Abstract
Once-daily administration of aminoglycosides is routinely used in many institutions. However, comparative efficacy data for patients with cystic fibrosis (CF) are lacking. The purpose of the present study was to compare the predicted pharmacodynamic activity of tobramycin at 10 mg/kg of body weight/day administered every 24 h (q24h), q12h, and q8h. Pharmacokinetic (PK) data were derived from analysis of data on the drug concentration in sera from 60 adult CF patients. Individual maximum a posteriori probability Bayesian PK parameter values were used to construct serum concentration-versus-time curves and to determine various indices (peak concentration/MIC ratio [peak/MIC], area under the concentration-time curve/MIC ratio [AUC/MIC], and time that the concentration was less than the MIC [T<MIC]) for the three regimens described above. MICs of 1, 2, and 4 μg/ml for Pseudomonas aeruginosa were assumed in the simulations. Irrespective of the MIC, significantly lower peak/MIC but shorter T<MIC were noted when regimens of q8h versus q12h (P < 0.001), q8h versus q24h (P < 0.001), and q12h versus q24h (P < 0.001) were compared. This analysis suggests that the potential benefit of achieving a greater peak/MIC with once-daily aminoglycoside administration may be offset by the significantly greater T<MIC in CF patients compared with that achieved with multiple-daily-dosing regimens. Clinical trials are necessary to determine if once daily aminoglycoside administration is efficacious in the CF population before its routine use can be recommended.
Lung disease is a major cause of morbidity and mortality in patients with cystic fibrosis (CF). Impaired mucociliary clearance and host defense mechanisms in the airway lead to eventual colonization with Pseudomonas aeruginosa and other organisms. The ensuing chronic infection and inflammation lead to bronchiectasis and progressive loss of lung function (8, 25, 30). Intermittent courses of intravenous antibiotics administered during acute pulmonary exacerbations significantly reduce the bacterial load and improve lung function (26). The combination of a beta-lactam and an aminoglycoside is frequently used due to their potential synergistic activity and for prevention of the development of resistance (27).
Traditionally, aminoglycosides have been administered by intermittent infusions three to four times daily. Recently, once-daily administration of aminoglycosides has been advocated (24). Several theoretical advantages to this dosing modality have been suggested. Data from in vitro studies and studies with animals have demonstrated that aminoglycosides exhibit concentration-dependent bactericidal activity (6, 17, 19, 33). The killing effect has therefore been correlated with the peak concentration achieved. These studies have also demonstrated that the aminoglycosides exhibit a short (1- to 3-h) in vitro postantibiotic effect (PAE) (10, 18) and a more prolonged (10-h) in vivo PAE (7). The presence of a PAE prevents organism regrowth from occurring for short periods of time after antibiotic concentrations fall below the MIC at the site of the infection. In addition, studies with animals have demonstrated saturable uptake mechanisms within the renal cortex and inner ear (23). Accumulation of aminoglycosides within these tissues has been demonstrated to cause nephrotoxicity and ototoxicity in these animals. Less frequent dosing but administration of larger doses reduces the relative accumulation of the aminoglycosides within these tissues; however, the degree of reduction is unknown. Taken together, these properties suggest that dosing strategies which maximize the peak concentration and allow trough concentrations to drop below the MIC for a short period of time should maximize bactericidal activity and minimize the risk of nephrotoxicity and ototoxicity.
Various pharmacodynamic indices have been proposed to predict clinical and bacteriological responses to antibiotic therapy. Data from in vitro models and studies with animals suggest that the aminoglycoside peak concentration/MIC ratio (peak/MIC) and the area under the concentration-versus-time curve/MIC ratio (AUC/MIC) are significantly correlated with microbiological outcome (17, 19, 33). However, if the dosing interval is sufficiently long compared to the half-life of the antibiotic, the time below the MIC (T<MIC) also becomes a significant predictor of microbiological outcome (17, 33). Evaluations with humans confirm these relationships (15). Moore et al. (22) demonstrated that aminoglycoside peak/MICs that exceed 10 provide optimal clinical outcomes for patients with pneumonia caused by gram-negative organisms. Furthermore, others noted that subinhibitory levels of gentamicin were associated with recurrent bacteremia (1). On the basis of these data, it has been suggested that the dosing interval for aminoglycosides should allow concentrations to fall below the MIC only for a short duration of time (i.e., the duration of the PAE). Considering the relatively short elimination half-life of aminoglycosides in patients with CF (9), which may lead to the possibility of organism regrowth at the end of the dosing interval, some have recommended against the use of extended-interval aminoglycoside dosing in this population (29).
