Abstract
Intervertebral disc degeneration (IVDD) is one of the main triggers of low back pain, which is most often associated with patient morbidity and high medical costs. IVDD triggers a wide range of pathologies and clinical syndromes like paresthesia, weakness of extremities, and intermittent/chronic back pain. Mesenchymal stem cells (MSCs) have demonstrated to possess immunomodulatory functions as well as the capability of differentiating into chondrocytes under appropriate microenvironment conditions, which makes them potentially epitome for intervertebral disc (IVD) regeneration. The IVD microenvironment is composed by niche of cells, and their chemical and physical milieus have been exhibited to have robust influence on MSC behavior as well as differentiation. Nevertheless, the contribution of MSCs to the IVD milieu conditions in healthy as well as degeneration situations is still a matter of debate. It is still not clear which factors, if any, are essential for effective and efficient MSC survival, proliferation, and differentiation. IVD microenvironment clues such as nucleopulpocytes, potential of hydrogen (pH), osmotic changes, glucose, hypoxia, apoptosis, pyroptosis, and hydrogels are capable of influencing the MSCs aimed for the treatment of IVDD. Therefore, clinical usage of MSCs ought to take into consideration these microenvironment clues during treatment. Alteration in these factors could function as prognostic indicators during the treatment of patients with IVDD using MSCs. Thus, standardized valves for these microenvironment clues are warranted.
1. Introduction
Intervertebral disc degeneration (IVDD) is one of the main triggers of low back pain, which is most often associated with patient morbidity and high medical costs [1, 2]. IVDD triggers a wide range of pathologies and clinical syndromes such as paresthesia, weakness of extremities, and intermittent/chronic back pain [2]. Cell senescence of intervertebral discs (IVDs), which is an indication of degeneration was detected as early as age of eleven [3–5]. Cell senescence is one of the most prominent pathways via which IVD cells decrease, resulting in IVDD [3]. Also, histological proof of reduced blood supply to the vertebral endplates was observed in the second decade of life and more predominant in advanced ages [3, 6].
The most current promising treatment modality for IVDD is cell-based therapy because it has demonstrated to possess disc regenerative potential with very minimally invasions compared to surgery [7, 8]. This therapeutic modality targets disc inflammation via blockade of anomalous cytokine generation, disc rehydration, and height restoration via the stimulation of matrix anabolism, repopulating and restoring the disc native cells [7]. Disc cells, notochordal cells, and stem cells are the key types of cell therapy for the treatment of IVD regeneration [7, 9]. Bach et al. demonstrated that healthy notochordal cell-derived NP tissue matrix was capable of harnessing the notochordal cells regenerative potential and implicated it for biological IVD repair [10].
Mesenchymal stem cells (MSCs) have demonstrated to possess immunomodulatory functions as well as the capability of differentiating into chondrocytes, which forms cartilage, making these cells potentially epitome for intervertebral disc (IVD) regeneration under appropriate microenvironment conditions [7, 11, 12]. Studies demonstrated that, MSCs obtained from bone marrow as well as adipose tissue were capable of differentiating into nucleus pulposus (NP)-like phenotype such as collagen fibrils and proteoglycan [11, 13]. Also, coculture trials of MSCs with NPCs revealed proliferation of the NPCs as well as differentiation of the MSCs into chondrogenic cells [7, 14]. Grafted MSCs were capable of restoring IVDD back to the normal disc milieu via the stimulation of the generation of extracellular matrix proteins such as aggrecan, proteoglycan, and collagen type-II, which constitute NPCs [7, 11, 15, 16].
The niche of the IVD milieu, which is often composed of the niche cells itself as well as their chemical and physical milieu, has been demonstrated to have a robust influence on MSC behavior as well as differentiation [17]. Nevertheless, the influence of MSCs by the IVD milieu conditions in healthy as well as IVDD situations is still a matter of debate. It is still not clear, which factors, if any, are essential for effective and efficient MSC survival, proliferation, and differentiation. Intradiscal injection of MSCs is a promising minimally invasive method of treating IVDD [18]. This mode of administration of MSCs determines therapeutic efficacy at different stages of IVDD [18].
This review thus edifies the immunomodulatory roles of MSCs at the microenvironment of IVDD. The “Boolean logic” was used to search for an article on the subject matter. Most of the articles were indexed in PubMed and/or PMC with strict inclusion criteria being the immune players that are influenced by MSCs during transplantation in human or animal models of IVDD. The search teams were a combination of MSCs and/or the immunome players, which constitute headings in the paper.
2. Anatomy of the Intervertebral Disc and Degeneration
The IVD is composed of three interrelated connective tissue components such as the NP, annulus fibrosus (AF), and hyaline cartilaginous endplates (CEPs) [1, 19]. The NP is specifically made up of hydrated tissue composed of copious proteoglycans and relatively low collagen content and located within the core of the disc for compression resistance and torsion, respectively. A study revealed that the glycosaminoglycan to collagen ratio was about 27:1, while the ratio of CEPs in contrast was about 2:1 in the NP of young adults [19]. It was also established that hydration of the NP triggered a swelling pressure that was opposed by the circumferential AF, which encloses the NP compartment and constitutes a sprouting junction with adjacent vertebrae [19].
The AF consists of concentric, collagenous lamellae intertwined with proteoglycan-rich septae [19, 20]. Histologically, the AF is separated into two zones including the inner collagen type-II as well as proteoglycan-rich zone and the outer collagen type-I-rich zone by a categorized modification [19, 20]. Morphologically dissimilar annulocytes have been observed at the cellular level [19, 20]. These cell categories include cells with widespread sinuous processes situated within the inner AF, cells with wide and branching processes precisely located in the interlamellar space of the outer AF, and interlocked cord-like cells situated at the outer zones [19, 20]. The hyaline CEPs are located at the boundary between the disc tissue and vertebral bodies. The vertebral bodies are porous and allow diffusion of molecules delivered via the extra-discal capillary beds [19, 21].
The NP, AF, and CEPs units as well as neighboring vertebral bodies form a polyaxial diarthrodial joint [19, 22]. This composition permits integrated movements and can deform along six degrees of freedom [19, 22]. The IVD is an avascular tissue and sections of the NP are found 8 mm from the closest blood supply [3, 6]. Nevertheless, the IVDs are fed via two distinct capillary plexuses. The first feeds the outer AF while the second originates from the vertebral bodies and feeds the bone-cartilage junction [3, 6]. Nutrition such as glucose, oxygen, and macromolecules are driven by a diffusion gradient through spinal movements. Anaerobic metabolism is often observed within the NP because the cells within it have low oxygen (O2) tension due to the limited blood supply [3]. Furthermore, the milieu of the NP has higher levels of lactic acid and a lower pH than other sections of the disk, which influence cell function negatively [3].
The degenerative process of the IVD often starts with disc herniation, which is depicted with protrusion of the NP as a result of wear and tear, repetitive mechanical overloading, and trauma/injury into the spinal canal [19, 23]. In severe situations, the endplate may rupture and protrude into the vertebral marrow [19, 23]. The extrusion into the spinal canal or the vertebral marrow compresses on vertebral marrow and triggers immune cascades [19, 23]. Subsequently, the central spinal canal or spinal foramen via which spinal nerves exit the column becomes narrower (spinal stenosis) as a result of bony overgrowth [19, 24].
Furthermore, displacement of a vertebra over another, which is often referred to as degenerative spondylolisthesis, occurs [19, 25]. Moreover, the spinal curvature digresses aberrantly as the discs collapse and facet joints degenerate in an asymmetric manner, which is also referred to as degenerative scoliosis[19, 26]. Finally, discogenic back pain, which is triggered by the ingrowth of nociceptive nerve fibers along the outer AF and annular tears, occurs, and the patients at this point need urgent attention because of pain [19, 27].
3. Nucleopulpocytes (NPCys)
Nucleopulpocytes (NPCys) are chondrocyte-like round cells usually located within the NP [4, 28]. These cells are depicted with precise markers such as hypoxia-inducible factor (HIF) 1α, glioma-associated oncogene (Gli) 1 and 3, glypican 3 (GPC3), paired box 1 and forkhead box 1, NOTO, and brachyury as well as keratin (KRT) 8, 18, and 19 (Figure 1) [4, 29, 30]. MSCs are capable of differentiating into NPCy-like phenotypes. MSCs sustainability, as well as differentiation into a NPCy-like phenotype, was augmented by growth factors like transforming growth factor beta (TGF-β), platelet-derived growth factor (PDGF), insulin-like growth factor 1 (IGF-1), growth differentiation factor (GDF)-5, and GDF-6 as well as basic fibroblast-like growth factor (bFGF) (Figure 1) [4].
