Abstract
A 59-year-old man with systemic sclerosis and interstitial pneumonia was referred to our department because he developed dyspnea and leg edema after receiving a first shot of coronavirus disease 2019 (COVID-19) vaccine. Transthoracic echocardiography showed moderate pericardial effusion with conspicuous fibrin deposition. Prednisolone was increased from 6 mg/day for systemic sclerosis to 20 mg/day. Thereafter, pericardial effusion gradually decreased. However, his symptoms continued. Transthoracic echocardiography showed disappearance of pericardial effusion and thickened pericardium. Pulsed-wave and tissue Doppler echocardiography revealed that the patient suffered from newly developed constrictive pericarditis. COVID-19 vaccination might have contributed to acute pericarditis and subsequent constrictive pericarditis in the present case of systemic sclerosis and pulmonary fibrosis.
Learning objective
Incidence of adverse effects after coronavirus disease 2019 vaccination is rare. The present case suggests the risk of pericarditis that may lead to constrictive pericarditis.
Keywords: Constrictive pericarditis, Coronavirus disease 2019 vaccine, Systemic sclerosis, Steroid
Introduction
On 23 June 2021, the US Centers for Disease Control and Prevention's safety committee stated there was a “likely association” between the Pfizer-BioNTech and Moderna coronavirus disease 2019 (COVID-19) vaccines and myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining around the heart) in some young adults [1]. We present a rare case of constrictive pericarditis in a 59-year-old man after the first shot of COVID-19 vaccine. He was successfully treated with increased prednisolone.
Case report
A 59-year-old man with systemic sclerosis and interstitial pneumonia was referred to our department because he developed dyspnea. He had been treated with 6 mg of prednisolone for the underlying diseases. He received the Pfizer mRNA vaccine a week before our consultation and had felt dyspnea five days after vaccination. His body temperature was 36.7 °C. Physical examination showed blood pressure was 123/83 mm Hg and pulse rate was 123 beats/min. Pulsus paradoxus was absent. Auscultation of the respiratory sounds revealed a fine crackle at the bilateral lung fields. The heart sounds were rather distant. There was evidence of pretibial edema.
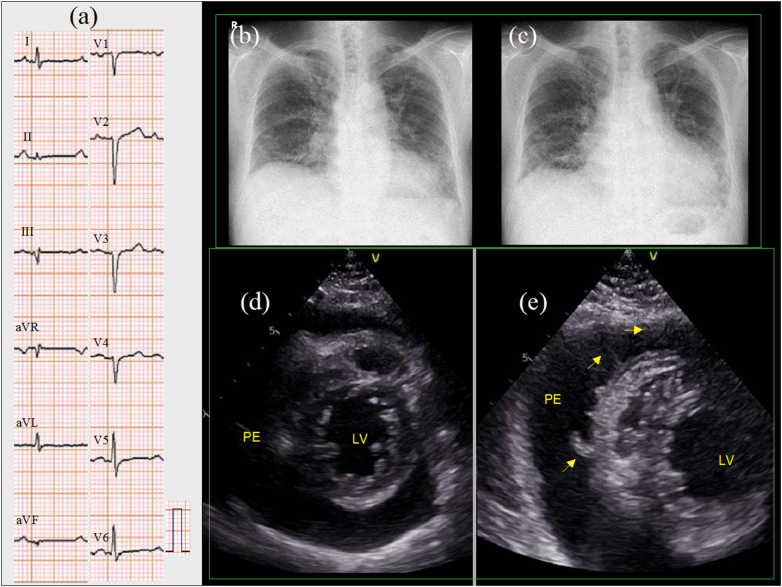
Results of blood tests showed hemoglobin level of 11.6 g/dL, serum creatinine phosphokinase level of 77 U/L (normal<248 U/L), serum C-reactive protein level of 3.4 mg/dL, and plasma brain natriuretic peptide level of 108 pg/mL. Plasma KL-6 level was 568 U/ml and did not increase from a month before. Serum cardiac troponin level was not measured. Thyroid function was normal. A 12-lead electrocardiogram (ECG) showed sinus rhythm with poor R wave progression in the precordial leads (Fig. 1a). One month before, a chest radiography showed pulmonary fibrosis with a cardiothoracic ratio (CTR) of 57% (Fig. 1b) that had not changed from six months previously. On admission, chest radiography showed generalized cardiac enlargement with a CTR of 61% (Fig. 1c). Transthoracic two-dimensional echocardiography showed normal left ventricular (LV) wall thickness (the interventricular septum and posterior wall were 8 mm, each). The LV diameter was small, and wall motion was normal (an LV end-diastolic dimension of 36 mm, end-systolic dimension of 22 mm, and LV ejection fraction of 62% by the modified Simpson method). There was moderate pericardial effusion (Fig. 1d). There was no collapse of cardiac chamber. A transthoracic echocardiography performed one year before showed that pericardial effusion was absent. Color flow imaging showed no significant valvular heart disease. Pulsed-wave Doppler echocardiography of mitral inflow showed an E wave of 65 cm/s, an A wave of 91 cm/s, and an E wave deceleration time of 152 ms. Tissue Doppler echocardiography at the septal annulus showed an e′ wave of 12 cm/s and an E/e′ ratio of 5.4 cm/s. The diameter of the inferior vena cava was 25 mm.
