Abstract
The SARS-CoV-2 virus has infected and killed millions of people, but little is known about the risk factors that lead to the development of severe, mild or asymptomatic conditions after infection. The individual immune response and the balance of cytokines and chemokines have been shown to be important for the prognosis of patients. Additionally, it is essential to understand how the production of specific antibodies with viral neutralizing capacity is established. In this context, this study aimed to identify positive individuals for IgG anti-SARS-CoV-2 in a large population of blood donors (n = 7837) to establish their immune response profile and to evaluate its viral neutralization capacity. The prevalence found for IgG anti-SARS-CoV-2 was 5.6% (n = 441), with male blood donors (61.9%) being more prevalent among the positive ones. The results showed that positive individuals for IgG anti-SARS-CoV-2 have high serum concentrations of chemokines, TNF, IFN-γ and IL-10. The analyses showed that the positivity index for IgG anti-SARS-CoV-2 is associated with the neutralizing capacity of the antibodies, which, in turn, is significantly related to lower serum concentrations of CCL5 and CXCL10. The results allow us to hypothesize that the development and maintenance of IgG anti-SARS-CoV-2 antibodies in infected individuals occurs in a pro-inflammatory microenvironment well regulated by IL-10 with great capacity for recruiting cells from the innate and adaptive immune systems.
Keywords: SARS-CoV-2, COVID-19, Blood donor, Chemokine, Cytokine, Neutralization
1. Introduction
In late 2019, an outbreak of SARS-CoV-2 infections was identified in Wuhan, Hubei Province, China. The disease caused by this new coronavirus, subsequently named COVID-19, was mainly characterized by flu-like symptoms such as fever, dry cough, runny nose, fatigue and anosmia [1]. Today we know that the clinical characteristics of COVID-19 are varied, from asymptomatic state to acute respiratory syndrome and multiple organ dysfunction, which led to millions of deaths worldwide [2]. Much remains to be studied about the risk factors leading to severe COVID-19. However, the role of the imbalanced immune responses contributing to disease symptoms and to more severe prognoses is becoming irrevocable. In this context, it is of critical importance to assess the immune response of infected individuals to better understand the balance of the immune responses capable of fighting the infection causing less harmful effects [3], [4]. Even more important is the verification of the profile of immune responses generated in asymptomatic individuals previously infected with SARS-Cov-2. This study aimed to evaluate the presence of IgG-type antibodies anti-SARS-CoV-2 in a large population of blood donors throughout 2020 and its viral neutralizing capacity alongside with the predominant type of immune responses in seropositive individuals.
2. Methods
2.1. Serum samples and data
Serum samples from 7837 blood donors from Fundação Hemominas, Minas Gerais, Brazil, were used in this study. Samples were randomly selected from March/2020 to December/2020. Demographic data of blood donors were accessed on institutional records.
2.2. Detection of IgG anti-SARS-CoV-2
All samples were tested for the presence of anti-SARS-CoV-2 IgG using the commercial SARS-CoV-2 IgG kit (Abbot Ireland Diagnostics, Sligo, Ireland). The experiments were performed as defined by the manufacturer using an Architect i4000 equipment (Abbot Ireland Diagnostics, Sligo, Ireland).
2.3. Dosage of immunological biomarkers
Positive (n = 160) and negative (n = 40) samples for IgG anti-SARS-CoV-2 had their serum concentrations of cytokines and chemokines measured. Commercial Th1/Th2/Th17 BD Cytometric Bead Array kit was used for IL-2 (interleukin 2), IL-4, IL-6, IL-10, TNF (tumour necrosis factor), IFN-γ (interferon γ) and IL-17A dosage. Chemokine BD Cytometric Bead Array kit was used to quantify CCL2 (MCP-1 or monocyte chemoattractant protein-1), CCL5 (regulated on activation, normal T cell expressed and secreted or RANTES), CXCL-8 (IL-8), CXCL9 (MIG or monokine induced by gamma interferon) and CXCL10 (IP-10 or Interferon gamma-induced protein 10). The experiments were performed as defined by the manufacturer. Beads were acquired in a FACSCanto flow cytometer and data were analysed in FCAP Array Software version 3.0. All kits, equipment and software were purchased from Becton Dickinson, San Jose, CA, USA.
