Abstract
Background:
The etiology of child and adolescent anxiety remains poorly understood. Although several previous studies have examined associations between prenatal maternal psychological functioning and infant and child health outcomes, less is known about the impact of maternal anxiety specific to pregnancy and cortisol during pregnancy on childhood anxiety outcomes.
Methods:
Participants included 496 mother-child pairs from the PROGRESS longitudinal birth cohort in Mexico City. Anxiety symptoms were assessed at age 8–11 years during 2018–2019 using the Revised Children’s Manifest Anxiety Scale. Pregnancy-specific anxiety was assessed using an expanded version of the Pregnancy Anxiety Scale. Maternal biological stress response during pregnancy was assessed using salivary cortisol measures (area under the curve, cortisol awakening response, and diurnal slope). Linear regression models were used to estimate associations between maternal anxiety and cortisol in relation to continuous child anxiety symptom T-scores. Models were adjusted for maternal age, socioeconomic status, child sex and age, and gestational age at saliva collection.
Results:
We found that higher levels of pregnancy-specific anxiety in the mother were associated with higher anxiety symptoms in the child (β: 1.30, 95% CI: 0.19, 2.41). We additionally observed an association between higher maternal total cortisol output during pregnancy and higher anxiety symptoms in the child (β: 1.13, 95% CI: 0.25, 2.01).
Discussion:
These findings highlight the importance of screening for maternal pregnancy-specific anxiety and the need to identify interventions and support for mothers during pregnancy in order to promote healthy outcomes for mothers and their children.
Keywords: Cortisol, pregnancy, anxiety, stress, fetal programming
1. Introduction
One in four people worldwide will be afflicted by a mental disorder at some point in their life (WHO, 2001), making it a major global contributor to excess morbidity and disability. Anxiety is one of the most common mental health disorders (Vigo et al., 2016) among children and adolescents, with a rapidly increasing prevalence over the past decade in this group (Bitsko et al., 2018; Twenge et al., 2019). Clinical and subclinical symptoms of anxiety often first appear early in life (Whalen et al., 2017) – with an average age of onset of 11 years (Bandelow and Michaelis, 2015). Mental health symptoms often continue into adulthood (Kessler et al., 2007), and can lead to substance abuse, suicide risk, and other chronic health conditions (Scott et al., 2011). Understanding risk factors for childhood/preadolescent onset may be critical for effective intervention programs as this life stage may represent a critical time period for prevention and early detection of anxiety symptoms (Fuhrmann et al., 2015), helping to prevent future mental and physical health burdens later in life, resulting in benefits to individuals and society.
Among the known risk factors for early life psychiatric disorders and subclinical symptoms in general are family-level stressors, including parental mental health and stress (Conger et al., 1994; Essex et al., 2006; Roustit et al., 2010). Research has identified the prenatal period as a particularly vulnerable time point for these maternal factors (McLean et al., 2018). Previous studies have assessed prenatal maternal psychological functioning on various infant and childhood health outcomes including associations between higher maternal psychological distress and dimensions of temperament behavior in infancy (Howland et al., 2020; Huizink et al., 2002). Additional studies have shown impacts between higher levels of maternal biologic markers of stress (i.e. cortisol) and slower rates of infant cognitive development (Davis and Sandman, 2010); while other studies have shown impacts between higher prenatal maternal stress and poorer child cognitive function (Grande et al., 2021). Most of these studies have either been cross-sectional or have focused on early offspring neurodevelopment, with few addressing impacts in late childhood and early adolescent mental health outcomes. Additionally, much of the literature has focused on childhood impacts of maternal depression during pregnancy (Galbally et al., 2020; Gjerde et al., 2017), with less attention given to the impacts of prenatal pregnancy specific anxiety. This is a critical gap, as a few recent studies have shown that stress and anxiety specifically related to pregnancy may be most strongly associated with birth outcomes and child health (Davis and Sandman, 2012; Huizink et al., 2003). Finally, women from lower socioeconomic status (SES) populations often face disproportionately higher stress exposure; however, the majority of previous studies have been located in high income countries with higher SES populations, with fewer studies from low and middle-income countries with lower SES populations (Buffa et al., 2018).
External stressors associated with poverty, violence exposure, nutritional deficiency among others are not processed uniformly. Mothers respond to stress in pregnancy through altered levels of stress hormones such as prenatal maternal cortisol concentrations. Variability in cortisol response may help to delineate mechanisms of the stress-mental health relationship that can occur across generations. Dysregulation of the maternal hypothalamic-pituitary-adrenal (HPA) axis in response to stressors during pregnancy can determine fetal exposure to stress hormones (Sandman, 2018). Maternal stress hormones can cross the placenta, reach the developing fetus, and may play a role in the development of anxiety in children and adolescents. A very limited number of previous studies have assessed the direct impacts of maternal cortisol concentrations during pregnancy on child mental health outcomes (Davis and Sandman, 2012; Isaksson et al., 2015), thus several research gaps remain. One major research gap is whether there is an interaction between maternal pregnancy-specific anxiety and cortisol on child anxiety symptoms.
In the current study, we address these research gaps by assessing the association between pregnancy specific anxiety, prenatal cortisol concentrations, and childhood anxiety symptoms at age 8–11 in a cohort of mothers and children living in Mexico City. We used commonly assessed indicators of diurnal cortisol activity including the area under the curve relative to ground (AUC), the cortisol awakening response (CAR), and the diurnal cortisol slope (slope). We additionally assessed if associations varied by sex of the child. We hypothesized that higher levels of maternal pregnancy specific anxiety would be related to higher childhood anxiety symptoms and that this would vary by child sex
2. Material and methods
2.1. Study population
Data for the current study came from the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) longitudinal birth cohort in Mexico City. Pregnant women were recruited between 2007 and 2011 at 12–24 weeks’ gestation in primary care clinics of the Mexican Social Security Institute. In order to be included in the study, women needed to be 18 years or older and plan to live in Mexico City. Women were additionally eligible if they were less than 20 weeks gestation, had completed primary education, had no medical history of heart or kidney disease, and did not consume alcohol daily. In total, 948 women enrolled in the 2nd trimester and delivered a live child who was then followed longitudinally. For the current study, we had complete exposure, covariate, and outcome data for 496 mother-child pairs (Supplemental Figure 1). Participant characteristics did not differ for those included and those in the original sample (Supplemental Table 1).