Evidence supporting the use of once-daily dosing of aminoglycosides in patients with CF is limited. The purpose of this study was to evaluate three different tobramycin dosing regimens for the treatment of Pseudomonas lung infections in patients with CF by comparing predicted pharmacodynamic indices.
MATERIALS AND METHODS
Patients.
CF patients from two centers were included in the study. Patients were included in the analysis if they met the following criteria: (i) were age 18 years or older, (ii) had acute pulmonary exacerbation due to Pseudomonas aeruginosa that required hospitalization, and (iii) had received therapy with tobramycin and had available at least one set of measured peak and trough serum tobramycin concentrations.
Study design and procedures.
The chart records of study patients admitted over a 1-year period were reviewed. The following information was recorded: tobramycin dosing history, serum tobramycin concentration data, age, height, weight, gender, and serum creatinine concentration. Blood samples for determination of peak tobramycin concentrations were obtained within 30 min after the end of a 30-min infusion. Trough concentrations were obtained within 30 min of the next scheduled administration time. The exact administration and sampling times were determined from the nursing medication administration record and the laboratory data sheets.
Pharmacokinetic model.
Pharmacokinetic analysis was performed for each patient by using the USC*PACK clinical programs (version 10.7; Laboratory of Applied Pharmacokinetics, University of Southern California School of Medicine, Los Angeles). Serum tobramycin concentrations for each individual were fitted to a one-compartment open model by using maximum a posteriori probability Bayesian (MAP-B) analysis. The Bayesian a priori parameter values were derived from an adult CF population (28), as follows: volume of distribution, 0.27 ± 0.05 liter/kg; elimination rate constant (in hours−1), 0.0028 (CLCR) + 0.01, where CLCR is creatinine clearance. CLCR was estimated on the basis of the patient's demographics and serum creatinine concentration(s) by the method developed by Jelliffe and Jelliffe (16).
Serum tobramycin concentrations were weighted by the inverse of the measured assay error variance. The individual MAP-B parameters were used to construct simulated serum concentration-time curves for three different tobramycin dosage regimens for each patient: 3.3 mg/kg of body weight every 8 h (q8h), 5 mg/kg q12h, and 10 mg/kg q24h. Simulations were performed by using individual rather than mean pharmacokinetic data in order to determine the variability in the concentrations and pharmacodynamic indices achieved.
Determination of pharmacodynamic indices.
The serum concentration-time curves for each regimen were used to compute the following pharmacodynamic indices: peak/MIC, AUC/MIC, and T<MIC. Since actual MICs were not available, values of 1, 2, and 4 μg/ml for P. aeruginosa were assumed in the simulations. These values are consistent with the range of tobramycin sensitivities reported in a recent clinical trial (12). A total of 540 simulations were performed (three dosage regimens and three different MICs with 60 patients). Successful tobramycin dosing regimens were defined as those which (i) achieved a peak/MIC greater than 10 and (ii) attained a T<MIC of not greater than the duration of the PAE (3 h in vitro or 10 h in vivo).
Tobramycin assay.
Serum tobramycin concentrations were determined by a fluorescence polarization immunoassay technique (TDx; Abbott Laboratories, Chicago, Ill.) at one institution and an enzyme immunoassay (ACA; Dupont, Wilmington, Del.) at the other. The assay error pattern for each method was determined by performing replicate measures of concentrations representing the expected range of measured concentrations. Curve fitting applied to the measured concentrations and standard deviations (SDs) at each concentration resulted in the following second-order polynomial equations: for TDx, SD (in milligrams/liter) = 0.059901 + 0.012634 [C] + 0.004375 [C2]; for ACA, SD (in milligrams/liter) = 0.09061 − 0.021630 [C] + 0.003129 [C2], where SD is the standard deviation of the assay and [C] and [C2] are the observed concentration and the concentration squared, respectively.