2.4. Plaque reduction neutralization test (PRNT)
The PRNT analyses were performed in a high-containment, biosafety level 4 facility (OIE BSL-4 – World Organization for Animal Health) at Laboratório Federal de Defesa Agropecuária, LFDA-MG, Pedro Leopoldo, MG, Brazil. SARS-CoV-2 isolate SP02/BRA (SARS.CoV2/SP02.2020.HIAE.Br) was kindly provided by Dr. Edison Luiz Durigon (Department of Microbiology, Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil) [5]. SARS-CoV-2 virus stock was collected from the supernatant of infected Vero CCL-81 cells and stored at −80 °C until further use in PRNT.
IgG anti-SARS-CoV-2 negative (n = 43) and positive (n = 432) serum samples from blood donors were heat-inactivated at 56 °C for 20 min and tested in duplicates through PRNT. Briefly, 1x105 Vero CCL-81 cells per well were seeded into 24-well plates 24 h prior to infection with SARS-CoV-2. The challenging virus suspension was serially diluted in DMEM-2 until obtaining 200 PFUs (plaque forming units) in 100 µL (2x103 PFU/mL). Each test serum sample was serially two-fold diluted starting at 1:20 in 100 µL of DMEM-2 until 1:80. For each serum dilution prepared, 100 μL of viral suspension containing 200 PFU was added (Vf = 200 µL). Reference, virus control sample was incubated with a non-reacting, human serum sample. The mixture was kept under agitation (200 rpm) at 37 °C for 1 h and then 100 µL was inoculated onto Vero cells. The infected plates were incubated at 37 °C for 1 h and then covered with 1 mL per well of DMEM-1% FCS supplemented with 1.2% CMC overlay media. The plates were incubated at 37 °C for 72 h until appearance of individual viral lysis plaques. The infected cells were fixed with 10% buffered formaldehyde solution for 15 min and stained with 1% Crystal Violet solution for 15 min. For each test performed, anti-SARS-CoV-2 horse-serum was used as PRNT-positive control. Neutralizing activity against SARS-CoV-2 particles was determined and expressed as serum concentration required to achieve 50% reduction in the number of PFUs relative to reference, virus control.
2.5. Statistical analysis
The number of events and their respective frequencies were calculated for categorical variables. Comparisons of these variables were made using Fisher's exact test. For continuous variables, medians with interquartile range (IQR) were calculated and comparisons were performed using the Mann-Whitney test. Differences were considered statistically significant when p < 0.05.
2.6. Ethical approval
This study was approved by the Research Ethics Committee of the institutions involved (CAAE 31087720.2.0000.5118).
3. Results
Among the 7837 samples tested from March to December 2020, 441 (5.6%) were positive for IgG anti-SARS-CoV-2. Data from donors included in the study are shown in Table 1 . Specific parameters such as age, the donation history (first-time or repeat donors) and the type of blood donation (invited, spontaneous or replacement) did not differ between positive and negative groups for IgG anti-SARS-CoV-2. Male donors were significantly more prevalent (61.9%) in the positive group (p = 0.002).
Table 1.
Characteristics of blood donors tested for IgG anti-SARS-CoV-2.
| Characteristics | IgG anti-SARS-CoV-2 status |
||
|---|---|---|---|
| Negative | Positive | p-value | |
| Number of blood donors (%) | 7396 (94.4) | 441 (5.6) | – |
| Age in years, median (IQR) | 35 (26–44) | 35 (27–44) | 0.814 |
| Sex, male, (%) | 4011 (54.2) | 273 (61.9) | 0.002 |
Significant p values are highlighted in bold. IQR, Interquartile range.
Among positive donors, the median index of positivity of IgG anti-SARS-CoV-2 in the chemiluminescence reaction was 3.65 (IQR 2.43–5.39). The index result was not different in relation to the sex of blood donors. However, Spearman test identified a weak significant positive correlation between the IgG index and the age of the donors (r = 0.16; 95% CI 0.07–0.25; p < 0.001). Older blood donors had significantly higher indexes. The IgG index of donors aged 41–50 years (median 3.97; IQR 2.74–5.82; p = 0.0097) and older than 50 years (median 4.71; IQR 2.83–6.40; p = 0.0115) was significantly higher when compared to the index of donors aged 16–30 years (median 3.35; IQR 2.26–4.89) (Fig. 1 ).
Fig. 1.
Anti-SARS-CoV-2 IgG index data in different age strata of blood donors. Each dot represents a donor. The horizontal line is the median index of each group. Grey area delimits dots with indexes below the median of all tested donors.
