Abstract
Androgen deprivation therapy (ADT) has been the main management strategy for prostate cancer for more than eight decades, nowadays achieved commonly by administration of luteinizing hormone-releasing hormone agonists. ADT markedly suppresses androgen hormones with the long-term risks of adverse events such as muscle weakness, impairment of glucose and lipid metabolism, impotence, osteoporosis, and secondary fractures. Extensive research has provided significantly better insight into the dynamics of ADT including identification of the benefits of sequential and combination therapies. This has led to the development of new pharmaceutical ADT modalities. This review provides a general overview of the evolution of ADT in the context of the new emerging pharmaceutical ADT modalities so that clinicians and medical providers have a better understanding of personalizing the available ADT options with their different risk-benefit profiles.
Keywords: prostate cancer, androgen deprivation therapy, historical review and update
Introduction
Prostate cancer (PCa) is the most common malignancy and the second most common cause of cancer-related deaths affecting men in developed countries.1 PCa incidence has risen over the recent decades. Factors responsible for this rise include aging population, obesity due to “western” dietary habits, and increasing use of prostate-specific antigen (PSA) testing.2 Prostate cells, normal or cancerous, are dependent upon androgens for survival and growth. Consequently, androgen deprivation therapy (ADT) (also called hormone therapy by some clinicians) is still the mainstay of PCa treatment. Surgical (bilateral orchiectomy) or chemical (pharmaceutical) interventions resulting in the reduction of serum testosterone or blockade of the androgen receptor, are referred to as ADT. Antiandrogens alone— like flutamide, enzalutamide, and apalutamide (the modern derivatives)— are not considered ADT but are used in combination with surgical or chemical castration. ADT was initially achieved by orchiectomy as the testes are the principal source of circulating androgens (producing nearly 95% of total); the remaining 5% are produced by the adrenal glands. Luteinizing hormone-releasing hormone (LHRH) agonists are the most widely used contemporary ADT modality usually offered when patients are diagnosed either with recurrent cancer after first-line treatment (such as radical prostatectomy or radiation treatment) for local disease or with advanced (incurable) disease at presentation.3 This review provides for the practicing clinician and medical provider not only a systemic overview on the evolution and the oncologic dynamics in patients undergoing traditional ADT, but also the clinical aspects and indications for the emerging new pharmaceutical ADT modalities.
Chapter 1: Historical Overview
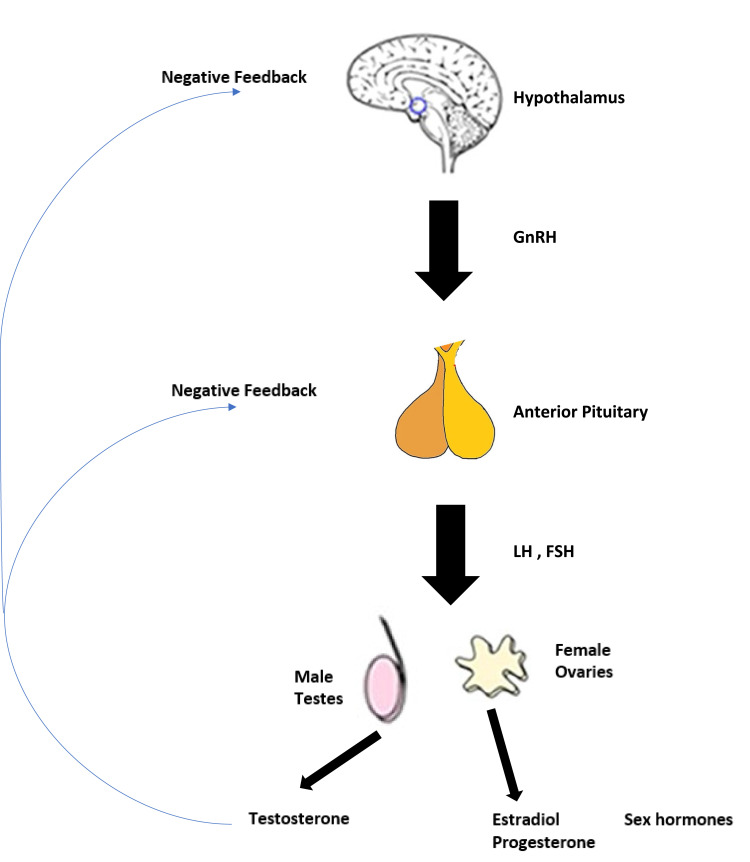
The Hypothalamic–Pituitary–Gonadal axis functions as a single system or entity due to direct interaction and feedback (Figure 1). Gonadotropin-releasing hormone (GnRH), also known as luteinizing hormone-releasing hormone (LHRH), is secreted from the hypothalamus by GnRH-expressing neurons and stimulates the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in the anterior portion of the pituitary gland which then stimulates the gonads for producing estrogen and testosterone. The therapeutic principle of Androgen Deprivation Therapy (ADT) in prostate cancer (PCa) has been established and not changed much over the last 80 years since Charles B. Huggins demonstrated the testosterone dependency of PCa.4 Huggins and Hodges first reported the dramatic clinical effects of suppressing serum testosterone levels (Figure 2) in men with advanced prostate cancer in 1941.5 Following bilateral orchiectomy of eight subjects with prostate cancer, serum acid phosphate levels decreased indicating decreased activity of the cancer. Five subjects with prostate cancer injected with stilbestrol also showed decreased serum levels of acid phosphatase while three subjects injected with testosterone propionate had an increase in serum acid phosphatase levels. For this pioneering work in treatment of advanced and metastatic prostate cancer Huggins was awarded the Nobel Prize for Medicine in 1966.
Figure 1.
The hypothalamus-pituitary-testicular axis.
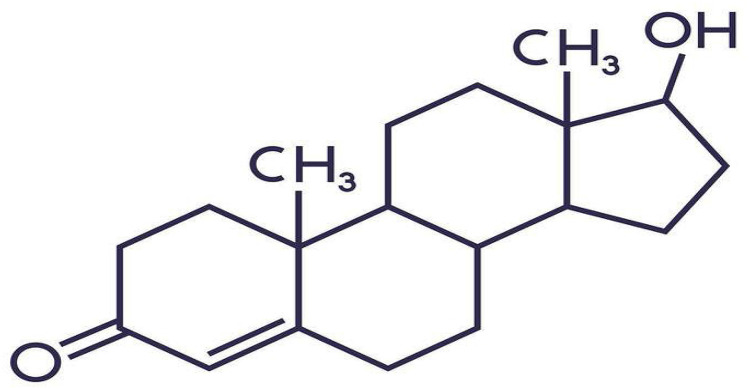
Figure 2.
Molecule structure of testosterone.
In 1938 diethylstilbestrol (DES, Figure 3) also known as stilbestrol or stilboestrol, was discovered and then introduced for medical use in 1939.6 DES is an estrogen and it suppresses luteinizing hormone-releasing hormone (LHRH) (Figure 4) production at the level of the hypothalamus. In the past, it was widely used for a variety of indications, including treatment of metastatic prostate cancer (“chemical castration”) and “hormonal support” during pregnancy for women with recurrent miscarriage. DES has excellent bioavailability and is well tolerated when taken by mouth.7
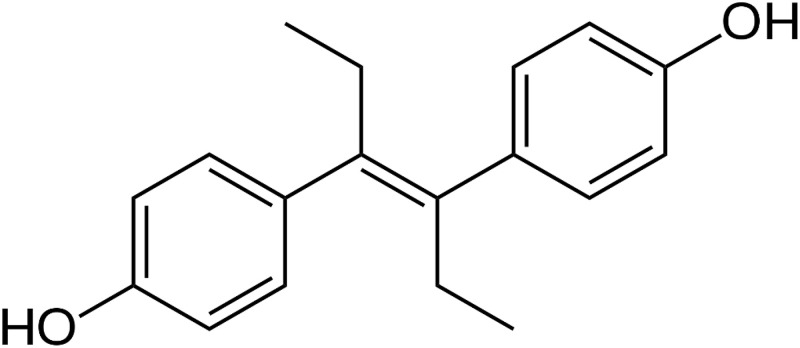
Figure 3.
Molecule structure of diethylstilbestrol (DES).
Figure 4.
Molecule structure of luteinizing hormone-releasing hormone (LHRH).
In 1959 the Veterans Administrative Cooperative Urological Research Group (VACURG) was established which further evolved ADT in the treatment of advanced prostate cancer. The VACURG used prospective randomized clinical trials for investigating the role of monotherapy of estrogen versus combined therapy of orchiectomy plus estrogen in advanced prostate cancer patients.8 The major conclusions were: 1.) A daily dose of 3 mg DES was considered the optimal dose and became the accepted regimen for pharmacologic castration. 2.) A lower DES dose (1 mg) was associated with reduced cardiovascular toxicity but did not reliably achieve castrate levels of testosterone. 3.) No survival advantage of early versus delayed initiation of hormonal therapy in advanced disease. Up to the 1970ʹs DES as hormonal therapy was widely accepted as the treatment for advanced and metastatic prostate cancer. However, its main adverse effects were increased risks of blood clots and cardiovascular events.9
Furthermore, reports in the 1970ʹs revealed that DES caused clear-cell carcinoma of the vagina, a rare tumor in girls and women who had been exposed to this medication in utero. Due to these discoveries and the significant cardiovascular side effects DES essentially disappeared from the pharmaceutical market.10
Both surgical and pharmacologic castration resulted in dramatic palliation of painful bony metastatic lesions, and improved quality of life in prostate cancer patients. In 1971 Schally and associates purified the decapeptide gonadotropin-releasing hormone (LHRH).9 Studies showed that constant exposure to LHRH ultimately suppressed testosterone serum levels by desensitizing pituitary cells through downregulation of the LHRH receptors.11 Modification of the sixth amino acid position of the LHRH molecule was significantly more potent.12 The monthly depot of leuprolide (Figure 5) was the first LHRH agonist granted FDA approval for advanced prostate cancer. In a randomized clinical trial, leuprolide was equivalent to 3 mg of DES in reducing serum testosterone to castrate levels, but with much lower cardiovascular toxicity.13 Ultimately in the 1980s Leuprolide replaced DES and orchiectomy as the preferred approach to androgen deprivation.
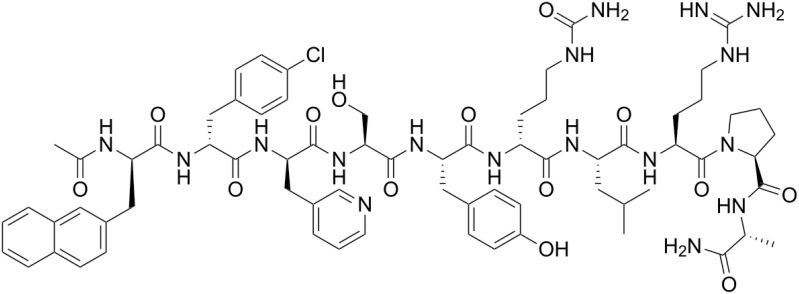
Figure 5.
Molecule structure of leuprolide.
Over the following decades substitutions at the sixth amino acid position yielded a variety of more LHRH agonists (goserelin, triptorelin, and histrelin), which then also became commercially available in the United States. These LHRH agonists are differentiated by their route of administration (intramuscular injection vs subcutaneous injection vs subcutaneous implant) and frequency of administration (1–12 months). All these LHRH agonists have similar therapeutic effectiveness in lowering serum testosterone to castrate levels, but also have a similar profile of adverse effects. One study directly compared different LHRH agonists.14 Overall improved survival with triptorelin compared with leuprolide, 97% vs 90.5% at 9 months (P = 0.033). Although not statistically significant, triptorelin seemed to better maintain castrate levels of testosterone over a 9-month interval.