Statistical analysis.
Statistical analysis was performed with GraphPad Prism software (version 3.00 for Windows; GraphPad Software, San Diego, Calif.). Differences in predicted peak and trough concentrations and pharmacodynamic indices between dosage regimens were determined by Kruskal-Wallis analysis of variance with Dunn's multiple comparisons test. Significance was defined as a P value of less than 0.05.
RESULTS
Patients.
Sixty adult CF patients treated with intravenous tobramycin at two different care centers were evaluated. Patient characteristics and clinical data are as follows: 33 males and 27 females were evaluated. The median age was 27 ± 6 years, the median weight was 54 ± 8.9 kilograms, and the estimated median CLCR was 129.5 ± 40 ml/min/1.73 m2 (range, 42.4 to 260 ml/min/1.73 m2). The median tobramycin dosage received was 3.4 mg/kg of body weight q8h.
Pharmacokinetic analysis.
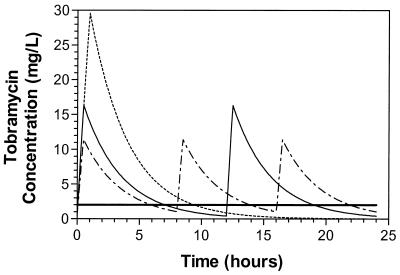
Bayesian analysis performed with the measured tobramycin concentrations resulted in the following fitted pharmacokinetic parameter values (median ± SD): total body clearance, 88.3 ± 21.7 ml/min/1.73 m2 (range, 43.7 to 167.3 ml/min/1.73 m2); volume of distribution 0.29 ± 0.11 liters/kg (range, 0.18 to 0.74 liters/kg); elimination rate constant, 0.32 ± 0.08 h−1 (range, 0.12 to 0.47 h−1); and half-life, 2.2 ± 0.8 h (range, 1.5 to 5.6 h). A comparison of the predicted peak and trough concentrations and the variability indicates significant differences between the three regimens (Table 1). These differences are shown graphically in Fig. 1, which was constructed by using the medians of the MAP-B fitted parameter values.
TABLE 1.
Predicted peak and trough concentrations and pharmacodynamic parametersa
| Parameter and concentration | 3.3 mg/kg q8h | 5 mg/kg q12h | 10 mg/kg q24h |
|---|---|---|---|
| Peak concn (μg/ml) | 11.6 ± 2.6bc | 16.4 ± 3.8bd | 29.3 ± 6.9cd |
| Trough concn (μg/ml) | 1.1 ± 0.9bc | 0.4 ± 0.7bd | 0.0 ± 0.2cd |
| MIC, 1 μg/ml | |||
| Peak/MIC | 11.6 ± 2.6bc | 16.4 ± 3.8bd | 29.3 ± 6.9cd |
| T<MIC | 0.0 ± 0.6bc | 2.6 ± 1.7bd | 12.5 ± 3.4cd |
| AUC/MIC | 110.5 ± 31.7 | 111.5 ± 32.3 | 110.5 ± 32.3 |
| MIC, 2 μg/ml | |||
| Peak/MIC | 5.8 ± 1.3bc | 8.2 ± 1.9bd | 14.7 ± 3.5cd |
| T<MIC | 2.0 ± 1.2bc | 4.9 ± 1.9bd | 14.6 ± 2.7cd |
| AUC/MIC | 55.3 ± 15.9 | 57 ± 16.1 | 55.2 ± 16.1 |
| MIC, 4 μg/ml | |||
| Peak/MIC | 2.9 ± 0.7bc | 4.1 ± 1.0bd | 7.3 ± 1.7cd |
| T<MIC | 4.2 ± 1.4bc | 7.1 ± 1.5bd | 16.8 ± 1.9cd |
| AUC/MIC | 27.6 ± 8.0 | 28.5 ± 8.1 | 27.6 ± 8.1 |
Values are medians ± SDs.
