Abstract
Triacylglycerol (TAG), an ester derived from glycerol and three fatty acids (FAs), is synthesized during seed development and controlled by transcriptional regulation. We examined the mechanism regulating the FA composition of developing Arabidopsis thaliana seeds. The seed‐specific DC3 PROMOTER‐BINDING FACTOR2 (DPBF2) transcription factor was upregulated by LEAFY COTYLEDON2 (LEC2). DPBF2 showed transcriptional activity in yeast and localized to the nucleus in Arabidopsis protoplast cells. The Arabidopsis dpbf2‐1 homozygous T‐DNA mutant and transgenic lines overexpressing of DPBF2 using a seed‐specific phaseolin promoter in wild‐type (WT) Arabidopsis and in dpbf2‐1 showed similar FA composition profiles in their seeds. Their 18:2 and 20:1 FA contents were higher, but 18:1 and 18:3 contents were lower than that of WT. Transcript levels of FATTY ACID DESATURASE2 (FAD2), FAD3, LYSOPHOSPHATIDYLCHOLINE ACYLTRANSFERASE1 (LPCAT1), LPCAT2, PHOSPHATIDYLCHOLINE DIACYLGLYCEROL CHOLINEPHOSPHOTRANSFERASE (PDCT), and FATTY ACID ELONGASE 1 (FAE1) are increased in DPBF2‐overexpressing seeds. Besides, PDCT and FAE1 were upregulated by DPBF2, LEC1‐LIKE (L1L), and NUCLEAR FACTOR‐YC2 (NF‐YC2) transcriptional complex based on tobacco protoplast transcriptional activation assay. These results suggest that DPBF2 effectively modulates the expression of genes encoding FA desaturases, elongase, and acyl‐editing enzymes for modifying the unsaturated FA composition in seeds.
Keywords: bZIP67, DPBF2, FAD3, FAE1, PDCT, seed fatty acid composition, transcription factor
1. INTRODUCTION
Triacylglycerol (TAG) is an oil molecule composed of three fatty acid (FA) chains esterified to a glycerol backbone. TAG accumulates during seed development and is an energy source for seed germination and seedling establishment (Graham, 2008; Li‐Beisson et al., 2013). TAG and FA biosynthesis have been studied at the molecular level using Arabidopsis thaliana as a model oilseed (Ohlrogge et al., 1991; Somerville, 1991; Wallis & Browse, 2010).
Biosynthesis of seed FAs begins in plastids. The first step is the conversion of acetyl‐CoA to malonyl‐CoA by acetyl‐CoA carboxylase followed by the conversion of malonyl‐CoA to malonyl‐acyl carrier protein (ACP) through malonyl‐CoA‐ACP transacylase. Acetyl‐CoA and malonyl‐ACP are then condensed by 3‐ketoacyl‐ACP synthase III (KASIII) and elongated by two reductases and one dehydratase. Condensation by KASI is followed by subsequent elongation reactions to yield 16:0‐ACP. Condensation from 16:0‐ACP to 18:0‐ACP occurs via KASII. Plastidial desaturase then converts 18:0‐ACP to 18:1‐ACP which is transported as 18:1 to the cytosol by fatty acyl‐ACP thioesterase A (FATA); 16:0‐ACP and 18:0‐ACP are released as 16:0 and 18:0, respectively, to the cytosol by FATB. The resulting FAs form an acyl‐CoA pool.
The 18:1‐CoA are elongated to 20:1‐CoA and 22:1‐CoA by fatty acid elongase 1 (FAE1) and which contribute to the acyl‐CoA pool. Fatty acid desaturase 2 (FAD2) acts on 18:1 on the sn‐2 position of phosphatidylcholine (PC) to create 18:2, which is converted to 18:3 by FAD3. The 18:2 and 18:3 (polyunsaturated fatty acid [PUFA]) are then released into the acyl‐CoA pool by acyl‐editing enzymes such as lysophosphatidylcholine acyltransferase (LPCAT). In addition, the PUFA‐PC is converted to PUFA‐diacylglycerol (DAG) via head group exchange by phosphatidylcholine diacylglycerol cholinephosphotransferase (PDCT) (Bates et al., 2012). The PUFA‐enriched acyl‐CoA pool and PUFA‐DAG are used for TAG biosynthesis in oilseeds (Bates & Browse, 2012).
TAG biosynthesis occurs in the endoplasmic reticulum (ER), usually via the Kennedy pathway. The first FA is attached to glycerol 3‐phosphate (G3P) at its sn‐1 position by glycerol‐3‐phosphate acyltransferase (GPAT), yielding lysophosphatidic acid (LPA). The second FA is attached to the sn‐2 position of LPA by lysophosphatidic acid acyltransferase (LPAT), resulting in phosphatidic acid (PA). PA phosphatase (PAP) removes the phosphate at the sn‐3 of PA to produce DAG. Finally, the third FA is attached to the sn‐3 position by diacylglycerol acyltransferase (DGAT) to produce TAG. Kennedy pathway utilizes FAs in the acyl‐CoA pool and is thus acyl‐CoA dependent. TAG can also be produced without incorporating FAs from the acyl‐CoA pool, in which phospholipid:DAG acyltransferase (PDAT) transfers the FA at the sn‐2 position of PC to the sn‐3 position of DAG (Dahlqvist et al., 2000; Ståhl et al., 2004). TAG is concentrated between the ER bilayer and surrounded by the hydrophobic protein oleosin, which accumulates in the embryo cell body in the form of a spherical oil body (Lacey et al., 1999; Napier et al., 1996; Wanner & Theimer, 1978).
Although the genes encoding enzymes involved in FA biosynthesis are well known, there are insufficient studies on the transcription factors that regulate the contents of TAG and unsaturated FAs during seed development. The master regulators LEAFY COTYLEDON1 (LEC1), ABSCISIC ACID INSENSITIVE3 (ABI3), FUSCA3 (FUS3), and LEC2 regulate seed development and TAG biosynthesis (Giraudat et al., 1992; Keith et al., 1994; Meinke, 1992; Stone et al., 2001; West et al., 1994). Mutations in these master regulator genes alter the FA composition and/or decrease the TAG content of seeds, whereas ectopic overexpression of master regulators promotes FA biosynthesis and TAG accumulation (Kim et al., 2014; Lemieux et al., 1990; Mu et al., 2008; Santos‐Mendoza et al., 2005). WRINKLED1 (WRI1) is a transcription factor regulating genes involved in glycolysis and FA biosynthesis (Baud et al., 2007; Cernac & Benning, 2004; Focks & Benning, 1998). LEC1, LEC2, and FUS3 bind to the promoter of WRI1, regulating its expression (Baud et al., 2007; Kong et al., 2019; Marchive et al., 2014; Mu et al., 2008).
Expression of LEC2 under the control of a senescence‐specific promoter results in TAG accumulation in leaves (Kim et al., 2014, 2015). LEC2 expression in leaves induces various seed‐specific transcription factor genes, presumed to be downstream targets of LEC2 (Kim et al., 2015). Among these seed‐specific transcription factors, we examined those that were coexpressed with FA and TAG biosynthesis genes. DC3 promoter‐binding factor 2 (DPBF2) is strongly upregulated in LEC2‐expressing leaf tissue and is coexpressed with FA biosynthesis genes, suggesting that DPBF2 is a transcription factor controlling FA biosynthesis (Kim et al., 2015). DPBF2 is also known as BASIC LEUCINE ZIPPER TRANSCRIPTION FACTOR 67 (bZIP67) (Jakoby et al., 2002). Genes encoding these DPBF proteins were initially isolated from a sunflower (Helianthus annuus) immature seed library using a modified yeast one‐hybrid system (Kim et al., 1997; Kim & Thomas, 1998). Of five DPBF genes expressed in Arabidopsis seeds, DPBF2 was confirmed to bind to the DC3 promoter in an electrophoretic mobility shift assay (EMSA) and showed transcriptional activity in a yeast one‐hybrid system (Kim et al., 2002).
DPBF2/bZIP67 has already been reported to be a transcription factor regulating FAD3 (Mendes et al., 2013). In this study, we aim to confirm that DPBF2 is a seed‐specific transcription factor and regulated transcriptionally by LEC2. We also analyzed the FA composition and FA‐related genes in a dpbf2‐1 knock‐out mutant and transgenic lines overexpressing DPBF2 in seeds. We confirmed that DPBF2 upregulates the expression of PDCT and FAE1 together with LEC1‐LIKE (L1L) and NUCLEAR FACTOR‐YC2 (NF‐YC2) by transcriptional activation assay in the tobacco protoplast.
2. MATERIALS AND METHODS
2.1. Plant materials and growth conditions
Plants including wild‐type (WT) A. thaliana (ecotype Col‐0), the T‐DNA insertion mutants, dpbf2‐1 (Salk_085497C) and lec2‐1 (CS3868) (Gaj et al., 2005; Meinke et al., 1994), F2 generation plants generated by WT and dpbf2‐1 crossing, and transgenic plants overexpressing DPBF2 under the control of cauliflower mosaic virus (CaMV) 35S promoter or phaseolin seed‐specific promoter (Slightom et al., 1983) were grown in potting soil in a growth chamber at 22 °C under a 16 h light/8 h dark period.
2.2. Transcriptional activity assay in protoplast and yeast
To perform the transcriptional activity assay in protoplast, recombinant effector and reporter plasmids were cotransformed into tobacco protoplasts by polyethylene glycol‐mediated transformation (Yoo et al., 2007). The effector plasmids were constructed with CaMV 35S promoter fused to LEC2, DPBF2, L1L, and NF‐YC2 cDNA genes, whereas the reporter plasmids were constructed with DPBF2, FAD2, LPCAT1/2, PDCT, FAE1, and CRU3 promoter, respectively, including 5′‐untranslated region (UTR) fused to luciferase reporter gene using listed primers (Table S1). To generate mutated DPBF2 promoter sequence, two primers including “AAAAAAAAAA” were designed, and it was amplified by two times PCR. GUS activity using pBI221 vector measured for normalization of the luciferase activity. These effector and reporter plasmids were cotransfected using polyethyleneglycol (PEG) solution (40% PEG 4000, 200 mM mannitol, 100 mM CaCl2) to protoplast isolated from Nicotiana benthamiana using enzyme solution (400 mM mannitol, 20 mM KCl, 20 mM MES [pH 5.7], 0.25% macerozyme, 1% cellulase). And this protoplast was incubated in dark for 16 h and measured luciferase and GUS activity with a luminometer (Glomax 20/20; Promega, USA).
