Abstract
Methods
The chemical ingredients of ANW were retrieved from TCMSP, TCMID, and literature. We predicted the potential targets of active ingredients by PubChem, Swiss Target Prediction, and STITCH databases. The targets related to ischemic stroke were retrieved using GeneCards, DisGeNET, DrugBank, TTD, and GEO databases. Subsequently, Venn diagrams were used to identify common targets of active ingredients and ischemic stroke. Protein-protein interaction (PPI) network was structured with STRING platform and Cytoscape 3.8.2. Gene ontology (GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses of key targets were performed in the Metascape database. Finally, molecular docking was conducted by AutoDock Tools and PyMOL software.
Results
A total of 2391 targets were identified for 230 active ingredients of ANW, and 1386 of them overlapped with ischemic stroke targets. The key active ingredients were mainly quercetin, β-estradiol, berberine, wogonin, and β-sitosterol, and the key targets were also identified, including IL-6, AKT1, MAPK3, PIK3CA, and TNF. The biological process (BP) results indicated that ANW may have therapeutic effects through response oxidative stress, inflammatory response, cellular response to lipid, and response to nutrient levels. Furthermore, the ingredients of ANW were predicted to have therapeutic effects on ischemic stroke via the HIF-1 signaling pathway, FoxO signaling pathway, chemokine signaling pathway, fluid shear stress and atherosclerosis, and neurotrophin signaling pathway. The molecular docking results all showed that the core ingredients were strong binding activity with the core targets.
Conclusion
In conclusion, the bioinformatics and pharmacological results reveal that counteracting oxidative stress, suppressing inflammation, inhibiting the development of AS, and even protecting neurological function are critical pathways for ANW in the treatment of ischemic stroke. These results may help to elucidate the mechanism of ANW on ischemic stroke for experimental studies and clinical applications.
1. Introduction
Ischemic stroke is a common cerebrovascular event due to an abrupt cerebral artery occlusion, resulting in insufficient perfusion, which then causes edema, inflammation, and necrosis of the affected tissue and severely damages to neurological function. The World Health Organization reports that ischemic stroke is the main cause of death and long-term disability in the world, which causes a tremendous psychological and financial burden on patients [1]. However, the pathological process of ischemic stroke involves multiple aspects, including energy metabolism disorder, oxidative stress, inflammation, and neuronal damage, and there is no sovereign remedy [2, 3]. Therefore, it is significantly important to explore drugs or active ingredients with multiple targets for the treatment of cerebral ischemia.
Notably, many of the Chinese herbs have been proven to produce therapeutic effects on ischemic stroke in clinical research [4]. As a famous Chinese herbal formula, Angong Niuhuang Wan (ANW) is widely used in clinical practice for the treatment of ischemic stroke, which contains 11 herbs, including Moschus, Realgar, Curcumae Radix, Borneolum, Scutellariae Radix, Coptidis Rhizoma, Gardeniae Fructus, Bovis Calculus, Bubali Cornu, Margarita, and Cinnabaris. Studies indicated that ANW had effect on reducing infarct size, protecting the integrity of the blood-brain barrier (BBB), improving antioxidant capacity, and inhibiting inflammation injury to produce neuroprotection; furthermore, it may improve the development of early atherosclerosis (AS) by suppressing inflammation [5–7]. However, the pharmacological effects of ANW on ischemic stroke have still not been elucidated.
In this study, we aim to elucidate the possible mechanism of ANW on ischemic stroke and reveal the interaction between ANW, target, and ischemic stroke from a holistic perspective through a network pharmacology approach. The workflow diagram of the study is presented in Figure 1.
Figure 1.
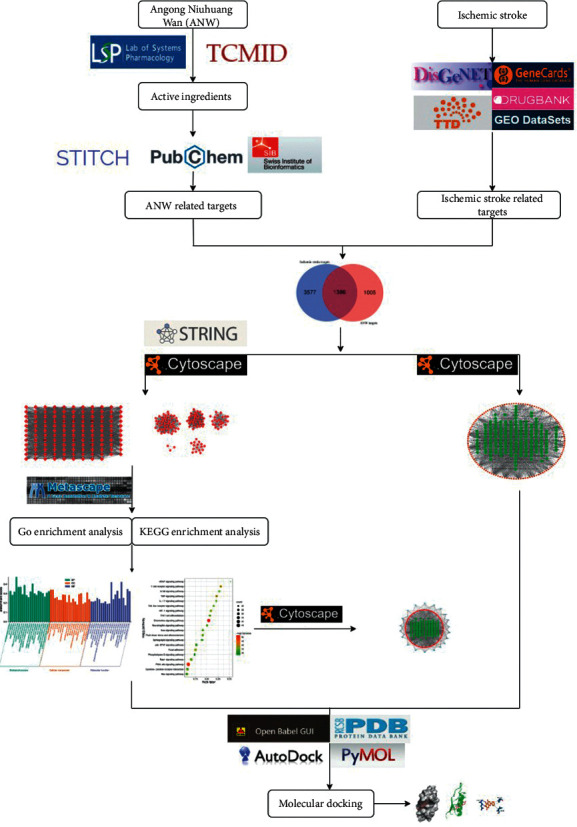
The workflow diagram of the study.
2. Material and Methods
2.1. Screening of Active Ingredients in ANW
The effective ingredients of ANW were searched through TCMSP (https://tcmspw.com/tcmsp.php) [8], TCMID (http://www.megabionet.org/tcmia/), and literature. The active compounds were screened for oral bioavailability (OB), drug-likeness (DL), and blood-brain barrier permeability (BBB) prediction. The selection of OB, DL, and BBB referred to the recommendations of the TCMSP database. Therefore, we finally screened the compounds with OB ≥ 0.2, DL ≥ 0.1, and BBB ≥ -0.3, which were considered as parameters for selecting potentially pharmacological ingredients [9–11], in addition, the ingredients with high content or pharmacological effects searched from literature and TCMID that did not contain the above parameters, which were also included in the further analysis. Besides, the threshold values were considered based on the following points: firstly, extracting more useful information from fewer compounds; secondly, maintaining concordance with the proven pharmacological data.
2.2. Prediction of Potential Targets of ANW
We retrieved SMILES number or 3D structure of each ingredient from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and TCMID and inputted them into the PubChem, Swiss Target Prediction (http://www.swisstargetprediction.ch/) [12], and STITCH (http://stitch.embl.de/) database to obtain potential targets of bioactive ingredients. The target was further standardized in UniProtKB database (http://www.uniprot.org) [13].
