Abstract
Hepatocellular carcinoma (HCC) is the fourth main reason of cancer-related death. Codonopsis pilosula is a commonly used traditional Chinese medicine (TCM) for patients with HCC. However, its potential mechanism for treatment of HCC remains unclear. Here, we used transcriptomics and network pharmacology to explore the potential molecular mechanisms of Codonopsis pilosula. In our study, twelve differentially expressed genes (DEGs) (5 upregulated and 7 downregulated) of Codonopsis pilosula treating HepG2 cells (a kind of HCC cell) were identified. Among the 12 DEGs, HMOX1 may play an essential role. Codonopsis pilosula mainly affects the mineral absorption pathway in HCC. We acquired 2957, 1877, and 255 targets from TCMID, SymMap, and TCMSP, respectively. Codonopsis pilosula could upregulate HMOX1 via luteolin, capsaicin, and sulforaphane. Our study provided new understanding of the potential pharmacological mechanisms of Codonopsis pilosula in treating HCC and pointed out a direction for further experimental research.
1. Introduction
There are multiple types of primary liver cancer, of which hepatocellular carcinoma (HCC), the fourth leading cause of cancer-related death overall worldwide, is the most predominant type [1, 2]. During the last few decades, HCC incidence has been increasing at a global level [3, 4], and it is estimated that more than 1 million people will die from HCC in 2030 [5, 6]. In addition to surgical treatments, drugs are the key to HCC therapy [7]. Sorafenib has been the global treatment standard for patients with HCC since 2007 [8], but its efficacy is unsatisfactory [9]. As a widely used alternative therapy, traditional Chinese medicine (TCM) can probably prolong the median survival time and improve the overall survival among patients with HCC [10]. Moreover, some TCMs have been reported to have the ability to assist in elevating the efficacy of sorafenib in the treatment of HCC [11–13].
Codonopsis pilosula, a kind of TCM, has anticancer activity and is widely used in adjuvant anticancer therapy [14]. A lot of evidence has shown that many ingredients of Codonopsis pilosula, such as Codonopsis pilosula polysaccharide (CPP) and atractylenolide III (ATL), have anti-HCC effects via different pathways. CPP is one of major active constituents in Codonopsis pilosula, and it could inhibit the proliferation and motility of HCC cells through the β-catenin/TCF4 pathway [15]. CPP1a and CPP1c are two water-soluble homogeneous polysaccharides isolated and purified from Codonopsis pilosula, and they could induce HepG2 cell apoptosis by upregulating the ratio of Bax/Bcl-2 and activating caspase-3 [16]. ATL, a sesquiterpenoid extracted from Codonopsis pilosula, exerts tumor-suppressive functions in liver cancer via the miR-195-5p/FGFR1 signaling axis [17]. However, Codonopsis pilosula, as a kind of Chinese herb, is often used as a whole in clinical practice. There are few reports on the mechanisms of Codonopsis pilosula in the treatment of HCC, and its application is greatly limited. The effects of TCM (or herbs of other nations) are not the sum of all active ingredients. In the mixed system of Codonopsis pilosula, new effects may emerge that the single active ingredients do not.
In this study, we integrated transcriptomics and network pharmacology to understand the mechanisms of Codonopsis pilosula in treating HCC. The differentially expressed genes (DEGs) of Codonopsis pilosula were derived from a previous study (GSE115506) [18]. The effective ingredients of Codonopsis pilosula and targets were assayed by TCMID, SymMap, and TCMSP [19–21]. The mechanisms of Codonopsis pilosula against HCC were assessed by Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis. Furthermore, we found that Codonopsis pilosula may regulate mineral absorption through luteolin, capsaicin, and sulforaphane directly targeting HMOX1.
2. Materials and Methods
2.1. Differentially Expressed Genes Screening
We obtained DEGs of Codonopsis pilosula from the GEO database (https://www.ncbi.nlm.nih.gov/geo/) (series: GSE115506; samples: GSM3179695, GSM3179696, GSM3179697, GSM3179698, GSM3179699, and GSM3179700). In GSE115506, total RNA was isolated from HepG2 cells 24 hours after 3 mg/mL Codonopsis pilosula aqueous extract treatment in vitro. We performed differential analysis by the Limma R packages [22], and the cutoff value for identifying DEG was set to |log2 fold change| >1 and adjusted pvalue <0.05.
2.2. Components and Targets Acquisition
The components and targets of Codonopsis pilosula were acquired from TCMID (http://www.megabionet.org/tcmid/) [20], SymMap (http://www.symmap.org/) [21], and TCMSP (https://tcmsp-e.com/) databases [19]. They are all integrative databases of traditional Chinese medicine.
2.3. Network Building
We performed the protein-protein interaction (PPI) network analysis using STRING (https://string-db.org/) [23]. The Codonopsis pilosula-gene network, protein-protein interaction (PPI) network, and Codonopsis pilosula-component-target network were visualized by Cytoscape software [24].
2.4. Functional Enrichment Analysis
We conducted Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis and biological process (BP) of Gene Ontology (GO) analysis by R package clusterProfiler [25].
