Abstract
Mutations in loci other than genes for the target topoisomerases of fluoroquinolones, gyrA and parC, may play a role in the development of fluoroquinolone resistance in Escherichia coli. A series of mutants with increasing resistance to ofloxacin was obtained from an E. coli K-12 strain and five clinical isolates. First-step mutants acquired a gyrA mutation. Second-step mutants reproducibly acquired a phenotype of multiple antibiotic resistance (Mar) and organic solvent tolerance and showed enhanced fluoroquinolone efflux. None of the second-step mutants showed additional topoisomerase mutations. All second-step mutants showed constitutive expression of marA and/or overexpressed soxS. In some third-step mutants, fluoroquinolone efflux was further enhanced compared to that for second-step mutants, even when the mutant had acquired additional topoisomerase mutations. Attempts to circumvent the second-step Mar mutation by induction of the mar locus with sodium salicylate and thus to select for pure topoisomerase mutants at the second step were not successful. At least in vitro, non-target gene mutations accumulate in second- and third-step mutants upon exposure to a fluoroquinolone and typically include, but do not appear to be limited to, mutations in the mar or sox regulons with consequent increased drug efflux.
Fluoroquinolones are potent bactericidal agents that are widely used for treatment of community-acquired and nosocomial infections. The activities of the currently available fluoroquinolone agents are particularly high against members of the family Enterobacteriaceae, and their clinical efficacies have accordingly been excellent in patients infected with these organisms. The emergence of high-level fluoroquinolone resistance, however, has been reported among different members of the family Enterobacteriaceae including Escherichia coli and Salmonella (9, 14, 17).
Virtually all high-level-resistant clinical isolates of E. coli show mutations in the so-called quinolone resistance-determining regions (QRDRs) of both the gyrA and the parC genes, which encode subunits of the target topoisomerases of the fluoroquinolones. On the basis of biochemical, genetic, and epidemiologic studies, it is likely that a single gyrA mutation is the first step during resistance development (10, 11, 15, 19, 30, 31; S. Conrad, L. Scheit, M. Oethinger, G. Klotz, R. Marre, and W. V. Kern, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-9, p. 47, 1996). For the expression of high-level resistance, subsequent acquisition of a second gyrA mutation and a parC mutation seems to be important; however, non-target gene mutations may also play a critical role. Many high-level fluoroquinolone-resistant clinical isolates show a multiple-antibiotic-resistance (Mar) phenotype and increased tolerance to organic solvents (10, 11, 30). They accumulate less ciprofloxacin than reference strains and/or lack outer membrane protein OmpF (11). A proportion of the high-level fluoroquinolone-resistant E. coli clinical isolates that display the Mar phenotype have been shown to constitutively express the regulatory marA or soxS genes (20, 25). Both MarA, a transcriptional activator negatively regulated by MarR, and SoxS, the regulator of the superoxide SoxRS regulon, confer increased resistance to chemically unrelated antibiotics by activating or depressing a number of genetic loci in E. coli that contribute to the Mar phenotype (21). It is unknown whether the non-target gene mutations that lead to a Mar phenotype in the resistant clinical isolates were a direct consequence of fluoroquinolone exposure or, rather, represented pre- or coselection events in a clinical setting of exposure to a variety of antimicrobial agents. E. coli K-12 mutants selected for resistance to tetracycline or chloramphenicol may mutate in a second step more easily to higher-level fluoroquinolone resistance than do control cells (8). Thus, at a given drug concentration, an initial Mar mutation could facilitate subsequent acquisition of target gene mutations. Other experiments have indicated that exposure of cells to a quinolone may select first- or second-step mutants with a Mar phenotype (16, 27). We investigated whether the occurrence and nature of non-target gene mutations that lead to a Mar phenotype upon fluoroquinolone exposure would be reproducible in a given E. coli strain at a certain step. We also tested whether induction of the mar regulon by pre- or coincubation of cells with sodium salicylate (7) is sufficient to obviate the need for non-target gene mutations.
(Part of this study has been presented at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, 18 September to 1 October 1997, Toronto, Ontario, Canada [W. V. Kern, M. Oethinger, A. S. Ritter, S. Conrad, R. Marre, and S. B. Levy, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-182, p. 77, 1997].)
MATERIALS AND METHODS
Bacterial strains.
E. coli AG100 (K-12 argE3 thi-1 rpsL xyl mtl Δ(gal-uvrB) supE44) has been described previously (13). AG112 is a marR mutant (5-bp deletion) derived from AG100 by selection on tetracycline (M. Oethinger, W. V. Kern, A. S. Jellen-Ritter, L. McMurry, and S. B. Levy, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-125, p. 58, 1998). The mar deletion mutant AG100MK was constructed by P1 transduction (28) from AG100/Kan as the donor strain into AG100. AG100/Kan was constructed by replacement of a chromosomal 1.24-kb BspHI fragment of the mar locus in AG100 by homologous recombination with the kanamycin resistance cassette (Kanr) from pKMN33 (20). E. coli 748k0.1 (serotype O101:K−:H9, with resistance to ampicillin and tetracycline) and 429II (serotype O19:K−:H−) were fluoroquinolone- and nalidixic acid-susceptible clinical isolates from cancer patients admitted to Ulm University Hospital and Medical Center. E. coli C175-92 (serotype O9:K−:H12), C482-92 (serotype O101:K103:H4), and C71-93 (serotype O101:K−:H9) were quinolone-susceptible clinical isolates obtained from the Statens Seruminstitut, Copenhagen, Denmark.
Chemicals and media.
Ofloxacin was obtained from Hoechst (Frankfurt, Germany). Nalidixic acid, sodium salicylate, and carbonyl cyanide m-chlorophenylhydrazone (CCCP) were purchased from Sigma Chemicals (St. Louis, Mo.). Hexane and cyclohexane were purchased from Aldrich (Milwaukee, Wis.). Trypticase soy (TS) broth, Mueller-Hinton (MH) broth, and MH agar were from Oxoid (Basingstoke, England). Luria-Bertani (LB) broth and agar were prepared by standard protocols. Radiolabeled [14C]ciprofloxacin was a gift from BAYER AG, Leverkusen, Germany.