The transcriptional activity of DPBF2 (AT3G44460) was assayed in budding yeast (Saccharomyces cerevisiae). A DPBF2 5′‐end primer containing a BamHI site (5′‐GCGGATCCGTTCGGTTTTCGAATCGGAGAC‐3′) and 3′‐end primer containing a PstI site (5′‐GGGCTGCAGTTACCACCCGGCACTGGCC‐3′) were used to PCR‐amplify DPBF2 cDNA. The DPBF2 cDNA was digested with BamHI and PstI and cloned into a pGBKT plasmid vector containing a GAL4 DNA‐binding (DB) domain to produce the pGBKT‐DB‐DPBF2 vector capable of expressing the DB‐DPBF2 fusion gene product. This vector was transformed into yeast strain PBN204, harboring ADE2 and URA3 reporter genes that were expressed under the control of various GAL promoters. pACT2 containing the GAL4 transcriptional activation domain was used as a positive control. Yeast transformants were selected on SD‐LW plates containing SD minimal media without leucine (L) or tryptophan (W). The selected colony was replicated to determine transcriptional activity on SD‐LWU medium without leucine, tryptophan, and uracil (U) and on SD‐LWA without leucine, tryptophan, and adenine (A).
2.3. Gene cloning and vector construction
To construct a plant transformation vector capable of overexpressing DPBF2, DPBF2 cDNA was amplified by RT‐PCR from RNA isolated from developing Arabidopsis siliques and cloned into the pENTR‐D/TOPO vector (Invitrogen, USA). The nucleotide sequence of the pENTR‐DPBF2 cDNA clone was determined by Sanger sequencing. Plant recombinant expression vectors 35S‐DPBF2 and Ph‐DPBF2, in which DPBF2 is overexpressed under the control of the CaMV 35S promoter and a seed‐specific phaseolin promoter, respectively, were generated by LR clonase cloning in pEarleyGate201 (11.7 kb) and pPhaseolin‐Gate plant expression vectors (Kim et al., 2020) (see Figures S3A and 6a). The Ph‐GUS transformation control vector was generated using the LR clonase cloning reaction between pENTR‐GUS and pPhaseolin‐Gate (Figure 6a). Agrobacterium tumefaciens GV3101 was transformed with the plant expression vectors, and the Agrobacterium‐mediated Arabidopsis transformation was carried out by the floral‐dip method (Clough & Bent, 1998). Transgenic Arabidopsis plants were selected on MS medium containing 50 μg ml−1 kanamycin for 35S‐DPBF2 and by spraying with BASTA herbicide at a concentration of 0.3% (v/v) for Ph‐DPBF2 and Ph‐GUS.
FIGURE 6.
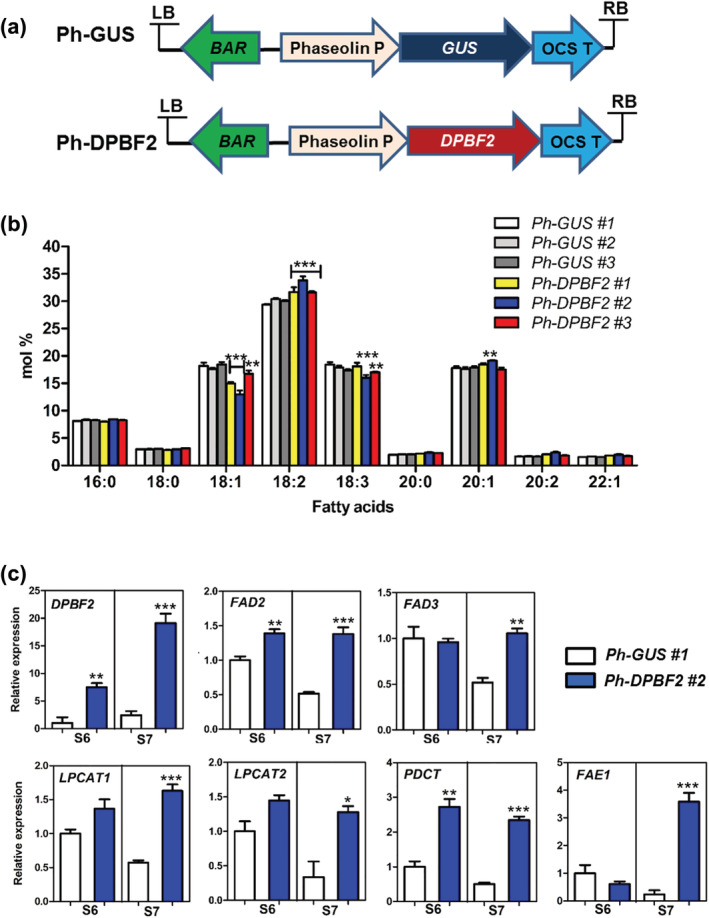
Fatty acid composition and gene expression changes in developing seeds of seed‐specific overexpressed GUS and DPBF2 in WT background. (a) Vectors used for seed‐specific heterologous expression of GUS and seed‐specific overexpression of DPBF2. (b) Comparison of FA composition in seeds of three independent Ph‐GUS and Ph‐DPBF2 T2 generation transgenic plants. FA content represents the mean (±SE) from three independent biological replicates. (c) Comparison of DPBF2 and FA biosynthesis gene expression in S6 and S7 stage of developing siliques between Ph‐GUS and Ph‐DPBF2 transformed plants. RT‐qPCR analysis of DPBF2, FAD2, FAD3, LPCAT1/2, PDCT, and FAE1 in the Ph‐GUS and Ph‐DPBF2 overexpression lines containing the phaseolin promoter. The S6 and S7 siliques used in this study included seeds of the walking‐stick embryo and curled cotyledon phase, respectively. Statistically significant differences by unpaired t test are indicated (*p < .05, **p < .01, ***p < .001). Relative expression values are given in comparison with the WT (WT = 1). Mean (±SE) from three independent biological replicates
2.4. Subcellular localization
DPBF2 cDNA was amplified by PCR from pENTR‐DPBF2 using primers with BamHI and SacI restriction sites and cloned into p326‐eGFP vector (Lee et al., 2016) carrying the enhanced green fluorescent protein (eGFP) to construct vector DPBF2‐GFP. This vector was transformed into Arabidopsis protoplasts using the PEG method with a red fluorescent protein (RFP) vector targeted at the nucleus as a control (Jin et al., 2001). Fluorescence images showing intracellular localization of gene products were obtained using a fluorescence microscope (Axioplan 2; Carl Zeiss, Germany).
2.5. T‐DNA mutant analysis
Salk_085497C seeds, with T‐DNA inserted into DPBF2, were purchased from ABRC (Arabidopsis Biological Resource Center). To obtain a homozygous dpbf2‐1 T‐DNA insertion mutant, DPBF2 gene‐specific primers targeting sequences either side of the T‐DNA insertion site (LP‐primer: 5′‐ACGATGTAATTTCAGCATCGG‐3′; RP‐primer: 5′‐CTCGGTTTTGGGAGAATCTTC‐3′) and the T‐DNA specific primer LBb1.3 (5′‐ATTTTGCCGATTTCGGAAC‐3′) were used for PCR. RT‐PCR was conducted using total RNA extracted from developing siliques of WT and dpbf2‐1 mutants using primers targeting the 5′‐end (5′‐ATGTCGGTTTTCGAATCGGAG‐3′) and 3′‐end (5′‐TTACCACCCGGCACTGGCCAT‐3′) to cover the full length of transcripts (see Figure S1A). ACT2 (At3g18780) forward (5′‐ATGATGCTCCCAGGGCTGTTT‐3′) and reverse (5′‐TTGTCACACACAAGTGCATCA‐3′) primers were used for control RT‐PCR amplification. Primer sequences for DPBF2 expression analysis in developing siliques of WT and dpbf2‐1 by reverse‐transcription quantitative PCR (RT‐qPCR) analysis were designed based on nucleotide sequences both 5′ and 3′ with the T‐DNA insertion centered in the DPBF2 genome. The forward primer sequence is 5′‐TTGATGGAGCGGAGACAACG‐3′ at the end of first exon of DPBF2 genomic DNA, and the reverse primer sequence is 5′‐CACTGGCCATCCTCCGAATC‐3′ at the end of fourth exon (Figure S1A). The DPBF2 cDNA size amplified by RT‐qPCR is 250 base pair.
2.6. Reverse‐transcription PCR and quantitative PCR
Total RNAs were isolated from S6 stage siliques containing walking‐stick embryo using the previously reported method (Onate‐Sanchez & Vicente‐Carbajosa, 2008) and from other tissues using TRIzol reagent (Invitrogen, USA). Quantitative PCR was performed with gene‐specific primers and cDNA prepared from 1 μg total RNA from developing siliques of WT, dpbf2‐1, 35S‐DPBF2, Ph‐DPBF2, and Ph‐GUS plants. Quantitative PCR was performed using SYBR Green Premix Ex Taq II (Takara, Japan) and the CFX96 Real‐Time PCR system (Bio‐Rad Laboratories), as specified by the manufacturer. Housekeeping genes ACTIN2 and elF4a were used for normalization in RT‐qPCR analysis. Primers for RT‐qPCR are listed in Table S1.
2.7. Fatty acid analysis
Ten milligrams of dry seeds and two leaf samples were transmethylated at 85°C for 90 min in 0.5 ml toluene and 0.5 ml 5% (v/v) H2SO4 in methanol. Heptadecanoic acid (17:0) was added to each sample as an internal standard to measure the amount of FAs. After transmethylation, 1.5 ml 0.9% (w/v) NaCl solution was added, and fatty acid methyl esters (FAMEs) were extracted with 0.5 ml n‐hexane. FAMEs were analyzed using gas chromatography (GC) on a GC‐2010 Plus Gas Chromatograph (Shimadzu, Japan) with a 30 m × 0.25 mm (inner diameter) HP‐FFAP column (Agilent, USA) while the oven temperature was increased from 190°C to 230°C at 3°C min−1. Nitrogen was used as the carrier gas at a flow rate of 1.4 ml min−1.
