Abstract
Siglec‐15, a novel immune checkpoint, is an emerging target for next‐generation cancer immunotherapy. However, the role of Siglec‐15 in pancreatic ductal adenocarcinoma (PDAC) remains poorly understood. We investigated the expression of Siglec‐15 and its association with clinicopathological characteristics, programmed cell death‐ligand 1 (PD‐L1), immune cells, and DNA damage repair (DDR) molecules in a cohort of 291 patients with PDAC. Positive tumour cell expression of Siglec‐15 and PD‐L1 was observed in 18.6 and 30.3% of the samples, respectively. We also detected Siglec‐15 positivity in macrophages in 3.4% of patients. Co‐expression of Siglec‐15 with PD‐L1 was observed in 6.1% of the patients. A total of 33 PD‐L1‐negative samples (18.0%) were Siglec‐15‐positive. Siglec‐15 was observed more frequently in moderate‐to‐well‐differentiated tumours. Siglec‐15 was associated with a low density of Tregs and CD45RO T cells, high BRCA1 expression, and improved survival. Both Siglec‐15 and PD‐L1 are independent factors of patient outcomes. The prognostic significance of Siglec‐15 for survival was more discriminative in lymph node‐negative, high BRCA1 expression, or low BRCA2 expression tumours than in lymph node‐positive, low BRCA1 expression, or high BRCA2 expression tumours. In conclusion, we identified Siglec‐15 as a promising predictor for prognosis combined with different DDR molecular statuses and complex tumour‐infiltrating cells in PDAC. Targeting Siglec‐15 may be a novel therapeutic option for patients who are unresponsive to anti‐PD‐1 therapy. Future studies are needed to validate the prognostic significance of Siglec‐15 and to investigate its regulatory mechanisms in this disease.
Keywords: pancreatic ductal adenocarcinoma, Siglec‐15, PD‐L1, immune infiltrates, DNA damage repair
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is currently the fourth leading cause of cancer‐related deaths, which causes over 227,000 deaths annually worldwide [1, 2]. The death rate for PDAC rose by 0.3% per year in males between 2011 and 2015 [3, 4]. The prognosis of most patients with PDAC is poor, with a 5‐year survival rate of approximately 8%, which is diagnosed at a late stage [1, 4]. To date, 80–85% of patients cannot be treated surgically [2, 5]. Moreover, PDAC responds poorly to currently available chemotherapeutic agents [5, 6, 7]. Thus, new therapies are urgently needed to treat patients with PDAC.
Cancer immunotherapies, including targeting of programmed cell death 1 (PD‐1) or its ligand PD‐L1, have achieved remarkable success in some types of solid tumour [8, 9]. Wainberg et al demonstrated an overall response rate of 18% in patients with advanced PDAC treated with anti‐PD‐1 in combination with nab‐paclitaxel plus gemcitabine. However, the clinical results of this study do not support further investigation [10]. In the KEYNOTE‐028 clinical trial, the median progression‐free survival (PFS) of patients with PDAC who were treated with pembrolizumab was 1.7 months across 20 cancer types [11]. In addition, only mismatch repair‐deficient or microsatellite instability‐high PDAC is responsive to immune checkpoint inhibitors, and together, they account for approximately 2% of all PDAC cases [12], indicating that only a small fraction of patients is eligible for treatment with this agent. Overall, the results of anti‐PD‐1 therapy are discouraging. Therefore, targeting other immune checkpoints may provide more immunotherapeutic options for patients with PDAC.
Siglec‐15, an emerging target for normalisation cancer immunotherapy, is a member of the sialic acid‐binding immunoglobulin‐like lectin family [13, 14, 15, 16]. Its role in tumour immunoregulation was first characterised in 2019 by Professor Lieping Chen [16]. Siglec‐15 is expressed in many human cancer cells and tumour‐infiltrating immune cells [16]. The expression of Siglec‐15 is mutually exclusive to that of PD‐L1 and independent of the PD‐L1/PD‐1 pathway in lung adenocarcinoma, indicating that targeting Siglec‐15 may be an effective immunotherapy choice for patients who do not respond to anti‐PD‐1 therapy [16, 17]. Wang et al revealed that Siglec‐15 expression in macrophages can inhibit the proliferation of antigen‐specific T cells, leading to tumour growth [16]. The interactions between Siglec‐15 expressed in macrophages and sialyl‐Tn‐expressing lung cancer cells promote TGF‐β secretion, resulting in immunosuppression through the DAP12/Syk pathway [18]. In a phase I clinical trial in advanced non‐small cell lung cancer (NSCLC), the results demonstrated promising efficacy in patients treated with Siglec‐15 inhibitors (NC318) (NCT03665285). Recent studies have found that Siglec‐15 is upregulated in the bladder, colon, endometroid, kidney, lung, liver, and thyroid cancers and revealed that Siglec‐15 may have prognostic implications [16, 19]. However, the role of Siglec‐15 in PDAC remains unknown and should be further explored.
DNA damage response maintains genomic stability through multiple repair pathways including and not limited to DNA double‐strand breaks initiated by homologous recombination repair and non‐homologous end joining [20]. Genomic analysis has shown that DNA repair is one of the core signalling pathways, and some of the key molecules in the DNA damage repair (DDR) pathways, including BRCA1, BRCA2, PALB2, and p53, play an important role in the development of PDAC [21, 22]. Park et al revealed that patients who are diagnosed with PDAC with mutations of the BRCA genes and PALB2 had improved PFS on first‐line platinum versus first‐line non‐platinum chemotherapy [23]. Patients who are diagnosed with advanced PDAC, which exhibits homologous recombination deficiency (usually caused by pathogenic BRCA mutations), had better clinical outcomes based on poly‐ADP ribose polymerase (PARP) inhibitor treatment [24]. Thus, combining immunotherapy with PARP inhibitor treatment may improve the prognosis of patients with this disease. Exploring the association between some DDR molecules and expression of immune checkpoints may be helpful in improving the outcomes of patients with PDAC.