2.3. Candidate Targets Collection of Ischemic Stroke
The disease targets correlated with “cerebral ischemic stroke” and “cerebral infarction” were identified through GeneCards (https://www.genecards.org/), DisGeNET (http://disgenet.org/), DrugBank (https://go.drugbank.com/), GEO (https://www.ncbi.nlm.nih.gov/geo/), and TTD (http://db.idrblab.net/ttd/) [14]. After deleting the duplicate targets of ischemic stroke, Venny 2.1 (http://bioinfogp.cnb.csic.Es/tools/venny/index.html) was used to identify common potential targets between ischemic stroke and the active ingredients of ANW.
2.4. Protein-Protein Interaction Network Construction and Analysis
Protein-protein interaction (PPI) network was constructed through the STRING database (https://string-db.org/) [15] with a confidence score >0.7. And topology analysis was performed by Cytoscape software. The key targets were sorted and screened according to the value of degree, betweenness centrality, and closeness centrality of the topological analysis results [16]. In addition, we screened important functional modules in PPI networks with the Cytoscape plugin MCODE.
2.5. Functional Enrichment and Pathways Analysis
The Gene ontology (GO) including biological process (BP), molecular function (MF), and cellular component (CC), and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were conducted using the Metascape database (https://metascape.org) [17]. The statistical significance threshold was set at the cutoff values of P < 0.01. In addition, the bioinformatics platform (http://www.bioinformatics.com.cn/) was used to visualize GO and KEGG enrichment analysis with the bubble charts.
2.6. Construction of Active Ingredients-Targets-Pathway Network
An ingredients-targets-network was constructed by Cytoscape software. The key active ingredients of ANW were sorted and screened according to the value of degree, betweenness centrality, and closeness centrality based on topological analysis.
2.7. Molecular Docking
The 3D structures of candidate ingredients were obtained from PubChem, which were transformed by Open Babel Toolkit (version 2.4.1) into a mol2 file format. The 3D structures of the core target were downloaded from the PDB database (http://www.rcsb.org/). The AutoDockTool 1.5.6 was used to add hydrogen and optimize protein structure for molecular docking after removing water and original ligands.
3. Results
3.1. Active Ingredients of ANW
A total of 230 active ingredients were obtained through the database after eliminating duplicates. These active ingredients were mainly derived from Borneolum (16 ingredients), Bovis Calculus (19 ingredients), Coptidis Rhizoma (17 ingredients), Moschus (32 ingredients), Bubali Cornu (22 ingredients), Realgar (3 ingredients), Curcumae Radix (44 ingredients), Margarita (16 ingredients), Gardeniae Fructus (22 ingredients), Cinnabaris (2 ingredients), and Scutellariae Radix (37 ingredients). Detailed active ingredients of ANW are shown in Table 1.
Table 1.
Information of the candidate active ingredients of ANW.
| Herb | Active ingredients |
|---|---|
| Bovis Calculus | Oleanolic acid, cherianoine, CLR, bilirubin, methyl(4R)-4-[(3R,5S,7S,8R,9S,10S,12S,13R,14S,17R)-3,7,12-trihydroxy-10,13-dimethyl- 2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoate, methyl desoxycholate, chenodeoxycholic acid, deoxycholic acid, ZINC01280365, biliverdin, cholic acid, choline, deoxycorticosterone, ergosterol, ergotamine, glycocholic acid, lithocholic acid, ursodeoxycholic acid, hyodeoxycholic acid |
| Coptidis Rhizoma | Berberine, columbamine, fagarine, berberrubine, DPEC(5,8-dihydroxy-2-(2-phenylethyl)chromone), epiberberine, groenlandicine, (R)-canadine, berlambine, magnograndiolide, palmatine, coptisine, tetrandrine, Worenine, Pycnamine, jatrorrhizine, quercetin |
| Scutellariae Radix | Acacetin, wogonin, (2R)-7-hydroxy-5-methoxy-2-phenylchroman-4-one, β-patchoulene, baicalein, 5,8,2′-Trihydroxy-7-methoxyflavone, dihydrobaicalin_qt |
| Salvigenin, 5,2′,6′-Trihydroxy-7,8-dimethoxyflavone, dihydrooroxylin A, skullcapflavone II, oroxylin a, panicolin, DIHYDROOROXYLIN(2beta-Phenyl-2,3- dihydro-5,7-dihydroxy-6-methoxy-4h-1-benzopyran-4-one), beta-sitosterol, sitosterol, norwogonin, 5,2′-dihydroxy-6,7,8-trimethoxyflavone, (-)-alpha-cedrene, linoleic acid, stigmasterol, dibutyl phthalate, coptisine, bis[(2S)-2-ethylhexyl] benzene-1,2-dicarboxylate, supraene, methyl palmitelaidate, methyl linolelaidate, Diop, epiberberine, patchoulene, 13-tetradecenyl acetate, moslosooflavone, 11,13-eicosadienoic acid, methyl ester, linolenic acid methyl ester, rivularin, neobaicalein, baicalin | |
| Bubali Cornu | Calcium carbonate, eukeratin, ssulfocysteine, serine, isoleucine, glutamic acid, phenylalanine, histidine, cholesterol, cysteine, proline, lysine, tyrosine, arginine |
| Ethanolamine, aspartic acid, glycine, alanine, methionine, threonine, guanidine derivatives, guanidine | |
