Abstract
Background
Both prescription and over-the-counter (OTC) drugs recently emerged among novel psychoactive substances (NPS) being reported as ingested for recreational purposes. Among them, benzydamine (BZY), normally prescribed as an OTC anti-inflammatory drug, is reportedly being diverted and recreationally used.
Objective
The aim of this study was to investigate how the misuse of BZY has been reported, illustrating its psychotropic molecular mechanism, and studying its psychopathological effects.
Methods
We firstly conducted a systematic review of the literature concerning the abuse of BZY and its effects. For data gathering purposes, both PRISMA and PROSPERO guidelines were followed. All research methods were approved by PROSPERO (identification code CRD42020187266). Second, we analysed BZY-related data from the European Monitoring Agency (EMA) Adverse Drug Reactions (ADRs) database recorded during 2005-2020 regarding its abuse.
Results
Eleven articles, published during 1997-2019, were included in our systematic review, including five case reports, four surveys, and two retrospective case series analyses. While nine articles dealt with the recreational use of BZY, two described an oral overdose of the drug. When specified, dosages of BZY consumed ranged from 500 to 1500mg. The EMA dataset contained three cases of BZY abuse.
Conclusion
Results from the systematic review showed BZY might be diverted for typical hallucinogenic properties occurring at high dosages. Healthcare professionals should be warned about a possible misuse/abuse of a commonly prescribed anti-inflammatory drug and be vigilant when prescribing it. Physicians working in emergency units should know that psychotic symptoms may be related to BZY abuse.
Keywords: Benzydamine, benzydamine misuse, over-the-counter drug abuse, NPS, novel psychoactive substances
1. INTRODUCTION
Benzydamine hydrochloride (BZY) is a non-steroidal anti-inflammatory drug (NSAID) with analgesic, antipyretic and antimicrobial properties [1], available since 1966 for the symptomatic treatment of acute inflammatory states of the oral and vaginal mucosae [2, 3]. It is dispensed without prescription and marketed for external use in various formulations, including mouthwash, vaginal douche preparations,
and capsule forms under the name of Tantum®, Difflam® or Opalgyne®, depending on the country of sale [4]. The absorption through skin and mucosa is typically low (e.g. less than 10% of total dose) [5], while after oral administration BZY is rapidly absorbed; approximately 64% of the dose is absorbed within 1 hour, and complete absorption occurs in 4 ± 6 hours. It presents with a half-life of 7.8 hours, persisting longer in the serum at higher doses [4]. Mechanisms of action of BZY are complex. Its anti-inflammatory mechanism of action seems to be associated with the inhibition of synthesis of tumour necrotizing factor-alpha, prostaglandins, and thromboxane [6] and, unlike other NSAIDs, it is not ulcerogenic due to the lack of inhibition of cyclooxygenase [3, 7]. Moreover, BZY has an affinity for membranes and shows membrane-stabilizing properties with local anaesthetic effects. It inhibits phagocyte degranulation and aggregation, production of reactive oxygen species by phagocytes, and leukocyte adhesion to vascular endothelium. The maximum oral daily dose of BZY is 150–200mg and psychiatric adverse effects have not been reported at this dosage [8].
Relatively little is known about the effects of BZY on the central nervous system (CNS). Neurological symptoms such as dizziness, hyperactivity, excitation, and convulsions have been reported because of unintended high-dosage (500 – 3,000 mg) systemic ingestion [2, 4, 9, 10]. Dose-dependent psychotropic effects, including anxiety, agitation, paranoia, and hallucinations; specifically visual hallucinations, with insects and monsters being reported [5, 9, 11, 12], have been described. So-called BZY “trips” are anecdotally characterized by feelings of happiness, euphoria, and ego-dystonic hallucinatory experiences [8], which generally appear to resolve spontaneously in 2-3 days [13].
1.1. Abuse of BZY
Over the last 20 years or so, evidence has emerged regarding the widespread recreational use of BZY in various countries, including Brazil, Italy, Romania, Poland and Turkey [6, 7, 9, 10, 12, 14-16]. BZY appears to be often taken together with alcohol, cannabinoids and dextromethorphan in order to intensify the overall psychotropic effects [7]. The first case of BZY intoxication was reported in 1999, describing a 6-year-old girl with a BZY accidental poisoning presenting with severe visual hallucinosis and resolving spontaneously in 17 hours [4]. Between December 2009 and January 2010, two northern Italian poison control centres recorded at least 50 cases of BZY misuse through ingestion of Tantum Rosa®, apparently related both to a misleading TV commercial and its recent reclassification as an over-the-counter (OTC) drug [17]. In Brazil, BZY has been abused by street youths since the early 1990s, probably due to its low price and ready availability [12]. Then, in the following two decades, its use has spread to the Brazilian club scene. This has occurred in parallel with the blossoming of themed rave parties called Benflogin® parties from the drug brand-name, and pop songs entitled ‘Benzydamine’. Associated with this, informal self-reports, hosted by Internet drug fora and social networks, provided information about routes of administration, dosages and substance preparation from commercial products, as well as advice about other psychotropic substances to be used together with BZY in order to both enhance its pleasurable effects and dampen undesired ones [12, 18]. BZY seemed to be particularly popular among young people with a previous history of drug/polydrug use [6, 9, 12, 16] and likely to be concomitantly consumed with other substances, such as alcohol, tobacco, cannabis, and medications like benzodiazepines and amphetamines [2, 6, 9, 12, 16, 19]. Websites and Internet fora have been contributing to BZY popularity, in illustrating its recreational power and sharing information regarding the best methods to obtain psychotropic effects [7, 16].
1.1.1. Aim of the Study
Given these premises, the purpose of this systematic review was to investigate how the misuse of BZY has been reported, illustrating its psychotropic molecular mechanism, and studying its psychopathological effects, in order to have a broad and updated picture of the issue. An analysis of benzydamine-abuse related data from the European Medicines Agency (EMA) has been performed as well.