The luteinizing hormone-releasing hormone (LHRH) agonists offered true medical castration but showed clinical issues due to testosterone surge and tumor flare when first administered. The “flare” phenomenon is attributed to the surge of serum testosterone levels caused by the initial stimulation of LHRH receptors. The introduction of antiandrogens was considered to ease this clinical issue by inhibiting the stimulation effect at the level of the androgen receptor.15 Despite initial expectations, the combined medical therapy of antiandrogens and LHRH agonists did not improve cancer-specific survival. The costs, inconvenience of therapy, added toxicity, and the introduction of the saturation model were considered the main reasons why the combined treatment never became standard of care. A 2017 literature review concluded that there is no evidence of testosterone flare following initiation of an LHRH agonist.16 It was proposed that this is due to the limited ability of androgens to stimulate prostate growth—also known as the saturation model.16 The saturation model, introduced in 2009, showed tumor growth increased when exposed to low levels of androgen, however after reaching a certain threshold, androgens no longer affected tumor growth. In the current literature there is still an ongoing and controversial debate on the validity of this saturation model.17
The introduction of Gonadotropin-releasing hormone (GnRH) antagonists offered a solution for the above mentioned “flare phenomenon”. This new drug class proved a rapid reduction of serum testosterone to castrate level without the initial testosterone surge and tumor flare. However, the long-term clinical benefits are still debated. A recent meta-analysis study comparing GnRH agonists and antagonists in patients with metastatic prostate cancer, showed that GnRH antagonist use was associated with a lower risk for all-cause mortality and cardiovascular events, respectively. However, this study did not find any significant differences between groups in PSA progression, musculoskeletal events, fractures or serious adverse events such as hepatic failure.18
In summary, ADT has come a long way since its introducing by Huggins for treatment of advanced and metastatic prostate cancer 80 years ago. Whereas surgical ADT via orchiectomy has become obsolete, medical ADT plays still a main role in the repertoire of treatment options for patients with recurrent, progressive and advanced prostate cancer.
Chapter 2: Different Types of ADT and Antiandrogen Therapy
LHRH/GnRH Agonists and Antagonists
LHRH/GnRH agonists, such as leuprorelin/leuprolide, goserelin, and triptorelin, are by far the most commonly utilized forms of ADT in clinical practice in the treatment of PCa, targeting the LHRH/GnRH receptor in the anterior pituitary gland and administered as an intramuscular or subcutaneous injection. They stimulate the receptor, creating a temporary surge in LH and testosterone levels followed by downregulation of the receptor over the next 2–3 weeks with a subsequent reduction in LH and suppression of testosterone production by the testes.19 They achieve serum testosterone levels below castration (<50 ng/dL) within 4–6 weeks with a subsequent reduction in the PSA level.20 The most common adverse effects (AE) associated with treatment are hot flashes, fatigue, sexual/erectile dysfunction, testicular atrophy, cognitive decline, increased risk of diabetes and cardiovascular events, and decreased bone mineral density associated with joint disorders and/or osteoporosis that needs to be monitored periodically with bone density scanning.21
LHRH/GnRH antagonists, such as degarelix, abarelix, and relugolix, are newer agents that competitively bind to the LHRH/GnRH receptor, inhibiting downstream LH signaling and suppressing testosterone secretion from the testes. LHRH/GnRH antagonists are not associated with an initial surge of serum testosterone, and castration levels are achieved within 2–3 days, so they are a good therapeutic option for the initiation of ADT in a newly diagnosed PCa patient.22 While degarelix is only available as a 1-month subcutaneous dose with a higher risk of adverse skin reactions, relugolix is a daily oral agent, although a food effect can reduce exposure by 50%.23 The side effect profile of LHRH/GnRH antagonists is similar to that of LHRH/GnRH agonists although since degarelix reduces FSH more than the LHRH/GnRH agonists (90 vs 50%), these lower levels of FSH may be cardioprotective, especially in men with preexisting cardiovascular disease, and may produce less muscle mass loss.24
Antiandrogens
Antiandrogens, such as bicalutamide, flutamide, and nilutamide, are some of the oldest drugs that inhibit binding of dihydrotestosterone (DHT) to the androgen receptor (AR).25 Since they do not reduce serum levels of testosterone, they can be considered as monotherapy in nonmetastatic patients who want to preserve libido and avoid the metabolic and sexual side effects of traditional ADT.26 They are, however, less effective than surgical castration or LHRH agonists/antagonists in metastatic prostate cancer (PCa) and are typically utilized concurrently with these agents for dual androgen blockade, especially during initiation of treatment with LHRH agonists to prevent clinical manifestations of testosterone surge within the first month.27 Table 1 summarizes single use versus combined use of ADT drugs and antiandrogens.
Table 1.
List of ADT Drugs and Antiandrogens That Can Be Used in Combination
| Drug Class | Drug Name | Combination |
|---|---|---|
| LHRH Agonists | Leuprolide | – |
| Goserelin | – | |
| Triptorelin | – | |
| LHRH Antagonists | Degarelix | – |
| Abarelix | – | |
| Relugolix | – | |
| Antiandrogens (Older) | Bicalutamide | + |
| Flutamide | + | |
| Nilutamide | + | |
| Antiandrogens (Newer) | Enzalutamide | + |
| Apalutamide | + | |
| Androgen Pathway Inhibitors | Abiraterone | + |
Notes: - Mainly single use. + Mainly used in combination.
Androgen Pathway Inhibitors
After long-term testosterone suppression, reactivation of AR pathways in some cell populations occur from multiple mechanisms including production of androgens by the adrenal glands and PCa cells themselves, androgen-independent activation of the AR, and AR gene amplification or overexpression.28 In this state, elimination of testosterone production from the testes is no longer sufficient to fully suppress PCa tumor cell growth, and another generation of antiandrogens is available for further testosterone suppression typically below 20 ng/dL.29
Abiraterone (in combination with traditional ADT) reduces androgen production from all sources including the testes, adrenal glands, and PCa cells through selective and irreversible inhibition of the enzyme 17α-hydroxylase/C17,20-lyase (CYP17), which can suppress testosterone levels even lower than with traditional LHRH/GnRH agonists alone. In the LATITUDE and STAMPEDE study, the addition of abiraterone acetate and prednisone to standard ADT significantly increased overall survival (OS) and radiographic progression-free survival (PFS) in men with newly diagnosed, metastatic, castration-sensitive PCa.30,31 In the LATITUDE study, OS was significantly longer in the abiraterone acetate plus prednisone group compared to the placebo group (median OS: 53.3 vs 36.5 months) when administered in combination with standard ADT.32 In a meta-analysis of both studies, abiraterone plus prednisone with standard ADT resulted in a 38% risk reduction of death compared to placebo with standard ADT for metastatic hormone-sensitive PCa.33 In addition to the expected AEs associated with testosterone suppression, abiraterone may also produce side effects associated with mineralocorticoid toxicity (ie, hypertension, hypokalemia, and fluid retention) and liver function abnormalities.34 Those patients with heart failure, recent myocardial infarction, cardiovascular disease, or ventricular arrhythmia need to be monitored more closely while on treatment.
Enzalutamide, another third-generation antiandrogen, competitively binds to the AR at the androgen-binding site, inhibiting nuclear translocation and interaction of the AR with chromosomal DNA, which prevents further transcription of tumor-associated Androgen genes. This prevention of AR-dependent transcription causes decreased cell proliferation and induces cell death. Enzalutamide blocks the action of testosterone at the cellular level regardless of where it is derived from and, in conjunction with traditional ADT, can be utilized for newly diagnosed, metastatic, castration-sensitive PCa.35 In the ENZAMET trial, enzalutamide with traditional ADT was associated with significantly longer PFS and OS compared to traditional ADT alone in men with metastatic, hormone-sensitive PCa (3-year OS: 80 vs 72%).36 In addition to the commonly expected AEs for an AR inhibitor, seizures and posterior reversible encephalopathy syndrome have also been seen on rare occasions, likely due to the drug crossing the blood−brain barrier.37 Seizure occurred in 0.4% of patients receiving treatment, but in patients with predisposing factors, seizures were reported in 2.2% of patients.
Apalutamide, another oral, nonsteroidal third-generation antiandrogen that blocks the action of testosterone by blocking the ligand-binding domain of the AR, was also designed to supersede the current androgen pathway inhibitors by overcoming AR-related resistance mechanisms. Like enzalutamide, it blocks androgen-receptor nuclear translocation, inhibits DNA binding, and obstructs AR-mediated transcription. In patients with non-metastatic, castrate-resistant PCa, according to the SPARTAN trial, metastasis-free survival (MFS) and time to symptomatic progression were significantly longer with apalutamide compared with placebo in combination with standard ADT (median MFS was 40.5 months in the apalutamide group versus 16.2 months in the placebo group).38 At median 52-month follow-up, median OS was markedly longer with apalutamide than placebo (73.9 vs 59.9 months).39 In the TITAN trial of patients with metastatic, castration-sensitive PCa, OS and PFS were significantly longer with the addition of apalutamide to standard ADT compared with placebo (OS at 24 months was 82.4% in the apalutamide group versus 73.5% in the placebo group).40 The most common adverse reactions with apalutamide were fatigue, hypertension, rash, and diarrhea.
The second and third generation antiandrogen drugs typically cost more than first-generation ADT drugs such as leuprolide or antiandrogens (bicalutamide) because they are not generic and usually patent-protected.
Surgical Castration
Surgical removal of both testicles (bilateral orchiectomy) remains a viable option as an alternative to chemical castration to eliminate testicular production of testosterone. Surgical castration may be associated with significantly lower risks of peripheral arterial disease and cardiac-related complications compared to chemical castration.41 Chemical castration, however, has largely replaced bilateral orchiectomy in clinical practice in the treatment of PCa because of the ease of administration, reversibility, and the avoidance of disfiguring surgery with its associated aesthetic and psychological consequences for patients.
