Abstract
Background:
Developing a causal graph is an important step in etiologic research planning and can be used to highlight data flaws and irreparable bias and confounding. As a case study, we consider recent findings that suggest human papillomavirus (HPV) vaccine is less effective against HPV-associated disease among girls living with HIV compared to girls without HIV.
Objectives:
To understand the relationship between HIV status and HPV vaccine effectiveness, it is important to outline the key assumptions of the causal mechanisms before designing a study to investigate the effect of the HPV vaccine in girls living with HIV infection.
Methods:
We present a causal graph to describe our assumptions and proposed approach to explore this relationship. We hope to obtain feedback on our assumptions prior to data analysis and exemplify the process for designing causal graphs to inform an etiologic study.
Conclusion:
The approach we lay out in this paper may be useful for other researchers who have an interest in using causal graphs to describe and assess assumptions in their own research prior to undergoing data collection and/or analysis.
Keywords: Directed acyclic graphs, causal graphs, human immunodeficiency virus, human papillomavirus, HPV vaccine
1. Introduction
1.1. Directed Acyclic Graphs in the literature
Analytic plans for estimating the magnitude of causal effects require a clear understanding of the relationship between the exposure and outcome of interest, as well as any common causes of exposure and outcome (potential confounders) or population sampling features (potential colliders). The appropriate choice of analytic method, including which covariates to include in the model, what sensitivity analyses to run, and what type of analysis to conduct, will depend on the research team’s pre-specified assumptions about the relationships between variables. Directed acyclic graphs (DAGs) are a useful tool for clarifying these assumptions and can inform discussion about the underlying theory both among the research team and within the wider research community. We believe that DAGs should be included in published research articles.
Although there is considerable literature on the formal mathematics of using DAGs for causal inference, there is very little literature on the development of DAGs for applied research 1,2. A recent review of the epidemiologic literature found very few published applied DAGs 1. Since these causal graphs represent the assumptions about underlying relationships between variables which must be true in order for the research conclusions to be valid, it is critical to include the graph and a discussion of the rationale for building the graph in order to ensure both reviewers and readers can accurately assess study conclusions.
In this paper, we attempt to address the lack of published DAGs by outlining the process for developing an evidence-based causal DAG. We use a case study approach to work through the creation and assumptions of this DAG and the evidence that supports its structure and discuss the next steps that will need to be taken to translate the DAG to the analytic phase. The goal of this case study is to formulate a plan to investigate an apparent decrease in the efficacy of the quadrivalent HPV vaccine in protecting against abnormal cytology in girls with perinatally-acquired human immunodeficiency virus (HIV) infection compared to girls who are not infected with HIV 3 4. There are numerous causal questions we could ask to address the efficacy of the HPV vaccine in this population of girls. Two causal questions we have decided to ask in this paper are1) “Does receiving the HPV vaccine decrease the incidence of abnormal cervical cytology compared with not receiving the HPV vaccine in girls with perinatal HIV infection?” and 2) “Does the number of doses of HPV vaccine received correspond to a decrease in abnormal cervical cytology in girls with perinatal HIV infection?” In Box 1, there are common terms that are frequently used when discussing DAGs in methods papers. Throughout this paper we intentionally avoid the use of jargon in an effort to make DAGs more accessible to the reader, who may not have experience with DAGs. This paper is aimed at researchers who are interested in using DAGs for research purposes but may not have a foundational understanding of DAGs. However, we feel that this paper can also be instructive for people who are familiar with DAGs and use them frequently in their research.
Box 1: Directed Acyclic Graphs Terminology and Definitions.
DAG: A type of diagram with nodes (variables) and edges/paths (arrows).
Causal DAG: A DAG with all common causes (known and unknown) of every pair of variables on the graph
Backdoor Paths: Any path from a node A to another node B that exists after closing (or blocking) all edges come out of node A
Example Figure 3: The path from Vaccine Initiation to Age to Abnormal Cytology
Collider: A node that has two (or more) edges directed to it. In other words, a node that is the child of two parents (or more).
Example Figure 4: Measured sexual history is a collider, since age and sexual history both have edges to that node.
Sharp null: The value of one variable has no effect on the value of another, for every individual. This is represented as the lack of an arrow between two variables in a DAG. The sharp null can be contrasted with the more common null hypothesis of no average causal effect.
Example Figure 3: The lack of a path from Contraindications to Insurance indicates that having contraindications for the HPV vaccine has no effect on any individual’s insurance status
Exchangeability: The counterfactual outcomes are independent of the exposure for all exposure values. In other words, the exposed had they been unexposed would have had the same average outcome as the unexposed did, and vice versa. On the DAG, this is represented by the only path from exposure to the outcome being the direct path from exposure to outcome (potentially after adjustment).
