Abstract
Background
Neural cells undergo functional or sensory loss due to neurological disorders. In addition to environmental or genetic factors, oxidative stress is a major contributor to neurodegeneration. In this context, there has been a growing interest in investigating the effects of EOs (EOs) in recent years, especially in the treatment of neuropathologies. The chemical and biological effects of EOs have led to important treatment tools for the management of various neurological disorders.
Objective
In the present study we performed a systematic review that sought to comprehend the neuroprotective effects of different EOs.
Method
This work is a systematic review where an electronic search was performed on PubMed, ScienceDirect, Cochrane Library and SciELO (Scientific Electronic Library Online) databases, covering the last 10 years, using “Essential oil” and “Neuroprotective effect” as reference terms.
Results
A total of 9 articles were identified, in which the efficacy of EOs was described in experimental models of anxiety, dementia, oxidative stress, cerebral ischemia, Alzheimer’s disease, and oxidative toxicity.
Conclusion
EOs from different species of medicinal plants have shown positive responses in neurological disorders such as anxiety, dementia, oxidative stress, cerebral ischemia, and oxidative toxicity. Thus, EOs emerges with the potential to be used as alternative agents in the treatment of neurological disorders.
Keywords: Neurodegenerative diseases, oxidative stress, essential oils, antioxidant, neuroprotection, brain
1. INTRODUCTION
Neurological disorders are related to functional and sensory loss of neuronal cells, with several factors contributing to this, such as the environment, genes, or oxidative toxicity. Oxidative stress is a major contributor to neurodegeneration, as the excess of reactive oxygen species (ROS), when not repaired, results in increased damage to biomolecules (DNA, lipids, proteins), compromising the functioning of cells. It can have repercussions on neurological disorders [1, 2].
Neurodegenerative diseases and traumatic injuries to the brain and spinal cord affect millions of people around the world every year. Recent estimates suggest that more than 6 million people die of stroke annually; about 70 million suffer some kind of traumatic brain injury whilst 47.5 million people live with dementia. Alzheimer’s disease (AD) is still the most common cause of dementia, accounting for 60-70% of cases and these numbers end up creating a dramatic economic impact on the health system [3].
In the United States alone, the combined annual costs of these neurological diseases reach nearly U$ 800 billion, although this number tends to increase each year due to population aging, resulting in a huge economic burden [4]. Therefore, alternative and lower-cost therapies should be investigated with the purpose of prevention and treatment of neurodegenerative diseases, which will ultimately improve the quality of life of affected patients and reduce the aforementioned economic burden.
The use of EOs (EOs) and their compounds has long been known in traditional medicine and aromatherapy for the treatment of various diseases [5]. In recent years, interest in medicinal plants and the study of their compounds has been growing, especially for the scientific community that deals with neurological disorders [5,6]. The metabolites present in EOs have biological and pharmacological properties such as antioxidant, anti-inflammatory and neuroprotective, in addition to being widely used as antibacterial, antifungal and insecticidal agents [7].
Natural antioxidants are molecules that protect cells from damage induced by ROS. Several of these compounds are present in herbaceous plants and have cytoprotective properties in vitro [6]. Oxidative stress has been associated with more than 100 pathologies, such as atherosclerosis, pancreatic diseases, cancer and neurological diseases [8]. Scientific evidence has demonstrated the importance of eliminating free radicals in the prevention and treatment of neurological disorders [7] and antioxidant therapy has shown a protective effect. In addition, medicinal plants not only protect oxidative damage, but also play an important role in maintaining health and preventing chronic degenerative diseases [8]. In this context, the antioxidants present in EOs have been considered a therapeutic alternative against neuronal loss, as they are able to neutralize the action of free radicals, providing a neuroprotective effect [9].
In view of the promising beneficial effects of natural EOs, such as better efficacy, safety, and cost-effectiveness [5], this current study aims to investigate, through a literature review, the neuroprotective effect of several EOs and their secondary metabolites in different neurological disorders.
2. MATERIALS AND METHODS
2.1. Databases and Keywords
This study comprises a systematic review of the literature which was designed based on the following steps: search, identification, selection and eligibility of published articles. The scientific guiding question that directed this study was, “Does EO treatment promote neuroprotection?”
The terms used for the search were previously selected considering the controlled vocabulary for articles’ indexing of the Health Sciences Descriptors. The terms “Essential oil” and “Neuroprotective effect” were selected. The boolean operator “and” was used to combine both terms, so that the association “EO and neuroprotective effect” was used. This systematic review of scientific studies followed the guidelines based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [10].
2.2. Eligibility/Exclusion Criteria
The search was performed on articles published between December 2017 and January 2018 using four databases: Science Direct, PubMed, LILACS, and Cochrane Library. The articles had to meet the following criteria: a) Use their own primary data; b) Experiments performed on rats or cultures of cells that were induced to neurological damage; c) The treated groups had some EO administered before or after the neurological damage; d) The activity of EOs and their mechanisms of action were investigated in neurological lesions; e) Articles were published in the last ten [10) years. We discarded all studies that: a) Used experimental models other than rats or cell culture; b) Did not use a specific group for the treatment with essential oil; c) Were not published in English.
Studies evaluated in this systematic review have limitations in important aspects. These include: an agent that induces neurological disorders, treatment durations and the use of different compounds to simulate neuronal damage in experimental models and present different mechanisms of action, aspects that reinforce the heterogeneity of the studies. Grouping statistics were not considered due to methodological heterogeneities between studies with different neurological disorders analyzed in different experimental models. Thus, the meta-analyzes were not performed for the accessed data.
2.3. Methodological Assessment of the Quality of Studies
The completed literature included in this systematic review was assessed for validity by an MQA, according to a modified version of the Downs and Black ‘Quality Index’ (IQ) [11]. The IQ consists of 27 questions applied to the MQA of randomized and non-randomized clinical trials, case-control, and cohort studies in humans. This tool (designed and published) is not suitable for evaluating animal studies that are essentially different from the clinical study design. However, with regard to animal studies, the areas assessed by the IQ that support internal validity and the fundamental scientific method are applicable and can be used for animal studies with the same accuracy [12].
Downs and Black IQ represents a model that can be applied to the development of a tool for assessing the quality of studies in animals with proven high internal consistency (KR-20: r = 0.89), good test-retest performance (r = 0.88), and inter-evaluated performance (r = 0.75) [11,12]. Therefore, the literature included in this review was assessed by a modified Downs and Black IQ.
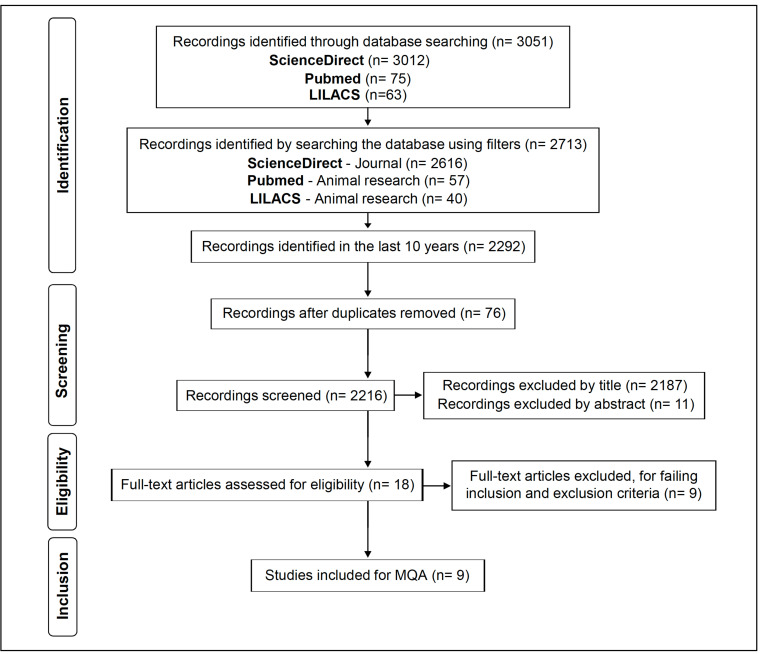
The questionnaire adapted by Ainge et al. had 19 questions, 12 of which assessed the quality of the reports (including specific questions for animals), six assessed the internal validity (three biased and confusing), and one question assessed the power of each study [12]. In the present review, 9 studies were selected using the search strategy; 7 of them were developed with animals and 2 with cell culture. For this purpose, 6 questions present in the questionnaire that report the characteristics of the animals were adapted to characteristics of cell culture (they are respectively from 3 to 7), and one question involving confusion (question 18) was adapted for the analysis of these 2 studies. This can be seen in Fig. (1).
Fig. (1).
Flowchart of the systematic selection of published articles developed based on PRISMA guidelines [10].
The independent MQA was conducted by two researchers. For each study, a 'yes' or 'no' answer was recorded for each question with a score of one or zero. The responses were added to give a total of 19 points, which was later represented as a percentage. This assessment consisted of questions that may have a greater influence on the reliability and validity of the studies evaluated. In addition, this tool not only evaluated all positive responses, but specific scores were also calculated for reports and internal validity, enabling a more accurate assessment of the reliability and validity of the studies [12].
