Abstract
Objective:
The neural mechanisms contributing to the social problems of pediatric brain tumor survivors (PBTS) are unknown. Face processing is important to social communication, social behavior, and peer acceptance. Research with other populations with social difficulties, namely autism spectrum disorder, suggests atypical brain activation in areas important for face processing. This case-controlled functional magnetic resonance imaging (fMRI) study compared brain activation during face processing in PBTS and typically developing (TD) youth.
Methods:
Participants included 36 age-, gender-, and IQ-matched youth (N = 18 per group). PBTS were at least 5 years from diagnosis and 2 years from the completion of tumor therapy. fMRI data were acquired during a face identity task and a control condition. Groups were compared on activation magnitude within the fusiform gyrus for the faces condition compared to the control condition. Correlational analyses evaluated associations between neuroimaging metrics and indices of social behavior for PBTS participants.
Results:
Both groups demonstrated face-specific activation within the social brain for the faces condition compared to the control condition. PBTS showed significantly decreased activation for faces in the medial portions of the fusiform gyrus bilaterally compared to TD youth, ps ≤ .004. Higher peak activity in the left fusiform gyrus was associated with better socialization (r = .53, p < .05).
Conclusions:
This study offers initial evidence of atypical activation in a key face processing area in PBTS. Such atypical activation may underlie some of the social difficulties of PBTS. Social cognitive neuroscience methodologies may elucidate the neurobiological bases for PBTS social behavior.
Keywords: Pediatric brain tumor, fMRI, Face processing, Fusiform gyrus
Pediatric brain tumor survivors (PBTS) represent a growing population who are at risk for significant social challenges associated with their disease and treatments (Hocking et al., 2015). While advances in tumor-directed therapy have improved survival rates, they also have led to the awareness of a variety of neurodevelopmental long-term consequences, termed late effects, due to their impact on the developing brain (Turner, Rey-Casserly, Liptak, & Chordas, 2009). A significant consequence of these sequelae for PBTS are difficulties related to social acceptance compared to healthy peers (Salley et al., 2015). Specifically, PBTS are more likely to not have close friends and have fewer interactions with friends compared to siblings and other childhood cancer survivors (Schulte et al., 2018).
Given the importance of developing and maintaining social relationships in childhood and later adulthood (Ladd & Troop-Gordon, 2003; Lansford, Dodge, Fontaine, Bates, & Pettit, 2014; Prinstein & Aikins, 2004; Prinstein, Boergers, Spirito, Little, & Grapentine, 2000), understanding the neurobiological factors associated with aberrant social behavior in PBTS is of great interest. Current conceptualizations of social behavior within the field of social cognitive neuroscience emphasize the interplay between brain areas or complex brain networks and fundamental social information processes (Beauchamp & Anderson, 2010; Herrington, Taylor, Grupe, Curby, & Schultz, 2011; Yeates et al., 2007). Social difficulties in childhood may be the result of a diminished ability to interpret and respond to different types of social information secondary to disruptions to essential areas or networks within the social brain.
Face processing refers to the ability to recognize and interpret nonverbal information communicated by faces. It is a foundational social information process that influences the quality of social interactions, and hence social relationships and acceptance (Blair, 2003; Erickson & Schulkin, 2003). Difficulties with recognizing identities and emotional expressions during face processing likely have a cascading effect on later sociocognitive processes and social behavior, including identifying and selecting appropriate responses in social situations (Beauchamp & Anderson, 2010; Yeates et al., 2007).
Several areas of the social brain are heavily involved during the processing of faces, including the fusiform gyrus, superior temporal sulcus, and amygdala (Allison, Puce, & McCarthy, 2000; Pelphrey & Carter, 2008). Recent evidence suggests that these individual areas comprise a larger, more complex face processing network where specific regions become activated during different aspects of facial processing (Nomi & Uddin, 2015). For example, the fusiform gyrus is implicated in face discrimination, identity recognition, and expression recognition (Winstion, Henson, Fine-Goulen, & Dolan, 2004). Additionally, increased activity in the amygdala and superior temporal sulcus are related to the perception of facial expressions (Hoffman & Haxby, 2000; Wang et al., 2014).
Several clinical groups experience significant difficulties with social behavior and social relationships despite differing neurodevelopmental etiologies, including youth with autism spectrum disorder (ASD) and those affected by 22q11.2 deletion syndrome (22q11DS). Disrupted face processing is common to both groups and a potential shared mechanism for their social difficulties (Beauchamp & Anderson, 2010). Among youth with ASD, a group with hallmark deficits in social behavior and communication, a large body of research provides evidence for impairments in face processing (Nomi & Uddin, 2015; Schultz, 2005; Speer, Cook, McMahon, & Clark, 2007) and diminished activation in the fusiform gyrus, superior temporal sulcus and amygdala during a variety of face processing tasks (Nomi & Uddin, 2015). Youth with 22q11DS also experience difficulties with interpreting facial expressions (Glaser et al., 2010) and reduced activation in the fusiform gyrus during face processing compared to typically developing (TD) controls (Azuma et al., 2015). Such findings suggest that impairments in face processing due to reduced activation in key brain areas may contribute to social impairments in a variety of pediatric populations.
Prior research underscores the potential role of impaired face processing abilities in PBTS social functioning. In a study comparing the face processing of PBTS to controls, survivors demonstrated increased errors in identifying facial expressions, while controlling for IQ (Bonner et al, 2008). Increased face processing difficulties also were related to more parent-reported social adjustment difficulties (Bonner et al., 2008). This finding of increased errors in face processing was recently replicated in youth treated for posterior fossa tumors (Moxon-Emre et al., 2019). Another study, showed face processing to be uniquely important to the social functioning of PBTS (Hocking et al., 2021). Further, recent evidence suggests that PBTS gaze less at faces during social situations than TD youth and in a manner consistent with youth with ASD (Hocking et al., 2020) suggesting reduced experience in processing facial information. Two recent studies have evaluated the neurobiological factors associated with the face processing abilities of PBTS (Moxon-Emre et al., 2019; Moxon-Emre et al., 2020), focusing on associations between the processing of facial expressions and structural connectivity (e.g., white matter). However, no studies have used functional neuroimaging modalities to evaluate neural activity during face processing. Given the findings of diminished neural activity in specialized face processing brain networks in other populations with similar social difficulties (e.g., youth with ASD) and the likelihood of disrupted brain networks in PBTS due to tumor and tumor-directed therapies, additional research is needed to evaluate the presence of diminished activity in face processing areas in PBTS to better understand the etiology of face processing difficulties in this population.