To date, Siglec‐15 expression patterns and their interactions with alternative immune checkpoints, immune cells, and DDR molecules remain unclear. Hence, we investigated the expression of Siglec‐15 and PD‐L1 as well as their associations with immune cells (CD3, CD4, CD8, Foxp3, CD45RO, CD68, and CD15), DDR molecules (p53 and BRCA1/2), and clinicopathological features and outcomes in a cohort of 291 patients with PDAC.
Materials and methods
Patient cohort and follow‐up
A total of 291 patients with primary PDAC who underwent surgical resection between January 2015 and July 2019 at Peking Union Medical College Hospital (Beijing, PR China) with available samples were consecutively included in our current retrospective study. Of these, 171 patients underwent classic pancreaticoduodenectomy, 47 underwent pylorus‐preserving pancreaticoduodenectomy, 78 underwent distal pancreatectomy, and 15 underwent total pancreatectomy. We excluded patients who died due to post‐operative complications, those with administration of neoadjuvant treatment, or those with inadequate formalin‐fixed and paraffin‐embedded tissue blocks for tissue microarrays (TMAs). To determine pathological variables, patients' histopathological slides were retrieved, scrutinised, and confirmed histologically by two pathologists (SY and ZL) according to the fifth edition of World Health Organization Classification of Tumors of the Digestive System. In case of discrepancies in results, a third expert pathologist (JC) made a final decision. Other clinical data, such as age, sex, tumour location, and so on, were collected from the medical records. Medical record reviews and telephone interviews were used to obtain survival and recurrence information. The time between surgery and tumour progression or the last follow‐up appointment was defined as PFS. Disease‐specific survival (DSS) was calculated from the date of surgery to the time of patient death caused by PDAC or the last follow‐up, which was 10 October 2020.
This retrospective study was approved by the Institutional Review Board of Peking Union Medical College Hospital (approval number: S‐K1593; date: 2 April 2021) and conformed to the ethical standards set forth in the Declaration of Helsinki. Informed consent was obtained from all patients.
TMA and IHC
Representative cancer tissues areas were marked on haematoxylin–eosin‐stained slides, and corresponding formalin‐fixed paraffin‐embedded blocks were sampled for TMA construction using a Manual Tissue Microarrayer (MiniCore, Mitogen, Hertford, UK). All tumour spots were punched out of the tumour centre.
The following primary antibodies were used for immunohistochemistry (IHC): PD‐L1 (E1L3N, Cell Signaling Technology, Danvers, MA, USA), Siglec‐15 (ab198684, Abcam, Cambridge, UK), CD3 (SP7, Abcam), CD4 (EPR19514, Abcam), CD8 (EPR21769, Abcam), Foxp3 (236A1E7, Abcam), CD45RO (UCH‐L1, Abcam), CD68 (KP1, Abcam), CD15 (SP159, Abcam), p53 (MX008, Maxim Biotechnology, Fuzhou, PR China), BRCA1 (MS110, Abcam), and BRCA2 (EPR23442‐43, Abcam). All slides were automatically stained using a BOND‐III immunostaining instrument (Leica Biosystems, Wetzlar, Germany) as per the manufacturer's instructions. Colon and prostate cancer tissues were used as positive controls for Siglec‐15 according to the antibody manufacturer's instructions and negative controls were prepared without the primary antibody.
Assessment of Siglec‐15, PD‐L1, and immune cell infiltration
Immunostaining was independently assessed by two investigators (XC and SM) who were blinded to the patients' clinicopathological data. In cases of discrepancy, the immunohistochemical staining slides were reviewed again, and a consensus was reached between the two investigators.
Siglec‐15 scoring was based on the percentage of Siglec‐15‐expressing tumour cells (TCs) with respect to the total tumour area. We identified positive Siglec‐15 expression on TCs through membrane staining and when ≥5% of the TCs expressed this protein. The 5% cut‐off point was set using X‐tile (Yale University, USA), and was the best value for prognosis discrimination through preliminary analysis in our cohort. PD‐L1 was evaluated based on the tumour proportion score (TPS), which was calculated as the sum of the number of PD‐L1‐expressing PDAC cells divided by the total number of viable PDAC cells. We also identified PD‐L1 positivity on TCs through membrane staining, and PD‐L1 staining was classified as positive when the TPS was ≥1% based on clinical practice and previous studies [25, 26]. We also identified 1% as the best cut‐off value for PD‐L1 through preliminary analysis using X‐tile. The expression of CD3, CD4, CD8, CD45RO, CD68, and CD15 in the stroma was quantified in ×40 fields using a computerised imaging system (KFBIO, Yuyao, PR China).
Evaluation of DDR molecules
Mutant p53 was defined by more than 50% nuclear staining or complete loss of nuclear expression in TCs, whereas wild‐type p53 was defined by weak and heterogeneous staining [27]. The expression of BRCA1 and BRCA2 was classified as high (≥50% of TCs stained) and low (<50% of TCs stained), as described by Beger et al [28].
mIF staining
Multiplexed immunofluorescence (mIF) staining was performed based on the manufacturer's protocol (Akoya Biosciences, Marlborough, MA, USA) to visualise the co‐expression of CD68 and Siglec‐15. Detailed experimental procedures for mIF staining are provided in Supplementary materials and methods.
Statistical analysis
The correlations between Siglec‐15, PD‐L1, and clinicopathological characteristics were assessed using the chi‐square test. Spearman's correlations were used to describe the association between the Siglec‐15 TPS and densities of immune infiltrates. Student's t‐test was used to analyse normally distributed continuous variables. Kaplan–Meier plots were generated and compared using the log‐rank test via Prism version 8.0.2 (GraphPad Software Inc., San Diego, CA, USA). Univariate and multivariate analyses were conducted using a Cox proportional hazards regression model to estimate the hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). Statistical analyses were two‐sided and performed using SPSS software (22.0; SPSS Inc., Chicago, IL, USA). Statistical significance was set at p < 0.05.