Chapter 3: ADT Alone and in Combination with Maximum Androgen Blockade
ADT is a mainstay second-line treatment of prostate cancer once primary curative treatment, such as radiotherapy or radical prostatectomy, has failed. ADT blocks the HPG axis (for details see chapter 1), upon which testosterone production depends – this in turn starves androgen-dependent elements of a tumor. However, patients’ response to ADT is not uniform. Klotz et al 2015 performed a secondary analysis on data from the PR-7 trial, which had examined intermittent vs continuous ADT. They first excluded all patients who underwent intermittent ADT, then stratified the remaining men into three groups based on their nadir testosterone levels: a) <20ng/dL, b) 20–50ng/dL, and c) >50ng/dL. They found that men who reached a nadir level of 20–50ng/dL and >50ng/dL were at hazard ratios of 1.62 (95% CI 1.20–2.18) and 1.90 (95% CI 0.98–4.70) of developing castrate resistant prostate cancer compared to men who had reached a nadir below 20ng/dL (P<0.015). The same group of researchers also found that the median time to development of CRPC for the three subgroups (<20ng/dL, 20–50ng/dL, and >50ng/dL) were 10.0, 7.21, and 3.62 years respectively.42 Morote et al 2007 studied patients with non-metastatic disease who were on ADT either as a primary treatment, or as an adjuvant treatment after radical prostatectomy. They showed that men who had testosterone levels above 50ng/dL after reaching their nadir were at higher risk for androgen-independent progression (defined as 3 consecutive rising PSA levels) (HR 2.8, 95% CI 1.3–5.9, P<0.008).43
It is a common clinical experience that patients on ADT will develop CRPC at some point in the future, even with low, castrate serum levels of testosterone. Montgomery et al 2008 demonstrated that testosterone levels within the tumor tissue of anorchid men had elevated intra-tumoral testosterone levels (0.74 ng/gm, 95% CI 0.59–0.89) when compared to tumor tissue samples from untreated eugonadal men (0.23ng/gm, 95% CI 0.03–0.44, P<0.0001). In addition, tumor tissue from anorchid men also had significantly increased levels of steroidogenic enzymes. The authors concluded that prostate tumors are capable of sustaining themselves through autocrine/paracrine signaling and endogenous androgen production, even when blocking the HPG axis with ADT.44
Adrenal androgens are another source of androgens missed by the central blockade of LHRH. Brendler 1973 reviewed cases of adrenalectomy and hypophysectomy in “reactivated prostate cancer after failing castration therapy” (nowadays we would call this CRPC). Symptomatic improvement in the adrenalectomy group was 65%, with a similar rate for hypophysectomy patients.45 However, these improvements were short lived, as the prostate cancer in these patients would apparently adapt to a more androgen poor environment. In 1983, Labrie et al revisited the clinical idea of total androgen blockade when studying 87 men with metastatic prostate cancer, some of whom had received previous hormonal therapy and some not. All patients were treated with LHRH and RU-23908, an AR blocker. The results showed that serum prostatic acid phosphatase (PAP) dropped to normal levels in 97% of patients who had not previously received hormonal treatment and had an elevated PAP prior. They also noted positive objective radiologic responses in 100% of treatment-naïve patients, with several patients experiencing a complete disappearance of all bone lesions on imaging. They also noted that patients with previous systemic estrogen therapy showed a 55% response with PAP drop and an 80% response on bone imaging, respectively. The authors also found that men who had previously undergone hormonal therapy had a fast diminishing response to total androgen blockade, and they concluded that this clinical phenomenon was likely due to a selection process of tumor cell clones less dependent on systemic testosterone support.46 In 1991, Labrie et al published results of a prospective study based on a similar regimen as described earlier by substituting flumatide for RU-23908. They found that 93% of patients had a positive objective response, with 30% of patients experiencing objective regression of all bone lesions.47 In 1997, Ansari et al conducted a trial in which 100 men with metastatic castrate sensitive prostate cancer were randomized into either orchiectomy alone or orchiectomy plus flutamide. The results showed no significant difference in overall survival at 3 years, with orchiectomy alone at 45.83% and orchiectomy plus flutamide at 48.07%. This pattern held to the 5 year follow-up, with orchiectomy and orchiectomy plus flutamide at 20.83% and 23.07%, respectively.48
The next step forward came with abiraterone, a CPY17 inhibitor. It was first described in 1994 by Barrie et al as one of several novel compounds found to inhibit 17α-hydroxylase/C17,20 lyase. When testing several of these compounds on mice, there were significant reductions in the weight of the prostate (50%), seminal vesicles (75%), and testes (25%) (P<0.01 for all).49 The first human trial was done by O’Donnell et al in 2004. They tested different doses of abiraterone in men with advanced CRPC who were either still receiving or had received ADT. They found significant reductions in serum testosterone levels in all patients, with no grade III adverse events.50 In 2008, Attard et al performed a Phase I clinical trial of 21 patients who had known mCRPC. They found that 66% of patients experienced a reduction of PSA by 30% or more. In addition, serum testosterone became undetectable in all patients within 8 days of their first dose, and 8/11 patients who had required analgesics at baseline had reduced analgesic requirements after receiving abiraterone.51 There was a further improved response rate reported by Tran et al in 2009 in a phase I/II trial for enzalutamide: 43% of patients had a sustained reduction in PSA by 50% or more.52
The idea of maximum androgen blockade has been a goal for men with metastatic prostate cancer for decades. As mentioned earlier, Brendler described some positive therapeutic results in both patients who had undergone hypophysectomy to inhibit ACTH and thereby adrenal androgens, and in patients who had undergone adrenalectomy for the same reason. However, this success typically came at a high cost. The next step forward on maximum androgen blockade came with Labrie in 1983, when he described the use of both LHRH and a first-generation AR blocker. His results in CRPC were impressive, with many patients experiencing both objective responses on imaging as well as symptomatic relief. Newer anti-androgen pharmaceuticals like abiraterone and enzalutamide have shown impressive objective response rates in prospective and randomized FDA trials, and have evolved to be part of the updated AUA guidelines in the treatment repertoire for asymptomatic and symptomatic mCRPC.
Chapter 4: Early vs Delayed ADT
The timing of androgen deprivation therapy (ADT) in the management of recurrent and advanced prostate cancer has been controversial for many years. This is mainly due to a lack of adequate randomized clinical trials comparing early vs delayed ADT in patients with recurrent PSA following failure of local curative treatment. To date the available studies and current guidelines are stating that early use of ADT to be only beneficial in symptomatic patients with recurrent or metastatic disease. The EAU recommends ADT only in symptomatic patients requiring palliative treatment.53 In the following we summarize the current practice guidelines on timing of ADT in patients with recurrent prostate cancer after failing local curative treatment.
Messing et al performed one of the first landmark studies addressing the timing of androgen deprivation therapy (ADT) and its effect on survival in patients with node positive prostate cancer following radical prostatectomy and pelvic lymphadenectomy. This randomized controlled trial enrolled 100 patients between 1988 and 1993 who had previously undergone surgery and had histologically proven nodal metastasis. The patients were randomly assigned to receive either immediate ADT or active surveillance with ADT intervention only given on proven symptomatic recurrences or detection of distant metastasis. This study revealed superiority of immediate ADT compared to delayed ADT: Significantly improved overall survival (hazard ratio 1.84 [95% CI 1.01–3.35], p=0.04), disease specific survival (4.09 [1.76–9.49], p=0.0004) and progression free survival (3.42 [1.96–5.98], p<0.0001). The main points of criticism for this study were the low number of recruited patients and that this study did not involve patients with high-risk local disease without node involvement leading to uncertainty of optimal ADT timing for this category of patients.54 Additionally, this debate emphasized the need for further clinical research on optimal ADT timing in patients with PSA recurrence following local curative treatment.
The EAU/ESTRO/SIOG guidelines for localized prostate cancer state that routine use of ADT should be avoided in nonmetastatic patients with the exception for symptom control. This clinical recommendation is based on the EORTC Trial 30,891 which compared immediate versus deferred ADT in T0-4 N0-2 M0 prostate cancer patients unsuitable for local curative treatment. While this trial did not address PSA recurrence following local curative intervention (i.e RP vs RT), it did, however, provide evidence for the benefit of immediate ADT in patients at increased risk of cancer-specific mortality. This study included patients with high baseline PSA (>50ng/mL) and/or a PSA Doubling Time <12 months. The authors stated that patients not meeting these high-risk inclusion criteria were indeed more likely to die of other causes unrelated to prostate cancer.55
Similar to the European guidelines, the AUA/ASTRO/SUO guidelines do also not recommend early initiation of ADT without proven metastatic disease in patients who have failed maximal local therapy.56 This recommendation is mainly based on the observational study by Garcia-Albeniz et al in 2006 where eligible patients had previously been recruited for the Cancer of the Prostate Strategic Urologic Research Endeavour (CaPSURE). These patients had failed prior local curative treatment and had been treated either with immediate or deferred ADT. The study revealed no survival benefits for immediate ADT vs deferred ADT initiation in patients with recurrent PSA. The adjusted mortality hazard ratio for immediate versus deferred ADT was 0.91 (95% confidence interval (CI), 0.52–1.60), which would be translated into a similar 5-year survival (difference between groups: 2.0% (95% CI: 10.0 to 5.9%). This suggests that in the absence of randomized control studies early ADT initiation does not provide a major survival benefit compared to deferred ADT therapy.57
Van den Bergh and colleagues performed a systematic literature review to assess the effectiveness of ADT in patients with PSA recurrence following local curative treatment. This meta-analysis found that the benefit of early/immediate ADT for nonmetastatic prostate cancer recurrence remains unproven. The conclusion was that early ADT should be reserved for patients with the highest risk of progression based on PSADT or Gleason Score, but having a long life expectancy. This falls in line with the current standard of care recommendations of the EAU/ESTRO/SIOG and AUA/ASTRO/SUO.58
Overall, currently there is a lack of randomized controlled trials (RCT) assessing the impact of early compared to delayed ADT in the management of recurrent prostate cancer following local curative therapy, which has led to the continued controversial debate on this subject. To date no RCT has addressed or shown the benefit of specific ADT timing in patients with recurrent PSA. Bruchovsky and colleagues proposed the idea of Intermittent Androgen Deprivation Therapy as a means to reduce side effects and improve quality of life. However, further research is needed for clarification of the optimal timing of reexposure of prostate cancer cells to androgens and its impact on delaying androgen resistance, which may also be influenced by early or delayed ADT.59 This situation has led to the clinical practice that early ADT is provided only to symptomatic patients in particular when weighing the long-term risks associated with ADT. Unfortunately, due to the lack of adequate RCTs we still are waiting for clear answers regarding the oncological benefits of early vs delayed ADT in asymptomatic patients with recurrent or advanced prostate cancer.53
Chapter 5: Intermittent vs Continuous ADT for Recurrent and Advanced/Metastatic Prostate Cancer
Since the 1940ʹs, androgen deprivation therapy (ADT) has been the foundational treatment for prostate cancer. Bilateral orchiectomy, the original form of ADT, is still used worldwide, in particular in the developing world. Medical ADT options are the standard of care when available. It is well documented in the literature that ADT is associated with numerous significant adverse effects which include hot flashes, loss of libido, erectile dysfunction, loss of muscle mass and strength, fatigue, anemia, breast enlargement and tenderness, mood swings, osteoporosis and bone fractures, obesity, cardiovascular disease, insulin resistance and diabetes. Some studies also see ADT associated with cognitive decline and dementia (for details see Chapter 9 of this review).
Intermittent androgen deprivation therapy (iADT) is a cyclic therapy with cessation of ADT (also called ‘treatment holidays’ by some clinicians) allowing serum androgen recovery. The clinical idea is based on animal studies showing that iADT delayed tumor progression.60 Furthermore, the rationale for iADT is based on balancing drug-related toxicity and oncologic benefits. As continuous ADT (cADT) is associated with substantial side effects, which may increase with duration of therapy, many clinicians consider iADT as an alternative providing reduced morbidity, and thus improved quality of life, with the possible oncological benefit of delaying castration-resistant PCa.
The PR-7 trial, a landmark study, randomized patients for iADT and cADT with biochemical recurrence after either failing primary local treatment or salvage external radiotherapy.61 Eligible patients had PSA levels ≥ 3 ng/mL and no evidence for metastatic disease. The overall survival in patients that underwent iADT was 8.8 years compared to 9.1 years in patients that underwent cADT (HR=1.02, 95% CI= 0.86–1.21) indicating no significant difference. Furthermore, this study showed a non-inferiority P-value of 0.009 for iADT in overall survival. The study also revealed that other causes of death unrelated to PCa were more common in those receiving cADT, leading to the conclusion that intermittent therapy may not only reduce drug-related morbidity, but even mortality associated with cADT toxicity. Furthermore, patients with high-grade disease showed no improved overall cancer-specific survival when receiving cADT.