The objectives of this study were: 1) to compare activity within face processing areas of the social brain during a face identity discrimination task of PBTS and TD youth; and 2) to determine associations between brain activation during the facial identity discrimination task and indices of social behavior and impairments among PBTS. We hypothesized that a) PBTS would have decreased activation in the fusiform gyrus, amygdala, and superior temporal sulcus during the facial processing task compared to TD youth; and b) decreased activation in these areas would be associated with higher levels of social impairments among PBTS.
METHODS
Participants
Participants consisted of 36 English-speaking youth between the ages of 8 and 17 years: PBTS (N = 18) and TD youth (N = 18). Descriptive data for the sample are presented in Table 1. Participants were approximately 14 years old and matched on sex ratio (47% female). Groups were matched in terms of age (t(34) = .496, p = .623) and general intelligence, as reflected by the Differential Abilities Scale General Conceptual Ability Score (described below; t(34) = 1.171, p = .290). Across groups, participant IQ was in the average range (sample mean = 99). Groups did not differ in terms of sex, race, or ethnicity. TD youth and PBTS were 100% and 77.8% right hand dominant, respectively.
Table 1.
Participant characteristics
| Brain Tumor (n = 18) |
Typically Developing (n = 18) |
|
|---|---|---|
| Variables | n (%) or M ± SD | n (%) or M ± SD |
|
| ||
| Age in years | 13.9 ± 2.9 | 13.5 ± 2.2 |
| Female sex | 8 (44.4%) | 7 (38.9%) |
| Race | ||
| Caucasian | 14 (77.8%) | 8 (44.4%) |
| African-American | 2 (11.1%) | 5 (27.8%) |
| Asian | 1 (5.6%) | 0 |
| Multi-ethnic | 0 | 3 (16.7%) |
| Other | 0 | 1 (5.6%) |
| Unreported | 1 (5.6%) | |
| Hispanic/Latinx | ||
| Hispanic/Latinx | 2 (11.1%) | 4 (22.2%) |
| Not Hispanic/Latinx | 13 (72.2%) | 12 (66.7%) |
| Unreported | 3 (16.7%) | 0 |
| Handedness | ||
| Right dominant | 14 (77.8%) | 18 (100%) |
| Left dominant | 3 (16.7%) | 0 |
| Unreported | 1 (5.6%) | 0 |
| Tumor characteristics | ||
| Age at diagnosis | 6.1 ± 3.3 | |
| Tumor types | ||
| Low grade glioma | 8 (44.4%) | |
| Medulloblastoma | 4 (22.2%) | |
| Ependymoma | 2 (11.1%) | |
| Ganglioglioma | 1 (5.6%) | |
| Glioma | 1 (5.6%) | |
| Primitive neuro-ectodermal tumor | 1 (5.6%) | |
| WHO grade | ||
| I | 10 (55.6%) | |
| II | 2 (11.1%) | |
| III | 0 | |
| IV | 5 (27.8%) | |
| Tumor location | ||
| Supratentorial | 5 (27.8%) | |
| Infratentorial | 12 (66.7%) | |
| Unreported | 1 (5.6%) | |
| Treatment | ||
| Surgery only | 7 (38.9%) | |
| Radiation only | 0 | |
| Chemo only | 2 (11.1%) | |
| Surgery + radiation | 2 (11.1%) | |
| Surgery + chemo | 1 (5.6%) | |
| Radiation + chemo | 0 | |
| All three | 5 (27.8%) | |
| Unreported | 1 (5.6%) | |
PBTS included those who had received any combination of surgical resection, chemotherapy, and/or cranial radiation therapy, who were diagnosed at least 5 years prior, and completed all tumor-directed treatments at least 2 years prior to study participation. Exclusion criteria for PBTS included any genetic condition affecting neurocognitive functioning (e.g., Neurofibromatosis, Down Syndrome), cognitive or developmental delay prior to brain tumor diagnosis, and visual defects that could not be corrected through corrective lenses (e.g., field cuts). Mean age at the time of brain tumor diagnosis was 6.10 years old. 67% of the PBTS sample had an infratentorial tumor and 33% had a supratentorial tumor, reflecting both low- and high-grade pathologies. The majority of the PBTS participants underwent surgical resection (71%; 12 gross total resections, 4 subtotal resections) with 41.2% receiving radiation therapy and 47.1% receiving some combination of tumor-directed therapies (e.g., surgical resection, chemotherapy, radiation therapy). Two participants had postoperative hydrocephalus and required shunt placement, two had hemiparesis, one participant had posterior fossa syndrome, and one had ataxia.
TD controls from this study were selected from a pool of 67 children and adolescents who had completed the same research protocol at the Center for Autism Research (CAR) at the Children’s Hospital of Philadelphia (CHOP), using the same scanner and experimental task. These data were collected between June of 2010, and October of 2012, prior to the data on PBTS, which were collected between August of 2016 and March of 2018. Participants were selected on a case-control basis to match the PBTS group on age, general intelligence (IQ), and sex. Exclusion criteria for TD youth included a) visual defects that could not be corrected with lenses; b) a history of traumatic brain injury or other neurological abnormality; c) delays suggestive of autism-like impairments on screening by study personnel; d) family history of a first- or second-degree relative with ASD; and e) a Diagnostic and Statistical Manual, 4th. Edition (DSM-IV)-TR Axis I disorder or significant symptoms of attention deficit hyperactivity disorder (ADHD) or mood, anxiety, substance-related, or conduct disorders. Of note, three PBTS participants had a diagnosis of an anxiety disorder, though this was not an exclusion criterion for the PBTS group.
Measures
Cognitive function
General cognitive ability was measured using the Differential Ability Scales, Second Edition (DAS-II) (Elliott, 2007). The DAS-II evaluates verbal and nonverbal reasoning abilities and provides norm-referenced overall cognitive ability scores that can be considered a measure of overall IQ due to its high correlation with other IQ tests (Elliott, 2007). Standard scores have a mean of 100 and standard deviation of 15.