Figure 1.
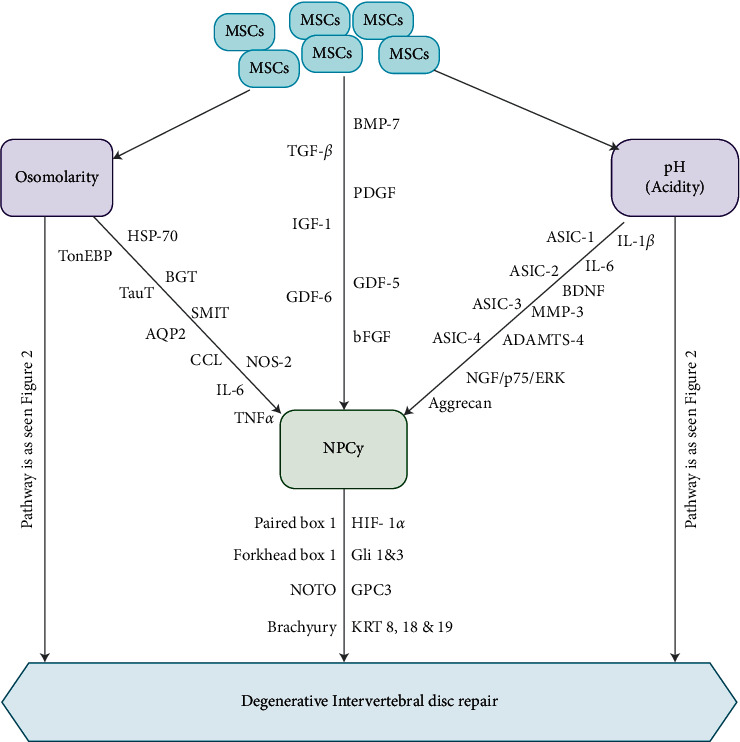
Illustration showing the pathway via which MSCs influence NPCy at the microenvironment leading to repair of IVDD. Factors such as TGF-β, GDF-5 and 6, BMP-7, PDGF, IGF-1, and βFGF are responsible for the differentiation of MSCs towards NPC-like cells. Also, acidity and osmolarity via diverse signaling pathways are key factors influencing the MSCs/NPC phenotype.
These molecules above are typically secreted by IVD resident cells. Nevertheless, cell density reduced and nutrient diffusion hampered; the extracellular membrane/matrix (ECM) turns out to be disrupted and their anabolic influence becomes nearly totally diminished [31]. Therefore, presubjecting MSCs in vitro to these growth factors before grafting or implanting them in MSC-loaded bio-frameworks could preserve their biological activity as well as enhance cell proliferation, differentiation, and synthetic potential [4, 31]. It is established that the potential of hydrogen (pH) changes are often detected by IVD cells via acid-sensing ion channels (ASICs), which regulate Ca2+ transmembrane influx upon instabilities of extracellular H+ levels [4].
It was observed that human IVD cells were capable of secreting ASIC isoforms such as ASIC-1, ASIC-2, and ASIC-3 as well as ASIC-4. ASIC-1 stimulation resulted in augmented Ca2+ cellular influx, leading to apoptosis of CEP chondrocytes (Figure 1) [4, 32]. Furthermore, ASIC-3 was implicated in NPCy survival under acidic as well as hyperosmolar microenvironments via the nerve growth factor (NGF)/p75/extracellular signal-related kinases (ERK) pathway (Figure 1) [4, 33]. A study revealed that ASIC secreted by NPCy and AF cells is mostly elevated in IVDD [34]. Gilbert et al. demonstrated that human NPCy viability was high, while proliferation was abrogated in an in vitro pH of 6.8 [35].
It was also noted that cells undergo necrosis at lower pH and express substantial quantities of proinflammatory cytokines such as IL-1β and ΙL-6, neurotrophic pain-related factors such as NGF, and brain-derived neurotrophic factor (BDNF) (Figure 1) [4]. Nevertheless, highly acidic culture milieu was associated with a reduction in aggrecan levels together with elevation of matrix metalloproteinases (MMP)-3, a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4, and ASIC-3, whilst ASIC-1 and ASIC-2 quantities remained unchanged. MSCs were capable of expressing IGF-1 and bone morphogenetic protein (BMP)-7, which protected NPCy against apoptosis (Figure 1) [4, 36].
NPCys are capable of detecting variations of osmolarity by stimulating the secretion of tonicity enhancer-binding protein (TonEBP) [4, 37, 38]. In a hypertonic milieu, the TonEBP, in turn, binds to the tonicity-responsive enhancer element (TonE) motif, leading to the elevation of genes like heat shock protein (HSP)-70, betaine/γ-aminobutyric acid transporter (BGT), sodium myo-inositol transporter (SMIT), taurine transporter (TauT), and aquaporin-2(AQP2), which are essential for the regulation of intracellular osmotic stress (Figure 1) [4, 37, 38]. TonEBP has been associated with the upregulation of proinflammatory cytokines stress such as C-C motif chemokine ligand (CCL), nitric oxide synthase 2 (NOS-2), interleukin (IL)-6, and TNFα under hypertonic (Figure 1) [4, 39].
It is affirmed that the hypertonic culture microenvironment decreased bone marrow-derived mesenchymal stem cell (BM-MSC) proliferation, anabolic marker secretion, and chondrogenic differentiation [4, 17]. Furthermore, adipose-derived mesenchymal stem cell (AD-MSC) viability and proliferation in addition to aggrecan and collagen type-I secretion were inhibited [4, 40]. Li et al. demonstrated that introduction of nucleus pulposus mesenchymal stem cells (NP-MSCs) to osmotic pressures that mimicked the healthy IVD environment led to a reduction in cell proliferation and chondrogenic differentiation via stimulation of the ERK pathway, while comparatively, the hypoosmotic microenvironment of mild IVDD revealed an upsurge of NP-MSC proliferation as well as chondrogenic potential [41].
4. Potential of Hydrogen (pH)
Several in vitro studies at low pH levels demonstrated that the frequencies of proteoglycan synthesis in IVD were reduced significantly [40, 42–44]. Furthermore, studies demonstrated that IVD-like pH triggered a substantial reduction in cell viability, cell proliferation, and aggrecan secretion of AD-MSCs, which means that pH was an essential factor inhibiting biological and metabolic potency of AD-MSCs [40, 42–44]. Also, the secretion of collagen type-I was not altered or marginally elevated under IVD-like pH, though the acidic pH triggered cell viability as well as proliferation (Figure 2) [17]. Moreover, the secretion of collagen type-I in AD-MSCs was not different when grafted into the degenerative IVD under a low pH [17]. Another study revealed that pH was an essential factor, restricting the use of MSCs for disc repair because in most cases, the pH in a sternly degenerated disc is usually as low as 5.7 [45].
Figure 2.
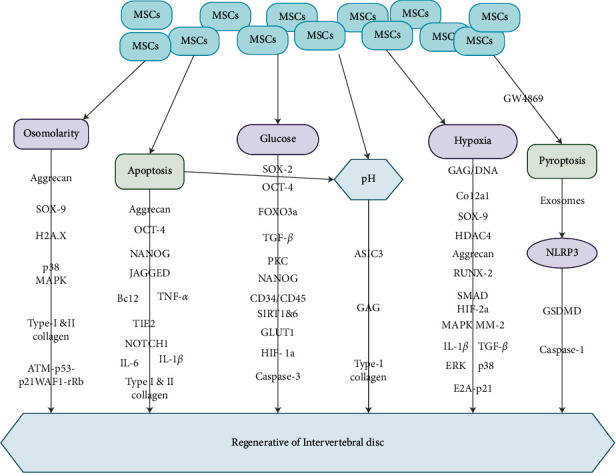
Illustration showing the various microenvironment clues and the pathways via which MSCs influence the repair of IVDD. MSCs influence osmolarity, apoptosis, glucose, pH, hypoxia, and pyroptosis via diverse signaling pathways contributing to the regeneration of intervertebral disc.