Results
Expression of Siglec‐15 and PD‐L1 in PDAC
A total of 291 patients with PDAC were included in this study. The clinicopathological characteristics of these patients are summarised by Zhang et al [29]. Given that some cores in the TMAs were lost during immunostaining, 263 and 264 cases were available for analysis of Siglec‐15 and PD‐L1, respectively.
Positive Siglec‐15 was observed in 18.6% (49/263) of PDAC samples, and PD‐L1 was observed in the TCs of 30.3% (80/264) of them. Of the cohort of 263 patient samples in which data for both proteins were available, 16 (6.1%) were double‐positive for Siglec‐15 and PD‐L1, and 150 (57.0%) were double‐negative. Furthermore, 64 of the Siglec‐15‐negative samples (29.9%) were PD‐L1‐positive, and 33 of the PD‐L1‐negative samples (18.0%) were Siglec‐15‐positive (supplementary material, Table S1). Representative images are presented in Figure 1. Additionally, we detected Siglec‐15 positivity in macrophages in 3.4% (9/263) of patients. Representative images of Siglec‐15 positivity in macrophages are presented in supplementary material, Figure S1.
Figure 1.
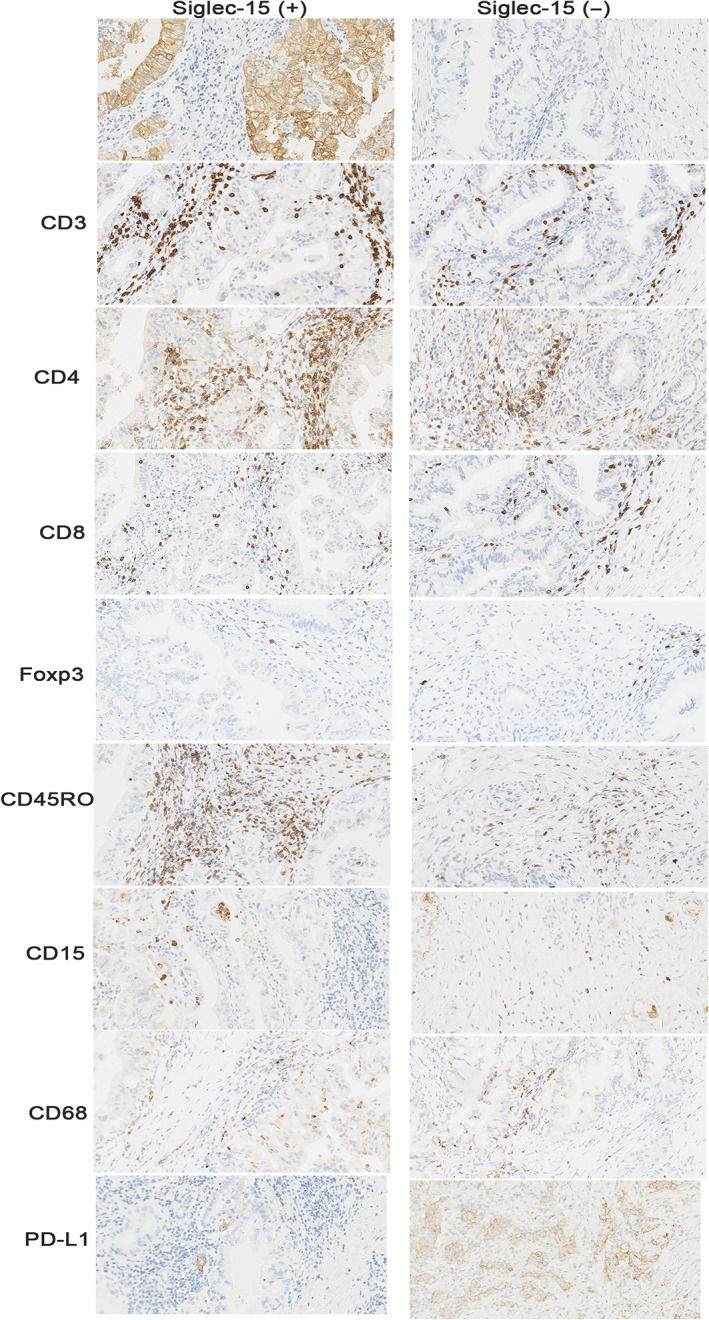
Expression of CD3, CD4, CD8, Foxp3, CD45RO, CD15, CD68, and PD‐L1 from the same TMA spot in Siglec‐15 positive/negative cases.
Expression of Siglec‐15 and PD‐L1 across pathological and DDR molecular subgroups
The associations between Siglec‐15 and patient clinicopathological characteristics are presented in supplementary material, Table S1. In all patients, Siglec‐15 positivity was significantly more frequent in patients with well and moderately differentiated tumours (p = 0.018), and positive expression of Siglec‐15 was associated with the presence of perineural invasion (p = 0.042). Additionally, there were no significant correlations between Siglec‐15 expression and other clinicopathological variables. Positive PD‐L1 expression was significantly associated with positive lymph nodes in our separately reported study [29].
The positive expression of Siglec‐15 on TCs was significantly associated with high BRCA1 expression (p = 0.001), as shown in supplementary material, Table S2. However, no significant correlations were found between Siglec‐15 expression and other DDR molecules (p53 and BRCA2).