2. METHODS
2.1. Systematic Review
A systematic electronic search was completed in May 2020 and was set without a time-frame on the following scientific search engines: PubMed, Scopus, and Web of Science (WoS). The following search strategies were used, respectively in PubMed: (benzydamine OR benzydamine hydrochloride OR tantum OR difflam) AND (abuse OR use OR misuse OR craving OR addiction) NOT review NOT (animal OR rat OR mouse); in Scopus: (TITLE-ABS-KEY (benzydamine) OR TITLE-ABS-KEY (benzydamine AND hydrochloride) OR TITLE-ABS-KEY (tantum) OR TITLE-ABS-KEY (difflam) AND TITLE-ABS-KEY (abuse) OR TITLE-ABS-KEY (use) OR TITLE-ABS-KEY (misuse) OR TITLE-ABS-KEY (craving) OR TITLE-ABS-KEY (addiction) AND NOT TITLE-ABS-KEY (review) AND NOT TITLE-ABS-KEY (animal) OR TITLE-ABS-KEY (rat) OR TITLE-ABS-KEY (mouse)) ; and WoS: ((benzydamine OR benzydamine hydrochloride OR tantum OR difflam) AND (abuse OR use OR misuse OR craving OR addiction) NOT review NOT (animal OR rat OR mouse)).
The systematic review was structured in accordance with the PRISMA [20] and PROSPERO guidelines [21]. Identified studies were assessed at title/abstract and full text screening against eligibility criteria. Studies were also manually searched to identify additional citations. All the research methods were approved by PROSPERO (identification code CRD42020187266).
2.1.1. Data Synthesis Strategy
Data were extracted by n=2 reviewers (AM, AMo), while n=3 senior researchers (SC, GM, MP) supervised all stages of the process and were consulted to resolve any possible disagreement. The exclusion criteria for both selection phases were: 1) a document being of the following category: review, commentary, editorial, book chapter; 2) non full-text articles (e.g. conference abstract); 3) language other than English; 4) animal/in vitro studies; 5) articles where BZY was mentioned only as an example in the context of OTC drug misuse; 6) articles which were not dealing with the misuse of BZY or its physical/psychotropic effects. The whole process has been conducted individually by both AM and Amo, who created an Excel database; for doubtful cases, the eligibility was discussed with GM, MP, SC and MdG. The assessment of risk of bias was made in accordance with the Cochrane risk of bias 2 (RoB 2) tool [22].
2.2. Data from EMA
2.2.1. Source of Data
The EMA is responsible for the EudraVigilance (EV) dataset recording Adverse Drug Reactions (ADR) reported for all medicinal products authorised in the European Economic Area (EEA) according to Directive 2001/83/EC and Regulation (EC) No 726/2004 [23]. An ADR was considered a voluntary and unsolicited communication reported by both Regulatory Authorities of the EU Member States where the reaction occurred and/or by the Marketing Authorisation Holders for those ADRs occurring outside the EEA [24]. Suspected ADRs alone are not always sufficient to prove that a certain suspected reaction has indeed been caused by a specific medicine; this could be a symptom of another illness, or it could be associated with another medicinal product taken by the patient at the same time. The suspected ADRs are presented using the ‘Preferred Terms’ (PTs) of the Medical Dictionary for Regulatory Activities (MedDRA), which is the internationally agreed list of terms that support the coding of ADR [25]. Upon a formal request to access data regarding cases of BZY-abuse/misuse/dependence, within three months, all data regarding the requested ADRs submitted to EV up to 6 May 2019, presented as large Excel files divided into information sections reported in a standardized format, were sent through a hyperlink [25]. Such listings showed all information related to the ADR, the patient, the drug, the reporter, and the diagnosis. Regarding the content of the ADR, according to the standardised MedDRA Query (SMQ) [25], all case reports of the Broad SMQ ’Drug abuse, dependence and withdrawal’ of any source except for clinical trials for both single and multi-ingredient preparations were considered. Specifically, abuse was here defined as “the intentional, non-therapeutic use by a patient or consumer of a product, OTC or prescription, for a perceived reward or desired non-therapeutic effect including, but not limited to, getting high (euphoria)” [25].
2.2.2. Analysis of Data
Due to the limited number of cases, a descriptive analysis was performed. The information is provided in line listings with the names ‘Safety Report’, ‘Reporter’, Literature_Study’, ‘Patient’, ‘Parent’, ‘Reaction’, ‘Test’, ‘Drug’, ‘Diagnosis Summary’. The line listing(s) ‘Safety Report’ provides the total number of cases. Each case report may refer to one or more reporter, study, or suspected ADR(s) as well as to one or more medicinal product(s). Therefore, a single case may be represented by more than one row in the other line listings, having the same “EV Local Report Number”, assigned by the EV, by which the listings are sorted. The received data files were searched for duplicates by report ID through the ‘EV Local Report Number’, which unequivocally identified an individual case [23]. The number of case reports for a particular medicinal product or ADR depends, inter alia, on its availability on the market and its extent of use, the nature of the reaction as well as the public awareness of a safety concern. This means that comparing reporting rates of the number of case reports per time unit for different reactions in relation to the same product or the same reaction in relation to different products may be misleading. Thus, any case report should be seen as a piece of a jigsaw puzzle, with consideration given to all available data to complete the picture. These data included case reports world-wide, clinical trials, epidemiological studies and toxicological investigations. Only the assessment of all available data allows for robust conclusions. Case reports reflect the information as provided to EV by the reporter. Due to the nature of such reports, not all data fields are provided for all reports.