3. RESULTS
3.1. Search Strategy
Initially, 3012 articles were found in ScienceDirect, 75 in PubMed, 63 in LILACS, and 0 in the Cochrane Library, totaling 3,150 publications. In the ScienceDirect database, when the “Journal” filter was used, the number of articles dropped to 2616. In PubMed, the “animal search” filter was selected, which reduced the number of articles to 57, being dropped to 46 when the “last 10 years” filter was also applied. At LILACS, the “animal research” filter was used with 40 articles which reduced to 37 after using the “last 10 years” filter. Therefore, the four databases initially yielded a total of 2,292 articles after being evaluated by the filter over the last 10 years, which is summarized in the flowchart.
The evaluation of the records was carried out by matching the title of the articles for the purpose of this review, and a total of 2187 articles were excluded. A total of 11 articles were excluded by abstracts. Then, a total of 18 full-text articles were accessed for eligibility and completely read. Subsequently, 9 articles were excluded for failing in inclusion and exclusion criteria, and 9 articles met the inclusion criteria mentioned above and were included for MQA (Methodological Quality Assessment). The complete article selection process is summarized in Fig. (1).
The process of extracting data from the selected articles was performed independently by two evaluators. It was guided by a previously developed standard analysis form, which was used to evaluate the selected articles through all the aforementioned search strategies (Table 1). In case of disagreements between the two evaluators regarding the collected data, the inclusion of the data in this study was conditioned to a consensus between the pair.
Table 1.
Methodological quality assessment questions (modified from (11) and (12)).
| Reporting | |
|---|---|
| General | 1 Were the hypotheses/aims/objectives of the study clearly described within the introduction? 2 Were the main outcomes to be measured clearly described in the introduction or methods section? |
| Animal characteristics/ cell culture | 3 Was animal species/ strain identified? / Have cell lines been identified? 4 Has the animal's age been specified? / Was a cell viability test performed? 5 Have animal weights been specified? / Have the dilutions and time taken for cultures been specified? 6 Have the initial animal numbers been specified? / Have the culture medium and supplementation percentages been specified? 7 Have the housing details been specified? / Have the incubator and/or cultivation details been specified? |
| Design and outcomes | 8 Were the interventions of interest clearly described? 9 Were the interventions of interest clearly described? 10 Were estimates of the random variability in the data for the main outcomes provided? 11 Have all important adverse events that may be consequences of the intervention been reported? 12 Have the current probability values been reported for the main outcomes, except where probability value is less than 0.001? |
| Internal validity bias | 13 Was an attempt made to blind those measuring the main outcomes of the intervention? 14 Were the statistical tests used to assess the main outcomes appropriate? 15 Were the main outcomes measures used accurate (valid and reliable)? |
| Confounding | 16 Was it stated in the text that rats were randomized to intervention groups? 17 Was there adequate adjustment for confounding in the analyses from which the main findings were drawn? 18 Were losses of animals explained? / Has cell lysis in the medium supernatant been calculated? |
| Power | 19 Was the paper of sufficient power to detect a clinical important effect where the probability value for a difference being due to chance is less than 5%? |
3.2. MQA (Methodological Quality Assessment)
The MQA performed on the 9 articles included for review is provided in Table 2. The total score for the IQ of each article can be seen below. The total score of the works varied widely, from 68 [13-16] to 79% [17, 18], with an average quality rating of 74%.
Table 2.
Methodological quality assessment conducted on all articles included for evaluation in this systematic review.
| Refs. | Questions | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | Total (%) | |
| [ 6 ] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 84 |
| [ 13 ] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 68 |
| [ 14 ] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 68 |
| [ 15 ] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 68 |
| [ 16 ] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 68 |
| [ 17 ] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 79 |
| [ 18 ] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 79 |
| [ 19 ] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 74 |
| [ 20 ] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 74 |
| Reporting | Internal validity (Bias) | Internal validity (Confounding) |
Power | Average | ||||||||||||||||
| Total/9 | 9 | 9 | 9 | 2 | 9 | 7 | 9 | 9 | 9 | 8 | 3 | 9 | 3 | 8 | 8 | 6 | 0 | 1 | 8 | 74 |
The evaluations of the total classification for reports and internal validity identified that the general reports within the manuscripts were of a higher quality than the internal validity (Table 3).
Table 3.
Methodological quality assessment results.
| Refs. | Reporting x/12 | Total % | Internal x/7 | Validity % | Total x/19 | Rating % |
|---|---|---|---|---|---|---|
| [ 6 ] | 12 | 100 | 4 | 57 | 16 | 84 |
| [ 13 ] | 10 | 83 | 3 | 43 | 13 | 68 |
| [ 14 ] | 10 | 83 | 3 | 43 | 13 | 68 |
| [ 15 ] | 8 | 67 | 5 | 71 | 13 | 68 |
| [ 16 ] | 10 | 83 | 3 | 43 | 13 | 68 |
| [ 17 ] | 11 | 92 | 4 | 57 | 15 | 79 |
| [ 18 ] | 12 | 100 | 3 | 43 | 15 | 79 |
| [ 19 ] | 9 | 75 | 5 | 71 | 14 | 74 |
| [ 20 ] | 10 | 83 | 4 | 57 | 14 | 74 |
| AVERAGE | 8.1 | 85 | 3.1 | 54 | 11.2 | 74 |
All studies (9/9) adequately report lineages and details of the intervention. Most studies were able to detect an important clinical effect (8/9). However, there were few reports of blinding (3/9) of those who measure the main results of the intervention. Not all adverse events as a result of the intervention were mentioned (3/9). There is a complete absence in studies of adequate adjustment for confusion in the analyses from which the main results were extracted (0/9).
4. DISCUSSION
The studies that were analyzed presented some differences regarding several aspects. For instance, the studies differed with respect to the EO that was investigated. Salvia lavandulifolia (5), Propolis (1), Bergamot (1), Pistacia lentiscus (1), Aloysia citrodora (1), and Rosa damascena (1) were investigated and different neurological disorders were evaluated such as oxidative stress (1), anxiety (2), dementia (1), cerebral ischemia (3), AD (1) and oxidative toxicity (1), including different tests performed in vivo (7) and in vitro (2). The method of administering the EOs also differed among the studies: orally (1), gavage (1), intraperitoneal (4), and inhalation (1).
Regarding the genus of the animals, all in vivo studies used male rats (7). The experimental models of neuropathological conditions were induced through stress (2), H2O2 (2),
MQA modified QI results for reporting, internal validity and overall score for the studies reviewed. Result shown as total out of 12, 7 and 19 respectively and as a percentage.
MCAO (2), BCCAO (1), scopolamine (1), β-amyloid (1), and L-dopa (1).
Regarding the behavioral analysis, the studies used different experimental tests such as locomotor activity test (1), high cross maze test (2), open field test (1), and forced swimming test (1). Other studies evaluated the following biochemical parameters: ROS (2), MNDA (2), MDA (4), GSH (4), GSSH (2), CAT (3), SOD (5), GPx (2), GR (1), HO-1 (1), NRF2 (1), Caspase-3 (1), nitric oxide (1), GSHPx (2), CORT (1), ACTH (1), PTN (2), PCC (2) ecBs (1), measurement of fatty acids (1), chelation activity of ferrous ions (1), and cell viability (1).
The studies also evaluated the following neurological outcomes: myocardial infarct size (2), measurement of cerebral edema (1), neurological deficits (1), and histopathological changes (1), where the tests used were Western blot (3), DPPH (1), MTT (1), and non-radioactive CytoTox 96 assay (1).
The selected articles were reviewed and described in Table 4 in terms of EOs and active compounds, mentioning the main chemical compounds present in EOs, neurological disorder, experimental model, experimental groups, posology, experimental analyzes, and the main findings.
Table 4.
In vivo and in vitro models of studies neurological disorders and the implications of the essentials oils in the treatment.