P < 0.001.
P < 0.001.
P < 0.001.
FIG. 1.
Computer simulations of concentration-time curves of tobramycin at 10 mg/kg/24 h during intermittent infusions. — - —, 3.3 mg/kg q8h over 30 min; —, 5 mg/kg q12h over 30 min; - - - - -, 10 mg/kg q24h over 60 min; and ————, MIC of 2 μg/ml. Simulations were performed with the USC∗PACK package of programs with the median MAP-B fitted parameter values.
Pharmacodynamic comparisons.
Differences in pharmacodynamic indices paralleled the differences in predicted peak and trough concentrations. Significantly lower peak/MICs but shorter T<MICs were noted when the q8h versus q12h, q8h versus q24h, and q12h versus q24h regimens were compared, regardless of the MIC (Table 1; Fig. 1). Similarly, with increasing MICs, the peak/MIC decreased significantly while the T<MIC increased significantly within each dosing group (data not shown). As expected, since the same total daily dose was provided by all three dosing regimens, no differences in AUC/MIC between regimens were observed.
A comparison of pharmacodynamic indices between dosing regimens in relationship to achievement of the desired goals (peak/MIC of 10 and T<MIC not to exceed the duration of the PAE) was also performed (Table 1). Regardless of the MIC, all once-daily regimens resulted in prolonged drug-free intervals that exceeded the duration of the PAE (both in vitro and in vivo). Only the two multiple-daily-dosing regimens (q8h and q12h) at an MIC of 1 μg/ml met the desired goals. However, at higher MICs the multiple-daily-dosing regimens failed to achieve the desired peak/MIC. Somewhat favored is the q12h regimen, which more effectively balances achievement of the desired peak/MIC while minimizing T<MIC.
DISCUSSION
The objective of the present investigation was to compare the predicted pharmacodynamic activities of three tobramycin dosing regimens in adult patients with CF. While few studies describe the pharmacokinetics and safety (3, 5) of this dosing modality in patients with CF, data on clinical efficacy are scant.
Vic et al. (31) performed a prospective open-label evaluation of once-daily amikacin with 20 pediatric CF patients with acute pulmonary exacerbations. Patients received amikacin at 35 mg/kg q24h in combination with either ceftazidime or imipenem at 200 mg/kg/day for 14 days. Significant improvements in respiratory and inflammatory indices were observed. However, without a comparison group, the efficacy of once-daily aminoglycoside administration remains in question. In a subsequent study, the same investigators performed a prospective comparative trial of tobramycin given once daily versus three times daily to 22 pediatric CF patients experiencing acute pulmonary exacerbations (32). Patients received ceftazidime at 200 mg/kg/day in combination with tobramycin at 5 mg/kg q8h or 15 mg/kg/day for 14 days. Improvements in pulmonary function and inflammatory indices were not different between the two groups. However, that study was underpowered and therefore does not provide definitive evidence in support of once-daily dosing of aminoglycosides in CF patients.
Powell et al. (23) evaluated the efficacies of three different tobramycin dosages with 52 adult CF patients with moderately severe pulmonary exacerbations. Twenty-six patients received 9 mg/kg q24h, 10 patients received 15 mg/kg q24h, and 16 patients received 11 mg/kg as a continuous infusion. Efficacy was evaluated by using a 15-point scale for paroxysmal cough, nocturnal cough, orthopnea, dyspnea at rest, and dyspnea with exertion. There were no differences in the rate of resolution of the five symptoms between the patients receiving the different dosages. Although these data are interesting, subsequent studies have shown that clinical symptom scores do not correlate with objective measures of pulmonary function during acute pulmonary exacerbations (4).
Various pharmacodynamic indices such as peak/MIC, AUC/MIC, and T<MIC have been suggested as useful indicators of the clinical and microbiological effectiveness of an antibiotic regimen. In particular, the aminoglycoside peak/MIC has been demonstrated to correlate significantly with successful treatment of pneumonia (15). In the present study we showed that peak/MIC is significantly greater with once-daily administration than with either q12h or q8h regimens. However, our data also show that T<MIC is significantly prolonged with once-daily administration than with either q12h or q8h regimens (Table 1). These two factors would be expected to have opposing pharmacodynamic effects. A greater peak/MIC would provide greater bactericidal activity, while a longer T<MIC would allow greater organism regrowth to occur within the dosing interval.