Selection of mutants.
Organisms were grown overnight in TS or LB broth. Inocula of 1010 to 1012 CFU were added to MH or LB agar containing inhibitory concentrations (2 to 16 times the MIC) of ofloxacin or nalidixic acid (for some of the first-step selections). Plates were incubated for 24 to 48 h at 37°C in air, and mutational frequencies were determined. Three to six colonies from each selecting concentration were purified and examined for antimicrobial susceptibility. In some experiments, minimal medium (Na2HPO4, 6 g/liter; KH2HPO4, 3 g/liter; NH4Cl, 1 g/liter; 2 mM MgSO4; 0.1 mM CaCl2; 0.004% thiamine; 0.4% glucose) instead of rich medium was used for overnight cultures before plating, or selection was done in liquid culture by passaging the cells in broth with increasing concentrations of ofloxacin (0.5, 2, 8, and 16 times the MIC for 16 h each) before plating.
Selection of second-step mutants was also done after pretreatment of cells with sodium salicylate. A suspension of first-step mutant 1-748MM cells incubated at 37°C overnight in LB broth containing 2.5 mM sodium salicylate was used for this experiment, yielding strain 2-748SL; 2-748SS was obtained from 1-748MM after incubation at 37°C overnight and short-term (30-min) induction with sodium salicylate (final concentration, 5 mM).
Coincubation experiments with salicylate plus ofloxacin were done as follows. An overnight culture in 200 ml of LB broth was split, and ofloxacin with or without 5 mM sodium salicylate was added. Viable counts were determined at 24 and 48 h of incubation. After 48 h, 106 to 107 cells from each flask were transferred into fresh medium containing the same concentrations of ofloxacin with or without salicylate. The remaining cell suspension was centrifuged, the pellet was plated onto LB agar containing the same concentration of ofloxacin, and the plate was read after 48 h. The broth cultures were incubated for 48 h and were then quantitatively plated onto LB agar with or without ofloxacin.
Antimicrobial susceptibility testing.
The MICs of the selected antimicrobial agents were determined by a standard broth microdilution procedure with cation-adjusted MH broth and a final inoculum of 5 × 105 CFU/ml according to the performance and interpretive guidelines of the National Committee for Clinical Laboratory Standards (22). Microtiter plates were purchased from Merlin Diagnostics (Bornheim, Germany).
Organic solvent tolerance testing.
Tolerance to hexane and cyclohexane was tested in an agar overlay assay, as described previously (3, 24). All wild-type strains were inhibited by cyclohexane, while strains with increased organic solvent tolerance grew in the presence of cyclohexane.
Fluoroquinolone uptake.
A whole-cell fluoroquinolone accumulation assay was performed with radiolabeled ciprofloxacin. Cells were grown to the logarithmic phase in LB broth at 30°C, washed twice in 50 mM potassium phosphate (pH 6.0) at room temperature, and resuspended in the same buffer. Cells were energized with 0.2% glucose for 20 min, and [14C]ciprofloxacin (specific activity, 59 mCi/mmol) was added to 10 μM. Steady-state accumulation at 30°C was measured at 5 and 15 min by diluting 50 μl of the cell-labeling suspension into 10 ml of 100 mM LiCl–50 mM potassium phosphate (pH 6.0) and immediately collecting the cells on Gelman mixed-cellulose ester membrane filters (pore size, 0.45 μm) and determining the radioactivity on the filters. Binding of radiolabel to the filters was subtracted. All uptake experiments included measurements after the addition of CCCP (200 μM). All measurements were done in duplicate. Results were expressed as picomoles of ciprofloxacin per A600 unit, and the drug accumulation by mutants relative to that of the wild-type parent strain was calculated in percent. Experiments were repeated three times, with values for the relative accumulation differing by less than 10 percent.
RNA extraction and Northern blot analysis.
For Northern blot analysis, a 387-bp marA probe and a 432-bp soxRS probe were used, as described previously (25). Briefly, overnight cultures were diluted 1:100 in fresh LB broth and were grown to the mid-logarithmic phase at 30°C with shaking. Where indicated, sodium salicylate (final concentration, 5 mM) or paraquat (final concentration, 1.3 mM; Sigma) were added as inducers for 45 min before the cells were harvested by centrifugation. Total RNA was extracted by cesium chloride ultracentrifugation. Hybridization of the [32P]dCTP-labeled DNA probes to the membrane-bound RNA (20 μg/lane) was performed at 65°C overnight, the blots were washed, and the membranes were exposed on a PhosphoImager screen with visualization with ImageQuant (Molecular Dynamics, Sunnyvale, Calif.).
Reverse transcription of total RNA and PCR of cDNA.
A total of 500 ng of total RNA (50 ng/μl) was mixed with 1 μl of pd(N)6 random hexamer (100 ng/μl; Pharmacia), and the mixture was incubated for 5 min at 65°C. The RNA was then immediately cooled on ice. A total of 9 μl of a reverse transcription reaction mixture containing 4 μl of 5× Superscript buffer, 2 μl of 10 mM dithiothreitol, 1 μl of 10 mM deoxynucleoside triphosphates, 10 U of RNasin (Promega), and 50 U of Superscript II reverse transcriptase (Gibco BRL) was added to the RNA-hexamer mixture, and the mixture was incubated at 42°C for 30 min.
A total of 2 μl of the cDNA was used for the amplification of marA-specific cDNA in a standard PCR. Primer sequences were 5′-TATGACGATGTCCAGACGCA-3′ (sense) and 5′-GATGTAAAAAGCGCGATTCG-3′ (antisense). The PCR was performed in a thermocycler (Biometra, Göttingen, Germany) with the following program: one cycle at 5 min at 94°C; 28 cycles of 1 min at 94°C, 1 min at 51.5°C, and 1 min 72°C; and finally, one cycle of 5 min at 72°C. The resulting PCR products (361 bp for marA) were detected on a 1.5% agarose gel containing ethidium bromide. The bands were analyzed densitometrically (Image Master 1D; Pharmacia, Freiburg, Germany). Expression of gapA (coding for d-glyceraldehyde-3-phosphate dehydrogenase [5]) in the same cDNA preparation was used as the standard. Primer sequences were 5′-GTATCAACGGTTTTGGCCG-3′ (sense) and 5′-AGCTTTAGCAGCACCGGTA-3′ (antisense), creating a PCR product of 626 bp. PCR was performed as described above with annealing at 55°C.