Association between Siglec‐15, PD‐L1, and immune cells
We determined the densities of stromal CD3, CD4, CD8, Foxp3, CD45RO, CD68, and CD15 neutrophils in 254, 249, 250, 217, 252, 256, and 250 primary tumours, respectively. The immune cell subset densities were as follows: CD3, median 146, interquartile range (IQR) 71–268; CD4, median 52.5, IQR 27–90.25; CD8, median 67, IQR 27–117; Foxp3, median 22, IQR 10–50; CD45RO, median 34, IQR 14–80.5; CD68, median 54, IQR, 34–84; and CD15, median 11.5, IQR, 3–30. According to Spearman's correlations and t‐tests, we found that the positive expression of Siglec‐15 was significantly associated with the low density of CD45RO T cells and Foxp3+ Tregs, and CD3, CD4, CD68, and CD15 immune cell densities were not significantly related to Siglec‐15 expression (supplementary material, Figures S2 and S3, and Table S3). PD‐L1 positivity on TCs did not correlate with these immune cells in our separately reported study [29].
Prognostic values of Siglec‐15 and PD‐L1 in PDAC
After excluding 19 patients lost to follow‐up, 272 patients (93.5%) were subjected to survival analysis. After a median follow‐up of 18 months (range 3–65 months), 206 (75.7%) patients had relapsed and 174 (64.0%) had died of PDAC as of October 2020. The 5‐year PFS and DSS rates were 15.5 and 19.4%, respectively.
Kaplan–Meier curves showed that Siglec‐15 positivity was significantly associated with better PFS and DSS (Figure 2). Univariate analysis showed, in our separately reported study, that poor differentiation, high T stage, high N stage, advanced American Joint Committee on Cancer (AJCC) stage, and positive PD‐L1 were associated with shorter PFS and DSS, whereas adjuvant chemotherapy was associated with improved PFS and DSS [29]. Multivariate analyses revealed that positive Siglec‐15 expression was a predictor of improved PFS (HR 0.604, 95% CI 0.403–0.905, p = 0.015) and DSS (HR 0.563, 95% CI 0.348–0.911, p = 0.019) independent of PD‐L1 expression, AJCC stage, or grade. Additionally, PD‐L1 positivity was an independent prognostic factor for shorter DSS, but not for PFS (Table 1).
Figure 2.
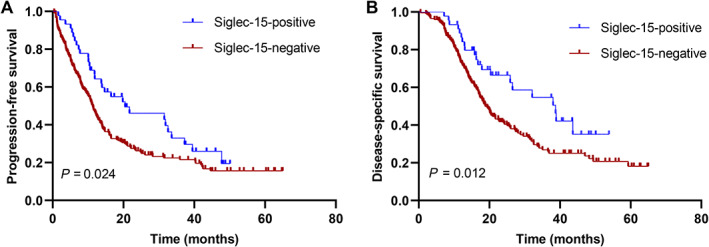
Kaplan–Meier curves according to Siglec‐15 expression. (A) PFS and (B) DSS .
Table 1.
Multivariate analyses of factors potentially predictive of survival in patients with PDAC.
| Variables | PFS | DSS | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Differentiation (grade) | ||||
| Well and moderate versus poor | 1.465 (1.072–2.001) | 0.016 | 1.633 (1.166–2.288) | 0.004 |
| AJCC stage | ||||
| I–II versus III–IV | 1.852 (1.314–2.609) | <0.001 | 2.024 (1.409–2.907) | <0.001 |
| Siglec‐15 | ||||
| Negative versus positive | 0.604 (0.403–0.905) | 0.015 | 0.563 (0.348–0.911) | 0.019 |
| PD‐L1 | ||||
| Negative versus positive | 1.290 (0.936–1.774) | 0.119 | 1.485 (1.054–2.093) | 0.024 |
Siglec‐15 stratification by DDR and clinically important subgroups
It is possible that the prognostic value of Siglec‐15 can be modified by DDR molecules and several clinically important parameters. Therefore, Siglec‐15 was stratified by lymph node status, grade, AJCC stage, p53 mutation, and BRCA1/2 status to assess the modification effect of these important parameters on Siglec‐15. The prognostic value of Siglec‐15 calculated by multivariate Cox regression analysis showed the most discriminative effect of positive Siglec‐15 expression in tumours with negative lymph nodes compared with that in tumours with positive lymph nodes. The same discriminative effect was observed in well and moderately differentiated compared with poorly differentiated tumours, in tumours with high BRCA1 expression compared with tumours with low BRCA1 expression, and in tumours with low BRCA2 expression compared with that in tumours with high BRCA2 expression for PFS and DSS. Additionally, we observed the most pronounced prognostic value of Siglec‐15 positivity in AJCC stage I–II or p53‐wild‐type patients compared with AJCC stage III–IV or p53‐mutant for DSS, but not for PFS (Table 2). The Kaplan–Meier curves of PFS and DSS for Siglec‐15 stratified by lymph node status, grade, and BRCA1/2 status are presented in Figure 3.
Table 2.
Results of the multivariate Cox regression analyses of Siglec‐15 per clinically important and DDR molecules subgroups.
| Siglec‐15 stratified by group | Subgroups | PFS | DSS |
|---|---|---|---|
| Lymph node status | Positive | HR 0.668, 95% CI 0.425–1.048, p = 0.079 | HR 0.622, 95% CI 0.368–1.051, p = 0.076 |
| Negative | HR 0.257, 95% CI 0.090–0.740, p = 0.012 | HR 0.173, 95% CI 0.040–0.743, p = 0.018 | |
| Differentiation (grade) | Well and moderate | HR 0.556, 95% CI 0.340–0.909, p = 0.019 | HR 0.529, 95% CI 0.298–0.939, p = 0.030 |
| Poor | HR 0.600, 95% CI 0.282–1.278, p = 0.186 | HR 0.491, 95% CI 0.234–1.467, p = 0.253 | |
| AJCC stage | I–II | HR 0.645, 95% CI 0.406–1.023, p = 0.062 | HR 0.494, 95% CI 0.277–0.879, p = 0.017 |
| III–IV | HR 0.491, 95% CI 0.203–1.186, p = 0.114 | HR 0.716, 95% CI 0.297–1.730, p = 0.459 | |
| p53 status | Wild type | HR 0.454, 95% CI 0.193–1.070, p = 0.071 | HR 0.244, 95% CI 0.080–0.749, p = 0.014 |
| Mutant | HR 0.625, 95% CI 0.392–1.096, p = 0.054 | HR 0.680, 95% CI 0.396–1.169, p = 0.163 | |
| BRCA1 | Low | HR 0.642, 95% CI 0.409–1.006, p = 0.053 | HR 0.703, 95% CI 0.425–1.165, p = 0.171 |
| High | HR 0.215, 95% CI 0.052–0.888, p = 0.034 | HR 0.046, 95% CI 0.005–0.429, p = 0.007 | |
| BRCA2 | Low | HR 0.565, 95% CI 0.330–0.967, p = 0.037 | HR 0.519, 95% CI 0.280–0.962, p = 0.037 |
| High | HR 0.664, 95% CI 0.353–1.248, p = 0.204 | HR 0.718, 95% CI 0.329–1.571, p = 0.407 |
Figure 3.