| Moschus | β-Estradiol, 3,5-dihydroxybenzoic acid, 3alpha,17-dihydroxy-5beta-androstane, 3alpha-hydroxy-5alpha-androstan-17-one, 3beta,17alpha-dihydroxy-5alpha-androstane, 3beta-hydroxy-5alpha-androstan-17-one, 3beta-hydroxy-androst-5-ene-17-one, 3α-hydroxy-5β-androstan-17-one, testosterone, allantoin, serine |
| 3β-Hydroxy-5α-androstan-17-one, 3β-hydroxy-androst-5-ene-17-one, 5 alpha-androstan-3,17-dione, 5beta-androstan-3 alpha,17beta-diol, 5α-androstan- 3,17-dione, 5α-androstane-3β,17α-diol, 5β-androstan-3,17-dione, 5β-androstan-3α,17α-diol, 5β-androstan-3α,17β-diol, alpha-estradiol, androst-4,6-diene-3,17-dione, androst-4-ene-3,17-dione, androsterone, cholesterol, decamine, estragole, morin, n-nornuciferine, normuscone, s-methyl cysteine, aspartic acid | |
| Cinnabaris | Mercuric sulfide, HgCl2 |
| Gardeniae Fructus | (4aS,6aR,6aS,6bR,8aR,10R,12aR,14bS)-10-Hydroxy-2,2,6a,6 b,9,9,12a-heptamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid |
| Ammidin, sudan III, linoleic acid, oleanolic acid, beta-sitosterol, stigmasterol, oleic acid, mandenol, supraene, methyl linoleate, methyl vaccinate, isoimperatorin | |
| Exceparl M-OL, chrysin, ethyl oleate (NF), 5-hydroxy-7-methoxy-2-(3,4,5-trimethoxyphenyl)chromone, PANA(N-Phenyl-1-naphthylamine), gardenoside_qt, quercetin, shanzhiside_qt, kaempferol | |
| Margarita | Aluminium, calcium carbonate, cuprum, iron, manganese, silicon, zinc, magnesium, strontium, alanine, aspartic acid, leucine, serine, taurine, selenium, valine |
| Borneolum | Oleanolic acid, caryophyllene oxide, dipterocarpol, asiatic acid, bornyl acetate, beta-caryophyllene, borneol, isocembrol, D-borneol, erythrodiol, beta-humulene |
| Oleanolic acid-28-O-beta-D-glucopyranoside, dryocrassin, camphor, elemicin, alphitolic acid | |
| Realgar | Realgar, as2s3, As4S4 |
| Curcumae Radix | Furanodienon, linoleic acid, beta-sitosterol, sitosterol, dibutyl phthalate, oleic acid, calarene, copaene, ()-aromadendrene, aromadendrene oxide 2, alnusone |
| (1Ar,4aS,7R,7aR,7bR)-1,1,7-Trimethyl-4-methylidene decahydro-1h-cyclopropa(e)azulen-7-olTrans-1,7-diphenyl-1-hepten-5-ol, Junipene, ()-ledene, (4aR,5R,8 R, 8aR)-5,8-dihydroxy-3,5,8a-trimethyl-6,7,8,9-tetrahydro-4ah-benzo[f]benzofuran-4-one, curcumol, epicurzerenone, germacrone-4,5-epoxide, glechomanolide, furanodienone, isospathulenol, patchoulene, 1-phenylnaphthalene, pyrocurzerenone, trans,trans-1,7-diphenyl-1,3-heptadien-5-ol, zederone, bisdemethoxycurcumin, 1,7-diphenyl-6(E)-hepten-3one, calarenepoxide, caryophyllene oxide, (1S,3aR,4R,8aS)-7-isopropyl-1,4-dimethyl- 2,3,3a,5,6,8a- hexahydroazulene-1,4-diol, Isocurcumenol, (1S,6R,7R)-4-isopropylidene-1-methyl-7-(3-oxobutyl)norcaran-3-one, (5R,6 R)-5-isopropenyl-3,6-dimethyl-6-vinyl- 5,7-dihydrobenzofuran-4-one, (-)-isoledene, gweicurculactone, curcumenol, (3S,3aS,8aR)-3-hydroxy-5-isopropylidene-3-methyl-8-methylene-2,3a,4,8a- tetrahydro-1h-azulen-6-one, zedoarondiol, procurcumadiol, (3S,3aS,8aR)-3-hydroxy-5-isopropylidene-3,8-dimethyl-2,3a,4,8a-tetrahydro- 1h-azulen-6-one, 3-octadecenoic acid, demethoxycurcumin |
3.2. Protein-Protein Interaction Network Analysis
A total of 4963 potential targets were obtained of ischemic stroke, and 1386 common targets were obtained after intersecting with 2391 potential targets of the active ingredients (Figure 2). The topological results of 1386 targets were obtained 130 significant targets according to the degree, betweenness centrality, and closeness centrality. The PPI network included 130 nodes and 2946 edges, among which 25 genes were more relevant to the ischemic stroke according to the MalaCards database (https://www.malacards.org/) [18], so they were identified as key targets (Figure 3, Table 2). MCODE has screened 5 functional modules according to the 130 targets (Figure 4). The biological functions of the subnetwork are shown in Table 3. The BP analysis revealed that the subnetworks were mainly associated with inflammatory response, response to lipid, neuroapoptosis, and development.
Figure 2.
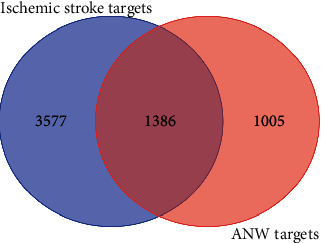
Venn diagram of ANW and ischemic stroke common targets.
Figure 3.
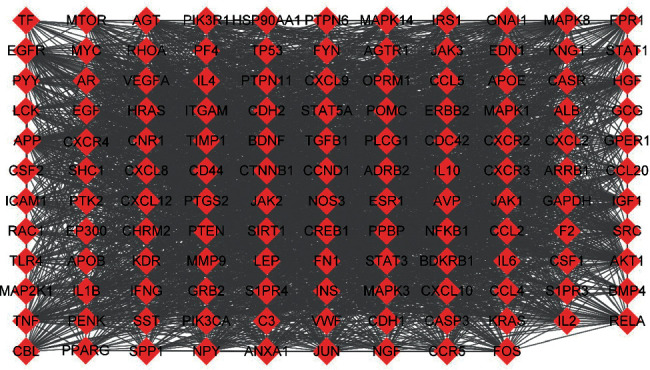
Protein-protein interaction network of core targets.
Table 2.
The information of the core targets.
| Gene | Degree | Betweenness centrality |
|---|---|---|
| IL-6 | 91 | 0.030325537 |
| AKT1 | 81 | 0.018339264 |
| CXCL12 | 73 | 0.018125697 |
| MAPK3 | 68 | 0.009734912 |
| CXCR4 | 66 | 0.015168905 |
| PIK3CA | 65 | 0.012205642 |
| TNF | 61 | 0.00919471 |
| AGT | 59 | 0.008882338 |
| MMP9 | 53 | 0.006325729 |
| IL1B | 51 | 0.0066625 |
| ALB | 51 | 0.007447992 |
| PPBP | 45 | 0.003930831 |
| PF4 | 42 | 0.002706756 |
| BDNF | 40 | 0.003798139 |
| NOS3 | 39 | 0.003217693 |
| TLR4 | 38 | 0.002216187 |
| AGTR1 | 37 | 0.004279922 |
| CREB1 | 33 | 0.0019083 |
| F2 | 32 | 0.002232586 |
| CASP3 | 31 | 0.000874129 |
| APOB | 29 | 0.002920764 |
| SIRT1 | 28 | 0.000753076 |
| APOE | 25 | 0.001338523 |
| VWF | 25 | 0.001087882 |
| AVP | 22 | 0.000773649 |
Figure 4.