Studies have demonstrated that substantial MSCs reactions at a lower pH were observed in moderately or severely IVDD, especially considering the log nature of the pH scale [17, 45]. Nevertheless, when MSCs were subjected to pH values as low as 6.5 in a follow-up study, stern influence on gene expression, proliferation, and viability was observed [46]. Studies have also shown that bovine NPCs reduced the synthesis of sulfated GAG below pH 6.8 (Figure 2) [42, 44]. Bibby and Urban found a reduction in cell viability at pH to 6.7 and a clearer reduction at pH 6.2, particularly, when culturing cells under nutrient deficit microenvironments [47]. Therefore, ECM acidity was fundamental for MSC gene secretion and proliferation and may also speedup IVDD by negatively influencing resident cells [47]. Moreover, ASIC3 secretion by IVD cells was capable of facilitating IVD cells to adapt more easily to an acidic microenvironment compared to undifferentiated MSCs (Figure 2).
5. Osmolarity
Osmotic changes are evident in the physicochemical microenvironment with IVD cells or NPCs, as changes in disc mechanical burden resulted in substantial fluctuations in tissue hydration. Thus, osmotic pressure has a significant influence on the IVD cells' milieu [48, 49]. Mavrogonatou and Kletsas showed that high osmolarity reduced the proliferation frequency of NP IVD cells without loss of viability by a value of 500 mOsm/kg H2O, as well as their capability of novel DNA synthesis in a reversible manner [48]. They explained that the obvious blockade consequence could be due to a G2/M interruption taking place early after the introduction of high salinity complemented with a reduced S-phase population and G1 arrest [48].
The ECM of annulus in the physiological microenvironment contains aggrecan, which is the major type of proteoglycan and gylcosaminoglycan, which are negatively charged [40, 50]. These negative charges explain the high osmotic pressure of the IVD [40, 50]. It was established that compressing the IVD resulted in modifications in its hydration and osmolarity. The osmotic pressure in the NP ranges from 450–550 mOsm [51]. Mavrogonatou and Kletsas observed that augmented osmolarity triggered p38 MAPK and ATM-p53-p21WAF1-pRb pathway in IVD cells (Figure 2), which stemmed the cells in G2 and G1 phases of the cell cycle [48].
Mavrogonatou and Kletsas demonstrated that proliferation reduced considerably under the IVD-like high osmolarity [48]. They indicated that high osmolarity was capable of activating a p53-dependent DNA repair reactions such as phosphorylation and collection of histones H2A.X at the IVDD site [48]. Liang et al. observed that high osmolarity demonstrated to be an essential nutrient for the survival of IVD cells after AD-MSCs grafting [40]. They indicated that IVD-like high osmolarity substantially decreased the viability and proliferation of AD-MSCs [40]. They further stated that high osmolarity severely blocked the synthesis of aggrecan and collagen type-I (Figure 2) [40]. Nevertheless, initial studies demonstrated that hyperosmolarity had no substantial influence on proteoglycan content but upmodulated collagen type-I secretion in NP and AF cells [52].
Another study revealed that the quantity of proteoglycans was absolutely associated with the osmolality of ECM in IVD and the loss of proteoglycans resulted in the reduction of osmotic pressure during IVDD [53]. Furthermore, high osmolarity reduced the secretion of proteins that preserve the function of NP [53]. Tao et al. found a reduction of SRY-related HMG box (SOX)-9, aggrecan secretion in NPCs, downregulation of collagen type-II, and aggrecan secretion in NP-MSCs in high osmolarity (Figure 2) [54]. Their data suggest that IVD-like high osmolarity stimulated IVDD via the blockade of cell proliferation and ECM synthesis [54]. Similar studies observed the reduction in the number and viability of the IVD cells, particularly, NPCs together with loss of the IVD ECM as the triggers of IVDD [55, 56]. Further studies are needed in this direction to rectify conflicting finding on the effect of osmolarity on MSCs at the IVD microenvironment.
6. Glucose
Glucose levels are very crucial in adenosine triphosphate (ATP) production and ECM protein synthesis in the IVD because the cellular energy metabolism is predominant via anaerobic glycolysis [47, 57, 58]. Discrepancies in nutrient supply often result in the development of IVDD-associated pathological features such as cartilage endplate calcification and reduction in glucose concentrations [57]. A study revealed that glucose was capable of triggering senescence and diminishing osteogenic differentiation potential in classical plastic-adherent MSC [59]. Studies have shown that high glucose media or elevated plasma glucose concentrations trigger senescence and reduce proliferation frequencies and augmented apoptosis in rat MSCs [59–61].
Currently, media composed of 5.5 mM glucose are recommended and most often used for human MSCs in culture [62]. It is worth noting that serum glucose concentration in normal human beings is 5.55 mM [60, 63]. An augmented proliferation was observed in 25 mM glucose compared to 5 mM glucose in a study involving human umbilical cord blood-derived passaged MSC [64, 65]. Furthermore, SOX-2 secretary levels were augmented in 25 mM glucose, while octamer-binding transcription factor (OCT)-4 and FOXO3a secretory levels were not changed. Also, glucose triggered the TGF-β section via the protein kinase C (PKC) phosphorylation, resulting in the augmented proliferation of MSC (Figure 2) [60, 66]. Moreover, high glucose was capable of inhibiting growth factor initiation in rat multipotent adult progenitor cells, which are almost like MSCs [63].
Al-Qarakhli et al. demonstrated that MSCs under hyperglycemia exhibited insignificant NANOG, OCT-4 pluripotency, and CD34/CD45 hematopoietic markers (Figure 2) [67]. Liu et al. also demonstrated that NP-derived MSCs cultured in high glucose exhibited negligible secretion of stemness genes, mRNA, as well as protein secretion of silent information regulator protein 1 (SIRT1), SIRT6, glucose transporter 1(GLUT1), and HIF-1α, but augmented cell senescence, cell apoptosis, and caspase-3 secretion (Figure 2) [68].
Cheng et al. demonstrated that both adipose-derived stem cells (ADSCs) from diabetic donors (dADSCs) and nondiabetic donors (nADSCs) showed possibilities of transdifferentiation into neuron-like cells via a reactive oxygen species (ROS)-mediated mechanism when cultured in a suitable stimulation medium [1]. They further indicated that dADSCs or high-glucose-preserved nADSCs exhibited higher secretion of the pluripotent markers SOX-2, OCT-4, and nanog homeobox (NANOG) (Figure 2), whose effects were associated with ROS-mediated Akt inhibition [1].
Cheng et al. concluded that hyperglycemia has a slight influence on the identification markers but reduces the stemness of MSCs in most instances [1]. Markers for identification and stemness are key subjects of importance within the field of MSC senescence under hyperglycemic microenvironment [69]. Cheng et al. further established that the secretion of cell surface markers in dADSCs and nADSCs was analogous to that of MSCs [1]. Liang et al. demonstrated that IVD-like low glucose in the in vivo microenvironments may be a positive factor for AD-MSCs transplantation because it conserved cell viability as well as proliferation at comparatively normal levels and enriched the secretion of ECM proteins [40]. Further studies are needed in this direction to rectify conflicting finding on the effect of glucose on MSCs at the IVD microenvironment.
7. Hypoxia
Hypoxia is often referred to low O2 tension at the microenvironment of cells [70, 71]. The hypoxic microenvironment was capable of supporting the multipotentiality of a subpopulation of human bone marrow stromal cells (BMSCs) throughout osteogenic differentiation [70, 71]. Studies have demonstrated that human BMSCs boosted proliferative activity under the hypoxic microenvironment of 1.5–3% O2, compared to normoxia [71, 72]. It was further established that hypoxia enriched BMSC chondrogenic differentiation potential compared to normoxia [70, 71].
Also, there was an upsurge in proliferation rate compared to cells expansion under normoxia when ovine BMSCs were isolated and propagated under the hypoxia microenvironment of 5% O2 [70]. Furthermore, it was observed that ovine BMSCs isolated and expanded in hypoxic microenvironments, and successive chondrogenic differentiation in normoxia microenvironments showed an augmented chondrogenic phenotype relative to their counterparts after normoxia-intermediated isolation and expansion [70, 73].