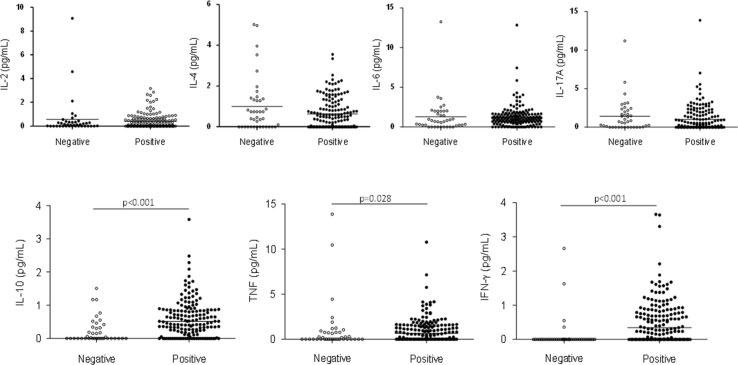
Serum cytokine measurements revealed that donors positive for IgG anti-SARS-CoV-2 had significant higher concentrations of IL-10 (median 0.51 pg/mL; IQR 0.18–0.86; p < 0.001), TNF (median 0.65 pg/mL; IQR 0.00–1.57; p = 0.028) and IFN-γ (median 0.35 pg/mL; IQR 0.00–0.87; p < 0.001) when compared to negative donors (IL-10 median 0.00 pg/mL; IQR 0.00–0.34; TNF median 0.00 pg/mL; IQR 0.00–0.77; IFN-γ median 0.00 pg/mL; IQR 0.00–0.00) (Fig. 2 ). Dosages did not show significant differences between negative and positive donors for serum concentrations of IL-2 (median 0.11 pg/mL; IQR 0.00–0.35 and median 0.10 pg/mL; IQR 0.00–0.61, respectively), IL-4 (median 0.60 pg/mL; IQR 0.00–1.37 and median 0.43 pg/mL; IQR 0.00–1.03, respectively, IL-6 (median 0.70 pg/mL; IQR 0.17–2.00 and median 1.02 pg/mL; IQR 0.56–1.61, respectively) and IL-17A (median 0.74 pg/mL; IQR 0.00–2.14 and median 0.26 pg/mL; IQR 0.00–1.65, respectively).
Fig. 2.
Comparison of cytokines (IL-2, IL-4, IL-6, IL-10, TNF, IFN-γ and IL-17A) dosage in blood donors negative (white dots) and positive (black dots) for IgG anti-SARS-CoV-2. Horizontal lines represent the median concentration of each cytokine.
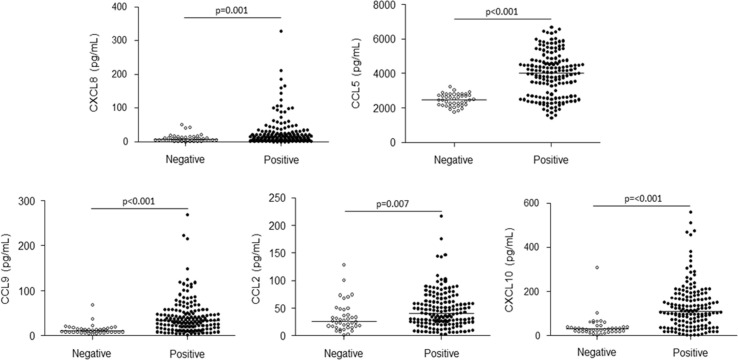
Serum chemokine measurements revealed that donors positive for IgG anti-SARS-CoV-2 had significant higher concentrations of all tested biomarkers (CXCL8 median 13.60 pg/mL; IQR 5.98–28.04; p = 0.001; CCL5 median 4017 pg/mL; IQR 2674–4736; p < 0.001; CXCL9 median 33.08 pg/mL; IQR 17.88–54.14; p < 0.001; CCL2 median 40.39 pg/mL; IQR 23.38–61.52; p = 0.007; CXCL10 median 111.70 pg/mL; IQR 56.98–178.00; p < 0.001) when compared to negative donors (CXCL8 median 7.90 pg/mL; IQR 3.99–14.34; CCL5 median 2467 pg/mL; IQR 2179–2764; CXCL9 median 10.23 pg/mL; IQR 6.90–15.31; CCL2 median 26.00 pg/mL; IQR 15.76–44.63; CXCL10 median 30.63 pg/mL; IQR 19.96–43.88) (Fig. 3 ).
Fig. 3.