2.8. Data repetition and statistical analysis
Expression analysis of LEC2 and DPBF2 was performed using the results of three repeated microarray analyses from two independent transgenic lines, OIL21 and OIL25, which express LEC2 under the control of senescence‐inducible promoter (Kim et al., 2015). The subcellular localization experiments using DPBF2‐GFP construct were performed twice. Seed fatty acid analysis of WT and dpbf2‐1 T‐DNA mutants was performed in 14 independent lines. Fatty acid composition of 35S‐DPBF2 overexpressing transgenic line seeds was analyzed in nine T1 independent lines. Seed fatty acid composition of WT + Ph‐GUS, WT + Ph‐DPBF2, and dpbf2‐1 + Ph‐DPBF2 was analyzed from T3 seeds obtained from three T2 independent lines. RT‐qPCR analysis for genes between WT and dpbf2‐1 mutant and between WT + Ph‐GUS and WT + Ph‐DPBF2 transformed plants was analyzed from three independent samples. Each data represents the mean (±SE) from three independent biological replicates. Asterisks indicate significant changes compared with control (*p < .05, **p < .01, ***p < .001).
3. RESULTS
3.1. DPBF2 is upregulated by LEC2
To examine if DPBF2 is regulated by LEC2, we used the Arabidopsis OIL21 and OIL25 transgenic lines in which LEC2 is expressed under the control of a senescence‐inducible promoter, resulting in TAG biosynthesis and accumulation in the leaf tissue (Kim et al., 2015). We found that DPBF2 was strongly upregulated in the senescing leaves of the transgenic plants compared with those of the WT (Figure 1a). Besides, DPBF2 expression was significantly lower in the developing seeds of lec2‐1 mutant than WT seeds (Figure 1b). These results suggest that DPBF2 may be regulated directly or indirectly by LEC2. Our results are also consistent with the microarray analysis of senescing leaves performed by Kim et al. (2015) which reveals 45 seed‐specific transcription factors including DPBF2 that were upregulated in the leaves of OIL21 compared with those of WT Arabidopsis (Table S2).
FIGURE 1.
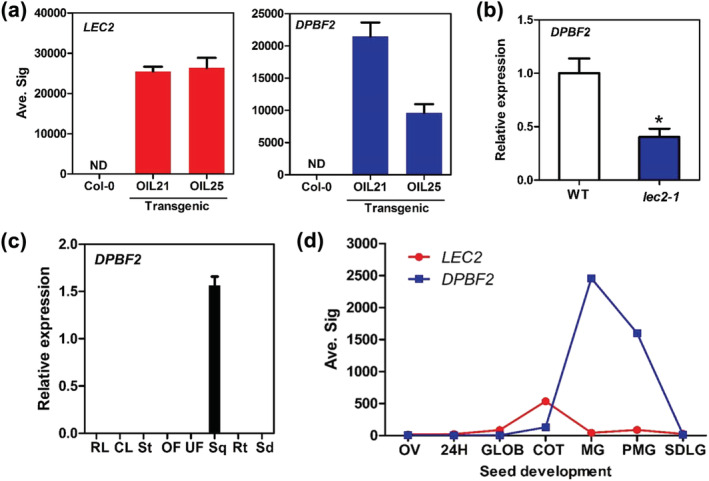
DPBF2 expression in transgenic lines OIL21 and OIL25, harboring senescence‐inducible LEC2, and wild‐type (WT) Arabidopsis plants. (a) Senescence‐inducible LEC2 upregulates DPBF2 transcript levels in senescing leaves. Senescent leaves of WT and two independent transgenic lines, OIL21 and OIL25, were used for microarray analysis. Relative expression values are mean (±SE) from three independent biological replicates. ND: none detected. (b) DPBF2 expression in developing siliques of the lec2‐1 mutant. Relative expression values are given in comparison with the WT (WT = 1). Mean (±SE) values are obtained from three independent biological replicates. *p < .05 (unpaired t test). (c) Seed‐specific expression of DPBF2. RL: rosette leaf; CL: cauline leaf; St: stem; OF: open flower; UF: unopen flower; Sq: developing silique S6 stage (containing walking‐stick embryo stages); Rt: root; Sd: seedling. Relative expression values are represented with a mean (±SE) from three independent biological replicates. (d) LEC2 and DPBF2 expression before, during, and after seed development. Average signal intensity (Avg. Sig.) of transcripts detected by GeneChip (Le et al., 2010). OV: embryo sac; 24H: pre‐globular embryo; GLOB: globular embryo; COT: linear and bent embryo; MG: mature embryo; PMG: post mature embryo; SDLG: seedling
To analyze the expression of DPBF2, we performed RT‐qPCR using a DPBF2 gene‐specific primer set and total RNA samples from several tissues of WT Arabidopsis (Table S1). DPBF2 transcripts were detected only in developing seeds, confirming that DPBF2 is a seed‐specific gene (Figure 1c). We investigated the expression pattern of DPBF2 before, during, and after seed development using GeneChip microarray data from Le et al. (2010). LEC2 expression was detected from the globular stage and peaked during cotyledon development (linear and bent stage of embryo). DPBF2 transcripts were detected later than those of LEC2 and peaked at the mature embryo stage when TAG had accumulated (Figure 1d).
To confirm that LEC2 regulates DPBF2 transcriptional activity by binding to its promoter, we investigated the luciferase activity driven by the promoter of 1092 bp including the 5′‐UTR portion of DPBF2 in transformed Nicotiana benthamiana protoplast (Figure 2). As a result, when LEC2 was expressed as an effector, DPBF2 promoter activity was increased by 130 times compared with the controls without effector expression. However, when all 10 base pairs of the RY motif (CATGCATGCA) predicted as the binding site of LEC2 (Braybrook et al., 2006) were replaced with A, the activity was greatly reduced by six to seven times compared with the native promoter (Figure 2b). This result suggests the possibility that LEC2 directly binds to the RY motif present in the −36 to −46 region of the DBPF2 promoter, resulting in regulating the transactivation of DPBF2 during seed development. Taken all together, these results showed that LEC2 directly regulates the expression of DPBF2.
FIGURE 2.
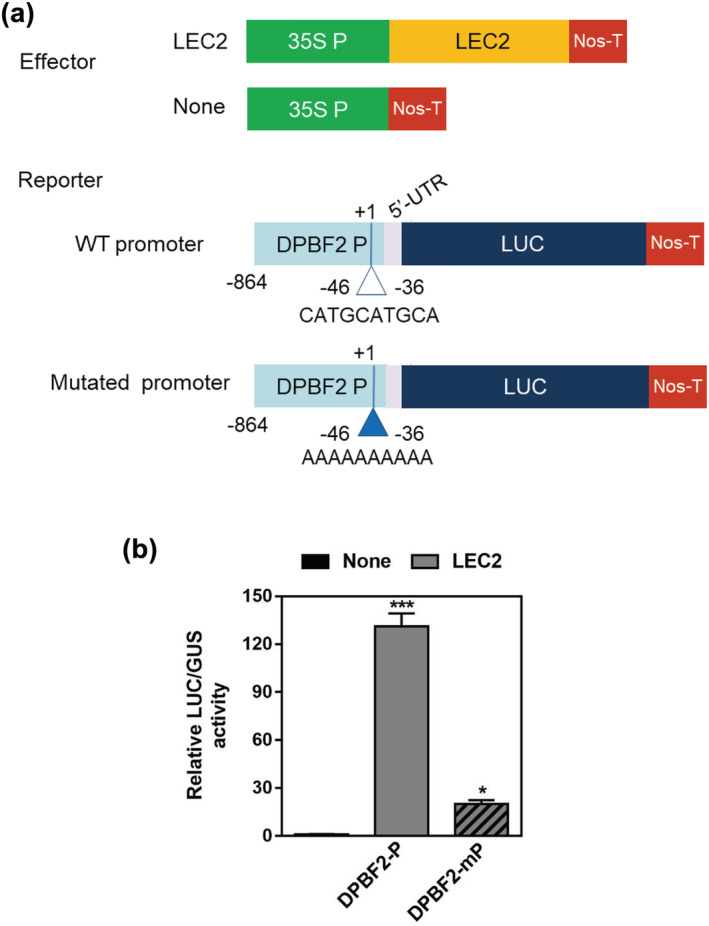
DPBF2 transcriptional activation assay by LEC2 in Nicotiana benthamiana leaf protoplasts. (a) Schematic diagrams of reporter and effector constructs. In the effector construct, LEC2 was cloned between the CaMV 35S promoter and the terminator of the nopaline synthase gene (Nos‐T). In the reporter constructs, the WT and RY motif‐mutated promoter of DPBF2 were fused to the luciferase gene. (b) WT and DPBF2 promoter activation assay in Nicotiana benthamiana leaf protoplasts. The effector and reporter constructs shown in (a) were cotransfected into protoplasts, and luciferase activities were determined fluorometrically. GUS gene expression was used to normalize the luciferase activities, and five measurements were averaged (t test, *p < .05). The bars indicate the SEM
3.2. DPBF2 has transcriptional regulatory activity and is present in the nucleus
To further investigate the function of DPBF2 as a transcription factor, DPBF2 was fused to the GAL4 DB domain of yeast and transformed into yeast PBN204, a nutrient‐requiring strain. The DB‐DPBF2‐expressing yeast cells grew as normal as those harboring the positive control clone (pACT2) under culture conditions without adenine or uracil, demonstrating that DPBF2 has transcriptional activity. By contrast, the negative control yeast transformed with the pBGKT7 vector did not grow under the same conditions (Figure 3a). To investigate if DPBF2 is localized in the nucleus, we introduced a construct containing GFP fused to DPBF2 together with a nuclei marker RFP vector into Arabidopsis protoplasts. In two independent experiments, the green fluorescence of DPBF2‐GFP was present in the nucleus and colocalized with the nuclear‐located red fluorescence of the marker RFP (Figure 3b). Thus, we have demonstrated that DPBF2 is targeted to the nucleus and has transcriptional activity. It is evident that DPBF2 is a functional transcription factor.
