetLogo is a programming language similar in nature to MATLAB specifically designed for agent-based modeling.
Very recently, an agent-based approach for developing a simulation model was also taken by Park et al.[115] The agent-based system was an adaptation of the Vicsek model of predicting ECM alignment from collisions between cancer-associated fibroblasts, and the researchers utilized software such as CT-FIRE, MATLAB, and METAMORPH as well as coding in C++ to obtain and analyze biological data. CT-FIRE and METAMORPH are both software used in analyzing cell images, with CT-FIRE being specialized for extracting data about individual collagen fibers such as angle and length. In a study done by Ahmadzadeh et al., computational interpretation was used to predict the invasion of tumorous cells into the surrounding ECM by designing the model around the experimental observation that the polarization of cells increased with increasing concentrations of collagen in the ECM.[116] Several groups of scientists have developed algorithms for anticipating cell migration through a 3D matrix by specifically observing the morphology of filopodia and the interplay between cell and ECM fibers.[61] Moreover, Hall et al.[63] also used MATLAB to analyze 3D photos of fluorescent beads and implemented a tracking algorithm to determine the cell-dependent deformation of a 3D collagenous matrix.[63]
Combining expertise from chemical, biological, and molecular engineering, Gjorevski et al., used various experimental and computational techniques, such as fluorescence beads and Imaris (Bitplane) software, to track and correlate cell movement with the deformations of the 3-D collagenous ECM.[31] Real-time 3D confocal microscopy images of these beads and GFP-labelled cells were obtained in undeformed and deformed states during migration of the tissue cohort obtained from bead displacements using Autoregressive Motion tracking protocol in Imaris. The Amira Division suite from Visage Imaging was utilized to create a 3D surface from the images before transoforming them into a Parasolid file using Mesh2Solid (Sycode). In Comsol Multiphysics 4.2a (Comsol Inc., Stockholm, Sweden), the solid tissue construct was introduced inside a cylindrical geometry representing the collagen ECM and converted into a finite element mesh.
Due to the nuances of cell-ECM interactions coupled with constant evolution of computing architecture, a common programming language or image processing tool still eludes the scientific community pursuing this research.
Where is the field headed?
This review describes the advancements in the development of smart materials used to reversibly manipulate cell activity and thestate-of-the-art means to study cell activity on static substrates with a focus on employing those means to investigate potential remodeling of a smart ECM by cell activity. Continued existence of this dichotomy represents a hurdle to the study of interactions between cells and the ECM. Research efforts in the direction of mimicking the in vivo environment of cells and examinations of the effect of physiologically relevant stimuli on the cell-ECM interaction forefront have led to tremendous growth in the development of smart materials. Although some smart platforms have demonstrated dynamic and reversible perturbations for a limited number of reversible and repeatable cycles,[14, 18–20, 24, 29, 36, 39, 50, 53] the progress to date still does not permit the levels of consistent repeatability nor the longevity of the polymer network with respect to sustaining repeated triggers as might be required by biological systems.[117]
The understanding of mechanical properties related to ECM remodeling established by Hall et al.[63] can be extrapolated to concepts such as measurements of rheological properties of a biopolymer with physical crosslinks that are dependent on the applied frequency and strain. This can then be compared to a covalently crosslinked polymer whose rheological properties are expected to be independent of applied strain and frequency (in the linear, elastic regime. While the majority of cell survivability and migration in 2D and 3D extracellular networks has focused on ECM stiffness and crosslinking, ECM viscoplasticity, a phenomenon in which the ECM undergoes plastic deformation under constant stress, still remains to be fully explored.[118, 119] The migration strategy of cancer cells through a given ECM could be proteolytically driven or independent of it. Recent studies have revealed that in a high plasticity polymer network, encapsulated cancer cells manufacture longer invasive protrusions.[119] In addition to this, they also apply larger actomyosin-originated contractile forces to create permanent pathways to support migration. A modeling-based approach has also been previously used to confirm that in case of popular biomaterials such as collagen and fibrin used to prepare 3D networks for cell encapsulation, in addition to covalent crosslinking, irreversible (plastic) remodeling of the ECM was affected by of events including cellular forces generated by actin contraction, and its transmission to the ECM by integrins. Mechanical properties of a biopolymer also point to the idea that even though biological network of tissues can represent isotropic mechanical properties at the macro scale, the local micro/nanoscopic microenvironment is generally anisotropic, highlighting the importance of focusing on the utility of the 3D architecture recreated to represent the native structural component of the ECM at a relevant length scale.