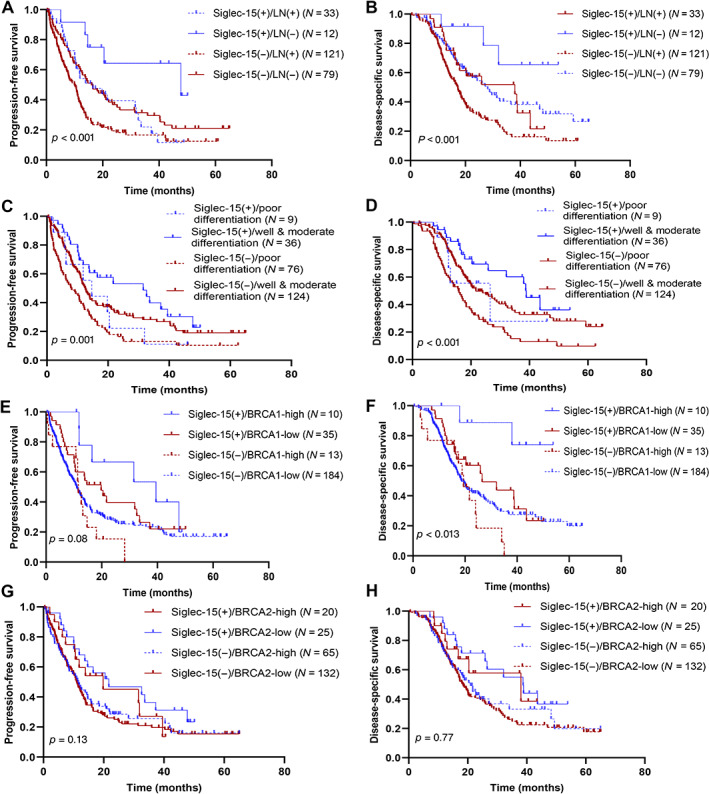
Kaplan–Meier curves for (A, C, E, and G) PFS stratified by Siglec‐15 expression combined with lymph node status, grade, and BRCA1/2 status, and (B, D, F, and H) DSS stratified by Siglec‐15 expression combined with lymph node status, grade, and BRCA1/2 status.
Discussion
To the best of our knowledge, this is the first study to focus on Siglec‐15 expression in patients with PDAC. We investigated the Siglec‐15 expression pattern and its interactions with PD‐L1, tumour‐infiltrating immune cells, and some DDR molecules in a relatively large cohort. We further analysed the prognostic value of Siglec‐15 in clinicopathologically significant and DDR molecular subgroups. Positive Siglec‐15 expression was an independent factor for improved PFS and DSS. We also found that the prognostic significance of Siglec‐15 was modified by the lymph node status, grade, and BRCA1/2 status in PDAC. Our results showed the most discriminative effect of positive Siglec‐15 expression in tumours with negative lymph nodes, well and moderately differentiated tumours, tumours with high BRCA1 expression, or tumours with low BRCA2 expression.
Based on a pan‐cancer analysis from The Cancer Genome Atlas (TCGA) database, upregulated Siglec‐15 was observed in breast invasive carcinoma, bladder cancer, cholangiocarcinoma, oesophageal carcinoma, thyroid cancer, head and neck squamous cell carcinoma, kidney cancer, hepatocellular carcinoma, gastric cancer, and endometrial carcinoma [19, 30]. Li et al demonstrated that Siglec‐15 overexpression was associated with worse overall survival (OS) and relapse‐free survival (RFS) in kidney cancer, and they also found that Siglec‐15 was correlated with poor PFS, but not OS, in lung adenocarcinoma [30]. Quirino et al found that Siglec‐15 expression was not related to OS and RFS in gastric cancer [31], and Hao et al similarly demonstrated that Siglec‐15 was not associated with OS in early NSCLC [32]. In contrast, high Siglec‐15 expression was correlated with better OS in bladder cancer, invasive breast carcinoma, thyroid cancer, endometrioid carcinoma, and head and neck squamous cell carcinoma and with better RFS in ovarian cancer, liver cancer, and endometrioid carcinoma, which was consistent with our results in PDAC [30]. In the present study, our findings also revealed that Siglec‐15 positivity was related to improved PFS and DSS, and was an independent predictor of prognosis in PDAC. In addition, a previous study demonstrated that Siglec‐15 expression was significantly more frequent in AJCC II stage and well‐differentiated gastric cancer [31], while positive expression of Siglec‐15 was associated with the presence of perineural invasion and well and moderate differentiation in our study. The pan‐cancer analysis and our data suggest that the expression of Siglec‐15 may have distinct prognostic implications in different types of cancer.