2.5. Expression Analysis of HMOX1
The expression of HMOX1 in HCC was obtained through UALCAN, which is a comprehensive and interactive web resource for analyzing cancer OMICS data [26].The expression of HMOX1 after apigenin, luteolin, capsaicin, 4-methylsulfinyl butyl isothiocyanate (sulforaphane), and geniposide treatment was obtained from the GEO database (apigenin series: GSE119552, samples: GSM3377483, GSM3377484, GSM3377485, GSM3377486, GSM3377495, GSM3377496, GSM3377497, and GSM3377498; luteolin series: GSE18740, samples: GSM465440, GSM465441, GSM465442, GSM465443, GSM465444, and GSM465445; capsaicin series: GSE59727, samples: GSM1442972, GSM1442973, GSM1442974, GSM1442975, GSM1442976, GSM1442977, GSM1442978, and GSM1442979; sulforaphane series: GSE28813, samples: GSM713517, GSM713518, GSM713519, GSM713520, GSM713521, GSM713522, GSM713523, and GSM713524; and geniposide series: GSE85871, samples: GSM2286350, GSM2286351, GSM2286248, GSM2286249, GSM2286316, GSM2286317, GSM2286398, and GSM2286399). In GSE18740, mouse BV-2 microglia were treated with 50 μM luteolin for 24 hours; in GSE59727, rat TRPV1-positive neurons were treated with 10 μM capsaicin for 30 minutes; in GSE119552, MCF-7 cells were treated with 10 μM apigenin for 24 hours; in GSE28813, MCF10A cells were treated with 15 μM sulforaphane for 24 hours; and in GSE85871, MCF-7 cells were treated with 10 μM geniposide for 12 hours. We extracted the expression level of the HMOX1 gene from these expression matrices and compared its significance with the t-test.
2.6. Molecular Docking
The structure of HMOX1 protein was obtained from PDB (https://www.rcsb.org/) [27], and the structures of luteolin, capsaicin, and sulforaphane were acquired from ZINC (https://zinc.docking.org/) [28]. We used AutoDock 4.2 to prepare the PDBQT file and perform molecular docking [29]. Finally, molecular docking maps were visualized through PyMOL.
3. Results
3.1. Codonopsis pilosula-Gene Network and PPI Analysis
We identified 12 DEGs (5 upregulated and 7 downregulated) from the GSE115506 data set (Table S1). A volcano plot (Figure 1(a)) and a heatmap (Figure 1(b)) were established to show the distribution of DEGs in HepG2 cells after treating Codonopsis pilosula. The cutoff value for identifying DEG was set to |log2 fold change| >1 and the adjusted pvalue <0.05. Accordingly, we built a Codonopsis pilosula-gene network (Figure 1(c)). In order to further explore the potential association among these DEGs, we performed a PPI network analysis for the 12 DEGs by STRING [23]. The final PPI network includes 11 nodes and 13 edges (Figure 1(d)). Furthermore, we identified HMOX1, an upregulated gene, as the hub gene because it has the highest degree.
Figure 1.
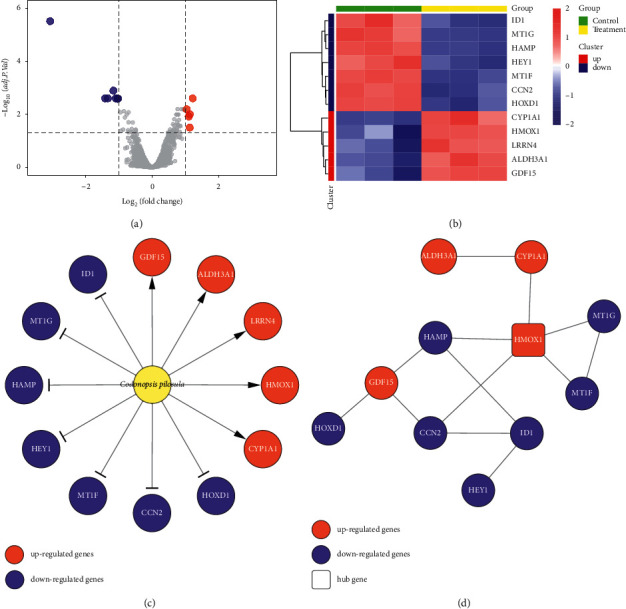
Codonopsis pilosula-gene network and PPI analysis. (a) Volcano plot and (b) heatmap of DEGs showed that genes with dramatic changes after Codonopsis pilosula treatment. (c) Codonopsis pilosula-gene network. (d) The PPI analysis of DEGs.
3.2. GO and KEGG Analysis
Through the clusterProfiler R package for KEGG enrichment analysis, we found that only 1 pathway was significantly affected (padjust <0.05) during Codonopsis pilosula treatment of HepG2 cells (Figure 2(a)). HMOX1 (hub gene), MT1F, and MT1G were enriched in mineral absorption. In total, 61 biological processes (GO terms) were notably enriched (padjust <0.05) (Table S2). The top 5 biological processes are shown in Figure 2(b). The highly enriched biological processes include responses to iron ions, cellular responses to copper ions, cellular transition metal ion homeostasis, transition metal ion homeostasis, and response to metal ions. These biological processes and pathways are closely related to the metabolism and homeostasis of metal ions, suggesting that Codonopsis pilosula mainly affects the mineral absorption pathway in HCC cells.
Figure 2.
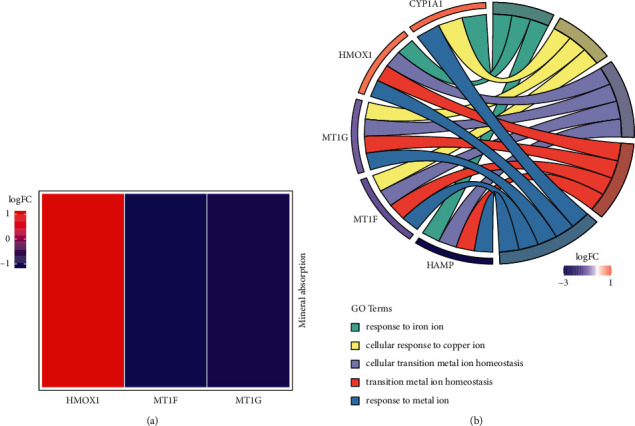
KEGG and GO analysis. (a) KEGG pathway enrichment of Codonopsis pilosula treating HepG2 cells. (b) The top 5 biological process in GO terms of Codonopsis pilosula treating HepG2 cells.