Mueller et al. demonstrated that, under hypoxic milieu, the expansion culture of human BMSCs triggered chondrogenesis even under normoxic milieu [74]. Adesida et al. demonstrated that the BMSCs obtained via propagation, under low O2 tension, underwent a more vigorous chondrogenesis than their counterparts under normal O2 tension, albeit with dependence on the donor [70]. The robust chondrogenic capabilities were depicted with higher GAG per DNA content, enhanced transcript secretion of a group of chondrogenic genes such as aggrecan, collagen type-X alpha 1 chain (Col2a1). and SOX-9 and an extreme as well as even dissemination of collagen type-II and safranin O staining for sulfated proteoglycans (Figure 2) [70].
Studies have shown that transcriptional actions of SOX-9 augmented the gene promoter behaviors of aggrecan in chondrocytes [75, 76]. Furthermore, studies have demonstrated that transcriptional factors such as SOX-9, L-SOX-5, and SOX-6 are very crucial for the regulation of the secretion of Col2a1 and other genes associated with chondrogenesis (Figure 2) [75, 77]. Also, enhancement of chondrogenic potential under hypoxia was complemented with a simultaneous inhibition of Col10a1 secretion and a marker of hypertrophic chondrogenesis and a terminal differentiation of chondrocytes [70]. Hirao et al. demonstrated that, under hypoxia of 5% O2, enhanced chondrogenic differentiation of pluripotent MSCs line-like C3HT10T1/2 rather than osteogenesis via the machinery associated with simultaneous reduction of Col10a1 and runt-related transcription factor (RUNX)-2 activity through SMAD6 inhibition and histone deactylase 4 activation (HDAC4) (Figure 2) [78].
Adesida et al. indicate that the reaction of BMSCs to the hypoxic milieu is associated with the augmentation of transcriptional secretion of HIF-2α rather than HIF-1α, which remained uninterrupted [70]. Also, HIF-1α was involved in the hypoxia-mediated blockade of senescence and maintenance of human MSC properties [79]. It was further established that the reaction of the BMSCs to hypoxia was mediated by the transcriptional activity of HIF-2α [70]. Moreover, in the hypoxic milieu of 5% O2, HIF-2α triggered the secretion of chondrogenic genes such as Col2a1, aggrecan, and SOX-9 during chondrogenesis of AD-MSCs (Figure 2) [80].
Studies have shown that BM-MSCs and ADSCs are capable of augmenting cell viability and synthesis of ECM components in the hypoxic microenvironment of 1–5% O2 and low glucose microenvironment in vitro [4, 17, 40]. Studies have further demonstrated that BM-MSCs had a greater capability of developing colony-forming units, reduced osteogenic differentiation, and inhibited IL-1β (Figure 2) chondrogenic differentiation in the hypoxic microenvironment [81, 82]. Also, MSC senescence and conserved cell stemness via stimulation of telomerase activity through the HIF1α-twist-mediated downregulation of the E2A-p21 pathway (Figure 2) were inhibited in hypoxic microenvironment [4, 79].
These adaptive modalities above may enhance MSC survival in the hostile IVD milieu after grafting. Also, persistent exposure to stern hypoxia with O2 <1% together with serum deprivation led to total cell death [4, 83]. Hypoxia together with TGF-β1 (Figure 2) simultaneously triggered gene cassettes within the NPCs that encode for ECM and cell surface receptors. Furthermore, low O2 tension together with TGF-β1 activated ERK and p38 signaling pathways (Figure 2), resulting in eventual differentiation of MSC [4, 84].
Risbud et al. established that in the presence of hypoxia and TGF-β1, cultured MSCs secreted a phenotype coherent with NPCs via mitogen-activated protein kinase (MAPK) signaling pathways. Hypoxia triggered an upsurge of MM-2, collagen type-II and IX, and aggrecan secretion, while TGF-β1 treatment augmented collagen type-II and aggrecan gene secretion (Figure 2) [13].
8. Apoptosis
IVD cells undergo programmed cell death through one of three apoptosis pathways such as mitochondrial permeabilization of the outer membrane, death receptor, and endoplasmic reticulum pathways [3]. The maintenance of the IVD often depends on the integrity of the ECM [3]. It is well established that degenerative activities within the ECM microenvironment trigger an upsurge in catabolism, which internally triggers several biological modifications in the IVD cells, resulting in the stimulation of the different apoptosis pathways [3]. The various IVD cell apoptosis pathways are usually stimulated at different levels of the IVDD [3].
It was established that the mild stage of IVDD usually involves the endoplasmic reticulum pathway. Also, the mild/moderate stage of IVDD was usually characterized by the death receptor pathway, while the severe stage of IVDD was characterized by the mitochondrial pathway [3]. The stimulation of bcl-2 and blockade of caspase-3 are the parameters for measuring reduction in apoptosis, while higher HIF-1α and CXCR4 mRNA secretion are the parameters for measuring improvement in cell migration [85, 86].
Liu et al. established that human NP-MSCs exhibited a decrease in cell proliferation, augmented apoptosis, and decreased gene secretion of type-I and II collagen, aggrecan, and SOX-9, as well as of stemness-associated genes such as OCT-4, NANOG, JAGGED, and NOTCH1 in an in vitro study at a decreased pH of 7.4–6 (Figure 2) [36]. It was further established that NPCy underwent irreversible apoptosis via caspase-dependent mitochondrial pathways. Moreover, a specific kind of programmed cell death with necrosis-like qualities (necroptosis) was implicated in the compression-stimulated death of NPCy [4, 87].
It was also observed that the presence of AD-MSCs triggered a decrease in NPCy apoptosis via the blockade of caspase-3 and 9 as well as augmentation of ECM genes with decreased secretion of metalloproteinases and proinflammatory cytokines like IL-1β, IL-6, and TNFα in a coculture experiment involving AD-MSCs and NPCy at a prolonged compressive loading of 3 MPa for 48 h (Figure 2) [4, 88]. Although Wangler et al. did not indicate any higher fraction of apoptotic IVD cells following MSC migration [85], an upsurge in apoptotic cells was seen in bovine IVDs at day 5 of culture compared to newly (day 0) isolated IVDs, which could be due to associated nonphysiological ex vivo culture and potential nutrient deprivation under free swelling microenvironment [89].
Wangler et al. established a minimal but inconsequential decrease in apoptotic cells after MSC treatment, signifying a marginal effect of MSCs on IVD cell apoptosis [85]. They demonstrated that MSC homing augmented a proportion of tyrosine kinase endothelial receptor (TEK)-positive IVD cells in bovine as well as human IVD samples [85]. They further revealed that a proliferative reaction, as well as lower proportion of dead cells, was seen after MSC homing in both bovine as well as human IVD tissues [85]. Nevertheless, a positive correlation was detected between the upregulation of TEK as well as B-cell lymphoma 2 (Bcl2) transcripts (Figure 2) [86]. It was established that Bcl2 was observed as an antagonist of the apoptosis cascade because it counteracted the apoptosis-promoting factor Bax resulting in an upregulation of Bcl2 secretion, thus contributing to an enhanced survival of phenotypic IVD cells upon MSC homing [86].
9. Pyroptosis
Pyroptosis is another kind of programmed cell death, which is activated by inflammasomes like NOD-, LRR-, and pyrin domain-containing proteins (NLRP) and absent in melanoma 2 (AIM2)-like receptor proteins and tripartite motif-containing proteins [90]. Studies have shown that the cleavage of inflammatory mediators such as caspase-1 or caspase-11 results in the stimulation of cells to express proinflammatory cytokines such as IL-1β and IL-18 once these inflammasomes are activated [90, 91]. Zhang et al. established an IVDD model by IVD puncture and discovered that NLRP3-mediated NP cell pyroptosis was triggered in the succession of IVDD, with the activation of the NLRP-3 inflammasome and the elevation of caspase-1, cleaved gasdermin D (GSDMD), and augmented expression of cytokines IL-18 and IL-1β [90].
He et al. demonstrated that NLRP3-mediated NP cell pyroptosis was involved in the IVDD progression both in vivo and in vitro under the propionibacterium acnes activation [92]. Tang et al. also demonstrated that NLRP3 inflammasome was stimulated in NPCs after H2O2 activation [93]. Song et al. detected stimulation of NLRP-3 in patients. They indicated that dysregulation of NLRP3 was responsible for the development of IVDD [94]. Tavakoli Dargani et al. observed that doxorubicin exposure appreciably triggered the secretion of NLRP-3, which, in turn, stimulated pyroptosis in H9c2 cells [95, 96]. They indicated further that pyroptosis was inhibitable with the treatment of exosomes derived from embryonic stem cells [95]. They further established that exosome treatment exhibited an analogous effect in vivo, which was advantageous in ameliorating doxorubicin-stimulated cardiomyopathy [96].