The report by Kim et al.[120] lays down the foundation for a quantitative understanding of cell-ECM interdependence in that – (i) the microstructural organization of cytoskeletal components of a cell (e.g., actin) can be impacted by the cues presented to it by the ECM (nanostructured PUA), and (ii) higher contractile forces exerted by the cell can cause significant remodeling of the enveloping microenvironment (collagen type I). These findings point toward the existence of a ‘positive feedback,’ which essentially emphasizes the fact that the mechanical forces transmitted from the ECM topography to the cellular framework cause conformational changes within the cell. The cell in turn can also reorganize the complex structure that provides support to them. Overall, probing the interdependence between cells and their surrounding ECM stiffness can further contribute towards the understanding of the metastasis of highly invasive cells, especially those related to the spread of different types of cancer.
Cell-ECM interactions are also greatly influenced by the interplay between cell and nascent proteins, and the production of these proteins relies on a complex sequence including their origin in mRNAs, specialized folding into its designated shape, and dispatch to its intended function-related location. The design of an appropriate ECM network is crucial for studying and modulating this cell-nascent protein interplay. In case of certain ECMs, cells have been shown to probe the physical properties of their pericellular space and determine whether it needs stiffening or softening to create conditions conducive to processes such as preferential differentiation into a specific lineage. By means of degradable and dynamic viscoelastic hydrogel platforms and YAP/TAZ assays, researchers have also demonstrated the role of nascent proteins secreted by cells in cell-ECM interactions.[121] In the case of a proteolytically-degradable ECM, in addition to synthetic cell-adhesion ligands incorporated into the ECM, the nascent proteins deposited by cells were critical in determining their preference towards osteogenic differentiation. For dynamic viscoelastic hydrogels, nascent proteins deposited by cells combined with a predominant dissipative network offered a moldable platform which contributed to favorable cell spreading. The remodeling of the nascent proteins also played a key role in determining cell fate.[122]
Overall, only a handful of studies have been able to assimilate physiologically relevant time scales that dictate biological processes such as stiffening of native tissue and the associated cellular behavior such as differentiation, into the design of smart materials.[37, 39] It is thus clear that an all-encompassing model would include perspectives from both the synthetic smart material and the native cell environment.
To achieve this, we envision the intersection of two areas —
Smart biomaterials that have been employed to control reversibly cell activity. These materials tend to be of synthetic origin and allow for the robust control of cell activity. Generally, they are modified with either biomimetic polymers or cell adhesive ligands to promote cell adhesion. As such, even if the change in properties of the smart material is reversible and repeatable, it is currently limited to the stimuli applied by user inputs. Moreover, this platform does not allow the characterization of the impacts of cell activity on the smart materials.
Static biomaterials used to examine dynamic changes in cell behavior. Traditionally, cell culture scientists have relied on collagen- or Matrigel-based hydrogels because they offer favorable protein sites for cell attachment. In these cases, the scientists are interested in probing the dynamicity of cell activities while maintaining the substrate under a fixed or passive state.
Concluding Remarks and Future Outlook
A. Design of smart ECM based on feedback loops that impact the cell-ECM microenvironment
In the context of biologically derived materials that are used as ECM constructs, a better understanding of signaling pathways that dominate such feedback loops in the cell-cell[56] and cell-ECM systems holds immense potential in unraveling significant advances such as predicting the development of the metastases pathways and the consequent remodeling of native ECM in case of cancer.[123] Although the progression of cancer involves an intricate web of several oncogenic pathways, it is also an exemplary case of a positive feedback mechanism between tumor cells, normal cells, and the ECM.[124] It is well-known that a majority of cancers have their origins in epithelial cells. Moreover, the deposition of collagenous ECM fibers by cancer-activated fibroblasts leads to continued ECM stiffening, causing an increase in the contractile forces generated, which, in turn, promotes further hardening. Mutations in the growth factors peptide sequences can also lead to the silencing of signal regulators that would function to suppress the tumor under normal circumstances.[124] If negative feedback played a dominating role in cancer, one of many consequences would mean that the ECM could become softer and arrest the aggressive spread of tumor cells. However, this may not always be the case. Previous research shows that certain proteins responsible for transforming normal cells into cancer cells can be non-responsive to negative feedback.[125, 126] As such, it is not the absence, but the desensitization of such systems to negative feedback that could lead to rapid disease progression.
Smart materials that can recreate the native environment of cells and provide a snapshot of the potential biochemical activity in response to engineered cues — generally referred to as bioresponsive polymers, are of great interest. For example, the polymer platform introduced in Loebel et al. describes a smart 3-D platform that allows the encapsulated cells to (i) degrade existing peptide crosslinks and (ii) deposit their own ECM proteins.[121] This dual effect enhances cells spreading. Another innovative platform introduced by Fonseca et al. comprised of an injectable MMP-sensitive alginate hydrogel.[127] They demonstrated that hMSC-encapsulated hydrogels deposited their own ECM and facilitated accelerated wound healing in mouse models. The conception of smart materials that can either inculcate feedback from cells or allow for external triggers that can emulate or contradict the feedback bear tremendous significance in the advance of this multi-disciplinary research arena.