Social behavior
The Social Responsiveness Scale, Second Edition (SRS-2) (Constantino & Gruber, 2012) is a 65-item parent-report measure of participant social behavior. The SRS-2 evaluates the frequency of reciprocal social behaviors, communication, and repetitive and stereotypic behaviors and yields a sex-normed T score that provides an index of social deficits (M =50, SD = 10). The SRS-2 has high internal consistency, test–retest reliability, and inter-rater reliability and has strong associations with other measures of social difficulties (Constantino & Gruber, 2012).
The Vineland Adaptive Behavior Scale-Second Edition: Parent/Teacher Rating Form (Vineland-II; Sparrow, Cicchetti, & Balla, 2005) is a well-researched, highly reliable, well-normed measure of adaptive behavior functioning. The questionnaire assesses adaptive functioning across the domains of communication, daily living, socialization, and motor functioning. The Socialization domain standard score was used to evaluate parental perceptions of participant functioning in social situations.
The Children’s Communication Checklist-2 (CCC-2; Bishop, 2006) is a parent-report measure of aspects of communication, such as speech, vocabulary, sentence structure, and social language skills of children and adolescents. It is a reliable tool for the assessment of social communication skills. The Social Relations score, which measures social behavior with others, will be used in analyses.
Procedures
All study procedures were approved by the institutional review board at CHOP and were conducted in accordance with the Helsinki Declaration. Potentially eligible PBTS were identified through tumor registry and electronic medical records and sent a letter describing the study. Study personnel contacted families via phone to provide more information about the study and conduct a verbal screening to determine eligibility. Those who met eligibility criteria and were interested in participating were invited to a one-time, in-person evaluation. PBTS participants were given the option of completing a 1-hour research magnetic resonance imaging (MRI) scan as part of the study. Written informed consent was obtained from parents before enrollment, and child assent was obtained. The protocol with PBTS mirrored that used with TD youth participants that were collected as part of a larger study. Parents of participants completed behavioral informant reports while youth completed the assessment. PBTS data were combined with data on TD youth for analyses. In total, 207 PBTS were contacted to participate in this study and 97 were screened for eligibility. Of the 97 PBTS, 90 met criteria and 54 completed the cognitive assessment. 23 participants opted to undergo the MRI task. For 5 of the participants who completed the MRI, either the face identity task did not work during the scan or there was excessive movement, leading to a total of 18 participants. Among the total number of PBTS who participated in the larger study, there were no differences on main study measures (DAS-II General Conceptual Ability, SRS-2, CCC-2, Vineland Socialization) between those who completed an MRI scan and those who did not.
MRI scanning
All participants were scanned on a Siemens Verio 3-Tesla scanner at CHOP. Data reported in this paper were collected as part of a multimodal imaging protocol lasting one hour per participant. Functional MRI (fMRI) data consisted of a gradient-echo echo-planar imaging sequence. While initial scanning of TD participants used TR/TE/Flip Angle/Voxel Size parameters of 2340 ms/25 ms/60 degrees/3.55 mm isotropic, the PBTS sample was acquired after a scanner software upgrade with slightly different parameters (2110 ms/25 ms/60 degrees/3.5 mm isotropic (with a .35 mm gap between slices). High-resolution structural data were also collected on all participants (TR/TE/Flip Angle/Voxel Size parameters of 1900 ms/2.54 ms/90 degrees/.8 × .8 × .9 mm isotropic), and were used here to facilitate registration of fMRI data into standard space.
Face identity discrimination task
The experimental paradigm closely followed prior studies from our group, and has been shown to reliably yield activity in “social brain” areas including fusiform gyrus (Herrington et al., 2017; Herrington, Miller, Pandey, & Schultz, 2016; Herrington et al., 2011). All participants completed a 5-minute face identity task where they were asked to identify whether two side-by-side photographs were of the same person or different people (i.e., subordinate-level identity judgment). Control stimuli consisted of pairs of side-by-side houses. All images were presented in grayscale. Participants were shown pairs of faces presented side-by-side for 3500 ms, followed by a blank screen of 500 ms, in blocks of six trials. A total of four face and four house blocks were presented during each run, yielding 24 trials per stimulus type. Participants pressed a button to indicate whether the faces depicted are of the same person. In the control trials, participants completed a task with an identical type of response (same or different) for pairs of houses instead of faces. Accuracy data were missing for two PBTS participants due to computer error.
fMRI data preprocessing
fMRI time series data were despiked using the program 3dDespike (Cox, 1996). All other preprocessing and analysis steps were implemented using either the Functional Magnetic Resonance Imaging of the Brain Software Library (FSL; Smith et al., 2004) or custom software. After despiking, fMRI data were motion corrected using FSL’s Motion Correction using FMRIB’s Linear Image Registration Tool. Participants with a maximum frame-to-frame motion exceeding 1.5 mm were excluded from analyses, leading to the exclusion of 1 PBTS participant and a final sample size of 18 per group (all TD participants selected for comparison met the motion criterion). Final groups did not differ in mean volume-to-volume displacement (average max relative displacement for PBTS/TD groups: .48 mm/.36 mm; t(34) = 1.264, p = .214). All time series data were prewhitened to meet the assumption of noncorrelated error terms in general linear model-based analyses (GLM). Functional data were spatially filtered with a 4 mm (full width at half-maximum) Gaussian kernel, and temporally filtered with a 108-second high-pass temporal filter (to remove linear trends in MRI signal stabilization). While all statistical analyses were conducted in each participant’s native fMRI space, statistical maps were resampled to 2 mm (isotropic) for visualization and localization of activation clusters.
Statistical analyses
After preprocessing, each participant’s time series was submitted to per-voxel GLM analysis. Explanatory variables (EVs) in the analysis including parameters representing face and house conditions (convolved with a gamma function), and temporal derivatives of these (in order to account for variability in hemodynamic responses). Six EVs representing head rotation and translation were also added as nuisance parameters. These analyses resulted in parameter estimate (beta) maps that were then used in group analysis. The primary beta map used in all subsequent analyses was the contrast of Faces > Houses.