Example Figure 4: The only remaining pathways are the primary pathway of causal inference (the dotted paths represent closed paths)
Consistency: The exposure is well defined; all mechanisms for getting exposed result in the same outcome for being exposed.
Positivity: The probability of being exposed within each level of covariates is between 0 and 1, exclusive.
Four types of EMM: direct effect modification, indirect effect modification, effect modification by proxy and effect modification by common cause.
D-separation: Two nodes A and B are d-separated if there is no open path from A to B, conditional on a set of covariates C (which may be the empty set). Under the null hypothesis of no effect of A on B, these nodes are d-separated when no open backdoor paths exist.
Faithfulness: If a variable A is independent of a variable B (no association), perhaps conditional on a set of covariates C, then A is d-separated from B, conditional on C, in the DAG. This implies that there are no arrows on the DAG for which subgroup effects operate in the exact opposite direction and magnitude.
1.2. Background on Causal Directed Acyclic Graphs
A causal DAG is a nonparametric causal diagram that represents the data generating process and can be useful for creating diagrams of real-world problems to increase understanding of the mechanisms, as well as inform the data analysis phase 5. The basic components of a DAG are nodes and arrows representing variables and assumptions about their inter-relationships. Importantly, the mathematical assumptions underlying DAGs are encoded in the absence of arrows between nodes – the presence of an arrow allows the possibility of a relationship but does not require it; in contrast, the absence of an arrow assumes that there cannot ever be a causal relationship between the two disjoint variables6. We recognize that causal DAGs are not the only causal graphs used for causal analysis, however we will use causal graphs and DAGs interchangeably throughout this paper7. In all of our DAGs in this paper, we assume faithfulness holds (see Box 1 for definition). A full explanation of the technical details for reading causal DAGs and translating their assumptions into appropriate data analysis plans is beyond the scope of the current paper, and we refer interested readers to Greenland et al 1999 and Hernan & Robins 2020 6,7.
1.3. Background on HPV and HIV
HPV is one of the most common sexually transmitted diseases, infecting over 79 million people in the United States at any given time 8. Most HPV infections are asymptomatic and clear on their own (without medical treatment); however, persistent infection with certain HPV genotypes have been found to cause disease, including genital warts and cervical cancer 8,9. Two high-risk HPV genotypes, HPV16 and HPV18, account for the majority of HPV-related cancer burden in the United States 9.
A person can be infected with multiple genotypes at one time, and viral persistence and progression of HPV infection are influenced by a variety of factors, including genotype and an individual’s immunologic capabilities 10. People living with HIV are at increased risk of HPV infection, are more likely to have multiple-type infections and are at greater risk of HPV persistence and development of HPV associated morbidities compared to those who do not have HIV 11. Girls with perinatal HIV infection are suspected to be at higher risk for future cervical cancer, an AIDS-defining illness 12–14 The World Health Organization (WHO) currently recommends age appropriate HPV vaccination paired with cervical cancer screenings and treatment to reduce the incidence and prevalence of cervical cancer, including among people living with HIV 15. In designing our proposed DAG we focused on the sharp null can be contrasted with the more common null hypothesis of no average causal effect 3,10.
Current guidelines recommend vaccination before the age of sexual debut (around 11 years of age), since the vaccines are most effective when delivered before exposure to the virus 16. Gardasil, the brand name quadrivalent HPV vaccine from Merck, has recommended a three-dose schedule for immunocompromised individuals and those over the age of 15, with the second and third doses given 2 and 6-12 months after the first dose, respectively 16. The HPV vaccines have been found to decrease HPV-associated cancer risk in the general population; however the efficacy of HPV vaccines in people living with HIV has more recently come into question 3,11,17,18. While researchers observed a higher incidence of abnormal cytology in HPV vaccinated girls with perinatal HIV infection, the causal pathway remains unknown.
The efficacy of a vaccine, which is the ability of the vaccine to trigger an immune response that generates enough antibodies to protect against future infection, is generally determined using serological assays that measure HPV antibody titers.
1.4. Research Aim and Preliminary DAG
To investigate the causal relationship between HPV vaccination and abnormal cytology, it is necessary to understand the potential mediating and confounding variables, as these covariates will inform our data analysis and interpretation. To accomplish this, we begin with a basic (non-causal) DAG representing the causal path of our research question (Figure 1). Note that our exposure, quadrivalent HPV vaccination, is depicted in two separate nodes in order to consider the effect of vaccine initiation (i.e. one dose) separate from vaccine continuation (i.e. two or three doses) on abnormal cervical cytology (a precursor to cervical cancer). We have chosen abnormal cervical cytology as the primary outcome of interest, since it is an indicator of cervical cancer. Vaccine response is a mediator on the pathway from vaccination to abnormal cytology.