Nucleotide sequencing.
The QRDRs of gyrA (nucleotides 123 to 366) and parC (nucleotides 145 to 492) in the mutants were amplified by PCR and were purified by use of Qiaquick spin columns (Qiagen, Hilden, Germany), as described previously (10). marOR was amplified from base pairs 1311 to 1858 with primer pair ORAB2 and RK3 as described earlier (25). Direct cycle sequencing was performed in an automatic 373A DNA Sequencer (Applied Biosystems, Weiterstadt, Germany).
RESULTS
Acquisition of gyrA mutations in first-step mutants.
First-step mutants were obtained from all parental strains at similar frequencies, ranging from 5 × 10−9 to 1 × 10−11. They exhibited roughly 10-fold decreased susceptibilities to ofloxacin and ciprofloxacin and a 32-fold decreased susceptibility to nalidixic acid compared to those of the wild type. The clones that were obtained with the different selecting concentrations and that were examined for susceptibility did not show increased resistance to unrelated antibiotics. The clones obtained with the highest selecting concentration were used for further investigation and experiments (Table 1). All first-step mutants had acquired a gyrA mutation at either amino acid position 83 or amino acid position 87 (Table 1). At four times the MIC, the frequencies of obtaining a first-step gyrA mutant were similar for AG100, its mar mutant AG112, and the mar-deleted strain AG100MK (data not shown). First-step mutants retained their susceptibilities to tetracycline, chloramphenicol, and cefoxitin (Table 1). No changes in organic solvent tolerance were observed between wild-type strains and first-step mutants.
TABLE 1.
Characterization of stepwise-selected E. coli mutants with increasing levels of resistance to fluoroquinolonesa
| Parental strain and mutantb | Selecting concn (fold MIC) | Growth under cyclohexane | Amino acid changec
|
Expression ofd:
|
MIC (μg/ml)e
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| gyrA | parC | marA | soxS | Nal | Ofl | Cip | Cefx | Tet | Cm | |||
| AG100 | − | WT | − | 2 | 0.06 | 0.03 | 4 | 2 | 4 | |||
| 1-AG100 | 16 | − | D87G | WT | − | 64 | 0.25 | 0.25 | 4 | 1 | 4 | |
| 2-AG100 | 4 | + | D87G | + | − | 256 | 1 | 0.5 | 16 | 4 | 16 | |
| 2-AG100b | 4 | + | D87G | + | − | ND | 2 | 0.5 | 32 | 8 | 32 | |
| 3-AG100b | 2 | + | S83L, D87G | + | − | ND | 4 | 2 | ND | 8 | 32 | |
| AG100MKf | − | − | − | ND | 0.03 | 0.015 | ND | 0.5 | 2 | |||
| 1-AG100MKX | 4 | − | S83L | − | − | ND | 0.5 | 0.25 | ND | 0.5 | 4 | |
| 2-AG100MKX | 2 | − | S83L | − | − | ND | 1 | 0.5 | ND | 1 | 4 | |
| 3-AG100MKX | 4 | + | S83L | − | − | ND | 2 | 1 | ND | 4 | 16 | |
| AG112g | + | + | − | 8 | 0.125 | 0.06 | 16 | 8 | 16 | |||
| 1-AG112 | 8 | + | D87G | + | − | 256 | 1 | 0.5 | 16 | 8 | 32 | |
| 2-AG112 | 4 | + | D87G | + | − | 512 | 4 | 2 | 32 | 32 | 64 | |
| 748k0.1 | − | WT | − | 2 | 0.06 | 0.03 | 4 | >64 | 4 | |||
| 1-748 | 8 | − | S83L | WT | − | 64 | 0.5 | 0.5 | 4 | ND | 4 | |
| 2-748 | 8 | + | S83L | + | − | 512 | 2 | 1 | 16 | ND | 16 | |
| 3-748 | 4 | + | S83L | + | − | >1,024 | 8 | 4 | 32 | ND | 32 | |
| 2-748Lh | NAi | + | S83L | + | − | 512 | 2 | 1 | 4 | ND | 8 | |
| 3-748L | NA | + | S83L | + | − | 1,024 | 8 | 8 | 8 | ND | 32 | |
| 1-748MM | 16 | − | D87Y | WT | − | 64 | 1 | 0.25 | 4 | ND | 4 | |
| 2-748MM | 4 | + | D87Yj | + | − | >1,024 | 4 | 2 | 32 | ND | 32 | |
| 3-748MM | 4 | + | S83L, D87Nj | S80I | + | − | >1,024 | 32 | 32 | 32 | ND | >64 |
| 429II | − | WT | − | 2 | 0.06 | 0.015 | <2 | 1 | 4 | |||
| 1-429 | 8 | − | D87G | WT | − | 64 | 0.5 | 0.125 | <2 | 2 | 4 | |
| 2-429 | 4 | + | D87G | WT | + | 128 | 1 | 0.25 | 8 | 2 | 8 | |
| 3-429 | 4 | + | S83L D87G | WT | + | 512 | 4 | 1 | 8 | 4 | 16 | |
| 1-429MM | 8 | − | S83L | WT | − | 64 | 1 | 0.25 | 2 | 1 | 8 | |
| 2-429MM | 4 | + | S83L | WT | + | >1,024 | 8 | 2 | 32 | 8 | 32 | |
| C175-92 | − | WT | − | ND | 0.03 | 0.015 | ND | 1 | 4 | |||
| 1-C175 | 4 | − | S83L | WT | − | ND | 0.5 | 0.25 | ND | 1 | 4 | |
| 2-C175 | 4 | + | S83L | + | − | ND | 2 | 1 | ND | 4 | 16 | |
| C482-92 | − | WT | − | ND | 0.015 | 0.015 | ND | 0.5 | 2 | |||
| 1-C482 | 8 | − | D87Y | WT | − | ND | 0.25 | 0.06 | ND | 0.5 | 4 | |
| 2-C482 | 4 | + | D87Y | + | + | ND | 0.5 | 0.25 | ND | 2 | 8 | |
| C71-93 | − | WT | − | ND | 0.03 | 0.015 | ND | 16 | 32 | |||
| 1-C71 | 8 | − | S83L | WT | − | ND | 0.25 | 0.06 | ND | 16 | 32 | |
| 2-C71 | 4 | + | S83L | + | − | ND | 1 | 0.25 | ND | 64 | >64 | |
All mutants except 1-748MM were selected with ofloxacin, 1-748MM was selected with nalidixic acid.