Comparison of CXCL8, CCL5, CXCL9, CCL2 and CXCL10 dosage in blood donors negative (white dots) and positive (black dots) for IgG anti-SARS-CoV-2. Horizontal lines represent the median concentration of each cytokine.
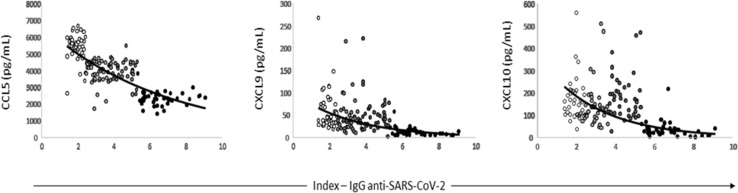
Correlation analysis between cytokine/chemokine and index results revealed significant negative correlation between all biomarkers except IL-4, IL-6 and IL-17A. Despite the high levels of TNF, IFN-γ and IL-10 in positive individuals for IgG anti-SARS-CoV-2 these cytokines presented slightly negative correlation with antibody index (Supplementary Table S1). Especially for CCL5 (r = −0.79; 95% CI −0.84 to −0.72; p < 0.001), CXCL9 (r = −0.57; 95% CI −0.67 to −0.45; p < 0.001) and CXCL10 (r = −0.51; 95% CI −0.62 to −0.38; p < 0.001), the result of the correlation was strong or moderate (r value below −0.5) (Fig. 4 ).
Fig. 4.
Result of the correlation test between the anti-SARS-CoV-2 IgG index and the chemokines CCL5, CXCL9 and CXCL10 for each donor tested. The colour gradation of the points represents the different index interquartiles in which each sample is inserted.
The neutralization test of SARS-CoV-2 revealed that, among those positive for IgG in chemiluminescence, 322 (74.5%) had the capacity to neutralize the virus. Otherwise, among the negative donors that were tested, only five donors (11.6%) presented viral neutralization capacity. Among the negative samples with neutralizing capacity, two neutralized 50% of the viral particles when diluted 80X and another three when diluted 160X. Among those positive samples, 50 (19.9%) neutralized 50% of the viral particles when diluted 40X, 98 (30.4%) when diluted 80X and another 160 (49.7%) when diluted 160X.
The data obtained reveal that the donors with the highest IgG indexes are those with the greatest capacity of viral neutralization. Samples with a viral neutralizing capacity when diluted 80X (median index 3.58; IQR 2.62–5.08) have a significantly higher median index when compared to those that do not neutralize (median index 2.90; IQR 2.01–4.32; p = 0.005). Additionally, samples with a viral neutralizing capacity when diluted 160X (median index 4.68; IQR 2.91–6.49) have a significantly higher median index when compared to those that do not neutralize (p < 0.001), to those that neutralize when diluted 40X (median index 3.14; IQR 2.00–4.99; p < 0.001) and 80X (p = 0.001) (Fig. 5 A). Analysis of the concentration of cytokines and chemokines in different serum dilutions capable of neutralizing at least 50% of the viral particles revealed some significant results (Supplementary Table S2). Importantly, the results of the dosage of the CCL5 and CXCL10 chemokines show that lower concentrations of these chemokines are related to the greater neutralizing capacity of anti-SARS-CoV-2 IgG antibodies (Fig. 5B and 5C).
Fig. 5.
Neutralizing capacity of SARS-CoV-2 by serum IgG from blood donors. (A) Anti-SARS-CoV-2 IgG index results in the different groups of samples without (Neg) and with neutralizing capacity of the viral particle. Serum dosage of CCL5 (B) and CXCL10 (C) in different groups of samples with and without viral particle neutralization capacity. Blood donors are grouped into Neg, 40X, 80X and 160X accordingly the neutralizing capacity detected.
4. Discussion
SARS-CoV-2 infection and the development of COVID-19 in millions of people around the world have challenged science due to the limitation of adequate treatments to decrease the number of deaths. Fortunately, viral infection causes the production of antibodies that considerably reduce the risk of reinfections. Despite this, it is still unclear how long the antibodies generated against SARS-CoV-2 remain in circulation after exposure to the virus [6], [7]. In this context, it is extremely important to study the types of immune response triggered by SARS-CoV-2 infection, as well as their roles in the development and maintenance of specific antibodies in circulation. In this study we evaluated a large population of blood donors (n = 7837) and identified that 441 (5.6%) had IgG anti-SARS-CoV-2 in their serum samples throughout the 2020.
