Abstract
Background:
Middle-aged subjects with meniscal tear treated with arthroscopic partial meniscectomy (APM) experience greater progression of damage to joint structures on imaging than subjects treated nonoperatively. It is unclear whether these changes are clinically relevant.
Methods:
We used data from the MeTeOR (Meniscal Tear in Osteoarthritis Research) Trial of APM vs. physical therapy for subjects ≥ 45 years with knee pain, cartilage damage, and meniscal tear. We assessed whether change in cartilage surface area damage score (and other structural measures) from baseline to 18 months, assessed on MRI with MOAKS (MRI Osteoarthritis Knee Score), was associated with change in KOOS Pain (Knee Injury and Osteoarthritis Outcome Score; 0–100, 100=worst) from 18–60 months.
Results:
The primary analysis included 168 subjects with complete MRI data at baseline and 18 months and KOOS data at 18 and 60 months. We did not observe clinically important associations between change in cartilage surface area score between baseline and 18 months and change in pain scores from 18–60 months. Pain scores in the worst tertile for cartilage surface area damage score progression worsened by 0.45 points more than in the best tertile (95% CI −4.45, 5.35). Similarly, we did not observe clinically important associations between changes in bone marrow lesions, osteophytes, or synovitis and subsequent pain.
Conclusions:
We did not observe clinically important associations between early changes in cartilage damage and other structural measures and worsening in pain over the subsequent 3.5 years. Further follow-up is required to assess this association over a longer follow-up period.
Introduction:
Knee pain with associated structural findings of non-traumatic damage of cartilage and other knee structures is prevalent and costly. Over 400,000 persons with this constellation of findings (which we shall term meniscal tear with osteoarthritic damage) undergo arthroscopic partial meniscectomy (APM) each year (1), and many more are managed nonoperatively. Trials comparing APM with conservative therapy in this clinical setting show that both approaches are associated with substantial and comparable symptom relief in intention to treat analyses (2–8). These trials provide some support for the recommendation to offer physical therapy (PT) as initial management for patients with knee pain, meniscal tear and osteoarthritic damage, with APM offered to patients who do not respond to PT (8).
Several reports have suggested that patients with knee pain, meniscal tear and osteoarthritic damage who undergo APM experience greater degenerative changes in imaging features after surgery than patients with the same condition treated with PT. It is unclear whether these changes are clinically meaningful. Collins et al. demonstrated that subjects in the MeTeOR (Meniscal tear in Osteoarthritis Research) Trial cohort who received APM had greater progression in cartilage surface area damage, osteophytes, and effusion-synovitis – though not in bone marrow lesions, or Hoffa synovitis -- than those who received PT (9). Similarly, Sonesson et al. reported that five years after randomization to APM or PT, 60% of those who received APM progressed by one grade or more on the Kellgren-Lawrence scale, as compared with 37% of those who received PT (10).
These findings are difficult to interpret because it is not clear whether short-term structural damage leads to subsequent worsening in knee pain or function. Further, if structural damage does ultimately translate to symptomatic worsening, the time course between structural change and subsequent clinical deterioration is unknown.
The question of whether imaging changes observed in cartilage, bone, or synovium portend clinically important symptomatic worsening is pertinent not just to patients with meniscal tear, but also to the larger population of persons with osteoarthritis (OA). The goal of this study is to use data from the MeTeOR Trial (Meniscal Tear in OA Research) -- a randomized controlled trial of APM vs. PT in persons with knee pain, meniscal tear, and osteoarthritic changes -- to assess whether worsening in MRI-assessed tissue damage over 18 months leads to subsequent worsening in knee pain over the subsequent 3.5 years.
Methods:
Design and sample:
MeTeOR is a seven-center randomized controlled trial of APM and PT vs. PT alone for subjects with knee pain, meniscal tear, and imaging (radiograph or MRI) evidence of damage to cartilage or an osteophyte. We did not require presence of radiographic features of OA (osteophytes or joint space narrowing) because MRI evidence of cartilage damage and other pathoanatomical features of OA may occur in the absence of radiographic changes. The MeTeOR entry and exclusion criteria, interventions, and data collection protocols have been described previously (3, 11). In brief, to be eligible individuals had to be ≥ 45 years old with ≥ 4 weeks of knee pain, at least one symptom often associated with meniscal tear (clicking, popping, catching, giving way, swelling, pain with pivot), meniscal tear documented on MRI, and evidence on radiographs or MRI of some cartilage damage or an osteophyte. We excluded persons with more than 50% joint space narrowing on standing radiographs, inflammatory arthritis, and those receiving Worker’s Compensation. Subjects were randomized to receive either APM with concomitant PT or PT alone. Subjects randomized to the PT arm were allowed to cross-over to APM, and some subjects randomized to APM did not undergo surgery. The PT regimen was clinic-based and focused primarily on open and closed chain strengthening exercises as well as mobilization and stretching. The program lasted 6–8 weeks and involved one or two visits to the therapist per week. (3, 11). The APM consisted of trimming damaged meniscus back to a stable rim (3, 11). We sent participants questionnaires at baseline, three months, and every six months thereafter through five years. The questionnaires included the Pain, Symptoms, and ADL scales from the Knee Injury and Osteoarthritis Outcome Score (KOOS) (12–14).