3.3. Codonopsis pilosula Reverses HMOX1 Expression in HCC
We acquired 2957 targets from TCMID, 1877 targets from SymMap, and 255 targets from TCMSP (Table S3). According to the intersection of these targets and 12 DEGs, we found that HMOX1 is the direct target of Codonopsis pilosula in three databases (Figure 3(a)). Our results showed that the expression of HMOX1 was significantly enhanced (Figure 1). Interestingly, HMOX1 was significantly decreased in HCC patients (Figure 3(b)). The abovementioned results suggest that Codonopsis pilosula may resist HCC by reversing HMOX1 expression in HCC patients.
Figure 3.
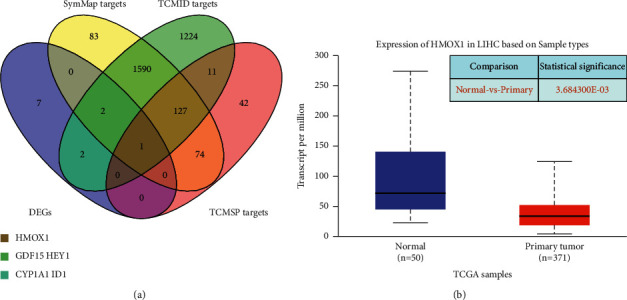
Codonopsis pilosula could target HMOX1. (a) Among all targets and 12 DEGs of Codonopsis pilosula, HMOX1 is the only common gene. (b) HMOX1 expression is significantly decreased in HCC.
3.4. Codonopsis pilosula Could Upregulate HMOX1 via Luteolin, Capsaicin, and Sulforaphane
To explore how Codonopsis pilosula promotes the expression of HMOX1, we established a Codonopsis pilosula-component-target network (Figure 4(a)). The result showed that Codonopsis pilosula may directly target HMOX1 through apigenin, luteolin, capsaicin, 4-methylsulfinyl butyl isothiocyanate (sulforaphane), and geniposide. In addition, we detected the expression of HMOX1 after treating with these components (Figures 4(b)–4(f)). Figure 4(b) showed that luteolin could upregulate Hmox1 in BV-2 cells, although the p value is 0.081. Figure 4(c) showed that capsaicin could significantly promote the expression of Hmox1 in dorsal root ganglia neurons. Figure 4(d) showed that apigenin could not affect the expression of HMOX1 in MCF-7 cells. Figure 4(e) manifested that sulforaphane could dramatically enhance the expression of HMOX1 in MCF10A cells. Figure 4(f) showed that geniposide could not affect the expression of HMOX1 in MCF-7 cells. These results imply that Codonopsis pilosula could upregulate HMOX1 in HCC via luteolin, capsaicin, and sulforaphane.
Figure 4.
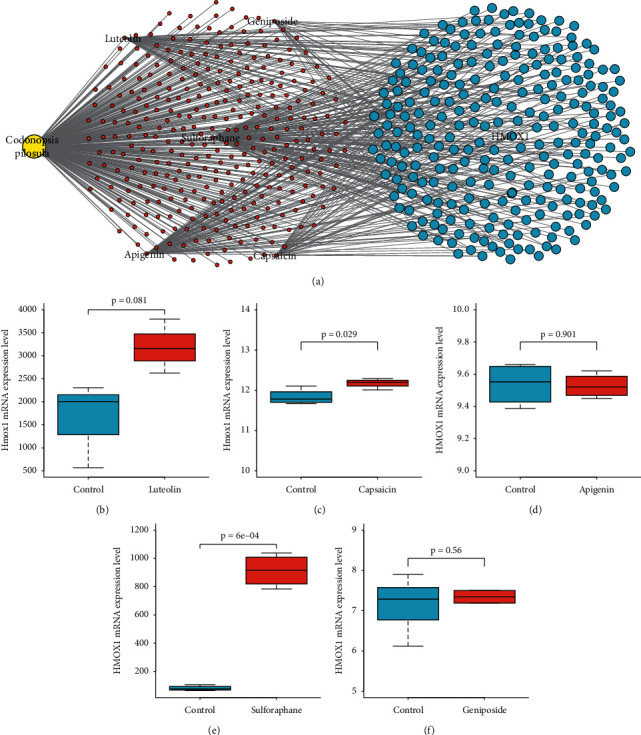
Codonopsis pilosula targeted HMOX1 via luteolin, capsaicin, and sulforaphane. (a) Codonopsis pilosula-component-target network showed that Codonopsis pilosula may target HMOX1 directly through apigenin, luteolin, capsaicin, sulforaphane, and geniposide. (b) Luteolin trends to upregulate Hmox1 in BV-2 cells (p=0.081). (c) Capsaicin could significantly upregulate Hmox1 in dorsal root ganglia neurons (p=0.029). (d) Apigenin could not affect the expression of HMOX1 in MCF-7 cells (p=0.901). (e) Sulforaphane could significantly enhance HMOX1 expression in MCF10A cells (p=0.0006). (f) Geniposide has no effect on the expression of HMOX1 in MCF-7 cells (p=0.56).
3.5. Potential Binding Site between Active Ingredients of Codonopsis pilosula and HMOX1 Protein
For exploring potential interaction between active ingredients of Codonopsis pilosula (luteolin, capsaicin, and sulforaphane) and HMOX1 protein, we predicted the potential binding site of them via molecular docking. As shown in Figure 5(a), luteolin may directly bind ASP-140, LEU-141, GLN-145, ALA-173, SER-174, and ALA-175 to promote the expression level of HMOX1. Capsaicin may combine with HMOX1 by ARG-44, LYS-48, and PHE-95, thus enhancing the expression level of HMOX1 (Figure 5(b)). Sulforaphane may target HMOX1 via binding PHE-167 and ALA-175, resulting in increased expression of HMOX1 (Figure 5(c)). These results suggest that luteolin, capsaicin, and sulforaphane may promote HMOX1 expression through direct binding.