Positive blood donors were predominantly male (61.9%). Association of SARS-CoV-2 infection with sex is controversial, but other studies have found similar result [8], [9]. The results of this study also showed that older blood donors, especially those over 40 years, have higher serum levels of IgG anti-SARS-CoV-2 when compared to those <30 years. On the other hand, another study did not identify association between antibody levels and age of the seropositive individuals [10], [11]. Despite the lack of consistent data regarding the production of anti-SARS-CoV-2 antibodies and the age of the infected individuals, other recent published studies showed that infected children and adults have differential antibody production, the latter having higher circulating levels of IgG [12] and that SARS-CoV-2 IgG levels are positively correlated with age in adult COVID-19 patients [13], [14], [15], [16]. Additionally, the repertoire of recognized viral antigens appears to be larger in adults. The authors hypothesize that this reduction in functional antibodies in paediatric patients could be explained by the greater capacity for viral clearance in children, reducing the availability of antigens that could induce a humoral response. In this study there is not such a wide range in the age of the tested blood donors, but the results seem to confirm that older individuals have a greater capacity to produce anti-SARS-CoV-2 antibodies. However, it is important to emphasize that the oldest blood donors had 70 years.
The neutralization test results showed that the majority of individuals positive for IgG anti-SARS-CoV-2 (80.1%) have antibodies with viral neutralizing capacity at dilutions above 80X. As expected, individuals with greater neutralizing capacity also had higher indexes in the chemiluminescence test. In this sense, samples diluted 160X (dilution required, for these samples, to promote a 50% reduction in the number of plaque forming units) are those with significantly higher indexes (Fig. 5A). Interestingly, five negative donors in the serological test showed high viral neutralization capacity even with indexes well below the cutoff point of the commercial anti-SARS-CoV-2 IgG detection kit (mean 0.42; variation of 0.04–1.13). A previous study had already shown that individuals who were infected had no detectable IgG in a serological test after 90 days post infection but maintained the capacity for viral neutralization [5]. These results indicate that negative test for IgG anti-SARS-CoV-2 cannot rule out previous infection. Additionally, the incidence of other coronaviruses does not rule out the possibility of cross-reacting antibodies [17]. The collection approach in the present work considering blood donors does not permit us to know the time after the SARS-CoV-2 or another coronavirus infection. However, it is important to highlight that a recent study comparing neutralization response in individuals presenting severe and mild disease from 0 to 210 days from symptoms onset and the same period in days after test confirmation for asymptomatic individuals, showed comparable neutralization antibody titres in individuals presenting asymptomatic infection and those with mild COVID-19 [18].
The cytokine profile of the samples showed that positive donors for IgG anti-SARS-CoV-2 had significantly higher serum concentrations of IL-10, TNF and IFN-γ. Other studies that evaluated the immunological profile of asymptomatic or mild COVID-19 patients and convalescent plasma samples also reported elevated levels of these cytokines [19], [20], [21]. On the other hand, IL-6 levels in positive donors were similar to those in the negative group, reinforcing that this cytokine seem to have a key role in progress to severe COVID-19 [22], [23]. In general, regardless of the individual's symptoms, SARS-CoV-2 infection induces a pro-inflammatory response, evidenced in this study by the high levels of TNF and IFN-γ [20]. However, in individuals without clinical signs of COVID-19 or those with mild symptoms with a good prognosis, there seems to exist a regulation of inflammation by IL-10 [22].
Chemokines are involved in attraction of leukocytes to the site of an inflammatory immune response mainly in the beginning of the stimulus. The dosage of these biomarkers in the samples showed that individuals positive for IgG anti-SARS-CoV-2 had significantly higher concentrations of CCL2, CCL5, CXCL8, CXCL9 and CXCL10 when compared to negative ones. Other studies have reported increased serum concentrations of CCL2, CXCL8 and CXCL10 in patients with severe COVID-19 [20], [23], [24], [25]. However, due to its inflammatory nature, it is believed that asymptomatic or mild symptomatic infections can also lead to an increased secretion of these chemokines. Despite the high concentration of chemokines in samples from positive donors, there are differences in the levels of these biomarkers among individuals in this group. Interestingly, there is a significant inverse correlation between CCL5, CXCL9 and CXCL10 concentrations and anti-SARS-CoV-2 IgG levels. These chemokines are involved in the recruitment and activation of monocytes, neutrophils, Natural Killer and T cells [26], [27]. In this study, CCL5 and CXCL10 are found in lower concentration in individuals with greater capacity for viral neutralization. We hypothesize that the decrease in serum concentration of these chemokines is related to the time elapsed since infection as described for other chemokines elsewhere [20]. Meanwhile, another study showed higher concentrations of CXCL10 in convalescent plasma samples with high neutralization capacity of SARS-CoV-2 [19].