Protocols were approved by the institutional review boards at the Icahn School of Medicine at Mount Sinai, Harvard School of Public Health, and Mexican National Institute of Public Health. All women provided informed consent and all children provided assent.
2.2. Childhood anxiety symptoms
Late childhood anxiety was assessed in children at 8–11 years using the Spanish version of the validated (Ferrando, 1994) Revised Children’s Manifest Anxiety Scale (RCMAS-2) (Reynolds and Richmond, 2008). The Spanish version of the RCMAS was administered to children by trained psychologists. The RCMAS short form has 12 items rated as yes/no. Higher scores indicate greater anxiety symptoms. Individual items on the RCMAS were summed to create a total raw score. Raw scores on the RCMAS-2 assessment ranged from 0–10 (Cronbach’s alpha = 0.70). Raw total anxiety scores were converted to age and sex standardized T-scores based on normative samples. We analyzed scores as continuous and also dichotomized scores based on cut-offs recommended by the RCMAS manual (Reynolds and Richmond, 2008). A RCMAS T-score of ≥60 represents scores 1 SD higher than the mean and indicates clinically elevated anxiety symptoms.
2.3. Maternal prenatal psychological functioning and cortisol measures
2.3.1. Cortisol.
Maternal biological stress response during pregnancy was assessed using salivary cortisol measures. Women were trained and instructed to provide samples into Salicaps using the passive drool technique: a) upon awakening, b) 45 minutes after awakening, c) 4 hours after awakening, d) 10 hours after awakening, and e) at bedtime. Saliva samples were collected across two consecutive days during pregnancy (mean ± SD: 18.7 ± 2 weeks, range: 16–34 weeks). After sample collection, women recorded the collection time on the tube and in their diary, and refrigerated samples until pickup, after which they were stored at −70C until shipment on dry ice to the laboratory of Dr. Clemens Kirschbaum in Dresden, Germany for assay. Samples were assayed in duplicates using a chemi-luminescence assay (IBL; Hamburg, Germany) with sensitivity of ~0.16 ng/ml. Intra- and interassay coefficients of variation were less than 8%. Cortisol samples were excluded if the timing of assessment could not be determined or if the sample was missing.
From these samples, we derived three features of the daily cortisol curve: 1) the total daily cortisol output, area under the curve relative to ground (AUC); 2) the cortisol awakening response (CAR); and 3) the diurnal slope across the day (slope). We assessed associations with these three different measures because they each represent different aspects of the diurnal cortisol pattern and may have different associations with health outcomes (Adam and Kumari, 2009). We examined associations with daily cortisol production using the AUC of the salivary cortisol levels; the AUC was calculated using the trapezoidal rule. A higher AUC is indicative of a greater cortisol output over the day. The CAR, or the rapid increase in cortisol in the morning, was estimated from the change in cortisol between the first and second saliva samples of the day. Finally, the slope represents the decline in cortisol concentrations over the day. In general, steeper slopes indicate a more rapidly declining cortisol output over the course of the day, while flatter slopes represent a slower decline. Each cortisol measure was calculated for each day and then averaged across the two days of collection.
2.3.2. Pregnancy-specific anxiety
Pregnancy-specific anxiety was assessed using an expanded set of items based on those developed by Wadhwa et al. (1993). The Pregnancy Anxiety Scale assesses a woman’s feelings about her health during pregnancy, the health of her baby, and her feelings about labor and delivery. Answers were given on a 4-point scale (0=not at all, 4=a lot) and include items such as “I am concerned or worried about losing my baby,” and “I am worried about developing medical problems during my pregnancy.” The Spanish version of the scale was administered to women by trained psychologists during either the second or third trimester of pregnancy. This scale was previously validated and linked to health outcomes in a Latino population (Rini et al., 1999). The scale had good internal consistency in our study population (Cronbach’s alpha = 0.86). A continuous and dichotomized measure (at the median) was used in analyses.
2.3.3. Additional measures of maternal stress and psychological functioning during pregnancy.
Symptoms of trait generalized anxiety were measured with the 10-item trait anxiety scale of the Spielberger State-Trait Anxiety Scale (STAI) (Cronbach’s alpha = 0.75) (Spielberger, 1983). Responses were summed to create a total trait anxiety score. Perceived stress was measured using the 4-item Perceived Stress Scale (PSS-4) (Cronbach’s alpha = 0.72) (Cohen et al., 1983; Lee, 2012; Vallejo et al., 2018). Responses were summed to create an overall score. Negative life events within the previous six months were assessed using the Crisis in Family Systems-Revised survey, which inquires about 11 life event domains experienced in the last 6 months (finances, legal problems, career, home events, relationships, safety in the home, safety in the neighborhood, personal medical issues, medical issues with others, prejudice, and authority figures/institutions) (Berry et al., 2006; Shalowitz et al., 1998). A summary score (range 0 to 11) was calculated by summing the number of domains that women endorsed as having at least one negative life event. Depressive symptoms were assessed using the Edinburgh Postnatal Depression Scale (Cronbach’s alpha = 0.87) (Cox et al., 1987; Flom et al., 2018b).
2.4. Covariates
A directed acyclic graph (DAG) was used to identify the minimally sufficient adjustment set that resulted in the least biased estimates. This adjustment set included maternal age, SES, gestational week of saliva collection, child age at anxiety assessment, and child sex. Maternal age (years) and SES were assessed at enrollment. Thirteen variables derived from questionnaire results were used to classify study participants into six levels based on the SES index created by the Asociación Mexicana de Agencias de Investigación de Mercados y Opinión Pública (Carrasco, 2002). We further collapsed these six levels into lower, medium, and higher SES based on the distribution in our study population. Maternal pre-pregnancy body mass index (BMI) was derived using weight and height measurements taken during the second trimester. Given the differences in week of gestation for saliva collection, we additionally adjusted for this variable in analyses. The gestational week of saliva sample collection during pregnancy was calculated based on date of last menstrual period. Tanner staging was used to determine pubertal status (Tanner, 1986). Trained study pediatricians performed tanner staging on all PROGRESS children. Visual inspection was used to assess girls breast development, and boys and girls pubic hair development. Boys genital development was assessed by inspection and use of the orchidometer. Tanner staging (1–5) was categorized as 2+ (development) vs 1 (no development).