Fig. 1.
A 12-lead electrocardiogram showed sinus rhythm and poor R wave progression in the precordial leads (a). One month before, a chest radiography showed pulmonary fibrosis with a cardiothoracic ratio (CTR) of 57% (b). On admission, a chest radiography showed generalized cardiac enlargement with a CTR of 61% (c). Two-dimensional transthoracic echocardiography showed moderate pericardial effusion (d). One week after treatment with diuretics, a follow-up transthoracic echocardiography showed an increased pericardial effusion volume and conspicuous fibrin deposition (e, arrows).
PE, pericardial effusion; LV, left ventricle.
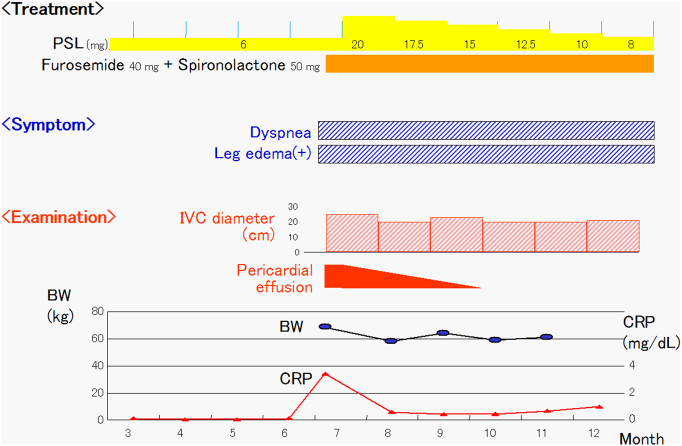
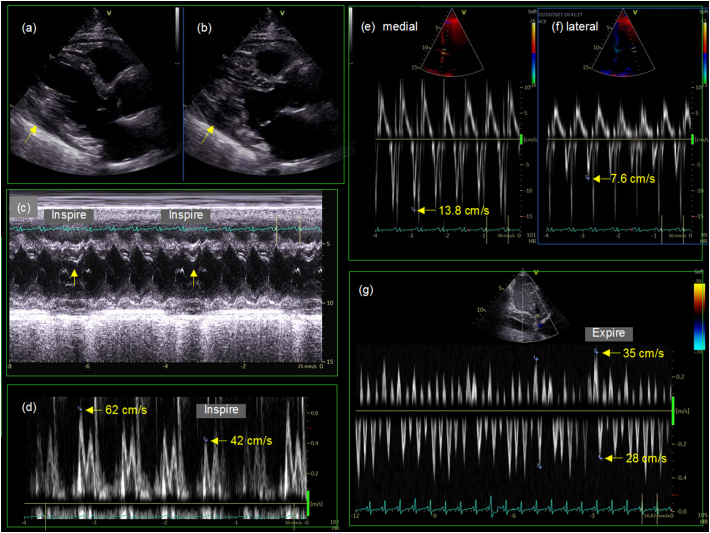
One week later, a follow-up transthoracic echocardiography showed an increased volume of pericardial effusion and conspicuous fibrin deposition (Fig. 1e, arrows). Prednisolone was increased from 6 mg/day for systemic sclerosis to 20 mg/day. Thereafter, his body weight decreased, C-reactive protein (CRP) level decreased, and the pericardial effusion eventually disappeared within 3 months (Fig. 2). A 12-lead ECG showed increased voltage of QRS complex. However, his symptoms did not improve and inferior vena cava remained dilated. A transthoracic echocardiography showed that pericardial effusion had completely disappeared and that pericardium was thickened to 8 mm (Fig. 3a, b, arrows). An M-mode echocardiography at the left ventricle showed septal bounce, which means movement of the interventricular septum to the left ventricle during inspiration (Fig. 3c). A pulsed-wave Doppler echocardiography at the mitral inflow showed inspiratory decrease in E wave velocity (Fig. 3d). Medial (Fig. 3e) and lateral (Fig. 3f) mitral annular tissue Doppler recording showed increased early relaxation velocity (e′ wave) with medial velocity greater than lateral (annulus reversus). A pulsed-wave Doppler recording within the hepatic vein showed prominent diastolic flow reversal in expiration (Fig. 3g). These findings made the diagnosis of constrictive pericarditis [2].
Fig. 2.
Clinical course in this case. In July, the patient complained of dyspnea and leg edema. A transthoracic echocardiography showed pericardial effusion. After treatment with diuretics and increased prednisolone, the body weight decreased and the pericardial effusion disappeared. However, symptoms and dilatation of inferior vena cava continued.
PSL, prednisolone; IVC, inferior vena cava; BW, body weight; CRP, C-reactive protein.
Fig. 3.