| Refs. | Medicinal Plant/ Essential Oil | Neurological Disorder | Experimental Model | Experimental Groups | Posology |
Experimental
Analyzes |
Main Findings |
|---|---|---|---|---|---|---|---|
| [6] | Aloysia citrodora (limonene, geranial, neral, 1, 8-cineole, curcumene, spathulenol and caryophyllene oxide) | Alzheimer’s disease induced by H2O2 and β-amyloid | CAD cells | Experiment I: (1) Control; (2) 10µM β-amyloid; (3) Aloysia citrodora EO (EO) at 0.001 mg/ml; (4) EO at 0.01 mg/ml; (5) EO at 0.001 mg/ml + β-amyloid (6) EO at 0.01 mg/ml + β-amyloid Experiment II: (1) Control; (2) 250µM H2O2; (3) EO 0.001 mg/ml; (4) EO 0.01 mg/ml; (5) EO 0.001 mg/ml + 250µM H2O2; (6) EO 0.01 mg/ml + 250µM H2O2; |
Cells were treated for 24h | - DPPH assay; - MTT assay; - CytoTox 96 non-radioactive assay; - Ferrous ion chelating activity; |
Aloysia citrodora EO inhibited nicotine binding to membranes and increased ferrous ion chelation in vitro. A. citrodora exhibited antioxidant and radical elimination activities with significant protective properties, compared to hydrogen peroxide and β-amyloid induced neurotoxicity. |
| [13] | Propolis Brazilian green própolis (Apis mellifera L.) (terpenoids, aromatic and aliphatic compounds) | Anxiety-like behavior induced in restraint stressed rats In vivo |
Rats | (1) Normal control; (2) Model control; (3) Low dose of propolis EO (PEO) (50 mg/kg); (4) Intermediate dose of PEO (100 mg/kg); (5) High dose of PEO (200 mg/kg); (6) Positive control (DZP 2 mg/kg) |
Orally administered PEO and DZP at the dose of 0.1 mL per gram of body weight during 14 days before exposure to stress | - Locomotor activity test; - High cross maze test; - CORT; - ACTH; - GSH; - MDA; - SOD |
Pretreatment with PEO significantly reversed anxiety-like behavior in rats. In addition, PEO significantly decreased plasma levels of CORT, ACTH and MDA, while increasing SOD activity. |
| [14] |
Lavandula angustifólia ssp. angustifolia Mill. and Lavandula hybrida Rev. (Linalool, linalyl acetate, terpinen-4-ol, lavandulyl acetate and traces of camphor and borneol) |
Dementia induced by scopolamine In vivo/ inhalation |
Wistar rats | (1) Control; (2) Positive control (Silexan); (3) Scopolamin (4) L. angustifólia (5) L. hybrida |
Silexan and Lavandula: - The animals were exposed to vapors (200 μL) for 1h daily during 7 continuous days; Scopolamin: - Daily injection (i.p.) for 7 continuous days at 30 min after exposure to Silexan and Lavandula |
-SOD; - GPx; - CAT; - GSH; - MNDA; - Apoptosis - Protein assay |
Subacute exposures to Lavandula EOs significantly increased the activity of antioxidant enzymes (SOD, GPX and CAT) and reduced total GSH content, while decreasing MDA levels, suggesting an important antioxidant activity. In addition, DNA cleavage patterns were absent in the groups treated with Lavandula, suggesting an antiapoptotic activity. |
| [15] | Lavandula angustifolia Mill. (linalool, linalil acetate, camphor, 1,8-cineole) | Focal cerebral ischemia with reperfusion induced by transient occlusion of the middle cerebral artery | Kunming mice | (1) Control; (2) Lavandula EO (LEO) (50 mg/kg); (3) LEO (100 mg/kg); (4) LEO (200 mg/kg). (5) Positive control (Edavarone 3 mg/kg, i.p.) |
LEO was administered 3 days before surgery | -Measurement of neurological deficits; -Measurement of the infarcted area; - Histopathological changes; - PCC; - MDA; - SOD; - CAT; -GSH-Px - GSH/GSSG; - ROS |
LEO treatment significantly decreased neurological deficit scores, infarct size, MDA levels, carbonylated PTN and ROS. In addition, it regulated the activity of SOD, CAT, GSH-Px and GSH/GSSG. |
| Refs. | Medicinal Plant/ Essential Oil | Neurological Disorder | Experimental Model | Experimental Groups | Posology |
Experimental
Analyzes |
Main Findings |
| [16] |
Rosa damascena Mill. (flavonoids, anthocyanins, terpenes and glycosides) and Lavandula angustifolia Mill (linalool, linalil acetate, camphor, 1,8-cineole) |
L-dopa-induced oxidative toxicity | Albino rats | (1) Control; (2) L-dopa; (3) Vitamin C + L-dopa; (4) Trolox + L-dopa; (5) Rose oil + L-dopa; (6) Lavender oil + L-dopa. |
The animals were first pretreated with injections (i.p) of vitamin C (400 mg/kg), Trolox and Rose or Lavender oil, followed by injections of L-dopa (100 mg/kg) and benserazide (10 mg/kg) | -MDA; - Nitric oxide; - PCC |
Rose and lavender oils protected against acute oxidative toxicity induced by L-dopa. Combining L-dopa therapy with antioxidants may reduce related side effects and provide symptomatic relief. Since natural antioxidants are rich in phenols, they can slow the oxidative degradation of lipids, proteins and DNA. |
| [17] |
Pistacia lentiscus L. (terpenes and sesquiterpenes) |
Focal cerebral ischemia with reperfusion (R) induced by transient bilateral common carotid artery occlusion (BCCAO/R) | Wistar rats | (1) Simulated operation + control; (2) Simulated operation + Pistacia lentiscus L. EO (3) BCCAO/R + control (4) BCCAO/R + Pistacia lentiscus L. EO |
200 mg of Pistacia lentiscus L. E.O. was administered by gavage | - Measurement of fatty acid composition; - Analysis of eCBs and congeners; - Western blot. |
BCCAO/R induced a decrease in docosahexaenoic acid (DHA) in the frontal cortex. Pre-treatment with Pistacia lentiscus L. EO reduced the levels of cyclooxygenase-2 (COX-2). In plasma, only after BCCAO/R that EO administration increased both the ratio of DHA-to-its precursor, eicosapentaenoic acid (EPA) and levels of palmytoylethanolamide (PEA) and oleoylethanolamide (OEA). |
| [18] |
Salvia lavandulifolia Vahl (Spanish sage). (1,8-cineole, camphor, α-pinene, β-pinene and camphene) |
Oxidative stress induced by H2O2 In vitro |
PC12 cells | (1) Control; (2) H2O2; (3) Triton; (4) 5 µg/mL; (5) 15 µg/mL; (6) 25 µg/mL; (7) 50 µg/mL. |
- Cells pre-treated with 5 and 50 µg/mL (24 hs); - H2O2 0,1 mM (30min). |
- Cell viability; - Intracellular measurement of ROS; - MDMA; - GSH/GSSG; - CAT; - SOD; - GPx; -GR; -HO-1; - Nrf2; - Western blot; - Caspase- 3 |
Pretreatment with Spanish sage EO decreased MNDA levels and intracellular ROS production while increased the GSH/GSSG ratio. This EO increased antioxidant status as evidenced by increased activity of antioxidant enzymes (CAT, SOD, GPx, GR and HO-1), protein expression and inhibition of caspase-3 activity. In addition, this EO was capable of activating transcription factor Nrf2. |
| [19] | Bergamot Citrus bergamia Risso and Poiteau (monoterpene hydrocarbons d-limonene, the monoterpene ester linalyl acetate, monoterpene alcohol linalool) |
Anxiety induced by stress In vivo |
Wistar rats | (1) Control; (2) Bergamot EO (BEO) (100 L/kg) (3) BEO (250 L/kg) (4) BEO (500 L/kg) (5) DZP (1.2mg/kg) (6) DZP (1.2mg/kg) |
Pre-treated with intraperitoneal injections at 30 minutes prior to each test | - Open field test; - Labyrinth test; - Forced swimming test. |
Data analysis suggests that the EOs extracted from bergamot attenuated anxiety-like behavior in rats. |
| [20] |
Lavandula angustifolia (Linalool, linalyl acetate, α-pinene, limonene,1,8-cineole,cis-andtrans-ocimene, 3 octanone, camphor, caryophyllene, terpinen-4-ol and lavendulyl acetate, cineole and flavonoids) |
Focal cerebral ischemia induced by transient middle cerebral artery occlusion (MCAO) | Wistar rats | Infarct: (1) Control; (2) Lavandula EO (LEO) (50 mg/kg); (3) LEO (100 mg/kg); (4) LEO (200 mg/kg). Edema: (1) Control; (2) LEO (100 mg/kg); (3) LEO (200 mg/kg); (4) LEO (400 mg/kg). |
LEO was administered intraperitoneally (i.p) prior to MCAO | - Neurobehavioral test; - Measurement of infarct size; - Measurement of cerebral edema; - MDA; -SOD; -GSH-px - PTN; - Western blotting |
Treatment with LEO at doses of 200 and 400 mg/kg significantly reduced infarct size, cerebral edema and improved functional performance after cerebral ischemia. LEO (200 mg/kg) also reduced the MDA content and increased the activities of SOD, GSH-px and total antioxidant capacity. Analysis by western blotting showed that treatment with LEO (200 mg/kg) resulted in a significant increase of inactive vascular endothelial growth factor (VEGF) expression (18%) when compared to that of the control group. |
4.1. Anxiety
Anxiety is a state of psychological and physiological disorder manifested by cognitive, emotional, behavioral, and somatic elements. Its onset is sudden and unexpected, without any triggering stimulus. In addition, by dealing with the unusual situation of panic, the body is subjected to symptoms such as tension, sweating, palpitations, pupillary dilation, and shortness of breath [21, 22].
EOs are widely used in aromatherapy towards mild mood disorders, minimizing the symptoms of anxiety [23, 24]. In fact, several studies have shown that species of Citrus [25, 26] induce sedative and antidepressant effects in behavioral analysis. Bergamot EO (BEO) extracted from Citrus bergamia has been used to alleviate such symptoms [19].