The PAE is frequently cited as one of the principal factors that enables extended-interval aminoglycoside dosing. While the PAE has been described in numerous in vitro studies and several studies with animals, the reported duration varies significantly between studies. The most likely explanation for this variation relates to the experimental methods used including exposure to fixed versus fluctuating antibiotic concentrations and differences in the availability of nutritional factors necessary for microorganism recovery. Many studies measured the PAE in vitro by comparing growth curves following dilution to remove the antibiotic, with the duration reported to be 2 to 3 h. A limitation of this approach is that it does not mimic in vivo conditions. In vivo, aminoglycoside concentrations decline exponentially. Den Hollander et al. (10) have recently demonstrated a more prolonged growth inhibition of tobramycin against P. aeruginosa (6 h) using an in vitro pharmacokinetic-pharmacodynamic model, which provided fluctuating tobramycin concentrations similar to those achieved under in vivo conditions. The investigators attributed the more prolonged growth inhibition to a postantibiotic sub-MIC effect which would not be detected by standard methods for measurement of PAE. More recently, Mouton et al. (J. W. Mouton, N. J. Jacobs, P. DeGraaf, J. G. Hollander Den, and A. M. Horrevorts, Abst. 39th Intersci. Conf. Antimicrob. Agents Chemother. abstr. 539, p. 22, 1999) have demonstrated that the in vitro PAE varies with the concentration of nutritional factors from the broth used for microorganism recovery. The results of these studies correlate better with the limited in vivo data available from studies with animals. Craig et al. (7) measured a PAE of 10 h with amikacin using a once-daily regimen in a murine neutropenic thigh infection model. These data suggest that the PAE in humans may be longer than the 2 to 3 h obtained by standard in vitro techniques.
The tobramycin pharmacokinetic parameters found in the present study are similar to those reported by other investigators evaluating adult CF patients (3, 28). Considering the relatively short elimination half-life of approximately 2 h in our CF population, concentrations in serum would be expected to be undetectable within 10 h. If a short PAE (i.e., 3 h) is assumed, a prolonged drug-free interval (or T<MIC) is observed. The expected result is significant organism regrowth within the dosing interval that may more than offset any benefit achieved from a higher peak associated with once-daily dosing. In contrast, in general medicine patients, a slower clearance and longer half-life would be expected to result in a substantially reduced drug-free interval compared with that in our CF population (13). Thus, less potential for organism regrowth within the dosing interval would be expected. These observations offer a possible explanation for the similar efficacy seen with once-daily and multiple-daily-dosing regimens in general medicine patients (24), while the same may or may not be seen with CF patients. Alternatively, if a more prolonged PAE is assumed (i.e., 10 h), the greater peak-associated bactericidal activity of the once-daily regimen may outweigh the relatively short period of time when the PAE has been exceeded. Comparative bactericidal activity data for CF patients are lacking. On the basis of our analysis, it is unclear whether once-daily administration would offer improved or poorer outcomes compared to those achieved with traditional multiple-daily-dosing regimens.
The analysis applied in this study does not consider the effects of concomitant antibiotic therapy. In clinical practice, combination therapy with an aminoglycoside and a beta-lactam is frequently prescribed in order to provide potentially synergistic activity and to reduce the likelihood for development of antibiotic resistance. In an experimental model of P. aeruginosa pneumonia in neutropenic guinea pigs, once-daily dosing of tobramycin was less active than every 4 h for dosing a similar overall dose. The addition of mezlocillin to both regimens resulted in similar killing activities (17). These data suggest that combination therapy is likely to obscure differences in aminoglycoside regimens at lower MICs (i.e., an MIC of 0.8 μg/ml was used in the earlier study [17]). It would be important to know whether this relationship holds true for the isolates for which MICs are relatively higher and which are commonly encountered in patients with CF. Additionally, once-daily administration of aminoglycosides in our patients would result in drug concentrations below 0.5 mg/liter for nearly half of the dosing interval (Fig. 1). Whether this provides similar activity in terms of synergy and deterring the development of resistance as conventional multiple daily dosing regimens is unclear.