Figure 1:

A directed acyclic graph (DAG) depicting the relationship driving the research aim; the association between HPV vaccine dose and abnormal cervical cytology.
2. Conceptual Causal Graph
Designing a DAG often requires a review of the literature to understand established mediators and confounders. Ideally, building a DAG also involves qualitative expert and stakeholder consultations, although current methods to guide such an activity do not exist 19. We used a combination of systematic literature review and expert discussion in designing our initial DAG. Importantly, threats to internal validity can only arise through variables which are causes or consequences of two or more other variables already on the existing DAG 6. In contrast, variables which cause only one other variable can be important for determining external validity (i.e. ensuring that you can extrapolate your findings to a population outside of your study population)20. Therefore, in building our DAG we began with the structure in Figure 1 and created a list of covariates to take into consideration in building the DAG. After consulting the literature and discussing with experts, we added in covariate nodes that were related to at least two other covariates in the list or on the causal pathway of interest. We collapsed some categories into one (e.g. history of chlamydia and history of unprotected sex with multiple partners were collapsed into one node for sexual history). We kept contraindications for the HPV vaccine (detailed in section 2.2) on the DAG, even though it is only related to one other variable (vaccine initiation) because it is important for external validity. In other words, the causal effect that we want to estimate is dependent on whether we are looking at a causal effect in the general population or within the population of people eligible to receive the HPV vaccine. Figure 2 represents the resulting DAG illustrating the important covariate relationships that we identified from this process.
Figure 2:
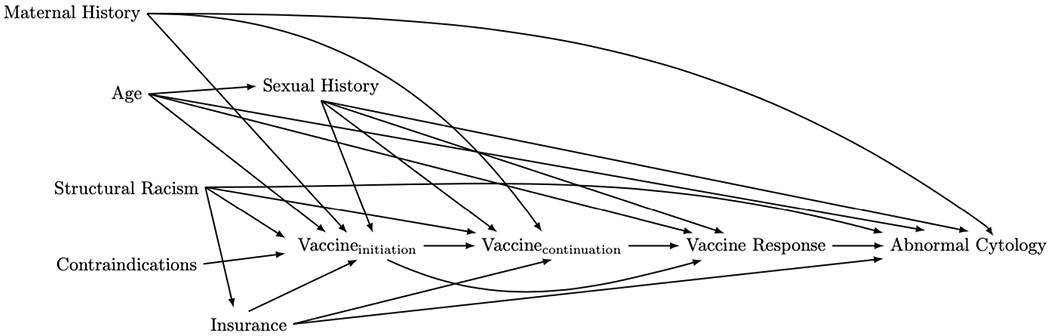
A more-complete directed acyclic graph (DAG) depicting the relationship between HPV vaccine dose and abnormal cervical cytology among people living with HIV, incorporating additional relationships between our variables that we initially left out.
In addition to the variables depicted on our DAG, several studies indicated that CD4 T-cell count, HIV viral load and antiretroviral therapy (ART) may act as effect measure modifiers (EMMs) in this relationship3,21–23. These variables are not included on the DAG since we do not believe they will be confounders for our analysis since clinicians in the PHACS cohort do nto consider markers of HIV severity in determining HPV vaccination schedules 19. However, in the general public, there may be a link between HIV severity and HPV vaccine initiation or continuation, and in an analysis of routinely collected data these variables could be important confounders. After conducting our primary analysis comparing abnormal cytology incidence in vaccinated girls with HIV infection to unvaccinated girls with HIV infection and looking at a dose-response relationship, it is possible that we may investigate this relationship within different levels of CD4 T-cell count or HIV RNA levels. We could also look for differences in vaccine efficacy between those taking ART and those not taking ART.
In the next section, we discuss the evidence supporting inclusion or exclusion of arrows and nodes from our proposed DAG in Figure 2. The reader is reminded that omitted arrows represent a stronger assumption than included arrows. As such, arrows with mixed or limited evidence are included by default. We focus here on key arrows required for our analysis. For a full list of supporting evidence for each arrow, see the eAppendix.
2.1. Discussion on Included Arrows
The inclusion of an arrow between two variables indicates either: 1) a well-established relationship between the two variables (generally verified by the literature or through conversations with experts) or, 2) the lack of strong evidence supporting an assumption of no relationship between the two variables. In this section we will walk through the rationale for including a few of the key arrows in our DAG. A complete rationale for each arrow in our DAG can be found in eTable 1.