Figure 5.
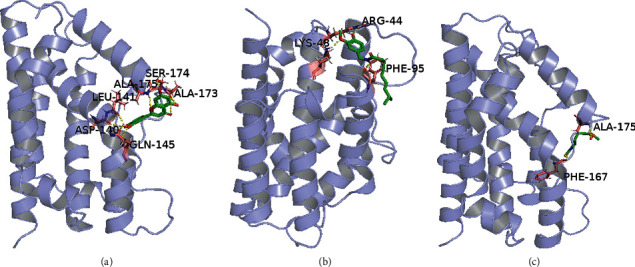
The molecular docking results of luteolin, capsaicin, and sulforaphane. (a) Luteolin may bind to HMOX1 with ASP-140, LEU-141, GLN-145, ALA-173, SER-174, and ALA-175. (b) Capsaicin may combine with HMOX1 by ARG-44, LYS-48, and PHE-95. (c) Sulforaphane may target HMOX1 via PHE-167 and ALA-175.
4. Discussion
TCMs are widely used during HCC treatment in China [30]. As with traditional medicine in other nations, herbal medicines are the main form of TCM [31]. Unlike small molecule drugs, herbal medicines contain many components and possess complex targets. Besides, some studies have revealed that miRNAs of herbal medicines may be ingested by the body and regulate the process of disease [32–34]. Complex components and targets limit the exploration of mechanisms in herbal medicines. Although multiple active components of Codonopsis pilosula were proved to have anti-HCC potential [15–17], the overall mechanism of Codonopsis pilosula is unclear.
In the present study, the Codonopsis pilosula-gene network was built by 12 striking DEGs (Figure 1(c)), and we identified HMOX1 as the hub gene via the PPI network (Figure 1(d)). HMOX1 was significantly enriched in the mineral absorption pathway, and the biological processes of its enrichment are primarily related to the metabolism of metal ions (Figures 2(a) and 2(b)). A study reported that there is a remarkable correlation between mineral absorption pathways and HCC development [35]. Furthermore, metal ion metabolism plays an essential role in the progression and treatment of HCC [36, 37]. Consequently, Codonopsis pilosula is highly likely to treat HCC via targeting HMOX1 to affect the mineral absorption pathway.
HMOX1 (heme oxygenase-1) is a stress-induced enzyme that catalyzes the degradation of heme to carbon monoxide, iron, and biliverdin [38]. The byproducts of HMOX1 enzymatic activity are cytoprotective because of their antioxidant and anti-inflammatory properties, showing that HMOX1 is a potential therapeutic target in many diseases [39]. Our results revealed that HMOX1 was significantly decreased in HCC patients (Figure 3(b)), and Codonopsis pilosula could distinctly enhance the expression of HMOX1 in HepG2 cells (Figure 1), suggesting that Codonopsis pilosula may reverse the expression pattern of HMOX1 in the HCC environment. Interestingly, HMOX1 overexpression could inhibit the growth, migration, and invasion in vivo, as well as higher HMOX1 expression was also associated with favorable disease-free survival of HBV-HCC patients who underwent hepatectomy [40]. These results indicate that Codonopsis pilosula is likely to improve the survival of HCC patients by promoting the expression of HMOX1, and is a potential adjuvant therapy for HCC.
Through the network pharmacology strategies, we built a Codonopsis pilosula-component-target network (Figure 4(a)). The network showed that Codonopsis pilosula may directly target HMOX1 via apigenin, luteolin, capsaicin, sulforaphane, and geniposide. To verify this result, we examined the effect of luteolin on Hmox1 expression in mouse BV-2 microglia (GSE18740), capsaicin on Hmox1 expression in rat TRPV1-positive neurons (GSE59727), apigenin on HMOX1 expression in human breast cancer cells MCF-7 (GSE119552), sulforaphane on HMOX1 expression in human breast epithelial cells MCF10A (GSE28813), and geniposide on HMOX1 expression in human breast cancer cells MCF-7 (GSE85871) (Figures 4(b)–4(f)). The results indicated that luteolin, capsaicin, and sulforaphane could increase the expression of HMOX1 expression in vitro, although not in HCC cells. Luteolin, a natural flavonoid, plays multiple roles in the anti-HCC process. Growth inhibition of luteolin on HCC cells is induced via multiple signaling pathways of TGF-β1 pathways, p53 pathways, Fas/Fas-ligand pathways [41], ER stress [42], and AKT/OPN pathway [43]. Besides, a recent study reported that luteolin could significantly inhibit HCC growth and cause apoptosis and cell cycle arrest in vitro and significantly suppress HCC growth in vivo via upregulating miR-6809-5p [44]. Capsaicin is a natural vanilloid and may inhibit the growth of SK-Hep-1 hepatocellular carcinoma cells by inducing apoptosis via Bcl-2 downregulation and caspase-3 activation [45]. Moreover, capsaicin could induce apoptosis in HepG2 cells by reducing the levels of xIAP and cIAP1 proteins, which are inhibitors of caspase-3 activation [46]. Interestingly, both luteolin and capsaicin are able to assist sorafenib to produce better anti-HCC therapeutic effects [47, 48]. Sulforaphane, a member of the isothiocyanate family, has exhibited promising inhibitory effects on breast cancer, lung cancer, liver cancer, and other malignant tumors [49]. Some studies revealed that sulforaphane could induce apoptosis [50] and enhance the radiation sensitivity [51] in HCC.