Currently, a phase II clinical trial is underway to assess the efficacy of anti‐Siglec‐15 therapy in some tumours including colorectal cancer, ovarian cancer, melanoma, and breast cancer. The recent progress of this phase II clinical trial has been slow, casting doubt on the validity of anti‐Siglec‐15 in unselected cancer types, although anti‐Siglec‐15 showed promising efficacy in NSCLC in a phase I trial [15, 17]. It is critical to note that the potential of Siglec‐15 as a broad‐spectrum therapeutic target was not validated in pan‐cancers before initiating this phase II clinical trial. According to these results and the pan‐cancer analysis, Siglec‐15 appears to exert an immunosuppressive function in ‘hot tumours’ like NSCLC, and anti‐Siglec‐15 also showed efficacy in these tumours. However, Siglec‐15 correlated with a better prognosis and may play an important role in inhibiting tumourigenesis in ‘cold tumours’ such as luminal A/B breast cancer [30] and PDAC in this study. Importantly, the association between Siglec‐15 expression and better prognosis suggests that using anti‐Siglec‐15 therapy to treat cancer may be detrimental to patients. Alternatively, administration of agonistic antibodies for Siglec‐15 may be beneficial for patients with these cancers. We hope that agonistic antibodies for Siglec‐15 can be developed. Clinical trials are warranted to validate these data.
Different mechanisms for modulating Siglec‐15 expression may reflect the varied roles on different cell types. Siglec‐15 on macrophages could modulate TLR‐induced cytokine responses to activate the chemokine signalling pathway including CXCR3, which is mainly expressed in Treg and TCs [33, 34, 35]. Li et al revealed that there was an increasing trend for CXCR3 expression in patients with overexpressed Siglec‐15 on macrophages, which may recruit Treg [30]. Siglec‐15 expression on macrophages inhibited the proliferation of activated CD8+ T cells, leading to tumour growth [16]. In our study, Siglec‐15 was mainly expressed on TCs, but not on macrophages. The expression of Siglec‐15 on TCs and macrophages may impact different functions. Siglec‐15 expression on TCs may inhibit CXCR3 expression resulting in lesser Tregs infiltration and no effect on the CD8+ T‐cell responses in PDAC. Our studies also demonstrated that Siglec‐15 on TCs was significantly related to lesser Tregs infiltration. This may partly explain why Siglec‐15 expression on TCs was related to a favourable prognosis. However, the exact mechanisms that regulate Siglec‐15 need to be elucidated in further studies in PDAC.
Siglec‐15 shows a similar domain composition and high homology with PD‐L1 based on a genome‐scale T‐cell activity array by Wang et al [16]. The association between Siglec‐15 and PD‐L1 has been investigated in several cancers [17, 30, 32]. Hu et al demonstrated that Siglec‐15 expression was mutually exclusive from PD‐L1 in bladder cancer [17], and Wang et al also found similar results in lung adenocarcinoma [16]. Siglec‐15, which is induced by macrophage colony‐stimulating factor on macrophages, can be downregulated by IFN‐γ [16] and, to our knowledge, IFN‐γ is a major positive factor for PD‐L1. This may partly explain why the expression of PD‐L1 and Siglec‐15 is mutually exclusive in these types of cancer. In this study, 64 of the Siglec‐15‐negative samples (29.9%) were PD‐L1‐positive, and 33 of the PD‐L1‐negative samples (18.0%) were Siglec‐15‐positive. These data indicate that there was complementarity of PD‐L1 and Siglec‐15 in about 20% of the PDAC patients with PD‐L1 negativity. According to our results, Siglec‐15 may be an effective target. Detection of the expression of Siglec‐15 in PD‐L1‐negative patients may therefore offer more options for immunotherapy for some of the PD‐L1‐negative patients, which is a subgroup that is resistant to anti‐PD‐1 therapy. Thus, for the subgroup with PD‐L1−Siglec‐15+ phenotype, administration of Siglec‐15‐targeted immunotherapy may be beneficial. However, future clinical trials are needed for validation.
Different infiltrating patterns of immune cells can influence the response to immunotherapy [36, 37]. Therefore, we assessed the associations between Siglec‐15 expression and the densities of stromal CD3, CD4, CD8, Foxp3, CD45RO, CD15, and CD68. We found that Siglec‐15 expression on TCs was negatively correlated with the density of CD45RO T cells and Tregs. In addition, Siglec‐15 on TCs was not associated with the macrophage density. However, a very small proportion of cases showed Siglec‐15 positivity on macrophages, different from some previous studies in other types of cancers, including NSCLC, bladder cancer, breast cancer, and thyroid cancer [16, 17, 19, 30, 32]. First, their results were based on NGS data, with most of the previous studies focusing on the relationships between Siglec‐15 and immune infiltrates, which are from bulk cells. This limited their analysis of the exact differentiation of TCs and immune infiltrates. However, in our study, we used IHC analysis focusing on the expression of proteins. Second, differences in Siglec‐15 expression on macrophages and TCs remain unknown, and Siglec‐15 expression on macrophages and dendritic cells can increase the infiltration of Tregs [30]. Siglec‐15 on TCs may exert an opposite effect, leading to lesser densities of Tregs. Third, although Siglec‐15 expression in the membrane was considered as positive, we cannot exclude the simultaneous expression of Siglec‐15 in the cytoplasm and membrane. Additionally, the tumour microenvironment was complex and variable due to spatial and temporal heterogeneity [38]. However, whether this immune infiltrating pattern is derived from Siglec‐15 overexpression on TCs, and not macrophages, remains unclear. Future work should be conducted to explore the mechanism.