Group-level analyses included per-voxel t-tests comparing each group separately for the Faces > Houses contrast, and unpaired t-tests comparing the groups to one another. Family-wise error was controlled via the application of FSL’s Threshold-Free Cluster Enhancement (TFCE; Smith &Nichols, 2009) As our strongest a priori hypotheses related to fusiform gyrus, amygdala, superior temporal sulcus, TFCE was conducted within masks (i.e., a small volume correction) subsuming these area bilaterally, as defined by the Harvard-Oxford Cortical Atlas (fusiform gyrus and amygdala) and the Destrieux atlas (superior temporal sulcus) (Destrieux, Fischl, Dale, & Halgren, 2010; Flitney et al., 2007). Inferences for all other brain areas were conducted using the whole-brain gray matter segmentation from the Montreal Neurological Institute (MNI) template brain. Bivariate Pearson correlations evaluated the associations between select neuroimaging metrics and indices of social behavior and adjustment for the PBTS participants only.
Although none of the participants had tumors or resections that extended into the a priori brain areas of interest, we nevertheless conducted post hoc analyses to establish that groups did not have morphometric differences in areas where significant differences in activity were found. This analysis was conducted via FSLVBM (Douaud et al., 2007; Good et al., 2001). This procedure involved the nonlinear registration of each participant’s brain to MNI space to create a template for the entire study sample. Each participant’s structural image is then nonlinearly registered to this template and “modulated” to correct for local expansion (or contraction) due to the nonlinear component of the spatial transformation. As in Voxel-Based Morphometry generally, group-specific differences are estimated on a per-voxel basis by testing for differences in the Jacobean of the warp field. As with the fMRI data, significant clusters were identified using FSL’s TFCE. No significant clusters were identified that overlapped spatially with significant fMRI clusters, suggesting that observed fMRI differences could not be attributed to morphometric differences.
RESULTS
Preliminary analyses
PBTS participants had more parent-reported social impairments on the SRS-2 Total T score (m = 50.82, SD = 6.64) than the TD participants (m = 42.33, SD = 4.36), t(33) = 4.45, p < .01. Parents also rated PBTS participants (m = 9.65, SD = 2.74) as having lower quality social relations on the CCC-2 compared to TD participants (m = 12.31, SD = 1.08), t(31) = 3.72, p < .01. There were no significant differences on the Vineland Socialization score between the PBTS group (m = 104.75, SD = 22.00) and the TD group (m = 114.28, SD = 15.14), t(32) = −1.49, p = .14. Notably, ratings of PBTS social behavior were in the average range across these measures. Among PBTS, there were no significant differences on IQ or any of the social or behavioral measures based on tumor location (supratentorial vs. infratentorial).
fMRI face identity discrimination task
Accuracy across the experiment was 76% for the PBTS group and 87% for the TD group. These data indicate that, as predicted, both groups attended to the task, but the PBTS group was significantly less accurate than TD, t(31) = 2.21, p = .03.
Family-wise error-corrected analyses were conducted within region of interests representing bilateral fusiform gyrus, amygdala, superior temporal sulcus, and whole brain. Both groups presented with significant increases in activity in fusiform gyrus, amygdala, and superior temporal sulcus (see Table 2 for a listing of all significant activation clusters in this study). Additionally, the TD group showed a significant increase in right inferior gyrus. When comparing the TD and PBTS statistical maps, no significant group differences were observed for amygdala, superior temporal sulcus, or whole-brain analyses. However, as predicted, this activation was significantly less in the PBTS group than the TD group (Figure 1 bottom row). The peak group difference was centered on −30, −54, −10 in the left hemisphere, with a cluster volume of 51 2 mm voxels; in the right hemisphere the coordinates were 30, −36, −22 will a cluster volume of 206 2 mm voxels. Peak p-values were .004 and <.001 in the left and right hemispheres, respectively (both p-values corrected for multiple comparisons).
Table 2.
fMRI activation clusters
| Contrast | Region | LH Coords/Size/Peak p-Value | RH Coords/Size/Peak p-Value |
|---|---|---|---|
|
| |||
| TD > PBTS | |||
| Fusiform gyrus | −30, −54, −10/51/p = .014 | 30, −36, −22/206/p < .001 | |
| TD Only | Fusiform gyrus | −40, −56, −18/170/p < .001 | 48, −48, −22/296/p < .001 |
| Amygdala | −22, −8, −10/78/p = .008 | 22, −6, −12/131/p = .001 | |
| STS | 58, −40, 9/222/p = .005 | N.S. | |
| IFG | N.S. | 46, 16, 30/966/p < .001 | |
| PBTS only | Fusiform gyrus | −42, −50, −22/57/p = .004 | 42, −48, −22/65/p = .008 |
| Amygdala | −18, −6, −18/95/p = .002 | 20, −12, −16/114/p = .001 | |
| STS | −48, −56, 18/25/p =.026 | 52, −40, −4/644/p < .001 | |
Note. Results included statistical maps representing the contrast of Faces > Houses. All reported cluster were significant at a family error-corrected threshold of p < .05 and cluster sizes > 10 voxels. Sizes represent number of 2 mm voxels in the cluster. Where clusters were nonsignificant in one hemisphere (but significant in the other), N.S. is listed in the table. STS: superior temporal sulcus. IFG: inferior frontal gyrus
Fig. 1.