FIGURE 3.
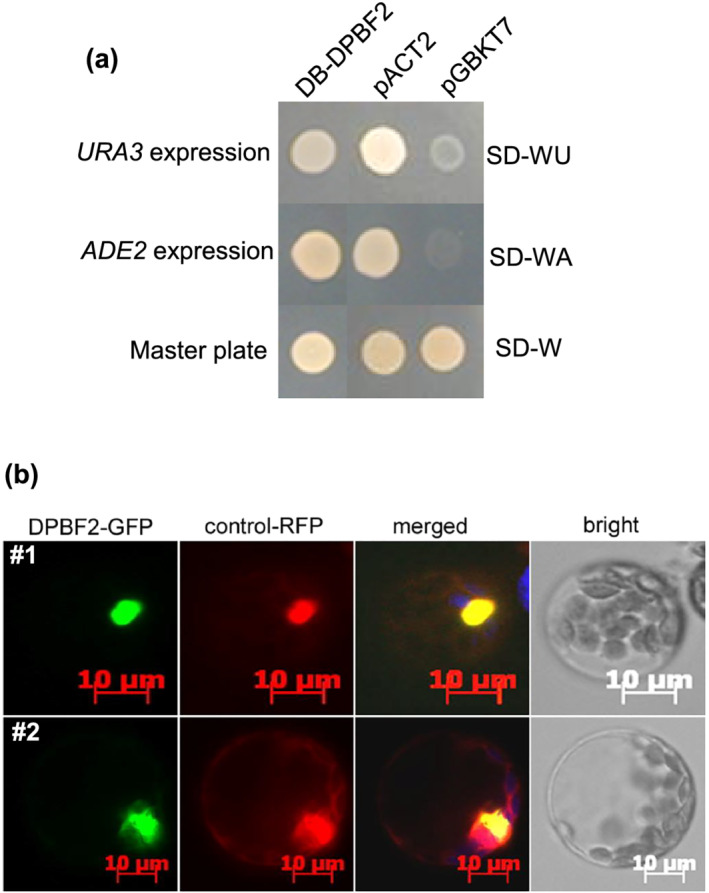
Transcriptional activity and subcellular localization of DPBF2. (a) Transcription activity test of DPBF2 in yeast. DB‐DPBF2, associated with the GAL4 DNA‐binding (DB) domain, induces URA3 and ADE2 expression in transgenic yeast, allowing growth in medium lacking uracil and adenosine. A positive control vector (pACT2) expressing the GAL4 activation domain (AD) allows the expression of URA3 and ADE2 in yeast. Negative control yeast expressing the pGBKT7 vector lacking the GAL4 DB domain and the GAL4 AD did not grow because URA3 and ADE2 were not expressed. SD‐W: minimum medium without tryptophan; SD‐WA: minimum medium without tryptophan and adenosine; SD‐WU: minimum medium without tryptophan and uracil. (b) DPBF2 localizes in the nucleus of Arabidopsis protoplasts. A nuclear‐targeted RFP construct (control‐RFP) was used as a control. Two independent experiments (#1 and #2) showed the same results
3.3. A dpbf2‐1 T‐DNA insertion mutant lacks DPBF2 expression in developing seeds
To investigate the function of DPBF2, we identified homozygous Arabidopsis T‐DNA insertion mutants from seeds of Salk_085497C line (Figure S1). DPBF2 contains four exons and three introns. We selected a mutant with the T‐DNA inserted into the second intron, which we named dpbf2‐1 (Figure S1A). As expected, PCR analysis using DPBF2 gene‐specific primers located on the left and right sides of the T‐DNA insertion position amplified a 1.2‐kb band in WT, whereas the LBb1.3 primers located within the T‐DNA yielded a 0.6‐kb band in the dpbf2‐1 mutant (Figure S1B).
We further analyzed DPBF2 expression by RT‐PCR of total RNA isolated from the developing seeds of WT and dpbf2‐1 mutant plants using DPBF2 specific primers that recognize cDNA containing the full‐length sequence of DPBF2. As shown in Figure S1C, the DPBF2 transcript was detected in WT, but not in dpbf2‐1. Thus, although the T‐DNA is inserted into the second intron, splicing did not occur correctly, resulting in the absence of DPBF2 expression. In addition, RT‐qPCR was performed using primers of the sequence corresponding to the exon portion of left and right regions of T‐DNA insertion to investigate whether the DPBF2 transcript was expressed in the developing seed of the dpbf2‐1 mutant. As a result, DPBF2 transcript was not detected in the dpbf2‐1 mutant (Figure S1D).
3.4. Fatty acid composition is changed in dpbf2‐1 mutant seeds
To examine the effect of expressing DPBF2 in Arabidopsis, we compared the seed FA composition of dpbf2‐1 with that of the WT (Figure 4a). There was no difference in the ratio of 16:0 to 18:0 saturated FAs in dpbf2‐1 seeds compared with WT seeds, but the contents of unsaturated FAs such as 18:1, 18:2, 18:3, and 20:1 were significantly changed at p < .001 by two‐way ANOVA. The 18:1 and 18:3 FAs showed a decrease (3% and 4%) in dpbf2‐1 compared with the WT. The 18:2 and 20:1 showed an increase of 5 and 2%, respectively, in dpbf2‐1 compared with the WT (Figure 4a). Total FA content representing the TAG content was not significantly different between the WT and mutant at p < .05 by t test (Figure 4B). There was also no difference in FA composition of leaves between the WT and dpbf2‐1 mutant (Figure S2). Therefore, DPBF2 affected unsaturated FA composition in seed oil without change of its total TAG content. DPBF2 did not affect FA composition in leaves.
FIGURE 4.
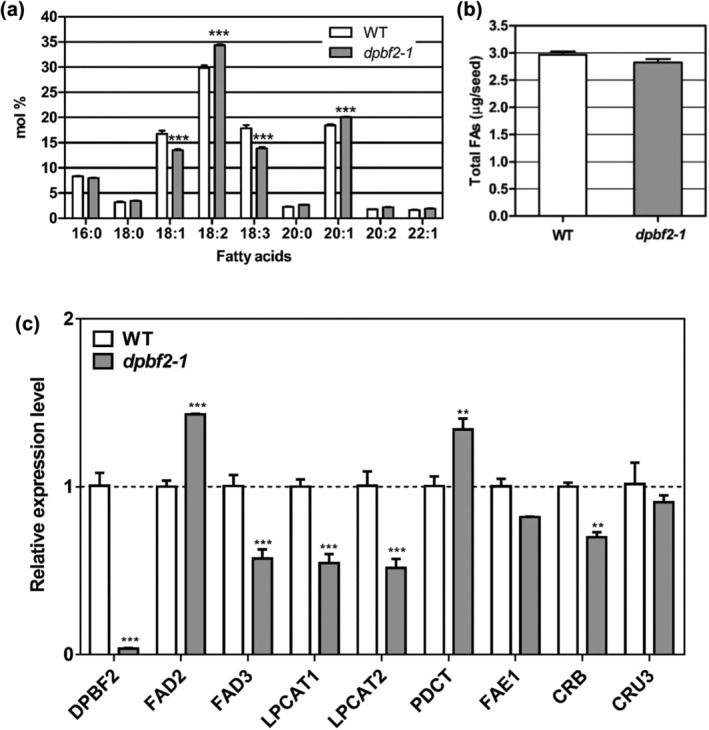
Seed fatty acid content analysis and gene expression changes in WT and dpbf2‐1 knock‐out mutant. (a) Seed FA composition in WT and dpbf2‐1 line. Statistically significant differences are indicated by two‐way ANOVA with Bonferroni posttests (***p < .001). (b) Total fatty acid amount in WT and dpbf2‐1 line. A statistically significant test for total fatty acid amount was done by t test with Wilcoxon matched pairs test in 95% confidence intervals. Data represent the mean (±SE) from 10 independent biological replicates. (c) Gene expression changes in developing seeds of the WT and dpbf2‐1 line by RT‐qPCR analysis of DPBF2, FAD2, FAD3, LPCAT1/2, PDCT1, FAE1, CRB, and CRU3 transcript levels. The measurements are normalized to eIF4a. Statistically significant differences are indicated by one‐way ANOVA with Tukey t tests (**p < .01, ***p < .001)
Because the unsaturated FA composition of seeds was altered relative to the WT in the dpbf2‐1 mutant line (Figure 4a), the DPBF2 transcription factor may regulate the expression of genes involved in unsaturated FA biosynthesis in developing seeds. We thus compared the expression of genes involved in unsaturated FA biosynthesis and acyl‐editing pathway in the 13‐day‐old developing seeds after pollination of dpbf2‐1 knock‐out mutant and the WT (Figure 4c). To determine the accuracy of RT‐qPCR analysis, CRUCIFERIN B (CRB) and CRUCIFERIN 3 (CRU3) were used as controls among the seed storage protein genes that were previously reported to have decreased expression in the developmental seeds of dpbf2‐1/bzip67 knock‐out mutant (Mendes et al., 2013). Gene expression analysis was performed using samples of developing WT and dpbf2‐1 mutant seeds at the same stage. In developing seeds of the dpbf2‐1 mutant that did not express DPBF2. Similar to a previous report (Mendes et al., 2013), the expression of CRB and CRU3 was decreased compared with the WT. The expression of the unsaturated FA biosynthesis gene FAD2 slightly increased and FAD3 decreased. The decrease in FAD3 expression in the dpbf2‐1 mutant was consistent with results from previously reported dpbf2‐1/bzip67 mutants (Mendes et al., 2013). The increase of FAD2 expression and decrease of FAD3 expression in the dpbf2‐1 mutant was also consistent with the 18:2 FA increase and 18:1 and 18:3 FA decrease phenotype (Figure 4a). Among the genes encoding enzymes in the acyl‐editing pathway between PC and TAG, expression of LPCAT1 and LPCAT2 was lower in the dpbf2‐1 mutant than in the WT, while PDCT expression was slightly increased (Figure 4c). The change of the unsaturated FA composition in dpbf2‐1 developing seeds was obvious, but the change in the FA synthesis genes showed less than onefold.