2.1.1. Age to Vaccine Response
There is substantial evidence that the antibody response to the HPV vaccination is much stronger and longer lasting in people who are vaccinated at a younger age. In an analysis of data from clinical trials of the quadrivalent vaccine, researchers reported an inverse relationship with age at vaccination and geometric mean titers 7 months post-vaccination 24. In an analysis of HPV4 response in women over the age of 24, lower titers were observed in older women, although they were still high enough to indicate vaccine efficacy 25. The same has been observed in both HPV9 and the HPV2 vaccines 26,27. Consequently, the high vaccine titers seen in young individuals does not necessarily translate to “better” protection.
2.1.2. Structural Racism to Vaccine Initiation
A meta-analysis of 29 studies, conducted in 2013, found strong evidence that Black women were less likely to initiate HPV vaccination compared to White women 28. Differences between Asian women compared to White women were varied, but some studies reported that White women were more likely to initiate HPV vaccination compared to Asian women 28. Investigators found a larger number of studies on HPV vaccine initiation in Latina women compared to White women, but these studies reported contradictory evidence as well 28. Embedded in this arrow are social, economic and political factors that explain this relationship, including medical racism, differences in access to healthcare as a result of inequitable healthcare policies, and other factors.
2.2. Discussion on Excluded Arrows
As we have stated before, excluding an arrow from a DAG is a stronger assumption than including an arrow, as it assumes the two disconnected nodes can never have a direct relationship. Our DAG excludes very few arrows, and this section will discuss two of the excluded arrows that we believe are appropriate assumptions to make.
2.2.1. Contraindications to Abnormal Cytology
The quadrivalent HPV vaccine has one contraindication that would prevent an individual from receiving the vaccine: hypersensitivity to baker’s yeast 16. We feel confident that hypersensitivity to baker’s yeast, while potentially leading to severe allergic reaction, does not lead to the development of abnormal cervical cytology other than via a lack of vaccination. While there have been no studies to our knowledge on this relationship, we feel this comfortable assuming no relationship and thus excluding an arrow between these nodes from the DAG.
2.2.2. Structural Racism to Vaccine Response
The vaccine response node represents the immunological response to the vaccine. A relationship between structural racism and vaccine response would indicate a biological mechanism at play. We believe that such a mechanism does not exist, and so we have excluded the potential for a relationship between structural racism and biological vaccine response. One of the benefits of explicitly laying out our DAG in detail is that we may receive feedback on our assumptions via the review process. A reviewer pointed out that, while race is a social construct and thus independent from biological characteristics, structural racism may impact the immune system (for example, through chronic stress). The inclusion of this pathway would not change our analytic plan or adjustment set, as systemic racism is an established confounder related to both our exposure and our outcome, but the reviewer raises an important point that deserves further consideration
3. Analytic Causal Graph
The first step in identifying the analytic causal model is to identify the causal relationship of interest. An appropriate adjustment set can then be identified from a DAG by assessing all sequences of arrows between the exposure and outcome. Potential sources of bias are present through ‘backdoor paths’ (see Box 1 for definition). If we are interested in identifying the causal effect of vaccine initiation (vs. no vaccine initiation) on development of abnormal cytology, the adjustment set that blocks (or closes) all backdoor paths includes age, insurance, structural racism, maternal history, and sexual history. In identifying this adjustment set, we are assuming that our DAG is correct. The DAG illustrated in Figure 3 represents the resulting pathways after adjustment for these variables.
Figure 3:
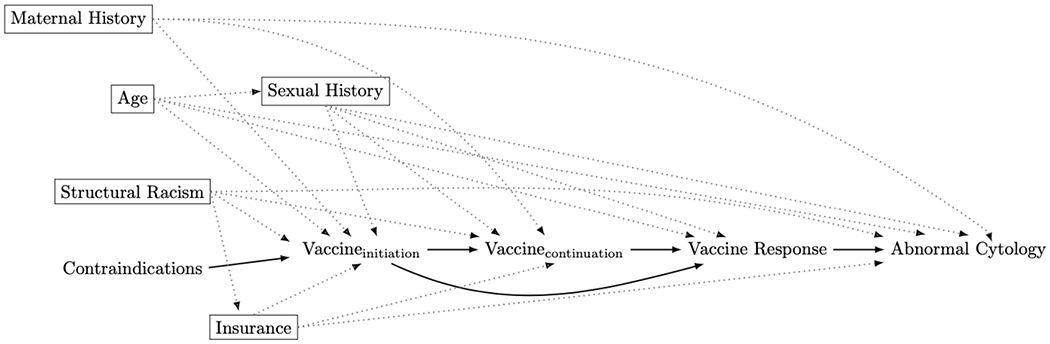
Our final adjusted directed acyclic graph (DAG) depicting the relationship between vaccine dose and abnormal cervical cytology among people living with HIV after revisiting our assumptions. Rectangles indicate variables adjusted for and the dotted gray lines indicate complete removal of a path. There are no confounding pathways in this model.
