Abstract
One of the most urgent needs worldwide is to vaccinate against SARS-CoV-2 as many people as possible. We evaluated humoral response to Comirnaty vaccine in Thalassemia Major patients (TM). We measured SARS-CoV-2–specific antibodies against Spike protein in 57 TM patients and 58 healthy blood donors (HBD). TM and HBD subjects revealed a homogeneous serological response to the Comirnaty (Mean ± SD; TM = 1917,21 ± 1384,49; HBD = 2039,81 ± 1064,44; p = 0,5884). No statistically significant differences were observed among two groups. Interestingly, we observed in 73.3% of asplenic patients Ab-S titres above 800 BAU, whereas only in 26% of non splenectomized patients showed Ab-S titres above 800 BAU). This differences were statistically significant p < 0.039. Further measurement on other Ab types was needed for better understanding humoral response to Comirnaty.
Keywords: Thalassemia major, Sars-Cov 2, Vaccination, Humoral response
The efficiency of the humoral response to the new vaccines against SARS-CoV-2 is currently a topic of great scientific relevance and represents an important aspect to monitor viral spread and reduce the access in intensive care unit [1,2] Anti-Spike antibodies (anti-S ab) and anti-Nucleocapsid antibodies (anti-N ab) are, at the current time, the best tools to monitor humoral response to SARS-CoV-2 infection [3,4]. Vaccination-induced immunity in comparison to natural infection-induced immunity, provides a strong reason why in vulnerable patients vaccination is needed.
Most of the studies describing humoral response to natural infection suggest that the persistence of these antibodies is limited to few months [5,6].
Therefore, one of the most urgent needs worldwide is to vaccinate against SARS-CoV-2 as many people as possible.
Although previous studies do not indicate a greater severity of Covid-19 in β-thalassemia patients, it is necessary to limit infection risk, not only for virus pathogenic effects, but to avoid in symptomatic patients the interruption of iron chelation therapy [7].
Moreover, an increased susceptibility to SARS-CoV-2 infection has been described in unvaccinated β-thalassemia heterozygous subjects, this evidence highlights the importance of vaccination in TM patients [8].
Furthermore, monitoring the vaccinated population is the main tool to understand the effectiveness of vaccines and particularly their usability by the most fragile categories of patients. In the population affected by cancer, diabetes, as well as in cardiovascular, hematological, or lung diseases, it is essential to evaluate and monitor the eventually side effects of vaccine.
Understanding the extent of the humoral response in these patients may also represent an important element to evaluate the effectiveness of vaccines recall. Within the subjects affected by hematological disorders, Thalassemia Major patients (TM), characterized by severe chronic hemolytic anemia and multiple organs impairment, can be included among fragile populations.
Pathophysiological alterations observed in TM patients, such as iron overload or the relative frequent splenectomy, combined with the side effects induced by repeated blood transfusion have been linked to a greater risk of infections [9,10].
1. Study design and results
In our study, TM patients were boosted with BNT162b2 (Comirnaty) a mRNA vaccine, produced by Pfizer-Biontech. The study was approved by the local Ethics Committee (0498/2021), conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice. Written informed consent was obtained from all study participants before enrolment. We proposed anti-Covid 19 vaccination to 105 Thalassemic patients (median age 39,4 range 26–70; F/M 54/51). Among 105 patients, 90 of them were - transfusion-dependent (TDT) and 15 were Non-transfusion-dependent (NTDT) -. Only 76 of the 105 patients (72%) accepted to join the vaccination program.
29 out of 105 patients didn't get vaccinated:10 were under 18 years, 9 patients couldn't get the vaccine for unknown reasons and 10 disagreed to the immunization protocol. Among the 76 vaccinated patients, 11 were previously infected with Sars-Cov2 and received only a single-dose of vaccine. Thus, they have been excluded from the study.
Finally, 57 patients all TDT (median age of 41.3 ± 9.05; 33 male and 24 female) were enrolled in our study.