Profiles of the concentration in serum were used to predict the efficacy of tobramycin on the basis of pharmacodynamic indices. The tobramycin concentrations within the lung airways have been demonstrated to be significantly lower than those in serum due to decreased penetration (21). In addition, binding to sputum mucins also contributes to reduced tobramycin activity (20). While these factors would predict decreased killing activity in the lungs compared with that in the serum, results from a murine pneumonitis model indicate that aminoglycoside elimination is slower from the lungs than from serum (19). The prolonged elimination from the lungs resulted in a more sustained antibacterial activity within the lungs. While the net effect of these factors in patients with CF is not clear, it would be expected to similarly effect the activities of all tobramycin dosing regimens. Therefore, the relative differences between dosing regimens as suggested by the current pharmacodynamic analysis are likely to be retained.
The results of this study are equivocal with regard to the predicted comparative efficacy of once-daily and multiple-daily tobramycin dosing regimens in adult CF patients. In the presence of a short PAE the potential benefit of achieving a greater peak/MIC with once-daily aminoglycoside dosing may be more than offset by the significantly greater T<MIC in CF patients compared with that achieved with either a q12h or a q8h regimen. In contrast, with a more prolonged PAE, as observed in studies with animals, once-daily dosing regimens may provide greater bactericidal activity.
Comparative evaluations of administration of single daily doses versus multiple daily doses of an aminoglycoside in CF patients are necessary to determine definitively the efficacy of the once-daily dosing modality. A comparison of pulmonary function between treatment groups would enable objective comparison of the efficacies of the dosing regimens and should serve as the primary outcome parameter. Reduced bacterial densities in sputum have been demonstrated to correlate with improvement in pulmonary function during acute exacerbations in patients with CF and would provide a useful secondary measure of the comparative activity of once-daily and traditional multiple-daily dosing regimens (26). Additionally, development of standardized measurements of pulmonary inflammation (i.e., cytokine measurements) may provide useful measures of outcome besides lung function itself. The dosages and timing of administration of concomitantly administered antibiotics (e.g., beta-lactams and fluoroquinolones) in relation to the aminoglycoside administration should be documented since these are also likely to affect the relationship (2). Routine use of once-daily aminoglycoside administration should be avoided in patients with CF until such data are available to confirm the efficacy of this dosing modality.
REFERENCES
- 1.Anderson E T, Young L S, Hewitt W L. Simultaneous antibiotic levels in “break through” gram-negative bacteremia. Am J Med. 1976;61:493–497. doi: 10.1016/0002-9343(76)90328-4. [DOI] [PubMed] [Google Scholar]
- 2.Barclay M L, Begg E J, Chambers S T, Boswell D R. Improved efficacy with nonsimultaneous administration of first doses of gentamicin and ceftazidime in vitro. Antimicrob Agents Chemother. 1995;39:132–136. doi: 10.1128/aac.39.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates R D, Nahata M C, Jones J W, McCoy K, Young G, Cox S, Barson W J. Pharmacokinetics and safety of tobramycin after once-daily administration in patients with cystic fibrosis. Chest. 1997;112:1208–1213. doi: 10.1378/chest.112.5.1208. [DOI] [PubMed] [Google Scholar]