Several clinical studies involving aromatherapy with BEO have shown promising results, such as stress reduction, anti-depression, pain relief, and blood pressure/heart rate reduction. In addition, BEO has minimal side effects, and it has been suggested that its inhalation may have potential therapeutic benefits [27]. In a study conducted by Saiyudthong and Marsden (2011), BEO was administered to rats previously subjected to anxiety-related situations, such as the high-labyrinth test, in which plasma corticosterone levels were measured [28]. The authors demonstrated that BEO at a concentration of 2.5% exhibited a similar anxiolytic effect as that of diazepam; in addition, it attenuated the corticosterone response to acute stress.
Although the mechanisms by which BEO induces its effects on the central nervous system have not yet been fully understood, it is suggested that they are mediated by the release of amino acids that interact with the mechanisms that modulate synaptic plasticity. According to a more recent study, BEO reduced anxiety behavior in rats through the stress response pathway by decreasing hypothalamic-pituitary-adrenocortical (HPA) activity [29]. The major secondary metabolites of BEO are limonene, linalyl acetate, and linalool. Limonene has been reported to exert sedative and anxiolytic effects in mice [30]. Another study showed that the subchronic treatment with BEO’s secondary metabolites increased the concentration of GABA in the rats’ brain, suggesting an anti-stress effect [31]. On the other hand, inhalation of linalool induced relaxation and reduced anxiety in mice [32]. In addition, linalool decreased human heart rate and exerted a sedative effect on autonomic nervous activity [33].
Similar to BEO, EOs from other plant species of the Citrus genus are used as sedatives, hypnotics, and tranquilizers, showing potential to be used as alternative therapy for anxiety disorders. Citrus aurantium L., whose main component is limonene, is popularly used to treat insomnia, nervousness, anxiety, and hysteria [34]. Carvalho-Freitas and Costa (2002) demonstrated that the EO of C. aurantium has a potential anxiolytic activity after a single administration in mice during elevated-plus labyrinth tests [35]. EOs from Citrus latifolia and Citrus reticulata also induced anxiolytic and sedative effects after oral administration in mice 30 min before each experimental procedure. They proved to be effective in increasing the duration of sleep induced by inhalation with ether [36].
In a recent study conducted by Faturi et al., an anxiolytic effect was observed for another Citrus species, sweet orange (Citrus sinensis) [37]. The study showed that the rats exposed to 200 and 400 μl of the Citrus sinensis EO showed increased exploration of the open arms of the elevated plus-maze and the lit chamber of the light/dark paradigm. Park et al. found that limonene binds directly to adenosine A2A and may induce sedative effects [38]. These results suggest that limonene may act as a linker and an agonist for A2A adenosine receptors.
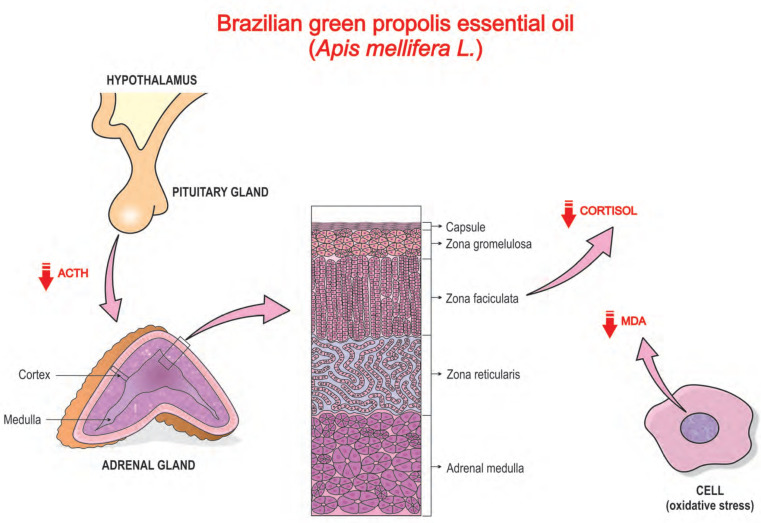
Another EO worth mentioning is the one extracted from propolis (PEO) due to its broad spectrum of biological properties, such as antioxidant, antimicrobial, anti-inflammatory, and anticancer activities [39]. Similar to most of the EOs covered in this review, PEO has terpenoids as its main secondary metabolites. Recent studies have pointed out that PEO has neuroprotective effects on retinal ganglion cells, neuronal, pheochromocytoma cells (PC12), and human neuroblastoma cells (SH-SY5Y) [40]. The anxiolytic effect of PEO was unknown until the pioneering study of Li et al. who, by using restriction-stress as an experimental model, observed that PEO at a dose of 100 mg/kg had therapeutic effects on anxiety, whose activity seemed to be attributed to the inhibition of HPA axis hyperactivity in mice [13] (Fig. 2). These authors also showed that treatment with PEO had a significant attenuation of lipid peroxidation and partial restoration of SOD activity in the mice’s brain tissue.
Fig. (2).
Anxiolytic and antioxidant mechanisms of Brazilian Green propolis essential oil. Propolis EO (PEO) has shown therapeutic effects on anxiety through the antagonism of the hypothalamic-pituitary-adrenal (HPA) axis and by enhancing the activity of antioxidant enzymes in the brain. In addition, it significantly decreased plasma cortisol levels (CORT) secreted by cells from the fasciculate zone. PEO decreased the levels of adrenocorticotropic hormone (ACTH) and malondialdehyde (MDA), while increasing the activity of the antioxidant enzyme superoxide dismutase (SOD), therefore, avoiding oxidative stress in brain tissue. (A higher resolution/colour version of this figure is available in the electronic copy of the article).
4.2. Dementia
Dementia is a clinical syndrome of progressive nature that usually leads to disorders of upper cortical functions, including memory, thinking, orientation, comprehension, calculus, learning ability, abstraction, language, and judgment. Cognitive deficits include memory impairment, aphasia, apraxia, agnosia, and executive functioning disorders; all of these severely impact the daily life of the patients, especially the elderly population [41].
Oxidative stress, an important damaging factor during aging and pathological conditions, is involved in the onset and progression of various neurodegenerative disorders [42,43,140,141]. In addition, evidence suggests that the activation of caspases and the onset of apoptosis have an important function in the pathophysiology of AD [44]. In this way, EO has been identified as a promising approach in the prevention of neurological disorders, enabling benefits to cognitive health [5].
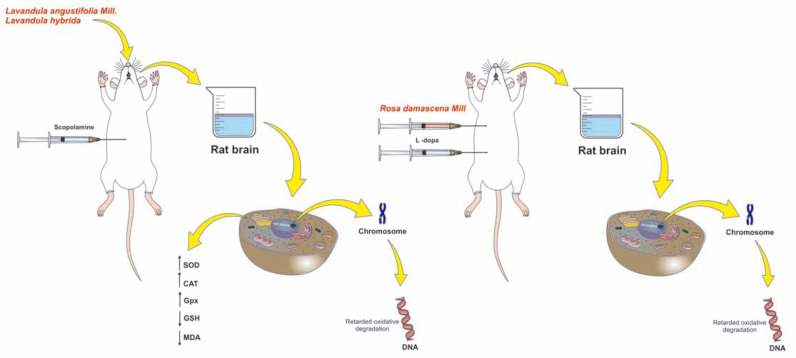
Lavender EO has shown to be active in the treatment of inflammation, depression, stress, and headache. The antioxidant and antiapoptotic activities of the species Lavandula angustifolia ssp., angustifolia Mill., and Lavandula hybrida Rev have been investigated in rats using the scopolamine-induced dementia model. The results indicated that after 7 days of continuous subacute exposure to lavender EO, a significant increase in the activity of antioxidant enzymes (superoxide dismutase, glutathione peroxidase, and catalase), a marked reduction in the total glutathione content, and reduced lipid peroxidation (malondialdehyde level) were observed, suggesting a potential antioxidant activity. The DNA cleavage patterns were absent in the lavender-treated groups, indicating an antiapoptotic activity. Thus, the authors suggest that the antioxidant and antiapoptotic activities of lavender EO are the main mechanisms involved in their neuroprotective effect against scopolamine-induced oxidative stress in rats [14]. The main mechanisms of action of both EOs are shown in Fig. (3).
Fig. (3).
Mechanisms of action proposed for Lavandula angustifolia Mill EOs Lavandula hybrida Rev. and Rosa Damascena Mill. and in in vivo models for neurological disorders. The study was conducted through an in vivo model of dementia induced by scopolamine. Treatment with EOs of Lavandula angustifolia Mill. and Lavandula hybrida significantly increased the antioxidant activities of enzymes SOD, GPX and CAT and reduced total GSH content while decreasing MDA levels. In addition, these EOs showed activity on DNA fragmentation, thus the cleavage patterns were absent in the treated groups suggesting an antiapoptotic effect. The EO of Rosa damascene Mill. demonstrates a similar effect because it acts to prevent important oxidative damage caused by intraperitoneal injection with high doses of L-dopa, thus demonstrating the antioxidant effect that is possibly mentioned as preventing damage to DNA. (A higher resolution/colour version of this figure is available in the electronic copy of the article).