2.5. Statistical analyses
We first report descriptive characteristics of the study population, including the average pregnancy-specific anxiety and total cortisol output levels by maternal characteristics. We additionally assess distributions of all exposure measures and correlations between measures using Pearson correlation coefficients.
Our main analyses focused on associations between pregnancy-specific anxiety, maternal cortisol, and continuous anxiety symptoms in the child. We a priori focus on pregnancy-specific anxiety as previous studies have found this measure to be most strongly associated with child health outcomes (Davis and Sandman, 2012). We additionally assess associations with several salivary cortisol summary indicators including the AUC, CAR, and slope. We first use linear regression approaches and report beta coefficients and 95% confidence intervals (CI) for the associations with continuous anxiety T-scores. Linear regression assumptions were assessed in all models. Scatterplots were used to detect outliers and visually inspect linearity. Normality of residuals was checked visually using QQ plots. We additionally plotted residuals against fitted values to confirm homoscedascity. Finally, multicollinearity was assessed by examining the variable inflation factors (VIF) coefficient. All VIFs ranged from 1.01–1.08.
We assessed if exposure-outcome associations differed by child sex by using an augmented product term approach (Buckley et al., 2017). Using this approach, we specified a model that included product terms between sex and all covariates – this model is equivalent to a fully stratified model. From this model we were then able to evaluate the sex-exposure product term; a p<0.20 cut-off was used to indicate significant effect measure modification by child sex (Buckley et al., 2017).
We conducted several sensitivity analyses. Previous studies have found relations between prenatal maternal stress and age of onset of puberty (Brauner et al., 2021); mental health outcomes additionally differ by pubertal status (Oldehinkel et al., 2011). Thus, given the potential mediating role of pubertal status, we did not adjust for this in our main models. In sensitivity analyses we assessed the influence of adjustment for pubertal status by adjusting for tanner stage (2+ vs 1). Additionally, given the impact of concurrent maternal depressive symptoms we additionally adjusted for this measure in sensitivity analyses. Next, we assessed if associations held with different categorizations of AUC, including categorizing continuous levels into quartiles. We also assessed associations between the CAR and anxiety symptoms, excluding women with negative CAR values. Previous studies have shown an interactive effects between maternal psychosocial measures and cortisol levels (Campbell et al., 2019; Enlow et al., 2017; Flom et al., 2018a). In order to examine this in our study population we assessed effect modification between the pregnancy-specific anxiety and child anxiety association by maternal prenatal AUC levels. For these analyses, we dichotomized total cortisol output into high and low levels, based on the median value. Logistic regression was additionally used to assess associations between exposures and the odds of clinically relevant RCMAS T-scores, using the cut points provided in the RMCAS-2 manual. Children were categorized as being in the clinically elevated group for the RCMAS-2 if the T-scores were ≥ 60. For logistic models, we report odds ratios (OR) and 95% CIs.
Our main analyses focused on associations with pregnancy-specific anxiety. We additionally assessed associations with other measures of psychological functioning during pregnancy to assess if the impacts were specific to pregnancy-specific anxiety or if we observed independent associations across all psychological functioning measures during pregnancy. We compared associations with additional measures including trait generalized anxiety, negative life events, perceived stress, and depressive symptoms. For these analyses, we report beta coefficients and 95% CI.
3. Results
3.1. Sample characteristics
Table 1 shows descriptive characteristics of the 496 mother-child pairs included in the current analysis. Mothers in the study population were on average of low SES and had less than a high school education at study enrollment; the mean maternal age at birth was 28 years. The majority of women had their saliva collected during the second trimester (mean: 18.5 weeks, min: 16, max: 33 weeks). There was an equal distribution of male and female children in the study population (51% males). Children were on average 9.7 years old at the anxiety symptom assessment study visit and had a mean anxiety symptom T-score of 51.2 (SD: 8).
Table 1.
Characteristics of the 496 mother-child pairs in the PROGRESS (Programming Research in Obesity, Growth, Environment and Social Stressors) study
| No. (%) or Mean ± SD | |
|---|---|
| Overall | 496 |
| Child sex | |
| Male | 252 (51) |
| Female | 244 (49) |
| Child age (years) | 9.7 ± 0.7 |
| Maternal age at delivery (years) | 28.1 ± 6 |
| Maternal pre-pregnancy body mass index | 26.5 ± 4 |
| Gestational age at saliva collection (weeks) | 18.5 ± 2 |
| Socioeconomic status | |
| Low | 267 (54) |
| Medium | 181 (36) |
| High | 48 (10) |
| Maternal education | |
| Less than high school | 202 (41) |
| High school | 175 (35) |
| More than high school | 119 (24) |
| Average child anxiety T-score | 51.2 ± 8 |
3.2. Distribution of maternal exposures across participant characteristics
Women who were younger, had lower SES, and reported a higher number of negative life events during pregnancy tended to have higher pregnancy-specific anxiety levels (Supplemental Table 1). There were no major differences for maternal AUC levels during pregnancy across the maternal characteristics, though higher educated women tended to have lower AUC levels during pregnancy compared to low or medium educated women (AUC high educated 8.9 vs 9.7 low educated) (Supplemental Table 2).
3.3. Salivary cortisol summary measures
Table 2 includes descriptive characteristics for the prenatal maternal cortisol and psychological functioning measures. Mean concentrations of the five cortisol samples followed the expected daily circadian pattern of the HPA-axis. Specifically, average cortisol levels were highest upon awakening (Time 1 mean: 20.5 nmol/L) and shortly after (Time 2 mean: 20.8 nmol/L), and declined throughout the day with lower levels seen four (Time 3 mean: 10.1 nmol/L) and ten hours after awakening (Time 4 mean: 6.0 nmol/L), and the lowest at bedtime (Time 5 mean: 5.2 nmol/L) (Table 2). Overall, total cortisol output was significantly correlated with all cortisol samples throughout the day (Times 1–4), except the bedtime sample (Time 5) (0.26–0.53). Further, AUC was significantly positively correlated with CAR (0.34) and negatively correlated with the diurnal cortisol slope (−0.24) (Supplemental Table 3). Pregnancy-specific anxiety was negatively correlated with awakening cortisol (−0.11) and positively correlated with each of the other psychological functioning measures (0.22–0.35).