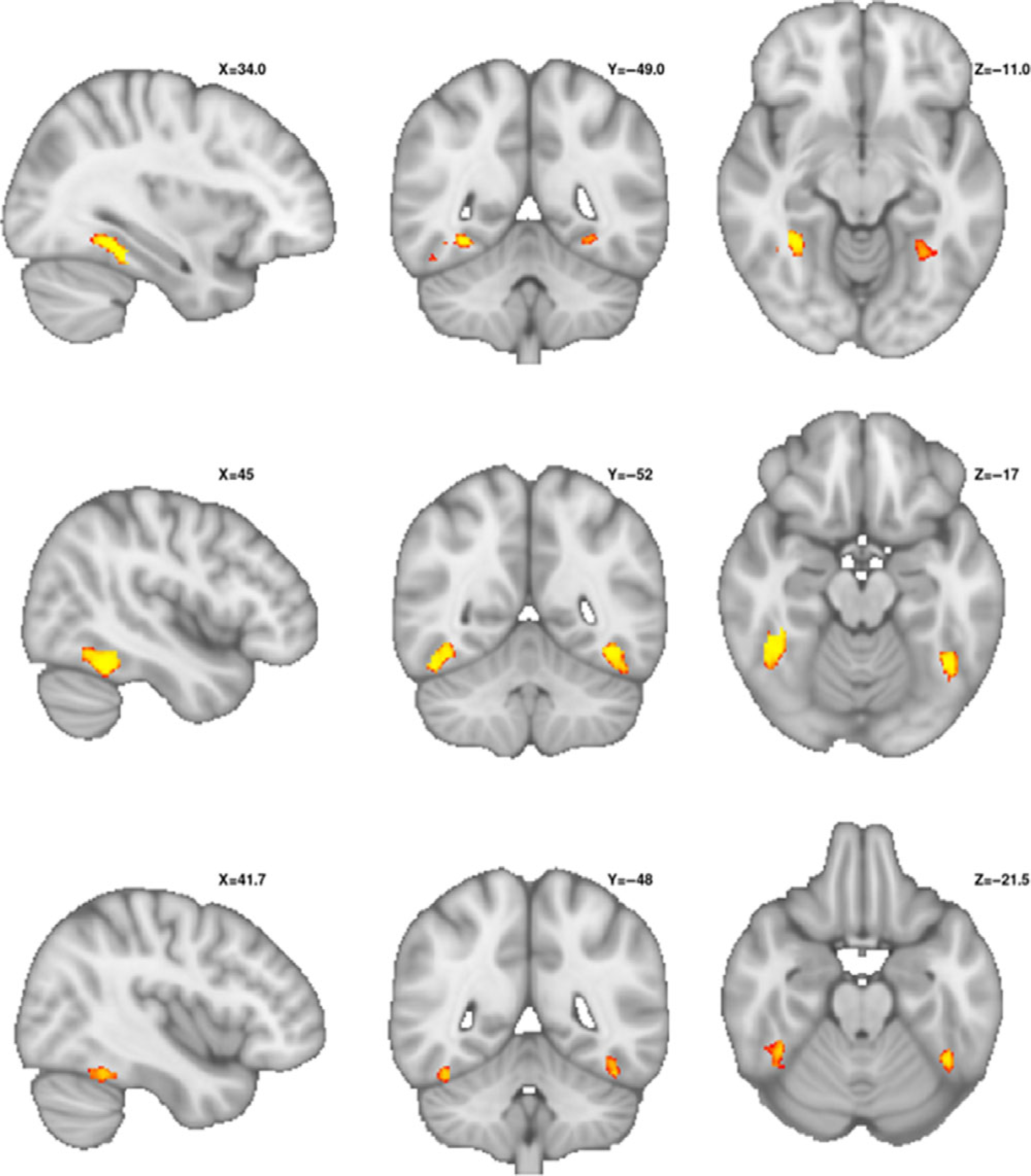
Decreased fusiform gyrus activation during face processing in pediatric brain tumor survivors.
Medical characteristics and imaging metrics
Among the PBTS participants only, some of the neuroimaging metrics were related to medical factors. PBTS whose treatment consisted of chemotherapy had lower peak activation in the right fusiform gyrus (m = 69.34, SD = 39.43) compared to those who did not receive chemotherapy (m = 143.83, SD = 52.83), t[15] = 3.25, p < .01. Additionally, those whose received multimodal tumor-directed therapy (m = 77.36, SD = 49.15) had lower peak activation in the right fusiform gyrus compared to those who only received one type of tumor-directed treatment (m = 136.80, SD = 55.64), t[15] = 2.32, p < .05. Age at diagnosis was unrelated to neuroimaging metrics. Additionally, there were no differences in neuroimaging metrics based on tumor locations (supratentorial vs. infratentorial) or history of radiation therapy, surgical resection, or recurrence.
Neuroimaging metrics and behavior
Correlational analyses presenting associations between neuroimaging metrics and aspects of social behavior and social acceptance are presented in Table 3. Higher peak activity strength in the left fusiform gyrus was associated with better Socialization scores on the Vineland-II (r = .53, p < .05). Activity strength on either side was not related to total level of social impairments on the SRS-2 or social relations on the CCC-2.
Table 3.
Pearson correlations
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1. Age at Evaluation | 1 | – | – | – | – | – | – | – |
| 2. SRS Total T-Score | −.204 | 1 | – | – | – | – | – | – |
| 3. Vineland Socialization Standard Score | −.231 | −.453** | 1 | – | – | – | – | – |
| 4. CCC-2 Social Relations Score | −.327 | −.630** | .535** | 1 | – | – | – | – |
| 5. rffa_peakpe_fvh | .227 | .297 | .050 | −.128 | 1 | – | – | – |
| 6. lffa_peakpe_fvh | .194 | −.275 | .530* | −.018 | .537* | 1 | – | – |
| 7. rffa_meanpe_fvh | .203 | −.119 | .165 | −.260 | .191 | .427 | 1 | – |
| 8. lffa_meanpe_fvh | −.05 | −.177 | .085 | −.097 | −.095 | .166 | .532* | 1 |
p < .05
p < .01
DISCUSSION
Diminished activation in key “social brain” areas during face processing is a notable feature among youth with a variety of developmental conditions that are characterized by social difficulties (Azuma et al., 2015; Nomi & Uddin, 2015; Schultz, 2005). Consistent with these studies, results from the present study suggest reduced activation in the bilateral fusiform gyrus areas among youth treated for a pediatric brain tumor compared to TD controls during a face identity task. These novel findings provide evidence of the potential neural underpinnings for poor social information processing in PBTS that could underlie their social difficulties. Further, among PBTS participants, decreased fusiform gyrus activation was related to notable medical risk factors, including history of multimodal tumor-directed therapy, and an index of social behavior.
Similar to previous studies using the face identity discrimination task (Herrington et al., 2017; Herrington et al., 2016; Herrington et al., 2011), the paradigm in the current study successfully activated areas in the social brain known to be involved in face processing, particularly bilateral fusiform gyrus, in both PBTS and TD controls. As expected, both groups showed higher activation bilaterally in the fusiform gyrus for faces compared to houses during the paradigm. However, PBTS showed less activation in the bilateral fusiform gyrus for faces compared to TD controls, suggesting abnormalities in the neural processes associated with processing faces in survivors. While these neural irregularities during face processing are also seen in other neurodevelopmental conditions with social difficulties (e.g., ASD, 22q11DS), these data represent the first in youth with an acquired brain injury secondary to tumor and tumor-directed treatments.