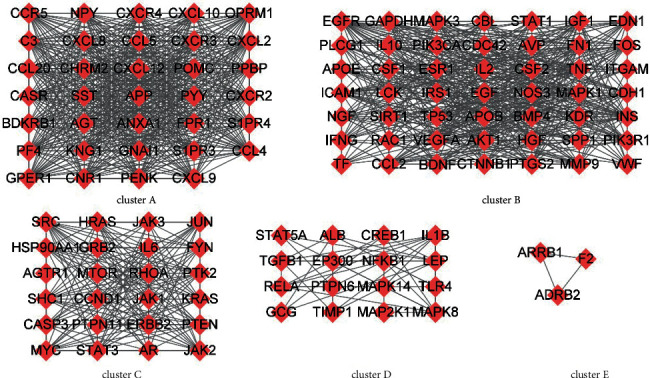
Subnetwork of targets PPI network.
Table 3.
The biological functions of subnetworks.
| MCODE | GO | Description |
|---|---|---|
| A | GO:0006954 | Inflammatory response |
| A | GO:0070098 | Chemokine-mediated signaling pathway |
| A | GO:0006874 | Cellular calcium ion homeostasis |
| B | GO:0070997 | Neuron death |
| B | GO:0050900 | Leukocyte migration |
| B | GO:0001568 | Blood vessel development |
| C | GO:0007169 | Transmembrane receptor protein tyrosine kinase signaling pathway |
| C | GO:0022407 | Regulation of cell-cell adhesion |
| C | GO:0061564 | Axon development |
| D | GO:1901652 | Response to peptide |
| D | GO:0071396 | Cellular response to lipid |
| D | GO:0002521 | Leukocyte differentiation |
| E | GO:0008277 | Regulation of G protein-coupled receptor signaling pathway |
| E | GO:0033674 | Positive regulation of kinase activity |
| E | GO:0051347 | Positive regulation of transferase activity |
3.3. Construction of Active Ingredients-Targets Network
As shown in Figure 5, we constructed a network of active ingredients-targets using Cytoscape software (version 3.8.0). The active ingredients-targets network contained 310 nodes (including 180 ingredients and 130 genes) and 2110 edges. The top 20 active ingredients were screened by topology analysis (Table 4).
Figure 5.
The active ingredients-targets network. Green represents active ingredients, and red represents the potential targets.
Table 4.
List of core ingredients in the top 20.
| Active components | Herbs | Degree | Betweenness centrality |
|---|---|---|---|
| Quercetin | Coptidis Rhizoma, Gardeniae Fructus | 54 | 0.056874662 |
| β-estradiol | Moschus | 51 | 0.078777286 |
| Tyrosine | Bubali Cornu | 44 | 0.045643145 |
| Berberine | Coptidis Rhizoma | 40 | 0.018811059 |
| Wogonin | Scutellariae Radix | 39 | 0.021360805 |
| Beta-sitosterol | Scutellariae Radix, Gardeniae Fructus, Curcumae Radix | 37 | 0.019391773 |
| Baicalein | Scutellariae Radix | 36 | 0.016063119 |
| Tetrandrine | Coptidis Rhizoma | 35 | 0.013666927 |
| chrysin | Gardeniae Fructus | 34 | 0.013739355 |
| Baicalin | Scutellariae Radix | 32 | 0.008324522 |
| Acacetin | Scutellariae Radix | 31 | 0.013057 |
| Oroxylin a | Scutellariae Radix | 31 | 0.022607 |
| Kaempferol | Gardeniae Fructus | 31 | 0.009087 |
| Demethoxycurcumin | Curcumae Radix | 30 | 0.014592 |
| Stigmasterol | Scutellariae Radix, Gardeniae Fructus | 29 | 0.017506 |
| Oleanolic acid | Bovis Calculus, Gardeniae Fructus, Borneolum | 28 | 0.008257 |
| Serine | Bubali Cornu, Moschus, Margarita | 28 | 0.006694 |
| Linoleic acid | Scutellariae Radix, Gardeniae Fructus, Curcumae Radix | 27 | 0.010942 |
| Oleic acid | Gardeniae Fructus, Curcumae Radix | 26 | 0.012292 |
| Ammidin | Gardeniae Fructus | 26 | 0.011124 |
3.4. GO Enrichment Analysis
GO enrichment results include 296 BP terms, 99 MF terms, and 92 CC terms. The key items of BP mainly included response to oxidative stress, inflammatory response, cellular response to lipid, and response to nutrient levels. The main results of MF included oxidoreductase activity, cytokine receptor binding, lipid binding, and neurotransmitter receptor activity, and CC mainly included neuronal cell body, dendritic tree, axon, and postsynapse. We individually selected top 20 remarkably enriched terms in BP, MF, and CC classification as presented in Figure 6.
Figure 6.
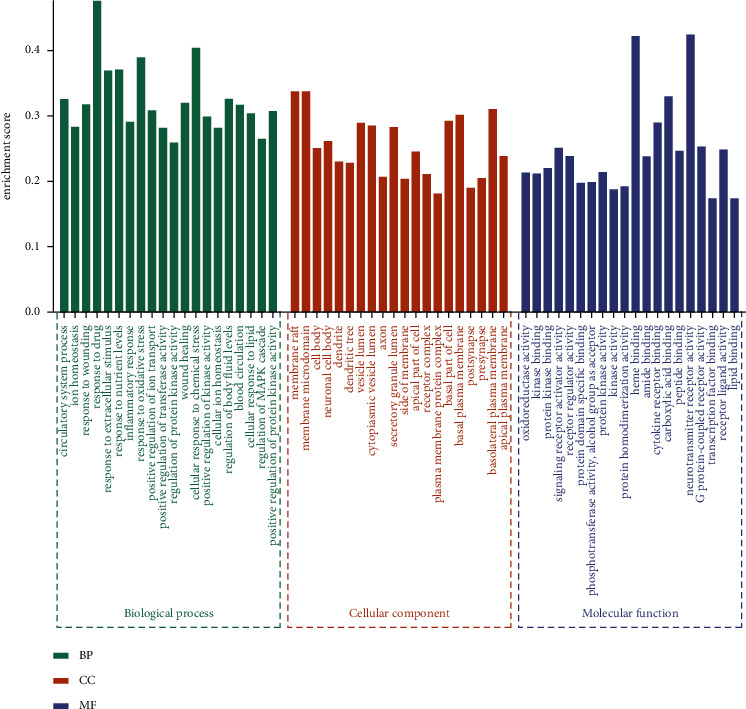
The GO enrichment analysis of 130 targets.