3.1. Adapting the DAG to the Data
Although Figure 3 represents the data generating mechanism we suspect is operating in our population of girls with perinatal HIV, some additional considerations are needed to ensure we can account for bias in our analysis. Previous studies exploring the relationship between HPV vaccination and abnormal cytology experienced issues with missing data, misclassification introduced by self-report or measurement error, inaccurate chart reviews and lack of timing between sexual history and blood samples 29. These are not currently nodes on our DAG. We are planning on using data from AMP and AMP Up, two studies from the U.S. based Pediatrics HIV/AIDS Cohort Study (PHACS) network, and we can updated our DAG to represent the anticipated challenges with conducting the analysis used these specific data (Figure 4)3,30.
Figure 4:
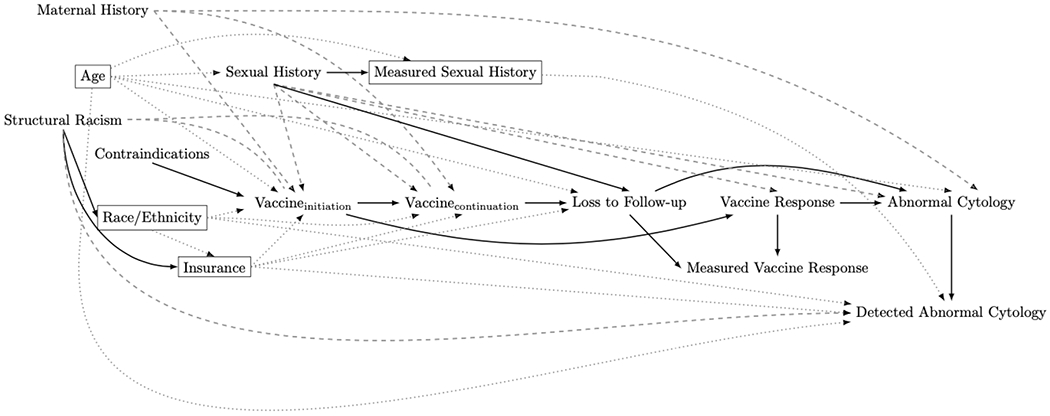
Our final adjusted directed acyclic graph (DAG) depicting the relationship between vaccine dose and abnormal cervical cytology among people living with HIV that incorporates potential data issues. Rectangles indicate variables adjusted for, the dotted gray lines indicate complete removal of a path, and the dashed lines indicate confounding pathways.
Misclassification of sexual behavior is likely the largest source of residual confounding in this new diagram. One recent study found that between 14% and 39% of a cohort of children and adolescents with perinatal HIV exposure were inconsistent in their reports of lifetime number of partners and condom use 31. These inconsistencies differed by age and type of sexual behavior. Importantly, in our DAG misclassification in sexual history does not lead to additional bias (due to “measured sexual history” operating as a collider) as long as age is controlled for but may result in residual confounding by true sexual history 29. Since we do not have a measure for structural racism available in the dataset and will use race and ethnicity data as an imperfect proxy, this is another likely source of residual confounding. For our specific dataset, maternal history of cervical cancer is another source of potential confounding, because our data does not contain this information. By including maternal history on our DAG, we are made aware of the necessity of including in our analytic plan quantitative bias analyses or other sensitivity analyses assessing the impact of this residual confounding 32,33.
Additionally, vaccine response (as measured using antibody titers in the PHACS dataset) does not necessarily equate to immune function, since an individual is considered seropositive if their titer level is above a somewhat arbitrary type-specific cut-off 34–36 Consequently, antibody titers alone are associated with measurement error in immune response (see the “Measured Vaccine Response” node in Fig. 4). This is another important potential source of bias and will require further sensitivity analyses to understand the impact of this misclassification and measurement error on our analysis. A final consideration in adapting the conceptual DAG to our data is whether or not to restrict (e.g. adjust for) HPV vaccine contraindication. Since this variable is only related to the exposure, adjustment is not necessary for internal validity. Instead, the adjustment decision depends on our research question; if we want to ask, “what is the average effect of HPV vaccination on incidence of abnormal cervical cytology among all girls with perinatally-acquired HIV infection?” we would not restrict on contraindications because girls with contraindications are included in our target population of interest; however, if our question is, “what is the average effect of HPV vaccination on incidence of abnormal cervical cytology among girls who are eligible to receive the HPV vaccine and have perinatally-acquired HIV infection?” then restriction on contraindications would be required.
4. Conclusions and Next Steps
Here we present a case study describing the creation of our DAG based on our assumptions about the data generating model which may give rise to the observed decreased HPV vaccine efficacy in girls with perinatal HIV infection. This DAG can help provide a clear foundation for the creation of an analytic plan, highlight potential sources of residual biases that require sensitivity analyses, and describe the target population for inference. In our DAG building process, we discovered that analysis using the data available to answer our causal questions will likely be susceptible to confounding by sexual history, structural racism and maternal history due to measurement error or lack of available data.