B. Complexity of algorithms and simulations limits the exploration of smart reversible constructs to examine cell mechanobiology
It is clear that because of the complexity involving ECM composition, specific cell type response to these ECMs, and period of maturity of structures involved in cell migration, there is currently no consensus on the software or algorithms used for predicting cell-ECM interactions. Despite substantial progress in this area, the predictive data desired from machine learning algorithms still often rely on 3D images of fluorescent beads and cell-specific protrusions obtained from confocal microscopy. Since many of these studies are performed on polymer constructs with fixed properties, it would be constructive to have worldwide access to a consolidated online database of these images so that any errors stemming from 3D confocal microscopy could be eliminated. Furthermore, efforts may be directed at the inclusion of more parameters such as maturity of lamellipodia in the predictive simulation models with an aim of refining the current computation algorithms and leveraging the new data to obtain more accurate cell migration and consequent ECM remodeling results. An optimal representation of the cell-ECM interdependence could involve a system that permits on-demand or dynamic remodeling or responsiveness while cells interact with the synthetic ECM microstructure; its characterization is a crucial challenge in the design of smart ECM materials to emulate the local and global environment of native tissue.[128–130]
C. Potential design of a smart material that can sense cells’ signals and be triggered repeatedly
Over the past decade, smart polymeric platforms with tunable properties have been exploited to mirror physiological conditions and exercise reversible control over cellular activity. Furthermore, remodeling of the ECM architecture by resident cells has also been increasingly documented. However, although several mechanobiological responses of cells have been documented by utilizing modulation of polymeric properties, the role played by the cells in remodeling smart materials in combination with repeated stimuli being applied to the materials is not understood yet. Taken together, we currently do not have control over restoring the properties of the ECM which was remodeled due to cellular activity. Under such circumstances, smart materials with dynamically tunable properties that can counteract or promote the remodeling performed by the cells can be envisaged. Such a smart material could repeatedly sense an external trigger and return to its original state by sensing an external stimulus and will also be capable of sensing other biological signals generated due to cellular activity. The cyclic changes thus produced in the biomaterial will enable continuous monitoring, data collection, and analysis. Moreover, large volumes of data collected will strengthen the possibilities of applying machine learning algorithms and modeling of complex cell mechanotransduction operations in the analysis. This will help coalesce the fields of smart biomaterials and cell biology to create advanced biomaterials with tailored properties.
Scheme 2.
The interdependence between polymeric materials and cells used in static culture experiments.
Highlights.
Smart materials with dynamic properties that offer reversible control over cell adhesion, proliferation, or differentiation.
The disconnect between dynamic and static biomaterials with respect to evaluating cell-ECM interactions.
Utilizing oft-used tools and combining them with seldom used techniques to offer a new perspective towards evaluating the bi-directional interplay between smart biomaterials and cells.
Acknowledgements
We gratefully acknowledge funding from the National Science Foundation’s Biomaterials and Advanced Manufacturing programs (DMR-1609523 and CMMI-2022421). This work was also partially supported by NIH R21AR076645 and R21AR076642. We also express gratitude towards Kiersten Edwards for scientific discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Mohamed MA, et al. , Stimuli-responsive hydrogels for manipulation of cell microenvironment: From chemistry to biofabrication technology. Progress in Polymer Science, 2019. 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Place ES, Evans ND, and Stevens MM, Complexity in biomaterials for tissue engineering. Nat Mater, 2009. 8(6): p. 457–70. [DOI] [PubMed] [Google Scholar]