The ICELAND study published in 2016 is considered one of the main studies on iADT vs cADT in patients with relapsing prostate cancer.62 This prospective study included 102 different locations in 20 European countries and followed more than 700 participants randomized either to iADT or cADT. The authors found no difference in overall survival between the two groups, and they also emphasized the obvious reduced drug cost benefit of iADT over cADT.
The SWOG trial initiated by Dr. Hussain enrolled patients with metastatic, hormone-sensitive disease.63 All patients received an initial 7-month induction course of ADT. Patients with responding PSA levels ≤ 4 ng/mL were subsequently randomized to iADT or cADT (770 to iADT and 765 to cADT). The Kaplan-Meier curves were very similar, the hazard ratio was 1.10 with a confidence interval of 0.99 to 1.23. The pre-specified upper boundary of the confidence interval for non-inferiority was 1.20. Therefore, neither superiority nor non-inferiority could be concluded. However, the study data showed improved quality of life outcomes in the iADT arm: Better erectile function, improved bone density, less ischemic and thrombotic events.
A meta-analysis of seven studies with a total of 4810 patients treated with iADT or cADT between the years 2009 and 2015 showed no significant difference regarding cardiovascular events (risk ratio (RR): 0.95; confidence interval (CI) 95%=0.83–1.08) and thromboembolic events (RR: 1.05; CI 95%=0.85–1.30). However, iADT was associated with lower cardiovascular-related mortality.64
Another meta-analysis of randomized controlled trials (RCTs) assessed the risks of disease progression, all-cause, and cancer-specific mortality.65 Eight RCTs with 4664 patients were included in this report. It did not find any statistical difference in overall mortality and cancer-specific mortality between iADT and cADT. Again, the authors observed a better quality of life outcome for iADT, and therefore, they concluded that patients should be informed of the potential benefits of iADT.
Dason et al found that iADT was non-inferior to cADT in the primary setting of biochemical recurrence after radiation treatment of non-metastatic local prostate cancer. In the metastatic prostate cancer setting, iADT was also found to be non-inferior to cADT. Additionally, the authors reported that many ADT-related symptoms improved or resolved during the off-cycle with iADT.66
The results of a retrospective Japanese study on PSA recurrence after radical prostatectomy supported the hypothesis that iADT may delay castration-resistance in PCa.67 The iADT group had a significantly higher 5-year non-recurrence rate (92.9% vs 57.9%, p <0.001) and a better 10-year overall survival rate (95.9% vs 84.3%, p = 0.47) than the cADT group.
When summarizing the presented and pooled data, iADT provides better quality of life, whereas cancer-specific mortality shows interchangeable findings for iADT and cADT. Some studies even show improved overall survival for iADT patients likely due to reduced medication-related toxicity. Significantly reduced drug costs are also a strong rationale for iADT. Over more than one decade, iADT has evolved as the first option for patients after failing first-line local treatment in the absence of extensive metastatic disease. Although there are no well-defined recommendations or guidelines, the AUA/ASTRO/SUO website makes the following statement:
… if ADT is initiated in the absence of metastatic disease, intermittent ADT may be offered in lieu of continuous ADT. (Conditional Recommendation; Evidence Level: Grade B)68
iADT may be considered after at least 9–12 months of ADT or until PSA nadir has been reached (in an ideal clinical scenario with PSA < 0.1 ng/mL). The “off-treatment” period varies depending on PSA monitoring and PSA rising, but also on patient’s and physician’s preference. Again, there are no well-defined recommendations or guidelines at what PSA threshold ADT should be resumed. Some clinicians restart ADT when PSA doubling time (PSADT) is less than 6 months or when serum PSA has reached a level of 6–10 ng/mL. Some clinicians and medical providers set this threshold even far above 10 ng/mL.
Chapter 6: Combination of ADT with Radiation Therapy in the Management of Prostate Cancer
Radiation Therapy (RT) and its part in prostate cancer (PCa) management has continued to evolve as this Technology has improved over time, and research has led to a better understanding of the oncologic disease dynamics. Radiation therapy for prostate cancer consists of targeted energy beams such as External Beam Radiation Therapy (EBRT) or implantable radioactive seeds such as Brachytherapy, which destroy and thus eliminate neoplastic cells. While there are diverse subcategories of radiation-based therapies (such as Intensity Modulated Radiation Therapy, Proton Beam Therapy, and Low/High Dose Rate Implants), the risk and benefits of each as well as other factors such as the patient’s preference ultimately guide the decision of the selected type of therapy. There are varying degrees of early and late mainly gastrointestinal and genitourinary complications caused by RT which underscore the need for shared decision making between treating physicians and PCa patients.56,69
The current AUA/ASTRO/SUO guidelines for management of advanced prostate cancer recommend primary radiotherapy in combination with ADT as a treatment option for selected patients with hormone sensitive prostate cancer and low volume metastatic disease (mHSPC). This is only a conditional recommendation with Grade C (low) level evidence and is mainly based on two preliminary Phase III trials (HORRAD and STAMPEDE Arm H) showing some benefit of combined therapy in these patients.56 These studies are still ongoing and may provide additional information to guide clinical practice in the upcoming years.
However, the AUA/ASTRO/SUO do not recommend combined therapy on localized, low risk prostate cancer except for the management of prostatic size reduction in selected patients undergoing brachytherapy.69 This recommendation stems from the randomized, phase III trial by Jones et al in 1979 which failed to show any overall survival benefit in low risk prostate cancer patients receiving EBRT with ADT vs EBRT alone; the 10-year overall survival was 64% to 67% (hazard ratio, 1.07; 95% CI, 0.83 to 1.39). In this trial the greatest clinical benefit of combined therapy was seen in the intermediate prostate cancer group: overall survival improved from 54% to 61% (hazard ratio, 1.23; 95% CI, 1.02 to 1.49).70
In the HORRAD trial prostate cancer patients with bone metastasis showed no overall survival benefit with the combination of ADT and Radiation Therapy. This study was a multi-Center randomized controlled trial on primary metastatic prostate cancer (2004–2014), and 432 patients were randomized to ADT with Radiation Therapy vs ADT alone. The median survival was not statistically different: 45 months (95% confidence interval [CI], 40.4–49.6) in the ADT plus radiation therapy group, and 43 months (95% CI: 32.6–53.4) in the control group (p=0.4). The overall survival was also not found different (hazard ratio [HR]: 0.90; 95% CI: 0.70–1.14; p=0.4).71
In the STAMPEDE phase III trial (Arm H) more than 2000 patients with metastatic prostate cancer on lifelong ADT were randomized to receiving additional radiation therapy. Radiation Therapy did improve failure-free survival (HR 0.76, 95% CI 0.68–0.84; p<0.0001), but not overall survival (0.92, 0.80–1.06; p=0.266). However, radiation therapy showed a trend to improve overall survival in those patients with low metastatic burden (73% vs 81% at 3 years).72
Shipley et al performed the landmark trial on the benefits of combining ADT and Salvage Radiation Therapy for post prostatectomy patients with persistent/recurrent PSA. These patients had either pT2 disease with positive surgical margins or pT3 disease without nodal involvement. This double blind, placebo-controlled trial recruited 760 eligible patients between 1998 and 2003 who underwent 24 months of ADT (Bicalumatide) in addition to salvage radiation therapy. This study showed in the ADT plus radiation arm significant higher rates in overall survival, but also decreased incidence of metastasis and cancer-related deaths compared to radiation therapy alone.73
The clinical trial done by Warde et al confirmed the unequivocal benefits of combining ADT and Radiation therapy in the management of nonmetastatic, locally advanced high-risk prostate cancer. This randomized, phase III trial included 1205 patients with high-risk pT2 and pT3/T4 prostate cancer over a 10-year period (1995–2005) who were randomized either to Radiation Therapy + ADT or ADT alone. The results showed an explicit overall survival benefit when receiving the combination therapy (74% vs 66%).74
Based on current guidelines Low and Intermediate Risk local Prostate Cancer may be treated with EBRT alone.69 However, the still ongoing EORTC 22991 trial with over 800 patients randomized to radiation therapy alone vs radiation therapy plus ADT showed at 7.2 years median follow-up that the radiation therapy plus ADT arm had significantly improved biochemical disease-free survival (DFS) (HR, 0.52; 95% CI, 0.41 to 0.66; P = 0.001) as well as clinical progression free survival (PFS) (HR, 0.63; 95% CI, 0.48 to 0.84; P = 0.001). Overall survival data is still pending. These results suggest an overall benefit with combination therapy in improving biochemical and clinical disease-free survival.75
A meta-analysis by Spratt et al revealed that ADT and radiation treatment sequencing may also be an important aspect. Adjuvant ADT post radiation therapy improved Metastasis Free Survival (MFS) and Progression Free Survival (PFS) compared to Neoadjuvant ADT without increasing long-term toxicity. The authors reviewed two randomized Phase II Trials (Ottawa 0101 and RTOG 9413) in the management of localized prostate cancer, and concluded that delaying radiation therapy to perform neoadjuvant ADT did not lead to additional tumor control or reduced toxicity and may actually be inferior to adjuvant ADT.76
Summarizing the present and available data, it appears that the combined therapy (RT + ADT) is of greatest benefit in patients with high-risk local disease. In addition, some patients with hormone sensitive prostate cancer and low volume metastatic disease may also benefit from combination therapy.