- 4.Bosso J A, Walker K B. Lack of correlation between objective indicators and clinical-response scores during antimicrobial therapy for acute pulmonary exacerbations of cystic fibrosis. Clin Pharm. 1988;7:897–901. [PubMed] [Google Scholar]
- 5.Canis F, Husson M O, Turck D, Vic P, Launay V, Ategbo S, Vincent A, Courcol R J. Pharmacokinetics and bronchial diffusion of single daily dose amikacin in cystic fibrosis patients. J Antimicrob Chemother. 1997;39:431–433. doi: 10.1093/jac/39.3.431. [DOI] [PubMed] [Google Scholar]
- 6.Craig W A, Ebert S C. Killing and regrowth of bacteria in vitro: a review. Scand J Infect Dis. 1991;74(Suppl.):63–70. [PubMed] [Google Scholar]
- 7.Craig W A, Redington J, Ebert S C. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J Antimicrob Chemother. 1991;27(Suppl. C):29–40. doi: 10.1093/jac/27.suppl_c.29. [DOI] [PubMed] [Google Scholar]
- 8.Davis P B, Drumm M, Konstan M. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–1256. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- 9.De Groot R, Smith A L. Antibiotic pharmacokinetics in cystic fibrosis: differences and clinical significance. Clin Pharmacokinet. 1987;13:228–253. doi: 10.2165/00003088-198713040-00002. [DOI] [PubMed] [Google Scholar]
- 10.Den Hollander J G, Fuursted K, Verbrugh H A, Mouton J W. Duration and clinical relevance of postantibiotic effect in relation to the dosing interval. Antimicrob Agents Chemother. 1998;42:749–754. doi: 10.1128/aac.42.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DuBois D, DuBois E F. A formula to estimate appropriate surface area if height and weight are known. Arch Intern Med. 1916;17:263–271. [Google Scholar]
- 12.Eisenberg J, Pepe M, Williams-Warren J, Vasiliev M, Montgomery A B, Smith A L, Ramsey B W. A comparison of peak sputum tobramycin concentration in patients with cystic fibrosis using jet and ultrasonic nebulizer systems. Chest. 1997;111:955–962. doi: 10.1378/chest.111.4.955. [DOI] [PubMed] [Google Scholar]
- 13.Garrelts J C. Exploration of once-daily dosing of aminoglycosides through Bayesian simulation. Pharmacotherapy. 1996;16:286–294. [PubMed] [Google Scholar]
- 14.Hallynck T H, Soep H H, Thomis J A, Boelaert J, Daneels R, Dettli L. Should clearance be normalized to body surface or to lean body mass? Br J Clin Pharmacol. 1981;11:523–525. doi: 10.1111/j.1365-2125.1981.tb01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyatt J M, McKinnon P S, Zimmer G S, Schentag J J. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome: focus on antibacterial agents. Clin Pharmacokinet. 1995;28:143–160. doi: 10.2165/00003088-199528020-00005. [DOI] [PubMed] [Google Scholar]
- 16.Jelliffe R W, Jelliffe S M. A computer program for estimation of creatinine clearance from unstable serum creatinine levels, age, sex and weight. Math Biosci. 1972;14:17–24. [Google Scholar]
- 17.Kapusnik J E, Hackbarth C J, Chambers H F, Carpenter T, Sande M A. Single, large, daily dosing versus intermittent dosing of tobramycin for treating experimental Pseudomonas pneumonia. J Infect Dis. 1988;158:7–12. doi: 10.1093/infdis/158.1.7. [DOI] [PubMed] [Google Scholar]
- 18.Karlowsky J A, Zhanel G G, Davidson R J, Hoban D J. Postantibiotic effect in Pseudomonas aeruginosa following single and multiple aminoglycoside exposures in vitro. J Antimicrob Chemother. 1994;33:937–947. doi: 10.1093/jac/33.5.937. [DOI] [PubMed] [Google Scholar]
- 19.Leggett J E, Fantin B, Ebert S, Totsuka K, Vogelman B, Calame W, Mattie H, Craig W A. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J Infect Dis. 1989;159:281–292. doi: 10.1093/infdis/159.2.281. [DOI] [PubMed] [Google Scholar]