3.5. DPBF2/dpbf2‐1 heterozygous showed intermediate FA composition levels between WT and dpbf2‐1 mutant in seeds
To further elucidate the effect of DPBF2 on seed FA composition, we crossed WT Arabidopsis with dpbf2‐1 to create DPBF2/dpbf2‐1 heterozygous lines in the F1 generation. F2 segregating progenies were generated by F1 selfing, and 30 F2 lines were randomly selected. We determined the DPBF2 genotype of 30 F2 plants and analyzed their seed FAs (Figure 5). In an F2 generation of 30 individuals, null‐segregated WT lines, heterozygous lines, and homozygous lines segregated 7:16:7, respectively, close to a 1:2:1 ratio (Figure 5a). We compared the average FA compositions of seeds obtained from WT, heterozygous, and homozygous individuals. In dpbf2‐1 homozygous seeds, 18:1 and 18:3 FAs were decreased and 18:2 FA was increased compared with those of the WT (Figure 5b). Intriguingly, the seed FA composition of the DPBF2/dpbf2‐1 heterozygous genotype showed a FA composition precisely intermediate between that of WT and dpbf2‐1 homozygous mutants (Figure 5b). These results show that DPBF2 has a dosage‐dependent effect on FA composition.
FIGURE 5.
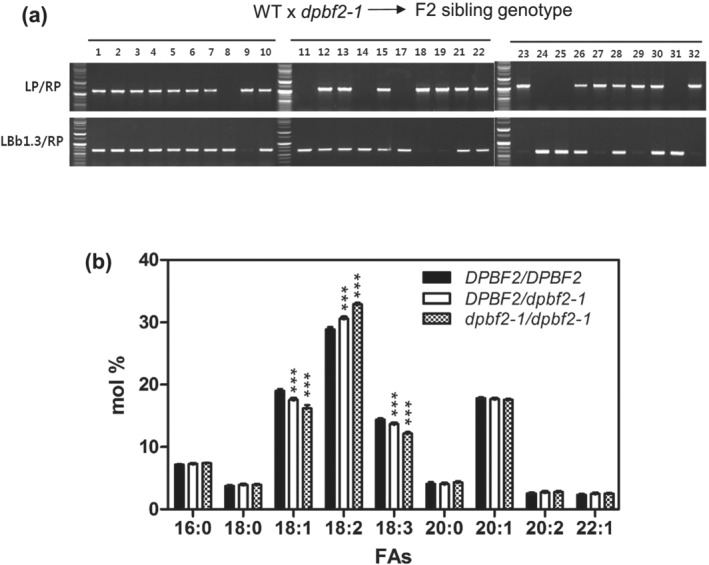
Progeny segregation test for DPBF2 and its effect on FA composition. (a) The dpbf2‐1 homozygous mutant was crossed with WT and a progeny F1 heterozygous plant was selfed to produce F2 lines. The F2 plant seeds were germinated, and 30 individual plants were genotyped using genomic DNA PCR to identify WT and heterozygous or homozygous dpbf2‐1 T‐DNA genotypes. Genotyping of 30 F2 offspring resulted in 7 WT (DPBF2/DPBF2), 16 heterozygous (DPBF2/dpbf2‐1), and 7 homozygous (dpbf2‐1/dpbf2‐1). The dpbf2‐1 T‐DNA insertion locus segregates as a single copy. (b) The fatty acid composition in F2 segregants of DPBF2/dpbf2‐1 F1 heterozygous plants. Statistically significant differences from WT were determined by two‐way ANOVA with Bonferroni posttests (***p < .001)
3.6. Seed‐specific DPBF2 overexpressor regulates the expression of seed unsaturated FA biosynthesis genes
Because the seed FA composition of the dpbf2‐1 knock‐out mutant decreased in 18:1 and 18:3 FAs and increased in 18:2 and 20:1 FAs compared with the WT (Figure 4a), we investigated changes in seed FA when DPBF2 was overexpressed. First, the 35S‐DPBF2 vector containing DPBF2 cDNA expressed under the control of the CaMV 35S promoter was transformed into WT Arabidopsis (Figure S3A). We randomly selected nine T1 transgenic lines showing resistance to kanamycin and analyzed the FAs of their T2 seeds in comparison with the WT line. In all 35S‐DPBF2 T2 transgenic lines, DPBF2 transcripts level was higher than that of WT (Figure S3B). 35S‐DPBF2 transgenic lines showed changes in 18:1, 18:2, 18:3, and 20:1 compared with those of WT. When the FA composition of 35S‐DPBF2 was compared with the FA composition of WT, the decrease of 18:1 and increase of 20:1 were not consistent in all transgenic lines, but the decrease of 18:2 and increase of 18:3 was consistent (Figure S3C).
To investigate the role of DPBF2 in seed, DPBF2 was overexpressed during seed development under the control of seed‐specific phaseolin promoter (Figure 6). Transgenic plants heterologously expressing the GUS gene from the phaseolin promoter were used as controls (Figure 6a). T3 seed FA analysis was performed on three T2 independent lines of Ph‐GUS or Ph‐DPBF2. As a result, seed‐specific DPBF2 overexpression increased 18:2 and 20:1 and decreased 18:1 and 18:3 compared with the control GUS overexpression (Figure 6b). The result was a change in FA composition similar to the dpbf2‐1 knock‐out mutant (Figure 4a). In addition, seed FA composition of dpbf2‐1 transformed with Ph‐DPBF2 was analyzed (Figure S4). FA composition of T3 seed harvested from three independent T2 transgenic lines showed the same change in FA composition as dpbf2‐1 (Figure 4a) and transgenics overexpressing Ph‐DBPF2 (WT + Ph‐DBPF2) (Figure 6b).
To find the cause of the change in the composition of seed FAs in WT + Ph‐DBPF2 lines, the expression of unsaturated FA synthesis‐related genes was analyzed in S6 siliques containing walking‐stick embryo stages and S7 stage siliques containing curled cotyledon stages in Ph‐DPBF2 #2 line and Ph‐GUS #1 control. Ph‐DPBF2 #2 line, showed a 7.2‐fold in S6 and 8.1‐fold in S7 increase in DPBF2 expression compared with Ph‐GUS #1 line (Figure 6c). Although DPBF2 increased very subtly upon transition from S6 to S7, the expression of FAD2, FAD3, LPCAT1, LPCAT2, PDCT, and FAE1 decreased from S6 to S7 stage in the Ph‐GUS #1 line. In contrast, in Ph‐DPBF2 #2, these genes were upregulated in the S6 stage and remained high through the S7 stage (Figure 6c). These results indicated that during seed development, seed‐specific overexpression of DPBF2 increased FAD2, FAD3, LPCAT1, and LPCAT2 slightly and PDCT and FAE1 highly. Taken together, increased expression of DPBF2 during different stages of seed development can affect the regulation of many FA synthesis genes and contribute to changes in the unsaturated FA composition of seed TAG.
3.7. DPBF2/L1L/NF‐YC2 complex upregulates PDCT and FAE1 expression
In previous reports, it has been identified that DPBF2 regulates seed storage protein and FA biosynthesis genes (CRU3 and FAD3) with LEC1‐LIKE (L1L), NF‐YC2 by binding G‐Box ACGT core sequence (Mendes et al., 2013; Yamamoto et al., 2009). Therefore, we performed a transcriptional activity assay with DPBF2, L1L, and NF‐YC2 transient coexpression to explain how DPBF2 can regulate FA biosynthesis genes (Figure 7a). The FAD2 and LPCAT1/2 promoter were not activated by expression of DPBF2 alone and DPBF2 with L1L and NF‐YC2 (Figure 7b). But PDCT and FAE1 promoters exhibited 5‐ and 8.6‐fold activation by coexpression of DPBF2 with L1L and NF‐YC2 compared with control (None), nor by DPBF2 alone (Figure 7b). The CRU3 promoter used for positive control was activated 8.1‐fold compared with control, which is consistent with results in Yamamoto et al. (2009). The promoter in all FA synthesis genes and CRU3 was included at least three or more putative DPBF2‐binding motifs (ACGT) although FAD2 and LPCAT1/2 had not changed in promoter activation (Figure S5). These results reveal that DBPF2 regulates PDCT and FAE1 together with L1L and NF‐YC2 to control FA composition.
FIGURE 7.