Studies have shown that lipopolysaccharide (LPS) was capable of triggering an inflammatory response and facilitating proinflammatory cytokines buildup in NP cells [90, 97]. Lebeaupin et al. established that LPS was capable of stimulating NLRP3-induced pyroptosis in vivo [98]. Thus, NP cell pyroptosis is very crucial in the formation of IVDD. Zhang et al. detected that MSCs were capable of inhibiting NP cell pyroptosis blocking the secretion of NLRP3 in the LPS-induced model [90]. They indicated further that the anti-pyroptosis effect of MSCs was abrogated when they treated the MSCs with GW4869 to inhibit the secretion of exosomes (Figure 2) [90]. They concluded that the effect of MSCs on pyroptosis was mainly caused by its derived exosomes [90]. Zhang et al. ,therefore, determined novel machinery via which the downregulation of NLRP3 by exosomal miR-410 led to a reduction in caspase-1 and GSDMD, thereby blocking NP cell pyroptosis to ameliorate IVDD (Figure 2) [90].
10. Hydrogel
Hydrogels are the common biomaterial scaffolds for tissue engineering purposes due to their hydrated nature [99, 100]. Most hydrogels have the capability of mimicking the natural structure of in vivo tissue milieus [99, 100]. Hydrogels possess properties such as biocompatibility, a flexible method of formation, estimated physical features, offering of structural integrity to tissue building, fundamental structural, and compositional resemblances to ECM and a widespread framework, which offers cellular proliferation and survival [99, 100]. These properties make them the ultimate tissues for cell delivery and transient support for NP tissue regeneration [99, 100].
The amalgamation of human MSC at low O2 tension as well as mimicking the three-dimensional (3-D) ECM milieu via hydrogels was capable of augmenting the differentiation capabilities of human MSC towards an NP cell variety [99, 100]. Kumar et al. established that novel synthetic p(HEMA-co-APMA) gPAA hydrogel was able to encapsulate human MSC in situ and enhance the growth and differentiation of cartilage-like cells [99]. They observed a significant reduction in stiffness of NP tissue and detected modulus when human MSCs were cultured in human MSC or chondrogenic media after being encapsulated in hydrogels [99]. The reason for such occurrence was as a result of polymer degradation transpiring via hydrolysis [99].
Photocurable hydrogel together with hypoxic and chondrogenic culture media supported differentiation of human MSC towards a chondrocyte-like lineage and augment secretory levels of markers such as collagen II and aggrecan [99, 101]. Thus, by mimicking the structural milieu as well as the hydrated state of NP tissue using the hydrogel and by providing TGF-β1 in chondrogenic media together with a hypoxic milieu led to transcriptional changes in human MSC which resulted in an augmented secretion of aggrecan and collagen II leading to outside-in and inside-out signaling between the cells and their milieu, which ultimately resulted in the expression as well as production of NP-like tissue ECM [99, 101].
11. Conclusion
IVD microenvironment clues such as nucleopulpocytes, pH, osmotic changes, glucose, hypoxia, apoptosis, pyroptosis, and hydrogels are capable of influencing the MSCs during the treatment of IVDD. Therefore, clinical usage of MSCs ought to take into consideration these microenvironment clues during treatment. These microenvironment clues could function as prognostic indicators during the treatment of patients with IVDD using MSCs.
Abbreviations
- AF:
Annulus fibrosus
- ASICs:
Acid-sensing ion channels
- ADAMTS:
A disintegrin and metalloproteinase with thrombospondin motifs
- AQP2:
Aquaporin-2
- AD-MSC:
Adipose-derived mesenchymal stem cells
- ADSCs:
Adipose-derived stem cells
- ATP:
Adenosine triphosphate
- AIM2:
Absent in melanoma 2
- bFGF:
Basic fibroblast-like growth factor
- BDNF:
Brain-derived neurotrophic factor
- BMP:
Bone morphogenetic protein
- BGT:
Betaine/γ-aminobutyric acid transporter
- BMSCs:
Bone marrow stromal cells
- Bcl2:
B-cell lymphoma 2
- CEPs:
Cartilaginous endplates
- CCL:
C-C motif chemokine ligand
- Col2a1:
Collagen type-X alpha 1 chain
- ECM:
Extracellular membrane/matrix
- ERK:
Extracellular signal-related kinases
- Gli:
Glioma-associated oncogene
- GPC3:
Glypican 3
- GDF:
Growth differentiation factor
- GLUT1:
Glucose transporter 1
- GSDMD:
Gasdermin D
- HIF:
Hypoxia-inducible factor
- HSP:
Heat shock protein
- HDAC4:
Histone deactylase 4 activation
- IVD:
Intervertebral disc
- IVDD:
Intervertebral disc degeneration
- IGF-1:
Insulin-like growth factor 1
- IL:
Interleukin
- KRT:
Keratin
- LPS:
Lipopolysaccharide
- MSCs:
Mesenchymal stem cells
- MMP:
Matrix metalloproteinases
- MAPK:
Mitogen-activated protein kinase
- NP:
Nucleus pulposus
- NPCs:
Nucleus pulposus cells
- NPCys:
Nucleopulpocytes
- NGF:
Nerve growth factor
- NOS-2:
Nitric oxide synthase 2
- NP-MSCs:
Nucleus pulposus mesenchymal stem cells
- NANOG:
Nanog homeobox
- NLRP:
NOD-, LRR-, and pyrin domain-containing proteins
- OCT:
Octamer-binding transcription factor
- pH:
Potential of hydrogen
- PDGF:
Platelet-derived growth factor
- PKC:
Protein kinase C
- SMIT:
Sodium myo-inositol transporter
- SOX:
SRY-related HMG box
- SIRT1:
Silent information regulator protein 1
- ROS:
Reactive oxygen species
- RUNX:
Runt-related transcription factor
- TGF-β:
Transforming growth factor beta
- TonEBP:
Tonicity enhancer-binding protein
- TonE:
Tonicity-responsive enhancer element
- TauT:
Taurine transporter
- TEK:
Tyrosine kinase endothelial receptor
- 3-D:
Three-dimensional.
Data Availability
No data were used to support this paper.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
All authors contributed towards literature search, drafting, and critical revision of the paper and agreed to be accountable for all aspects of the work.