- 20.Levy J, Smith A L, Kenny M A, Ramsey B, Schoenknecht F D. Bioactivity of gentamicin in purulent sputum from patients with cystic fibrosis or bronchiectasis: comparison with activity in serum. J Infect Dis. 1983;148:1069–1076. doi: 10.1093/infdis/148.6.1069. [DOI] [PubMed] [Google Scholar]
- 21.Mendelman P M, Smith A L, Levy J, Weber A, Ramsey B, Davis R L. Aminoglycoside penetration, inactivation, and efficacy in cystic fibrosis. Am Rev Respir Dis. 1985;132:761–765. doi: 10.1164/arrd.1985.132.4.761. [DOI] [PubMed] [Google Scholar]
- 22.Moore R D, Smith C R, Lietman P S. Association of aminoglycoside plasma levels with therapeutic outcome in gram-negative pneumonia. Am J Med. 1984;77:657–662. doi: 10.1016/0002-9343(84)90358-9. [DOI] [PubMed] [Google Scholar]
- 23.Powell S H, Thompson W L, Luthe M A, Stern R C, Grossniklaus D A, Bloxham D D, Groden D L, Jacobs M R, DiScenna A O, Cash H A, Klinger J D. Once-daily vs. continuous aminoglycoside dosing: efficacy and toxicity in animal and clinical studies of gentamicin, netilmicin, and tobramycin. J Infect Dis. 1983;5:918–932. doi: 10.1093/infdis/147.5.918. [DOI] [PubMed] [Google Scholar]
- 24.Preston S L, Briceland L L. Single daily dosing of aminoglycosides. Pharmacotherapy. 1995;15:297–316. [PubMed] [Google Scholar]
- 25.Ramsey B W. Management of pulmonary disease in patients with cystic fibrosis. N Engl J Med. 1996;335:179–188. doi: 10.1056/NEJM199607183350307. [DOI] [PubMed] [Google Scholar]
- 26.Regelmann W E, Elliott G R, Warwick W J, Clawson C C. Reduction of sputum Pseudomonas aeruginosa density by antibiotics improves lung function in cystic fibrosis more than do bronchodilators and chest physiotherapy alone. Am Rev Respir Dis. 1990;141:914–921. doi: 10.1164/ajrccm/141.4_Pt_1.914. [DOI] [PubMed] [Google Scholar]
- 27.Saiman L, Mehar F, Niu W W, Neu H C, Shaw K J, Miller G, Prince A. Antibiotic susceptibility of multiply resistant Pseudomonas aeruginosa isolated from patients with cystic fibrosis, including candidates for transplantation. Clin Infect Dis. 1996;23:532–537. doi: 10.1093/clinids/23.3.532. [DOI] [PubMed] [Google Scholar]
- 28.Touw D J, Vinks A A T M M, Neef C. Pharmacokinetic modelling of intravenous tobramycin in adolescent and adult patients with cystic fibrosis using the nonparametric expectation maximization (NPEM) algorithm. Pharm World Sci. 1997;19:142–151. doi: 10.1023/a:1008633526772. [DOI] [PubMed] [Google Scholar]
- 29.Touw D J, Vinks A A T M M, Mouton J W, Horevorts A M. Pharmacokinetic optimisation of antibacterial treatment in patients with cystic fibrosis. Clin Pharmacokinet. 1998;35:437–459. doi: 10.2165/00003088-199835060-00003. [DOI] [PubMed] [Google Scholar]
- 30.Turpin S V, Knowles M R. Treatment of pulmonary disease in patients with cystic fibrosis. In: Davis P B, editor. Lung biology in health and disease. 64. Cystic fibrosis. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 277–344. [Google Scholar]
- 31.Vic P, Ategbo S, Turck D, Husson M O, Tassin E, Loeuille G A, Deschildre A, Druon D, Elian J C, Arrouet-Lagandre C, Farriaux J P. Tolerance, pharmacokinetics and efficacy of once daily amikacin for treatment of Pseudomonas aeruginosa pulmonary exacerbations in cystic fibrosis patients. Eur J Pediatr. 1996;155:948–953. doi: 10.1007/BF02282885. [DOI] [PubMed] [Google Scholar]
- 32.Vic P, Ategbo S, Turck D, Husson M O, Launay V, Loeuille G A, Sardet A, Deschildre A, Druon D, Arrouet-Lagande C. Efficacy, tolerance, and pharmacokinetics of once daily tobramycin for pseudomonas exacerbations in cystic fibrosis. Arch Dis Child. 1998;78:536–539. doi: 10.1136/adc.78.6.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig W A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]