2.2.3. Ethical Issues
In compliance with applicable Personal Data Protection legislation (Regulation (EC) No 45/2001 and Regulation (EC) No 1049/2001, the protection of privacy and integrity of individuals was guaranteed, and in order to safeguard the identity of individuals certain data elements, including names/identifiers or country-specific information were not disclosed by the EMA [23]. The study was ethically approved in March 2018 by the University of Hertfordshire Ethics’ Committee, with reference number LMS/PGR/UH/03234.
3. FINDINGS
3.1. Systematic Review
In removing duplicate articles (n = 21), from a total of 341 papers (PubMed = 22; Scopus = 17; WoS = 302), a number of 320 records were screened, and, among these, the majority (n = 282) were not relevant due to: the subject reading title and abstract (animal/in vitro studies not dealing with the misuse of BZY); 11 were not written in English; and 6 were non-original articles (e.g. review, metanalysis, commentary, letter to the editor without data available). Of the 20 full-text articles assessed for eligibility, 5 were not relating to the misuse of BZY or its physical/psychotropic effects; 1 was relating to OTC drugs other than BZY; and 3 were not available. Finally, 11 articles were taken into consideration in order to be analysed (all the operative method is synthesized in Fig. 1) and included in the present systematic review. Table 1 summarizes the main findings emerging from the retrieved papers organized consistent with the design of the study. They included five case reports [4, 6, 8, 9, 19]; four surveys (one conducted in Poland [26] among pharmacists and three in Brazil among users [12, 16, 27]); and two retrospective case series analyses [10, 28].
Fig. (1).
PRISMA flow-diagram regarding the retrieved studies on Benzydamine abuse/misuse.
Table 1.
Main findings of retrieved studies.
| References | Main Outcome | Type of Use | Participants (N tot, Age) | Administration (mg) | Psychiatric Comorbidity | Physical Symptoms | Psychiatric Symptoms | Poly-abuse (Substance) | Notes |
|---|---|---|---|---|---|---|---|---|---|
| CASE REPORTS | |||||||||
| [4] | Describing a case of BZY intoxication | Overdose | N tot: 1; age: 6 years | Oral (500 mg) | None | None | Visual and somatic hallucinations | None | Spontaneous resolution |
| [6] | Describing a case of BZY intoxication | Misuse | N tot: 1; age: 22 years | Oral (500 mg) | History of SUDs | Hyperreactivity; muscleweakness | Excitation; visual hallucinations | None | Hallucinations lasted for 10 hours |
| [8] | Describing a case of BZY intoxication | Misuse | N tot: 1; age: 20 years | Oral (500 mg) | None | None | Chronic psychosis, visual and auditory hallucinations. Reference delusions, dissociative symptoms | None | Symptoms recovered spontaneously in another two episodes. He was treated for 15 days with olanzapine 10 mg/day |
| [9] | Describing a case of BZY intoxication | Misuse | N tot: 1; age: 20 years | Oral (1000 mg) | None | None | Visual hallucinations | Alcohol | - |
| [19] | Describing a case of BZY intoxication | Misuse | N tot: 1; age: 22 years | Oral (750 mg) | History of SUDs, suicide attempts | Reduced rhythm of speech | Referential concerns, visual illusions | Alcohol | - |
| SURVEY | |||||||||
| [12] | Describing the pattern of use among youngsters in Brazilian cities | Misuse | N tot: 2,807; BZY N: 78 (79.5% male); age range: 15-18 years | Oral/Intravenous (100mg-2000mg) | None | Nausea and vomiting (N: 6); hunger (N: 3); headache (N: 3) | Hallucinations (N: 15);animal visions (N: 2);slowness (N: 2); sadness (N: 2); and psychotic symptoms (N: 1) | Tobacco and/or solvents (N: 27); marijuana and/or cocaine(N: 26); alcohol (N: 25) | - |
| [16] | Studying the recreational use of BZY among adolescents | Misuse | N tot: 5,208; age range: 12-18 years | Oral | None | None | None | Increased use of alcohol, tobacco, marijuana, and inhalants | BZY recreational use was detected in 2.3% of the sample |
| [26] | Studying the misuse of OTC drugs in Poland administrating anonymous questionnaires to pharmacists | Misuse | N tot: 680, N BZY users: NS; age: 20 years | NS | None | NS | NS | NS | Most pharmacists think that the misuse of OTC drugs has increased |
| [27] | Describing patterns of drug use in Brazilian cities | Misuse | N tot: 565; BZY N: 31 (76.6% male); age range: 6-17 years | Oral (N/A) | None | None | None | None | 13.5% of users in Fortaleza and 6.5% in Porto Alegre |
| RETROSPECTIVE STUDY | |||||||||
| [28] | Treating drug dependence with the aid of ibogaine | Misuse | N tot: 75; BZY N: 1; age: NS | NS | NS | NS | NS | NS | - |
| RETROSPECTIVE CASE SERIES | |||||||||
| [10] | Describing BZY effects after its ingestion | Overdose | N tot: 724; age range̴̴: 6-80 years | Oral (10-1500 mg; mean 500 mg) | None | GI (N: 110);GI and Neurological (N: 48) | Not specified (N:71) | N/A | - |
Abbreviations: BZY: benzydamine; GI: gastrointestinal; N: number; N/A: not applicable; NS: not specified; OTC: over-the-counter; SD: Standard Deviation; SUD: Substance Use Disorder.