These findings suggest neurobiological similarities in specialized face processing areas between PBTS and youth with other neurodevelopmental conditions with social difficulties, such as ASD, despite differing etiologies. Additionally, our findings are consistent with broader disturbances across both structural (e.g., white matter) and functional brain networks in PBTS that have been documented in prior neuroimaging studies with survivors (Dockstader, Wang, Bouffet, & Mabbott, 2014; Gauvreau et al., 2019; Scantlebury et al., 2016) and could impact face processing networks. The importance of white matter connectivity to face processing brain areas has been established in TD individuals (Gschwind, Pourtois, Schwartz, Van De Ville, & Vuilleumier, 2012) and related to social processing and behavioral impairments in youth with ASD (Ameis & Catani, 2015; Fitzgerald, Gallagher, & McGrath, 2016). Further, abnormalities in social information processing networks and diminished connectivity strength both within topological systems and across global networks have been documented in youth with ASD (Yerys et al., 2015; Yerys et al., 2017). Additionally, preliminary research has highlighted a link between white matter structure and attention regulation toward processing of emotional information communicated by faces among PBTS (Moxon-Emre et al., 2020). Pediatric brain tumors and their related tumor-directed therapies likely disrupt neurodevelopment and negatively impact brain connectivity both globally and within specialized networks (Beauchamp & Anderson, 2010; Yeates et al., 2007), contributing to diminished activation in areas essential to face processing. This interpretation of the current results is supported by the finding that use of multimodal tumor treatments was associated with reduced peak activity in the right fusiform gyrus. Additional neuroimaging research, particularly longitudinal studies and those that have sufficient samples to evaluate the effects of specific treatment variables (e.g., radiation field and dose), is needed to determine the neurobiological processes underlying the social difficulties of PBTS.
Findings from the current study provide an important perspective for prior research with PBTS showing reduced facial affect recognition accuracy (Bonner et al., 2008; Moxon-Emre et al., 2019) and diminished gaze preference for faces when observing social interactions (Hocking et al., 2020). Hypoactivation in the specialized face processing areas of the fusiform gyrus may underlie these social information processing difficulties in survivors and contribute to a negative feedback loop. In such a loop, reduced activation in face processing areas could underlie difficulties with accurately encoding facial information, which, in turn, could contribute to reduced gaze preferences for faces and less opportunity for processing faces, thus leading to diminished activation in face processing areas. Longitudinal research is needed to disentangle these potential causal associations between these factors, particularly with respect to tumor treatments that affect tissue outside of the immediate tumor area (i.e., craniospinal irradiation).
Within the PBTS participants in this study, higher activation levels in the left fusiform gyrus were associated with better ratings on a measure of behavior in social situations. This is the first evidence that individual differences in neural activation in face processing areas is related to variability in social behavior in PBTS. While a study on youth with 22q11DS also found associations between brain activation during face processing and behavior (Azuma et al., 2015), similar research in youth with ASD have not found consistent associations between activation strength in face processing areas and social behavior (Nomi & Uddin, 2015). It should be noted that ratings of PBTS social behavior were in the average range across measures. This is consistent with other studies that used informant reports of survivor social behavior (e.g., Barrera et al., 2017; Bonner et al., 2008; Willard, Berlin, Conklin, & Merchant, 2019) and raises the question about the suitability of these measures with this population given findings of social deficits in studies using more robust sociometric methods (e.g., Desjardins et al., 2019; Salley et al., 2015). However, variability in face processing abilities has been associated with ratings of survivor social behavior in studies from two different research groups (Bonner et al., 2008; Hocking et al., 2021), underscoring the importance of evaluating potential neurobiological factors underlying the variability of face processing in PBTS. Future studies should seek to replicate these findings in larger samples of PBTS to determine if hypoactivation in the fusiform gyrus is a key neurobiological process that contributes to variability in the social behavior of PBTS.
This study has several strengths. First, it employed a case-controlled design that compared a clinical group with TD controls matched in terms of age, sex, and IQ. Second, it employed a strong, previously used paradigm to elicit specialized face processing regions during imaging. Third, we used a conservative analytical approach for evaluating differences in activation between groups despite a small sample size. Limitations include a small sample size, a wide age range in participants, and a heterogenous sample of PBTS in terms of diagnoses and treatment histories. The current sample size precluded further evaluation of several medical factors (e.g., radiation does and field) that are important to consider in their associations with face processing and social behavior.
An important future direction in this line of research will be to identify the exact neural mechanisms mediating pediatric brain tumor treatment and decreased activity in fusiform gyrus. The structural MRI analyses from this study established that the PBTS sample did not appear to have gross morphometric differences in fusiform gyrus. However, PBTS treatments have known consequences for neuronal function and development that may not appear clearly in structural imaging data. Radiation has been shown to reduce synaptic density and dendritic length, increase neuronal inflammation, and alter cerebral vasculature (Acharya et al., 2016; Chakraborti, Allen, Allen, Rosi, & Filke, 2012; Hinkle, Olschowka, Love, Williams, & O’Banion, 2019; Wake, Moorhouse, Jinno, Kohsaka, & Nabekura, 2009). Chemotherapy also has significant consequences for central nervous system functioning, including decreased myelination and increased microglial activation (a sign of neuronal inflammation) (Gibson et al., 2019; Han et al., 2008). With the exception of diffusion-weighted imaging correlates of myelination, few in vivo neuroimaging techniques have the sensitivity to precisely distinguish between these different potential sources of deficit. Clearly this is an important area of future studies as imaging techniques continue to advance.
This study represents an important step in employing methods from the field of social cognitive neuroscience to understand the social functioning of PBTS. Future research is needed with larger samples that are more homogenous in terms of diagnosis, tumor location, and treatment history to better account for these variables. It also is important to determine connections between activation in face processing areas and global structural and functional connectivity metrics obtained during diffusion-weighted imaging (Na et al., 2018) and resting-state fMRI to link impairments in global connectivity to hypoactivation during face processing.
ACKNOWLEDGEMENTS
The authors thank all the participants who provided their time for this study.