Notably, there are about 200 phytometabolites in Codonopsis pilosula, and the main bioactive ingredients include polysaccharides, polyyne and polyacetylene glycosides, lignans, penoids, alkaloids, flavonoids, and lactones [52]. Polysaccharides are large-molecule components in Codonopsis pilosula, which have a significant inhibitory effect on gastric cancer and lung cancer, in addition to liver cancer [53]. Although luteolin, capsaicin, and sulforaphane are not the most abundant ingredients of Codonopsis pilosula, they are essential for understanding the pharmacological effects of Codonopsis pilosula. Tang et al. found that Codonopsis pilosula may play an antigastric cancer role through luteolin [54], suggesting that luteolin may play an important role in the anticancer effects of Codonopsis pilosula. Furthermore, several studies showed that luteolin [55], capsaicin [56], and sulforaphane [57] could target HMOX1 and significantly enhance its expression level. Luteolin, capsaicin, and sulforaphane are components of Codonopsis pilosula, but their quantitative studies in Codonopsis pilosula are insufficient. It is reported that the content of luteolin in Codonopsis thalictrifolis is 0.7% via HPLC [58]. It is important to notice that Codonopsis thalictrifolis is not Codonopsis pilosula, although they belong to the Codonopsis genus, and there may be great differences in chemical composition between them. Nonetheless, as a reference, the data implied that the content of the luteolin in Codonopsis pilosula may be less than 0.1% or even 0.01%. The lowest dose of luteolin that has been reported to produce anti-HCC effects in rats is 0.2 mg/kg via intraperitoneal injection [59]. In addition, orally administered luteolin (0.2 mg/kg) could produce anticolon cancer effects in rats [60]. Fuzheng Jiedu Xiaoji formulation (including 15 g of Codonopsis pilosula) could inhibit HCC progression in patients [61]. Jian Pi Li Qi Decoction (including 20 g of Codonopsis pilosula) could improve the prognosis of patients with HCC [62]. Therefore, it is likely that the effective concentration of luteolin can be reached in the application of Codonopsis pilosula. At present, there are no quantitative studies on capsaicin and sulforaphane in Codonopsis pilosula. Studies showed that capsaicin (2 mg/kg) [63] and sulforaphane (50 mg/kg) [64] could inhibit the growth of HCC in xenograft mice. Compared with the effective dosage of capsaicin and sulforaphane, the application of Codonopsis pilosula (15–20 g) is higher. Consequently, luteolin, capsaicin, and sulforaphane are likely to reach effective concentrations in the clinical application of Codonopsis pilosula. These studies suggest that Codonopsis pilosula is most likely to exert anti-HCC effects via luteolin, capsaicin, and sulforaphane (Figure 6). In fact, although our study showed that luteolin, capsaicin, and sulforaphane may play roles in the adjuvant treatment of HCC by Codonopsis pilosula, they may not be the main active ingredients of Codonopsis pilosula. As an herbal medicine, Codonopsis pilosula contains a variety of ingredients. An inulin fructan from Codonopsis pilosula possessed potential anti-HCC effects (inhibiting proliferation and inducing apoptosis) on Huh-7 and HepG2 cells without side effects on normal cells [65]. In addition, a novel fructose-enriched polysaccharide from Codonopsis pilosula inhibited HepG2 cell proliferation and promoted apoptosis [66]. Taken together, apoptosis may be one of the anti-HCC pathways of Codonopsis pilosula.
Figure 6.
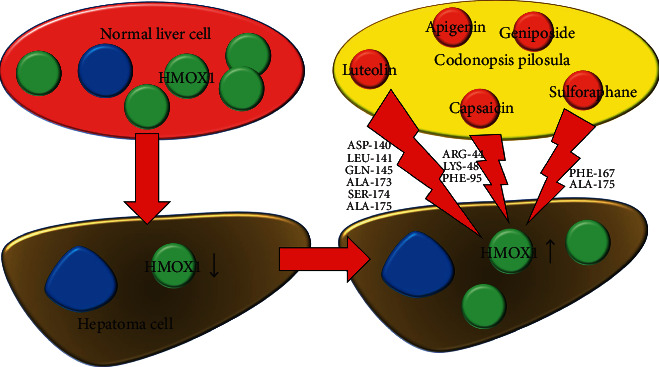
Schematic diagram of Codonopsis pilosula for HCC treatment.
5. Conclusions
The present study explored the effects of Codonopsis pilosula in the treatment of HCC via transcriptomics and network pharmacology. We revealed the transcriptome changes of HCC cells induced by Codonopsis pilosula. In addition, Codonopsis pilosula is likely to upregulate HMOX1 directly through luteolin, capsaicin, and sulforaphane, thus affecting the mineral absorption pathway in HCC cells. This study provides clues to comprehend the potential mechanisms of Codonopsis pilosula in treating HCC. Of course, these conclusions require further experimental support.
Acknowledgments
The authors thank the Shenzhen Key Lab of Neurogenomics (BGI-Shenzhen) for support in the analysis. The study was supported by the National Key Research and Development Program of China (No. 2020YFC2002902) and the Science, and Technology and Innovation Commission of Shenzhen Municipality under Grant (No. JCYJ20180507183615145).
Contributor Information
Hefu Zhen, Email: zhenhf@genomics.cn.
Chao Nie, Email: niechao@genomics.cn.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
There are no conflicts of interest.
Supplementary Materials
Tables S1–S3 in the Supplemental files. Table S1: twelve DEGs in HepG2 cells after Codonopsis pilosula treatment. Table S2: all significantly enriched biological processes after Codonopsis pilosula treatment. Table S3: targets of Codonopsis pilosula.