Other studies with EOs corroborate these findings. Hritcu et al. evaluated the effect of lavender oil inhalation on scopolamine-induced spatial memory impairment in rats [45]. The EOs of Lavandula angustifolia ssp., angustifolia Mill., and Lavandula hybrida Rer. were also evaluated after continuous 7-day exposure to rats submitted to the scopolamine-induced dementia model (0.7 mg/kg). The results showed a significant reduction in anxiety-like behavior and an inhibition of depression in elevated plus-maze and forced swimming tests, suggesting both anxiolytic and antidepressant effects. The spatial memory performance in the Y-maze and radial arm-maze was improved, suggesting positive effects on memory formation. The authors conclude that chronic exposure to lavender EOs can effectively reverse spatial memory deficits induced by cholinergic system dysfunction in the rat’s brain, therefore, providing an opportunity for the management of neurological abnormalities in dementia conditions [45].
Lavender EO has protective properties for the nervous system, as it has been shown to be effective in controlling neurological disorders such as depression, cerebral ischemia, and depression [46,47]. Experimental models of AD treated with lavender EO showed its neuroprotective effect and improved cognition, whose mechanism of action was attributed to its antioxidant function [14,45]. Linalool, the main component of lavender EO (40%) and the secondary metabolite responsible for its therapeutic properties, has shown to have antioxidant, anti-inflammatory, antitumor, antidepressant, and antimicrobial activities [48,49]. In addition, its antioxidant properties have been shown to protect neurons from neurotoxicity, and neuropathological and behavioral deficits in preclinical studies [50].
Xu et al. investigated the effects and related mechanisms of lavender EO on cognitive deficits in mice induced by aluminum trichloride and D-galactose using the Morris aquatic labyrinth and step-through tests [51]. The treatment with 100 mg/kg of Lavender EO demonstrated protection against the cognitive deficits analyzed, which can be explained by its antioxidant action, protecting against the decrease in the activity of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase and avoiding the increase of the action acetylcholinesterase and malondialdehyde content. It has been shown that lavender EO and its bioactive compound Linalool prevent oxidative stress, protecting the cholinergic function and the expression of proteins involved in synaptic plasticity, as well as the Nrf2/HO-1 pathway. Thus, it is proposed that linalool extracted from lavender consists of a complementary treatment to improve cognitive function, mainly in AD [51].
4.3. Oxidative Stress
Oxidative stress has been reported as a major contributor to neuronal cell death. It occurs when the balance between antioxidants and reactive oxygen species (ROS) is disrupted due to depletion of the antioxidants or excess of ROS [52]. The imbalance in this homeostasis leads to the production of ROS, such as hydrogen peroxide (H2O2), which is involved in most cellular oxidative stresses [2,53]. Numerous antioxidant compounds are present in plants and have a protective effect, as they have the ability to inhibit or attenuate oxidative degradation caused by ROS [18,54]. It has been shown that the EO of medicinal plants such as Salvia lavandulifolia has a neuroprotective action, preventing oxidative stress induced by H2O2 in PC12 cells [18]. This mechanism of action may be related to the ability of the EO of Salvia lavandulifolia to activate the Nrf2 factor, so the pre-treatment resulted in a reduction in lipid peroxidation, caspase-3 activity, and ROS levels, enabling viability and recovery of cell morphology.
Additional studies with other EOs corroborate these findings. Bak et al. evaluated the antioxidant activity of Panax ginseng EO (red ginseng), where pretreatment with this EO also reduced H2O2-mediated ROS formation [55]. The authors also measured the activity of SOD, GPx, and CAT enzymes, which are considered the first line of antioxidant defense against ROS generated during oxidative stress [56]. It was observed that the treatment with ginseng EO restored the activity of these enzymes. The authors also found that sesquiterpenes are the main secondary metabolites present in ginseng EO, which are strongly involved in the antioxidant activity of EOs from both P. ginseng and S. lavandulifolia.
It is well known that astrocytes, the most abundant type of glial cells in the brain, protect neurons from ROS and damages that would affect neuronal survival. Elmann et al. compared the ability of EOs to protect astrocytes from H2O2-induced death. They found that EO from Salvia fruticosa (SF) showed remarkable protective activity [57]. This effect was attributed to both R-humulene and R-pinene, which belonged to the class of terpenes and attenuated H2O2-induced cell death. Therefore, they show the potential to be used as an alternative treatment for neurodegenerative diseases. The components of the SF EO exerted their protective effect by interfering with signals and processes induced by H2O2, either directly or through receptor-mediated signaling.
Recently published systematic reviews have demonstrated the neuroprotective effect of phytotherapics, especially those with phenolic compounds, in Parkinson’s disease (PD) models. The extracts of Camellia sinenses, Cucuma longa, Astragalus membranaceus, and Withania somnifera were able to improve the cellular antioxidant defense system and therefore, avoid oxidative damages [58,139]. In addition, the neuroprotective and antioxidant activities of Mucuna pruriens have been demonstrated, which have been attributed to its phenolic compounds, especially L-DOPA [59,60].
Other natural compounds with antioxidant action can also be found in olive oil and a diet enriched with fish oil or coconut oil; their administration demonstrated neuroprotective action when evaluated in experimental models of rats submitted to these diets, followed by exposure to filtered air or ozone (0.8 ppm) for 4h/day for 2 days. The results show that the mitochondrial complex I enzyme activity was significantly decreased in the cerebellum, hypothalamus, and hippocampus by diets, and the complex II enzyme activity was significantly lower in the frontal cortex and cerebellum of rats maintained in all test diets [61].
Extra virgin olive oil contains phenols in its composition that represent an important part of the antioxidants present in the Mediterranean diet [62-64], especially hydroxytyrosol and tyrosol. The oleaginine and oleocantal secairidoides perform functions as neuroprotective, anti-inflammatory, and anticancer agents [64-66]. In addition, a powerful antioxidant molecule, Oleuropein, present in the olive leaf has also demonstrated antioxidant properties. Its action on neuronal stress induced by hydrogen peroxide in vitro in human glioblastoma cells (U87) pretreated with oleuropein EO was evaluated, acting on the regeneration of total antioxidant capacity and cell survival [67].
As mentioned, dietary factors influence neuronal function and synaptic plasticity. A phenolic compound present in olive oil, hydroxytyrosol (HTyr), had a neuroprotective effect. It was observed that HTyr treatment activates neurogenesis in the dentate gyrus of the adult, elderly and null Btg1 mice, increasing the survival of new neurons and decreasing apoptosis. The treatment also stimulates the proliferation of stem cells and progenitors and reduces the aging markers of lipofuscin and Iba1. Thus, the results show that treatment with HTyr may indicate a promising therapy, as it neutralizes the decline in neurogenesis during aging [68].
4.4. Cerebral Ischemia
Cerebral ischemia (CI) is one of the main causes of morbidity and mortality in society nowadays, as it affects millions of people and results in high health costs [69]. CI occurs with an interruption of regional cerebral blood flow, causing hypoxia and subsequently an insufficient supply of glucose, whose persistence can impair the development of brain functions. It is generally associated with the onset and progression of neurodegenerative diseases of cognitive impairment [70, 71]. The result is free radical-mediated toxicity, resulting in neurological deficit and neuronal death [72, 73].
Current treatments for reducing post-ischemic neuronal damage have limitations, so it is necessary to study complementary treatments [74]. Terpenoids constitute the largest and structurally the most diverse group of volatiles released by plants. These compounds are present in EO and have neurological properties such as antidepressant, antinociceptive, and sedative [75, 76].
Linalool consists of a monoterpene present in the lavender EO which has therapeutic properties and acts on nervous disorders [77, 78]. Pharmacological studies have demonstrated that linalool has a broad spectrum of action in experimental models of epilepsy in mice, such as protection against convulsions induced by pentilenotetrazole, picrotoxin, and electroshock [79]. Linalool also acts as a competitive antagonist of NMDA receptors involved in the loss of memory [15].
A recent study reported the neuroprotective activity of lavender oil in transient focal cerebral ischemia in mice [20]. Lavender oil attenuated MCAO-induced cerebral edema in rats. These results provided new evidence of the neuroprotective potential of lavender oil in a model of focal cerebral ischemia in rats.