Throughout this paper we have highlighted the importance of ensuring the assumptions in your DAG are justified (especially concerning assumptions about the lack of an edge between two variables). We have also identified areas of future research to bring us closer to conducting a more accurate causal analysis, including identifying how maternal history and HPV vaccination are related, understanding racial disparities in abnormal cytology, and creating better methods for accurately measuring sexual activity. We hope that publishing our DAG will generate productive debate and discussion on our assumptions and choices that will improve our analysis of this research question. To facilitate this discussion, we have also posted an electronic version at http://dagitty.net/mM-raKq. With this tool, readers can explore the relationships and analytic consequences of our assumptions themselves. We have also created a google form to obtain feedback on our DAG from the medical and public health community, and we invite readers to send us their feedback (see Note below for link). An added benefit of this process is that future projects examining the same relationship can cite and build upon our DAG. This will further our mission of creating dialogue around study assumptions and results in order to improve the quality of published studies.
Some features of our proposed DAG for which community feedback is particularly encouraged include our omission of HIV-related variables such as CD4 cell count and viral load as confounders, and the lack of strain-specific effects. Omitting these factors assumes that they are not relevant for either internal or external validity, and we invite community feedback on the appropriateness of that assumption. Our rationale for excluding them is that, although we intend to model CD4 cell count and viral load as potential effect modifiers of vaccine efficacy among girls with perinatal HIV, we do not anticipate that these elements are confounders of the relationship between HPV vaccination and abnormal cytology, since, to the best of our knowledge, clinicians are not currently making HPV vaccination decisions on the basis of HIV-related immune function.
Additionally, there are at least 12 oncogenic genotypes of HPV, as well as a number of possibly oncogenic subtypes and several which can cause genital warts. While HPV16 and HPV18 are the most prevalent oncogenic types in the general population, some strains seem to be more common in people living with HIV 37. The quadrivalent vaccine, which protects against four strains of HPV has been shown to induce some additional protection against other (non-vaccine specific) strains in people living with HIV 11,38,39. However, existing studies have reported lower immune response for HPV18 than for the other three strains and some studies have found that HPV16 infection is less impacted by HIV status, compared to other strains 40,41. We were unable to capture these strain-specific effects in our DAG and are open to suggestions for improving this aspect of our model.
DAGs are a useful tool for designing analyses and exploring covariate relationships. Since they can be designed with minimal understanding of the underlying formal mathematical theory, DAGs are particularly valuable for facilitating interdisciplinary collaboration. While protocols exist for conceptual models and pre-registered reports, we are not aware of established protocols or guidelines for the publication of DAGs. Additionally, authors rarely publish their DAGs and the DAGs that are published vary widely in terms of design and adjustment 42. This can make it more difficult to judge the assumptions made by published studies and understand the validity of adjustment techniques. By setting a precedent for DAG publication in this case study we hope to increase the transparency of published studies, especially pertaining to analytic decisions, and encourage productive debate in the name of advancing knowledge 43. We welcome feedback on our proposed causal DAG and we hope to demonstrate that pre-registration or pre-publication of the DAG before embarking on an analysis can improve research methods.
Supplementary Material
Highlights.
Key findings:
There is a lack of information on the development of DAGs for applied research. Potential sources of bias and misclassification should be represented on any DAG applied to a dataset.
What this adds to what is known:
We demonstrate how to modify a DAG so that the data features essential for accurate effect estimation are taken in to account.
What is the implication/what should change now:
Causal DAGs are a tool for conveying researchers’ assumptions, and we demonstrate best practices for establishing an evidence-base for these assumptions. By demonstrating best practices for discussing and publishing DAGs, we fill an existing gap in the causal graph literature and provide a resource for future epidemiologic researchers to reference for guidance on how to utilize DAGs in applied research settings.