References
- 1.Craig A. J., von Felden J., Garcia-Lezana T., Sarcognato S., Villanueva A. Tumour evolution in hepatocellular carcinoma. Nature Reviews Gastroenterology & Hepatology . 2020;17(3):139–152. doi: 10.1038/s41575-019-0229-4. [DOI] [PubMed] [Google Scholar]
- 2.Sia D., Villanueva A., Friedman S. L., Llovet J. M. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology . 2017;152(4):745–761. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z., Jiang Y., Yuan H., et al. The trends in incidence of primary liver cancer caused by specific etiologies: results from the global burden of disease study 2016 and implications for liver cancer prevention. Journal of Hepatology . 2019;70(4):674–683. doi: 10.1016/j.jhep.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Liu J., Tang W., Budhu A., et al. A viral exposure signature defines early onset of hepatocellular carcinoma. Cell . 2020;182(2):317–328. doi: 10.1016/j.cell.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villanueva A., Carcinoma H. Hepatocellular carcinoma. New England Journal of Medicine . 2019;380(15):1450–1462. doi: 10.1056/nejmra1713263. [DOI] [PubMed] [Google Scholar]
- 6.Nault J. C., Villanueva A. Biomarkers for hepatobiliary cancers. Hepatology . 2021;73(S1):115–127. doi: 10.1002/hep.31175. [DOI] [PubMed] [Google Scholar]
- 7.Foerster F., Galle P. R. The current landscape of clinical trials for systemic treatment of HCC. Cancers . 2021;13(8) doi: 10.3390/cancers13081962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rimassa L., Personeni N., Czauderna C., Foerster F., Galle P. Systemic treatment of HCC in special populations. Journal of Hepatology . 2021;74(4):931–943. doi: 10.1016/j.jhep.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Busche S., John K., Wandrer F., et al. BH3-only protein expression determines hepatocellular carcinoma response to sorafenib-based treatment. Cell Death & Disease . 2021;12(8):p. 736. doi: 10.1038/s41419-021-04020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X., Li M., Wang X., et al. Effects of adjuvant traditional Chinese medicine therapy on long-term survival in patients with hepatocellular carcinoma. Phytomedicine . 2019;62 doi: 10.1016/j.phymed.2019.152930.152930 [DOI] [PubMed] [Google Scholar]
- 11.Yang Y., Sun M., Yao W., et al. Compound kushen injection relieves tumor-associated macrophage-mediated immunosuppression through TNFR1 and sensitizes hepatocellular carcinoma to sorafenib. Journal for immunotherapy of cancer . 2020;8(1) doi: 10.1136/jitc-2019-000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam W., Jiang Z., Guan F., et al. PHY906(KD018), an adjuvant based on a 1800-year-old Chinese medicine, enhanced the anti-tumor activity of Sorafenib by changing the tumor microenvironment. Scientific Reports . 2015;5(1):p. 9384. doi: 10.1038/srep09384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhai B., Hu F., Yan H., et al. Bufalin reverses resistance to sorafenib by inhibiting akt activation in hepatocellular carcinoma: the role of endoplasmic reticulum stress. PLoS One . 2015;10(9) doi: 10.1371/journal.pone.0138485.e0138485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye L., Jia Y., Ji K., et al. Traditional Chinese medicine in the prevention and treatment of cancer and cancer metastasis. Oncology Letters . 2015;10(3):1240–1250. doi: 10.3892/ol.2015.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y., Zhang Y., Xu H. Effect of Codonopsis pilosula polysaccharides on the growth and motility of hepatocellular carcinoma HepG2 cells by regulating β-catenin/TCF4 pathway. International Journal of Polymer Science . 2019;2019:7. doi: 10.1155/2019/7068437.7068437 [DOI] [Google Scholar]
- 16.Bai R., Li W., Li Y., et al. Cytotoxicity of two water-soluble polysaccharides from Codonopsis pilosula Nannf. var. modesta (Nannf.) L.T.Shen against human hepatocellular carcinoma HepG2 cells and its mechanism. International Journal of Biological Macromolecules . 2018;120:1544–1550. doi: 10.1016/j.ijbiomac.2018.09.123. [DOI] [PubMed] [Google Scholar]
- 17.Sheng L., Li J., Li N., et al. Atractylenolide III predisposes miR-195-5p/FGFR1 signaling axis to exert tumor-suppressive functions in liver cancer. Journal of Food Biochemistry . 2021;45(5) doi: 10.1111/jfbc.13582.e13582 [DOI] [PubMed] [Google Scholar]
- 18.Ko P.-H., Huang C.-W., Chang H.-H., Chuang E. Y., Tsai M.-H., Lai L.-C. Identifying the functions and biomarkers of Codonopsis pilosula and Astragalus membranaceus aqueous extracts in hepatic cells. Chinese Medicine . 2019;14(1):p. 10. doi: 10.1186/s13020-019-0233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ru J., Li P., Wang J., et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics . 2014;6(1):p. 13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L., Xie D., Yu Y., et al. TCMID 2.0: a comprehensive resource for TCM. Nucleic Acids Research . 2018;46:D1117–D1120. doi: 10.1093/nar/gkx1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y., Zhang F., Yang K., et al. SymMap: an integrative database of traditional Chinese medicine enhanced by symptom mapping. Nucleic Acids Research . 2019;47:D1110–D1117. doi: 10.1093/nar/gky1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritchie M. E., Phipson B., Wu D., et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research . 2015;43(7):p. e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szklarczyk D., Gable A. L., Lyon D., et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Research . 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shannon P., Markiel A., Ozier O., et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Research . 