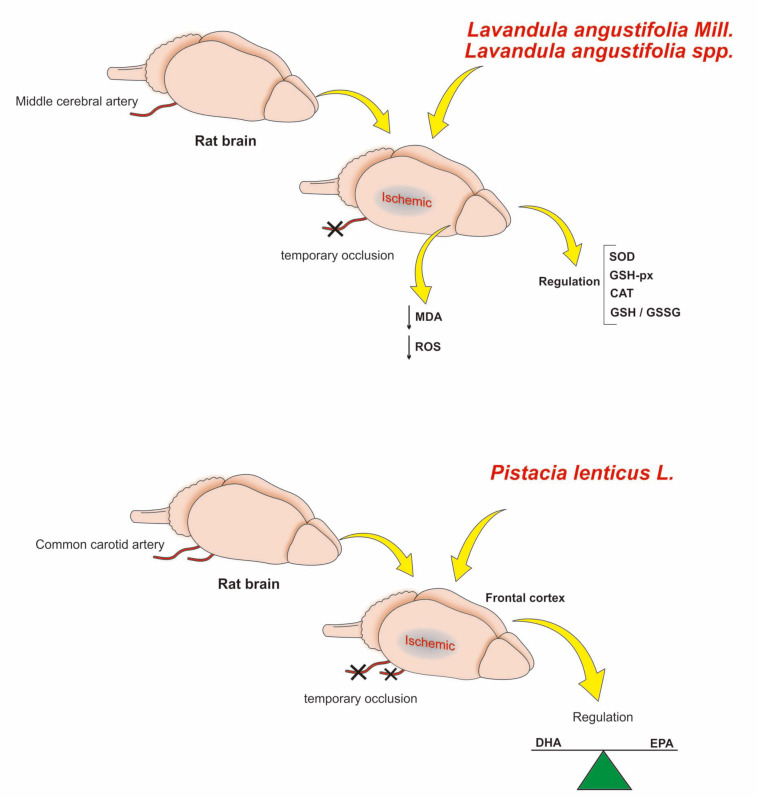
Lavandula angustifolia also had a neuroprotective effect on focal cerebral ischemia in experimental models with Wistar rats, mainly the groups treated with doses of 200 and 400 mg/kg of EO. After treatment, it was observed that the functional performance improved after ischemia, reduction of the area of infarction, and cerebral edema [20]. In addition, it enhanced the total antioxidant defense system [20]. Neuroprotective mechanisms of both Lavandula and Pistacia are depicted in Fig. (4).
Fig. (4).
Main mechanisms of action of Lavandula angustifolia and Pistacia lenticus L. in experimental models of focal cerebral ischemia. The mechanisms of action of Lavandula angustifolia were investigated using an in vivo experimental model of focal cerebral ischemia induced by transient occlusion of the middle cerebral artery. The Lavandula angustifolia oil at 200 and 400 mg/kg significantly decreased both infarct size and cerebral edema. In addition, it reduced the levels of MDA and ROS, as well as increased the activities of SOD and GSH-px and the total antioxidant capacity. In an experimental model of cerebral ischemia induced by occlusion of the common carotid artery followed by reperfusion (BCCAO/R), it was observed that such occlusion triggered a decrease of docosahexaenoic acid (DHA) in the frontal cortex. However, the experimental groups treated with Pistacia lenticus L. showed an increase in the ratio of DHA to its precursor eicosapentaenoic acid (EPA). Moreover, it increased the levels of palmitoylethanololamide (PEA) and oleoylethanolamine (OEA) in the brain, decreasing its susceptibility to oxidation. (A higher resolution/colour version of this figure is available in the electronic copy of the article).
Another EO present in the leaves of Pistacia lentiscus L. showed a relatively high concentration of terpenes and sesquiterpenes [17]. Chang et al. demonstrated that β-caryophyllene, another natural bicyclic sesquiterpene, was able to inhibit cortical neuronal damage when administered prior to the initial injury in rat reperfusion models [80]. The authors also suggested that the neuroprotection induced by this sesquiterpenoid may be partially related to the modulation of inflammatory mediators. Thus, treatment with β-caryophyllene decreased reperfusion-induced ischemia, cortical cell death, production and expression of inflammatory mediators (NO and PGE2/COX-2) in vitro, and cortical infarction and neurological deficit in vivo.
Chang et al., working with several terpenoids in an in vitro simulated ischemia model using human neuroblastoma SH-SY5Y cell line, verified that the sesquiterpenoid transcariophilene was the most potent neuroprotective compound [81]. In addition, it proved to be even more potent than MK 801 (positive control).
Partenolide (PN), a sesquiterpene lactone found in EOs extracted from Indian plants, has demonstrated a wide range of biological activities, including anti-inflammatory, anti-apoptosis, and anti-oxidation. PN is a secondary metabolite of a lipophilic character that favors a good permeability through the blood-brain barrier (BBB) [82]. Dong et al., using a classic model of cerebral ischemia induced by transient occlusion of the middle cerebral artery (MCAO), demonstrated that the systemic administration of this sesquiterpene relieved the ischemic brain injury due to a marked reduction of the inflammatory response, which occurred through the negative regulation of caspase-1, phospho-p38MAPK, and NF-κB, therefore, improving the blood-brain barrier through the positive regulation of claudin-5 [83, 84]. This regulation had a positive aspect in the study as caspases are known to mediate cell death and inflammation. Thus, it is expected that the blockage of caspase-1 might, in fact, reduce the inflammatory responses to focal ischemia. In addition, the improvement of the blood-brain barrier strength proved to be important since its rupture after cerebral ischemia is considered the initial step in the development of brain lesions.
EO of cinnamon powder called trans-cinnamaldehyde (TCA) also showed neuroprotective action in an animal model of brain injury induced by ischemia/reperfusion (I/R), and the neuroprotective mechanism was verified in LPS-induced inflammation of microglia cells BV-2. The findings of this study demonstrated that TCA at concentrations of 10-30 mg/kg significantly reduced the area of infarction and the neurological deficit score. In addition, it inhibited NO production induced by LPS of 0.5 µg/ml without affecting cell viability. It improved brain damage through inhibition of neuroinflammation, attenuation of iNOS, expression of COX-2, and NFκ-B signaling pathway [85].
Other compounds have also been investigated that showed neuroprotective activity, such as resveratrol, a phytochemical that can be found naturally in red wine, grapes, berries, chocolate, and peanuts [86]. This polyphenol also demonstrated positive action in an experimental model of cerebral ischemia. In cerebral ischemia, there is an increase in oxidative stress due to the overproduction of ROS, and it was investigated whether the neuroprotective effect of resveratrol was related to its modulation of the concentrations of metallic lead and trace elements [87]. To carry out the experiments, rats received resveratrol (20 mg/kg) once a day for 10 consecutive days. Cerebral ischemia was induced by ligating the right middle cerebral artery and the right common carotid artery for 1 h. Then, the cerebral cortex tissues were homogenized, and the supernatants were collected for biochemical analysis [87]. It was observed that rats pretreated with resveratrol before ischemia had significantly higher concentrations of trace elements of Mg, Zn, and Se and greater antioxidant activity in the cerebral cortex when compared to untreated rats, in addition to the pretreatment having attenuated lipid peroxidation and concentrations of toxic metal, thus demonstrating that the mechanisms underlying the neuroprotective effect of resveratrol involve the modulation of brain levels of trace elements, antioxidant activity, toxic metallic lead, and lipid peroxidation [87]. Resveratrol has also been shown to mitigate neuronal cell death, inflammation, and oxidative stress [88]. In addition to being indicated as a potential alternative for neuroinflammatory diseases, based on in vitro and in vivo studies, it is also revealed as an important compound with neuroprotective action [89].
A recently published review also demonstrated the neuroprotective effect of an EO extracted from black cumin seeds (Nigella sativa), and its main active component, thymoquinone, as a neuroprotective potential in acute and chronic forms of brain pathology, including neuroprotective action demonstrated in ischemia models/cerebral reperfusion, PD, AD and traumatic brain injury [90].
4.5. Alzheimer’s Disease
As previously reported, the antioxidants present in some medicinal plants work by inhibiting or attenuating oxidative stress, as these compounds are capable of combating free radicals. Some flavonoids and other phenolic compounds are included among these natural antioxidants. A recently published systematic review showed that curcumin acts through several neuroprotective mechanisms that ultimately prevent the neurotoxic and behavioral damages in AD models [91]. Curcumin acts on the antioxidant defense system, avoiding the formation of senile plaques due to its anti-amyloidogenic activity, significantly attenuating the formation of neurofibrillary tangles of TAU protein [92].
The EO of medicinal plants such as Aloysia citrodora provided partial and complete protection against oxidative stress in neuroblastoma cells, in an experimental model with AD after H2O2 induction and β-amyloid-induced neurotoxicity. The study demonstrated that the induction of toxicity with 250 μm H2O2 did not result in a neurotoxic effect in the treatment group with 0.01 and 0.001 mg/mL of A. citrodora EO, as it showed neuroprotective action in both concentrations [6].
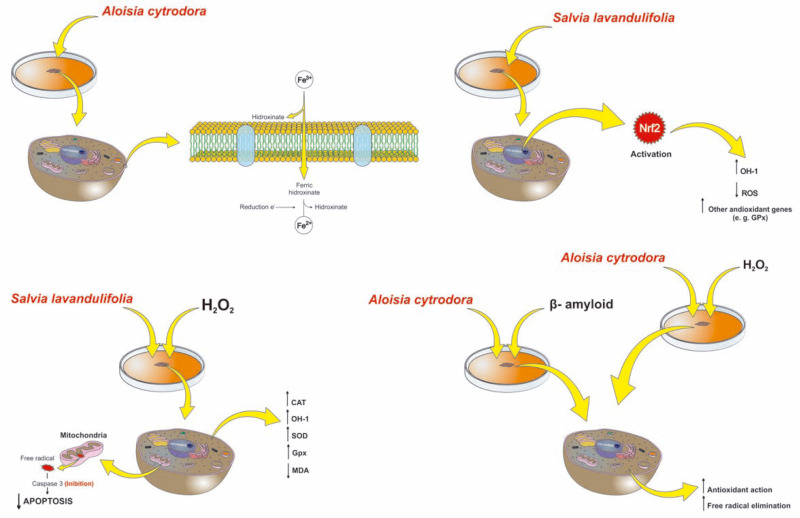
Evidence indicates that the neuroprotective activity shown by A. citrodora may be related to its chelating action, since one of the antioxidant mechanisms is the chelation of transition metals, preventing the catalysis of H2O2 decomposition through the Fenton reaction [93]. Primordial mechanisms proposed for the action of EO of A. cytrodora and Salvia lavandulifolia in models of neurological disorders in vitro are described in Fig. (5).