The most important limitations of this study are the impossibility of characterizing the studied population regarding the development of symptoms of COVID-19 before and after blood donation and the possible date of infection by SARS-CoV-2. However, the results obtained are quite informative as to the type of immune response that is predominant in the development and maintenance of IgG anti-SARS-CoV-2 in asymptomatic individuals. Considering the results found in this study, we hypothesize that the development of anti-SARS-CoV-2 IgG, as well as its maintenance in circulation, is dependent on a pro-inflammatory immune response mediated by chemokines, TNF and IFN-γ, but regulated by IL-10. In this microenvironment, there is favoured recruitment and activation of antigen-presenting cells, as well as T and B lymphocytes. On the other hand, the results indicate that the maintenance of high levels of neutralizing antibodies depends on lower serum concentrations of CCL5 and CXCL10. This result is compatible with a late immune response against SARS-CoV-2, when the activation of specific T and B cells overlaps the recruitment of cells of the innate immune system.
Authors contributions
D.G.C., L.C.O. and I.R.O performed the experiments; E.F.B-S. and M.M.T. contributed with study design and revised the article; M.C.F.S.M collected clinical data and revised the article; D.G.C. and M.L.M. designed the research and wrote the article. All authors critically revised the article and approved the final version.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank all the professionals who contributed to the enrollment of blood donors in the study and the technical support from Laboratory of Immunopharmacology, Department of Biochemistry and Immunology, Instituto de Ciências Biológicas, UFMG. We are thankful to Andrea Garcia de Oliveira, Marcelo Camargos, Kelly Nascimento and Laboratório Federal de Defesa Agropecuária LFDA-MG, Ministry of Agriculture, Livestock and Food Supply (MAPA), for allowing and supporting our activities in the BSL-4-OIE laboratory. We also thank Fundação Hemominas for the financial support. EFB-S and IRO are fellows from CNPq. L.C.O. is recipient of a postdoctoral fellowship from Capes.
Footnotes
IgG anti-SARS-CoV-2 seroprevalence was evaluated in a large number of blood donors. The seropositive individuals showed a pro-inflammatory environment regulated by IL-10. Antibodieśneutralizing capacity was related to lower serum concentrations of CCL5 and CXCL10.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cyto.2022.155874.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Wu Y.C., Chen C.S., Chan Y.J. The outbreak of COVID-19: An overview. J. Chin. Med. Assoc. 2020;83(3):217–220. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev.Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Notz Q., Schmalzing M., Wedekink F., Schlesinger T., Gernert M., Herrmann J., Sorger L., Weismann D., Schmid B., Sitter M., Schlegel N., Kranke P., Wischhusen J., Meybohm P., Lotz C. Pro- and anti-inflammatory responses in severe COVID-19-induced acute respiratory distress syndrome – an observational pilot study. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.581338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamal M., Bangash H.I., Habiba M., Lei Y., Xie T., Sun J., Wei Z., Hong Z., Shao L., Zhang Q. Immune dysregulation and system pathology in COVID-19. Virulence. 2021;12(1):918–936. doi: 10.1080/21505594.2021.1898790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araújo D.B., Machado R.R.G., Amgarten D.E., Malta F.M., de Araújo G.G., Monteiro C.O., Candido E.D., Soares C.P., de Menezes F.G., Pires A.C.C., Santana R.A.F., Viana A.O., Dorlass E., Thomazelli L., Ferreira L.C.S., Botosso V.F., Carvalho C.R.G., Oliveira D.B.L., Pinho J.R.R., Durigon E.L. SARS-CoV-2 isolation from the first reported patients in Brazil and establishment of a coordinated task network. Mem. Inst. Oswaldo Cruz. 2020;23(115):e200342. doi: 10.1590/0074-02760200342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marklund E., Leach S., Axelsson H., Nyström K., Norder H., Bemark M., Angeletti D., Lundgren A., Nilsson S., Andersson L.M., Yilmaz A., Lindh M., Liljeqvist J.