TM have been matched with 58 healthy blood donors (HBD), (median age 38.28 ± 1.616; 31 male and 27 female). All participants signed informed consent at the beginning of the study. According to the international protocol, all participants were subjected to two doses vaccine inoculation separated by 21 days. A month after receiving the second dose, blood samples were collected to evaluate antibody titers in response to vaccine. We measured SARS-CoV-2–specific antibodies against the receptor binding domain (RBD) in the S1 subunit of the Spike protein (pan-Ig anti–S1-RBD) by using quantitative Elecsys anti SARS-CoV-2 ROCHE automated system with a sensitivity of 98.8% (95% CI 98.1–99.3) and a specificity of 99,98% (95%CI 99,91%). Measurement range 0,4<>250 U/mL. A cut-off index ≥0,8 U/ml is regarded as positive. All results were expressed as WHO international standard binding antibody unit (BAU) for mL. Descriptive data were presented as mean, standard deviation, frequency, and percentage. Chi-square test were used to compare qualitative variables between the two groups. Quantitative variables were compared by Student t-test between the two groups; p-value of 0.05 or less was considered statistically significant. Analyses were done using Statistical Package for the Social Science (SPSS) software version 24 (SPSS Inc., Chicago, Illinois, USA) or GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA, USA). TM and HBD subjects revealed a homogeneous serological response to the Comirnaty (Mean ± SD; TM = 1917,21 ± 1384,49; HBD = 2039,81 ± 1064,44; p = 0,5884). No statistically significant differences were observed among two groups (Fig. 1 ). Next, we investigated a possible correlation between anti-S Ab titres and pathophysiological alterations detectable in thalassemic patients. For this purpose, we arbitrarily grouped Thalassemic patients in two clusters: one with anti-S Ab titer below 800 BAU/mL and the second one with anti-S ab above 800 BAU/mL [3]. According to this partition we compared Ab titers (above and below 800 BAU/mL) vs biochemical parameters such as: ferritin, hemoglobin, vitamin D value neutrophils and lymphocytes counts. No statistically significant variation has been observed. Interestingly, among the TDT patients enrolled in the study, we observed that 73.3% of splenectomized TDT patients showed anti-S ab titers in the second quartile, while non-splenectomized TDT patients have anti-S ab titers below 800 BAU/mL (I quartile). Data reported are summarized in Table 1 . Overall, anti Sars-CoV-2 vaccination appeared to be well accepted in the assayed population of thalassemic patients.
Fig. 1.
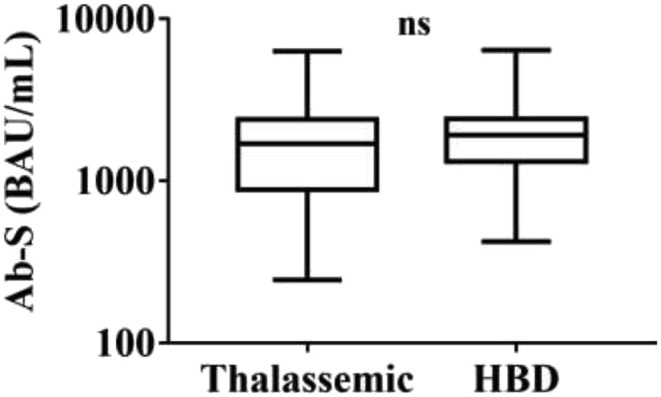
Ab-S (BAU/mL) Statistical analysis was carried out using t test. No statistically significant differences was observed among two groups.
Table 1.
Data and characteristics of patients.
| Parameters | Ab-S < 800 (n = 12) | Ab-S > 800 (n = 45) | P-value |
|---|---|---|---|
| Transfusion-Dependent (%) | 100 | 95.6 | 0.457 |
| Splenectomy (%) | 41.7 | 73.3 | 0.039∗ |
| No Splenectomy (%) | 58.3 | 26.7 | 0.039∗ |
| Supplemented vitamin D (%) | 100 | 88.9 | 0.227 |
|
Hemoglobin (g/dl) Mean ± SD |
9.60 ± 0.42 | 11.47 ± 12.75 | 0.340 |
|
Serum ferritin (ng/mL) Mean ± SD |
815.25 ± 613.59 | 825.33 ± 631.63 | 0.835 |
| >2000 (%) | 8.3 | 6.7 | 0.841 |
∗Statistically significance difference between groups.
After receiving the first dose, TDT patients experienced no symptoms, except for a local swelling in the deltoidal region. After receiving the second dose, only five patients presented fever and, among them, two patients showed superficial ancillary lymphadenopathy.
There were no notable side effects in the patients, one month following the second vaccine dose. In the present study, we observed that the administration of Comirnaty vaccine against SARS-Cov-2 induced a robust production of immunoglobulin levels, especially in asplenic patients arising several issues concerning the unusual humoral immune response in this vulnerable population. It is known that the immune response after splenectomy, as the main biological alteration, prompts the deficiency of memory B cells [11]. Spleen and peripheral lymphoid tissue show common immunological properties therefore, in asplenic individuals, peripheral lymphatic tissue and bone marrow could compensate for its missing immunological functions [12]. Indeed, increased production of antibodies in asplenic thalassemia patients could be supported by unknown biological pathways of the immune system. It's notable that to date the knowledge available on the new mRNA vaccine technology are limited. Thus, future studies to design optimal vaccination protocol in fragile individuals including TM patients are needed.
2. Conclusion
The spleen plays an important role in regulating innate and adaptive immunity and protecting against infections. Therefore, in asplenic individuals, peripheral lymphatic tissue and bone marrow could compensate for the missing immunological functions of the spleen. Low levels of anti SARS-CoV-2 Spike-ab in response to Comirnaty observed in no-splenectomized TDT patient could be a consequence of functional hyposplenism caused by disease itself [13]. Summarizing, this pilot study demonstrated that TDT patients are good responders to Comirnaty in terms of clinical outcomes and humoral response. No correlation has been observed with common biomarkers used in the evaluation of Sars-CoV-2 infected subjects and with usual parameters used in thalassemia evaluation disease (data not shown). Interestingly, splenectomy seems to correlate with a higher titer of antibodies to the Spike viral protein, although further studies are needed to confirm this finding. In the six months following the vaccination plan, no patient turned subsequently infected with Sars-Cov 2.