Fig. (5).
Mechanisms of action proposed for the EOs of Aloysia cytrodora and Salvia lavandulifolia on in vitro models for neurological disorders. Subtitle. Aloisia cytrodora presented important mechanisms of action in an in vitro model for Alzheimer's disease induced by H2O2 and β-amyloid. Its EO exhibited antioxidant and radical scavenging activities in additional to significant protective properties when compared to H2O2 and β-amyloid-induced neurotoxicity. It shows an iron chelation activity in vitro through hydroximation of Fe3+ to Fe2+, which is an important mechanism as the transition metal ions contribute to the oxidative damage involved in neurodegenerative disorders. Therefore, the chelation of transition metals avoids decomposition catalysis of H2O2 via the Fenton type reaction. Conversely, Salvia lavandulifolia presented protective mechanisms even after induction of H2O2 toxicity. Salvia lavandulifolia is able to activate the transcription factor Nrf2 (regulator of antioxidant genes) as the protein expression and the activity of CAT, HO-1, SOD and GPX are reduced, which correlates with the decrease in the levels of Nrf2. Thus, treatment with S. lavandulifolia regulated Nrf2 and concomitantly increased HO-1, which results in decreased ROS formation and increased cell viability when compared with the group exposed to H2O2 without S. lavandulifolia essential oil. In addition, it reduced the levels malondialdehyde (MDA) induced by H2O2 and avoided mitochondrial damage due to its inhibition effect over caspase 3 (effector of apoptosis). (A higher resolution/colour version of this figure is available in the electronic copy of the article).
One of the mechanisms that may be involved in the antioxidant activity of EO is its ability to chelate iron II, because as reported in the literature, chelating agents act as secondary antioxidants, reducing the redox potential of transition metals by regulating the metallic ion in its form oxidized [94].
In addition, the effect of A. citrodora EO on two major binding sites at GABAA receptor was investigated in order to detect its ability to modulate this receptor. The results demonstrated that A. citrodora EO inhibited the [3H] nicotine binding, which contradicts the results for the European lemon balm oil where no significant effect on [3H] nicotine binding activity was detected (up to 0.1 mg/mL). However, this EO showed a significant inhibitory effect on [35 S] TBPS (Tert-Butylbicyclophosphorothionate) binding to the GABAA receptor channel [95]. These findings helped to understand that nicotinic acetylcholine receptors represent recent therapeutic targets for the treatment of pathologies associated with the Central Nervous System.
Other EO that had a neuroprotective effect and may be natural agents of therapeutic interest against Aβ 1-42-induced neurotoxicity were the lavender and coriander
EOs, and their main active constituent is linalool. Lavender and coriander EOs at a concentration of 10 μg/mL, as well as linalool at the same concentration, acted to improve the viability and reduce nuclear morphological abnormalities in cells treated with Aβ 1-42 oligomers for 24 hours. In addition, EOS and linalool have also been shown to neutralize the increased production of intracellular ROS and the activation of the pro-apoptotic enzyme caspase-3. Together, these findings indicated possible protective agents [96].
The EO of Zataria multiflora (ZMEO) has been shown to act against memory impairment in a rat model of AD. For this, four groups were treated as follows: Negative Control (NC): no treatment, Simulated Control (sham): distilled water by intracerebroventricular injection (ICV), AD control performed with Aβ 1-42 by ICV injection, and the ZMEO group established with Aβ 1-42 by ICV injection and ZMEO at 100 μL/kg orally for 20 days [97].
The results of this study demonstrated that ZMEO has a protective effect against memory impairment in rats with AD, at least in part, by reducing the activity of AchE in the hippocampus and increasing BDNF levels without altering antioxidant status. Thus, the authors indicate that these findings may pave the way for future studies on the usefulness of ZMEO in preventing AD [97].
As mentioned previously, a substance indicated with neuroprotective and antioxidant capacity was resveratrol, also presenting great prospects as a therapeutic candidate for the treatment of AD [86]. This polyphenol demonstrated neuroprotective activity in several in vivo and in vitro models of AD, since its effects include its antioxidant and anti-inflammatory function and non-amyoloidogenic degradation of amyloid precursor protein (APP) acting in the removal of toxic amyloid beta peptides, a mechanism that prevents and slows pathology [86]. However, as indicated in the study, it is unlikely to be effective as monotherapy in AD due to its low bioavailability, biotransformation, and necessary synergism with other dietary factors [86].
Resveratrol crosses the blood-brain barrier and acts to increase anti-oxidant enzymes and is also involved in Sirtuin-mediated life extension activity (SIRT1), thus involved in aging. Thus, resveratrol reduced glial activation and helped to increase hippocampal neurogenesis, decreasing the expression of amyloid precursor protein. It is associated with the improvement of spatial working memory. Despite all these beneficial mechanisms, the study also highlights that resveratrol alone may not be an effective therapy when compared to resveratrol coupled with other compounds [98].
The neuroprotective effect of resveratrol was also observed in a mouse model with diabetes associated with AD. Two pathologies that share pathophysiological mechanisms, such as inflammatory pathways, apoptosis and oxidative stress, demonstrate that the protective mechanisms of resveratrol act on these mechanisms. Therefore, it was demonstrated that resveratrol may have a neuroprotective action by activating Sirt1 signaling in diabetes and AD with concomitant onset [99].
It is worth noting that even with EOs and chemical compounds with beneficial action in experimental models of AD, there are no effective human studies available for AD and dementia to date since the search for drugs for treatment has not resulted in effective therapies [100, 101]. In light of this, in order to obtain satisfactory results in therapies aimed at AD, it is essential to establish critical goals for the disease process, conduct rigorous tests, and develop effective agents [100].
4.6. Oxidative Toxicity
Oxidative stress is one of the main contributors to toxicity towards brain cells [102]. Therefore, substances like Levodopa which has been considered as the gold standard for treating PD, have been questioned in some in vitro studies due to the toxicity for dopaminergic neurons [103]. This finding usually results from the prolonged use of L-dopa, which has been associated with motor complications that end up obscuring its clinical efficacy. Thus, it seems reasonable to assume that the use of natural antioxidants might retard the progression of PD or even avoid the side effects of L-dopa [104].
Cellular oxidative degradation has been hindered by EOs from Rosa damascena Mill and Lavandula angustifolia [16], whose antioxidant activity has been attributed to their high content of different terpenes. In addition, Nikolova et al. investigated the ability of EOs isolated from Lavandula angustifolia Mill. and Rosa damascena Mill. in combination with vitamin C and Trolox in reducing the oxidative stress induced by L-dopa in healthy rats [16]. The protective effect against oxidative toxicity of L-dopa were evaluated by measuring the levels of OS-MDA, carbonylated protein content (CPC), and NO radicals in the rats’ brain homogenate and blood, where the dose of 400 mg/kg of the EOs showed the best protective results. In addition, the results indicated that Rosa damascena EO showed the highest scavenging activity of 2, 2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) free radical.
It has been shown that natural terpenes present in Rosa damascena Mill and Lavandula angustifolia have protective effects in neurodegenerative diseases [16]. The neuroprotective effects of these substances are due to their ability to cross the blood-brain barrier and directly capture the chelated metal ions and ROS/RNS [105,106], as well as through their antioxidant effects on the brain [106].
Hancianu et al. investigated the neuroprotective effects of inhaled lavender oils on the toxicity induced by scopolamine [14]. The authors found that after subacute exposure (daily for 7 continuous days) to lavender oil, a significant increase in the activities of the antioxidant enzymes GPX and CAT and a reduction of lipid peroxidation (MDA level) were observed, which suggests a potential antioxidant activity.
A study on the action of EO of Selinum vaginatum (Edgew) investigated its neuroprotective effect after inducing oxidative damage caused by methylmercury (MeHg). The analysis showed that there was a significant inhibition of the levels of glutathione (GSH) and CAT activity and an increase in the levels of substances reactive to thiobarbituric acid (TBARS) in the sample treated with MeHg. These changes were prevented by co-incubating with EO extracted from Selinum vaginatum, consisting of an important defense mechanism against oxidative damage [107].
The EO of Eplingiella fruticosa also demonstrated a neuroprotective effect and was used in association with cyclodextrins, which are used to improve the pharmacological profile of the EO. EPL was obtained from leaves complexed with β-cyclodextrin (EPL-βCD). The effects of EPL and EPL-βCD at a concentration of 5 mg/kg, for 40 days in male were evaluated since this EO has anti-inflammatory and antioxidant activities. EPL delayed the onset of catalepsy and decreased the levels of membrane lipid peroxides in the striatum. In addition, EPL-βCD also delayed the onset of catalepsy, reducing the frequency of oral dyskinesia, restoring memory deficit, producing anxiolytic activity and protecting against the depletion of dopaminergic depletion in the striatum and Substantia nigra pars compacta (SNpc). Taken together, such results indicate that this EPL has an important neuroprotective effect in an animal model of progressive PD, possibly because of its anti-inflammatory and antioxidant actions [108].