3.5. KEGG Pathway Enrichment Analysis and Ingredients-Targets Pathway Network Construction
KEGG pathway enrichment analysis may elaborate the mechanism of ANW on ischemic stroke. 139 signal pathways were obtained based on the 130 core targets. After removing pathways associated with cancer and unrelated to disease, the main results of KEGG pathways included the HIF-1 signaling pathway, FoxO signaling pathway, chemokine signaling pathway, fluid shear stress and atherosclerosis, and neurotrophin signaling pathway. 20 significantly enriched pathways were selected as shown in Figure 7. An ingredients-targets pathway network was built involving pathways, targets, and corresponding ingredients to further elucidate the molecular biological process of ANW for cerebral ischemic stroke (Figure 8). A total of 292 nodes (163 ingredients, 109 targets, and 20 pathways) and 2285 edges were obtained.
Figure 7.
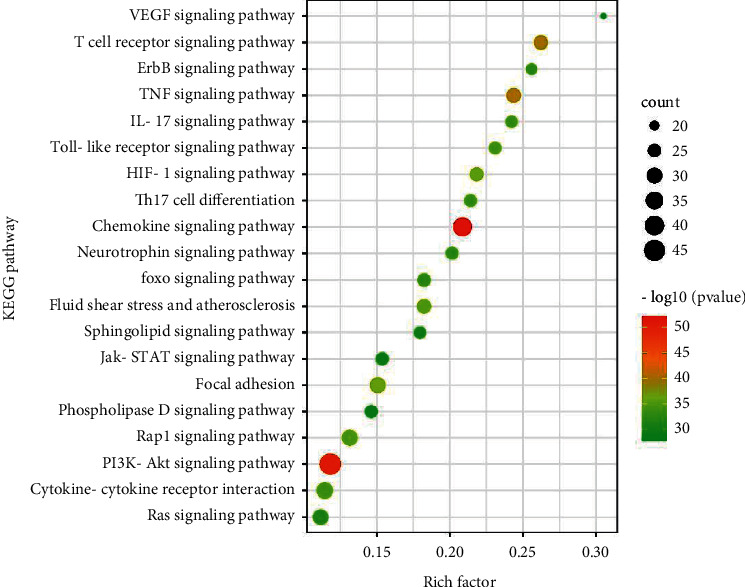
The KEGG enrichment analysis of 130 targets.
Figure 8.
An ingredients-targets pathway network (green represents active ingredients, red represents potential targets, and blue represents the pathway).
3.6. Docking Results Analysis
We selected the core targets, including IL-6, AKT1, MAPK3, PIK3CA, and TNF for molecular docking with the quercetin, β-estradiol, berberine, wogonin, and β-sitosterol. The results suggested that the 5 key ingredients all had a strong affinity with IL-6, AKT1, MAPK3, PIK3CA, and TNF, and the results of the docking were visualized by PyMOL software (Table 5, Figure 9).
Table 5.
Docking results of core active ingredients with core targets (kcal/mol).
| Ingredients | IL-6 | AKT1 | MAPK3 | PIK3CA | TNF |
|---|---|---|---|---|---|
| Quercetin | −5.68 | −7.41 | −6.39 | −5.96 | −6.39 |
| β-Estradiol | −6.18 | −9.05 | −8.44 | −8.28 | −7.28 |
| Berberine | −7.02 | −8.69 | −7.83 | −8.8 | −6.27 |
| Wogonin | −5.37 | −7.82 | −6.79 | −7.4 | −6.62 |
| β-Sitosterol | −6.54 | −10.34 | −8.54 | −8.38 | −7.65 |
Figure 9.
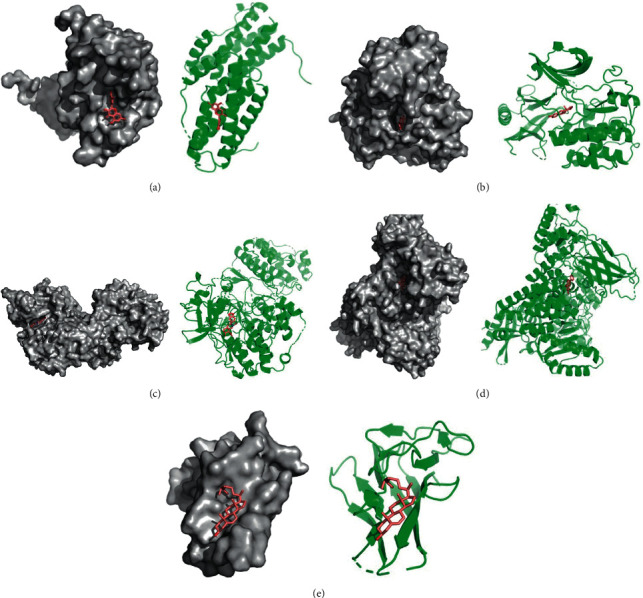
(a) Action mode of quercetin with target IL-6. (b) Action mode of β-estradiol with target AKT1. (c) Action mode of berberine with target MAPK3. (d) Action mode of wogonin with target PIK3CA. (e) Action mode of β-sitosterol with target TNF.
4. Discussion
Stroke is classified as ischemic or hemorrhagic. Cerebral hemorrhage and cerebral ischemia have the possibility to cause serious inflammatory response, cerebral edema, and neurological deficits [19, 20]. The studies found that ANW reduced brain edema and intracranial pressure in cerebral ischemia and cerebral hemorrhage by regulating the expression of MMP-9 and AQP4 which were closely related to the formation of brain edema and the disruption of the BBB [21, 22]; in addition, it was able to exert neuroprotective function by reducing the inflammatory response and inhibiting oxidative stress and neurotoxicity in brain tissue of cerebral ischemia and cerebral hemorrhage [23, 24]. At present, the incidence of cerebral ischemia is far higher than cerebral hemorrhage; therefore, the paper is focused on the mechanism of Angong Niuhuang Wan in the treatment of cerebral ischemia. Network analysis increases the understanding of multiple mechanisms of drug action. Systems pharmacology may provide new avenues for drug discovery in complex diseases. Thus, network pharmacology may be helpful in excavating the potential mechanism of ANW for ischemic stroke.