Participants had an MRI at baseline and were invited to undergo MRI at 18 months following randomization. The MRI protocol at each center included water-sensitive sequences. MRIs were read by experienced musculoskeletal radiologists who were blinded to treatment assignment but were aware of the order in which the scans were done. Readers used the MOAKS (MRI Osteoarthritis Knee Score) assessment scheme (15), which assesses a range of tissues (cartilage damage, bone marrow lesions, osteophytes, synovitis and effusion) at the subregion level. Most structures are assessed as 0 (no damage) to 3 (greater damage).
Outcomes:
The primary outcome for this analysis was the difference in KOOS Pain score between 18 and 60months following randomization. The KOOS Pain Scale contains 9 items scored 0 to 4; we summed the items (unweighted) and transformed them to a 0–100 scale, with 100 representing the most severe pain. Thus, a greater difference (60-month score minus 18-month score) indicates worsening pain. A secondary outcome was the difference in KOOS ADL score between 18 and 60 months following randomization.
Measures of structural damage:
The primary predictor was change in cartilage surface area score from baseline to 18-months post-randomization. We chose cartilage damage as the primary structural predictor because it generally changes unidirectionally, with gradual worsening. Synovitis and bone marrow lesions may wax and wane, such that subtracting baseline score from 18-month score may fail to capture the actual changes that occurred in these structures over 18 months. We did not include meniscus structure because over half of the cohort had arthroscopic partial meniscectomy, which could have influenced these meniscal structure ratings, conflating the structural variable with the treatment.
Each subregion was rated using MOAKS (15), according to the percentage of the total cartilage surface area in that subregion affected by cartilage damage of any depth (from superficial to full thickness): 0=none; 1=< 10% of surface area affected; 2=10–75%; 3= >75% of surface area affected. The cartilage surface area summary score is the sum of these 0–3 ratings across all 14 subregions in the tibiofemoral and patellofemoral joints, yielding a potential range of 0–42. Secondary predictors included the change from baseline to 18 months in total cartilage thickness score (14 subregions, range 0–42), total osteophyte score (12 subregions, range 0–36), bone marrow lesion score (14 subregions, range 0–42), and effusion- and Hoffa-synovitis (single score for the knee, range 0–3).
Potential confounders:
We considered a number of potential confounders (factors conceivably associated with both initial structural change and subsequent change in pain score) including participant age, sex, body mass index, 5-item mental health index (16, 17), KOOS Pain at 18 months, and self-report of other musculoskeletal areas limiting activity (e.g. contralateral knee, hips, back, neck, shoulders, upper extremities) (18).
Statistical analyses:
We performed our primary analysis in subjects with complete data on MRI scores at baseline and 18 months and on KOOS Pain at 18 and 60 months. The primary goal was to assess the relationship between change in cartilage surface area score between baseline and 18 months and subsequent change in KOOS Pain score from 18 to 60 months. To assess for selection bias, we examined baseline and 12-month demographic and pain score values on those excluded from the analysis (no MRI data), those with incomplete data due to loss-to-follow-up, total knee arthroplasty (TKA) or death, and those with complete data for this analysis.
We first examined the relationship between change in cartilage surface area damage score from baseline to 18 months and change in KOOS Pain from 18 to 60 months with scatterplots and assessed this association with the Pearson correlation coefficient. In addition, we presented the distribution of crude change in KOOS Pain from 18–60 months stratified by level of the predictor variables using boxplots. To facilitate this analysis, we categorized early change in cartilage surface area score into three groups -- 0–1, 2–3 and 4+ points -- with roughly equal number of subjects in each group. We consider a difference on the KOOS Pain score of 8–10 points as clinically meaningful(19).
We built a multiple linear regression model with the three-level specification of the cartilage surface area damage score as the predictor of interest, and the continuous difference from 18 to 60 months in KOOS Pain as the outcome. The regression model adjusted for age, sex, BMI, treatment arm, KOOS Pain at 18 months, and baseline levels of mental health (MHI-5) and number of musculoskeletal areas limiting activity.
In secondary analyses, we used the same approach outlined above to examine relationships between early changes in other structural indicators (cartilage thickness damage, osteophytes, bone marrow lesions, effusion-synovitis and Hoffa-synovitis) and change in KOOS Pain score. Effusion and Hoffa-synovitis are rated for the whole knee (not in multiple subregions); changes were categorized as improvement vs. no change vs. worsen. In addition, we examined the relationship between progression in each of the structural variables and change (from 18 months to 60 months) in KOOS ADL score, the secondary outcome, and in two specific items on the KOOS Pain scale – frequency of knee pain (scored from never to always) and amount of knee pain the last week when twisting or pivoting (scored from none to extreme).