Whilst nine articles described a recreational use of BZY [6, 8, 9, 12, 16, 19, 26-28], two of them dealt with an oral overdose of the drug, and described both physical and mental side-effects, the latter including visual and somatic hallucinations [4, 10]. Out of the five case-reports here identified, four referred to the recreational use of BZY and involved male subjects with a mean age of 18±6.1 years [6, 8, 9, 19], while one case report described the accidental BZY intoxication of a 6-years old girl [4]. In most of these cases, the dosage ingested was 500 mg [4, 6, 8]). Conversely, in two case reports BZY dosages were reportedly higher and taken
together with alcohol [9, 19]. Two articles reported psychiatric comorbidities, e.g. a substance use disorder or suicidal behaviour [6, 19]. In all cases, visual or auditory hallucinations were reported, in the form of terrifying images of aliens, symmetrical geometric forms, animals and worms crawling on the skin. Two case reports also described paranoid delusional symptoms [8, 19]. In one case, the BZY intoxication led to a chronic psychosis and loss of thought association [8]. These symptoms began immediately after taking the substance for the first time and worsened in the following three months during which the subject reported the BZY consumption to have occurred on some 3-4 occasions; the symptoms decreased after a 3 month-period free from BZY. Besides psychiatric symptoms, physical symptoms included slowed speech [19]; hyperreactivity; and muscle weakness [6]. In terms of treatment/management, in four cases, the symptoms resolved spontaneously [4, 6, 9, 19], whilst in one case olanzapine 10 mg/day prescribing was associated with a partial improvement of the psychotic features [8].
Three out of the four surveys selected [12, 16, 27] were conducted in Brazil and involved high numbers of youngsters. Noto et al. [27] analysed data collected from 565 subjects (mostly male, 76.6%), with 23 subjects having reported at least a single use of BZY, seven a recent use and one subject a problematic use of this substance. High prevalence levels were reported from Fortaleza (13.5%) and Porto Alegre (6.5%) areas. During 2009, Opalaye et al. [12] conducted a survey in 27 Brazilian cities, identifying 78 BZY users (79.5% male) among the 2,807 adolescents enrolled. About 80% of the subjects were street users, consuming BZY even at high dosages, with a maximal recorded level of 2,000 mg, often together with alcohol, marijuana, tobacco, cocaine, benzodiazepines and coffee. In most cases, the route of administration was oral, but intravenous intake was recorded as well. Hallucinations (e.g. seeing animals, lights, and geometric shapes) and gastrointestinal symptoms (e.g. nausea and vomiting) were the most typical psychiatric effects recorded. In the following survey conducted in Sao Paulo; the same authors [16] enrolled a bigger sample (N: 5,208) of young students (12-18 years old), overall confirming previous results.
Analysing the epidemiological characteristics of BZY misuse from another point of view, Zaprutko et al. [26] conducted a survey in Poland among pharmacists focussing on their perception of the prevalence of OTC drugs misuse; significant levels of perception of BZY misuse were identified. In a retrospective case series [10], a high number (N: 724) of cases of BZY oral intoxication was recorded from 1991 to 2003, mostly involving females (73.4%) older than 14 years (86.2%). Interestingly in 94.3% of cases, the intoxication occurred at home, with the mean amount of BZY ingested having been 500 mg (range: 10 mg – 1,500 mg); and the mean time of exposure before calling a clinician of 30 minutes (range: 5 minutes-24 hours). Nearly one-third (31.6%) reported a range of side-effects, including visual or auditory hallucinations (e.g. seeing animals and parasites, coloured lights, cartoon characters) in 15% of cases. Remaining psychiatric symptoms included agitation, dizziness, drowsiness, and tiredness. Non-psychiatric symptoms were mainly gastrointestinal (48%). The only study dealing with the treatment of BZY misuse here identified was a retrospective study by Schenberg et al. [28], who used ibogaine on 75 drug-dependent patients, and one of them was a BZY misuser.
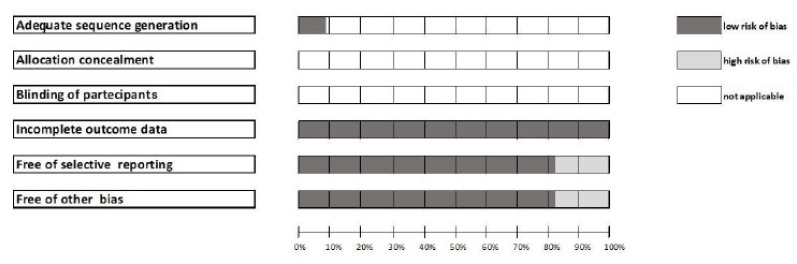
3.1.1. Risk of Bias
Considering the heterogeneity of the articles selected, we found a low risk of bias for almost all parameters (Fig. 2). For most studies, three selected parameters (e.g., “adequate sequence generation”, “allocation concealment” and “blinding of participants”) were not applicable to the selected articles (Fig. 2).
Fig. (2).
Risk of bias.
3.2. EMA Data
The dataset obtained from the EMA reported a total of 14 ADRs, corresponding to 3 single cases recorded during the 2003-2019 timeframe, two of them being reported in the literature available [17, 29]. They related to BZY abuse/misuse/dependence issues. Safety reports were all voluntarily submitted, two from a regulatory authority and one from a pharmaceutical company. Countries involved included Portugal, Italy, and Romania. Cases were recorded using the following PTs: ‘Product use for unknown indication’; ‘Accidental overdose’; ‘Drug abuse’; ‘Intentional drug misuse’. Subjects reported included a 4-years-old child; an adolescent (16- years-old), and a young adult. BZY was reported as having been ingested as a lone molecule in all cases, with dosages ranging from 500 mg to 1,250 mg. In one case, a medical history of polydrug abuse was recorded [17]; no psychiatric diagnoses were reported. Reactions associated with abuse/misuse/dependence issues included: psychomotor hyperactivity; agitation; tremor; ataxia; asphyxia; dysphoria; and visual hallucinations. The outcome in all reactions reported was ‘recovered/resolved’. No suicidal or self-harm behaviour was recorded (Table 2).
Table 2.