The results of pharmaceutical ingredient analyses and molecular docking showed that the main ingredients quercetin, β-estradiol, berberine, and β-sitosterol showed strong binding activity to the IL-6, AKT1, MAPK3, PIK3CA of the core targets. IL-6 is a pleiotropic cytokine that plays a crucial role in host defense [25]. However, trans-signaling of IL-6 induces vascular endothelial cells to express and release the pro-inflammatory chemokine MCP-1, which is mediated through the JAK/STAT3 and PI3K/AKT pathways [26]. The studies found that administration of β-estradiol from Moschus reversed neuronal damage by regulating the JAK-STAT3 pathway and protected neurons from acidosis-mediated neurotoxicity and ischemic cerebral injury, thus promoting remodel and repair after brain injury [27, 28]. Liao et al. [29] demonstrated that β-sitosterol inhibited the secretion of inflammatory factors such as TNF-α, IL-1β, IL-6 to suppress the inflammatory response. TNF is a versatile pro-inflammatory cytokine involved in all stages of ischemic stroke. The study confirmed that quercetin from Coptidis Rhizoma and Gardeniae Fructus attenuated TNF-induced inflammation by suppressing the NF-κB pathway [30]. MAPK is involved in inflammatory and apoptotic processes in cerebral ischemia-reperfusion injury. Studies had shown that quercetin inhibited inflammation and regulated JNK and ERK signaling pathways to produce antiapoptosis, thereby improving ischemic brain injury [31, 32].
AKT1, as a threonine protein kinases, is an important regulator of the AKT-mTOR signaling pathway that controls the tempo of newborn neurons during adult neurogenesis. PIK3CA is involved in the cell signaling of various growth factors. Yan et al. [33] demonstrated that activation of the PI3K/Akt/mTOR pathway inhibited oxidative stress-related neuronal autophagy and exerted neuroprotective functions. The research showed that berberine can reduce the apoptosis of striatum and mitochondrial through regulating PI3K/Akt signaling pathway and reducing intracellular ROS levels to exert neuroprotective effects [34, 35].
According to the results of KEGG enrichment analysis, ANW is considered to affect important pathways that are closely related to the pathogenesis of ischemic stroke, including HIF-1 signaling pathway, FoxO signaling pathway, chemokine signaling pathway, fluid shear stress and atherosclerosis, and neurotrophin signaling pathway. The results of GO enrichment were also closely related to response to oxidative stress, inflammatory response, cellular response to lipid, and response to nutrient levels. Furthermore, the BP analysis revealed that the subnetworks were mainly associated with inflammatory response, response to lipid, neuroapoptosis, and development.
HIF-1α is a primary modulator of cellular and systemic homeostatic reactions to hypoxia. Evidence showed that HIF-1 facilitated the transcription of various prosurvival proteins engaged in energy metabolism, angiogenesis, and neurogenesis, exerting a neuroprotective effect against ischemic stroke in ischemic conditions [36]. Research showed that estradiol facilitated neurogenesis in rats after stroke, possibly via increasing HIF-1α and VEGF protein expression [37]. The FoxO family of transcription factors is a critical regulator of cellular stress responses and facilitated the antioxidant defense of cells. Akt and p38MAPK are known stress-responsive kinases targeted to FoxO and are involved in the regulation of FoxO activity [38]. Zhang et al. [39] found that regulation of PI3K/Akt/FoxO-3a signaling pathway facilitated the proliferation of neural stem/progenitor cells and reduced ischemia-reperfusion injury. The inflammatory immune reaction needs leukocytes to be recruited to the site of inflammation. Chemokines are critical in protecting the host response by providing directional cues for cellular transport. Research confirmed that ANW downregulated the expression of chemokine receptors CCR2, CXCR3, and cell adhesion molecules in the arterial vasculature and alleviated the development of atherosclerosis by suppressing inflammation [7].
Atherosclerosis is a major cause of stroke onset or recurrence, and blood flow-induced shear stress has become an essential characteristic of atherosclerosis. The fluid resistance exerted on the vessel wall is mechanically translated into biochemical signals that lead to alter vascular behavior. Therefore, the maintenance of physiological laminar shear stress is essential for normal vascular function [40]. Quercetin alleviates vascular calcification by suppressing oxidative stress and mitochondrial division [41]. Moreover, Fan et al. [42] stated that ANW suppressed the development of atherosclerosis by regulating immune homeostasis and suppressing chronic inflammation. Neurotrophins have been proved to control survival, development, and function of neurons in the central nervous system. Studies asserted that quercetin and berberine alleviated neuronal apoptosis of ischemic stroke in the rat by activating the BDNF-TrkB-PI3K/Akt signaling pathway to increase the expression of BDNF [34, 43]. This suggests a potential application of neurotrophins in the therapy of ischemic stroke.
This research based on a pharmacological network explored the potential mechanisms of ANW for the treatment of ischemic stroke. The findings highlighted the improvement of the inflammatory response, immune defense, and neuroprotection of ANW against ischemic stroke. Our results were consistent with published studies that upregulation of HIF-1 signaling pathway, FoxO signaling pathway, and neurotrophin signaling pathway and downregulation of chemokine signaling pathway had positive effects on cerebral ischemia [42, 44–47]. In addition, we also provided some potential targets for treating ischemic stroke, which would contribute to the exploitation of new therapeutic strategies.
5. Conclusion
In conclusion, the bioinformatics and pharmacological results reveal that counteracting oxidative stress, suppressing inflammation, inhibiting the development of AS, and even protecting neurological function are critical pathways for ANW in the treatment of ischemic stroke. These results may help to elucidate the mechanism of ANW on ischemic stroke for experimental studies and clinical applications.
Acknowledgments
This study was supported by the Foundation of He'nan Educational Committee (ID: 18A360012), the key scientific and technological project of Henan Province (ID: 192102310424), and the National Natural Science Foundation of China (ID: 81704135).
Data Availability
All data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Yasu Zhang and Xiaomin Liu conceived and designed the research; Junzi Long, Xue Cheng, and Xinyu Wang analyzed the data. Xiaodong Feng reviewed the paper for intellectual content. All authors read and approved the final version of the manuscript. Yasu Zhang and Xiaomin Liu contributed equally to this work.