FINANCIAL SUPPORT
This research was supported in part by the National Cancer Institute (K07CA178100, to MCH), National Institute of Mental Health (RC1MH08879, to RTS); the Intellectual and Developmental Disabilities Research Center funded by the National Institute of Child and Human Development (I5U54HD086984, to RTS; principal investigator, M. Robinson), the Pennsylvania Department of Health (SAP 4100042728 and SAP 4100047863, to RTS), Pfizer (to RTS); Robert Wood Johnson Foundation (6672, to RTS); and Shire Pharmaceuticals (to JDH).
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest.
REFERENCES
- Acharya MM, Green KN, Allen BD, Najafi AR, Syage A, Minasyan H, ... Limoli CL (2016). Elimination of microglia improves cognitive function following cranial irradiation. Scientific Reports, 6(1), 31545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T, Puce A, & McCarthy G (2000). Social perception from visual cues: role of the STS region. Trends in Cognitive Neuroscience, 4, 267–278. [DOI] [PubMed] [Google Scholar]
- Ameis SH, & Catani M (2015). Altered white matter connectivity as a neural substrate for social impairment in Autism Spectrum Disorder. Cortex, 62, 158–181. [DOI] [PubMed] [Google Scholar]
- Azuma R, Deeley Q, Campbell LE, Daly EM, Giampietro V, Brammer MJ, ... Murphy DG (2015). An fMRI study of facial emotion processing in children and adolescents with 22q11.2 deletion syndrome. Journal of Neurodevelopmental Disorders, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera M, Atenafu EG, Schulte F, Bartels U, Sung L, Janzen L, ... Zelcer S (2017). Determinants of social competence in pediatric brain tumor survivors who participated in an intervention study. Supportive Care in Cancer, 25, 2891–2898. doi: 10.1007/s00520-017-3708-6 [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, & Anderson V (2010). SOCIAL: An integrative framework for the development of social skills. Psychological Bulletin, 136(1), 39–64. [DOI] [PubMed] [Google Scholar]
- Bishop D (2006). Children’s Communication Checklist-2. San Antonio, TX: Pearson. [Google Scholar]
- Blair RJR (2003). Facial expressions, their communicatory functions and neuro-cognitive substrates. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358, 561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner MJ, Hardy KK, Willard VW, Anthony KK, Hood M, & Gururangan S (2008). Social functioning and facial expression recognition in survivors of pediatric brain tumors. Journal of Pediatric Psychology, 33, 1142–1152. [DOI] [PubMed] [Google Scholar]
- Chakraborti A, Allen A, Allen BD, Rosi S, & Filke JR (2012). Cranial irradiation alters dendritic spine density and morphology in the hippocampus. PLoS One, 7(7), e40844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, & Gruber CP (2012). (SRSTM −2) Social Responsiveness ScaleTM (Second Ed.). Torrance, CA: Western Psychological Services. [Google Scholar]
- Cox R (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computations in Biomedical Research, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- Desjardins L, Barrera M, Chung J, Cataudella D, Janzen L, Bartels U, ... Fairclough D (2019). Are we friends? Best friend nominations in pediatric brain tumor survivors and associated factors. Supportive Care in Cancer. doi: 10.1007/s00520-019-04706-3 [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, & Halgren E (2010). Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage, 53(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockstader C, Wang F, Bouffet E, & Mabbott DJ (2014). Gamma deficits as a neural signature of cognitive impairment in children treated for brain tumors. The Journal of Neuroscience, 34(26), 8813–8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, ... James A (2007). Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain, 130(9), 2375–2386. [DOI] [PubMed] [Google Scholar]
- Elliott CD (2007). Differential Ability Scales - Second edition (DAS-II). San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Erickson K, & Schulkin J (2003). Facial expressions of emotion: A cognitive neuroscience perspective. Brain and Cognition, 52, 52–60. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J, Gallagher L, & McGrath J (2016). Widespread disrupted white matter microstructure in autism spectrum disorders. Journal of Autism and Developmental Disorders. doi: 10.1007/s10803-016-2803-8 [DOI] [PubMed] [Google Scholar]
- Flitney D, Webster M, Patenaude B, Seidman LJ, Goldstein J, Tordesillas Gutierrez S, & Eickhoff SB (2007). Anatomical Brain Atlases and Their Application in the FSLView Visualisation Tool. Paper presented at the Annual Meeting of the Organization for Human Brain Mapping, Chicago, IL. [Google Scholar]
- Gauvreau S, Lefebvre J, Bells S, Laughlin S, Bouffet E, & Mabbott DJ (2019). Disrupted network connectivity in pediatric brain tumor survivors is a signature of injury. The Journal of Comparative Neurology. doi: 10.1002/cne.24717 [DOI] [PubMed] [Google Scholar]
- Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, ... Monje ML (2019). Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. Cell, 176(1), 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser B, Debbane M, Ottet M-C, Vuilleumier P, Zesiger P, Antonarakis SE, & Eliez S (2010). Eye gaze during face processing in children and adolescents with 22q11.2 deletion syndrome. Journal of American Academy of Child and Adolescent Psychiatry, 49, 665–674. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, & Frackowiak RS (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage, 14(1), 21–36. [DOI] [PubMed] [Google Scholar]
- Gschwind M, Pourtois G, Schwartz S, Van De Ville D, & Vuilleumier P (2012). White-matter connectivity between face-responsive regions in the human brain. Cerebral Cortex, 22, 1564–1576. [DOI] [PubMed] [Google Scholar]
- Han R, Yang YM, Dietrich J, Luebke A, Mayer-Proschel M, & Noble M (2008). Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. Journal of Biology, 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington JD, Maddox BB, McVey AJ, Franklin ME, Yerys BE, Miller JS, & Schultz RT (2017). Negative valence in autism spectrum disorder: The relationship between amygdala activity, selective attention, and co-occurring anxiety. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(6), 510–517. doi: 10.1016/j.bpsc.