First-, second-, and third-step mutants are designated by the prefixes 1, 2, and 3, respectively.
Deduced amino acid changes based on nucleotide sequencing of the QRDRs of gyrA and parC.
Based on Northern blotting and reverse transcription-PCR analysis; WT, wild type (inducible, but weak or no constitutive expression); −, no expression; +, constitutive expression.
Nal, nalidixic acid; Ofl, ofloxacin; Cip, ciprofloxacin; Cefx, cefoxitin; Tet, tetracycline; Cm, chloramphenicol; ND, not determined.
mar-deleted strain AG100.
mar mutant derived from AG100 by selection on tetracycline.
Derived from 1-748 after passage in liquid culture.
NA, not applicable.
Nucleotide change from TAC to AAC.
Acquisition of a Mar phenotype and cyclohexane tolerance in second-step mutants.
Second-step mutants were obtained at frequencies ranging from 10−8 to 10−10. Compared to the first-step mutants, susceptibility to nalidixic acid and fluoroquinolones decreased further (2- to >10-fold), and these mutants now showed cyclohexane tolerance (Table 1). In most second-step mutants, the MICs of tetracycline, chloramphenicol, and cefoxitin had also increased (Table 1), while no change in susceptibility to broad-spectrum cephalosporins, imipenem, and aminoglycosides was observed (data not shown). No further mutations in the QRDR of gyrA or parC were detected in any of the second-step mutants. In contrast to AG100, the mar-deleted strain AG100MK acquired cyclohexane tolerance and a Mar phenotype in the third selection step. Further increases in the MICs of tetracycline, chloramphenicol, and cefoxitin were found for mar mutant AG112 in the second selection step (Table 1).
Increased fluoroquinolone efflux in second-step mutants.
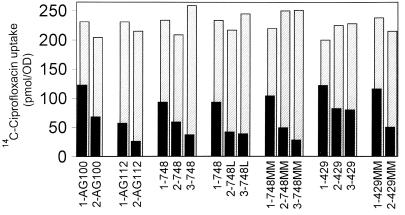
Ciprofloxacin uptake in energized cells did not differ between wild-type 748k0.1 and its first-step mutants. Second-step mutants, however, accumulated less drug (ranging between 42 and 73% of that accumulated by the wild type). The addition of CCCP abolished this reduced uptake, indicating enhanced active efflux that is dependent on maintenance of the proton gradient (Fig. 1). Similar data were obtained with mutants derived from AG100 and 429II (Fig. 1). Similar to mar mutant AG112, second-step mar mutant 2-AG100 accumulated less drug than AG100 (58% of that accumulated by AG100) and 1-AG100. Drug uptake in 2-AG112 decreased to 21% of that of the wild type, whereas drug uptake was 36% of that accumulated by wild-type AG100 in AG112 and 40% of that accumulated by the wild type in 1-AG112. Reduced ciprofloxacin uptake in second-step mutants derived from E. coli wild-type strain 429II ranged between 49% (2-429MM) and 82% (2-429) of that for wild-type cells.
FIG. 1.
Radiolabeled ciprofloxacin uptake in energized cells with (▨) and without (■) CCCP (200 μM). First-, second-, and third-step quinolone-resistant mutants are designated by the prefixes 1, 2, and 3, respectively. Results are expressed as picomoles of ciprofloxacin per A600 unit (OD, optical density) and represent the mean of duplicate steady-state accumulation measurements. Experiments were repeated three times, with values for the accumulation relative to that of the parental cells differing by less than 10 percent. The results shown here are the results of one representative experiment.
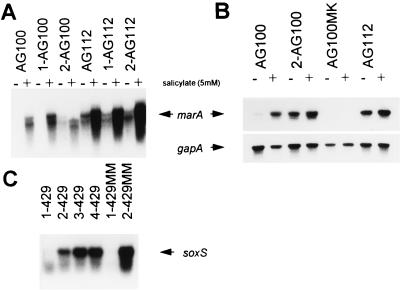
Expression of marA or soxS.
Northern blot analysis and reverse transcription-PCR were used to determine the expression of marA and soxS. Some baseline expression of marA in uninduced wild-type cells and first-step mutants was seen (Fig. 2). Both analyses showed constitutive expression of marA in all second-step mutants derived from strains AG100, C175-92, 748k0.1, C482-92, and C71-93, similar to that seen for mar mutant AG112. Neither 748k0.1, AG100, C175-92, nor C-71-93, nor any of the mutants derived from these strains (including the mar deletion mutants), expressed soxS, as determined by Northern blot analysis. In contrast, in both independently obtained second-step mutants of 429II, second-step mutants 2-429 and 2-429MM, soxS overexpression was observed (Fig. 2), while the level of marA expression was the same as that of the wild type. Second-step mutant 2-C482 showed a weak constitutive expression of soxS, in addition to overexpression of marA.
FIG. 2.
Northern blot analysis of marA (A) and soxS (C) and reverse transcription-PCR analysis of marA and gapA (B). Results are shown for uninduced cells and for cells after induction of marA with sodium salicylate (5 mM).
Mutant selection after pretreatment or coincubation with sodium salicylate.