References
- 1.World Health Organization (Who), corp-author. Top 10 Global Causes of Death . Geneva, Switzerland: WHO; 2016. [Google Scholar]
- 2.Orellana-Urzúa S., Rojas I., Líbano L., Rodrigo R. Pathophysiology of ischemic stroke: role of oxidative stress. Current Pharmaceutical Design . 2020;26(34):4246–4260. doi: 10.2174/1381612826666200708133912. [DOI] [PubMed] [Google Scholar]
- 3.Maida C. D., Norrito R. L., Daidone M., Tuttolomondo A., Pinto A. Neuroinflammatory mechanisms in ischemic stroke: focus on cardioembolic stroke, background, and therapeutic approaches. International Journal of Molecular Sciences . 2020;21(18):p. 6454. doi: 10.3390/ijms21186454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L., Zhang B., Saatman K. Therapeutic potential of natural compounds from Chinese medicine in acute and subacute phases of ischemic stroke. Neural Regeneration Research . 2020;15(3):416–424. doi: 10.4103/1673-5374.265545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsoi B., Chen X., Gao C., et al. Neuroprotective effects and hepatorenal toxicity of Angong Niuhuang wan against ischemia-reperfusion brain injury in rats. Frontiers in Pharmacology . 2019;10:p. 593. doi: 10.3389/fphar.2019.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li A., Zhang J.-Y., Xiao X., et al. Hepatorenal protective effects of medicinal herbs in An-Gong-Niu-Huang Wan (AGNH) against cinnabar- and realgar-induced oxidative stress and inflammatory damage in mice. Food and Chemical Toxicology . 2018;119:445–456. doi: 10.1016/j.fct.2017.11.054. [DOI] [PubMed] [Google Scholar]
- 7.Chai Y., Yin Z., Fan Q., et al. Protective effects of Angong Niuhuang pill on early atherosclerosis in ApoE-/- mice by reducing the inflammatory response. Evidence-based Complementary and Alternative Medicine . 2019;2019:1–13. doi: 10.1155/2019/9747212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ru J., Li P., Wang J., et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics . 2014;6(1):p. 13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X., Zhang W., Huang C., et al. A novel chemometric method for the prediction of human oral bioavailability. International Journal of Molecular Sciences . 2012;13(6):6964–6982. doi: 10.3390/ijms13066964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu T., Xu F. F., Li S. H. Exploration on mechanisms of simiao wan in treating rheumatoid arthritis and eczema with concept of “treating different diseases with same method” based on network pharmacology. Traditional Chinese Drug Research & Clinical Pharmacology . 2021;32(12):1825–1832. [Google Scholar]
- 11.Qiu Y., Zhang Z. Y., Zhang X. Analysis on similarities and differences of sedative and hypnotic mechanisms between Ziziphi Spinosae Semen and Platycladi Semen based on systems pharmacology. Chinese Journal of New Drugs and Clinical Remedies . 2021;40(12):850–857. [Google Scholar]
- 12.Gfeller D., Grosdidier A., Wirth M., Daina A., Michielin O., Zoete V. SwissTargetPrediction: a web server for target prediction of bioactive small molecules. Nucleic Acids Research . 2014;42(W1):W32–W38. doi: 10.1093/nar/gku293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UniProt Consortium T. U. P. UniProt: the universal protein knowledgebase. Nucleic Acids Research . 2018;46(5):p. 2699. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y., Zhang S., Li F., et al. Therapeutic target database 2020: enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Research . 2020;48:D1031–D1041. doi: 10.1093/nar/gkz981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szklarczyk D., Gable A. L., Lyon D., et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Research . 2019;47(D1):D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu G., Zhang Y., Ren W., et al. Network pharmacology-based identification of key pharmacological pathways of Yin–Huang–Qing–Fei capsule acting on chronic bronchitis. International Journal of Chronic Obstructive Pulmonary Disease . 2016;Volume 12:85–94. doi: 10.2147/COPD.S121079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y., Zhou B., Pache L., et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nature Communications . 2019;10(1):p. 1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rappaport N., Twik M., Plaschkes I., et al. MalaCards: an amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic Acids Research . 2017;45(D1):D877–D887. doi: 10.1093/nar/gkw1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng Z., Gong X., Hu Z. L-3-n-butylphthalide attenuates inflammation response and brain edema in rat intracerebral hemorrhage model. Aging . 2020;12(12):11768–11780. doi: 10.18632/aging.103342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo H., Zhu L., Tang P., et al. Carthamin yellow improves cerebral ischemia‑reperfusion injury by attenuating inflammation and ferroptosis in rats. International Journal of Molecular Medicine . 2021;47(4):p. 52. doi: 10.3892/ijmm.2021.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J. Z., Jiang J., Zeng Y. Effect of An-gong Niu-huang Wan on the expression of MMP9 and AQP4 in rats with acute cerebral ischemia-reperfusion. Journal of Zunyi Medical University . 2019;42(04):412–415. [Google Scholar]
- 22.Feng S. Y., Sun J. N. Influences of an Gong Niu Huang Wan on expressions of MMP-9 and AQP-4 in rats with experimental intracerebral hemorrhage. Journal of Beijing University of Traditional Chinese Medicine . 2011;34(08):535–538+578. [Google Scholar]
- 23.Yan J. W., Chen L., Wang Y. Y. Protective effects of An-gong niu-huang Wan on cerebral ischemia-reperfusion injury and traumatic brain injury in rats. Journal of Zunyi Medical University . 2017;40(03):249–253. [Google Scholar]
- 24.Li P. Y., Wang X. P. Effect of Angong Niuhuang Pill on acute intracerebral hemorrhage and its effect on serum BNP and hs CRP levels. Studies of Trace Elements and Health . 2019;36(2):37–38. [Google Scholar]
- 25.Tanaka T., Narazaki M., Masuda K., Kishimoto T. Regulation of IL-6 in immunity and diseases. Advances in Experimental Medicine & Biology . 2016;941:79–88. doi: 10.1007/978-94-024-0921-5_4. [DOI] [PubMed] [Google Scholar]