Chapter 7: ADT in Treatment-Naïve Metastatic PCa, Alone vs Combination
Docetaxel
Docetaxel, a taxane-based systemic chemotherapy agent, was one of the first additional agents to emerge as a treatment with strong evidence for an overall survival (OS) benefit in patients with metastatic, castrate-sensitive prostate cancer in addition to standard ADT. In the CHAARTED trial, patients with metastatic, hormone-sensitive prostate cancer were randomized to treatment with either traditional ADT alone (which consisted of surgical castration with orchiectomy or medical castration with LHRH agonists such as leuprolide) versus ADT plus docetaxel, which they received as 75 mg/m2 every 21 days for six cycles.77 Median OS was 13.6 months longer (57.6 vs 44.0 months, HR: 0.61) in the ADT plus docetaxel group compared to traditional ADT alone.77 The survival benefit of adding docetaxel to ADT was even more pronounced in high-volume disease (defined as the presence of visceral metastases and/or greater than or equal to four bone lesions with at least one beyond the spine and/or pelvis) with median survival increased by 17.0 months compared to ADT alone (HR: 0.60).77 Additional benefits in the ADT plus docetaxel group included a longer time to the development of castrate-resistant disease, higher rate of decline of the PSA to <0.2 ng/mL at 12 months, and a lower incidence of prostate cancer-related death.77
Another trial called the STAMPEDE trial was published assessing the role of docetaxel in the metastatic hormone-sensitive prostate cancer space.78 When comparing the ADT plus docetaxel group to ADT alone, STAMPEDE again suggested an overall survival benefit with ADT and docetaxel for the subset of patients with metastatic disease (HR: 0.80).78 Patient selection in this trial notably included not only men with metastatic disease (61% of participants), but also patients with node-positive and high-risk localized disease.78
In a meta-analysis performed with the combined data from GETUG-AF15, CHAARTED, and STAMPEDE, men with metastatic castrate-sensitive disease across all three studies had a statistically significant overall survival benefit with the addition of docetaxel to traditional ADT (HR: 0.72).79 The combined data from all three trials yielded an overall 27% risk reduction of death for prostate cancer patients with metastatic disease (HR: 0.73), and a 33% risk reduction of death in high-volume, metastatic, castrate-sensitive prostate cancer patients (HR: 0.67).79
Abiraterone
Abiraterone acetate is an androgen biosynthesis inhibitor. Another analysis of the STAMPEDE trial utilized standard ADT alone versus ADT with abiraterone (1000 mg) daily with prednisolone (5 mg daily) to assess its role in men with metastatic, castrate-sensitive prostate cancer, nodal disease, or high-risk localized disease.31 Over a three-year follow-up, overall survival was 83% in the abiraterone plus ADT group vs 76% in the ADT alone group (HR: 0.63).31
In the LATITUDE trial, patients were randomly assigned to ADT alone versus ADT with abiraterone (1000 mg) daily with prednisolone (5 mg daily).30 After a median follow-up of 30.4 months, overall survival was significantly longer in the abiraterone + ADT group than in the ADT alone group (not reached versus 34.7 months, HR: 0.62).30 Progression-free survival was 33.0 months in the abiraterone + ADT group and 14.8 months in the ADT along group (HR: 0.47).30
Enzalutamide
Enzalutamide is a potent androgen-receptor inhibitor. In the ARCHES trial, 1150 men with metastatic, hormone-sensitive prostate cancer were randomized 1:1 to receive either 160 mg enzalutamide daily plus ADT or ADT plus placebo.80 Sixty-three percent of the study participants had high-volume disease, defined as either visceral metastases or ≥4 bony metastases with at least one outside the spine/pelvis.80 The risk of radiographic progression or death was significantly reduced with enzalutamide plus ADT compared to placebo plus ADT (HR: 0.39).80 This benefit was similarly seen regardless of disease volume or prior docetaxel chemotherapy. Enzalutamide plus ADT significantly reduced the risk of PSA progression, initiation of new antineoplastic therapy, first symptomatic skeletal-related event, castration resistance, and reduced risk of pain progression compared to ADT with placebo.80
Another contemporary trial evaluating enzalutamide in the metastatic, hormone-sensitive prostate cancer space, ENZAMET, randomized 1125 men with metastatic, castrate-sensitive prostate cancer to treatment with enzalutamide 160 mg daily + ADT versus traditional ADT alone.36 At median follow-up of 34 months, overall survival was improved in the enzalutamide + ADT group compared to the ADT alone group (HR: 0.67).36 Better outcomes with enzalutamide + ADT were also seen in PSA and clinical progression-free survival (HR: 0.39 and 0.40, respectively) compared to ADT alone.36 Enzalutamide was associated with significantly longer progression-free and overall survival than standard care with traditional ADT in men with metastatic, hormone-sensitive prostate cancer.36
Apalutamide
Apalutamide is an inhibitor of the ligand-binding domain of the androgen receptor. In the TITAN trial, over 1000 patients with metastatic, castration-sensitive prostate cancer were randomized to receive apalutamide (240 mg per day) with traditional ADT or placebo with ADT.40 A total of 10.7% had received previous docetaxel therapy, 62.7% had high-volume disease, and 37.3% had low-volume disease.40 Progression-free survival at 24 months was 68.2% in the apalutamide with ADT group versus 47.5% in the placebo with ADT group (HR: 0.48).40 Overall survival at 24 months was also greater with apalutamide + ADT than with placebo + ADT (82.4% versus 73.5%, HR: 0.67).40
Future Directions
All four agents (docetaxel, abiraterone, enzalutamide, and apalutamide) have been FDA-approved for the treatment of metastatic, castration-sensitive prostate cancer and are now listed as category 1 recommendations within the NCCN guidelines.81 Treatment choice between agents for metastatic, hormone-sensitive prostate cancer is a challenge, and there is currently no clear consensus on preferential initial selection or sequencing of these agents. There is, however, a moderate degree of uncertainty in the role of chemotherapy in low-volume disease patients.82 Marchioni et al systematically reviewed the literature according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) criteria and found that no treatment was superior to docetaxel in terms of overall survival.83 However, abiraterone (HR: 0.89), enzalutamide (HR: 0.90), and apalutamide (HR: 0.90) showed nonstatistically significant lower overall mortality rates when compared to docetaxel.83 Abiraterone (HR: 0.71), enzalutamide (HR: 0.61), and apalutamide (HR: 0.74) also showed statistically significant lower disease progression rates when compared to docetaxel.83
Chapter 8: ADT in Castrate-Resistant PCa
Overview of CRPC
Despite its effectiveness, nearly all patients on ADT will progress to castrate resistant prostate cancer (CRPC).84 CRPC is defined as biochemical or radiologic progression of disease in spite of castrate level testosterone (<50ng/dL). In the past, ADT was stopped after a cancer became castrate resistant – however, Taylor showed in 1993 that there was a survival benefit of 2–6 months with continued ADT.85 Since then, the AUA has recommended continuing ADT in both metastatic and non-metastatic CRPC.86 In 2015, Merseburger reviewed the rationale for continuing ADT as a backbone of treatment after development of CRPC, and found no trials to this point that compared either abiraterone or enzalutamide with ADT versus without. The SPARE trial done in Germany is comparing abiraterone monotherapy with abiraterone plus ADT – however, the final results are still pending. Hen comparing clinical studies Merseburger et al noticed that patients with CRPC who were started on abiraterone with prednisolone benefited from continued ADT, as the LH surge after discontinuing ADT overcame the inhibition of abiraterone. Similarly, they found that there was limited data comparing enzalutamide alone versus enzalutamide with ADT – all previously published phase III trials on enzalutamide had continuation of ADT as an inclusion criteria.87
In this context the NCCN guidelines state that “androgen receptor activation and autocrine/paracrine androgen synthesis are potential mechanisms of recurrence of prostate cancer during ADT (CRPC),” and they recommend continuation of traditional ADT.88 Harris et al 2009 studied mechanisms of androgen persistence in prostate cancer despite ADT – they found that prostate cancer cells could bypass castration with multiple mechanisms, including upregulation of the androgen receptor and synthesis of DHT in prostate cancer cells.89 Dai et al 2017 discusses changes to the androgen receptor itself as prostate cancer is treated. The androgen receptor is an intracytoplasmic steroid hormone receptor that functions as a nuclear transcription factor after an androgen binds to its ligand binding domain. In men with prostate cancer treated with ADT, mutations in the androgen receptor are seen in 10–30%. On of the most common involves the loss of the ligand binding domain (the target of enzalutamide), which uncouples transcription of the targets of the androgen receptor from its activation by androgens.90 As outlined below, these pathways provide targets for newer hormonal therapies.
Non-Metastatic CRPC
For a CRPC to be considered non-metastatic, it must meet the criteria of castrate resistance without radiologic evidence of metastasis on CT or technetium-99m bone scan. Once non-metastatic CRPC (nmCRPC for short) has been confirmed, the next important clinical variable is PSA doubling time (PSADT).
In patients with a PSADT greater than 10 months, the AUA and NCCN recommend continued ADT to maintain castrate level. Serial PSAs should be drawn every 3–6 months for adequate monitoring PSADT. Additionally, imaging should be obtained every 6–12 months for re-staging and to rule out development of metastatic disease.
In patients with a PSADT less than 10 months, the AUA and NCCN recommend continued ADT with a non-steroidal anti-androgen such as apalutamide, darolutamide, or enzalutamide. Smith et al 2018 showed a difference in median metastasis-free survival of 40.5 months vs 16.2 months in the apalutamide vs placebo group (P<0 0.001), as well as a significant benefit in time to symptomatic progression, with a hazard ratio of 0.45 (95% CI, 0.32–0.63 - P<0.001).91 Similar benefits for enzalutamide were shown by Hussain et al in 2018, with a difference in median metastasis free survival of 36.6 months for enzalutamide versus 14.7 months for placebo (P<0.001).92 Fizazi et al 2019 showed a similar benefit for darolutamide, with median metastasis free survival at 40.4 months versus 18.4 months for placebo (hazard ratio 0.41, 95% CI 0.34–0.50, P<0.001).93 Finally, a review by Mori et al 2020 showed that apalutamide and enzalutamide were more effective than darolutamide in regards to metastasis free survival and PSA-progression free survival, while darolutamide had an overall lower rate of adverse events.94
Metastatic CRPC
A patient is considered to have progressed from nmCRPC to metastatic CRPC (mCRPC) when metastatic disease is confirmed on imaging. Depending on previous treatments, the patient may have several options for ADT. Importantly, both the AUA and NCCN recommend continuation of traditional ADT with either GnRH/LHRH agonists or antagonists. Further treatment options depend on prior treatment.
In men with mCRPC without any prior novel hormone therapies (including abiraterone, apalutamide, enzalutamide, or darolutamide) several different treatment options are recommended - these include abiraterone with a steroid, docetaxel, and enzalutamide. Abiraterone is an inhibitor of cytochrome P-450c17, which ultimately manifests in inhibition of 17α-hydroxylase and 17,20-lyase. Ryan et al 2013 examined abiraterone with steroid versus placebo in patients with mCRPC on ADT, who had not been previously treated with chemotherapy. Median overall survival was not reached in the abiraterone plus steroid group, while it was 27.2 months in the placebo group (HR 0.75, 95% CI 0.61–0.93, P<0.01). They also benefit in radiographic progression free survival, with 16.5 months versus 8.3 months in the placebo group (Hazard ratio 0.53, 95% CI 0.45–0.62, P<0.001).95 De Bono et al 2011 examined the role of abiraterone in mCRPC after treatment with chemotherapy and noted an overall survival of 14.8 months for abiraterone plus prednisolone versus 10.9 months for placebo, with a hazard ratio of 0.66 (95% CI 0.56–0.79, P<0.001). The same study also noted a benefit to progression free survival according to radiographic imaging, with 5.6 months on abiraterone versus 3.6 months with placebo (HR 0.67, 95% CI 0.59–0.78, P<0.001).96
Similar results were found with the AR blocker enzalutamide in mCRPC. Beer et al 2014 examined enzalutamide in mCRPC before use of chemotherapy. They showed a benefit in in cancer-specific survival, where 72% on enzalutamide and 65% in the placebo group (Hazard ratio 0.71, 95% CI 0.60–0.84, P<0.001). Additionally, radiographic progression free survival was 65% in the enzalutamide group versus 14% in the placebo group (Hazard ratio 0.19, 95% CI 0.15–0.23, P<0.001).97 Scher et al showed similar benefits when enzalutamide was used after receiving chemotherapy for mCRPC, with a difference in survival of 18.4 months for enzalutamide versus 13.6 months for placebo (HR 0.63, 95% CI 0.53–0.75, P<0.001). The same study showed a benefit in radiographic progression free survival, with 8.3 months versus 2.9 months (HR 0.40, 95% CI 0.35–0.47, P<0.001).98
The conclusion of the above a data is that ADT is still standard of care in nmCRPC and mCRPC alike, even with the introduction of new modalities.
Chapter 9: Toxicity and Adverse Events of ADT
ADT has been associated with undesirable side effects ranging from musculoskeletal decline to autoimmune disorders. The aim of this narrative is to summarize and update the adverse effects of ADT with studies published within the past 6 years.