Induction of the mar locus by pretreatment of first-step mutant 1-748MM with sodium salicylate before plating did not prevent the emergence of a mutation in the mar locus at the second step. The resulting mutants, 2-748SL and 2-748SS, had a Mar phenotype very similar to that of 2-748MM and likewise overexpressed marA (Table 1).
In initial coincubation experiments, we found that viable counts after 48 h of incubation in ofloxacin with or without salicylate were similar, and the largest difference between cultures with and without salicylate was <1 log10 CFU/ml (data not shown). Further coincubation experiments were done at concentrations of ofloxacin that were slowly bactericidal (2 μg/ml for 1-748MM and 1 μg/ml for 748k0.1). No mutants were obtained after 48 h of incubation in ofloxacin with or without salicylate and plating on ofloxacin agar. After transfer of 106 to 107 cells preexposed to ofloxacin with or without salicylate into fresh medium containing ofloxacin with or without salicylate, growth occurred only in the presence of salicylate, presumably due to an effective induction of the mar operon, and growth was independent of preexposure to salicylate.
After 48 h of growth in fresh ofloxacin-salicylate medium, plating of 1-748MM cells onto LB agar with and without ofloxacin showed that significant subpopulations (10−2 to 10−4 in three independent experiments) grew on ofloxacin agar plates in the absence of salicylate. Replica plating (98 colonies from each plate) on agar containing different concentrations of ofloxacin and chloramphenicol and confirmatory MIC tests indicated that cells for which the ofloxacin MIC was unchanged (compared to the MIC for parental strain 1-748MM, i.e., 1 μg/ml) had remained susceptible to chloramphenicol (MIC, 4 μg/ml), whereas the emergent mutant cells for which the ofloxacin MIC increased (MIC, 2 μg/ml) had decreased susceptibility to chloramphenicol (MIC, 32 μg/ml). Two such clones (clones 2-748SM-2 and 2-748SM-3) obtained in independent experiments were further studied. Both had become organic solvent tolerant and overexpressed marA according to Northern blot analysis and reverse transcription-PCR (Table 2). None of them had new topoisomerase mutations. The Mar phenotype was stable in the absence of antibiotic, and both mutants showed decreased levels of fluoroquinolone accumulation (data not shown).
TABLE 2.
Characterization of mutants obtained after pre- and coincubation experiments with sodium salicylate as mar inducer
| Parental strain and mutant | Growth under cyclohexane | gyrA mutationa | Expression of marAb | MIC (μg/ml)c
|
||
|---|---|---|---|---|---|---|
| Ofl | Cip | Cm | ||||
| 1-748MM | − | D87Y | WT | 1 | 0.25 | 4 |
| 2-748SLd | + | D87Y | + | 4 | 1 | 32 |
| 2-748SSd | + | D87Y | + | 2 | 2 | 32 |
| 2-748SM-2e | + | D87Y | + | 2 | 0.5 | 32 |
| 2-748SM-3e | + | D87Y | + | 2 | 1 | 32 |
| 748k0.1 | − | WT | 0.06 | 0.03 | 4 | |
| 2T-If | + | S83L | + | 4 | 1 | 16 |
| 2T-IVf | + | S83L | + | 2 | 1 | 8 |
| 2T-Vf | + | S83L | + | 2 | 1 | 8 |
| 2T-VIIf | + | S83L | + | 4 | 2 | 8 |
Deduced amino acid changes based on nucleotide sequencing of the QRDRs; none of the mutants had mutations in the QRDR of parC.
Based on Northern blotting and reverse transcription-PCR analysis; WT, wild-type (inducible, but weak or no constitutive expression); +, constitutive expression.
Ofl, ofloxacin; Cip, ciprofloxacin; Cm, chloramphenicol.
Derived from 1-748MM after pretreatment with sodium salicylate as mar inducer before plating.
Derived from 1-748MM after coincubation in 5 mM sodium salicylate and ofloxacin (2 μg/ml).
Derived from 748k0.1 after coincubation in 5 mM sodium salicylate and ofloxacin (1 μg/ml).
Experiments with wild-type E. coli 748k0.1 gave similar results. No growth was observed in cultures without salicylate, while cells grew in the ofloxacin-salicylate medium. After 48 h of incubation, plating of the cells revealed a subpopulation (10−5) that formed colonies on ofloxacin agar. Replica plating (98 colonies from each plate) and confirmatory MIC tests revealed that the ofloxacin MICs for these mutants were increased (MIC range, 1 to 4 μg/ml), and for some of them chloramphenicol MICs increased (MICs, 8 to 16 μg/ml). Ten clones obtained in two independent experiments (six from ofloxacin plates and four from LB agar plates) were further studied. All had a gyrA mutation (S83L), and all four clones for which chloramphenicol MICs were increased (clones 2T-I, 2T-IV, 2T-V, 2T-VII) had become organic solvent tolerant and constitutively expressed marA (Table 2).
marOR mutations.
First- and second-step mutants as well as several clones obtained in the coincubation experiments were sequenced to detect mutations in the marOR region. All but two second-step mutants that overexpressed marA had new mutations or deletions in marOR (Table 3), with two exceptions: 2-748SS, the mutant strain obtained after preincubation with 5 mM sodium salicylate for 30 min before the cells were plated, and 2-748SM-2, one of the mutants obtained from 1-748MM after coincubation in ofloxacin-salicylate (Table 3). The reason for the Mar phenotype and increased marA expression in these mutants remains unclear.
TABLE 3.
Mutations in marOR in E. coli mar mutants selected with ofloxacin
| Mutant | Nucleotide change | Mutation |
|---|---|---|
| 2-AG100 | 1485+1 | Frameshift |
| 2-AG100b | 1834+1; Δ1835−1847 | Frameshift, 13-bp deletion |
| 2-748 | Δ1610 | Frameshift |
| 2-748L | Δ1800 | Frameshift |
| 2-748MM | Δ1408−1422 | 15-bp deletion |
| 2-748SL | Δ1835−1847 | 13-bp deletion |
| 2-748SS | Wild type | |
| 2-748SM-2 | Wild type | |
| 2-748SM-3 | Δ1694 | Frameshift |
| 2T-I | 1673C→A | R77S |
| 2T-IV | 1609+2 | Frameshift |
| 2T-V | Δ1639 | Frameshift |
| 2T-VII | Δ1425-1444 | 20-bp deletion |
| 2-C175 | 1751G→Aa; Δ1831−1853 | G103Sa; 23-bp deletion |
| 2-C482 | Δ1748−1758 | 11-bp deletion |
| 2-C71 | Δ1519 | Frameshift |
Previously shown to be a genotypic variation in marR without loss of repressor activity (25).