We performed two sensitivity analyses. While the summary score approach allows us to capture all changes across all subregions, a consequence is that several small changes across multiple subregions may have the same difference in score as a larger change across one subregion. In sensitivity analysis we instead quantified the number of subregions with any worsening and created the categories of no subregions with worsening, 1 subregion, and 2+ subregions with worsening (9). Also, since the primary analysis was restricted to subjects with complete imaging and outcome data, we performed a sensitivity analysis including subjects who had at least one of the three MRIs done at baseline, 18 months and 60 months post-randomization. Using a multiple imputation approach (20), we created 10 sets of imputed data based on participants’ baseline information (age, sex, race, body mass index, intervention arm, Kellgren-Lawrence grade) and longitudinal follow-up data (self-reported scores from 0, 3, 6, 12, 18, 24, 30, 36, 42, 48, and 60 months and MRI data from 0, 18 and 60 months).
All analyses were conducted using SAS version 9.4 (SAS Institute, Cary NC).
Results:
Sample characteristics:
Of 351 subjects randomized to APM or PT arms of the MeTeOR Trial, 34 were excluded because they did not provide any MRI data. Among the remaining 317 subjects, 168 had complete data on both change in KOOS Pain from 18 to 60 months and change in MRI parameters from baseline to 18 months and were included in the primary analysis. 317 subjects were included in the sensitivity analysis, in which we imputed missing MRI and KOOS Pain data.
Subjects included in the primary analysis had mean age (SD) of 58 (7), 59% were female. Baseline KOOS Pain score was 46 (16). Twenty-nine percent had KL 0 or 1 radiographs, 40% had KL-2 radiographs and 31% had KL-3 (Table 1). Baseline demographic and KOOS Pain and KOOS ADL scores were similar among those included in the primary analysis (N=168), those excluded because they had no MRI data (N=34) and those included in the sensitivity analysis, but not the primary analysis because they did not have complete MRI data at baseline and 30 months or lacked KOOS Pain at either 18 or 60 months (N=149, Appendix Table 1). The 34 excluded subjects had worse 12-month pain and function scores and were more likely to have KL-3 radiographs than those included in the primary or sensitivity analyses (Appendix Table 1).
Table 1:
Baseline features of the study sample (N=168)
| Categorical Variables | N | % |
|---|---|---|
| Female | 99 | 59 |
| 3 | 52 | 31 |
| None* | 3 | 2 |
| Continuous Variables | Mean | SD |
| Age | 58 | 7 |
| Body mass index | 29 | 6 |
| KOOS Pain at baseline | 46 | 16 |
| KOOS ADL at baseline | 36 | 18 |
3 subjects were randomized to APM but did not receive surgery or formal PT
Changes in structural damage and in pain: Table 2 shows the mean summary scores for cartilage surface area damage, cartilage thickness, bone marrow lesions, osteophytes, and both Hoffa- and effusion-synovitis at baseline and 18 months, as well as the change in these parameters between baseline and 18 months. Table 2 also shows the mean KOOS Pain scores at baseline, 18 months and 60 months and the change in KOOS Pain between 18 and 60 months.
Table 2:
MOAKS scores for structural measures (mean, SD) at baseline and 18 months and KOOS Pain and ADL scores (mean, SD) at baseline, 18 months and 60 months
| Predictors | Baseline | 18 mo | 18 mo minus baseline |
|---|---|---|---|
| Cartilage surface area score | 8.8 (5.1) | 11.4 (5.9) | 2.6 (3.1) |
| Cartilage thickness score | 2.3 (2.8) | 3.8 (3.7) | 1.5 (2.1) |
| Osteophyte score | 6.5 (5.2) | 9.8 (5.9) | 3.3 (3.2) |
| Bone marrow lesion score | 2.9 (2.6) | 3.5 (3.5) | 0.7 (3.1) |
| Hoffa synovitis | 1.1 (0.7) | 0.9 (0.8) | −0.1 (0.7) |
| Effusion synovitis | 1.4 (0.9) | 1.2 (0.7) | −0.2 (0.8) |
| Outcomes | 18 mo | 60 mo | 60 mo minus 18 mo |
| KOOS Pain Score | 19.0 (17.2) | 16.8 (16.1) | −2.2 (13.9) |
| KOOS ADL Score | 13.0 (15.3) | 12.5 (14.8) | −0.5 (12.9) |
Cell values for cartilage surface area, cartilage thickness, bone marrow lesion, and osteophyte scores reflect mean scores (SD) across subregions
A scatterplot showing the relationship between change in cartilage surface area damage score from baseline to 18 months and the change in KOOS Pain between 18 and 60 months (Figure 1) shows that the correlation coefficient was −0.12 (95% −0.27, 0.028). Figure 2 shows the relationship between change in cartilage surface area damage score from baseline to 18 months and subsequent change in KOOS Pain using the ordinal specification in cartilage score. The mean differences in KOOS Pain scores were similar across categories of cartilage surface area damage change (mean difference 2.3 points (SD 12.1) for change in surface score of 0–1; mean difference −1.4 points (13.5) for change in surface area score of 2–3 and mean difference −2.6 points (17.1) for change in surface area ≥4).. These small differences observed across groups (Figure 2) were neither statistically significant, nor clinically meaningful (19).