Table 2.
Prenatal maternal cortisol and psychological functioning measures
| Mean ± SD | Median | Min | Max | |
|---|---|---|---|---|
| Cortisol Measures (nmol/L) | ||||
| Time 1 (Awakening) | 20.5 ± 9 | 20.2 | 0.07 | 130.9 |
| Time 2 (45 minutes after awakening) | 20.8 ± 9 | 18.9 | 1.97 | 131.6 |
| Time 3 (4 hours after awakening) | 10.1 ± 5 | 9.2 | 0.97 | 51.3 |
| Time 4 (10 hours after awakening) | 6.0 ± 4 | 5.1 | 0.10 | 41.3 |
| Time 5 (Bedtime) | 5.2 ± 5 | 3.8 | 0.54 | 67.2 |
| Area Under the Curve (AUC) | 9.5 ± 3 | 9.1 | 1.7 | 21.4 |
| Cortisol Awakening Response (CAR) | 0.07 ± 8 | −1.01 | −19.9 | 30.8 |
| Diurnal Cortisol Slope (slope) | −1.01 ± 0.4 | −1.00 | −2.22 | 0.65 |
| Maternal Psychological functioning During Pregnancy | ||||
| Pregnancy-specific anxiety | 18.8 ± 5 | 18 | 7 | 28 |
| Trait anxiety | 19.1 ± 5 | 18 | 10 | 34 |
| Perceived stress | 5.2 ± 3 | 5 | 0 | 13 |
| Negative life events | 3.2 ± 2 | 3 | 0 | 10 |
| Depressive symptoms | 8.4 ± 6 | 8 | 0 | 28 |
3.4. Main associations between pregnancy-anxiety, cortisol, and child anxiety symptoms
Figure 1 shows the adjusted associations between pregnancy-specific anxiety, cortisol, and continuous childhood anxiety symptom T-scores, overall and stratified by child sex (numeric results are shown in Supplemental Table 4). Results are reported per interquartile range (IQR) increase for each measure. Overall, we observed significant associations between increased pregnancy-specific anxiety in the mother and higher anxiety symptoms in the child (β: 1.30, 95% CI: 0.19, 2.41). These associations did not differ by sex of the child. We additionally observed associations between higher maternal AUC during pregnancy and increased anxiety symptoms in the child (β: 1.13, 95% CI: 0.25, 2.01) (Figure 1, Supplemental Table 4). We observed significant differences by sex for total cortisol output, with associations seen in boys (β: 2.18, 95% CI: 0.88, 3.47), but not girls (β: 0.39, 95% CI: −0.82, 1.61; p-int: 0.06). There were no observed associations for any of the other prenatal maternal cortisol measures. Effect estimates were similar when adjusting for pubertal status (tanner stage) and concurrent maternal depressive symptoms (Supplemental Table 5). When excluding women with negative CAR values we still did not detect an association between CAR and anxiety symptoms (β: 0.70, 95% CI: −0.55, 1.94). We additionally observed an interaction between pregnancy-specific anxiety and total cortisol output, with higher anxiety symptoms seen in children of mothers with higher (β: 1.99, 95% CI: 0.42, 3.56) compared to lower (β: 0.41, 95% CI: −1.20, 2.02) AUC levels during pregnancy (p-int: 0.16) (Figure 2, Supplemental Table 6).
Figure 1.

Associations between interquartile range increases in maternal pregnancy-specific anxiety, cortisol measures (total cortisol output, AUC; cortisol awakening response, CAR; and diurnal cortisol slope), and continuous childhood anxiety symptom T-scores, overall and stratified by child sex. Models are adjusted for maternal age, SES, child age at anxiety assessment, child sex, and gestational age at saliva collection.
AUC: area under the curve; CAR: cortisol awakening response; CI: confidence interval, Slope, diurnal slope across the day.
Figure 2.

Effect modification of the association between maternal pregnancy-specific anxiety and childhood anxiety symptoms, by total cortisol output (high/low levels dichotomized at the median).
AUC: area under the curve; CI: confidence interval
3.5. Associations between categories of AUC and anxiety symptoms
We conducted a few additional sensitivity and sub-analyses. First, we assessed associations by quartiles of total cortisol output. Overall, we saw stronger associations in the fourth (β: 2.41, 95% CI: 0.53, 4.30) compared to the first quartile of AUC (Supplemental Table 7). Additionally, in boys we saw an increasing trend with stronger associations seen in the fourth (β: 4.13, 95% CI: 1.48, 6.77) compared to the first quartile of AUC.
3.6. Impact of other maternal psychological functioning measures
Next, we investigated whether the associations were specific to pregnancy-specific anxiety, or if there were independent associations with other measures of psychological functioning during pregnancy. Figure 3 shows the results for each of the maternal psychological functioning measures (modeled separately) during pregnancy in relation to anxiety symptoms in the child (see Supplemental Table 8 for numeric results). Results are reported per IQR increase in each psychological functioning measure. We did not observe any overall associations with any of the other measures. We additionally assessed if these associations differed by child sex. Overall, results were similar for males and females; however, for depressive symptoms, there were stronger associations seen in girls (β: 1.55, 95% CI: −0.06, 3.17) compared to boys (β: 0.37, 95% CI: −1.13, 1.88)(interaction p-value: 0.19).
Figure 3.