References
- 1.Chen X., Zhu L., Wu G., Liang Z., Yang L., Du Z. A comparison between nucleus pulposus-derived stem cell transplantation and nucleus pulposus cell transplantation for the treatment of intervertebral disc degeneration in a rabbit model. International Journal of Surgery . 2016;28:77–82. doi: 10.1016/j.ijsu.2016.02.045. [DOI] [PubMed] [Google Scholar]
- 2.Maher C., Underwood M., Buchbinder R. Non-specific low back pain. The Lancet . 2017;389(10070):736–747. doi: 10.1016/s0140-6736(16)30970-9. [DOI] [PubMed] [Google Scholar]
- 3.Dowdell J., Erwin M., Choma T., Vaccaro A., Iatridis J., Cho S. K. Intervertebral disk degeneration and repair. Neurosurgery . 2017;80(3s):S46–S54. doi: 10.1093/neuros/nyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vadalà G., Ambrosio L., Russo F., Papalia R., Denaro V. Interaction between mesenchymal stem cells and intervertebral disc microenvironment: from cell therapy to tissue engineering. Stem Cells International . 2019;2019:15. doi: 10.1155/2019/2376172.2376172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acosta F. L., Jr., Lotz J., Ames C. P. The potential role of mesenchymal stem cell therapy for intervertebral disc degeneration: a critical overview. Neurosurgical Focus . 2005;19(3):p. E4. doi: 10.3171/foc.2005.19.3.5. [DOI] [PubMed] [Google Scholar]
- 6.Benneker L. M., Heini P. F., Alini M., Anderson S. E., Ito K. Young investigator award winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine . 2004;30(2):167–173. doi: 10.1097/01.brs.0000150833.93248.09. [DOI] [PubMed] [Google Scholar]
- 7.Meisel H. J., Agarwal N., Hsieh P. C., et al. Cell therapy for treatment of intervertebral disc degeneration: a systematic review. Global Spine Journal . 2019;9(1 Suppl):39s–52s. doi: 10.1177/2192568219829024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei A., Shen B., Williams L., Diwan A. Mesenchymal stem cells: potential application in intervertebral disc regeneration. Translational Pediatrics . 2014;3(2):71–90. doi: 10.3978/j.issn.2224-4336.2014.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn J., Park E.-M., Kim B. J., et al. Transplantation of human Wharton’s jelly-derived mesenchymal stem cells highly expressing TGFβ receptors in a rabbit model of disc degeneration. Stem Cell Research & Therapy . 2015;6(1):p. 190. doi: 10.1186/s13287-015-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bach F. C., Tellegen A. R., Beukers M., et al. Biologic canine and human intervertebral disc repair by notochordal cell-derived matrix: from bench towards bedside. Oncotarget . 2018;9(41):26507–26526. doi: 10.18632/oncotarget.25476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakai D., Mochida J., Iwashina T., et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine (Phila Pa 1976) . 2005;30(21):2379–2387. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 12.Croft A. S., Illien-Jünger S., Grad S., Guerrero J., Wangler S., Gantenbein B. The application of mesenchymal stromal cells and their homing capabilities to regenerate the intervertebral disc. International Journal of Molecular Sciences . 2021;22(7) doi: 10.3390/ijms22073519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risbud M. V., Albert T. J., Guttapalli A., et al. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine (Phila Pa 1976) . 2004;29(23):2627–2632. doi: 10.1097/01.brs.0000146462.92171.7f. [DOI] [PubMed] [Google Scholar]
- 14.Vadalà G., Studer R. K., Sowa G., et al. Coculture of bone marrow mesenchymal stem cells and nucleus pulposus cells modulate gene expression profile without cell fusion. Spine (Phila Pa 1976) . 2008;33(8):870–876. doi: 10.1097/BRS.0b013e31816b4619. [DOI] [PubMed] [Google Scholar]
- 15.Hohaus C., Ganey T. M., Minkus Y., Meisel H. J. Cell transplantation in lumbar spine disc degeneration disease. European Spine Journal . 2008;17(Suppl 4):492–503. doi: 10.1007/s00586-008-0750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henriksson H. B., Svanvik T., Jonsson M., et al. Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine (Phila Pa 1976) . 2009;34(2):141–148. doi: 10.1097/BRS.0b013e31818f8c20. [DOI] [PubMed] [Google Scholar]
- 17.Wuertz K., Godburn K., Neidlinger-Wilke C., Urban J., Iatridis J. C. Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc. Spine (Phila Pa 1976) . 2008;33(17):1843–1849. doi: 10.1097/BRS.0b013e31817b8f53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shu C. C., Dart A., Bell R., et al. Efficacy of administered mesenchymal stem cells in the initiation and co-ordination of repair processes by resident disc cells in an ovine (Ovis aries) large destabilizing lesion model of experimental disc degeneration. JOR Spine . 2018;1(4) doi: 10.1002/jsp2.1037.e1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tessier S., Risbud M. V. Understanding embryonic development for cell-based therapies of intervertebral disc degeneration: toward an effort to treat disc degeneration subphenotypes. Developmental Dynamics . 2021;250(3):302–317. doi: 10.1002/dvdy.217. [DOI] [PubMed] [Google Scholar]
- 20.Bruehlmann S. B., Rattner J. B., Matyas J. R., Duncan N. A. Regional variations in the cellular matrix of the annulus fibrosus of the intervertebral disc. Journal of Anatomy . 2002;201(2):159–171. doi: 10.1046/j.1469-7580.2002.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fields A. J., Ballatori A., Liebenberg E. C., Lotz J. C. Contribution of the endplates to disc degeneration. Current Molecular Biology Reports . 2018;4(4):151–160. doi: 10.1007/s40610-018-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro I. M., Vresilovic E. J., Risbud M. V. Is the spinal motion segment a diarthrodial polyaxial joint: what a nice nucleus like you doing in a joint like this? Bone . 2012;50(3):771–776. doi: 10.1016/j.bone.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorth D. J., Shapiro I. M., Risbud M. V. Transgenic mice overexpressing human TNF-α experience early onset spontaneous intervertebral disc herniation in the absence of overt degeneration. Cell Death & Disease . 2018;10(1):p. 7. doi: 10.1038/s41419-018-1246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroeder G. D., Kurd M. F., Vaccaro A. R. Lumbar spinal stenosis. Journal of the American Academy of Orthopaedic Surgeons . 2016;24(12):843–852. doi: 10.5435/jaaos-d-15-00034. [DOI] [PubMed] [Google Scholar]
- 25.Bydon M., Alvi M. A., Goyal A. Degenerative lumbar spondylolisthesis. Neurosurgery Clinics of North America . 2019;30(3):299–304. doi: 10.1016/j.nec.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Wong E., Altaf F., Oh L. J., Gray R. J. Adult degenerative lumbar scoliosis. Orthopedics . 2017;40(6):e930–e939. doi: 10.3928/01477447-20170606-02. [DOI] [PubMed] [Google Scholar]
- 27.Fujii K., Yamazaki M., Kang J. D., et al. Discogenic back pain: literature review of definition, diagnosis, and treatment. JBMR Plus . 2019;3(5) doi: 10.1002/jbm4.10180.e10180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clouet J., Fusellier M., Camus A., Le Visage C., Guicheux J. Intervertebral disc regeneration: from cell therapy to the development of novel bioinspired endogenous repair strategies. Advanced Drug Delivery Reviews . 2019;146:306–324. doi: 10.1016/j.addr.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y.-C., Leung V. Y. L., Lu W. W., Luk K. D. K. The effects of microenvironment in mesenchymal stem cell-based regeneration of intervertebral disc. The Spine Journal . 2013;13(3):352–362. doi: 10.1016/j.spinee.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Li K., Kapper D., Youngs B., et al. Potential biomarkers of the mature intervertebral disc identified at the single cell level. Journal of Anatomy . 2019;234(1):16–32. doi: 10.1111/joa.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang F., Shi R., Cai F., Wang Y.-T., Wu X.-T. Stem cell approaches to intervertebral disc regeneration: obstacles from the disc microenvironment. Stem Cells and Development . 2015;24(21):2479–2495. doi: 10.1089/scd.2015.0158. [DOI] [PubMed] [Google Scholar]
- 32.Li X., Wu F. R., Xu R. S., et al. Acid-sensing ion channel 1a-mediated calcium influx regulates apoptosis of endplate chondrocytes in intervertebral discs. Expert Opinion on Therapeutic Targets . 2014;18(1):1–14. doi: 10.1517/14728222.2014.859248. [DOI] [PubMed] [Google Scholar]
- 33.Uchiyama Y., Cheng C.-C., Danielson K. G., et al. Expression of acid-sensing ion channel 3 (ASIC3) in nucleus pulposus cells of the intervertebral disc is regulated by p75NTR and ERK signaling. Journal of Bone and Mineral Research . 2007;22(12):1996–2006. doi: 10.1359/jbmr.070805. [DOI] [PubMed] [Google Scholar]