Figure 1:
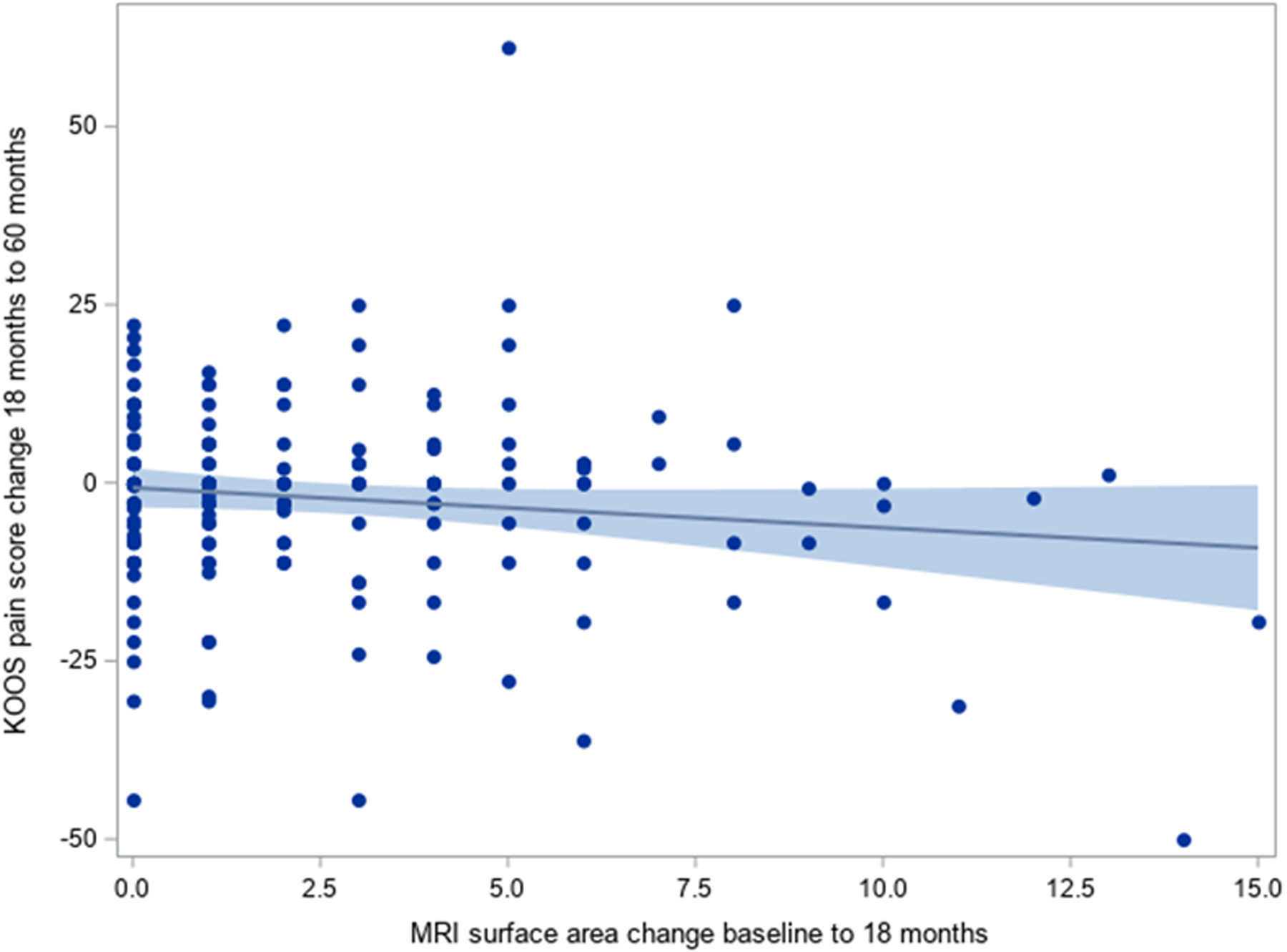
Association between change in cartilage surface area score (18 mo – baseline) and change in KOOS Pain (60 mo – 18 mo)
Higher values on the y-axis imply greater worsening in KOOS Pain from 18 to 60 months.