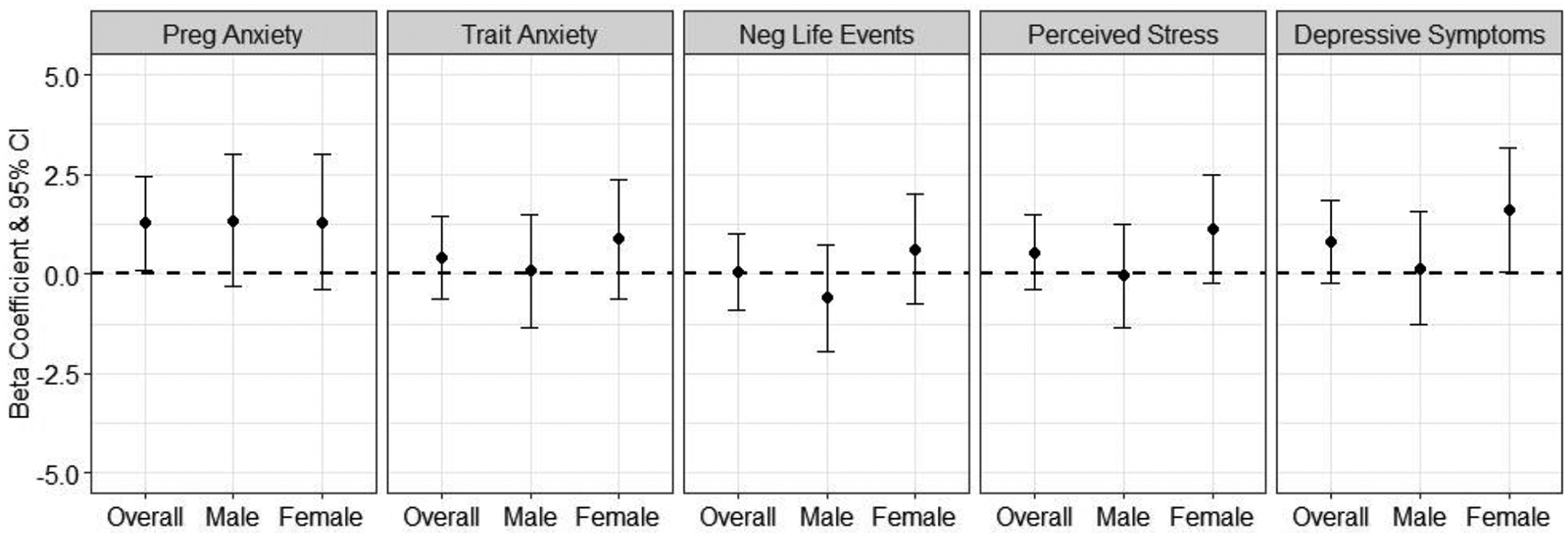
Associations between pregnancy-specific anxiety and other maternal psychological functioning measures during pregnancy (trait anxiety, negative life events, perceived stress, and depressive symptoms) in relation to continuous childhood anxiety symptom T-scores, overall and stratified by child sex. Results are presented per IQR increase in the continuous measure. Models are adjusted for maternal age, SES, child age, child sex, and gestational age at saliva collection.
3.7. Associations with dichotomized measures of child anxiety symptoms
Finally, we assessed associations between maternal pregnancy-specific anxiety, cortisol, and childhood anxiety, with anxiety symptoms dichotomized at clinically elevated cutpoints. Overall, we observed associations between higher maternal pregnancy-specific anxiety and increased odds of the child having clinically elevated anxiety symptoms (OR: 1.64, 95% CI: 1.05, 2.57) (Supplemental Table 9). These associations were stronger in girls (OR: 2.02, 95% CI: 1.10, 3.69) compared to boys (OR: 1.29, 95% CI: 0.65, 2.58), though the interaction p-value did not reach significance. We additionally observed increased odds of clinically elevated anxiety for each of the cortisol measures, including total cortisol output (OR: 1.38, 95% CI: 0.99, 1.92), the cortisol awakening response (OR: 1.29, 95% CI: 0.95, 1.77), and diurnal slope (OR: 1.25, 95% CI: 0.91, 1.73), though we note that the 95% CI includes the null value for each of these results (Supplemental Table 9).
4. Discussion
In the current study, we analyzed the association between prenatal maternal pregnancy-specific anxiety, total cortisol output during pregnancy, and childhood anxiety symptoms at age 8–11 in a cohort of mothers and children living in Mexico City. We found that higher levels of pregnancy-specific anxiety in the mother were associated with higher anxiety symptoms in the child. We additionally observed an association between total cortisol output during pregnancy and higher anxiety symptoms in the child. We did not observe associations with the other cortisol measures, including the CAR or diurnal cortisol slope.
The observed association with higher levels of pregnancy-specific anxiety is in line with previous studies on the topic (Davis and Sandman, 2012). Several previous studies have found increased childhood impacts particularly for pregnancy-specific anxiety, compared to other measures of psychological functioning during pregnancy (Buss et al., 2011; Davis and Sandman, 2012; Szekely et al., 2021). This may be because stressors related specifically to pregnancy may physiologically impact the mother over general trait anxiety or perceived stress (Blackmore et al., 2016). In analyses with other psychological functioning measures during pregnancy, we did observe associations between greater maternal depressive symptoms and increased child anxiety symptoms, only among girls. This is additionally in line with previous studies that have found childhood health impacts with maternal depressive symptoms during pregnancy (Capron et al., 2015; Galbally et al., 2020; Sandman et al., 2015). Several different studies have found sex-specific impacts of maternal stress and psychological functioning; however, further research is needed to completely delineate the sex-specific mechanisms (Sandman et al., 2013). An accumulating body of literature demonstrates a greater female vulnerability for early life stress exposure, with several studies pointing specifically to the amygdala as a target for prenatal stress exposure to impact later life mental health outcomes including anxiety (Cattarinussi et al., 2021; Sikes-Keilp and Rubinow, 2021). In fact, recent studies have shown that associations between prenatal maternal depressive symptoms and variations in amygdala functional connectivity among girls only (Soe et al., 2018; Wen et al., 2017).
We assessed associations with three different cortisol measures, as they each represent different aspects of the diurnal cortisol pattern. Overall, our findings were restricted to associations with maternal AUC during pregnancy. AUC represents the daily cortisol production; several studies have found poor correlations between AUC and CAR measures, and that the CAR may represent something separate from daily cortisol exposure (Golden et al., 2013). We observed associations with higher levels of total cortisol output during pregnancy and higher anxiety levels in the child. Previous studies have found associations between cortisol levels during pregnancy and several birth outcomes (Johnston et al., 2020) including birth weight (Peterson et al., 2020) and fetal growth (Hompes et al., 2012). Additionally, other studies have found associations with later child outcomes including child brain network properties and (Kim et al., 2017) neurodevelopment (Davis et al., 2017; Ram et al., 2019). To our knowledge, only two previous studies have examined associations between maternal cortisol during pregnancy and anxiety outcomes in children (Davis and Sandman, 2012; Isaksson et al., 2015). Our study improves on these two prior studies by having over ten years of follow-up, using an outcome ascertainment instrument specific to anxiety, and measuring cortisol multiple times, instead of a one-time sample as is often done in studies. In the current study, women provided saliva samples five times throughout the day, over the course of two days, thus more comprehensively assessing maternal biologic stress response during pregnancy. In addition, our saliva samples were predominantly from the second trimester of pregnancy. Studies have found associations may differ by trimester of saliva sample, with stronger associations seen during the second compared to the third trimester in one study (Buss et al., 2012).