- 26.Zegeye M. M., Lindkvist M., Fälker K., et al. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Communication and Signaling . 2018;16(1):p. 55. doi: 10.1186/s12964-018-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou R., Leng T., Yang T., Chen F., Hu W., Xiong Z.-G. β-Estradiol protects against acidosis-mediated and ischemic neuronal injury by promoting ASIC1a (Acid-Sensing ion channel 1a) protein degradation. Stroke . 2019;50(10):2902–2911. doi: 10.1161/STROKEAHA.119.025940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J., Sareddy G. R., Lu Y., et al. Astrocyte-derived estrogen regulates reactive astrogliosis and is neuroprotective following ischemic brain injury. Journal of Neuroscience . 2020;40(50):9751–9771. doi: 10.1523/JNEUROSCI.0888-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao P.-C., Lai M.-H., Hsu K.-P., et al. Identification of β-sitosterol as in vitro anti-inflammatory constituent in moringa oleifera. Journal of Agricultural and Food Chemistry . 2018;66(41):10748–10759. doi: 10.1021/acs.jafc.8b04555. [DOI] [PubMed] [Google Scholar]
- 30.Granado-Serrano A. B., Martín M. Á., Bravo L., Goya L., Ramos S. Quercetin attenuates TNF-induced inflammation in hepatic cells by inhibiting the NF-κB pathway. Nutrition and Cancer . 2012;64(4):588–598. doi: 10.1080/01635581.2012.661513. [DOI] [PubMed] [Google Scholar]
- 31.Ashrafizadeh M., Samarghandian S., Hushmandi K., et al. Quercetin in attenuation of ischemic/reperfusion injury: a review. Current Molecular Pharmacology . 2021;14(4):537–558. doi: 10.2174/1874467213666201217122544. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa Y., Kitamura M. Anti-apoptotic effect of quercetin: intervention in the JNK- and ERK-mediated apoptotic pathways. Kidney International . 2000;58(3):1078–1087. doi: 10.1046/j.1523-1755.2000.00265.x. [DOI] [PubMed] [Google Scholar]
- 33.Yan B. C., Wang J., Rui Y., et al. Neuroprotective effects of gabapentin against cerebral ischemia reperfusion-induced neuronal autophagic injury via regulation of the PI3K/Akt/mTOR signaling pathways. Journal of Neuropathology & Experimental Neurology . 2019;78(2):157–171. doi: 10.1093/jnen/nly119. [DOI] [PubMed] [Google Scholar]
- 34.Yang J., Yan H., Li S., Zhang M. Berberine ameliorates MCAO induced cerebral ischemia/reperfusion injury via activation of the BDNF-TrkB-PI3K/akt signaling pathway. Neurochemical Research . 2018;43(3):702–710. doi: 10.1007/s11064-018-2472-4. [DOI] [PubMed] [Google Scholar]
- 35.Zhou X.-Q., Zeng X.-N., Kong H., Sun X.-L. Neuroprotective effects of berberine on stroke models in vitro and in vivo. Neuroscience Letters . 2008;447(1):31–36. doi: 10.1016/j.neulet.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 36.Zhu T., Zhan L., Liang D., et al. Hypoxia-inducible factor 1α mediates neuroprotection of hypoxic postconditioning against global cerebral ischemia. Journal of Neuropathology & Experimental Neurology . 2014;73(10):975–986. doi: 10.1097/NEN.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 37.Zheng J., Zhang P., Li X., et al. Post-stroke estradiol treatment enhances neurogenesis in the subventricular zone of rats after permanent focal cerebral ischemia. Neuroscience . 2013;231:82–90. doi: 10.1016/j.neuroscience.2012.11.042. [DOI] [PubMed] [Google Scholar]
- 38.Maiese K. FoxO proteins in the nervous system. Analytical Cellular Pathology . 2015;2015:1–15. doi: 10.1155/2015/569392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang K., Yang Y., Ge H., et al. Artesunate promotes the proliferation of neural stem/progenitor cells and alleviates Ischemia-reperfusion Injury through PI3K/Akt/FOXO-3a/p27kip1 signaling pathway. Aging . 2020;12(9):8029–8048. doi: 10.18632/aging.103121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunningham K. S., Gotlieb A. I. The role of shear stress in the pathogenesis of atherosclerosis. Laboratory Investigation . 2005;85(1):9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 41.Cui L., Li Z., Chang X., Cong G., Hao L. Quercetin attenuates vascular calcification by inhibiting oxidative stress and mitochondrial fission. Vascular Pharmacology . 2017;88:21–29. doi: 10.1016/j.vph.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Fan Q., Liu Y., Rao J., et al. Anti-atherosclerosis effect of Angong Niuhuang pill via regulating Th17/treg immune balance and inhibiting chronic inflammatory on ApoE-/- mice model of early and mid-term atherosclerosis. Frontiers in Pharmacology . 2020;10:p. 1584. doi: 10.3389/fphar.2019.01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao R.-Q., Qi D.-S., Yu H.-L., Liu J., Yang L.-H., Wu X.-X. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochemical Research . 2012;37(12):2777–2786. doi: 10.1007/s11064-012-0871-5. [DOI] [PubMed] [Google Scholar]
- 44.Wu X., Liu S., Hu Z., Zhu G., Zheng G., Wang G. Enriched housing promotes post-stroke neurogenesis through calpain 1-STAT3/HIF-1α/VEGF signaling. Brain Research Bulletin . 2018;139:133–143. doi: 10.1016/j.brainresbull.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y., Yang R., Yan F., Jin Y., Liu X., Wang T. Medicarpin protects cerebral microvascular endothelial cells against oxygen-glucose deprivation/reoxygenation-induced injury via the PI3K/Akt/FoxO pathway: a study of network pharmacology analysis and experimental validation. Neurochemical Research . 2021;47(2):347–357. doi: 10.1007/s11064-021-03449-0. [DOI] [PubMed] [Google Scholar]
- 46.Li L., Lou W., Li H., Zhu Y., Huang X. a. Upregulated C-C motif chemokine ligand 2 promotes ischemic stroke via chemokine signaling pathway. Annals of Vascular Surgery . 2020;68:476–486. doi: 10.1016/j.avsg.2020.04.047. [DOI] [PubMed] [Google Scholar]
- 47.Li Z., Wang H., Xiao G., et al. Recovery of post-stroke cognitive and motor deficiencies by Shuxuening injection via regulating hippocampal BDNF-mediated Neurotrophin/Trk Signaling. Biomedicine & Pharmacotherapy . 2021;141:p. 111828. doi: 10.1016/j.biopha.2021.111828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used to support the findings of this study are included within the article.