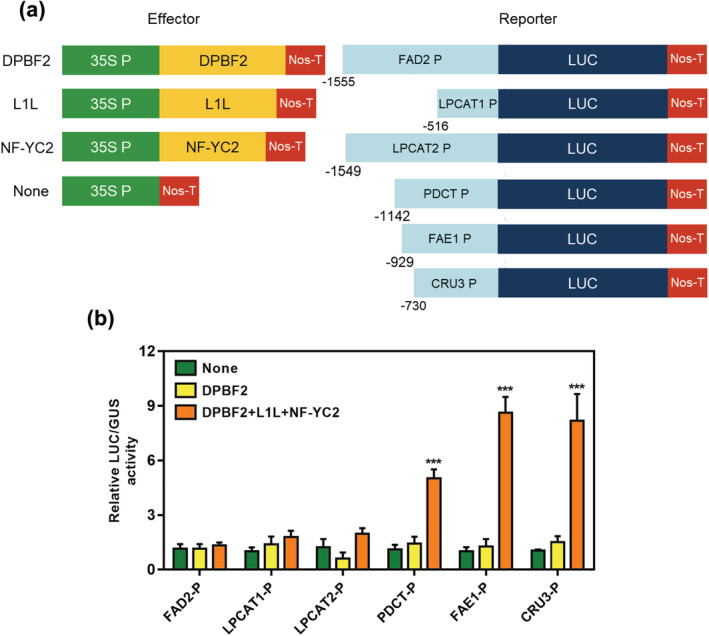
FA biosynthesis genes transcriptional activation assay by DPBF2 and DPBF2/L1L/NF‐YC2 complex in Nicotiana benthamiana leaf protoplasts. (a) Schematic diagrams of reporter and effector constructs. In the effector construct, DPBF2, L1L, and NF‐YC2 were cloned between the CaMV 35S promoter and the terminator of the nopaline synthase gene (Nos‐T). In the reporter constructs, the promoter of FA biosynthesis genes was fused to the luciferase gene. (b) Transcriptional activation assay in Nicotiana benthamiana leaf protoplasts. The effector and reporter constructs shown in (a) were cotransfected into protoplasts, and luciferase activities were determined fluorometrically. GUS gene expression was used to normalize the luciferase activities, and four measurements were averaged (t test, ***p < .001). The bars indicate the SEM
4. DISCUSSION
TAG, which accumulates during seed development, mediates the storage of a large amount of unsaturated FAs in Arabidopsis seeds. In this study, we report that DPBF2 transcription factor regulates the FA composition in the TAG of seeds. Spatial–temporal expression of DPBF2 was seed specific and started during cotyledon and axis development in seeds (Figure 1c,d). This suggests that DPBF2 is a seed‐specific gene and related to genes involved in FA modification. DPBF2/bZIP67 has the highest expression level in siliques at 10–13 days after pollination (DAP) (Bensmihen et al., 2002), and transgenic Arabidopsis embryos carrying bZIP67 promoter:GFP‐tagged bZIP67 exhibit fluorescence from 8 DAP to 13DAP (Bensmihen et al., 2005). In addition, the eFP browser (http://bar.utoronto.ca/efp2/Arabidopsis/Arabidopsis_eFPBrowser2.html) describes locus AT3G44460 for DPBF2 as being seed‐specific and most strongly expressed in the middle stages of seed development (Winter et al., 2007).
DPBF2 is regulated by the master regulator LEC2. DPBF2 expression began at the embryo stage when LEC2 expression reached its peak (Figure 1d). In addition, the expression of DPBF2 was decreased in developing seeds of lec2‐1 mutant (Figure 1b). LEC2 expression significantly increased DPBF2 expression in transgenic lines (Figure 1a) and protoplasts (Figure 2). Mutation of the RY motif region present in the DPBF2 promoter reduced the transcriptional activation of DPBF2 by LEC2, and this result showed that LEC2 directly regulated the expression of DPBF2 (Figure 2).
The demonstration that DPBF2 was targeted to the nucleus and had transcriptional activity when transgenically expressed in yeast supports the notion that DPBF2 is a transcription factor (Figure 3). Transformation of DPBF2 fused with the GAL4 DB domain into yeast harboring GAL4‐binding sites fused to a lacZ gene produces strong β‐galactosidase activity, indicating that DPBF2 has transcriptional activity in yeast (Kim et al., 2002). bZIP67 is localized to the nucleus in Arabidopsis containing bZIP67 promoter:GFP‐tagged bZIP67 (Bensmihen et al., 2005). In vitro and in vivo experiments have confirmed that DPBF2/bZIP67 binds to the FAD3 promoter region (Mendes et al., 2013).
The dpbf2‐1 mutant used in this study represents the same T‐DNA inserted mutant to bzip67‐1 in the same DPBF2/bZIP67 gene. In dpbf2‐1/bzip67‐1 (Salk_085497C), a T‐DNA is located in the second intron (Figure S1), and in bzip67‐2 (GABI314D04), the T‐DNA is inserted into the third intron (Mendes et al., 2013). In dpbf2‐1, the proportion of 18:1 and 18:3 FAs decreased and that of 18:2 FA increased compared with those in the WT (Figure 4a). This is slightly different from the seed FA composition of bzip67‐1, but the tendency of each FA to increase and decrease is consistent (Mendes et al., 2013). Seed FA content of dpbf2‐1 was about 93% of the WT (Figure 4b). Similarly, the total FA content, protein content, and seed weight of bzip67‐1 seeds did not change compared with those of WT (Mendes et al., 2013). This showed that, unlike LEC2, DPBF2 did not have a critical effect on seed oil content but was a transcription factor that affects FA composition. Mendes et al. (2013) reported that DPBF2/bZIP67 regulates the transcription of FAD3. We also observed downregulation of FAD3 in developing seeds of the dpbf2‐1 mutant (Figure 4c).
DPBF2 exerted a dose‐dependent effect on the FA composition (Figure 5). The proportion of 18:1 and 18:3 FAs are decreased and 18:2 is increased in bzip67‐1. In bZIP67 overexpressors, 18:1 and 18:2 are increased and 18:3 is decreased compared with the WT (Mendes et al., 2013). Changes in FA composition of dpb2‐1 mutant seeds are the same as reported by Mendes et al. (2013). The increase of 18:2 and the decrease of 18:3 in the DPBF2 overexpressing seeds produced in this study were the same as those reported by Mendes et al. (2013), but the change of 18:1 was different. Such a subtle difference is likely due to the difference in expression timing and amount of DPBF2/bZIP67 by phaseolin or glycine seed‐specific promoters.
Compared with bzip67‐1, the expression level of FAD3 in dpbf2‐1 was reduced to a lesser extent than in the WT, but that the expression of PDCT/ROD1 was similarly increased compared with that of the WT (Figure 4c). Mendes et al. (2013) suggested that the decreased FAD3 transcript levels and increased PDCT transcript levels in developing stage 8 siliques of bzip67‐1 result in a decrease in 18:3 and 18:1 FAs content. We showed that transcript levels of LPCAT1 and LPCAT2 dropped to 68% relative to those of the WT in dpbf2‐1 (Figure 4c). lpcat1/2 show a slight decrease in 16:0 and 18:1 FA contents and a 2% decrease in both 18:2 and 18:3 FAs compared with those of WT (Bates et al., 2012; Wang et al., 2012), whereas C20–22 unsaturated FA contents are increased to 33.5% compared with 26.5% in the WT (Bates et al., 2012). The increase in C20–22 unsaturated FA content is most likely due to the increase in C20 unsaturated fatty acyl‐CoA, a substrate that can naturally acylate to TAG. Based on the above report, a slight increase of 20:1 in dpbf2‐1 mutant is likely due to decreased expression of LPCAT1 and LPCAT2 (Figure 4a,c).
In the Arabidopsis fad3 mutant, the 18:3 FA content is decreased, and the 18:1 and 18:2 FA contents are increased (James & Dooner, 1990; Lemieux et al., 1990). The expression levels of FAD2 were upregulated in dpbf2‐1, so the seed FA composition of dpbf2‐1 is likely to be a mix of the FA compositions of fad3 mutant and FAD2 overexpressor (Figure 4). Therefore, in slight contrast to the FA composition of fad3, the decrease of 18:1 in dpbf2‐1 seed may be due to downregulation of FAD3 and upregulation of FAD2 in the dpbf2‐1 knock‐out mutant.
Seed‐specific overexpression of DPBF2 in WT and dpbf2‐1 mutants showed a very similar phenotype to the FA composition of dpbf2‐1 knock‐out mutant seeds (Figures 4a, 6b, and S4). 18:1 and 18:3 decreased while 18:2 and 20:1 increased (Figures 4a, 6b, and S4). This may be due to that DPBF2 upregulated a number of genes related to the synthesis of unsaturated FAs, which ultimately control FA composition regardless of excessive DPBF2 expression during seed development.
Transcriptional activation assay was performed on the FAD2, FAD3, LPCAT1, LPCAT2, PDCT, and FAE1 genes, which showed changes in expression in dpbf2‐1 and Ph‐DPBF2 overexpressors (Figure 4c, 6c, and 7). As a result, PDCT and FAE1 expression was upregulated when DPBF2 was combined with L1L and NF‐YC2 rather than alone (Figure 7). We predicted DPBF2‐binding motif (ACGT) in the promoter region of PDCT and FAE1 genes, there were at least seven motifs in both of forward and reverse sequence (Figure S5). We speculate that DPBF2 may regulate PDCT and FAE1 transactivation by binding these sites. But it should be confirmed which position will be bound with DPBF2, in effect. Although FAD2, LPCAT1, and LPCAT2 expression showed no changes in transactivation, it is possible that DPBF2 regulates these genes with other transcription factors because it has been reported that DPBF2 functions with various transcription factors (Bryant et al., 2019; Jo et al., 2020; Yamamoto et al., 2009). Taken together, these results suggest that DPBF2 works together with other transcription factors, such as L1L and NF‐YC2 to regulate the synthesis of unsaturated fatty acids in TAG during seed development.
In conclusion, DPBF2 is a seed‐specific transcription factor regulated directly by LEC2 that controls the expression of genes regulating the degree of unsaturation of seed FAs, such as 18:1, 18:2, 18:3, and 20:1, in TAG accumulation (described in Figure 8). Our results demonstrated that DBPF2 with L1L and NF‐YC2 positively controls the expression of PDCT and FAE1 together with the previously reported FAD3. However, it remains to be determined whether DPBF2 directly or indirectly modulates FAD2, LPCAT1, and LPCAT2 genes in combination with various transcription factors.
FIGURE 8.