Pearson correlation −0.12 (95% CI −0.27, 0.028)
Figure 2:
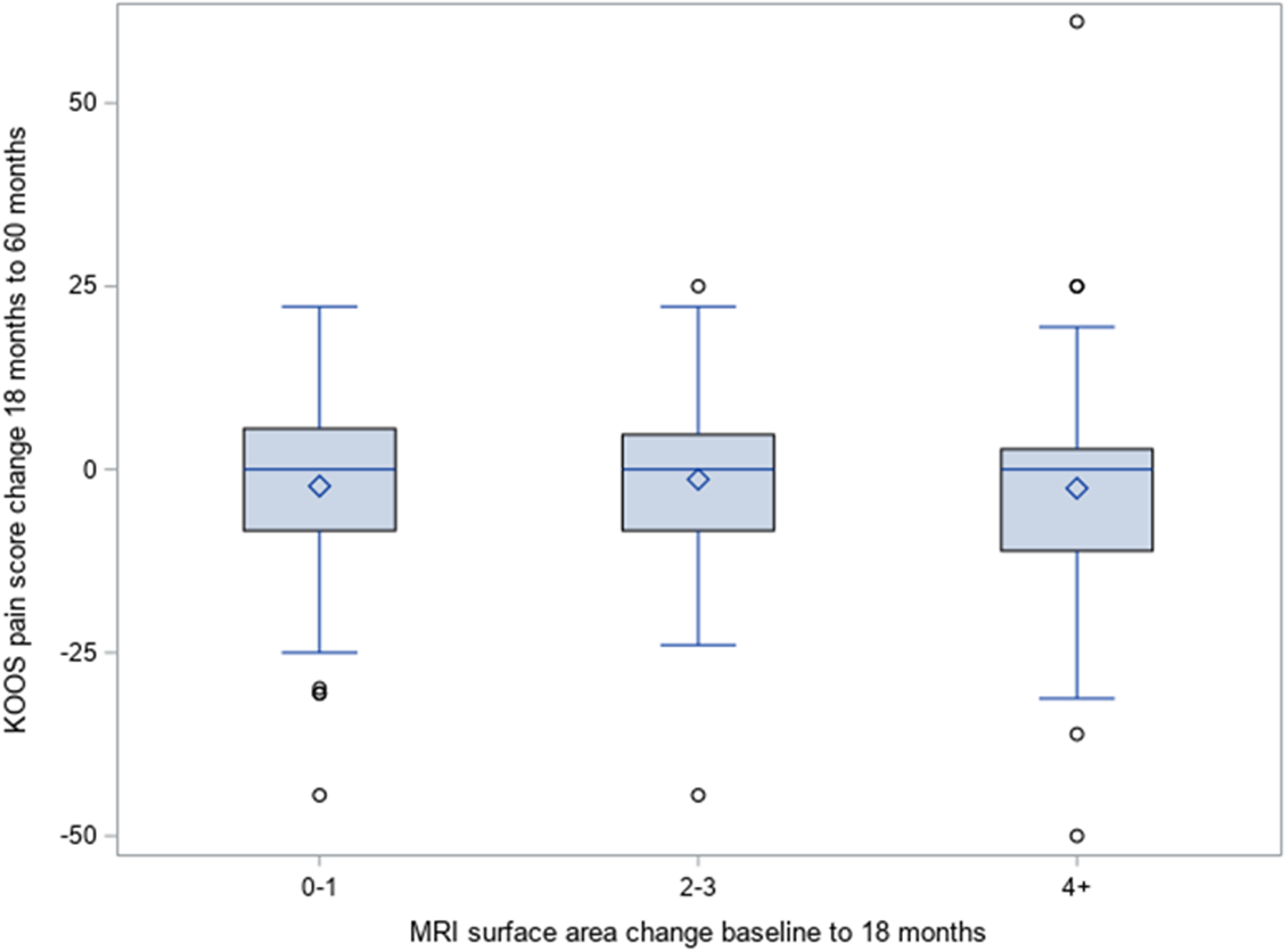
Association between change in cartilage surface area score between 0 and 18 months and worsening in KOOS Pain score between 18 and 60 months
* The length of the box represents the 25th and 75th percentiles; the horizontal line in the box interior represents the group median; the diamond symbol in the box interior represents the group mean.
Table 3 shows results of the multiple linear regression models of the association between changes in structural measures (cartilage surface area damage, cartilage thickness damage, osteophytes, bone marrow lesions, effusion-and Hoffa-synovitis) from 0–18 months and change in KOOS Pain from 18–60 months. We did not observe statistically significant associations between the change in any of the structural variables and subsequent change in KOOS Pain. For example, those with a 2–3 point increase (worsening) in cartilage surface area damage score from baseline to 18 months had a slightly greater increase (worsening) in KOOS Pain score between 18 and 60 months (1.36 points,(95% CI −3.91, 6.64)) as compared with subjects whose cartilage surface area damage score worsened by just 0–1 points from 0–18 months. Similarly, those with more than 4 points of worsening in their cartilage surface area damage score in the first 18 months experienced 0.45 points more worsening in KOOS Pain between 18 and 60 months (95% CI −4.45, 5.35), as compared with subjects whose cartilage surface area damage score worsened by just 0–1 points. In each of these comparisons, the confidence intervals included 0 and the magnitude of difference in KOOS Pain score was considerably less than the 8–10 points considered clinically meaningful.