Our study has some strengths: this is the first study examining a large cohort of thalassemia patients with no comorbidities affecting humoral response; the same vaccine protocol was adopted for all patients and finally identical withdrawal times were applied to all patients.
On the other hand, limitation of this study is owed to the short endpoint after the second dose of vaccine thus, the long-term immune response was not investigated. To this purpose, further studies are ongoing to evaluate cell-mediated immune response.
Conflict of interest statement
The authors declare no conflict of interests.
Acknowledgements
This study is founded by The University of Rome “Sapienza”. We are thankful to Giuseppina Gennarini, Barbara Colaprisca and Valentina Viggiani for their technical assistance.
References
- 1.Salvagno G.L., Henry B.M., di Piazza G., Pighi L., de Nitto S., Bragantini D., et al. Anti-spike S1 IgA, anti-spike trimeric IgG, and anti-spike RBD IgG response after BNT162b2 COVID-19 mRNA vaccination in healthcare workers. J Med Biochem. 2021;40(4):327–334. doi: 10.5937/jomb0-32373. PMID: 34616222; PMCID: PMC8451231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandini O., Criniti A., Ballesio L., Giglio S., Galardo G., Gianni W., et al. Serum ferritin is an independent risk factor for acute respiratory distress syndrome in COVID-19. J Infect. 2020;81(6):979–997. doi: 10.1016/j.jinf.2020.09.006. Epub 2020 Sep 15. PMID: 32946917; PMCID: PMC7490639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farina A., Labriola R., Ialongo C., Suppa M., Viggiani V., Lucarelli M., et al. Transient plasma cell dyscrasia in COVID-19 patients linked to IL-6 triggering. Microb Infect. 2021;23(4–5):104808. doi: 10.1016/j.micinf.2021.104808. Epub 2021 Mar 20. PMID: 33753206; PMCID: PMC7979272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anastasi E., Ialongo C., Labriola R., Ferraguti G., Lucarelli M., Angeloni A. Vitamin K deficiency and covid-19. Scand J Clin Lab Invest. 2020;80(7):525–527. doi: 10.1080/00365513.2020.1805122. Epub 2020 Aug 11. PMID: 32779537. [DOI] [PubMed] [Google Scholar]
- 5.Krishna E., Pathak V.K., Prasad R., Jose H., Kumar M.M. COVID-19 reinfection: linked Possibilities and future outlook. J Fam Med Prim Care. 2020;9(11):5445–5449. doi: 10.4103/jfmpc.jfmpc_1672_20. PMID: 33532377; PMCID: PMC7842419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021 Feb 18;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. Epub 2021 Jan 12. PMID: 33497610; PMCID: PMC7803150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bou-Fakhredin R., Daadaa H., Koussa S., Abou Nasr T., Noun P., Taher A.T. SARS-CoV-2 infection in patients with β-thalassemia: experience from Lebanon. Am J Hematol. 2021;96(8):E285–E288. doi: 10.1002/ajh.26211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sotiriou Sotirios, Samara Athina A., Vamvakopoulou Dimitra, Vamvakopoulos Konstantinos-Odysseas, Sidiropoulos Andreas, Vamvakopoulos Nikolaos, et al. Susceptibility of β-thalassemia heterozygotes to COVID-19. J Clin Med. 2021 Aug 18;10(16):3645. doi: 10.3390/jcm10163645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marziali M., Ribersani M., Losardo A.A., Taglietti F., Pugliese P., Micozzi A., et al. COVID-19 pneumonia and pulmonary microembolism in a patient with B-thalassemia major. Clin Case Rep. 2020 Sep 25;8(12):3139–3142. doi: 10.1002/ccr3.3275. Epub ahead of print. PMID: 33173583; PMCID: PMC7646639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motta I., Marcon A., Carrabba M.D., Cassinerio E., Migone De Amicis M., Branchi A., et al. Management of chronic patients during the COVID-19 pandemic: the experience of a referral center for rare hematological disorders in the hardest-hit region in Italy. Ann Hematol. 2021;100(8):2129–2131. doi: 10.1007/s00277-021-04442-x. Epub 2021 Feb 2. PMID: 33528610; PMCID: PMC7851802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Sabatino A., Carsetti R., Corazza G.R. Post-splenectomy and hyposplenic states. Lancet. 2011 Jul 2;378(9785):86–97. doi: 10.1016/S0140-6736(10)61493-6. Epub 2011 Apr 5. PMID: 21474172. [DOI] [PubMed] [Google Scholar]
- 12.Doğan S.M., Aykas A., Yücel E.Ş., Okut G., Şimşek C., Çayhan K., et al. Immune profile of asplenic patients following single or double vaccine administration: a longitudinal cross-sectional study. Ulus Cerrahi Derg. 2015 Apr 9;31(3):118–123. doi: 10.5152/UCD.2015.2822. PMID: 26504413; PMCID: PMC4605105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.William B.M., Corazza G.R. Hyposplenism: a comprehensive review. Part I: basic concepts and causes. Hematology. 2007;12(1):1–13. doi: 10.1080/10245330600938422. PMID: 17364987. [DOI] [PubMed] [Google Scholar]