Musculoskeletal
It is well documented that ADT is associated with decreased bone mineral density (BMD) and an increased risk of developing osteoporosis and bone fractures. In one study, BMD decreased by 2.5%, 4.28%, 5.34%, and 6.16% after 6, 12, 18 and 24 months respectively following initiation of ADT.99 Patients on ADT also had an increased risk for any fracture (HR=1.4, CI=1.28–1.53), hip fractures (HR= 1.38, CI=1.20–1.58), and major osteoporotic fractures (HR=1.44, CI 1.28–1.61).100
Subsequent studies also illustrated the effect of ADT on muscle strength and volume. A 2016 study showed a decrease in self-reported physical functioning in men receiving ADT. Objective measurements of both grip strength and chair rise showed that grip strength was significantly diminished after 12 months of receiving ADT (P=0.01), and chair rise performance was significantly worse at both 6 and 12 months (P=0.02, P=0.003).101 Another study has confirmed differences in muscle volumes, measured by MRI, in patients on ADT. The levator ani muscle volume in men receiving ADT was significantly lower than in men of the control group (P=0.002). These men lost 16% of their initial baseline muscle volume when compared to the control. Patients on ADT had significant muscle loss in the gluteus maximus, iliopsoas, and quadriceps (P=0.017, P=0.013, P=0.031) along with increased intramuscular fat in the gluteus maximus (P=0.003).102
Metabolic Syndrome and Cardiovascular Disease
Metabolic syndrome is a set of symptoms that increase the risk of stroke, cardiovascular disease, and type II diabetes mellitus (DM). There is an increased risk of developing DM in prostate cancer patients treated with ADT than patients not treated with ADT (HR=1.49, CI= 1.34–1.66).103 ADT was also associated with higher risk of complications in patients previously diagnosed with DM. Patients on ADT had a 17% increased risk of developing diabetic retinopathy, 14% higher risk for diabetic neuropathy, and twice as likely to have diabetic amputations.104
In longitudinal cohort study of 190 men undergoing ADT, mean triglycerides (P<0.001), HDL cholesterol (P<0.001), and waist circumference (P<0.001) were significantly increased 6 months and 12 months after initiating ADT.105 Although HDL cholesterol is known to improve cardiovascular Health, an increase in overall cholesterol and triglycerides have negative effects as shown in the next two studies. Patients on ADT were at a higher risk of coronary heart disease and myocardial infarctions (OR=2.07, P<0.01).106 ADT also increased the risk for ischemic stroke (HR= 3.32, CI-1.14–9.67, P=0.028) when compared to non ADT users.107
Cognitive and Psychiatric
The effect of ADT on cognitive function is still controversial. In one study, patients on ADT had more cognitive deficits in language ability, short-term memory, mental flexibility, and inhibitory control (P<0.05) when compared to a control group.108 A literature review and meta-analysis found an increased risk of new dementia onset (of any cause) and Alzheimer disease in patients on ADT (HR=1.21, CI=1.11–1.33; HR=1.16, CI=1.07–1.72).109 However, study results are conflicting as another retrospective study using the Taiwan Longitudinal Health Insurance Database in 5340 patients found no significant difference in Alzheimer or Parkinson’s disease between patients receiving ADT and patients who did not receive ADT (HR=1.76, CI=0.55–5.62; HR=1.13, CI=0.58–2.20).110 When summarizing the reported data, several investigators think that there is a trend of cognitive decline under ADT, but they also agree that further prospective clinical studies are necessary.
A diagnosis of prostate cancer can be devastating patients and their families, and published data has shown that ADT can further increase psychiatric stress, and therefore, its impact must be considered when choosing the best alternative of therapy. The self-reported depression scores were higher in patients on ADT at 12 and 15 months when compared to patients with BPH or post prostatectomy.111 Furthermore, 43.1% of patients on ADT experienced higher incidence of anxiety when compared to control (P<0.001). This study also showed a correlation between longer duration of therapy and higher risk of anxiety (HR= 1.16, CI=2.04–1.29, P=0.01).112
Hematology and Immunology
Androgens can affect hematopoiesis and immunology. Patients on ADT are at increased risk of developing iron deficiency anemia (HR=1.62, CI 1.24–2.12).113 A retrospective study showed the association between ADT and the risk of developing any hematologic disorder including anemia and hematologic malignancy when compared to patients who underwent radical prostatectomy (HR=1.60, CI=1.29–1.97; HR 1.98, CI=1.62, 2.42). This associated risk even increased with longer duration of ADT (P<0.001).114
A study in 2016 analyzed the association between ADT and community acquired pneumonia: 62.2% of patients on ADT had respiratory events compared to 54.5% patients who did not receive ADT and 47.8% of patients who underwent one-sided orchiectomy (P<0.001). Patients with more than 11 doses of ADT were at increased risk for developing sinusitis, bronchitis, and pneumonia (HR=1.13; HR=1.26; HR=1.15; all P<0.001).115
The association between ADT and autoimmune diseases apparently depend on the type of disease. Whereas patients on ADT had a 23% increased risk of developing rheumatoid arthritis (HR1.23, CI 1.09–1.40),116 ADT seems to have a protective role in developing inflammatory bowel diseases: A decreased risk for ulcerative colitis (HR= 0.52, CI= 0.28–0.99) and Crohn’s disease (HR=0.38, CI 0.11–1.37).117
Although ADT is an effective treatment for prostate cancer, it comes with many risks and potentially harmful side effects. Therefore, a detailed risk-benefit discussion should be provided to the patient before initiating this form of treatment.
Chapter 10: Maintaining Bone Health in the Setting of ADT
Patient Education
Bone mineral density (BMD) testing in patients on ADT is underutilized, and many men are unaware how to monitor and maintain good bone health. Clinical investigators have recommended to address patient education on risks of osteoporosis and strategies to improve bone health while on ADT. A 2018 study showed that a bone health pamphlet and support from a bone-health care coordinator resulted in a significantly higher percentage of men undergoing BMD testing when compared to men who underwent “usual care” (P<0.001). This bone health pamphlet given by the family physician also resulted in a significantly higher percentage of BMD testing (P=0.047).118
Lifestyle and Exercise
Lifestyle modifications including smoking cessation and reduced alcohol intake are recommended for patients on ADT.119 In addition, recent studies have confirmed the benefits of exercise on muscle and bone health for men on ADT. A 2019 study compared BMD in ADT patients who were randomized into immediate exercise and delayed exercise (6 months of “usual activity” followed by a 6-month exercise program). There was significant preservation of lumbar spine BMD in the immediate exercise group when compared to the delayed exercise group. There were no significant differences in whole body, spine, or hip BMD. Lean mass, appendicular skeletal muscle, and muscle density were preserved in the immediate exercise group after 6 months, while the delayed exercise group recovered after 12 months.120 A 2018 randomized and controlled trial evaluated the effect of home-based exercise intervention on bone-health outcomes. Although there was no difference in bone health, this study showed significantly improved muscle strength in the home-based exercise group when compared to the placebo intervention of stretching exercise.121 Improved muscle strength not only improves vitality in patients on ADT, but may also improve some of the metabolic side effects of ADT. There is some evidence of reduced risks of accidental falls and fractures in patients on ADT when participating in exercising programs, however there is a lack of robust prospective and randomized clinical trials to support this hypothesis.
Calcium and Vitamin D
The National Osteoporosis Foundation recommends a daily calcium intake of 1200 mg and vitamin D supplement of 800–1000 IU/d for all men over the age of 50.122 A 2015 study analyzed whether the recommended vitamin D supplementation of 800IU/d increased blood levels of 25-OH vitamin D in patients receiving ADT. Regression analysis showed vitamin D supplementation was associated with increased 25-OH vitamin D serum levels supporting the current recommendation of 800 IU/d for men receiving ADT.123
Bone Protective Drugs
Two controlled studies have analyzed the effect of zoledronic acid on BMD in patients undergoing ADT. In one study with 32 ADT patients diagnosed with nonmetastatic prostate cancer and osteopenia or osteoporosis received zoledronic acid for 12 months or no treatment. The patients on zoledronic acid treatment were significantly older than the control group and had lower BMD at baseline. BMD of the lumbar spine and hip were significantly increased in the patients on zoledronic acid following 12 months of treatment.124 Similar results were found in a 2-year trial of 76 men showing increased BMD in the lumbar spine and hip when on zoledronic acid versus the control. However, there was no difference in bone microarchitecture measured by high-resolution peripheral quantitative computed tomography indicating that zoledronic acid may slow but does not prevent unbalanced bone modeling.125 These studies have small sample size and inconsistencies in dosing of zoledronic acid, which limit the scientific value, and therefore, larger prospective and randomized clinical trials are needed.
Denosumab, a RANKL inhibitor, has been shown to have similar clinical efficacy when compared to alendronate (Fosamax). One study divided patients into 4 groups: 1) treated with denosumab, 2) treated with alendronate, 3) no treatment, 4) previously treated with alendronate and switched to denosumab. After 1 year, the patients who were treated with denosumab or alendronate had increased bone mass in the lumbar spine and femoral neck when compared to the control. Men treated with denosumab had significantly higher bone mass in the total hip while there was no significant change in men treated with alendronate.126 A subsequent 2017 study on denosumab showed significantly increased bone turnover markers and BMD when compared to alendronate; furthermore, a decreased rate of vertebral fractures were observed.127
Osteonecrosis of the jaw is the most common significant adverse effect of zoledronic acid and denosumab. A retrospective study in 2021 analyzed the incidence of agent-related jaw osteonecrosis in prostate cancer patients: 27.5% developed this feared osteonecrosis of the jaw within 5 years of treatment with a bone-modifying agent.128
Maintaining bone health in prostate cancer patients on ADT is an important clinical aspect as musculoskeletal side effects are common with ADT. The use of vitamin D, calcium, and bone modifying drugs should be properly discussed with patients on ADT in order to protect bone health.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. [DOI] [PubMed] [Google Scholar]
- 2.Ito K. Prostate cancer in Asian men. Nat Rev Urol. 2014;11(4):197–212. [DOI] [PubMed] [Google Scholar]
- 3.Connolly RM, Carducci MA, Antonarakis ES. Use of androgen deprivation therapy in prostate cancer: indications and prevalence. Asian J Androl. 2012;14(2):177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debruyne F. Hormonal therapy of prostate cancer. Semin Urol Oncol. 2002;20(3 Suppl 1):4–9. [DOI] [PubMed] [Google Scholar]
- 5.Huggins CHC. Studies on prostatic cancer: the effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–223. [Google Scholar]
- 6.Veurink M, Koster M, Berg LT. The history of DES, lessons to be learned. Pharm World Sci. 2005;27(3):139–143. [DOI] [PubMed] [Google Scholar]
- 7.Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8(Suppl 1):3–63. [DOI] [PubMed] [Google Scholar]