Table 3:
Effect of structural progression between 0 and 18 months and worsening in KOOS scores between 18 and 60 months in multivariate models
| Structural domain | Structural change from 0 to 18 mo. | Adjusted difference in Change in KOOS Pain Score from 18 to 60 mo. | Adjusted difference in Change in KOOS ADL Score from 18 to 60 mo. | ||
|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | ||
| Cartilage surface area | 0–1 point worsening | Reference | Reference | ||
| 2–3 point worsening | 1.36 | −3.91, 6.64 | 3.04 | −1.91, 7.99 | |
| 4+ point worsening | 0.45 | −4.45, 5.35 | 3.68 | −0.90, 8.25 | |
| Cartilage surface area subregion | 0 subregion worsening | Reference | Reference | ||
| 1 subregion worsening | −0.68 | −6.06, 4.70 | 0.40 | −4.65, 5.46 | |
| 2+ subregions worsening | 0.75 | −4.15, 5.66 | 4.19 | −0.37, 8.75 | |
| Cartilage thickness | No worsening | Reference | Reference | ||
| 1–2 point worsening | 3.06 | −1.63, 7.74 | 3.61 | −0.82, 8.05 | |
| 3+ point worsening | −0.96 | −6.39, 4.47 | 0.30 | −4.74, 5.33 | |
| Osteophyte | 0–1 point worsening | Reference | Reference | ||
| 2–4 point worsening | −0.46 | −5.28, 4.37 | 2.33 | −2.23, 6.90 | |
| 5+ point worsening | 2.34 | −2.86, 7.54 | 4.95 | 0.12, 9.77 | |
| Bone marrow lesion | Improved | Reference | Reference | ||
| 0–1 point worsening | −0.03 | −5.01, 4.95 | 1.73 | −2.97, 6.43 | |
| 2+ point worsening | 1.78 | −3.82, 7.38 | 4.04 | −1.18, 9.26 | |
| Hoffa synovitis | Improved | 0.99 | −3.63, 5.62 | −0.23 | −4.58, 4.12 |
| No change | Reference | Reference | |||
| Worsen | 3.85 | −1.79, 9.48 | 4.62 | −0.67, 9.92 | |
| Effusion synovitis | Improved | 1.77 | −2.72, 6.26 | 2.42 | −1.83, 6.66 |
| No change | Reference | Reference | |||
| Worsen | −2.40 | −7.97, 3.17 | −1.59 | −6.84, 3.65 | |
Models adjusted for KOOS score at 18 mon, treatment arm, age at enrollment, gender, body mass index, MHI-5 Mental Health index, MSK functional limitations.
We used an analogous approach to that noted above to examine the association between worsening in other structural measures (full thickness cartilage damage, osteophytes, bone marrow lesions, and Hoffa-and effusion-synovitis) and worsening in KOOS Pain, as well as worsening in these measures and in KOOS ADL score. We did not observe statistically significant or clinically meaningful associations between worsening in these measures and subsequent KOOS Pain scores. Participants with the most severe changes in several structural measures did have 4- to 5-point more worsening in KOOS ADL score from 18 to 60 months compared to those with minimal structural changes, including 4.95-points more worsening (95% CI 0.12, 9.77) in KOOS ADL for those with the most severe worsening category in osteophyte score compared to the group with 0–1 points worsening (Table 2, Table 3). Change in cartilage surface area score from baseline to 18 months and change had very weak correlations with change in frequency of knee pain (r=−0.16), and with change in severity of knee pain with twisting/pivoting (r=−0.16).
Sensitivity analyses:
The results of the sensitivity analyses, performed on 317 subjects using imputation for missing variables, were largely similar to those of the primary analyses performed on the 168 with complete MRI and KOOS data. Specifically, these analyses did not show clinically important or statistically significant associations between changes in structural measures between 0 and 18 months and changes in KOOS Pain from 18 to 60 months (Appendix Tables 2 and 3). We also performed analyses using the imputed samples with the difference in KOOS ADL score between 18 and 60 months as the dependent variable. These findings are presented in Appendix Tables 2 and 3, and demonstrate no clinically important or statistically significant associations between changes in MRI parameters between 0 and 18 months and changes in KOOS ADL between 18 and 60 months.
To address the concern that summarizing structural measures over all subregions may not be as clinically meaningful as assessing within-subregion changes, we assessed whether each of the 14 subregions worsened from 18–60 months, and summed the number of subregions with within-subregion worsening. Similar to the primary analysis, this sum of within-subregion changes occurring between baseline and 18 months was not meaningfully or statistically significantly associated with changes in KOOS Pain or ADL from 18 to 60 months (Table 3).