In addition to its role in immune regulation, Li et al revealed that Siglec‐15 was associated with some pathways (e.g. MAPK, PI3K‐Akt, Hippo, and p53) through Gene Set Enrichment Analysis [30]. Some of the molecules in these pathways play a role in DDR. In the present study, Siglec‐15 expression was associated with high BRCA1 status. Wei et al revealed that, through the MAPK pathway, BRCA1 protein expression can be induced in gastric cancer cells [39]. PDAC cells may overexpress Siglec‐15 through BRCA1 induced by MAPK pathways. Interestingly, we observed more pronounced prognostic effects of Siglec‐15 positivity in tumours with high BRCA1 expression than in tumours with low BRCA1 expression and in tumours with low BRCA2 expression than in tumours with high BRCA2 expression. Some studies suggested that BRCA1‐deficient breast cancers were associated with increased PD‐L1 expression and BRCA2 depletion promotes PD‐L1 upregulation in a Chk1‐dependent manner in lung cancer cells [40, 41]. Based on these data and our results, there may be a close relationship between the expression levels of BRCA1 and BRCA2 and the expression of PD‐L1 and Siglec‐15. Thus, combining immunotherapy with PARP inhibitor treatment may offer an option for patients with both BRCA deficiency and expression of Siglec‐15. However, the regulatory mechanism between Siglec‐15 and DDR molecules in PDAC remains to be elucidated.
This study had some limitations. First, it was a retrospective study and the results has not been validated in an independent cohort, which may limit the generalisability of our results. Second, tumour heterogeneity was inevitable owing to the use of TMAs. Third, the small number of cases may be considered as a further limitation to subgroup analysis. Therefore, clinical data from multiple centres and prospective studies are required to confirm our results.
In conclusion, we investigated the expression status of Siglec‐15 in PDAC and found that Siglec‐15 expression was observed in 18.6% of patients, particularly in well and moderately differentiated tumours. Siglec‐15 positivity was an independent factor for favourable clinical outcomes. Anti‐PD‐1 combined with targeting Siglec‐15 may offer a novel strategy for patients with PDAC. Furthermore, we also found that the prognostic significance of Siglec‐15 was modified by lymph node status, grade, and BRCA1/2 status in PDAC. Immunotherapy targeting Siglec‐15 and its regulatory mechanisms ought to be further investigated in future studies of PDAC.
Author contributions statement
XC contributed to sample and data acquisition and manuscript drafting. SM, YZ, HM, SY and ZL offered technical support. JC made substantial contributions to the conception and design of the study, funding of the study, and supervision. All authors read and approved the final manuscript.
Supporting information
Supplementary materials and methods
Figure S1. Representative mIF staining of Siglec‐15 positivity on macrophages in PDAC
Figure S2. Comparison between Siglec‐15 and stromal densities of (A) CD3+ T cells, (B) CD4+ T cells, (C) CD8+ T cells, (D) FOXP3+ T cells, (E) CD45RO+ T cells, (F) CD15+ neutrophils, (G) CD68+ macrophages, and (H) TPS of PD‐L1 in PDAC using t‐tests
Figure S3. Associations of TPS of Siglec‐15 with (A) CD3+ T cells, (B) CD4+ T cells, (C) CD8+ T cells, (D) FOXP3+ T cells, (E) CD45RO+ T cells, (F) CD15+ neutrophils, and (G) CD68+ macrophages using Spearman's correlation
Table S1. Association of clinicopathological features with Siglec‐15 expression
Table S2. Association of DDR molecules with Siglec‐15 expression
Table S3. Association of Siglec‐15 with densities of immune cells
Acknowledgements
We thank Junyi Pang and Mei Li for providing technical assistance.
This work was supported by grants from the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (CAMS‐2016‐I2M‐1‐001), the National Natural Science Foundation of China (Nos. 81472326 and 81672648), and the National Scientific Data Sharing Platform for Population and Health (NCMI‐YF01N‐201906). The funders of the study had no role in the design of the study; the collection, analysis, and interpretation of data; or in writing the manuscript.
No conflicts of interest were declared.
Data availability statement
The data sets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011; 378: 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74: 2913–2921. [DOI] [PubMed] [Google Scholar]
- 4. Simard EP, Ward EM, Siegel R, et al. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin 2012; 62: 118–128. [DOI] [PubMed] [Google Scholar]
- 5. Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 2004; 91: 586–594. [DOI] [PubMed] [Google Scholar]
- 6. Versteijne E, Suker M, Groothuis K, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin Oncol 2020; 38: 1763–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Louvet C, Philip PA. Accomplishments in 2007 in the treatment of metastatic pancreatic cancer. Gastrointest Cancer Res 2008; 2(3 Suppl): S37–S41. [PMC free article] [PubMed] [Google Scholar]
- 8. Luke JJ, Flaherty KT, Ribas A, et al. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 2017; 14: 463–482. [DOI] [PubMed] [Google Scholar]
- 9. Meng X, Liu Y, Zhang J, et al. PD‐1/PD‐L1 checkpoint blockades in non‐small cell lung cancer: new development and challenges. Cancer Lett 2017; 405: 29–37. [DOI] [PubMed] [Google Scholar]
- 10. Wainberg ZA, Hochster HS, Kim EJ, et al. Open‐label, phase I study of nivolumab combined with nab‐paclitaxel plus gemcitabine in advanced pancreatic cancer. Clin Cancer Res 2020; 26: 4814–4822. [DOI] [PubMed] [Google Scholar]
- 11. Ott PA, Bang YJ, Piha‐Paul SA, et al. T‐cell‐inflamed gene‐expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE‐028. J Clin Oncol 2019; 37: 318–327. [DOI] [PubMed] [Google Scholar]
- 12. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD‐1 blockade. Science 2017; 357: 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siglec‐15: an attractive immunotherapy target. Cancer Discov 2020; 10: 7–8. [DOI] [PubMed] [Google Scholar]