- 34.Cuesta A., Del Valle M. E., García-Suárez O., et al. Acid-sensing ion channels in healthy and degenerated human intervertebral disc. Connective Tissue Research . 2014;55(3):197–204. doi: 10.3109/03008207.2014.884083. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert H. T. J., Hodson N., Baird P., Richardson S. M., Hoyland J. A. Acidic pH promotes intervertebral disc degeneration: acid-sensing ion channel -3 as a potential therapeutic target. Scientific Reports . 2016;6:p. 37360. doi: 10.1038/srep37360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J., Tao H., Wang H., et al. Biological behavior of human nucleus pulposus mesenchymal stem cells in response to changes in the acidic environment during intervertebral disc degeneration. Stem Cells and Development . 2017;26(12):901–911. doi: 10.1089/scd.2016.0314. [DOI] [PubMed] [Google Scholar]
- 37.Tsai T.-T., Danielson K. G., Guttapalli A., et al. TonEBP/OREBP is a regulator of nucleus pulposus cell function and survival in the intervertebral disc. Journal of Biological Chemistry . 2006;281(35):25416–25424. doi: 10.1074/jbc.m601969200. [DOI] [PubMed] [Google Scholar]
- 38.Gajghate S., Hiyama A., Shah M., et al. Osmolarity and intracellular calcium regulate aquaporin2 expression through TonEBP in nucleus pulposus cells of the intervertebral disc. Journal of Bone and Mineral Research . 2009;24(6):992–1001. doi: 10.1359/jbmr.090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson Z. I., Shapiro I. M., Risbud M. V. RNA sequencing reveals a role of TonEBP transcription factor in regulation of pro-inflammatory genes in response to hyperosmolarity in healthy nucleus pulposus cells. Journal of Biological Chemistry . 2016;291(52):26686–26697. doi: 10.1074/jbc.m116.757732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang C., Li H., Tao Y., et al. Responses of human adipose-derived mesenchymal stem cells to chemical microenvironment of the intervertebral disc. Journal of Translational Medicine . 2012;10(1):p. 49. doi: 10.1186/1479-5876-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H., Wang J., Li F., Chen G., Chen Q. The influence of hyperosmolarity in the intervertebral disc on the proliferation and chondrogenic differentiation of nucleus pulposus-derived mesenchymal stem cells. Cells Tissues Organs . 2018;205(3):178–188. doi: 10.1159/000490760. [DOI] [PubMed] [Google Scholar]
- 42.Razaq S., Wilkins R. J., Urban J. P. G. The effect of extracellular pH on matrix turnover by cells of the bovine nucleus pulposus. European Spine Journal . 2003;12(4):341–349. doi: 10.1007/s00586-003-0582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ichimura K., Tsuji H., Matsui H., Makiyama N. Cell culture of the intervertebral disc of rats. Journal of Spinal Disorders . 1991;4(4):428–436. doi: 10.1097/00002517-199112000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Ohshima H., Urban J. P. The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine (Phila Pa 1976) . 1992;17(9):1079–1082. doi: 10.1097/00007632-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Diamant B., Karlsson J., Nachemson A. Correlation between lactate levels and pH in discs of patients with lumbar rhizopathies. Experientia . 1968;24(12):1195–1196. doi: 10.1007/bf02146615. [DOI] [PubMed] [Google Scholar]
- 46.Wuertz K., Godburn K., Iatridis J. C. PH dose response of mesenchymal stem cells: investigation of a critical factor for intervertebral disc repair. Translation Orthopedic Research Society . 2008;33 [Google Scholar]
- 47.Bibby S. R. S., Urban J. P. G. Effect of nutrient deprivation on the viability of intervertebral disc cells. European Spine Journal . 2004;13(8):695–701. doi: 10.1007/s00586-003-0616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mavrogonatou E., Kletsas D. High osmolality activates the G1 and G2 cell cycle checkpoints and affects the DNA integrity of nucleus pulposus intervertebral disc cells triggering an enhanced DNA repair response. DNA Repair . 2009;8(8):930–943. doi: 10.1016/j.dnarep.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Sivan S., Neidlinger-Wilke C., Würtz K., Maroudas A., Urban J. P. Diurnal fluid expression and activity of intervertebral disc cells. Biorheology . 2006;43(3-4):283–291. [PubMed] [Google Scholar]
- 50.Lai W. M., Mow V. C., Sun D. D., Ateshian G. A. On the electric potentials inside a charged soft hydrated biological tissue: streaming potential versus diffusion potential. Journal of Biomechanical Engineering . 2000;122(4):336–346. doi: 10.1115/1.1286316. [DOI] [PubMed] [Google Scholar]
- 51.Urban J. P. The role of the physicochemical environment in determining disc cell behaviour. Biochemical Society Transactions . 2002;30(Pt 6):858–864. doi: 10.1042/bst0300858. [DOI] [PubMed] [Google Scholar]
- 52.Haschtmann D., Stoyanov J. V., Ferguson S. J. Influence of diurnal hyperosmotic loading on the metabolism and matrix gene expression of a whole-organ intervertebral disc model. Journal of Orthopaedic Research . 2006;24(10):1957–1966. doi: 10.1002/jor.20243. [DOI] [PubMed] [Google Scholar]
- 53.Ishihara H., Warensjo K., Roberts S., Urban J. P. Proteoglycan synthesis in the intervertebral disk nucleus: the role of extracellular osmolality. American Journal of Physiology . 1997;272(5 Pt 1):C1499–C1506. doi: 10.1152/ajpcell.1997.272.5.C1499. [DOI] [PubMed] [Google Scholar]
- 54.Tao Y.-Q., Liang C.-Z., Li H., et al. Potential of co-culture of nucleus pulposus mesenchymal stem cells and nucleus pulposus cells in hyperosmotic microenvironment for intervertebral disc regeneration. Cell Biology International . 2013;37(8):826–834. doi: 10.1002/cbin.10110. [DOI] [PubMed] [Google Scholar]
- 55.Heathfield S., Le Maitre C., Hoyland J. Caveolin-1 expression and stress-induced premature senescence in human intervertebral disc degeneration. Arthritis Research and Therapy . 2008;10(4):p. R87. doi: 10.1186/ar2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Maitre C., Freemont A., Hoyland J. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Research and Therapy . 2007;9(3):p. R45. doi: 10.1186/ar2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cisewski S. E., Zhang L., Kuo J., et al. The effects of oxygen level and glucose concentration on the metabolism of porcine TMJ disc cells. Osteoarthritis and Cartilage . 2015;23(10):1790–1796. doi: 10.1016/j.joca.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holm S., Maroudas A., Urban J. P. G., Selstam G., Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connective Tissue Research . 1981;8(2):101–119. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- 59.Stolzing A., Sellers D., Llewelyn O., Scutt A. Diabetes induced changes in rat mesenchymal stem cells. Cells Tissues Organs . 2010;191(6):453–465. doi: 10.1159/000281826. [DOI] [PubMed] [Google Scholar]
- 60.Stolzing A., Bauer E., Scutt A. Suspension cultures of bone-marrow-derived mesenchymal stem cells: effects of donor age and glucose level. Stem Cells and Development . 2012;21(14):2718–2723. doi: 10.1089/scd.2011.0406. [DOI] [PubMed] [Google Scholar]
- 61.Stolzing A., Coleman N., Scutt A. Glucose-induced replicative senescence in mesenchymal stem cells. Rejuvenation Research . 2006;9(1):31–35. doi: 10.1089/rej.2006.9.31. [DOI] [PubMed] [Google Scholar]
- 62.Sotiropoulou P. A., Perez S. A., Salagianni M., Baxevanis C. N., Papamichail M. Cell culture medium composition and translational adult bone marrow-derived stem cell research. Stem Cells . 2006;24(5):1409–1410. doi: 10.1634/stemcells.2005-0654. [DOI] [PubMed] [Google Scholar]
- 63.Sugimoto R., Enjoji M., Kohjima M., et al. High glucose stimulates hepatic stellate cells to proliferate and to produce collagen through free radical production and activation of mitogen-activated protein kinase. Liver International . 2005;25(5):1018–1026. doi: 10.1111/j.1478-3231.2005.01130.x. [DOI] [PubMed] [Google Scholar]
- 64.Hong S. H., Gang E. J., Jeong J. A., et al. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochemical and Biophysical Research Communications . 2005;330(4):1153–1161. doi: 10.1016/j.bbrc.2005.03.086. [DOI] [PubMed] [Google Scholar]
- 65.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy . 2003;5(6):485–489. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 66.Ryu J. M., Lee M. Y., Yun S. P., Han H. J. High glucose regulates cyclin D1/E of human mesenchymal stem cells through TGF-beta1 expression via Ca2+/PKC/MAPKs and PI3K/Akt/mTOR signal pathways. Journal of Cellular Physiology . 2010;224(1):59–70. doi: 10.1002/jcp.22091. [DOI] [PubMed] [Google Scholar]
- 67.Al-Qarakhli A. M. A., Yusop N., Waddington R. J., Moseley R. Effects of high glucose conditions on the expansion and differentiation capabilities of mesenchymal stromal cells derived from rat endosteal niche. BMC Molecular and Cell Biology . 2019;20(1):p. 51. doi: 10.1186/s12860-019-0235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y., Li Y., Nan L. P., et al. The effect of high glucose on the biological characteristics of nucleus pulposus-derived mesenchymal stem cells. Cell Biochemistry and Function . 2020;38(2):130–140. doi: 10.1002/cbf.3441. [DOI] [PubMed] [Google Scholar]