Description of abuse/misuse cases reported to the European Medicines Agency dataset.
| ID | Year | Age | Gender | Needs heading | PT | Dosage | Country | Route Of Administration | Reactions Associated | Concomitant Drugs | Outcome | Reporter | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12291259 | N/A | 4 | M | Suspect | Product use for unknown indication; Accidental overdose | 500mg | Portugal | N/A | -Psychomotor hyperactivity-Agitation-Visual hallucinations-Tremor-Ataxia-Asphyxia | No other licit/illicit drugs | Recovered/resolved | Physician | [29] |
| 12724804 | 2014 | 24 | F | Suspect | Drug abuse | 500mg | Italy | Oral | -Medical history of polydrug abuse-Dysphoria-Hallucinations-Weakness-Memory deficit after recent recreational drug consumption | No other licit/illicit drugs | Recovered/resolved | Consumer or other Non-Health Professional | [17] |
| 10000561825 | N/A | 16 | M | Suspect | Intentional drug misuse; Drug abuse | 1250mg | Romania | Oral | Hallucination | N/A | Recovered/resolved | Consumer or other Non-Health Professional | N/A |
Abbreviations: F: female; M: male; N/A: not available; PT: Preferred Terms.
4. DISCUSSION
4.1. Misusing Issues Regarding BZY
To the best of our knowledge, this is the first systematic review focussing on a range of BZY misusing issues, with current findings having been further supported by an assessment of available pharmacovigilance data. Although limited in number, the identified papers and data are of clear relevance supporting the levels of concern relating to the misuse of BZY, a misuse which may be under-recognized, and may well occur in some vulnerable categories, including the youngsters, a category where the OTC drugs’ misusing intake is popular indeed [26, 30]. The remaining OTC drugs being reported in this age-group include: loperamide [31]; dextromethorphan; pseudoephedrine; and mixtures of promethazine and OTC codeine-containing cough suppressant syrups and alcohol and/or soft drinks, known as ‘Purple Drank’, ‘Lean’, ‘dirty Sprite’ or ‘Sizzurp’ [30, 32-35]. The phenomenon involving the non-medical use and misuse of high and super-high dosage prescription and OTC medications is called “pharming”; its popularity may be associated with a range of reasons, including prescription and OTC medications easy accessibility; low cost/affordability; being considered socially acceptable/lack of stigmatization issues; and overall safer than traditional illicit substances [26, 36-38]. Moreover, the web contributes to OTC drugs’ popularity as well. Indeed, it works as a proper platform to share intoxication experiences in chatrooms, blogs, and social media posts. OTC drugs may be available as well online from both proper vendor and 'rogue', no-prescription, websites [26, 39, 40]. Overall, the non-medical use of medications should not be underestimated, resulting in serious clinical effects with potential life-threatening complications, drug-seeking behaviour, dependence, and the occurrence of related withdrawal syndrome as well [37, 38]. For instance, loperamide, known as ‘the poor man’s methadone’, might cause cardiotoxicity, prolonged QT and fatal arrythmias [31, 41]; whilst a drug-induced psychosis may be associated with dextromethorphan use [42]. Moreover, in being completely legal, OTC drugs might not be detected through standard toxicology screenings, thus making a proper diagnosis challenging. In being a public health concern, the implementation of proper prevention campaigns to counteract the increasing levels of online drug acquisition and the related intake activities has been suggested [26, 39].
BZY was included in April 2010 in the list of Novel Psychoactive Substances (NPS) by the European Monitoring Agency for Drugs and Drug Abuse (EMCDDA) and by Europol [7]. Consistent with this, in Turkey BZY was moved in 2012 into prescription status [8].
4.2. BZY Psychotropic Molecular Mechanism
In being an NSAID, BZY has molecular mechanisms, which may be associated with other NSAIDs. In fact, acute poisoning with NSAIDs such as diclofenac, ibuprofen, and indomethacin has been associated with a variety of both neurological and psychiatric events, ranging from headache, vertigo, light-headedness, disturbed sensation, stroke, and neuronal degeneration, to mood disturbances, delirium, hallucinosis, paranoia, and abnormal behaviour [42-45]. Less frequently, these issues may be identified even at therapeutic dosages and especially so in the elderly or when pre-existing psychiatric disorders and concomitant psychiatric treatments are identified [4, 41, 43-45]. In fact, lithium, as well as serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs), show different degrees of pharmacokinetic interaction with NSAIDs, through the varieties of cytochrome P450 isoenzymes (2D6, 3A3/4, 1A2 and 2C19) in the liver [44, 45]. A range of neurobiological mechanisms may be involved in the BZY-related onset of these psychiatric symptoms, including:
Cross-sensitivity with other substances of abuse. Indeed, preclinical studies suggested that BZY may show a powerful reinforcing effect in animals previously administered with heroin and cocaine [2, 11].
BZY affinity with CB1 cannabinoid receptors, which may help in explaining some of cannabinoid-like BZY psychotropic effects and its abuse liability [2].
At high dosages (e.g. 2,000-2,500mg), BZY might influence the dopaminergic regulation of limbic-striatal interplay, increasing dopamine release and ultimately activating the reward pathway [11].
A BZY-related serotonergic 5HT-2A activation; this may be associated with an indole group within the BZY chemical structure [2, 6]. Hence, BZY effects may resemble those of remaining hallucinogenic drugs [19], including lysergic acid diethylamide/LSD [43]; and dimethyltryptamine/DMT [2, 11, 12], which is the psychoactive compound contained in ayahuasca, a hallucinogenic tea used in religious rituals in Brazil [46]. These observations may explain why the BZY-related visual hallucinations here reported in most cases were described as vivid animal images [4, 6, 8, 9, 12, 14, 19]. Indeed, similar to what may occur with BZY high-dosage ingestion [12], the main side-effects reported with ayahuasca are nausea and vomiting [47].
Alteration of prostaglandins and prostaglandin precursors in the CNS [44, 48].
Finally, benzydamine has been the subject as well of piloting docking/’in silico’ studies, an approach which may help in shedding further light on its observed biological effects [49].