Discussion:
Recent studies have suggested that middle-age and older persons having APM for knee pain and meniscal tear experience greater progression than persons treated nonoperatively in radiographic OA grade and in MRI evidence of cartilage damage, osteophytes, and effusion synovitis – though not bone marrow lesions and Hoffa synovitis (9, 10). To determine whether these short-term structural changes were clinically relevant, we examined the association between changes in cartilage damage, osteophytes, bone marrow lesions and Hoffa- and effusion-synovitis between baseline and 18 months and subsequent changes in knee pain from 18 to 60 months in the MeTeOR Trial cohort. We adjusted for potential confounders and did not identify clinically important or statistically significant associations between structural change over 18 months and subsequent (18 to 60 month) change in knee pain. We did observe that KOOS ADL scores worsened by 5 points in those with worsening of osteophyte score from baseline to 18 months. This difference is not generally regarded as clinically important(19).
We are not aware of other studies that have examined associations between structural damage following treatment of degenerative meniscal tear and subsequent changes in pain or functional status. Sonesson and colleagues documented greater progression over five years in Kellgren-Lawrence radiographic grade following APM than following PT but did not observe appreciable concomitant differences in symptoms between the APM and PT groups (10). Similarly, Sihvonen et al demonstrated greater radiographic progression (as assessed by the OARSI radiographic score) following APM than following sham meniscal resection, but did not observe differences in symptom severity between these two randomized groups over the same five year period (21). Collins et al noted greater progression in several MRI-defined structural parameters in MeTeOR trial subjects receiving APM than in those receiving PT. Symptomatic outcomes of the two treatment groups did not differ in as-treated or intention to treat analyses at five years (22). None of these studies directly assessed the question of whether those who progressed the most structurally had worse symptoms over a subsequent period of observation, irrespective of treatment assigned or received.
Our findings are relevant to discussions between clinicians and patients regarding treatment for knee pain, osteoarthritis and meniscal tear. While APM appears to be associated with greater MRI evidence of worsening in some (but not all) structural features (9), we did not find evidence that these structural changes are associated with worsening pain over the subsequent 3.5 years. We acknowledge that 3.5 years of follow up may be insufficient and that longer follow-up is necessary to document or exclude the possibility that structural damage gives rise to symptomatic worsening. We acknowledge that a third of MeTeOR participants did not meet the radiographic definition of OA -- grade two on the Kellgren-Lawrence scale -- requiring an osteophyte of joint space narrowing. Each subject had evidence on MRI of cartilage damage, indicating they had pathoanatomical features of OA.
These findings have implications not only for the management of meniscal tear but also for a foundational goal of OA treatment. The premise that structural damage portends subsequent clinical deterioration is fundamental to efforts to identify structure modifying osteoarthritis drugs. While cartilage is aneural, it is hypothesized (23) that breakdown of cartilage, meniscus and other load-bearing structures may increase load on subchondral bone, menisci and ligaments and inflame synovium – structures that are associated with pain (23). Longer follow-up of MeTeOR trial participants may further illuminate this key question of whether changes in structure are associated with subsequent changes in symptoms and help define the time course over which these changes in pain occur.
Our findings should be interpreted in the context of several limitations. First, 3.5 years may be insufficient follow-up to document clinical sequelae of structural change. Second, we had substantial missing MRI and KOOS Pain data, raising the question of bias. Appendix Table 1 demonstrates that those excluded from the analyses altogether, because they had no MRI data, had worse pain and function at 12 months than those included in the primary analysis. While these subjects had worse pain, it would seem unlikely that the association between structural worsening and pain would be different in this group. Recognizing the potential for biased loss to follow up, we performed an analysis of all 317 subjects with some MRI data and imputed values (20) for missing data, using the missing at random assumption. The results of these analyses mirrored those of the primary analysis. While it is possible that those with incomplete data (e.g. those undergoing TKR) would have had greater increases in pain and functional disability than predicted based on observed data alone, only 18 of these 317 subjects (6%) underwent TKR over the course of follow-up, and prior analyses showed that varying missing values over a wide range of assumptions did not influence estimates of KOOS Pain score in the MeTeOR cohort (23), so we consider this unlikely.
We found no evidence an association between structural damage occurring in the first 18 months following randomization in the MeTeOR Trial and clinically relevant worsening in symptoms over 3.5-years of follow-up. From a research standpoint these findings call for longer follow-up to ensure that early structural change does not portend symptomatic worsening over a longer time frame.
Supplementary Material
Significance and Innovation:
We did not observe clinically important or statistically significant associations between imaging changes in cartilage, synovitis, bone marrow lesions and osteophytes from baseline to 18 months and subsequent changes in pain scores between 18 and 60 months among participants in the MeTeOR trial.
The observation that structural worsening was not associated with worsening pain over 3.5 years of follow-up suggests these changes may not be clinically meaningful.
Acknowledgement:
The authors thank Claire McHugh, BS, for expert editorial assistance.