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu G., Wang L.-G., Han Y., He Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS: A Journal of Integrative Biology . 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandrashekar D. S., Bashel B., Balasubramanya S. A. H., et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia . 2017;19(8):649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berman H. M., Westbrook J., Feng Z., et al. The protein data bank. Nucleic Acids Research . 2000;28(1):235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterling T., Irwin J. J. Zinc 15—ligand discovery for everyone. Journal of Chemical Information and Modeling . 2015;55(11):2324–2337. doi: 10.1021/acs.jcim.5b00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris G. M., Huey R., Lindstrom W., et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. Journal of Computational Chemistry . 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao X., Bu Y., Jia Q. Traditional Chinese medicine as supportive care for the management of liver cancer: past, present, and future. Genes & Diseases . 2020;7(3):370–379. doi: 10.1016/j.gendis.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products. Molecules . 2016;21(5) doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou L.-K., Zhou Z., Jiang X.-M., et al. Absorbed plant MIR2911 in honeysuckle decoction inhibits SARS-CoV-2 replication and accelerates the negative conversion of infected patients. Cell Discovery . 2020;6(1):p. 54. doi: 10.1038/s41421-020-00197-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia C., Zhou H., Xu X., et al. Identification and investigation of miRNAs from gastrodia elata blume and their potential function. Frontiers in Pharmacology . 2020;11(1477) doi: 10.3389/fphar.2020.542405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Z., Li X., Liu J., et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Research . 2015;25(1):39–49. doi: 10.1038/cr.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y., Chen R., Yang J., et al. Integrated bioinformatics analysis reveals key candidate genes and pathways associated with clinical outcome in hepatocellular carcinoma. Frontiers in Genetics . 2020;11:p. 814. doi: 10.3389/fgene.2020.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wachsmann J., Peng F. Molecular imaging and therapy targeting copper metabolism in hepatocellular carcinoma. World Journal of Gastroenterology . 2016;22(1):221–231. doi: 10.3748/wjg.v22.i1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W., Xie Q., Zhou X., et al. Mitofusin-2 triggers mitochondria Ca2+ influx from the endoplasmic reticulum to induce apoptosis in hepatocellular carcinoma cells. Cancer Letters . 2015;358(1):47–58. doi: 10.1016/j.canlet.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 38.Dunn L. L., Kong S. M. Y., Tumanov S., et al. Hmox1 (heme oxygenase-1) protects against ischemia-mediated injury via stabilization of HIF-1α (Hypoxia-Inducible factor-1α) Arteriosclerosis, Thrombosis, and Vascular Biology . 2021;41(1):317–330. doi: 10.1161/ATVBAHA.120.315393. [DOI] [PubMed] [Google Scholar]
- 39.Hull T. D., Agarwal A., George J. F. The mononuclear phagocyte system in homeostasis and disease: a role for heme oxygenase-1. Antioxidants and Redox Signaling . 2014;20(11):1770–1788. doi: 10.1089/ars.2013.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeh C.-N., Wu R.-C., Cheng C.-T., et al. HO-1 is a favorable prognostic factor for HBV-HCC patients who underwent hepatectomy. Cancer Management and Research . 2018;10:6049–6059. doi: 10.2147/cmar.s186931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yee S. B., Choi H. J., Chung S. W., et al. Growth inhibition of luteolin on HepG2 cells is induced via p53 and Fas/Fas-ligand besides the TGF-β pathway. International Journal of Oncology . 2015;47(2):747–754. doi: 10.3892/ijo.2015.3053. [DOI] [PubMed] [Google Scholar]
- 42.Lee Y., Kwon Y. H. Regulation of apoptosis and autophagy by luteolin in human hepatocellular cancer Hep3B cells. Biochemical and Biophysical Research Communications . 2019;517(4):617–622. doi: 10.1016/j.bbrc.2019.07.073. [DOI] [PubMed] [Google Scholar]
- 43.Im E., Yeo C., Lee E.-O. Luteolin induces caspase-dependent apoptosis via inhibiting the AKT/osteopontin pathway in human hepatocellular carcinoma SK-Hep-1 cells. Life Sciences . 2018;209:259–266. doi: 10.1016/j.lfs.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 44.Yang P.-W., Lu Z.-Y., Pan Q., et al. MicroRNA-6809-5p mediates luteolin-induced anticancer effects against hepatoma by targeting flotillin 1. Phytomedicine . 2019;57:18–29. doi: 10.1016/j.phymed.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 45.Jung M.-Y., Kang H.-J., Moon A. Capsaicin-induced apoptosis in SK-Hep-1 hepatocarcinoma cells involves Bcl-2 downregulation and caspase-3 activation. Cancer Letters . 2001;165(2):139–145. doi: 10.1016/s0304-3835(01)00426-8. [DOI] [PubMed] [Google Scholar]
- 46.Huang S. P., Chen J. C., Wu C. C., et al. Capsaicin-induced apoptosis in human hepatoma HepG2 cells. Anticancer Research . 2009;29(1):165–174. [PubMed] [Google Scholar]