- 8.Treatment and survival of patients with cancer of the prostate. The Veterans Administration Co-operative Urological Research Group. Surg Gynecol Obstet. 1967;124(5):1011–1017. [PubMed] [Google Scholar]
- 9.Schally AV, Arimura A, Baba Y, et al. Isolation and properties of the FSH and LH-releasing hormone. Biochem Biophys Res Commun. 1971;43(2):393–399. [DOI] [PubMed] [Google Scholar]
- 10.Smith EK, White MC, Weir HK, Peipins LA, Thompson TD. Higher incidence of clear cell adenocarcinoma of the cervix and vagina among women born between 1947 and 1971 in the United States. Cancer Causes Control. 2012;23(1):207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conn PM, Rogers DC, Seay SG. Biphasic regulation of the gonadotropin-releasing hormone receptor by receptor microaggregation and intracellular Ca2+ levels. Mol Pharmacol. 1984;25(1):51–55. [PubMed] [Google Scholar]
- 12.van Loenen AC, Huirne JA, Schats R, Hompes PG, Lambalk CB. GnRH agonists, antagonists, and assisted conception. Semin Reprod Med. 2002;20(4):349–364. [DOI] [PubMed] [Google Scholar]
- 13.Leuprolide Study Group. Leuprolide versus diethylstilbestrol for metastatic prostate cancer. N Engl J Med. 1984;311(20):1281–1286. [DOI] [PubMed] [Google Scholar]
- 14.Heyns CF, Simonin MP, Grosgurin P, Schall R, Porchet HC. Comparative efficacy of triptorelin pamoate and leuprolide acetate in men with advanced prostate cancer. BJU Int. 2003;92(3):226–231. [DOI] [PubMed] [Google Scholar]
- 15.Schulze H, Senge T. Influence of different types of antiandrogens on luteinizing hormone-releasing hormone analogue-induced testosterone surge in patients with metastatic carcinoma of the prostate. J Urol. 1990;144(4):934–941. [DOI] [PubMed] [Google Scholar]
- 16.Krakowsky Y, Morgentaler A. Risk of Testosterone Flare in the Era of the Saturation Model: one More Historical Myth. Eur Urol Focus. 2019;5(1):81–89. [DOI] [PubMed] [Google Scholar]
- 17.Kim JW. Questioning the evidence behind the Saturation Model for testosterone replacement therapy in prostate cancer. Investig Clin Urol. 2020;61(3):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abufaraj M, Iwata T, Kimura S, et al. Differential Impact of Gonadotropin-releasing Hormone Antagonist Versus Agonist on Clinical Safety and Oncologic Outcomes on Patients with Metastatic Prostate Cancer: a Meta-analysis of Randomized Controlled Trials. Eur Urol. 2021;79(1):44–53. [DOI] [PubMed] [Google Scholar]
- 19.Miyazawa Y, Kato H, Arai S, et al. Clinical endocrinological evaluation of the gonadal axis (testosterone, LH and FSH) in prostate cancer patients switched from a GnRH antagonist to a LHRH agonist. Basic Clin Androl. 2015;25:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkateswaran S, Margel D, Yap S, Hersey K, Yip P, Fleshner NE. Comparison of serum testosterone levels in prostate cancer patients receiving LHRH agonist therapy with or without the removal of the prostate. Can Urol Assoc J. 2012;6(3):183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richard PO, Fleshner NE, Bhatt JR, et al. randomised, double-blind, placebo-controlled trial of methylphenidate for reduction of fatigue levels in patients with prostate cancer receiving LHRH-agonist therapy. BJU Int. 2015;116(5):744–752. [DOI] [PubMed] [Google Scholar]
- 22.Garnick MB. Important considerations in LHRH antagonist therapy for prostate cancer. Oncology. 2009;23(7):636–637. [PubMed] [Google Scholar]
- 23.Shore ND, Saad F, Cookson MS, et al. Oral Relugolix for Androgen-Deprivation Therapy in Advanced Prostate Cancer. N Engl J Med. 2020;382(23):2187–2196. [DOI] [PubMed] [Google Scholar]
- 24.Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102(11):1531–1538. [DOI] [PubMed] [Google Scholar]
- 25.Iversen P. Bicalutamide monotherapy for early stage prostate cancer: an update. J Urol. 2003;170(6 Pt 2):S48–52. [DOI] [PubMed] [Google Scholar]
- 26.Wadhwa VK, Weston R, Parr NJ. Bicalutamide monotherapy preserves bone mineral density, muscle strength and has significant health-related quality of life benefits for osteoporotic men with prostate cancer. BJU Int. 2011;107(12):1923–1929. [DOI] [PubMed] [Google Scholar]
- 27.Boccardo F, Barichello M, Battaglia M, et al. Bicalutamide monotherapy versus flutamide plus goserelin in prostate cancer: updated results of a multicentric trial. Eur Urol. 2002;42(5):481–490. [DOI] [PubMed] [Google Scholar]
- 28.Cai C, Wang H, Xu Y, Chen S, Balk SP. Reactivation of androgen receptor-regulated TMPRSS2: eRGgene expression in castration-resistant prostate cancer. Cancer Res. 2009;69(15):6027–6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu J, Wang G, Sun T. Dissecting the roles of the androgen receptor in prostate cancer from molecular perspectives. Tumour Biol. 2017;39(5):1010428317692259. [DOI] [PubMed] [Google Scholar]
- 30.Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017;377(4):352–360. [DOI] [PubMed] [Google Scholar]
- 31.James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377(4):338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, Phase 3 trial. Lancet Oncol. 2019;20(5):686–700. [DOI] [PubMed] [Google Scholar]
- 33.Rydzewska LHM, Burdett S, Vale CL, et al. Adding Abiraterone to androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: a systematic review and meta-analysis. Eur J Cancer. 2017;84:88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreira RB, Debiasi M, Francini E, et al. Differential side effects profile in patients with mCRPC treated with Abiraterone or enzalutamide: a meta-analysis of randomized controlled trials. Oncotarget. 2017;8(48):84572–84578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweeney CJ, Martin AJ, Stockler MR, et al. Overall Survival of Men with Metachronous Metastatic Hormone-sensitive Prostate Cancer Treated with Enzalutamide and Androgen Deprivation Therapy. Eur Urol. 2021;80(3):275–279. [DOI] [PubMed] [Google Scholar]
- 36.Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med. 2019;381(2):121–131. [DOI] [PubMed] [Google Scholar]
- 37.Slovin S, Clark W, Carles J, et al. Seizure Rates in Enzalutamide-Treated Men With Metastatic Castration-Resistant Prostate Cancer and Risk of Seizure: the UPWARD Study. JAMA Oncol. 2018;4(5):702–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith MR, Saad F, Chowdhury S, et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med. 2018;378(15):1408–1418. [DOI] [PubMed] [Google Scholar]
- 39.Smith MR, Saad F, Chowdhury S, et al. Apalutamide and Overall Survival in Prostate Cancer. Eur Urol. 2021;79(1):150–158. [DOI] [PubMed] [Google Scholar]
- 40.Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2019;381(1):13–24. [DOI] [PubMed] [Google Scholar]
- 41.Sun M, Choueiri TK, Hamnvik OP, et al. Comparison of Gonadotropin-Releasing Hormone Agonists and Orchiectomy: effects of Androgen-Deprivation Therapy. JAMA Oncol. 2016;2(4):500–507. [DOI] [PubMed] [Google Scholar]
- 42.Klotz L, O’Callaghan C, Ding K, et al. Nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: a secondary analysis of the PR-7 trial of intermittent versus continuous ADT. J Clin Oncol. 2015;33(10):1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morote J, Orsola A, Planas J, et al. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol. 2007;178(4 Pt 1):1290–1295. [DOI] [PubMed] [Google Scholar]
- 44.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brendler H. Adrenalectomy and hypophysectomy for prostatic cancer. Urology. 1973;2(2):99–102. [DOI] [PubMed] [Google Scholar]
- 46.Labrie F, Dupont A, Belanger A, et al. New approach in the treatment of prostate cancer: complete instead of partial withdrawal of androgens. The Prostate. 1983;4(6):579–594. [DOI] [PubMed] [Google Scholar]
- 47.Labrie F. Endocrine Therapy for Prostate Cancer. Endocrinol Metab Clin North Am. 1991;20(4):845–872. [PubMed] [Google Scholar]
- 48.Ansari MS, Gupta NP, Hemal AK, Dogra PN, Seth A. Combined androgen blockade in the management of advanced prostate cancer: a sensible or ostensible approach. Int J Urol. 2004;11(12):1092–1096. [DOI] [PubMed] [Google Scholar]
- 49.Barrie SE, Potter GA, Goddard PM, Haynes BP, Dowsett M, Jarman M. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase). J Steroid Biochem Mol Biol. 1994;50(5–6):267–273. [DOI] [PubMed] [Google Scholar]
- 50.O’Donnell A, Judson I, Dowsett M, et al. Hormonal impact of the 17α-hydroxylase/C17,20-lyase inhibitor Abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90(12):2317–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, Abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26(28):4563–4571. [DOI] [PubMed] [Google Scholar]
- 52.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol. 2017;71(4):630–642. [DOI] [PubMed] [Google Scholar]
- 54.Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7(6):472–479. [DOI] [PubMed] [Google Scholar]
- 55.Studer UE, Collette L, Whelan P, et al. Using PSA to guide timing of androgen deprivation in patients with T0-4 N0-2 M0 prostate cancer not suitable for local curative treatment (EORTC 30891). Eur Urol. 2008;53(5):941–949. [DOI] [PubMed] [Google Scholar]
- 56.Lowrance WT, Breau RH, Chou R, et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART I. J Urol. 2021;205(1):14–21. [DOI] [PubMed] [Google Scholar]
- 57.Garcia-Albeniz X, Chan JM, Paciorek A, et al. Immediate versus deferred initiation of androgen deprivation therapy in prostate cancer patients with PSA-only relapse. An observational follow-up study. Eur J Cancer. 2015;51(7):817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van den bergh RC, van Casteren NJ, van den Broeck T, et al. Role of Hormonal Treatment in Prostate Cancer Patients with Nonmetastatic Disease Recurrence After Local Curative Treatment: a Systematic Review. Eur Urol. 2016;69(5):802–820. [DOI] [PubMed] [Google Scholar]
- 59.Klotz L. The history of intermittent androgen deprivation therapy - A Canadian story. Can Urol Assoc J. 2020;14(6):159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruchovsky N, Rennie PS, Coldman AJ, Goldenberg SL, To M, Lawson D. Effects of androgen withdrawal on the stem cell composition of the Shionogi carcinoma. Cancer Res. 1990;50(8):2275–2282. [PubMed] [Google Scholar]
- 61.Crook JMMS, Horwitz E. A Phase III Randomized Trial of Intermittent vs. Continuous Androgen Suppression for PSA Progression after Radical Therapy (NCIC CTG PR.7/SWOG JPR.7/CTSU JPR.7/UK Intercontinental Trial CRUKE/01/013). Int J Rad Oncol. 2011;81(2):supplS5. [Google Scholar]
- 62.Schulman CCE, Matveev V. Intermittent Versus Continuous Androgen Deprivation Therapy in Patients with Relapsing or Locally Advanced Prostate Cancer: a 3b Randomised Study (Iceland). Eur Urol. 2016;69(4):720–727. [DOI] [PubMed] [Google Scholar]
- 63.Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368(14):1314–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin C, Fan Y, Meng Y, et al. A meta-analysis of cardiovascular events in intermittent androgen-deprivation therapy versus continuous androgen-deprivation therapy for prostate cancer patients. Prostate Cancer Prostatic Dis. 2016;19(4):333–339. [DOI] [PubMed] [Google Scholar]
- 65.Tsai HT, Penson DF, Makambi KH, Lynch JH, Van Den Eeden SK, Potosky AL. Efficacy of intermittent androgen deprivation therapy vs conventional continuous androgen deprivation therapy for advanced prostate cancer: a meta-analysis. Urology. 2013;82(2):327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dason S, Allard CB, Wang JG, Hoogenes J, Shayegan B. Intermittent androgen deprivation therapy for prostate cancer: translating randomized controlled trials into clinical practice. Can J Urol. 2014;21(2 Supp 1):28–36. [PubMed] [Google Scholar]
- 67.Maru S, Uchino H, Osawa T, Chiba S, Mouri G, Sazawa A. Long-term treatment outcomes of intermittent androgen deprivation therapy for relapsed prostate cancer after radical prostatectomy. PLoS One. 2018;13(5):e0197252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.AUA/ASTOR/SUO. Advanced Prostate Cancer Guidelines. 2020.