Three months later, a parasternal long-axis view during diastole (a) and systole (b) showed the disappearance of pericardial effusion. However, pericardium was thickened to 8 mm (a, b, arrows). An M-mode echocardiography at the left ventricle showed septal bounce, which means movement of the interventricular septum to the left ventricle during inspiration (c). A pulsed-wave Doppler echocardiography at the mitral inflow showed inspiratory decrease in E wave velocity (d). Medial (e) and lateral (f) mitral annular tissue Doppler recording showed increased early relaxation velocity (e′) with medial velocity greater than lateral (annulus reversus). A pulsed-wave Doppler recording within the hepatic vein showed prominent diastolic flow reversal in expiration (g). These findings were diagnostic of constrictive pericarditis.
Discussion
Immunosuppressive effect of corticosteroids may influence the effectiveness of vaccine response [3]. Doses >40 mg/day prednisone more than 1 week or ≥20 mg of prednisone for 2 weeks or more induce immunosuppression. As evidence showed that the response of inactivated vaccine could not be suppressed with the dose of up to 20 mg/day prednisolone, the present case safely received COVID-19 vaccine.
Although there was no ST-T change in ECG, pericardial effusion might be caused by acute pericarditis. Dyspnea and leg edema occurred 5 days after vaccination, cardiomegaly in chest radiography emerged within a month, high CRP level was present only on admission, and pericardial effusion was replaced by fibrotic tissue in 3 months, causing constrictive pericarditis.
The Vaccine Adverse Event Reporting System received 1226 preliminary cases of myocarditis and pericarditis after approximately 300 million doses (1 to 240,000) of the Pfizer or Moderna vaccines [1]. Although the incidence may be higher in male adolescents, a 59-year-old man with pericarditis after the first dose might be a rare case.
While the exact mechanisms of pericarditis following COVID-19 vaccinations are not elucidated, the vaccines might lead to cardiac inflammation. Vaccines for smallpox and influenza vaccines are known to cause myocardial and pericardial inflammation as well as systemic inflammation [4]. Moreover, pericarditis may occur in patients with systemic diseases such as autoreactive diseases or immune-mediated diseases [5]. In the present case of systemic sclerosis, there are possibilities that systemic sclerosis might have played a major role or have cooperated with COVID-19 vaccine in pericarditis.
Pericarditis caused by the COVID-19 vaccine may be mild and is treatable with non-steroid anti-inflammatory drugs. However, in the present case, a transthoracic echocardiography revealed multiple pericardial fibrin deposition within a week, suggesting massive inflammation and fibrotic change of pericardium. Although there has been no report about prevention of constrictive pericarditis, steroid has been used to treat it. Therefore, we increased prednisolone against worsening of fibrosis of pericardium. In the present case of systemic sclerosis that was under treatment with prednisolone (6 mg daily), an increment in prednisolone treatment might be preferential to non-steroidal anti-inflammatory drugs for acute pericarditis.
In spite of increased prednisolone, symptoms continued and findings of constrictive pericarditis developed. Constrictive pericarditis after COVID-19 vaccination might be very rare. Nakanishi et al. reported that a 70-year-old woman with pulmonary fibrosis developed constrictive pericarditis after severe acute respiratory syndrome coronavirus 2 vaccination [6]. Although the exact mechanisms of constrictive pericarditis after vaccination are unclear, it may be possible that pulmonary fibrosis might be a risk factor of constrictive pericarditis after COVID-19 vaccination.
Conclusion
We report a case of acute pericarditis and subsequent constrictive pericarditis following COVID-19 vaccination in a patient with systemic sclerosis in spite of increasing prednisolone administration.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgments
We would like to thank Mr Yoshiharu Saito and Ms Yuko Sakami for taking echocardiographic images.
References
- 1.Wise J. Covid-19: should we be worried about reports of myocarditis and pericarditis after mRNA vaccines? BMJ. 2021;373 doi: 10.1136/bmj.n1635. [DOI] [PubMed] [Google Scholar]
- 2.Welch T.D., Ling L.H., Espinosa R.E., Anavekar N.S., Wiste H.J., Lahr B.D., et al. Echocardiographic diagnosis of constrictive pericarditis: Mayo Clinic criteria. Circ Cardiovasc Imaging. 2014;7:526–534. doi: 10.1161/CIRCIMAGING.113.001613. [DOI] [PubMed] [Google Scholar]
- 3.Shokouhi S., Hakamifard A. COVID-19 vaccine and corticosteroids: a challenging issue. Eur J Inflamm. 2021;19:1–2. [Google Scholar]
- 4.Vidula M.K., Ambrose M., Glassberg H., Chokshi N., Chen T., Ferrari V.A., et al. Myocarditis and other cardiovascular complications of the mRNA-based COVID-19 vaccines. Cureus. 2021;13 doi: 10.7759/cureus.15576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khandaker M.H., Espinosa R.E., Nishimura R.A., Sinak L.J., Hayes S.N., Melduni R.M., et al. Pericardial disease: diagnosis and management. Mayo Clin Proc. 2010;85:572–593. doi: 10.4065/mcp.2010.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakanishi Y., Honda S., Yamano M., Kawasaki T., Yoshioka K. Constrictive pericarditis after SARS-CoV-2 vaccination: a case report. Int J Infect Dis. 2022;116:238–240. doi: 10.1016/j.ijid.2022.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]