Among the mar mutants obtained from 748k0.1 after coincubation in ofloxacin-salicylate, different marR mutations were detected (Table 3), while clones with wild-type expression of marA had no mutations (data not shown).
Third- and fourth-step mutants.
Third-step mutants 3-AG100b and 3-429 acquired a second gyrA mutation, and the MICs of ofloxacin and ciprofloxacin for the mutants increased two- to fourfold (Table 1). Quinolone MICs were also increased for third-step mutants 3-748 and 3-748L, and the mutants did not have a new mutation in the QRDRs of gyrA and parC, as was observed in 2-AG112. Third-step mutant 3-748MM was the only mutant that had acquired additional mutations in both gyrA and parC (Table 1).
Cyclohexane tolerance and the Mar phenotype remained stable in the third-step mutants. For four of five third-step mutants cefoxitin, tetracycline, and/or chloramphenicol MICs were increased, suggesting enhanced efflux as the mechanism (Table 1). In these mutants, ciprofloxacin uptake was compared with uptake in parental cells (Fig. 1). A decreased intracellular ciprofloxacin concentration compared with that in second-step mutants was found in one mutant (mutant 3-748) without a further gyrase mutation (uptake change, 73 to 59% of that for the wild type), but it was also found in one mutant (mutant 3-748MM), despite further target mutations (60 to 36% of that for the wild type), while no change in drug accumulation was measured in third-step mutants 3-429 (82 to 81% of that for the wild type) and 3-748L (42 to 41% of that for the wild type).
The gyrase double mutants 3-AG100b and 3-429 were used for further selection experiments. Fourth-step mutant 4-AG100b had no new target gene mutation (ofloxacin MIC, 16 μg/ml). Fourth-step mutant 4-429 acquired an unusual third target gene mutation in the parC gene (G78D), leading to an increase in the fluoroquinolone MIC but not in the MIC of nalidixic acid (ofloxacin MIC, 64 μg/ml; nalidixic acid MIC, 128 μg/ml). Such a parC mutation has been reported previously (15; P. Heisig, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-177, p. 76, 1997) and corresponds to an analogous mutation at codon 81 of the gyrA gene (6). None of the third- or fourth-step mutants which constitutively expressed marA overexpressed soxS.
DISCUSSION
In the initial experiments with wild-type E. coli 748k0.1, there was a reproducible sequence of mutations in first-step (gyrA) and second-step (mar) mutants obtained after exposure to ofloxacin. In contrast, an earlier study reported that only 2 of 10 second-step mutants derived from putative gyrA mutants displayed a Mar phenotype (27). Also, a few quinolone-resistant mutants with a putative non-target gene mutation in the first step have been described (27, 32, 33). Since these differences could reflect the different wild-type parental strains, we used other clinical isolates and the well-characterized E. coli K-12 strain AG100 in similar selection experiments. Our results show that the second-step acquisition of a Mar phenotype and increased organic solvent tolerance upon exposure to ofloxacin were reproducible with all other strains. The Mar phenotype was most likely linked to overexpression of the mar regulatory gene, but SoxS was an alternative mechanism. Whether one or the other locus was involved appeared to depend on the particular strain. We used ofloxacin in our experiments, but acquisition of a Mar phenotype in a second-step mutant after selection with ciprofloxacin has been reported (15, 16), and recent observations in this laboratory indicate that trovafloxacin also selects mar mutants (A. S. Jellen-Ritter and W. V. Kern, unpublished data).
There is a reasonable explanation for the consistent sequence of mutations first in gyrA and then in mar or sox. The initial gyrA mutation provides a decrease in susceptibility to fluoroquinolones (∼10-fold) which is greater than that associated with a mar or sox regulatory gene mutation (∼2- to 8-fold), and this might be a predisposition to selection of gyrA mutants and against mar mutants, at least when a concentration of two or more times the MIC is used. Selection experiments with drug at the MIC and by testing of multiple clones could prove this hypothesis. A second gyrA mutation apparently has a rather limited impact on the resistance level. We observed only a two- to fourfold decrease in susceptibility to fluoroquinolones in the mutants that had acquired another gyrA mutation, and this is consistent with other experimental work (4). On the basis of this and other studies (8, 20, 25, 26) a similar decrease can be provided by increased drug efflux in association with a mutation in the mar or sox regulatory genes. The greater likelihood for selection of mar or sox mutants than selection of gyrA mutants may be related to the variety of functionally effective mutations in this region as opposed to the highly specific mutations required in the QRDR. Whether particular aspects of quinolone action in a mutant gyrA background such as reduced bactericidal activity (18) or the recently observed increased doubling time in gyrase double mutants (4) play an additional role remains unknown. It is important that even in a mar mutant or a mar deletion strain, we observed efflux-associated mutations rather than a second gyrA mutation as the next step after an initial gyrA mutation.
There is an apparent discrepancy between the present findings and epidemiologic studies that show that only half of the high-level fluoroquinolone-resistant E. coli clinical isolates have an organic solvent tolerance and Mar phenotype, and only a proportion of them are overproducers of mar or sox regulatory genes (25). It is possible and we cannot exclude the possibility that necessity and the type of non-target gene mutations in the development of fluoroquinolone resistance are strain specific. Some strains may need mutations associated with drug efflux, while others may not. The increased tolerance to organic solvents in the absence of mar or sox mutations that was observed among clinical isolates (25) may well reflect altered drug accumulation due to mutations in other loci that affect the AcrAB-TolC efflux pump (2, 3, 12, 23) or in genes that code for other pumps. Our selected group of wild-type strains may be too limited and biased to account for the range of potential mutations associated with enhanced drug efflux and increased tolerance to organic solvents. AG100 is a laboratory strain known to readily acquire a mar mutation. Among the five clinical strains that we used, three were serotype O101, a serotype that was overrepresented for unknown reasons among high-level fluoroquinolone-resistant and multiple-antibiotic-resistant E. coli strains (29). Although we identified O101 strains with high-level fluoroquinolone resistance with and without mar mutations among a collection of resistant clinical isolates from the Ulm University Hospital and Medical Center (W. V. Kern and M. Oethinger, unpublished data, 1997), strains of this serotype may be more likely than other strains to acquire mar or sox mutations in response to fluoroquinolone exposure in vivo and in vitro.