- 14. Pan C, Liu H, Robins E, et al. Next‐generation immuno‐oncology agents: current momentum shifts in cancer immunotherapy. J Hematol Oncol 2020; 13: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun J, Lu Q, Sanmamed MF, et al. Siglec‐15 as an emerging target for next‐generation cancer immunotherapy. Clin Cancer Res 2021; 27: 680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J, Sun J, Liu LN, et al. Siglec‐15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med 2019; 25: 656–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu J, Yu A, Othmane B, et al. Siglec15 shapes a non‐inflamed tumor microenvironment and predicts the molecular subtype in bladder cancer. Theranostics 2021; 11: 3089–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takamiya R, Ohtsubo K, Takamatsu S, et al. The interaction between Siglec‐15 and tumor‐associated sialyl‐Tn antigen enhances TGF‐β secretion from monocytes/macrophages through the DAP12‐Syk pathway. Glycobiology 2013; 23: 178–187. [DOI] [PubMed] [Google Scholar]
- 19. Li QT, Huang ZZ, Chen YB, et al. Integrative analysis of Siglec‐15 mRNA in human cancers based on data mining. J Cancer 2020; 11: 2453–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson SP, Bartek J. The DNA‐damage response in human biology and disease. Nature 2009; 461: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016; 531: 47–52. [DOI] [PubMed] [Google Scholar]
- 22. Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008; 321: 1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park W, Chen J, Chou JF, et al. Genomic methods identify homologous recombination deficiency in pancreas adenocarcinoma and optimize treatment selection. Clin Cancer Res 2020; 26: 3239–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Golan T, Hammel P. Management of BRCA mutation carriers with pancreatic adenocarcinoma. J Natl Compr Canc Netw 2021; 19: 469–473. [DOI] [PubMed] [Google Scholar]
- 25. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015; 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- 26. Danilova L, Ho WJ, Zhu Q, et al. Programmed cell death ligand‐1 (PD‐L1) and CD8 expression profiling identify an immunologic subtype of pancreatic ductal adenocarcinomas with favorable survival. Cancer Immunol Res 2019; 7: 886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oshima M, Okano K, Muraki S, et al. Immunohistochemically detected expression of 3 major genes (CDKN2A/p16, TP53, and SMAD4/DPC4) strongly predicts survival in patients with resectable pancreatic cancer. Ann Surg 2013; 258: 336–346. [DOI] [PubMed] [Google Scholar]
- 28. Beger C, Ramadani M, Meyer S, et al. Down‐regulation of BRCA1 in chronic pancreatitis and sporadic pancreatic adenocarcinoma. Clin Cancer Res 2004; 10: 3780–3787. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Chen XL, Mo SW, et al. PD‐L1 and PD‐L2 expression in pancreatic ductal adenocarcinoma and their relation to immune infiltrates and DNA damage response molecules. J Pathol Clin Res 2022; 8: 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li B, Zhang B, Wang X, et al. Expression signature, prognosis value, and immune characteristics of Siglec‐15 identified by pan‐cancer analysis. Oncoimmunology 2020; 9: 1807291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quirino MWL, Pereira MC, Deodato de Souza MF, et al. Immunopositivity for Siglec‐15 in gastric cancer and its association with clinical and pathological parameters. Eur J Histochem 2021; 65: 3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hao JQ, Nong JY, Zhao D, et al. The significance of Siglec‐15 expression in resectable non‐small cell lung cancer. Neoplasma 2020; 67: 1214–1222. [DOI] [PubMed] [Google Scholar]
- 33. Briard JG, Jiang H, Moremen KW, et al. Cell‐based glycan arrays for probing glycan‐glycan binding protein interactions. Nat Commun 2018; 9: 880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boyd CR, Orr SJ, Spence S, et al. Siglec‐E is up‐regulated and phosphorylated following lipopolysaccharide stimulation in order to limit TLR‐driven cytokine production. J Immunol 2009; 183: 7703–7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang BQ, Zhang CM, Gao W, et al. Cancer‐derived matrix metalloproteinase‐9 contributes to tumor tolerance. J Cancer Res Clin Oncol 2011; 137: 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peng Q, Qiu X, Zhang Z, et al. PD‐L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat Commun 2020; 11: 4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Velez‐Delgado A, Mathew E, et al. Myeloid cells are required for PD‐1/PD‐L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut 2017; 66: 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kondratova M, Czerwinska U, Sompairac N, et al. A multiscale signalling network map of innate immune response in cancer reveals cell heterogeneity signatures. Nat Commun 2019; 10: 4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wei X, Liu X, Liu H, et al. BRCA1‐associated protein induced proliferation and migration of gastric cancer cells through MAPK pathway. Surg Oncol 2020; 35: 191–199. [DOI] [PubMed] [Google Scholar]
- 40. Wen WX, Leong CO. Association of BRCA1‐ and BRCA2‐deficiency with mutation burden, expression of PD‐L1/PD‐1, immune infiltrates, and T cell‐inflamed signature in breast cancer. PLoS One 2019; 14: e0215381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sato H, Niimi A, Yasuhara T, et al. DNA double‐strand break repair pathway regulates PD‐L1 expression in cancer cells. Nat Commun 2017; 8: 1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials and methods
Figure S1. Representative mIF staining of Siglec‐15 positivity on macrophages in PDAC
Figure S2. Comparison between Siglec‐15 and stromal densities of (A) CD3+ T cells, (B) CD4+ T cells, (C) CD8+ T cells, (D) FOXP3+ T cells, (E) CD45RO+ T cells, (F) CD15+ neutrophils, (G) CD68+ macrophages, and (H) TPS of PD‐L1 in PDAC using t‐tests
Figure S3. Associations of TPS of Siglec‐15 with (A) CD3+ T cells, (B) CD4+ T cells, (C) CD8+ T cells, (D) FOXP3+ T cells, (E) CD45RO+ T cells, (F) CD15+ neutrophils, and (G) CD68+ macrophages using Spearman's correlation
Table S1. Association of clinicopathological features with Siglec‐15 expression
Table S2. Association of DDR molecules with Siglec‐15 expression
Table S3. Association of Siglec‐15 with densities of immune cells
Data Availability Statement
The data sets used and/or analysed during the current study are available from the corresponding author upon reasonable request.