- 69.Yin M., Zhang Y., Yu H., Li X. Role of hyperglycemia in the senescence of mesenchymal stem cells. Frontiers in Cell and Developmental Biology . 2021;9 doi: 10.3389/fcell.2021.665412.665412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adesida A. B., Mulet-Sierra A., Jomha N. M. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Research & Therapy . 2012;3(2):p. 9. doi: 10.1186/scrt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.D’Ippolito G., Diabira S., Howard G. A., Roos B. A., Schiller P. C. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone . 2006;39(3):513–522. doi: 10.1016/j.bone.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 72.Grayson W. L., Zhao F., Bunnell B., Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochemical and Biophysical Research Communications . 2007;358(3):948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 73.Krinner A., Zscharnack M., Bader A., Drasdo D., Galle J. Impact of oxygen environment on mesenchymal stem cell expansion and chondrogenic differentiation. Cell Proliferation . 2009;42(4):471–484. doi: 10.1111/j.1365-2184.2009.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Müller J., Benz K., Ahlers M., Gaissmaier C., Mollenhauer J. Hypoxic conditions during expansion culture prime human mesenchymal stromal precursor cells for chondrogenic differentiation in three-dimensional cultures. Cell Transplantation . 2011;20(10):1589–1602. doi: 10.3727/096368910X564094. [DOI] [PubMed] [Google Scholar]
- 75.Lefebvre V., Huang W., Harley V. R., Goodfellow P. N., de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Molecular and Cellular Biology . 1997;17(4):2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bi W., Deng J. M., Zhang Z., Behringer R. R., de Crombrugghe B. Sox9 is required for cartilage formation. Nature Genetics . 1999;22(1):85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 77.Lefebvre V., Behringer R. R., de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis and Cartilage . 2001;9(Suppl A):S69–S75. doi: 10.1053/joca.2001.0447. [DOI] [PubMed] [Google Scholar]
- 78.Hirao M., Tamai N., Tsumaki N., Yoshikawa H., Myoui A. Oxygen tension regulates chondrocyte differentiation and function during endochondral ossification. Journal of Biological Chemistry . 2006;281(41):31079–31092. doi: 10.1074/jbc.m602296200. [DOI] [PubMed] [Google Scholar]
- 79.Tsai C.-C., Chen Y.-J., Yew T.-L., et al. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood . 2011;117(2):459–469. doi: 10.1182/blood-2010-05-287508. [DOI] [PubMed] [Google Scholar]
- 80.Khan W. S., Adesida A. B., Hardingham T. E. Hypoxic conditions increase hypoxia-inducible transcription factor 2α and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Research and Therapy . 2007;9(3):p. R55. doi: 10.1186/ar2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grayson W. L., Zhao F., Izadpanah R., Bunnell B., Ma T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. Journal of Cellular Physiology . 2006;207(2):331–339. doi: 10.1002/jcp.20571. [DOI] [PubMed] [Google Scholar]
- 82.Felka T., Schäfer R., Schewe B., Benz K., Aicher W. K. Hypoxia reduces the inhibitory effect of IL-1β on chondrogenic differentiation of FCS-free expanded MSC. Osteoarthritis and Cartilage . 2009;17(10):1368–1376. doi: 10.1016/j.joca.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 83.Potier E., Ferreira E., Meunier A., Sedel L., Logeart-Avramoglou D., Petite H. Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Engineering . 2007;13(6):1325–1331. doi: 10.1089/ten.2006.0325. [DOI] [PubMed] [Google Scholar]
- 84.Chakravarthy K., Chen Y., He C., Christo P. J. Stem cell therapy for chronic pain management: review of uses, advances, and adverse effects. Pain Physician . 2017;20(4):293–305. doi: 10.36076/ppj.2017.305. [DOI] [PubMed] [Google Scholar]
- 85.Wangler S., Peroglio M., Menzel U., et al. Mesenchymal stem cell homing into intervertebral discs enhances the tie2-positive progenitor cell population, prevents cell death, and induces a proliferative response. Spine (Phila Pa 1976) . 2019;44(23):1613–1622. doi: 10.1097/BRS.0000000000003150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adams J., Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Current Opinion in Immunology . 2007;19(5):488–496. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma K., Chen S., Li Z., et al. Mechanisms of endogenous repair failure during intervertebral disc degeneration. Osteoarthritis and Cartilage . 2019;27(1):41–48. doi: 10.1016/j.joca.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 88.Sun Z., Luo B., Liu Z.-H., et al. Adipose-derived stromal cells protect intervertebral disc cells in compression: implications for stem cell regenerative disc therapy. International Journal of Biological Sciences . 2015;11(2):133–143. doi: 10.7150/ijbs.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang F., Zhao X., Shen H., Zhang C. Molecular mechanisms of cell death in intervertebral disc degeneration (review) International Journal of Molecular Medicine . 2016;37(6):1439–1448. doi: 10.3892/ijmm.2016.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang J., Zhang J., Zhang Y., et al. Mesenchymal stem cells-derived exosomes ameliorate intervertebral disc degeneration through inhibiting pyroptosis. Journal of Cellular and Molecular Medicine . 2020;24(20):11742–11754. doi: 10.1111/jcmm.15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jorgensen I., Rayamajhi M., Miao E. A. Programmed cell death as a defence against infection. Nature Reviews Immunology . 2017;17(3):151–164. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.He D., Zhou M., Bai Z., Wen Y., Shen J., Hu Z. Propionibacterium acnes induces intervertebral disc degeneration by promoting nucleus pulposus cell pyroptosis via NLRP3-dependent pathway. Biochemical and Biophysical Research Communications . 2020;526(3):772–779. doi: 10.1016/j.bbrc.2020.03.161. [DOI] [PubMed] [Google Scholar]
- 93.Tang P., Gu J.-M., Xie Z.-A., et al. Honokiol alleviates the degeneration of intervertebral disc via suppressing the activation of TXNIP-NLRP3 inflammasome signal pathway. Free Radical Biology and Medicine . 2018;120:368–379. doi: 10.1016/j.freeradbiomed.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 94.Song Y., Wang Y., Zhang Y., et al. Advanced glycation end products regulate anabolic and catabolic activities via NLRP3-inflammasome activation in human nucleus pulposus cells. Journal of Cellular and Molecular Medicine . 2017;21(7):1373–1387. doi: 10.1111/jcmm.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tavakoli Dargani Z., Singla D. K. Embryonic stem cell-derived exosomes inhibit doxorubicin-induced TLR4-NLRP3-mediated cell death-pyroptosis. American Journal of Physiology—Heart and Circulatory Physiology . 2019;317(2):H460–H471. doi: 10.1152/ajpheart.00056.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singla D. K., Johnson T. A., Tavakoli Dargani Z. Exosome treatment enhances anti-inflammatory M2 macrophages and reduces inflammation-induced pyroptosis in doxorubicin-induced cardiomyopathy. Cells . 2019;8(10) doi: 10.3390/cells8101224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guo Y., Tian L., Liu X., He Y., Chang S., Shen Y. ERRFI1 inhibits proliferation and inflammation of nucleus pulposus and is negatively regulated by miR-2355-5p in intervertebral disc degeneration. Spine (Phila Pa 1976) . 2019;44(15):E873–E881. doi: 10.1097/BRS.0000000000003011. [DOI] [PubMed] [Google Scholar]
- 98.Lebeaupin C., Proics E., de Bieville C. H. D., et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death & Disease . 2015;6(9) doi: 10.1038/cddis.2015.248.e1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kumar D., Gerges I., Tamplenizza M., Lenardi C., Forsyth N. R., Liu Y. Three-dimensional hypoxic culture of human mesenchymal stem cells encapsulated in a photocurable, biodegradable polymer hydrogel: a potential injectable cellular product for nucleus pulposus regeneration. Acta Biomaterialia . 2014;10(8):3463–3474. doi: 10.1016/j.actbio.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 100.Slaughter B. V., Khurshid S. S., Fisher O. Z., Khademhosseini A., Peppas N. A. Hydrogels in regenerative medicine. Advanced Materials . 2014;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stoyanov J., Gantenbein-Ritter B., Gantenbein-Ritter B., et al. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. European Cells and Materials . 2011;21:533–547. doi: 10.22203/ecm.v021a40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this paper.