- 3.Cox TR and Erler JT, Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech, 2011. 4(2): p. 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piraino F and Selimović Š, A Current View of Functional Biomaterials for Wound Care, Molecular and Cellular Therapies. BioMed Research International, 2015. 2015: p. 403801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soufi A and Dalton S, Cycling through developmental decisions: how cell cycle dynamics control pluripotency, differentiation and reprogramming. 2016. 143(23): p. 4301–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uto K, et al. , Dynamically Tunable Cell Culture Platforms for Tissue Engineering and Mechanobiology. Prog Polym Sci, 2017. 65: p. 53–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratner BD, Biomaterials: Been There, Done That, and Evolving into the Future. Annu. Rev. Biomed. Eng, 2019. 21(1): p. 171–191. [DOI] [PubMed] [Google Scholar]
- 8.Narkar AR, et al. , pH Responsive and Oxidation Resistant Wet Adhesive based on Reversible Catechol–Boronate Complexation. Chem. Mater, 2016. 28(15): p. 5432–5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peppas NA, et al. , Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Advanced Materials, 2006. 18(11): p. 1345–1360. [Google Scholar]
- 10.Khan F and Tanaka M, Designing Smart Biomaterials for Tissue Engineering. Int. J. Mol. Sci, 2018. 19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu HY, et al. , Enzyme-mediated stiffening hydrogels for probing activation of pancreatic stellate cells. Acta Biomater, 2017. 48: p. 258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H-Y, Korc M, and Lin C-C, Biomimetic and enzyme-responsive dynamic hydrogels for studying cell-matrix interactions in pancreatic ductal adenocarcinoma. Biomaterials, 2018. 160: p. 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhan W, et al. , Sweet Switch: Sugar-Responsive Bioactive Surfaces Based on Dynamic Covalent Bonding. ACS Appl Mater Interfaces, 2018. 10(13): p. 10647–10655. [DOI] [PubMed] [Google Scholar]
- 14.Karimi F, et al. , Dynamic Covalent Hydrogels for Triggered Cell Capture and Release. Bioconjug Chem, 2017. 28(9): p. 2235–2240. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, et al. , Dual-responsive surfaces modified with phenylboronic acid-containing polymer brush to reversibly capture and release cancer cells. J Am Chem Soc, 2013. 135(20): p. 7603–9. [DOI] [PubMed] [Google Scholar]
- 16.Yoshikawa HY, et al. , Quantitative evaluation of mechanosensing of cells on dynamically tunable hydrogels. J Am Chem Soc, 2011. 133(5): p. 1367–74. [DOI] [PubMed] [Google Scholar]
- 17.Bian Q, et al. , Light-Triggered Specific Cancer Cell Release from Cyclodextrin/Azobenzene and Aptamer-Modified Substrate. ACS Appl Mater Interfaces, 2016. 8(40): p. 27360–27367. [DOI] [PubMed] [Google Scholar]
- 18.Edahiro J. i., et al. , In Situ Control of Cell Adhesion Using Photoresponsive Culture Surface. Biomacromolecules, 2005. 6(2): p. 970–974. [DOI] [PubMed] [Google Scholar]
- 19.Li W, et al. , Noninvasive and Reversible Cell Adhesion and Detachment via Single-Wavelength Near-Infrared Laser Mediated Photoisomerization. J Am Chem Soc, 2015. 137(25): p. 8199–205. [DOI] [PubMed] [Google Scholar]
- 20.Cui H, et al. , Near-infrared (NIR) controlled reversible cell adhesion on a responsive nano-biointerface. Nano Research, 2017. 10(4): p. 1345–1355. [Google Scholar]
- 21.Liu D, et al. , Using azobenzene-embedded self-assembled monolayers to photochemically control cell adhesion reversibly. Angew Chem Int Ed Engl, 2009. 48(24): p. 4406–8. [DOI] [PubMed] [Google Scholar]
- 22.Rosales AM, et al. , Hydrogels with Reversible Mechanics to Probe Dynamic Cell Microenvironments. Angew Chem Int Ed Engl, 2017. 56(40): p. 12132–12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stowers RS, Allen SC, and Suggs LJ, Dynamic phototuning of 3D hydrogel stiffness. Proc Natl Acad Sci U S A, 2015. 112(7): p. 1953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kollarigowda RH, et al. , Light-responsive polymer brushes: active topographic cues for cell culture applications. Polymer Chemistry, 2017. 8(21): p. 3271–3278. [Google Scholar]
- 25.Yoon J, et al. , Local switching of chemical patterns through light-triggered unfolding of creased hydrogel surfaces. Angew Chem Int Ed Engl, 2012. 51(29): p. 7146–9. [DOI] [PubMed] [Google Scholar]
- 26.Miyata T, Asami N, and Uragami T, A reversibly antigen-responsive hydrogel. Nature, 1999. 399(6738): p. 766–769. [DOI] [PubMed] [Google Scholar]
- 27.Levental KR, et al. , Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell, 2009. 139(5): p. 891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong T, et al. , Thermally activated reversible shape switch of polymer particles. J Mater Chem B, 2014. 2(39): p. 6855–6866. [DOI] [PubMed] [Google Scholar]
- 29.Uto K, Ebara M, and Aoyagi T, Temperature-responsive poly(epsilon-caprolactone) cell culture platform with dynamically tunable nano-roughness and elasticity for control of myoblast morphology. Int J Mol Sci, 2014. 15(1): p. 1511–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, et al. , Smart Thin Hydrogel Coatings Harnessing Hydrophobicity and Topography to Capture and Release Cancer Cells. Small, 2016. 12(34): p. 4697–701. [DOI] [PubMed] [Google Scholar]