- 47.Dai N., Ye R., He Q., Guo P., Chen H., Zhang Q. Capsaicin and sorafenib combination treatment exerts synergistic anti-hepatocellular carcinoma activity by suppressing EGFR and PI3K/Akt/mTOR signaling. Oncology Reports . 2018;40(6):3235–3248. doi: 10.3892/or.2018.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng X. Q., Rong L. W., Wang R. X., et al. Luteolin and sorafenib combination kills human hepatocellular carcinoma cells through apoptosis potentiation and JNK activation. Oncology Letters . 2018;16(1):648–653. doi: 10.3892/ol.2018.8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu G., Yan Y., Zhou Y., et al. Sulforaphane: expected to become a novel antitumor compound. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics . 2020;28(4):439–446. doi: 10.3727/096504020x15828892654385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kntayya S. B., Ibrahim M. D., Mohd Ain N., Iori R., Ioannides C., Abdull Razis A. F. Induction of apoptosis and cytotoxicity by isothiocyanate sulforaphene in human hepatocarcinoma HepG2 cells. Nutrients . 2018;10(6) doi: 10.3390/nu10060718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren K., Li Z., Li Y., Zhang W., Han X. Sulforaphene enhances radiosensitivity of hepatocellular carcinoma through suppression of the NF-κB pathway. Journal of Biochemical and Molecular Toxicology . 2017;31(8) doi: 10.1002/jbt.21917. [DOI] [PubMed] [Google Scholar]
- 52.Zeng X., Li J., Lyu X., Chen J., Chen X., Guo S. Untargeted metabolomics reveals multiple phytometabolites in the agricultural waste materials and medicinal materials of Codonopsis pilosula. Frontiers of Plant Science . 2022;12 doi: 10.3389/fpls.2021.814011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He J.-Y., Ma N., Zhu S., Komatsu K., Li Z.-Y., Fu W.-M. The genus Codonopsis (Campanulaceae): a review of phytochemistry, bioactivity and quality control. Journal of Natural Medicines . 2015;69(1):1–21. doi: 10.1007/s11418-014-0861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang L., Chen J., Yin J., Fang M. Screening of active components and key targets of radix Codonopsis in the treatment of gastric cancer. Journal of Chemistry . 2021;2021:10. doi: 10.1155/2021/6056636.6056636 [DOI] [Google Scholar]
- 55.Li L., Zhou R., Lv H., Song L., Xue X., Wu L. Inhibitive effect of luteolin on sevoflurane-induced neurotoxicity through activation of the autophagy pathway by HMOX1. ACS Chemical Neuroscience . 2021;12(18):3314–3322. doi: 10.1021/acschemneuro.1c00157. [DOI] [PubMed] [Google Scholar]
- 56.Magierowska K., Wojcik D., Chmura A., et al. Alterations in gastric mucosal expression of calcitonin gene-related peptides, vanilloid receptors, and heme oxygenase-1 mediate gastroprotective action of carbon monoxide against ethanol-induced gastric mucosal lesions. International Journal of Molecular Sciences . 2018;19(10) doi: 10.3390/ijms19102960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nowak W. N., Taha H., Markiewicz J., et al. Atorvastatin and conditioned media from atorvastatin-treated human hematopoietic stem/progenitor-derived cells show proangiogenic activity in vitro but not in vivo. Mediators of Inflammation . 2019;2019:15. doi: 10.1155/2019/1868170.1868170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X., Liang J., Peng S., Ding L., Gu Y. Analysis of luteolin from traditional tibetan medicine of Codonopsis thalictrifolis. Chinese Journal of Analysis Laboratory . 2009;28:263–264. [Google Scholar]
- 59.Balamurugan K., Karthikeyan J. Evaluation of luteolin in the prevention of N-nitrosodiethylamine-induced hepatocellular carcinoma using animal model system. Indian Journal of Clinical Biochemistry . 2012;27(2):157–163. doi: 10.1007/s12291-011-0166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osman N. H. A., Said U. Z., El-Waseef A. M., Ahmed E. S. A. Luteolin supplementation adjacent to aspirin treatment reduced dimethylhydrazine-induced experimental colon carcinogenesis in rats. Tumor Biology . 2015;36(2):1179–1190. doi: 10.1007/s13277-014-2678-2. [DOI] [PubMed] [Google Scholar]
- 61.Yang X., Feng Y., Liu Y., et al. Fuzheng Jiedu Xiaoji formulation inhibits hepatocellular carcinoma progression in patients by targeting the AKT/CyclinD1/p21/p27 pathway. Phytomedicine . 2021;87 doi: 10.1016/j.phymed.2021.153575.153575 [DOI] [PubMed] [Google Scholar]
- 62.Xu L., Wang S., Zhuang L., et al. Jian pi Li Qi decoction alleviated postembolization syndrome following transcatheter arterial chemoembolization for hepatocellular carcinoma. Integrative Cancer Therapies . 2016;15(3):349–357. doi: 10.1177/1534735415617020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie C., Liu G., Li M., et al. Targeting TRPV1 on cellular plasticity regulated by Ovol 2 and Zeb 1 in hepatocellular carcinoma. Biomedicine & Pharmacotherapy . 2019;118 doi: 10.1016/j.biopha.2019.109270.109270 [DOI] [PubMed] [Google Scholar]
- 64.Wu J., Han J., Hou B., Deng C., Wu H., Shen L. Sulforaphane inhibits TGF-β-induced epithelial-mesenchymal transition of hepatocellular carcinoma cells via the reactive oxygen species-dependent pathway. Oncology Reports . 2016;35(5):2977–2983. doi: 10.3892/or.2016.4638. [DOI] [PubMed] [Google Scholar]
- 65.Hu N., Gao Z., Cao P., et al. Uniform and disperse selenium nanoparticles stabilized by inulin fructans from Codonopsis pilosula and their anti-hepatoma activities. International Journal of Biological Macromolecules . 2022;203:105–115. doi: 10.1016/j.ijbiomac.2022.01.140. [DOI] [PubMed] [Google Scholar]
- 66.Yu J., Dong X.-D., Jiao J.-S., et al. The inhibitory effects of selenium nanoparticles modified by fructose-enriched polysaccharide from Codonopsis pilosula on HepG2 cells. Industrial Crops and Products . 2022;176 doi: 10.1016/j.indcrop.2021.114335.114335 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3 in the Supplemental files. Table S1: twelve DEGs in HepG2 cells after Codonopsis pilosula treatment. Table S2: all significantly enriched biological processes after Codonopsis pilosula treatment. Table S3: targets of Codonopsis pilosula.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