Our findings showed an interactive effect with pregnancy-related anxiety and AUC to further increase anxiety symptoms. This is broadly consistent with previous studies, which have found interactions between cortisol levels and several other maternal psychosocial measures to increase risk of health outcomes in the child (Campbell et al., 2019; Enlow et al., 2017; Flom et al., 2018a). In addition to effect modification by cortisol, it is also possible that cortisol could mediate the relationship between pregnancy specific anxiety and child health outcomes; however, maternal cortisol and pregnancy-specific anxiety were not correlated in our study population. Further, several previous studies have shown that maternal cortisol does not mediate the impact of psychosocial stress on child anxiety outcomes (Davis and Sandman, 2010; Harville et al., 2009). This could be because cortisol responds to other environmental factors and is a common mediator for multiple risk factors besides stress (diet, chemical exposures).
Our study has several strengths. Most notably, its longitudinal design allowed us to assess impacts over many years. We assessed the association between prenatal pregnancy-specific anxiety and cortisol levels in relation to childhood anxiety levels in 8–11 year old children, characterizing impacts on late childhood health outcomes, compared to birth or earlier life stages. We used the validated RCMS to assess anxiety symptoms in our cohort. Additionally, we had a comprehensive assessment of maternal psychological functioning, with an a priori focus on pregnancy-specific anxiety, but also compared these associations with other psychological functioning measures during pregnancy including trait anxiety, perceived stress, negative life events, and maternal depressive symptoms. Future analyses will assess the combined impact of these measures on childhood mental health outcomes using advanced mixture approaches. We additionally had comprehensive confounding control, with additional adjustment for concurrent maternal depressive symptoms and tanner staging in sensitivity analyses. Finally, most previous studies on the topic have been conducted in high income English-speaking countries. Few studies have reported on findings in lower income countries – this is a key gap as prevalence of mental health concerns are often higher in these countries (Buffa et al., 2018). We assessed this research question in a low-income Spanish-speaking population. Additional research is needed in other cultures to fully understand the differences across communities.
Our study is not without limitations. Currently, there is no standard measure for pregnancy-related anxiety (Brunton et al., 2015). We assessed associations with pregnancy-specific anxiety by using an expanded set of items based on those from Wadhwa et al. Though this measure has been used in other studies, we acknowledge that validation studies are limited. Given the prevalence and distinctness of pregnancy-specific anxiety, additional well-validated measures are needed. We assessed associations between pregnancy-specific anxiety and cortisol levels at one point in time during pregnancy. We acknowledge that stress is dynamic over time, and it is known that maternal cortisol levels vary over the course of pregnancy. We additionally assessed cortisol measured from saliva, future studies will assess associations with hair cortisol concentrations, a longer term marker of HPA axis activity (D’Anna-Hernandez et al., 2011). Additionally, saliva measurement timing varied across participants. We adjusted for gestational week of saliva collection to address this concern. We had a comprehensive confounding assessment, however residual confounding by unmeasured factors is always of concern, particularly by factors such as child social support and resilience. Finally, we used a validated assessment of child anxiety; however, this measure did not include different facets of anxiety including social anxiety, nor did we include measures of other mental health symptoms including depression. Future studies will include these specific subscales of child and adolescent anxiety, as well as depressive symptoms. We also acknowledge that these screening tools do not diagnose anxiety, but instead provide an indication of severity of symptoms.
In conclusion, we observed associations between both pregnancy-specific anxiety and total cortisol output during pregnancy and higher anxiety symptoms in the child. PROGRESS is continuing to follow up children over time into their adolescent years. We will continue to assess measures of anxiety and other mental health outcomes in the adolescent and will build upon this study by assessing impacts with later life stressors and individual cortisol measures.
Supplementary Material
Highlights.
The etiology of child and adolescent anxiety remains poorly understood
We studied impacts of maternal cortisol and anxiety on offspring anxiety symptoms
Maternal pregnancy-specific anxiety was associated with anxiety in the child
Higher maternal prenatal cortisol was related to greater childhood anxiety symptoms
These findings highlight the importance of screening for pregnancy-specific anxiety
Funding
This work was supported by the National Institutes of Health [grant numbers T32HD049311, R01ES013744, R01ES021357, P30ES023515, R00ES027496, R24ES028522, and K99ES032480].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interest Statement:
The authors declare no competing interests.