- 31.Gjorevski N, et al. , Dynamic tensile forces drive collective cell migration through three-dimensional extracellular matrices. Sci Rep, 2015. 5: p. 11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong JY, Langer Robert, and Ingber Donald E., Electrically conducting polymers can noninvasively control the shape and growth of mammalian cells. Proceedings of the National Academy of Sciences, 1994. 91(8): p. 3201–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng CC, et al. , Using an electrical potential to reversibly switch surfaces between two states for dynamically controlling cell adhesion. Angew Chem Int Ed Engl, 2012. 51(31): p. 7706–10. [DOI] [PubMed] [Google Scholar]
- 34.Kim YJ, et al. , Dual stimuli-responsive smart beads that allow “on-off” manipulation of cancer cells. Biomater Sci, 2016. 4(6): p. 953–7. [DOI] [PubMed] [Google Scholar]
- 35.Jiang X, Ferrigno R, Mrksich M, & Whitesides GM, Electrochemical Desorption of Self-Assembled Monolayers Noninvasively Releases Patterned Cells from Geometrical Confinements. J. Am. Chem. Soc, 2003. 125(9). [DOI] [PubMed] [Google Scholar]
- 36.Sui Z, King WJ, and Murphy WL, Dynamic Materials Based on a Protein Conformational Change. Advanced Materials, 2007. 19(20): p. 3377–3380. [Google Scholar]
- 37.Das RK, et al. , Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat Mater, 2016. 15(3): p. 318–25. [DOI] [PubMed] [Google Scholar]
- 38.Caliari SR, et al. , Gradually softening hydrogels for modeling hepatic stellate cell behavior during fibrosis regression. Integrative Biology, 2016. 8(6): p. 720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guvendiren M and Burdick JA, Stem cell response to spatially and temporally displayed and reversible surface topography. Adv Healthc Mater, 2013. 2(1): p. 155–64. [DOI] [PubMed] [Google Scholar]
- 40.Bao G and Suresh S, Cell and molecular mechanics of biological materials. Nature materials, 2003. 2(11): p. 715–725. [DOI] [PubMed] [Google Scholar]
- 41.Ingber DE, Cellular mechanotransduction: putting all the pieces together again. FASEB J, 2006. 20(7): p. 811–27. [DOI] [PubMed] [Google Scholar]
- 42.Kustra S and Bettinger CJ, Smart polymers and interfaces for dynamic cell-biomaterials interactions. MRS Bulletin, 2012. 37(9): p. 836–846. [Google Scholar]
- 43.Vats K and Benoit DS, Dynamic manipulation of hydrogels to control cell behavior: a review. Tissue Eng Part B Rev, 2013. 19(6): p. 455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alves NM, et al. , Controlling cell behavior through the design of polymer surfaces. Small, 2010. 6(20): p. 2208–20. [DOI] [PubMed] [Google Scholar]
- 45.Zhang K, et al. , Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res, 2018. 6: p. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang W, et al. , Tuning the Poisson’s Ratio of Biomaterials for Investigating Cellular Response. Adv Funct Mater, 2013. 23(25): p. 10.1002/adfm.201202666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosales AM and Anseth KS, The design of reversible hydrogels to capture extracellular matrix dynamics. Nat Rev Mater, 2016. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sternlicht MD and Werb Z, How Matrix Metalloproteinases Regulate Cell Behavior. Annu. Rev. Cell. Dev. Biol, 2001. 17(1): p. 463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winkler J, et al. , Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nature Communications, 2020. 11(1): p. 5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdeen AA, et al. , Temporal Modulation of Stem Cell Activity Using Magnetoactive Hydrogels. Adv Healthc Mater, 2016. 5(19): p. 2536–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corbin EA, et al. , Tunable and Reversible Substrate Stiffness Reveals a Dynamic Mechanosensitivity of Cardiomyocytes. ACS Appl Mater Interfaces, 2019. 11(23): p. 20603–20614. [DOI] [PubMed] [Google Scholar]
- 52.Vining KH, Stafford A, and Mooney DJ, Sequential modes of crosslinking tune viscoelasticity of cell-instructive hydrogels. Biomaterials, 2019. 188: p. 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu H, et al. , Hydrophobic interaction-mediated capture and release of cancer cells on thermoresponsive nanostructured surfaces. Adv Mater, 2013. 25(6): p. 922–7. [DOI] [PubMed] [Google Scholar]
- 54.Discher DE, Janmey P, & Wang YL, Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science, 2005. 310(5751): p. 1139–1143. [DOI] [PubMed] [Google Scholar]
- 55.Hannezo E and Heisenberg CP, Mechanochemical Feedback Loops in Development and Disease. Cell, 2019. 178(1): p. 12–25. [DOI] [PubMed] [Google Scholar]
- 56.Petridou NI, Spiro Z, and Heisenberg CP, Multiscale force sensing in development. Nat Cell Biol, 2017. 19(6): p. 581–588. [DOI] [PubMed] [Google Scholar]