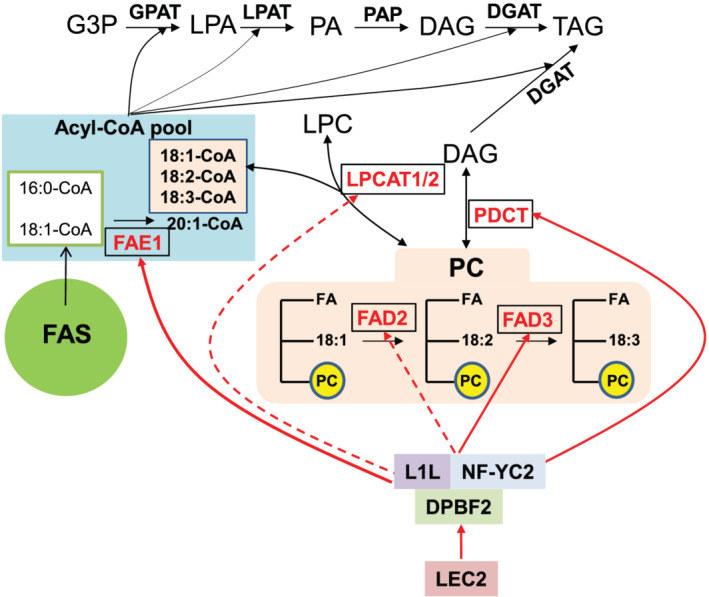
Model for the LEC2/DPBF2 network‐mediated regulation of polyunsaturated fatty acid biosynthesis and accumulation in triacylglycerol (TAG) in Arabidopsis seeds. DPBF2 regulates FAD2, FAD3, LPCAT1, LPCAT2, PDCT, and FAE1 expression. Black solid arrows represent metabolic reactions. Red solid arrows represent positive control by DPBF2/L1L/NF‐YC2 complex. Red dotted arrows represent possible positive control by DPBF2 together with other unknown factors. G3P: glycerol 3‐phosphate; LPA: lysophosphatidic acid; PA: phosphatidic acid; DAG: diacylglycerol; TAG: triacylglycerol; LPC: lysophosphatidylcholine; PC: phosphatidylcholine; GPAT: glycerol‐3‐phosphate acyltransferase; LPAT: lysophosphatidic acid acyltransferase; PAP: phosphatidic acid phosphatase; DGAT: diacylglycerol acyltransferase; FAS: fatty acid synthase; FAE1: fatty acid elongase 1; LPCAT1/2: lysophosphatidylcholine acyltransferase 1/2; PDCT: phospholipid:diacylglycerol cholinephosphotransferase; FAD2: fatty acid desaturase 2; FAD3: fatty acid desaturase 3
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
HUK, IK, and KRL performed the experiments and wrote the paper. MEP performed fatty acid analysis. All authors read and approved the final manuscript.
Supporting information
Table S1. Primers used for this study.
Table S2. Identities of transcription factors expressed at higher levels in senescing leaves at the S1 (100% green leaves, 30 days after germination) or S3 (50% yellow leaves) stages of the OIL21 line, which expresses senescence‐induced LEC2, than in wild‐type senescing leaves at the corresponding stages.
Figure S1. Identification of a dpbf2‐1 T‐DNA insertion knock‐out mutant.
Figure S2. Leaf fatty acid content analysis in WT and dpbf2‐1 line.
Figure S3. Overexpression of 35S:DPBF2 in WT plants.
Figure S4. Seed fatty acid composition of WT and three independent dpbf2‐1+Ph‐DPBF2 T2 generation transgenic plants.
Figure S5. The cis‐element in the promoter region of six genes.
ACKNOWLEDGMENTS
This work was supported by grants from the Mid‐Career Researcher Program of the National Research Foundation of Korea (NRF‐2020R1A2C2008175, HUK), the New Breeding Technologies Development Program (Project No. PJ016533, HUK), the Next Generation BioGreen21 associated program (Project No. PJ015714, HUK), the Rural Development Administration project (Project No. PJ01497102, KRL), Republic of Korea, and the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (iPET) (319107‐4, HUK), Republic of Korea.
Kim, I. , Lee, K.‐R. , Park, M.‐E. , & Kim, H. U. (2022). The seed‐specific transcription factor DPBF2 modulates the fatty acid composition in seeds. Plant Direct, 6(4), e395. 10.1002/pld3.395
Inyoung Kim and Kyeong‐Ryeol Lee contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data performed in this study are presented in the paper and the Supporting Information.
REFERENCES
- Bates, P. D. , & Browse, J. (2012). The significance of different diacylgycerol synthesis pathways on plant oil composition and bioengineering. Frontiers in Plant Science, 3, 147. 10.3389/fpls.2012.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, P. D. , Fatihi, A. , Snapp, A. R. , Carlsson, A. S. , Browse, J. , & Lu, C. (2012). Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiology, 160(3), 1530–1539. 10.1104/pp.112.204438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud, S. , Mendoza, M. S. , To, A. , Harscoët, E. , Lepiniec, L. , & Dubreucq, B. (2007). WRINKLED1 specifies the regulatory action ofLEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis. The Plant Journal, 50, 825–838. 10.1111/j.1365-313X.2007.03092.x [DOI] [PubMed] [Google Scholar]
- Bensmihen, S. , Giraudat, J. , & Parcy, F. (2005). Characterization of three homologous basic leucine zipper transcription factors (bZIP) of the ABI5 family during Arabidopsis thaliana embryo maturation. Journal of Experimental Botany, 56(412), 597–603. 10.1093/jxb/eri050 [DOI] [PubMed] [Google Scholar]
- Bensmihen, S. , Rippa, S. , Lambert, G. , Jublot, D. , Pautot, V. , Granier, F. , Giraudat, J. , & Parcy, F. (2002). The homologous ABI5 and EEL transcription factors function antagonistically to fine‐tune gene expression during late embryogenesis. Plant Cell, 14(6), 1391–1403. 10.1105/tpc.000869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braybrook, S. A. , Stone, S. L. , Park, S. , Bui, A. Q. , Le, B. H. , Fischer, R. L. , Goldberg, R. B. , & Harada, J. J. (2006). Genes directly regulated by LEAFY COTYLEDON2 provide insight into the control of embryo maturation and somatic embryogenesis. Proceedings of the National Academy of Sciences, 103(9), 3468–3473. 10.1073/pnas.0511331103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, F. M. , Hughes, D. , Hassani‐Pak, K. , & Eastmond, P. J. (2019). Basic LEUCINE ZIPPER TRANSCRIPTION FACTOR 67 transactivates DELAY OF GERMINATION 1 to establish primary seed dormancy in Arabidopsis. The Plant Cell, 31, 1276–1288. 10.1105/tpc.18.00892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernac, A. , & Benning, C. (2004). WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compoundbiosynthesis in Arabidopsis. The Plant Journal, 40, 575–585. 10.1111/j.1365-313X.2004.02235.x [DOI] [PubMed] [Google Scholar]
- Clough, S. J. , & Bent, A. F. (1998). Floral dip: A simplified method for Agrobacterium‐mediated transformation of Arabidopsis thaliana . The Plant Journal, 16(6), 735–743. 10.1046/j.1365-313x.1998.00343.x [DOI] [PubMed] [Google Scholar]
- Dahlqvist, A. , Stahl, U. , Lenman, M. , Banas, A. , Lee, M. , Sandager, L. , Ronne, H. , & Stymne, S. (2000). Phospholipid:diacylglycerol acyltransferase: An enzyme that catalyzes the acyl‐CoA‐independent formation of triacylglycerol in yeast and plants. Proceedings of the National Academy of Sciences, 97(12), 6487–6492. 10.1073/pnas.120067297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focks, N. , & Benning, C. (1998). wrinkled1: A novel, low‐seed‐oil mutantof Arabidopsis with a deficiency in the seed‐specific regulation ofcarbohydrate metabolism. Plant Physiology, 118, 91–101. 10.1104/pp.118.1.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj, M. D. , Zhang, S. , Harada, J. J. , & Lemaux, P. G. (2005). Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta, 222(6), 977–988. 10.1007/s00425-005-0041-y [DOI] [PubMed] [Google Scholar]
- Giraudat, J. , Hauge, B. M. , Valon, C. , Smalle, J. , Parcy, F. , & Goodman, H. M. (1992). Isolation of the Arabidopsis ABI3 gene bypositional cloning. The Plant Cell, 4(10), 1251–1261. 10.1105/tpc.4.10.1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, I. A. (2008). Seed storage oil mobilization. Annual Review of Plant Biology, 59, 115–142. 10.1146/annurev.arplant.59.032607.092938 [DOI] [PubMed] [Google Scholar]
- Jakoby, M. , Weisshaar, B. , Dröge‐Laser, W. , Vincente‐Carbajosa, J. , Tiedemann, J. , Kroj, T. , & Parcy, F. (2002). bZIP transcription factors in Arabidopsis. Trends in Plant Science, 7(3), 106–111. 10.1016/S1360-1385(01)02223-3 [DOI] [PubMed] [Google Scholar]
- James, D. W. , & Dooner, H. K. (1990). Isolation of EMS‐induced mutants in Arabidopsis altered in seed fatty acid composition. Theoretical and Applied Genetics, 80, 241–245. 10.1007/BF00224393 [DOI] [PubMed] [Google Scholar]
- Jin, J. B. , Kim, Y. A. , Kim, S. J. , Lee, S. H. , Kim, D. H. , Cheong, G. W. , & Hwang, I. (2001). A new dynamin‐like protein, ADL6, is involved in trafficking from the trans‐Golgi network to the central vacuole in Arabidopsis. The Plant Cell, 13(7), 1511–1526. 10.1105/TPC.000534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, L. , Pelletier, J. M. , Hsu, S. W. , Baden, R. , Goldberg, R. B. , & Harada, J. J. (2020). Combinatorial interactions of the LEC1 transcription factor specify diverse developmental programs during soybean seed development. Proceedings of the National Academy of Sciences, 117, 1223–1232. 10.1073/pnas.1918441117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith, K. , Kraml, M. , Dengler, N. G. , & McCourt, P. (1994). fusca3: A heterochronic mutation affecting late embryo development in Arabidopsis. The Plant Cell, 6, 589–600. 10.1105/tpc.6.5.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. U. , Jung, S. J. , Lee, K. R. , Kim, E. H. , Lee, S. M. , Roh, K. H. , & Kim, J. B. (2014). Ectopic overexpression of castor bean LEAFY COTYLEDON2 (LEC2) in Arabidopsis triggers the expression of genes that encode regulators of seed maturation and oil body proteins in vegetative tissues. FEBS Open Bio, 4(1), 25–32. 10.1016/j.fob.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. U. , Lee, K. R. , Jung, S. J. , Shin, H. A. , Go, Y. S. , Suh, M. C. , & Kim, J. B. (2015). Senescence‐inducible LEC2 enhances triacylglycerol accumulation in leaves without negatively affecting plant growth. Plant Biotechnology Journal, 13(9), 1346–1359. 10.1111/pbi.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. U. , Park, M.‐E. , Lee, K. R. , Suh, M. C. , & Grace, Q. C. (2020). Variant castor lysophosphatidic acid acyltransferases acylate ricinoleic acid in seed oil. Industrial Crops and Products, 150, 112245. 10.1016/j.indcrop.2020.112245 [DOI] [Google Scholar]