4.3. BZY Psychopathological Effects
BZY acute toxicity in oral dosages of 500–3,000 mg include signs and symptoms of CNS stimulation, including seizures, hyperreflexia, tachycardia, paraesthesias, hyper arousal/ hyperactivity, and convulsions [6, 8-10, 12]; moreover, frequent psychiatric symptoms after ingestion of BZY preparations include visual hallucinations, particularly in the form of zoomorphic visions, referential concerns, and paranoia [4-6, 8-10, 12, 13, 15, 17, 19, 29]. Furthermore, most frequent complaints associated with BZY ingestion are nausea, vomiting, and epigastric pain [10, 12], which are similar to the gastrointestinal toxicity described with other NSAIDs [8-10, 13]. BZY high-dose consumption may contribute to the development of psychiatric symptoms due to the high lipid solubility, which facilitates BZY passage into the CNS [9, 10, 12]. No BZY-related deaths have been reported in the literature; the only reported contraindications to the use of BZY included the existence of known hypersensitivity.
4.4. Management of BZY Intoxications
There was only little indication here regarding the optimal treatment and management approach for BZY intoxication. Most cases were here described as having been managed conservatively, including routine supportive therapy and gastric decontamination [8]; moreover, urinary alkalization and force diuresis were here considered to enhance the elimination of BZY [8, 9]. Focusing on psychiatric symptom resolution, one could argue that the administration of short-acting benzodiazepines or low-dosage antipsychotics, such as olanzapine, risperidone, quetiapine, and haloperidol may be required [7, 8, 14, 30]. Finally, even though no antidote against BZY intoxication has been recorded, Schenberg et al. [28] anecdotally reported the therapeutic use of the indole alkaloid ibogaine, which has been previously recorded in substance use misuse treatment [50].
CONCLUSION
The present systematic review confirmed that BZY misuse is an internationally recognised problem. As BZY is an easily available, typically inexpensive, OTC medication, this may be a reason for concern, requiring further investigations. Healthcare professionals should be aware of the drug’s potential for abuse, whilst being informed and prepared in order to avoid harm to those who purchase OTC medicines that may be liable to abuse. Also, as an agent of abuse that is not detected in standard urine drug screens, BZY abuse may represent an underdiagnosed cause of substance-induced psychosis. Thus, physicians should be aware of the psychiatric presentation of BZY abuse and consider this issue in carrying out a differential diagnosis for patients, especially teenagers and young adults, exhibiting acute onset of psychotic symptoms.
ACKNOWLEDGEMENTS
Declared none.
AUTHORS’ CONTRIBUTIONS
Schifano Fabrizio, Chiappini Stefania, Corkery John Martin, and Guirguis Amira conceived the idea of this paper; data were extracted by Chiappini Stefania, Miuli Andrea, Mosca Alessio, whilst Schifano Fabrizio, Martinotti Giovanni, Di Giannantonio Massimo supervised all stages of the process and were consulted to resolve any possible disagreement. Chiappini Stefania and Miuli Andrea drafted the first version and revised it after contributions from all authors, who approved the final version.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICTS OF INTEREST
Chiappini Stefania, Corkery John Martin, Miuli Andrea, Mosca Alessio, Pettorruso Mauro: nothing to be declared.
Martinotti Giovanni: has been a consultant and/or a speaker and/or has received research grants from Angelini, Doc Generici, Janssen-Cilag, Lundbeck, Otsuka, Pfizer, Servier, Recordati.
Di Giannantonio Massimo: has been a consultant and/or a speaker and/or has received research grants from Angelini, Janssen-Cilag, Lundbeck, Otsuka, Pfizer, Servier, Recordati.
Schifano Fabrizio was a member of the UK Advisory Council on the Misuse of Drugs (ACMD; 2011–2019) and is currently a member of the EMA Advisory Board (Psychiatry). Corkery John Martin is a member of the ACMD’s Novel Psychoactive Substances and Technical Committees.
REFERENCES
- 1.National Center for Biotechnology Information. PubChem Compound Summary for CID 12555, Benzydamine. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Benzydamine (Accessed Dec 12, 2020).