Funding:
National Institute of Arthritis and Musculoskeletal and Skin Diseases 1R01AR055557, K01AR075879, P30AR072577
References
- 1.Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. The Journal of bone and joint surgery American volume. 2011;93(11):994–1000. [DOI] [PubMed] [Google Scholar]
- 2.Herrlin SV, Wange PO, Lapidus G, Hållander M, Werner S, Weidenhielm L. Is arthroscopic surgery beneficial in treating non-traumatic, degenerative medial meniscal tears? A five year follow-up. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2013;21(2):358–64. [DOI] [PubMed] [Google Scholar]
- 3.Katz JN, Brophy RH, Chaisson CE, de Chaves L, Cole BJ, Dahm DL, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. The New England journal of medicine. 2013;368(18):1675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauffin H, Sonesson S, Meunier A, Magnusson H, Kvist J. Knee Arthroscopic Surgery in Middle-Aged Patients With Meniscal Symptoms: A 3-Year Follow-up of a Prospective, Randomized Study. Am J Sports Med. 2017;45(9):2077–84. [DOI] [PubMed] [Google Scholar]
- 5.Yim JH, Seon JK, Song EK, Choi JI, Kim MC, Lee KB, et al. A comparative study of meniscectomy and nonoperative treatment for degenerative horizontal tears of the medial meniscus. Am J Sports Med. 2013;41(7):1565–70. [DOI] [PubMed] [Google Scholar]
- 6.Kise NJ, Risberg MA, Stensrud S, Ranstam J, Engebretsen L, Roos EM. Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomised controlled trial with two year follow-up. BMJ. 2016;354:i3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van de Graaf VA, Noorduyn JCA, Willigenburg NW, Butter IK, de Gast A, Mol BW, et al. Effect of Early Surgery vs Physical Therapy on Knee Function Among Patients With Nonobstructive Meniscal Tears: The ESCAPE Randomized Clinical Trial. JAMA. 2018;320(13):1328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abram SGF, Hopewell S, Monk AP, Bayliss LE, Beard DJ, Price AJ. Arthroscopic partial meniscectomy for meniscal tears of the knee: a systematic review and meta-analysis. British journal of sports medicine. 2020;54(11):652–63. [DOI] [PubMed] [Google Scholar]
- 9.Collins JE, Losina E, Marx RG, Guermazi A, Jarraya M, Jones MH, et al. Early Magnetic Resonance Imaging-Based Changes in Patients With Meniscal Tear and Osteoarthritis: Eighteen-Month Data From a Randomized Controlled Trial of Arthroscopic Partial Meniscectomy Versus Physical Therapy. Arthritis Care Res (Hoboken). 2020;72(5):630–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonesson S, Kvist J, Yakob J, Hedevik H, Gauffin H. Knee Arthroscopic Surgery in Middle-Aged Patients With Meniscal Symptoms: A 5-Year Follow-up of a Prospective, Randomized Study. Orthop J Sports Med. 2020;8(1):2325967119893920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz JN, Chaisson CE, Cole B, Guermazi A, Hunter DJ, Jones M, et al. The MeTeOR trial (Meniscal Tear in Osteoarthritis Research): rationale and design features. Contemp Clin Trials. 2012;33(6):1189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roos EM. Knee Injury and Osteoarthritis Outcome Score. 2012. [cited; Available from: http://www.koos.nu/
- 13.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S208–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage. 2011;19(8):990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berwick DM, Murphy JM, Goldman PA, Ware JE Jr., Barsky AJ, Weinstein MC. Performance of a five-item mental health screening test. Med Care. 1991;29(2):169–76. [DOI] [PubMed] [Google Scholar]
- 17.Rumpf HJ, Meyer C, Hapke U, John U. Screening for mental health: validity of the MHI-5 using DSM-IV Axis I psychiatric disorders as gold standard. Psychiatry Res. 2001;105(3):243–53. [DOI] [PubMed] [Google Scholar]
- 18.Katz JN, Wright EA, Baron JA, Losina E. Development and validation of an index of musculoskeletal functional limitations. BMC Musculoskelet Disord. 2009;10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roos EM. The 2012 User’s Guide to: Knee injury and Osteoarthritis Outcome Score (KOOS) 2012 [cited 2021; Available from: http://www.koos.nu/KOOSusersguide2012.pdf
- 20.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 21.Sihvonen R, Paavola M, Malmivaara A, Itala A, Joukainen A, Kalske J, et al. Arthroscopic partial meniscectomy for a degenerative meniscus tear: a 5 year follow-up of the placebo-surgery controlled FIDELITY (Finnish Degenerative Meniscus Lesion Study) trial. British journal of sports medicine. 2020;54(22):1332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz JN, Shrestha S, Losina E, Jones MH, Marx RG, Mandl LA, et al. Five-Year Outcome of Operative and Nonoperative Management of Meniscal Tear in Persons Older Than Forty-Five Years. Arthritis Rheumatol. 2020;72(2):273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu K, Robbins SR, McDougall JJ. Osteoarthritis: the genesis of pain. Rheumatology (Oxford). 2018;57(suppl_4):iv43–iv50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