References
- Adam EK, Kumari M, 2009. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology 34, 1423–1436. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Michaelis S, 2015. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci 17, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry CA, Quinn KA, Portillo N, Shalowitz MU, 2006. Reliability and validity of the Spanish Version of the Crisis in Family Systems-Revised. Psychol Rep 98, 123–132. [DOI] [PubMed] [Google Scholar]
- Bitsko RH, Holbrook JR, Ghandour RM, Blumberg SJ, Visser SN, Perou R, Walkup JT, 2018. Epidemiology and Impact of Health Care Provider-Diagnosed Anxiety and Depression Among US Children. J Dev Behav Pediatr 39, 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore ER, Gustafsson H, Gilchrist M, Wyman C, G.O.C. T, 2016. Pregnancy-related anxiety: Evidence of distinct clinical significance from a prospective longitudinal study. J Affect Disord 197, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauner EV, Koch T, Juul A, Doherty DA, Hart R, Hickey M, 2021. Prenatal exposure to maternal stressful life events and earlier age at menarche: the Raine Study. Hum Reprod 36, 1959–1969. [DOI] [PubMed] [Google Scholar]
- Brunton RJ, Dryer R, Saliba A, Kohlhoff J, 2015. Pregnancy anxiety: A systematic review of current scales. J Affect Disord 176, 24–34. [DOI] [PubMed] [Google Scholar]
- Buckley JP, Doherty BT, Keil AP, Engel SM, 2017. Statistical Approaches for Estimating Sex-Specific Effects in Endocrine Disruptors Research. Environ Health Perspect 125, 067013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffa G, Dahan S, Sinclair I, St-Pierre M, Roofigari N, Mutran D, Rondeau JJ, Dancause KN, 2018. Prenatal stress and child development: A scoping review of research in low- and middle-income countries. PLoS One 13, e0207235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Hobel CJ, Sandman CA, 2011. Maternal pregnancy-specific anxiety is associated with child executive function at 6–9 years age. Stress 14, 665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA, 2012. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A 109, E1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RK, Devick KL, Coull BA, Cowell W, Askowitz T, Goldson B, Wright RO, Wright RJ, 2019. Prenatal cortisol modifies the association between maternal trauma history and child cognitive development in a sex-specific manner in an urban pregnancy cohort. Stress 22, 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron LE, Glover V, Pearson RM, Evans J, O’Connor TG, Stein A, Murphy SE, Ramchandani PG, 2015. Associations of maternal and paternal antenatal mood with offspring anxiety disorder at age 18 years. J Affect Disord 187, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco AJE, 2002. The Amai System of Classifying Households by Socio-economic Level: the Experience of Mexico and Its Comparison with Brazil and Argentina.
- Cattarinussi G, Aarabi MH, Sanjari Moghaddam H, Homayoun M, Ashrafi M, Soltanian-Zadeh H, Sambataro F, 2021. Effect of parental depressive symptoms on offspring’s brain structure and function: A systematic review of neuroimaging studies. Neurosci Biobehav Rev 131, 451–465. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J Health Soc Behav 24, 385–396. [PubMed] [Google Scholar]
- Conger RD, Ge X, Elder GH Jr., Lorenz FO, Simons RL, 1994. Economic stress, coercive family process, and developmental problems of adolescents. Child Dev 65, 541–561. [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R, 1987. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 150, 782–786. [DOI] [PubMed] [Google Scholar]
- D’Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML, 2011. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiol Behav 104, 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Head K, Buss C, Sandman CA, 2017. Prenatal maternal cortisol concentrations predict neurodevelopment in middle childhood. Psychoneuroendocrinology 75, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA, 2010. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev 81, 131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA, 2012. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology 37, 1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enlow MB, Devick KL, Brunst KJ, Lipton LR, Coull BA, Wright RJ, 2017. Maternal Lifetime Trauma Exposure, Prenatal Cortisol, and Infant Negative Affectivity. Infancy 22, 492–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Kraemer HC, Armstrong JM, Boyce WT, Goldsmith HH, Klein MH, Woodward H, Kupfer DJ, 2006. Exploring risk factors for the emergence of children’s mental health problems. Arch Gen Psychiatry 63, 1246–1256. [DOI] [PubMed] [Google Scholar]
- Ferrando PJ, 1994. Factorial Structure of the Revised Children Manifest Anxiety Scale in a Spanish Sample - Relations with Eysenck Personality Dimensions. Pers Indiv Differ 16, 693–699. [Google Scholar]
- Flom JD, Chiu YM, Hsu HL, Devick KL, Brunst KJ, Campbell R, Enlow MB, Coull BA, Wright RJ, 2018a. Maternal Lifetime Trauma and Birthweight: Effect Modification by In Utero Cortisol and Child Sex. J Pediatr 203, 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flom JD, Chiu YM, Tamayo-Ortiz M, Schnaas L, Curtin PC, Wright RJ, Wright RO, Tellez-Rojo MM, Rosa MJ, 2018b. Subconstructs of the Edinburgh Postpartum Depression Scale in a postpartum sample in Mexico City. J Affect Disord 238, 142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann D, Knoll LJ, Blakemore SJ, 2015. Adolescence as a Sensitive Period of Brain Development. Trends Cogn Sci 19, 558–566. [DOI] [PubMed] [Google Scholar]
- Galbally M, Watson SJ, van Rossum EFC, Chen W, de Kloet ER, Lewis AJ, 2020. The perinatal origins of childhood anxiety disorders and the role of early-life maternal predictors. Psychol Med, 1–9. [DOI] [PubMed] [Google Scholar]
- Gjerde LC, Eilertsen EM, Reichborn-Kjennerud T, McAdams TA, Zachrisson HD, Zambrana IM, Roysamb E, Kendler KS, Ystrom E, 2017. Maternal perinatal and concurrent depressive symptoms and child behavior problems: a sibling comparison study. J Child Psychol Psychiatry 58, 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SH, Sanchez BN, Wu M, Champaneri S, Diez Roux AV, Seeman T, Wand GS, 2013. Relationship between the cortisol awakening response and other features of the diurnal cortisol rhythm: the Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology 38, 2720–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande LA, Swales DA, Sandman CA, Glynn LM, Davis EP, 2021. Maternal caregiving ameliorates the consequences of prenatal maternal psychological distress on child development. Dev Psychopathol, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harville EW, Savitz DA, Dole N, Herring AH, Thorp JM, 2009. Stress questionnaires and stress biomarkers during pregnancy. J Womens Health (Larchmt) 18, 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hompes T, Vrieze E, Fieuws S, Simons A, Jaspers L, Van Bussel J, Schops G, Gellens E, Van Bree R, Verhaeghe J, Spitz B, Demyttenaere K, Allegaert K, Van den Bergh B, Claes S, 2012. The influence of maternal cortisol and emotional state during pregnancy on fetal intrauterine growth. Pediatr Res 72, 305–315. [DOI] [PubMed] [Google Scholar]