Another attractive hypothesis to explain the apparent discrepancy between in vitro and in vivo findings was that in vivo there is only rarely a need for mar, sox, and other efflux-associated mutations because E. coli is likely to be in an induced mar state in the natural environment (1, 25; V. Hüllen, P. Heisig, and B. Wiedemann, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-187, p. 123, 1998). This might obviate the need for mutations in the mar or sox system, i.e., for constitutive overexpression. Instead, fluoroquinolone exposure would readily select topoisomerase mutations from among induced E. coli cells and surpass the intermediate Mar mutation seen in the in vitro experiment. So far, experimental proof for this hypothesis is missing. Our pre- and coincubation experiments with sodium salicylate, a strong inducer of the mar regulon, did not resolve the issue. Despite pre- or coincubation with salicylate, ofloxacin in vitro selected mar mutants from both wild-type cells and first-step mutants. Nevertheless, salicylate had a significant effect in the experiment. Addition of salicylate enabled the cells to grow in the presence of an otherwise bactericidal fluoroquinolone concentration to a density which allowed spontaneous resistant mutants to emerge. Unexpectedly, these mutants were mar mutants and not double gyrase mutants. marA induction by salicylate may be more efficient in the presence of a mar mutation than in wild-type cells (7) and presumably provides a higher level of fluoroquinolone resistance through more efficient drug efflux. Together with the greater chance of selecting one of several functional marOR mutations, this may be the best explanation for the failure to select double gyrase mutants in the coincubation experiments. The relatively low frequency of mar mutants among clinical isolates might then be related to a more efficient mar induction in vivo by natural mar inducers compared with that achieved with 5 mM sodium salicylate in vitro in a wild-type mar background. This hypothesis, however, needs to be formulated with some caution, given that strain specificity cannot be excluded.
Exposure of second-step mutants to ofloxacin yielded a variety of phenotypes and genotypes, including further mutations in target genes but also further mutations in non-target genes. The nature of the latter mutations that leads to further enhancement of drug efflux is unknown. A presumed efflux-associated mutation was selectable in mar-deleted strain 2-AG100MKX. Furthermore, efflux in the 2-AG112, 3-748, and 3-748MM mutants was clearly enhanced over that provided by the mar mutation alone in the respective precursor cells. These observations indicate that the efflux pump(s) involved in fluoroquinolone resistance is under the incomplete control of the mar operon. Recent work has shown that deletion of acrAB, which encodes a multidrug efflux pump partially under the control of mar, reduces the fluoroquinolone MICs for 1-AG112 and 2-AG112 to the same low concentration and renders cells hypersusceptible to fluoroquinolones (Oethinger et al., 38th ICAAC).
In conclusion, at least in vitro, mutations associated with drug efflux appear to play a critical role in the development of fluoroquinolone resistance in E. coli. In vitro, they accumulate in second- and third-step mutants and typically include, but do not appear to be limited to, mutations in the mar and sox regulons. It is likely that modulation of fluoroquinolone efflux through regulatory genes and other mechanisms also contributes substantially to resistance development in vivo.
ACKNOWLEDGMENTS
We thank Cornelia Maunz for excellent technical assistance.
This study was supported by Deutsche Forschungsgemeinschaft grants Ke700/1-1 (to W.V.K.) and Oe195/1-1 (to M.O.), research grant P334/97 from the University of Ulm (to W.V.K.), and Public Health Service grant GM51661 (to S.B.L.).
REFERENCES
- 1.Alekshun M N, Levy S B. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob Agents Chemother. 1997;41:2067–2075. doi: 10.1128/aac.41.10.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aono R, Tsukagoshi N, Yamamoto M. Involvement of outer membrane protein TolC, a possible member of the mar-sox regulon, in maintenance and improvement of organic solvent tolerance of Escherichia coli K-12. J Bacteriol. 1998;180:938–944. doi: 10.1128/jb.180.4.938-944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asako H, Nakajima H, Kobayashi K, Konayashi M, Aono R. Organic solvent tolerance and antibiotic resistance increased by overexpression of marA in Escherichia coli. Appl Environ Microbiol. 1997;63:1428–1433. doi: 10.1128/aem.63.4.1428-1433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagel S, Hüllen V, Wiedemann B, Heisig P. Impact of gyrA and parC mutations on quinolone resistance, doubling time, and supercoiling degree of Escherichia coli. Antimicrob Agents Chemother. 1999;43:868–875. doi: 10.1128/aac.43.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branlant G, Branlant C. Nucleotide sequence of the Escherichia coli gap gene. Different evolutionary behaviour of the NAD+-binding domain and of the catalytic domain of d-glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1985;150:61–66. doi: 10.1111/j.1432-1033.1985.tb08988.x. [DOI] [PubMed] [Google Scholar]
- 6.Cambau E, Bordon F, Collatz E, Gutmann L. Novel gyrA point mutation in a strain of Escherichia coli resistant to fluoroquinolones but not to nalidixic acid. Antimicrob Agents Chemother. 1993;37:1247–1252. doi: 10.1128/aac.37.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen S P, Levy S B, Foulds J, Rosner J L. Salicylate induction of antibiotic resistance in Escherichia coli: activation of the mar operon and a mar-independent pathway. J Bacteriol. 1993;175:7856–7862. doi: 10.1128/jb.175.24.7856-7862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen S P, McMurry L M, Hooper D C, Wolfson J S, Levy S B. Cross-resistance to fluoroquinolones in multiple-antibiotic-resistant (Mar) Escherichia coli selected by tetracycline or chloramphenicol: decreased drug accumulation associated with membrane changes in addition to OmpF reduction. Antimicrob Agents Chemother. 1989;33:1318–1325. doi: 10.1128/aac.33.8.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cometta A, Calandra T, Bille J, Glauser M P. Escherichia coli resistant to fluoroquinolones in patients with cancer and neutropenia. N Engl J Med. 1994;330:1240–1241. doi: 10.1056/NEJM199404283301717. [DOI] [PubMed] [Google Scholar]