Å., Gisslén M. Serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG non-responders. PLoS ONE. 2020;15(10):e0241104. doi: 10.1371/journal.pone.0241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K.J.A., Hemmings O., O'Byrne A., Kouphou N., Galao R.P., Betancor G., Wilson H.D., Signell A.W., Winstone H., Kerridge C., Huettner I., Jimenez-Guardeño J.M., Lista M.J., Temperton N., Snell L.B., Bisnauthsing K., Moore A., Green A., Martinez L., Stokes B., Honey J., Izquierdo-Barras A., Arbane G., Patel A., Tan M.K.I., O'Connell L., O'Hara G., MacMahon E., Douthwaite S., Nebbia G., Batra R., Martinez-Nunez R., Shankar-Hari M., Edgeworth J.D., Neil S.J.D., Malim M.H., Doores K.J. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020;5(12):1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassallo R.R., Dumont L.J., Bravo M.D., Hazegh K., Kamel H. Progression and predictors of SARS-CoV-2 antibody seroreactivity in US blood donors. Transfus. Med. Rev. 2021;30 doi: 10.1016/j.tmrv.2021.07.003. S0887-7963(21)00028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rostami A., Sepidarkish M., Fazlzadeh A., Mokdad A.H., Sattarnezhad A., Esfandyari S., Riahi S.M., Mollalo A., Dooki M.E., Bayani M., Nazemipour M., Mansournia M.A., Hotez P.J., Gasser R.B. Update on SARS-CoV-2 seroprevalence: regional and worldwide. Clin. Microbiol. Infect. 2021;27(12):1762–1771. doi: 10.1016/j.cmi.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.M.K. Young, C. Kornmeier, R.M. Carpenter, N.R. Natale, J.M. Sasson, M.D. Solga, A.J. Mathers, M.D. Poulter, X. Qiang, W.A. Petri, IgG antibodies against SARS-CoV-2 correlate with days from symptom onset, viral load and IL-10. medRxiv [Preprint]. 2020 Dec 7:2020.12.05.20244541.
- 11.Garcia-Beltran W.F., Lam E.C., Astudillo M.G., Yang D., Miller T.E., Feldman J., Hauser B.M., Caradonna T.M., Clayton K.L., Nitido A.D., Murali M.R., Alter G., Charles R.C., Dighe A., Branda J.A., Lennerz J.K., Lingwood D., Schmidt A.G., Iafrate A.J., Balazs A.B. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021 Jan 21;184(2):476–488.e11. doi: 10.1016/j.cell.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisberg S.P., Connors T.J., Zhu Y., Baldwin M.R., Lin W.H., Wontakal S., Szabo P.A., Wells S.B., Dogra P., Gray J., Idzikowski E., Stelitano D., Bovier F.T., Davis-Porada J., Matsumoto R., Poon M.M.L., Chait M., Mathieu C., Horvat B., Decimo D., Hudson K.E., Zotti F.D., Bitan Z.C., La Carpia F., Ferrara S.A., Mace E., Milner J., Moscona A., Hod E., Porotto M., Farber D.L. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2021;22(1):25–31. doi: 10.1038/s41590-020-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H.S., Costa V., Racine-Brzostek S.E., Acker K.P., Yee J., Chen Z., Karbaschi M., Zuk R., Rand S., Sukhu A., Klasse P.J., Cushing M.M., Chadburn A., Zhao Z. Association of age with SARS-CoV-2 antibody response. JAMANetw Open. 2021;4(3):e214302. doi: 10.1001/jamanetworkopen.2021.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Racine-Brzostek S.E., Yang H.S., Jack G.A., Chen Z., Chadburn A., Ketas T.J., Francomano E., Klasse P.J., Moore J.P., McDonough K.A., Girardin R.C., Dupuis A.P., Payne A.F., Ma L.X., Sweeney J., Zhong E., Yee J., Cushing M.M., Zhao Z. Postconvalescent SARS-CoV-2 IgG and Neutralizing Antibodies are Elevated in Individuals with Poor Metabolic Health. J. Clin. Endocrinol. Metab. 2021;106(5):e2025–e2034. doi: 10.1210/clinem/dgab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.V. De Giorgi, K.A. West, A.N. Henning, L. Chen, M.R. Holbrook, R. Gross, J. Liang, E. Postnikova, J. Trenbeath, S. Pogue, T. Scinto, H.J. Alter, C.C. Cantilena, Anti-SARS-CoV-2 serology persistence over time in COVID-19 convalescent plasma donors. medRxiv [Preprint]. 2021 Mar 10:2021.03.08.21253093.