Funding
This project was supported by NICHD (R21HD098733) and through the Pediatric HIV/AIDS Cohort Study (PHACS) network. PHACS was supported by NICHD and other NIH institutes through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) and the Tulane University School of Medicine (HD052104). A full list of acknowledgements for the PHACS AMP and AMP Up studies is provided at https://phacsstudy.org/About-Us/AMP.Acknowledgements
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
To provide your feedback please fill out the google form (available at: https://forms.gle/rpmP585Kxmt2mz6f8) or email the corresponding author
Declarations of interest
none
References
- 1.Tennant PW, Harrison WJ, Murray EJ, et al. Use of directed acyclic graphs (DAGs) in applied health research: Review and recommendations. medRxiv. 2020;(January). doi: 10.1101/2019.12.20.19015511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearl J Causal Diagrams for Empirical Research. Biometrika. 1995;82(4):669. doi: 10.2307/2337329 [DOI] [Google Scholar]
- 3.Moscicki AB, Karalius B, Tassiopoulos K, et al. Human Papillomavirus Antibody Levels and Quadrivalent Vaccine Clinical Effectiveness in Perinatally Human Immunodeficiency Virus-infected and Exposed, Uninfected Youth. Clin Infect Dis. 2019;69(7):1183–1191. doi: 10.1093/cid/ciy1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moscicki A, Karalius B, Tassiopoulos K, Jacobson D, Al E HPV4 vaccine immunogencity/effectiveness in perinatally HIV-infected youth. In: Conference on Retroviruses and Opportunistic Infections (CROI 2018). ; 2018. [Google Scholar]
- 5.Munafò MR, Nosek BA, Bishop DVM, et al. A manifesto for reproducible science. Nat Hum Behav. 2017;1(1). doi: 10.1038/s41562-016-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenland S, Pearl J, Robins JM. Causal Diagrams for Epidemiologic Research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 7.Hernan MA, Robins JM. Causal Inference: What If. Chapman & Hill/CRC; 2020. [Google Scholar]
- 8.Fast Facts | American Sexual Health Association. Accessed September 16, 2019. http://www.ashasexualhealth.org/stdsstis/hpv/fast-facts/
- 9.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017.; 2018. doi: 10.15620/cdc.59237externalicon [DOI] [Google Scholar]
- 10.Schiffman M, Doorbar J, Wentzensen N, et al. Carcinogenic human papillomavirus infection. Nat Rev Dis Prim. 2016;2. doi: 10.1038/nrdp.2016.86 [DOI] [PubMed] [Google Scholar]
- 11.Lacey CJ. HPV vaccination in HIV infection. Papillomavirus Res. 2019;8. doi: 10.1016/j.pvr.2019.100174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10(4):321–322. doi: 10.1016/s1470-2045(09)70096-8 [DOI] [PubMed] [Google Scholar]
- 13.Lin C, Franceschi S, Clifford GM. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect Dis. 2018;18(2):198–206. doi: 10.1016/S1473-3099(17)30653-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults.; 1992. Accessed October 31, 2019. https://www.cdc.gov/mmwr/preview/mmwrhtml/00018871.htm
- 15.United Nations Population Fund (UNFPA). Comprehensive Cervical Cancer Prevention and Control Programme Guidance for Countries.; 2011. Accessed January 21, 2020. https://eeca.unfpa.org/sites/default/files/pub-pdf/ENGLISH-CervicalCancerGuidance.pdf
- 16.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. Published 2007. Accessed September 16, 2019. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5602a1.htm [PubMed] [Google Scholar]
- 17.AIDS Info. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Accessed October 8, 2019. https://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-opportunistic-infection/343/human-papillomavirus [PubMed]
- 18.Toft L, Tolstrup M, Storgaard M, Ostergaard L, Søgaard OS. Vaccination against oncogenic human papillomavirus infection in HIV-infected populations: review of current status and future perspectives. Sex Health. 2014;11(6):511–523. doi: 10.1071/SH14015 [DOI] [PubMed] [Google Scholar]
- 19.Barnard-Mayers RM, Childs E, Corlin L, et al. Assessing Knowledge, Attitudes, and Practices towards Causal Directed Acyclic Graphs among Epidemiologists and Medical Researchers: a qualitative research project. medRxiv. Published online January 18, 2020. doi: 10.1101/2020.01.17.20017939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray EJ, Robins JM, Seage GR, Freedberg KA, Hernan MA. A Comparison of Agent-Based Models and the Parametric G-Formula for Causal Inference. Am J Epidemiol. 2017;186(2):131–142. doi: 10.1093/aje/kwx091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly H, Weiss HA, Benavente Y, et al. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: a systematic review and meta-analysis. Lancet HIV. 2018;5(1):e45–e58. doi: 10.1016/S2352-3018(17)30149-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bratcher LF, Sahasrabuddhe VV. The impact of antiretroviral therapy on HPV and cervical intraepithelial neoplasia: Current evidence and directions for future research. Infect Agent Cancer. 2010;5(1). doi: 10.1186/1750-9378-5-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denny L, Hendricks B, Gordon C, et al. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in HIV-positive women in South Africa: A partially-blind randomised placebo-controlled study. Vaccine. Published online 2013. doi: 10.1016/j.vaccine.2013.09.032 [DOI] [PubMed] [Google Scholar]