- 69.Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: risk Stratification, Shared Decision Making, and Care Options. J Urol. 2018;199(3):683–690. [DOI] [PubMed] [Google Scholar]
- 70.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365(2):107–118. [DOI] [PubMed] [Google Scholar]
- 71.Boevé LMS, Hulshof M, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: data from the HORRAD Trial. Eur Urol. 2019;75(3):410–418. [DOI] [PubMed] [Google Scholar]
- 72.Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shipley WU. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. N Engl J Med. 2017;376(5):417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Warde P, Mason M, Ding K, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378(9809):2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bolla M, Maingon P, Carrie C, et al. Short Androgen Suppression and Radiation Dose Escalation for Intermediate- and High-Risk Localized Prostate Cancer: results of EORTC Trial 22991. J Clin Oncol. 2016;34(15):1748–1756. [DOI] [PubMed] [Google Scholar]
- 76.Spratt DE, Malone S, Roy S, et al. Prostate Radiotherapy With Adjuvant Androgen Deprivation Therapy (ADT) Improves Metastasis-Free Survival Compared to Neoadjuvant ADT: an Individual Patient Meta-Analysis. J Clin Oncol. 2021;39(2):136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373(8):737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tucci M, Bertaglia V, Vignani F, et al. Addition of Docetaxel to Androgen Deprivation Therapy for Patients with Hormone-sensitive Metastatic Prostate Cancer: a Systematic Review and Meta-analysis. Eur Urol. 2016;69(4):563–573. [DOI] [PubMed] [Google Scholar]
- 80.Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019;37(32):2974–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohler JL, Antonarakis ES. NCCN Guidelines Updates: management of Prostate Cancer. J Natl Compr Canc Netw. 2019;17(5):583–586. [DOI] [PubMed] [Google Scholar]
- 82.Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol. 2018;36(11):1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marchioni M, Di Nicola M, Primiceri G, et al. New Antiandrogen Compounds Compared to Docetaxel for Metastatic Hormone Sensitive Prostate Cancer: results from a Network Meta-Analysis. J Urol. 2020;203(4):751–759. [DOI] [PubMed] [Google Scholar]
- 84.Lokeshwar SD, Klaassen Z, Saad F. Treatment and trials in non-metastatic castration-resistant prostate cancer. Nat Rev Urol. 2021;18(7):433–442. [DOI] [PubMed] [Google Scholar]
- 85.Taylor CD, Elson P, Trump DL. Importance of continued testicular suppression in hormone-refractory prostate cancer. J Clin Oncol. 1993;11(11):2167–2172. [DOI] [PubMed] [Google Scholar]
- 86.Lowrance WT, Breau RH, Chou R, et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART II. J Urol. 2021;205(1):22–29. [DOI] [PubMed] [Google Scholar]
- 87.Merseburger AS, Hammerer P, Rozet F, et al. Androgen deprivation therapy in castrate-resistant prostate cancer: how important is GnRH agonist backbone therapy? World J Urol. 2015;33(8):1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Network NCC. Prostate Cancer (version 1.2022); 2021. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed August, 10 2021.
- 89.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6(2):76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dai C, Heemers H, Sharifi N. Androgen Signaling in Prostate Cancer. Cold Spring Harb Perspect Med. 2017;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith MR, Saad F, Chowdhury S, et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Eng J Med. 2018;378(15):1408–1418. [DOI] [PubMed] [Google Scholar]
- 92.Hussain M, Fizazi K, Saad F, et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med. 2018;378(26):2465–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fizazi K, Shore N, Tammela TL, et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N Eng J Med. 2019;380(13):1235–1246. [DOI] [PubMed] [Google Scholar]
- 94.Mori K, Mostafaei H, Pradere B, et al. Apalutamide, enzalutamide, and darolutamide for non-metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis. Int J Clin Oncol. 2020;25(11):1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. [DOI] [PubMed] [Google Scholar]
- 99.Miyazawa Y, Sekine Y, Syuto T, et al. Effect of Androgen-deprivation Therapy on Bone Mineral Density in Japanese Patients with Prostate Cancer. Vivo. 2018;32(2):409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wallander M, Axelsson KF, Lundh D, Lorentzon M. Patients with prostate cancer and androgen deprivation therapy have increased risk of fractures-a study from the fractures and fall injuries in the elderly cohort (FRAILCO). Osteoporos Int. 2019;30(1):115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gonzalez BD, Jim HSL, Small BJ, et al. Changes in physical functioning and muscle strength in men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Support Care Cancer. 2016;24(5):2201–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheung AS, Cunningham C, Ko DD, et al. Selective Loss of Levator Ani and Leg Muscle Volumes in Men Undergoing Androgen Deprivation Therapy. J Clin Endocrinol Metab. 2019;104(6):2229–2238. [DOI] [PubMed] [Google Scholar]
- 103.Drevinskaite M, Patasius A, Kincius M, Urbonas V, Smailyte G. Retrospective cohort study of androgen deprivation therapy and the risk of diabetes in men with prostate cancer in Lithuania. BMJ Open. 2021;11(7):e045797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bradley MC, Zhou Y, Freedman AN, et al. Risk of diabetes complications among those with diabetes receiving androgen deprivation therapy for localized prostate cancer. Cancer Causes Control. 2018;29(8):785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rezaei MM, Rezaei MM, Ghoreifi A, Kerigh BF. Metabolic syndrome in patients with prostate cancer undergoing intermittent androgen-deprivation therapy. Can Urol Assoc J. 2016;10(9–10):E300–e305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morgia G, Russo GI, Tubaro A, et al. Prevalence of Cardiovascular Disease and Osteoporosis During Androgen Deprivation Therapy Prescription Discordant to EAU Guidelines: results From a Multicenter, Cross-sectional Analysis From the CHOsIng Treatment for Prostate canCEr (CHOICE) Study. Urology. 2016;96:165–170. [DOI] [PubMed] [Google Scholar]
- 107.Teoh JY, Chiu PK, Chan SY, et al. Risk of ischemic stroke after androgen deprivation therapy for prostate cancer in the Chinese population living in Hong Kong. Jpn J Clin Oncol. 2015;45(5):483–487. [DOI] [PubMed] [Google Scholar]
- 108.Gunlusoy B, Ceylan Y, Koskderelioglu A, et al. Cognitive Effects of Androgen Deprivation Therapy in Men With Advanced Prostate Cancer. Urology. 2017;103:167–172. [DOI] [PubMed] [Google Scholar]
- 109.Sari Motlagh R, Quhal F, Mori K, et al. The Risk of New Onset Dementia and/or Alzheimer Disease among Patients with Prostate Cancer Treated with Androgen Deprivation Therapy: a Systematic Review and Meta-Analysis. J Urol. 2021;205(1):60–67. [DOI] [PubMed] [Google Scholar]
- 110.Chung SD, Lin HC, Tsai MC, Kao LT, Huang CY, Chen KC. Androgen deprivation therapy did not increase the risk of Alzheimer’s and Parkinson’s disease in patients with prostate cancer. Andrology. 2016;4(3):481–485. [DOI] [PubMed] [Google Scholar]
- 111.Zhang Z, Yang L, Xie D, Shi H, Li G, Yu D. Depressive symptoms are found to be potential adverse effects of androgen deprivation therapy in older prostate cancer patients: a 15-month prospective, observational study. Psychooncology. 2017;26(12):2238–2244. [DOI] [PubMed] [Google Scholar]
- 112.Dinh KT, Yang DD, Nead KT, Reznor G, Trinh QD, Nguyen PL. Association between androgen deprivation therapy and anxiety among 78 000 patients with localized prostate cancer. Int J Urol. 2017;24(10):743–748. [DOI] [PubMed] [Google Scholar]
- 113.Wu FJ, Li IH, Chien WC, et al. Androgen deprivation therapy and the risk of iron-deficiency anaemia among patients with prostate cancer: a population-based cohort study. BMJ Open. 2020;10(3):e034202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu JM, Liu YP, Chuang HC, Wu CT, Su YL, Hsu RJ. Androgen deprivation therapy for prostate cancer and the risk of hematologic disorders. PLoS One. 2020;15(2):e0229263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schmid M, Hanske J, Ravi P, et al. Relationship between androgen deprivation therapy and community-acquired respiratory infections in patients with prostate cancer. Int J Urol. 2016;23(4):305–311. [DOI] [PubMed] [Google Scholar]
- 116.Yang DD, Krasnova A, Nead KT, et al. Androgen deprivation therapy and risk of rheumatoid arthritis in patients with localized prostate cancer. Ann Oncol. 2018;29(2):386–391. [DOI] [PubMed] [Google Scholar]
- 117.Klil-Drori AJ, Tascilar K, Yin H, Aprikian A, Bitton A, Azoulay L. Androgen Deprivation Therapy and the Incidence of Inflammatory Bowel Disease in Patients With Prostate Cancer. Am J Epidemiol. 2016;184(1):15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alibhai SMH, Breunis H, Timilshina N, et al. Improving bone health in men with prostate cancer receiving androgen deprivation therapy: results of a randomized Phase 2 trial. Cancer. 2018;124(6):1132–1140. [DOI] [PubMed] [Google Scholar]
- 119.Owen PJ, Daly RM, Livingston PM, Fraser SF. Lifestyle guidelines for managing adverse effects on bone health and body composition in men treated with androgen deprivation therapy for prostate cancer: an update. Prostate Cancer Prostatic Dis. 2017;20(2):137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Taaffe DR, Galvão DA, Spry N, et al. Immediate versus delayed exercise in men initiating androgen deprivation: effects on bone density and soft tissue composition. BJU Int. 2019;123(2):261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim SH, Seong DH, Yoon SM, et al. The Effect on Bone Outcomes of Home-based Exercise Intervention for Prostate Cancer Survivors Receiving Androgen Deprivation Therapy: a Pilot Randomized Controlled Trial. Cancer Nurs. 2018;41(5):379–388. [DOI] [PubMed] [Google Scholar]
- 122.Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67(5):825–836. [DOI] [PubMed] [Google Scholar]
- 123.Mennen-Winchell LJ, Grigoriev V, Alpert P, Dos Santos H, Tonstad S. Determinants of vitamin D levels in men receiving androgen deprivation therapy for prostate cancer. J Am Assoc Nurse Pract. 2015;27(1):39–47. [DOI] [PubMed] [Google Scholar]
- 124.Kojima I, Naito Y, Yamamoto A, et al. Efficacy of zoledronic acid in older prostate cancer patients undergoing androgen deprivation therapy. Osteoporos Sarcopenia. 2019;5(4):128–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheung AS, Hoermann R, Ghasem-Zadeh A, et al. Differing Effects of Zoledronic Acid on Bone Microarchitecture and Bone Mineral Density in Men Receiving Androgen Deprivation Therapy: a Randomized Controlled Trial. J Bone Miner Res. 2020;35(10):1871–1880. [DOI] [PubMed] [Google Scholar]
- 126.Planas morin J, Celma Domenech A, Placer Santos J, et al. Bone mass behavior after 1 year of different treatment strategies in prostate cancer patients subjected to androgen deprivation therapy. Rheumatol Int. 2014;34(10):1419–1425. [DOI] [PubMed] [Google Scholar]
- 127.Doria C, Mosele GR, Solla F, Maestretti G, Balsano M, Scarpa RM. Treatment of osteoporosis secondary to hypogonadism in prostate cancer patients: a prospective randomized multicenter international study with denosumab vs. alendronate. Minerva Urol Nefrol. 2017;69(3):271–277. [DOI] [PubMed] [Google Scholar]
- 128.Nakai Y, Kanaki T, Yamamoto A, et al. Antiresorptive agent-related osteonecrosis of the jaw in prostate cancer patients with bone metastasis treated with bone-modifying agents. J Bone Miner Metab. 2021;39(2):295–301. [DOI] [PubMed] [Google Scholar]