- Howland MA, Sandman CA, Davis EP, Glynn LM, 2020. Prenatal maternal psychological distress and fetal developmental trajectories: associations with infant temperament. Dev Psychopathol 32, 1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, de Medina PG, Mulder EJ, Visser GH, Buitelaar JK, 2002. Psychological measures of prenatal stress as predictors of infant temperament. J Am Acad Child Adolesc Psychiatry 41, 1078–1085. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Robles de Medina PG, Mulder EJ, Visser GH, Buitelaar JK, 2003. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry 44, 810–818. [DOI] [PubMed] [Google Scholar]
- Isaksson J, Lindblad F, Valladares E, Hogberg U, 2015. High maternal cortisol levels during pregnancy are associated with more psychiatric symptoms in offspring at age of nine - A prospective study from Nicaragua. J Psychiatr Res 71, 97–102. [DOI] [PubMed] [Google Scholar]
- Johnston RC, Faulkner M, Carpenter PM, Nael A, Haydel D, Sandman CA, Wing DA, Davis EP, 2020. Associations Between Placental Corticotropin-Releasing Hormone, Maternal Cortisol, and Birth Outcomes, Based on Placental Histopathology. Reprod Sci 27, 1803–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB, 2007. Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry 20, 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Davis EP, Sandman CA, Sporns O, O’Donnell BF, Buss C, Hetrick WP, 2017. Prenatal Maternal Cortisol Has Sex-Specific Associations with Child Brain Network Properties. Cereb Cortex 27, 5230–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EH, 2012. Review of the psychometric evidence of the perceived stress scale. Asian Nurs Res (Korean Soc Nurs Sci) 6, 121–127. [DOI] [PubMed] [Google Scholar]
- McLean MA, Cobham VE, Simcock G, 2018. Prenatal Maternal Distress: A Risk Factor for Child Anxiety? Clin Child Fam Psychol Rev 21, 203–223. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Verhulst FC, Ormel J, 2011. Mental health problems during puberty: Tanner stage-related differences in specific symptoms. The TRAILS study. J Adolesc 34, 73–85. [DOI] [PubMed] [Google Scholar]
- Peterson AK, Toledo-Corral CM, Chavez TA, Naya CH, Johnson M, Eckel SP, Lerner D, Grubbs BH, Farzan SF, Dunton GF, Bastain TM, Breton CV, 2020. Prenatal Maternal Cortisol Levels and Infant Birth Weight in a Predominately Low-Income Hispanic Cohort. Int J Environ Res Public Health 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram S, Howland MA, Sandman CA, Davis EP, Glynn LM, 2019. Prenatal Risk for ASD: Fetal Cortisol Exposure Predicts Child Autism-Spectrum Disorder Symptoms. Clin Psychol Sci 7, 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Richmond BO, 2008. Revised Children’s Manifest Anxiety Scale–Second Edition (RCMAS-2): Manual. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA, 1999. Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health Psychol 18, 333–345. [DOI] [PubMed] [Google Scholar]
- Roustit C, Campoy E, Chaix B, Chauvin P, 2010. Exploring mediating factors in the association between parental psychological distress and psychosocial maladjustment in adolescence. Eur Child Adolesc Psychiatry 19, 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, 2018. Prenatal CRH: An integrating signal of fetal distress. Dev Psychopathol 30, 941–952. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Buss C, Head K, Davis EP, 2015. Fetal exposure to maternal depressive symptoms is associated with cortical thickness in late childhood. Biol Psychiatry 77, 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman, Glynn LM, Davis EP, 2013. Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J Psychosom Res 75, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KM, Von Korff M, Angermeyer MC, Benjet C, Bruffaerts R, de Girolamo G, Haro JM, Lepine JP, Ormel J, Posada-Villa J, Tachimori H, Kessler RC, 2011. Association of childhood adversities and early-onset mental disorders with adult-onset chronic physical conditions. Arch Gen Psychiatry 68, 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalowitz MU, Berry CA, Rasinski KA, Dannhausen-Brun CA, 1998. A new measure of contemporary life stress: development, validation, and reliability of the CRISYS. Health Serv Res 33, 1381–1402. [PMC free article] [PubMed] [Google Scholar]
- Sikes-Keilp C, Rubinow DR, 2021. In search of sex-related mediators of affective illness. Biol Sex Differ 12, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soe NN, Wen DJ, Poh JS, Chong YS, Broekman BF, Chen H, Shek LP, Tan KH, Gluckman PD, Fortier MV, Meaney MJ, Qiu A, 2018. Perinatal maternal depressive symptoms alter amygdala functional connectivity in girls. Hum Brain Mapp 39, 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, 1983. Manual for the State-Trait Anxiety Inventory STAI (form Y).. Consulting Psychologists Press, Inc. [Google Scholar]
- Szekely E, Neumann A, Sallis H, Jolicoeur-Martineau A, Verhulst FC, Meaney MJ, Pearson RM, Levitan RD, Kennedy JL, Lydon JE, Steiner M, Greenwood CMT, Tiemeier H, Evans J, Wazana A, 2021. Maternal Prenatal Mood, Pregnancy-Specific Worries, and Early Child Psychopathology: Findings From the DREAM BIG Consortium. J Am Acad Child Adolesc Psychiatry 60, 186–197. [DOI] [PubMed] [Google Scholar]
- Tanner JM, 1986. Normal growth and techniques of growth assessment. Clin Endocrinol Metab 15, 411–451. [DOI] [PubMed] [Google Scholar]
- Twenge JM, Cooper AB, Joiner TE, Duffy ME, Binau SG, 2019. Age, period, and cohort trends in mood disorder indicators and suicide-related outcomes in a nationally representative dataset, 2005–2017. J Abnorm Psychol 128, 185–199. [DOI] [PubMed] [Google Scholar]
- Vallejo MA, Vallejo-Slocker L, Fernandez-Abascal EG, Mananes G, 2018. Determining Factors for Stress Perception Assessed with the Perceived Stress Scale (PSS-4) in Spanish and Other European Samples. Front Psychol 9, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigo D, Thornicroft G, Atun R, 2016. Estimating the true global burden of mental illness. Lancet Psychiatry 3, 171–178. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ, 1993. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. Am J Obstet Gynecol 169, 858–865. [DOI] [PubMed] [Google Scholar]
- Wen DJ, Poh JS, Ni SN, Chong YS, Chen H, Kwek K, Shek LP, Gluckman PD, Fortier MV, Meaney MJ, Qiu A, 2017. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl Psychiatry 7, e1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen DJ, Sylvester CM, Luby JL, 2017. Depression and Anxiety in Preschoolers: A Review of the Past 7 Years. Child Adolesc Psychiatr Clin N Am 26, 503–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2001. Mental disorders affect one in four people.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