- 57.Beningo KA, Dembo M, and Wang Y.-l., Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proceedings of the National Academy of Sciences, 2004. 101(52): p. 18024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rijal G and Li W, A versatile 3D tissue matrix scaffold system for tumor modeling and drug screening. Science Advances, 2017. 3(9): p. e1700764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koch TM, et al. , 3D Traction forces in cancer cell invasion. PLoS One, 2012. 7(3): p. e33476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aung A, et al. , 3D traction stresses activate protease-dependent invasion of cancer cells. Biophys J, 2014. 107(11): p. 2528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim MC, et al. , Cell Invasion Dynamics into a Three Dimensional Extracellular Matrix Fibre Network. PLoS Comput Biol, 2015. 11(10): p. e1004535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reinhardt JW, Krakauer DA, and Gooch KJ, Complex matrix remodeling and durotaxis can emerge from simple rules for cell-matrix interaction in agent-based models. J Biomech Eng, 2013. 135(7): p. 71003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hall MS, et al. , Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs. Proc Natl Acad Sci U S A, 2016. 113(49): p. 14043–14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Legant WR, et al. , Microfabricated Tissue Gauges to Measure and Manipulate Forces from 3D Microtissues. Proceedings of the National Academy of Sciences of the United States of America, 2009. 106(25): p. 10097–10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brunato S, et al. , Thermosensitive “Smart” Surfaces for Biorecognition Based Cell Adhesion and Controlled Detachment. Macromol. Biosci, 2021. 21(2): p. 2000277. [DOI] [PubMed] [Google Scholar]
- 66.Masamichi N, et al. , Visible-light induced cell detachment system using smart fluoropolymer-coated surface. Frontiers in Bioengineering and Biotechnology, 2016. [Google Scholar]
- 67.Yoshikawa HY, et al. , Quantitative Evaluation of Mechanosensing of Cells on Dynamically Tunable Hydrogels. J. Am. Chem. Soc, 2011. 133(5): p. 1367–1374. [DOI] [PubMed] [Google Scholar]
- 68.Gong Y-H, et al. , Photoresponsive “Smart Template” via Host–Guest Interaction for Reversible Cell Adhesion. Macromolecules, 2011. 44(19): p. 7499–7502. [Google Scholar]
- 69.Wang N, et al. , High-Strength Photoresponsive Hydrogels Enable Surface-Mediated Gene Delivery and Light-Induced Reversible Cell Adhesion/Detachment. Langmuir, 2014. 30(39): p. 11823–11832. [DOI] [PubMed] [Google Scholar]
- 70.Ruskowitz ER and DeForest CA, Photoresponsive biomaterials for targeted drug delivery and 4D cell culture. Nature Reviews Materials, 2018. 3(2): p. 17087. [Google Scholar]
- 71.Canavan HE, et al. , Surface Characterization of the Extracellular Matrix Remaining after Cell Detachment from a Thermoresponsive Polymer. Langmuir, 2005. 21(5): p. 1949–1955. [DOI] [PubMed] [Google Scholar]
- 72.Cicotte KN, et al. , Optimization of electrospun poly(N-isopropyl acrylamide) mats for the rapid reversible adhesion of mammalian cells. Biointerphases, 2017. 12(2): p. 02C417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balint R, Cassidy NJ, and Cartmell SH, Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater, 2014. 10(6): p. 2341–53. [DOI] [PubMed] [Google Scholar]
- 74.Pranzetti A, et al. , An electrically reversible switchable surface to control and study early bacterial adhesion dynamics in real-time. Adv Mater, 2013. 25(15): p. 2181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma Y, et al. , Dynamic Synthetic Biointerfaces: From Reversible Chemical Interactions to Tunable Biological Effects. Acc Chem Res, 2019. 52(6): p. 1611–1622. [DOI] [PubMed] [Google Scholar]
- 76.Miyata T, Kawamura A, and Uragami T, Rational design of target biomolecule-responsive hydrogels using biomolecular complex crosslinks. Front. Bioeng. Biotechnol, 2016. [Google Scholar]
- 77.Nicolas J, et al. , 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules, 2020. 21(6): p. 1968–1994. [DOI] [PubMed] [Google Scholar]
- 78.Pardo A, et al. , Magnetic Nanocomposite Hydrogels for Tissue Engineering: Design Concepts and Remote Actuation Strategies to Control Cell Fate. ACS Nano, 2021. 15(1): p. 175–209. [DOI] [PubMed] [Google Scholar]
- 79.Van Huizen AV, et al. , Weak magnetic fields alter stem cell–mediated growth. Science advances, 2019. 5(1): p. eaau7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Homma K, et al. , Design of azobenzene-bearing hydrogel with photoswitchable mechanics driven by photo-induced phase transition for in vitro disease modeling. Acta Biomaterialia, 2021. 132: p. 103–113. [DOI] [PubMed] [Google Scholar]