- 10.Conrad S, Oethinger M, Kaifel K, Klotz G, Marre R, Kern W V. gyrA mutations in high-level fluoroquinolone-resistant Escherichia coli clinical isolates. J Antimicrob Chemother. 1996;38:443–455. doi: 10.1093/jac/38.3.443. [DOI] [PubMed] [Google Scholar]
- 11.Everett M J, Jin Y F, Ricci V, Piddock L J. Contributions of individual mechanisms to fluoroquinolone resistance in 36 Escherichia coli strains isolated from humans and animals. Antimicrob Agents Chemother. 1996;40:2380–2386. doi: 10.1128/aac.40.10.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fralick J A. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J Bacteriol. 1996;178:5803–5805. doi: 10.1128/jb.178.19.5803-5805.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George A M, Levy S B. Amplifiable resistance to tetracycline, chloramphenicol, and other antibiotics in Escherichia coli: involvement of a non-plasmid-determined efflux of tetracycline. J Bacteriol. 1983;155:531–540. doi: 10.1128/jb.155.2.531-540.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein F W, Acar J F. Epidemiology of quinolone resistance: Europe and North and South America. Drugs. 1995;49(Suppl. 2):36–42. doi: 10.2165/00003495-199500492-00007. [DOI] [PubMed] [Google Scholar]
- 15.Heisig P. Genetic evidence for a role of parC mutations in development of high-level fluoroquinolone resistance in Escherichia coli. Antimicrob Agents Chemother. 1996;40:879–885. doi: 10.1128/aac.40.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heisig P, Tschorny R. Characterization of fluoroquinolone-resistant mutants of Escherichia coli selected in vitro. Antimicrob Agents Chemother. 1994;38:1284–1291. doi: 10.1128/aac.38.6.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kern W V, Andriof E, Oethinger M, Kern P, Hacker J, Marre R. Emergence of fluoroquinolone-resistant Escherichia coli at a cancer center. Antimicrob Agents Chemother. 1994;38:681–687. doi: 10.1128/aac.38.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khodursky A B, Zechiedrich E L, Cozzarelli N R. Topoisomerase IV is a target of quinolones in Escherichia coli. Proc Natl Acad Sci USA. 1995;92:11801–11805. doi: 10.1073/pnas.92.25.11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehn N, Stower-Hoffmann J, Kott T, Strassner C, Wagner H, Krönke M, Schneider-Brachert W. Characterization of clinical isolates of Escherichia coli showing high levels of fluoroquinolone resistance. J Clin Microbiol. 1996;34:597–602. doi: 10.1128/jcm.34.3.597-602.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maneewannakul K, Levy S B. Identification of mar mutants among quinolone-resistant clinical isolates of Escherichia coli. Antimicrob Agents Chemother. 1996;40:1695–1698. doi: 10.1128/aac.40.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller P, Sulavik M C. Overlaps and parallels in the regulation of intrinsic multiple-antibiotic resistance in Escherichia coli. Mol Microbiol. 1996;21:441–448. doi: 10.1111/j.1365-2958.1996.tb02553.x. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-T. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1996. [Google Scholar]
- 23.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oethinger M, Kern W V, Goldman J D, Levy S B. Association of organic solvent tolerance and fluoroquinolone resistance in clinical isolates of Escherichia coli. J Antimicrob Chemother. 1998;41:111–114. doi: 10.1093/jac/41.1.111. [DOI] [PubMed] [Google Scholar]
- 25.Oethinger M, Podglajen I, Kern W V, Levy S B. Overexpression of the marA or soxS regulatory gene in clinical topoisomerase mutants of Escherichia coli. Antimicrob Agents Chemother. 1998;42:2089–2094. doi: 10.1128/aac.42.8.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okusu H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol. 1996;178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piddock L J V, Hall M C, Walters R N. Phenotypic characterization of quinolone-resistant mutants of Enterobacteriaceae selected from wild type, gyrA type and multiply-resistant (marA) type strains. J Antimicrob Chemother. 1991;28:185–198. doi: 10.1093/jac/28.2.185. [DOI] [PubMed] [Google Scholar]
- 28.Provence D L, Curtiss R., III . Gene transfer in gram-negative bacteria. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C.: American Society for Microbiology; 1994. pp. 317–347. [Google Scholar]
- 29.Threlfall E J, Cheasty T, Graham A, Rowe B. High-level resistance to ciprofloxacin in Escherichia coli. Lancet. 1997;349:403. doi: 10.1016/s0140-6736(97)80024-4. [DOI] [PubMed] [Google Scholar]
- 30.Vila J, Ruiz J, Marco F, Barcelo A, Goni P, Giralt E, Jimenez de Anta T. Association between double mutation in gyrA gene of ciprofloxacin-resistant clinical isolates of Escherichia coli and MICs. Antimicrob Agents Chemother. 1994;38:2477–2479. doi: 10.1128/aac.38.10.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vila J, Ruiz J, Marcos A, Jimenez de Anta T. Detection of mutations in parC in quinolone-resistant clinical isolates of Escherichia coli. Antimicrob Agents Chemother. 1996;40:491–493. doi: 10.1128/aac.40.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe M, Kotera Y, Yosue K, Inoue M, Mitsuhashi S. In vitro emergence of quinolone-resistant mutants of Escherichia coli, Enterobacter cloacae, and Serratia marcescens. Antimicrob Agents Chemother. 1990;34:173–175. doi: 10.1128/aac.34.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990;34:1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]