- 24.Giuliano AR, Lazcano-Ponce E, Villa L, et al. Impact of Baseline Covariates on the Immunogenicity of a Quadrivalent (Types 6, 11, 16, and 18) Human Papillomavirus Virus-Like-Particle Vaccine. J Infect Dis. 2007;196(8):1153–1162. doi: 10.1086/521679 [DOI] [PubMed] [Google Scholar]
- 25.Castellsagué X, Mũoz N, Pitisuttithum P, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24-45 years of age. Br J Cancer. 2011; 105(1):28–37. doi: 10.1038/bjc.2011.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castle PE, Xie X, Xue X, et al. Impact of human papillomavirus vaccination on the clinical meaning of cervical screening results. Prev Med (Baltim). 2019;118:44–50. doi: 10.1016/j.ypmed.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 27.Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis. 2017;17(12):1293–1302. doi: 10.1016/S1473-3099(17)30468-1 [DOI] [PubMed] [Google Scholar]
- 28.Fisher H, Trotter CL, Audrey S, MacDonald-Wallis K, Hickman M. Inequalities in the uptake of human papillomavirus vaccination: A systematic review and meta-analysis. Int J Epidemiol. 2013;42(3):896–908. doi: 10.1093/ije/dyt049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernán MA, Cole SR. Invited commentary: Causal diagrams and measurement bias. Am J Epidemiol. 2009;170(8):959–962. doi: 10.1093/aje/kwp293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tassiopoulos K, Patel K, Alperen J, et al. Following young people with perinatal HIV infection from adolescence into adulthood: The protocol for PHACS AMP Up, a prospective cohort study. BMJ Open. 2016;6(6). doi: 10.1136/bmjopen-2016-011396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cantos K, Franke Molly F., Tassiopoulos K, Williams PL, Moscicki A-B, Seage GR III. Inconsistent sexual behavior reporting among youth affected by perinatal HIV exposure living in the United States. In: IWHOD Conference. ; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lash T, Fox M, Fink A. Applying Quantitative Bias Analysis to Epidemiologic Data .; 2011. Accessed November 16, 2020. https://books.google.com/books?hl=en&lr=&id=BV599AQjiZQC&oi=fnd&pg=PR1&ots=Gyx8h2q6za&sig=o3wKB53wtAGS6pjyl2obiHzeADs
- 33.Van Der Weele TJ, Ding P. Sensitivity analysis in observational research: Introducing the E-Value. Ann Intern Med. 2017;167(4):268–274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 34.Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine. 2006;24(27-28):5571–5583. doi: 10.1016/j.vaccine.2006.04.068 [DOI] [PubMed] [Google Scholar]
- 35.Dias D, Van Doren J, Schlottmann S, et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol. 2005;12(8):959–969. doi: 10.1128/cdli.12.8.959-969.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner TB, Huh WK. HPV vaccines: Translating immunogenicity into efficacy. Hum Vaccines Immunother. 2016;12(6):1403–1405. doi: 10.1080/21645515.2015.1103936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kojic EM, Conley L, Bush T, et al. Prevalence and Incidence of Anal and Cervical High-Risk Human Papillomavirus (HPV) Types Covered by Current HPV Vaccines among HIV-Infected Women in the SUN Study. J Infect Dis. 2018;217(10):1544–1552. doi: 10.1093/infdis/jiy087 [DOI] [PubMed] [Google Scholar]
- 38.Dreyer G Clinical implications of the interaction between HPV and HIV infections. Best Pract Res Clin Obstet Gynaecol. 2018;47:95–106. doi: 10.1016/j.bpobgyn.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 39.Faust H, Toft L, Sehr P, et al. Human Papillomavirus neutralizing and cross-reactive antibodies induced in HIV-positive subjects after vaccination with quadrivalent and bivalent HPV vaccines. Vaccine. 2016;34(13): 1559–1565. doi: 10.1016/j.vaccine.2016.02.019 [DOI] [PubMed] [Google Scholar]
- 40.Cespedes MS, Kang M, Kojic EM, et al. Anogenital human papillomavirus virus DNA and sustained response to the quadrivalent HPV vaccine in women living with HIV-1. Papillomavirus Res. 2018;6:15–21. doi: 10.1016/j.pvr.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palefsky JM, Gillison ML, Strickler HD. Chapter 16: HPV vaccines in immunocompromised women and men. Vaccine. 2006;24 Suppl 3:S3/140–6. doi: 10.1016/j.vaccine.2006.05.120 [DOI] [PubMed] [Google Scholar]
- 42.Kupferschmidt K. More and more scientists are preregistering their studies. Should you? Science (80- ). Published online September 21, 2018. doi: 10.1126/science.aav4786 [DOI] [Google Scholar]
- 43.Warren M First analysis of ‘pre-registered’ studies shows sharp rise in null findings. Nature Published online October 24, 2018. doi: 10.1038/d41586-018-07118-1 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