- 81.Guvendiren M and Burdick JA, Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nature Communications, 2012. 3(1): p. 792. [DOI] [PubMed] [Google Scholar]
- 82.Prince E and Kumacheva E, Design and applications of man-made biomimetic fibrillar hydrogels. Nature Reviews Materials, 2019. 4(2): p. 99–115. [Google Scholar]
- 83.Jiang T, et al. , Engineering bioprintable alginate/gelatin composite hydrogels with tunable mechanical and cell adhesive properties to modulate tumor spheroid growth kinetics. Biofabrication, 2019. 12(1): p. 015024. [DOI] [PubMed] [Google Scholar]
- 84.Ort C, et al. , Bioprintable, Stiffness-Tunable Collagen-Alginate Microgels for Increased Throughput 3D Cell Culture Studies. ACS Biomaterials Science & Engineering, 2021. [DOI] [PubMed] [Google Scholar]
- 85.Lee H. p., et al. , The nuclear piston activates mechanosensitive ion channels to generate cell migration paths in confining microenvironments. Science Advances. 7(2): p. eabd4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kopeček J and Yang J, Smart Self-Assembled Hybrid Hydrogel Biomaterials. Angewandte Chemie International Edition, 2012. 51(30): p. 7396–7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ooi HW, et al. , Hydrogels that listen to cells: a review of cell-responsive strategies in biomaterial design for tissue regeneration. Materials Horizons, 2017. 4(6): p. 1020–1040. [Google Scholar]
- 88.Jeon O, Kim T-H, and Alsberg E, Reversible dynamic mechanics of hydrogels for regulation of cellular behavior. Acta Biomaterialia, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burdick JA and Murphy WL, Moving from static to dynamic complexity in hydrogel design. Nature Communications, 2012. 3(1): p. 1269. [DOI] [PubMed] [Google Scholar]
- 90.Young JL and Engler AJ, Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials, 2011. 32(4): p. 1002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gong Z, et al. , Matching material and cellular timescales maximizes cell spreading on viscoelastic substrates. Proc Natl Acad Sci U S A, 2018. 115(12): p. E2686–e2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chaudhuri O, et al. , Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature, 2020. 584(7822): p. 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Engler AJ, et al. , Matrix elasticity directs stem cell lineage specification. Cell, 2006. 126(4): p. 677–89. [DOI] [PubMed] [Google Scholar]
- 94.Kim D-H, et al. , Matrix nanotopography as a regulator of cell function. The Journal of Cell Biology, 2012. 197(3): p. 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tibbitt MW, et al. , Progress in material design for biomedical applications. Proceedings of the National Academy of Sciences, 2015. 112(47): p. 14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Galaev IY and Mattiasson B, ‘Smart’ polymers and what they could do in biotechnology and medicine. Trends in Biotechnology, 1999. 17(8): p. 335–340. [DOI] [PubMed] [Google Scholar]
- 97.Kumar A, et al. , Smart polymers: Physical forms and bioengineering applications. Progress in Polymer Science, 2007. 32(10): p. 1205–1237. [Google Scholar]
- 98.Matsuda N, et al. , Tissue Engineering Based on Cell Sheet Technology. Advanced Materials, 2007. 19(20): p. 3089–3099. [Google Scholar]
- 99.Nakanishi J, et al. , Spatiotemporal Control of Migration of Single Cells on a Photoactivatable Cell Microarray. J. Am. Chem. Soc, 2007. 129(21): p. 6694–6695. [DOI] [PubMed] [Google Scholar]
- 100.Shafiq Z, et al. , Bioinspired Underwater Bonding and Debonding on Demand. Angewandte Chemie International Edition, 2012. 51(18): p. 4332–4335. [DOI] [PubMed] [Google Scholar]
- 101.Ebara M, et al. , Shape-memory surface with dynamically tunable nano-geometry activated by body heat. Adv. Mater, 2012. 24(2): p. 273–8. [DOI] [PubMed] [Google Scholar]
- 102.Yu Q, et al. , Nanopatterned Smart Polymer Surfaces for Controlled Attachment, Killing, and Release of Bacteria. ACS Applied Materials & Interfaces, 2013. 5(19): p. 9295–9304. [DOI] [PubMed] [Google Scholar]
- 103.Guvendiren M, et al. , Hydrogels with differential and patterned mechanics to study stiffness-mediated myofibroblastic differentiation of hepatic stellate cells. Journal of the Mechanical Behavior of Biomedical Materials, 2014. 38: p. 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anguiano M, et al. , The use of mixed collagen-Matrigel matrices of increasing complexity recapitulates the biphasic role of cell adhesion in cancer cell migration: ECM sensing, remodeling and forces at the leading edge of cancer invasion. PLoS One, 2020. 15(1): p. e0220019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Colin-York H and Fritzsche M, The future of traction force microscopy. Current Opinion in Biomedical Engineering, 2018. 5: p. 1–5. [Google Scholar]