- 16.Schmidt J., Berghaus S., Blessing F., Wenzel F., Herbeck H., Blessing J., Schierack P., Rödiger S., Roggenbuck D. Serological and viral genetic features of patients with COVID-19 in a selected German patient cohort-correlation with disease characteristics. Geroscience. 2021;43(5):2249–2264. doi: 10.1007/s11357-021-00443-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen S.A., Kellogg C., Equils O. Neutralizing and cross-reacting antibodies: implications for immunotherapy and SARS-CoV-2 vaccine development. Hum. Vaccin. Immunother. 2021;17(1):84–87. doi: 10.1080/21645515.2020.1787074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau E.H.Y., Tsang O.T.Y., Hui D.S.C., Kwan M.Y.W., Chan W.H., Chiu S.S., Ko R.L.W., Chan K.H., Cheng S.M.S., Perera R.A.P.M., Cowling B.J., Poon L.L.M., Peiris M. Neutralizing antibody titres in SARS-CoV-2 infections. Nat. Commun. 2021;12(1):63. doi: 10.1038/s41467-020-20247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonny T.S., Patel E.U., Zhu X., Bloch E.M., Grabowski M.K., Abraham A.G., Littlefield K., Shrestha R., Benner S.E., Laeyendecker O., Shoham S., Sullivan D., Quinn T.C., Casadevall A., Pekosz A., Redd A.D., Tobian A.A.R. Cytokine and chemokine levels in coronavirus disease 20*19 convalescent plasma. Open Forum Infect. Dis. 2020;8(2):ofaa574. doi: 10.1093/ofid/ofaa574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon J.S., Kim J.Y., Kim M.C., Park S.Y., Kim B.N., Bae S., Cha H.H., Jung J., Kim M.J., Lee M.J., Choi S.H., Chung J.W., Shin E.C., Kim S.H. Factors of severity in patients with COVID-19: Cytokine/chemokine concentrations, viral load, and antibody responses. Am. J. Trop. Med. Hyg. 2020;103(6):2412–2418. doi: 10.4269/ajtmh.20-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tripathy A.S., Vishwakarma S., Trimbake D., Gurav Y.K., Potdar V.A., Mokashi N.D., Patsute S.D., Kaushal H., Choudhary M.L., Tilekar B.N., Sarje P., Dange V.S., Abraham P. Pro-inflammatory CXCL-10, TNF-α, IL-1β, and IL-6: biomarkers of SARS-CoV-2 infection. Arch Virol. 2021:1–10. doi: 10.1007/s00705-021-05247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McElvaney O.J., McEvoy N.L., McElvaney O.F., Carroll T.P., Murphy M.P., Dunlea D.M., Ní Choileáin O., Clarke J., O'Connor E., Hogan G., Ryan D., Sulaiman I., Gunaratnam C., Branagan P., O'Brien M.E., Morgan R.K., Costello R.W., Hurley K., Walsh S., de Barra E., McNally C., McConkey S., Boland F., Galvin S., Kiernan F., O'Rourke J., Dwyer R., Power M., Geoghegan P., Larkin C., O'Leary R.A., Freeman J., Gaffney A., Marsh B., Curley G.F., McElvaney N.G. Characterization of the inflammatory response to severe COVID-19 illness. Am. J. Respir. Crit. Care Med. 2020;202(6):812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horspool A.M., Kieffer T., Russ B.P., DeJong M.A., Wolf M.A., Karakiozis J.M., Hickey B.J., Fagone P., Tacker D.H., Bevere J.R., Martinez I., Barbier M., Perrotta P.L., Damron F.H. Interplay of antibody and cytokine production reveals CXCL13 as a potential novel biomarker of lethal SARS-CoV-2 infection. mSphere. 2021;6(1):e01324–e1420. doi: 10.1128/mSphere.01324-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y., Wang J., Liu C., Su L., Zhang D., Fan J., Yang Y., Xiao M., Xie J., Xu Y., Li Y., Zhang S. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol. Med. 2020;26(1):97. doi: 10.1186/s10020-020-00230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang N., Zhao Y.D., Wang X.M. CXCL10 an important chemokine associated with cytokine storm in COVID-19 infected patients. Eur. Rev. Med. Pharmacol. Sci. 2020;24(13):7497–7505. doi: 10.26355/eurrev_202007_21922. [DOI] [PubMed] [Google Scholar]
- 26.Schall T.J., Bacon K., Toy K.J., Goeddel D.V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347(6294):669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 27.Farber J.M. Mig and IP-10: CXC chemokines that target lymphocytes. J. Leukoc. Biol. 1997;61(3):246–257. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.