- Kim, S. Y. , Chung, H. J. , & Thomas, T. L. (1997). Isolation of a novel class of bZIP transcription factors that interact with ABA‐responsive elements and embryo‐specification elements in the Dc3 promoter using a modified yeast one‐hybrid system. The Plant Journal, 11(6), 1237–1251. 10.1046/j.1365-313X.1997.11061237.x [DOI] [PubMed] [Google Scholar]
- Kim, S. Y. , Ma, J. , Perret, P. , Li, Z. , & Thomas, T. L. (2002). Arabidopsis ABI5 subfamily members have distinct DNA‐binding and transcriptional activities. Plant Physiology, 130, 688–697. 10.1104/pp.003566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. Y. , & Thomas, T. L. (1998). A family of basic leucine zipper proteins bind to seed‐specification elements in the carrot Dc3 gene promoter. Journal of Plant Physiology, 152, 607–613. 10.1016/S0176-1617(98)80019-9 [DOI] [Google Scholar]
- Kong, Q. , Yuan, L. , & Ma, W. (2019). WRINKLED1, a “master regulator” in transcriptional control of plant oil biosynthesis. Plants (Basel), 8(7), E238. 10.3390/plants8070238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey, D. J. , Beaudoin, F. , Dempsey, C. E. , Shewry, P. R. , & Napier, J. A. (1999). The accumulation of triacylglycerols within the endoplasmic reticulum of developing seeds of Helianthus annuus . The Plant Journal, 17, 397–405. 10.1046/j.1365-313X.1999.00387.x [DOI] [Google Scholar]
- Le, B. H. , Cheng, C. , Bui, A. Q. , et al. (2010). Global analysis of gene activity during Arabidopsis seed development and identification of seed‐specific transcription factors. Proceedings of the National Academy of Sciences, 107, 8063–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K. R. , Lee, Y. , Kim, E. H. , Lee, S. B. , Roh, K. H. , Kim, J. B. , Kang, H. C. , & Kim, H. U. (2016). Functional identification of oleate 12‐desaturase and ω‐3 fatty acid desaturase genes from Perilla frutescens var. frutescens . Plant Cell Reports, 35, 2523–2537. 10.1007/s00299-016-2053-4 [DOI] [PubMed] [Google Scholar]
- Lemieux, B. , Miquel, M. , Somerville, C. , & Browse, J. (1990). Mutants of Arabidopsis with alterations in seed lipid fatty acidcomposition. Theoretical and Applied Genetics, 80, 234–240. [DOI] [PubMed] [Google Scholar]
- Li‐Beisson, Y. , Shorrosh, B. , Beisson, F. , Andersson, M. X. , Arondel, V. , Bates, P. D. , … Ohlrogge, J. (2013). Acyl‐lipid metabolism. Arabidopsis Book, 11, e0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchive, C. , Nikovics, K. , To, A. , Lepiniec, L. , & Baud, S. (2014). Transcriptional regulation of fatty acid production in higher plants: Molecular bases and biotechnological outcomes. European Journal of Lipid Science and Technology, 116(10), 1332–1343. 10.1002/ejlt.201400027 [DOI] [Google Scholar]
- Meinke, D. W. (1992). A homoeotic mutant of Arabidopsis thaliana with leafy cotyledons. Science, 258(5088), 1647–1650. 10.1126/science.258.5088.1647 [DOI] [PubMed] [Google Scholar]
- Meinke, D. W. , Franzmann, L. H. , Nickle, T. C. , & Yeung, E. C. (1994). Leafy cotyledon mutants of Arabidopsis. The Plant Cell, 6, 1049–1064. 10.1105/tpc.6.8.1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes, A. , Kelly, A. A. , Erp, H. , Shaw, E. , Powers, S. J. , Kurup, S. , & Eastmond, P. J. (2013). bZIP67 regulates the omega‐3 fatty acid content of Arabidopsis seed oil by activating FATTY ACID DESATURASE3 . The Plant Cell, 25(8), 3104–3116. 10.1105/tpc.113.116343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu, J. , Tan, H. , Zheng, Q. , Fu, F. , Liang, Y. , Zhang, J. , Yang, X. , Wang, T. , Chong, K. , Wang, X. J. , & Zuo, J. (2008). LEAFYCOTYLEDON1 is a key regulator of fatty acid biosynthesis in Arabidopsis. Plant Physiology, 148, 1042–1054. 10.1104/pp.108.126342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier, J. A. , Stobart, A. K. , & Shewry, P. R. (1996). The structure and biogenesis of plant oil bodies: The role of the ER membrane and the oleosin class of proteins. Plant Molecular Biology, 31(5), 945–956. 10.1007/BF00040714 [DOI] [PubMed] [Google Scholar]
- Ohlrogge, J. B. , Browse, J. , & Somerville, C. R. (1991). The genetics of plant lipids. Biochimicaet Biophysica Acta Lipids and Lipid Metabolism, 1082, 1–26. 10.1016/0005-2760(91)90294-R [DOI] [PubMed] [Google Scholar]
- Onate‐Sanchez, L. , & Vicente‐Carbajosa, J. (2008). DNA‐free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Research Notes, 1, 93. 10.1186/1756-0500-1-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos‐Mendoza, M. , Dubreucq, B. , Miquel, M. , Caboche, M. , & Lepiniec, L. (2005). LEAFY COTYLEDON 2 activation is sufficient to trigger the accumulation of oil and seed specific mRNAs in Arabidopsis leaves. FEBS Letters, 579(21), 4666–4670. 10.1016/j.febslet.2005.07.037 [DOI] [PubMed] [Google Scholar]
- Slightom, J. L. , Sun, S. M. , & Hall, T. C. (1983). Complete nucleotide sequence of a French bean storage protein gene: Phaseolin. Proceedings of the National Academy of Sciences, 80(7), 1897–1901. 10.1073/pnas.80.7.1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville, C. (1991). Plant lipids: Metabolism, mutants, and membranes. Science, 252(5002), 80–87. 10.1126/science.252.5002.80 [DOI] [PubMed] [Google Scholar]
- Ståhl, U. , Carlsson, A. S. , Lenman, M. , Dahlqvist, A. , Huang, B. , Banas, W. , Banas, A. , & Stymne, S. (2004). Cloning and functional characterization of a phospholipid:diacylglycerol acyltransferase from Arabidopsis. Plant Physiology, 135(3), 1324–1335. 10.1104/pp.104.044354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, S. L. , Kwong, L. W. , Yee, K. M. , Pelletier, J. , Lepiniec, L. , Fischer, R. L. , Goldberg, R. B. , & Harada, J. J. (2001). LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proceedings of the National Academy of Sciences, 98, 11806–11811. 10.1073/pnas.201413498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis, J. G. , & Browse, J. (2010). Lipid biochemists salute the genome. The Plant Journal, 61, 1092–1106. 10.1111/j.1365-313X.2010.04125.x [DOI] [PubMed] [Google Scholar]
- Wang, L. , Shen, W. , Kazachkov, M. , Chen, G. , Chen, Q. , Carlsson, A. S. , Stymne, S. , Weselake, R. J. , & Zou, J. (2012). Metabolic interactions between the Lands cycle and the Kennedy pathway of glycerolipid synthesis in Arabidopsis developing seeds. The Plant Cell, 24(11), 4652–4669. 10.1105/tpc.112.104604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner, G. , & Theimer, R. R. (1978). Membranous appendices of spherosomes (oleosomes). Possible role in fat utilisation in germinating oilseeds. Planta, 140(2), 163–169. 10.1007/BF00384916 [DOI] [PubMed] [Google Scholar]
- West, M. , Yee, K. M. , Danao, J. , Zimmerman, J. L. , Fischer, R. L. , Goldberg, R. B. , & Harada, J. J. (1994). LEAFY COTYLEDON1 is an essential regulator of late embryogenesis and cotyledon identity in Arabidopsis. The Plant Cell, 6, 1731–1745. 10.1105/tpc.6.12.1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, D. , Vinegar, B. , Nahal, H. , Ammar, R. , Wilson, G. V. , & Provart, N. J. (2007). An “electronic fluorescent pictograph” browser for exploring and analyzing large‐scale biological data sets. PLoS ONE, 2(8), e718. 10.1371/journal.pone.0000718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, A. , Kagaya, Y. , Toyoshima, R. , Kagaya, M. , Takeda, S. , & Hattori, T. (2009). Arabidopsis NF‐YB subunits LEC1 and LEC1‐LIKE activate transcription by interacting with seed‐specific ABRE‐binding factors. The Plant Journal, 58, 843–856. 10.1111/j.1365-313X.2009.03817.x [DOI] [PubMed] [Google Scholar]
- Yoo, S. D. , Cho, Y. H. , & Sheen, J. (2007). Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nature Protocols, 2(7), 1565–1572. 10.1038/nprot.2007.199 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers used for this study.
Table S2. Identities of transcription factors expressed at higher levels in senescing leaves at the S1 (100% green leaves, 30 days after germination) or S3 (50% yellow leaves) stages of the OIL21 line, which expresses senescence‐induced LEC2, than in wild‐type senescing leaves at the corresponding stages.
Figure S1. Identification of a dpbf2‐1 T‐DNA insertion knock‐out mutant.
Figure S2. Leaf fatty acid content analysis in WT and dpbf2‐1 line.
Figure S3. Overexpression of 35S:DPBF2 in WT plants.
Figure S4. Seed fatty acid composition of WT and three independent dpbf2‐1+Ph‐DPBF2 T2 generation transgenic plants.
Figure S5. The cis‐element in the promoter region of six genes.
Data Availability Statement
The data performed in this study are presented in the paper and the Supporting Information.