- 106.Patteson AE, et al. , Loss of Vimentin Enhances Cell Motility through Small Confining Spaces. Small, 2019. 15(50): p. 1903180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang JH and Lin JS, Cell traction force and measurement methods. Biomech Model Mechanobiol, 2007. 6(6): p. 361–71. [DOI] [PubMed] [Google Scholar]
- 108.Polacheck WJ and Chen CS, Measuring cell-generated forces: a guide to the available tools. Nat Methods, 2016. 13(5): p. 415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mulligan JA, et al. , Traction Force Microscopy for Noninvasive Imaging of Cell Forces, in Biomechanics in Oncology, Dong C, Zahir N, and Konstantopoulos K, Editors. 2018, Springer International Publishing: Cham. p. 319–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Holle AW, et al. , Cell-Extracellular Matrix Mechanobiology: Forceful Tools and Emerging Needs for Basic and Translational Research. Nano Lett, 2018. 18(1): p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maini PK, Olsen L, and Sherratt JA, MATHEMATICAL MODELS FOR CELL-MATRIX INTERACTIONS DURING DERMAL WOUND HEALING. International Journal of Bifurcation and Chaos, 2002. 12(09): p. 2021–2029. [Google Scholar]
- 112.Olsen L, et al. , A mathematical model for the capillary endothelial cell-extracellular matrix interactions in wound-healing angiogenesis. IMA journal of mathematics applied in medicine and biology, 1997. 14(4): p. 261–81. [PubMed] [Google Scholar]
- 113.Dallon JC, Sherratt JA, and Maini PK, Mathematical Modelling of Extracellular Matrix Dynamics using Discrete Cells: Fiber Orientation and Tissue Regeneration. Journal of Theoretical Biology, 1999. 199(4): p. 449–471. [DOI] [PubMed] [Google Scholar]
- 114.Kleinstreuer N, et al. , A computational model predicting disruption of blood vessel development. PLoS Comput Biol, 2013. 9(4): p. e1002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park D, et al. , Extracellular matrix anisotropy is determined by TFAP2C-dependent regulation of cell collisions. Nat Mater, 2020. 19(2): p. 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ahmadzadeh H, et al. , Modeling the two-way feedback between contractility and matrix realignment reveals a nonlinear mode of cancer cell invasion. Proc Natl Acad Sci U S A, 2017. 114(9): p. E1617–E1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lukashev ME and Werb Z, ECM signalling: orchestrating cell behaviour and misbehaviour. Trends in Cell Biology, 1998. 8(11): p. 437–441. [DOI] [PubMed] [Google Scholar]
- 118.Nam S, et al. , Viscoplasticity Enables Mechanical Remodeling of Matrix by Cells. Biophys. J, 2016. 111(10): p. 2296–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wisdom KM, et al. , Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nature Communications, 2018. 9(1): p. 4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim DH, et al. , Biomechanical interplay between anisotropic re-organization of cells and the surrounding matrix underlies transition to invasive cancer spread. Sci Rep, 2018. 8(1): p. 14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Loebel C, Mauck RL, and Burdick JA, Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nature Materials, 2019. 18(8): p. 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ferreira SA, et al. , Bi-directional cell-pericellular matrix interactions direct stem cell fate. Nature Communications, 2018. 9(1): p. 4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Groves JT, Learning the Chemical Language of Cell-Surface Interactions. Science's STKE, 2005.. 2005(301): p. pe45. [DOI] [PubMed] [Google Scholar]
- 124.Sever R and Brugge JS, Signal transduction in cancer. Cold Spring Harb Perspect Med, 2015. 5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pratilas CA, et al. , V600EBRAF is associated with disabled feedback inhibition of RAF–MEK signaling and elevated transcriptional output of the pathway. Proceedings of the National Academy of Sciences, 2009. 106(11): p. 4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Poulikakos PI, et al. , RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature, 2010. 464(7287): p. 427–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fonseca KB, et al. , Injectable MMP-Sensitive Alginate Hydrogels as hMSC Delivery Systems. Biomacromolecules, 2014. 15(1): p. 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Leight JL, et al. , Direct measurement of matrix metalloproteinase activity in 3D cellular microenvironments using a fluorogenic peptide substrate. Biomaterials, 2013. 34(30): p. 7344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Buffington SL, et al. , Enzymatically triggered shape memory polymers. Acta biomaterialia, 2019. 84: p. 88–97. [DOI] [PubMed] [Google Scholar]
- 130.Lin X, et al. , Recent progress in stem cell differentiation directed by material and mechanical cues. Biomedical materials, 2016. 11(1): p. 014109. [DOI] [PubMed] [Google Scholar]