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington JD, Miller JS, Pandey J, & Schultz RT (2016). Anxiety and social deficits have distinct relationships with amygdala function in autism spectrum disorder. Social Cognitive and Affective Neuroscience. doi: 10.1093/scan/nsw015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington JD, Taylor JM, Grupe DW, Curby KM, & Schultz RT (2011). Bidirectional communication between amygdala and fusiform gyrus during facial recognition. Neuroimage, 56(4), 2348–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle JJ, Olschowka JA, Love TM, Williams JP, & O’Banion MK (2019). Cranial irradiation mediated spine loss is sex-specific and complement receptor-3 dependent in male mice. Scientific Reports, 9(1), 18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking MC, Albee M, Brodsky C, Shabason E, Wang L, Schultz RT, & Herrington J (2021). Face processing and social functioning in pediatric brain tumor survivors. Journal of Pediatric Psychology, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking MC, McCurdy M, Turner E, Kazak AE, Noll RB, & Barakat LP (2015). Social competence in pediatric brain tumor survivors: Application of a model from social neuroscience and developmental psychology. Pediatric Blood & Cancer, 62, 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking MC, Parish-Morris J, Schultz RT, Minturn J, Brodsky C, Shabason E, & Herrington J (2020). Diminished social attention in pediatric brain tumor survivors: Using eye tracking technology during naturalistic social perception. Neuropsychology, 34(3), 350–358. doi: 10.1037/neu0000623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EA, & Haxby JV (2000). Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nature Neuroscience, 3, 80–84. [DOI] [PubMed] [Google Scholar]
- Ladd GW, & Troop-Gordon W (2003). The role of chronic peer difficulties in the development of children’s psychological adjustment problems. Child Development, 74(5), 1344–1367. [DOI] [PubMed] [Google Scholar]
- Lansford JE, Dodge KA, Fontaine RG, Bates JE, & Pettit GS (2014). Peer rejection, affiliation with deviant peers, delinquency, and risky sexual behavior. Journal of Youth and Adolescence, 43, 1742–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon-Emre I, Farb NAS, Oyefiade AA, Bouffet E, Laughlin S, Skocic J, ... Mabbott DJ (2019). Facial emotion recognition in children treated for posterior fossa tumours and typically developing children: A divergence of predictors. NeuroImage: Clinical, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon-Emre I, Taylor MJ, Farb NAS, Oyefiade AA, Taylor MD, Bouffet E, ... Mabbott DJ (2020). Eye movements and white matter are associated with emotional control in children treated for brain tumors. Journal of International Neuropsychological Society, 26(10), 978–992. [DOI] [PubMed] [Google Scholar]
- Na S, Li L, Crosson B, Dotson V, MacDonald TJ, Mao H, & King TZ (2018). White matter network topology relates to cognitive flexibility and cumulative neurological risk in adult survivors of pediatric brain tumors. NeuroImage: Clinical, 20, 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi JS, & Uddin LQ (2015). Face processing in autism spectrum disorders: From brain regions to brain networks. Neuropsychologia, 71, 201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, & Carter EJ (2008). Brain mechanisms for social perception: lessons from autism and typical development. Annals of New York Academy of Science, 1145, 283–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinstein MJ, & Aikins JW (2004). Cognitive mediators of the longitudinal association between peer rejection and adolescent depressive symptoms. Journal of Abnormal Child Psychology, 32(2), 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinstein MJ, Boergers J, Spirito A, Little TD, & Grapentine WL (2000). Peer functioning, family dysfunction, and psychological symptoms in a risk factor model for adolescent inpatients’ suicidal ideation severity. Journal of Clinical Child Psychology, 29(3), 392–405. [DOI] [PubMed] [Google Scholar]
- Salley CG, Hewitt LL, Patenaude AF, Vasey MW, Yeates KO, Gerhardt CA, & Vannatta K (2015). Temperament and social behavior in pediatric brain tumor survivors and comparison peers. Journal of Pediatric Psychology, 40(3), 297–308. doi: 10.1093/jpepsy/jsuo83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scantlebury N, Bouffet E, Laughlin S, Strother D, McConnell D, Hukin J, ... Mabbott DJ (2016). White matter and information processing speed following treatment with cranial-spinal radiation for pediatric brain tumor. Neuropsychology, 30, 425–438. [DOI] [PubMed] [Google Scholar]
- Schulte F, Brinkman TM, Li C, Fay-McClymont T, Srivastava DK, Ness KK, ... Krull KR (2018). Social adjustment in adolescent survivors of pediatric central nervous system tumors: A report from the Childhood Cancer Survivor Study. Cancer, 124, 3596–3608. doi: 10.1002/cncr31593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT (2005). Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience, 23(125–141). [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, ... Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23, S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM, & Nichols TE (2009). Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage, 44(1), 83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, & Balla DA (2005). Vineland Adaptive Behavior Scales - Second Edition. Circle Pines, MN: AGS. [Google Scholar]
- Speer LL, Cook AE, McMahon WM, & Clark E (2007). Face processing in children with autism: Effects of stimulus contents and type. Autism, 11, 265–277. [DOI] [PubMed] [Google Scholar]
- Turner CD, Rey-Casserly C, Liptak CC, & Chordas C (2009). Late effects of therapy for pediatric brain tumor survivors. Journal of Child Neurology, 24(11), 1455–1463. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, & Nabekura J (2009). Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. Journal of Neuroscience, 29(13), 3974–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Tudusciuc O, Mamelak AN, Ross IB, Adolphs R, & Rutishauser U (2014). Neurons in the human amygdala selective for perceived emotion. Proceedings of the National Academy of Science, 111, E3110–E3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard VW, Berlin KS, Conklin HM, & Merchant TE (2019). Trajectories of psychosocial and cognitive functioning in pediatric patients with brain tumors treated with radiation therapy. Neuro-oncology, 21(5), 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstion JS, Henson RN, Fine-Goulen MR, & Dolan RJ (2004). fMRI-adaptation reveals dissociable neural representations of identity and expression in face perception. Journal of Neurophysiology, 92, 1830–1839. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Bigler ED, Dennis M, Gerhardt CA, Rubin KH, Stancin T, ... Vannatta K (2007). Social outcomes in childhood brain disorder: A heuristic integration of social neuroscience and developmental psychology. Psychological Bulletin, 133, 535–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Gordon EM, Abrams DN, Satterthwaite TD, Weinblatt R, Jankowski KF, ... Vaidya CJ (2015). Default mode network segregation and social deficits in autism spectrum disorder: Evidence from non-medicated children. NeuroImage: Clinical, 9, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Herrington JD, Satterthwaite TD, Guy L, Schultz RT, & Bassett DS (2017). Globally weaker and topologically different: Resting-state connectivity in youth with autism. Molecular Autism, 8(39). doi: 10.1186/s13229-017-0156-6 [DOI] [PMC free article] [PubMed] [Google Scholar]