- 2.Avvisati R., Meringolo M., Stendardo E., Malavasi E., Marinelli S., Badiani A. Intravenous self-administration of benzydamine, a non-steroidal anti-inflammatory drug with a central cannabinoidergic mechanism of action. Addict. Biol. 2018;23(2):610–619. doi: 10.1111/adb.12516. [DOI] [PubMed] [Google Scholar]
- 3.Quane P.A., Graham G.G., Ziegler J.B. Pharmacology of benzydamine. Inflammopharmacology. 1998;6(2):95–107. doi: 10.1007/s10787-998-0026-0. [DOI] [PubMed] [Google Scholar]
- 4.Gómez-López L., Hernández-Rodríguez J., Pou J., Nogué S. Acute overdose due to benzydamine. Hum. Exp. Toxicol. 1999;18(7):471–473. doi: 10.1191/096032799678840264. [DOI] [PubMed] [Google Scholar]
- 5.Baldock G.A., Brodie R.R., Chasseaud L.F., Taylor T., Walmsley L.M., Catanese B. Pharmacokinetics of benzydamine after intravenous, oral, and topical doses to human subjects. Biopharm. Drug Dispos. 1991;12(7):481–492. doi: 10.1002/bdd.2510120702. [DOI] [PubMed] [Google Scholar]
- 6.Anand J.S., Glebocka M.L., Korolkiewicz R.P. Recreational abuse with benzydamine hydrochloride (tantum rosa). Clin. Toxicol. (Phila.) 2007;45(2):198–199. doi: 10.1080/15563650600981210. [DOI] [PubMed] [Google Scholar]
- 7.Schifano F., Corazza O., Marchi A., Di Melchiorre G., Sferrazza E., Enea A., Davey Z., Blaszko U., Deluca P. Psychonaut web mapping e rednet research projects. Analysis of online reports on the potential misuse of benzidamine. Riv. Psichiatr. 2013;48(3):182–186. doi: 10.1708/1292.14286. [DOI] [PubMed] [Google Scholar]
- 8.Gürü M., Şafak Y., Cengiz G.F., Kuru E., Örsel S. Chronic psychosis related to benzydamine hydrochloride abuse. Neurocase. 2019;25(3-4):156–158. doi: 10.1080/13554794.2019.1617318. [DOI] [PubMed] [Google Scholar]
- 9.Acar Y.A., Kalkan M., Cetin R., Cevik E., Cınar O. Acute psychotic symptoms due to benzydamine hydrochloride abuse with alcohol. Case Rep. Psychiatry. 2014;2014:290365. doi: 10.1155/2014/290365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballesteros S., Ramón M.F., Martínez-Arrieta R. Ingestions of benzydamine-containing vaginal preparations. Clin. Toxicol. (Phila.) 2009;47(2):145–149. doi: 10.1080/15563650801938670. [DOI] [PubMed] [Google Scholar]
- 11.Mota D.M., Costa A.A., Teixeira C.S., Bastos A.A., Dias M.F. Use abusive of benzydamine in Brazil: An overview in pharmacovigilance. Cien. Saude Colet. 2010;15(3):717–724. doi: 10.1590/S1413-81232010000300014. [DOI] [PubMed] [Google Scholar]
- 12.Opaleye E.S., Noto A.R., Sanchez Zv., Moura Y.G., Galduróz J.C., Carlini E.A. Recreational use of benzydamine as a hallucinogen among street youth in Brazil. Br. J. Psychiatry. 2009;31(3):208–213. doi: 10.1590/S1516-44462009000300005. [DOI] [PubMed] [Google Scholar]
- 13.Doksat M. Reversible worsening of psychosis due to benzydamine in a schizoaffective young girl who is already under treatment with antipsychotics. Klinik Psikofarmakol. Bülteni. 2009;19:280–285. [Google Scholar]
- 14.Can B., Oz I., Ozer H., Simsek T. Hallucinations after Ingesting a High Dose of Benzydamine Hydrochloride. Clin. Psychopharmacol. Neurosci. 2016;14(4):407–408. doi: 10.9758/cpn.2016.14.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doğan M., Yılmaz C., Çaksen H., Güven A.S. A case of benzydamine HCL intoxication. East. J. Med. 2006;11(1):26–28. [Google Scholar]
- 16.Opaleye E.S., Sanchez Z.M., Moura Y.G., Locatelli D.P., Noto A.R. An anti-inflammatory as a recreational drug in Brazil. Addiction. 2011;106(1):225. doi: 10.1111/j.1360-0443.2010.03196.x. [DOI] [PubMed] [Google Scholar]
- 17.Rotolo M.C., Pellegrini M., Solimini R., Pichini S., Pacifici R. ‘Smart drugs’, the new drugs on the web: two cases of acute intoxication. Biochim. Clin. 2014;38(3):268–271. [Google Scholar]
- 18.Souza J.F., Marinho C.L., Guilam M.C. Consumo de medicamentos e internet: análise crítica de uma comunidade virtual. Medicine consumption and the internet: critical evaluation of a virtual community. Rev. Assoc. Med. Bras. 2008;54(3):225–231. doi: 10.1590/S0104-42302008000300015. [Medicine consumption and the internet: critical evaluation of a virtual community]. [DOI] [PubMed] [Google Scholar]
- 19.Balaban O., Atagun M., Yilmaz H., Yazar M., Alpkan L. Benzydamine abuse as a hallucinogen: a case report. Klinik Psikofarmakol. Bülteni. 2013;23:276. doi: 10.5455/bcp.20111212083751. [DOI] [Google Scholar]
- 20.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernardo W.M. PRISMA statement and PROSPERO. Int. Braz. J. Urol. 2017;43(3):383–384. doi: 10.1590/s1677-5538.ibju.2017.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Medicines Agency (EMA) (2017) Guideline on good pharmacovigilance practices, Module VI—Collection, management and submission of reports of suspected adverse reactions to medicinal products (Rev 2). 2017. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2017/08/WC500232767.pdf(Accessed July 25, 2020).
- 24.European Medicines Agency (EMA) (2010) Note for guidance—EudraVigilance Human—Processing of safety messages and individual case safety reports (ICSRs) (EMA/H/20665/04/Final Rev. 2). 2010. Available from: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/note-guidance-eudravigilance-human-processing-safety-messages-individual-case-safety-reports-icsrs_en.pdf(Accessed July 25, 2020).
- 25.MedDRA. Version 22. 2019. Available from: https://www.meddra.org/sites/default/files/guidance/file/smq_intguide_22_0_english.pdf(Accessed July 25, 2020).
- 26.Zaprutko T., Koligat D., Michalak M., Wieczorek M., Józiak M., Ratajczak M., Szydłowska K., Miazek J., Kus K., Nowakowska E. Misuse of OTC drugs in Poland. Health Policy. 2016;120(8):875–881. doi: 10.1016/j.healthpol.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Noto A.R., Nappo S.A., Galduróz J.C., Mattei R., Carlini E.A. Use of drugs among street children in Brazil. J. Psychoactive Drugs. 1997;29(2):185–192. doi: 10.1080/02791072.1997.10400186. [DOI] [PubMed] [Google Scholar]
- 28.Schenberg E.E., de Castro C.M.A., Chaves B.R., da Silveira D.X. Treating drug dependence with the aid of ibogaine: A retrospective study. J. Psychopharmacol. 2014;28(11):993–1000. doi: 10.1177/0269881114552713. [DOI] [PubMed] [Google Scholar]
- 29.Lopes Dias A., Pereira H., Soares J., Campos T., Quaresma M. When the sachets get mixed-up – benzydamine hydrochloride ingestion. Nascer Crescer. 2015;24(3):133–135. [Google Scholar]
- 30.Cooper R.J. Over-the-counter medicine abuse - a review of the literature. J. Subst. Use. 2013;18(2):82–107. doi: 10.3109/14659891.2011.615002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schifano F., Chiappini S. Is there such a thing as a ‘lope’ dope? Analysis of loperamide-related European Medicines Agency (EMA) pharmacovigilance database reports. PLoS One. 2018;13(10):e0204443. doi: 10.1371/journal.pone.0204443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiappini S., Schifano F., Corkery J.M., Guirguis A. Beyond the purple drank. Study of promethazine abuse according to the European Medicines Agency (EMA) adverse drug reactions (ADR) reports. J. Psychopharmacol. 2021;35(6):681–692. doi: 10.1177/0269881120959615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miuli A., Stigliano G., Lalli A., Coladonato M., D’Angelo L., Esposito F., Cappello C., Pettorruso M., Martinotti G., Schifano F., Di Giannantonio M. “Purple Drank” (codeine and promethazine cough syrup): A systematic review of a social phenomenon with medical implications. J. Psychoactive Drugs. 2020;52(5):453–462. doi: 10.1080/02791072.2020.1797250. [DOI] [PubMed] [Google Scholar]
- 34.Burns J.M., Boyer E.W. Antitussives and substance abuse. Subst. Abuse Rehabil. 2013;4:75–82. doi: 10.2147/SAR.S36761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roussin A., Bouyssi A., Pouché L., Pourcel L., Lapeyre-Mestre M. Misuse and dependence on non-prescription codeine analgesics or sedative H1 antihistamines by adults: a cross-sectional investigation in France. PLoS One. 2013;8(10):e76499. doi: 10.1371/journal.pone.0076499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson K.M. Addicted media: Substances on screen. Child Adol. Psych. Clin. 2005;14(3):473–489. doi: 10.1016/j.chc.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Chiappini S., Guirguis A., Corkery J.M., Schifano F. Understanding the use of prescription and OTC drugs in obtaining highs and the pharmacist role in preventing abuse. Pharm J. 2020;305:7943. https://www.pharmaceutical-journal.com/research/perspective-article/misuse-of-prescription-and-over-the-counter-drugs-to-obtain-illicit-highs-how-pharmacists-can-prevent-abuse/20208538.article?firstPass=false [Google Scholar]
- 38.Chiappini S., Schifano F. What about “Pharming”? issues regarding the misuse of prescription and over-the-counter drugs. Brain Sci. 2020;10(10):736. doi: 10.3390/brainsci10100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen S., Barratt M.J. Prescription drug misuse: Is technology friend or foe? Drug Alcohol Rev. 2009;28(1):81–86. doi: 10.1111/j.1465-3362.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- 40.Orsolini L., Francesconi G., Papanti D., Giorgetti A., Schifano F. Profiling online recreational/prescription drugs’ customers and overview of drug vending virtual marketplaces. Hum. Psychopharmacol. 2015;30(4):302–318. doi: 10.1002/hup.2466. [DOI] [PubMed] [Google Scholar]
- 41.Dierksen J., Gonsoulin M., Walterscheid J.P. Poor man’s methadone: A case report of loperamide toxicity. Am. J. Forensic Med. Pathol. 2015;36(4):268–270. doi: 10.1097/PAF.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 42.Martinak B., Bolis R.A., Black J.R., Fargason R.E., Birur B. Dextromethorphan in cough syrup: The poor man’s psychosis. Psychopharmacol. Bull. 2017;47(4):59–63. doi: 10.64719/pb.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onder G., Pellicciotti F., Gambassi G., Bernabei R. NSAID-related psychiatric adverse events: who is at risk? Drugs. 2004;64(23):2619–2627. doi: 10.2165/00003495-200464230-00001. [DOI] [PubMed] [Google Scholar]
- 44.Auriel E., Regev K., Korczyn A.D. Nonsteroidal anti-inflammatory drugs exposure and the central nervous system. Handb. Clin. Neurol. 2014;119:577–584. doi: 10.1016/B978-0-7020-4086-3.00038-2. [DOI] [PubMed] [Google Scholar]
- 45.Jiang H.K., Chang D.M. Non-steroidal anti-inflammatory drugs with adverse psychiatric reactions: five case reports. Clin. Rheumatol. 1999;18(4):339–345. doi: 10.1007/s100670050114. [DOI] [PubMed] [Google Scholar]
- 46.Kometer M., Vollenweider F.X. Serotonergic hallucinogen-induced visual perceptual alterations. Curr. Top. Behav. Neurosci. 2018;36:257–282. doi: 10.1007/7854_2016_461. [DOI] [PubMed] [Google Scholar]
- 47.Graziano S., Orsolini L., Rotolo M.C., Tittarelli R., Schifano F., Pichini S. Herbal highs: review on psychoactive effects and neuropharmacology. Curr. Neuropharmacol. 2017;15(5):750–761. doi: 10.2174/1570159X14666161031144427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wongrakpanich S., Wongrakpanich A., Melhado K., Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9(1):143–150. doi: 10.14336/AD.2017.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao C., Catucci G., Di Nardo G., Gilardi G., Sadeghi S.J. Human flavin-containing monooxygenase 3: Structural mapping of gene polymorphisms and insights into molecular basis of drug binding. Gene. 2016;593(1):91–99. doi: 10.1016/j.gene.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 50.Corkery J.M. Ibogaine as a treatment for substance misuse: Potential benefits and practical dangers. Prog. Brain Res. 2018;242:217–257. doi: 10.1016/bs.pbr.2018.08.005. [DOI] [PubMed] [Google Scholar]