4.7. EOs Safety
EOs are generally safe with minimal adverse effects [109]. Several oils have been approved as food additives and fall into the category of generally recognized as safe by the Food and Drug Administration (FDA) [110]. The most common adverse effects reported are sensitization and irritation to the eyes, skin and mucous membranes, particularly oils containing aldehydes and phenols and EOs containing furocoumarins, such as Citrus bergamia [109, 111, 112]. In experimental models, there are some reports of genotoxic effect, and some examples such as a report proving the genotoxic effect on bone marrow in vivo of thymol and carvacrol [113].
Another study investigated the genotoxic and mutagenic potential of the EOs of Rosmarinus officinalis (rosemary) in rodents, using comet, micronucleus and chromosomal aberration assays. The animals received three dosages of rosemary oil (300, 1000 and 2000 mg/kg), with assessment of liver cells and peripheral blood collected 24 hours after treatment for testing. All doses of rosemary oil evaluated induced significant increases in DNA damage in mouse cells and there was also a significant increase in micronucleated cells and chromosomal aberrations at the two highest doses tested [114].
The genotoxicity of lavender oil, linalyl acetate, and linalool was investigated in human lymphocytes in vitro by the micronucleus test. In the range of non-toxic concentrations (0.5-100 μg/ml), linalyl acetate significantly increased the frequency of micronuclei and in a concentration-dependent manner; lavender oil did this only at the highest concentration tested, while linalool was free of genotoxicity. The study suggests that the mutagenic activity of lavender oil may be related to the presence of linalyl acetate, which appears to have an aneugenic agent profile [115]. These studies show that even with countless beneficial effects, a careful assessment of the safety of compounds and EOs is important.
4.8. Action of Neurotrophins and their Connections with Polyphenol Supplementation
Neurotrophins are proteins that are involved in the proper functioning and development of neurons, acting in the prevention of programmed cell death and thus allowing the survival of neurons [115, 116, 142], besides acting in neurogenesis processes [117, 118]. Nerve Growth Factor (NGF) and Brain-Derived Neurotrophic Factor (BDNF) are two well-studied neurotrophic factors [119, 120], playing important roles in the growth, development and survival of brain cells, also acting in behavioral processes associated with stress, as well as in learning and memory [116, 120-122].
Evidence indicates the role of BDNF in the pathophysiology of many neuropsychiatric, neurodegenerative disorders, depression, and anxiety. In addition, when present at low levels, it is associated with the etiology of Alzheimer’s and Huntington’s diseases [123]. A decrease in BDNF secretion leads to changes in anxiety-related memory and learning behavior [124, 125].
4.8.1. Olive Polyphenols
It was observed that the use of polyphenols can positively affect NGF and BDNF actions. One of the polyphenols that demonstrated positive action on NGF and BDNF was the polyphenol present in the olive extract [126]. The study was carried out with mice that were divided into two groups, animals administered with polyphenol and the control group. The polyphenol group received intraperitoneal injections for 10 consecutive days of a mixture of polyphenols extracted from the residues of the olive (bagasse) (10 mg/kg), obtained during the production of extra virgin olive oil in saline solution, and the control group received the same amount of saline solution in the same scheme of treatment. After 10 days, the growth factors NGF and BDNF were analyzed in the hippocampus, frontal cortex, striatum, and olfactory bulbs with enzyme immuno-absorption assay kits (ELISA), and behavioral tests were performed [126]. The results showed that olive polyphenols in mice could increase levels of NGF and BDNF in important areas of the limbic system and olfactory bulb, which play an essential role in the processes of memory and learning and in the proliferation and migration of endogenous progenitor cells present in the rodent brain [126].
4.8.2. Resveratrol
Resveratrol, a natural polyphenol enriched in Polygonum cuspidatum, prevents impaired cognition induced by chronic unpredictable mild stress (CUMS) in rats, an important finding since depression is a neuropsychiatric disorder associated with impaired cognition and changes in the neuroendocrine system and brain proteins [127, 128]. The results indicated that CUMS (5 weeks) produced cognitive deficits in rats, as indicated by the Morris water maze (LAM) and new object recognition task. In addition, exposure to CUMS significantly increased serum corticosterone levels and decreased BDNF levels in the prefrontal cortex (CPF) and hippocampus. Resveratrol may be an effective therapeutic agent for cognitive disorders, as seen in the stress model and its neuroprotective effect was mediated in part by the normalization of serum corticosterone levels, which increased regulation of BDNF, pCREB, and pERK levels [127].
In addition, resveratrol attenuated the neurotoxicity induced by the sevoflurane anesthetic by the SIRT1-dependent regulation of BDNF expression in developing mice. Pretreatment of neonatal mice with resveratrol almost reversed the reduction in the expression of SIRT1 in the hippocampus, which increased the expression of BDNF in developing mice exposed to sevoflurane. In addition, changes in CREB and MeCP2 levels, which were considered to interact with BDNF promoter IV, were also rescued by resveratrol. In this way, resveratrol improved the cognitive performance of adult mice [129].
Resveratrol is reported as a therapeutic potential in neuropsychiatric diseases and the neurotrophin NGF and the endocannabinol system are involved in its mechanisms of action. After single and 4-week intraperitoneal (i.p.) once-daily injections and of resveratrol (40, 80, and 100 mg/kg), amitriptyline (2.5, 5 and 10 mg/kg) or clonazepam (10, 20, and 40 mg/kg) into male Wistar rats, the contents of eCB and NGF were quantified in the brain regions involved in the modulation of emotions by isotope dilution liquid chromatography/mass spectrometry and Bio-Rad protein assay, respectively. The study concluded that resveratrol, like the classic antidepressant amitriptyline, affects brain NGF and eCB signaling under the regulatory action of CB1 receptors [130].
4.8.3. Green Tea Polyphenols
The polyphenol epigallocatechin-3-gallate (EGCG) found in green tea also showed a beneficial effect against sevoflurane-induced neuronal apoptosis involving the regulation of CREB/BDNF/TrkB signaling pathways, both crucial for neurogenesis and synaptic plasticity [131]. Distinct groups of C57BL/6 mice were used, which received EGCG (25, 50 or 75 mg/kg body weight) from postnatal day 3 (P3) to P21 and were exposed to sevoflurane (3%; 6 h) in P7. EGCG significantly inhibited sevoflurane-induced neuroapoptosis as determined by staining with Fluoro-Jade B and terminal labeling with dUTP nick end deoxynucleotidyl transferase (TUNEL) [131]. The study indicates that EGCG was able to effectively inhibit sevoflurane-induced neurodegeneration and improve mouse learning and memory retention by activating the CREB/BDNF/TrkB-PI3K/Akt signaling [131].
EGCG is also an important polyphenol with significant effects on anxiety and depression. Another study showed that rats administered intraperitoneally (i.p.) with EGCG for 14 successive days after the single prolonged stress process (SPS). This procedure stimulated the cognitive deficit in the Morris water maze test and in the object recognition task, and this impairment was improved by EGCG (25 mg/kg, i.p.). The daily administration of EGCG significantly decreased the freezing response to contextual fear conditioning; in addition, it moderated the memory-related decreases in the alternation of the binding protein to the cAMP and BDNF response element in the hippocampus. Thus, the results suggested that EGCG alleviated SPS-stimulated learning and memory deficit by inhibiting the increase in neuroinflammation in the rat brain [132].
A recent study found that tea polyphenols (TP) attenuate staurosporine-induced cytotoxicity and apoptosis by modulating the BDNF-TrkB/Akt and Erk1/2 signaling axis in hippocampal neurons. In addition, TP has shown beneficial effects to protect neurons from exogenous insults, such as STS-induced neural cytotoxicity and cell death. The study pointed that the BDNF-TrkB and Akt signaling axis was essential for the TP-mediated neuroprotective effects [133].
Another study also reported the beneficial effects of green tea in improving cognitive impairment induced by isoflurane by modulating oxidative stress; in addition, polyphenols administered at 25mg/kg per day significantly attenuated cognitive dysfunction on day 3 after anesthesia with isoflurane and effectively mitigated isoflurane-induced SOD declines, as well as levels of p-CaMKII, p-CREB and BDNF [134].
4.8.4. Cocoa Polyphenols
Cocoa powder also has polyphenols that triggered neuroprotective and preventive effects in a model of human Alzheimer’s disease by activating the BDNF signaling pathway in both cells treated with Aβ plaque and cells treated with Aβ oligomers, resulting in the counter-reaction of neuritis dystrophy, in addition to confirming the antioxidant properties present [135], indicating the use of cocoa powder as a neurodegeneration preventive agent [135].
A recently published systematic review investigated the effects of cocoa and cocoa products on cognitive performance in young adults and the results of individual studies confirm that acute and chronic cocoa intake has a positive effect on various cognitive outcomes [136]. After acute consumption, these beneficial effects appear to be accompanied by an increase in cerebral blood flow or cerebral blood oxygenation. After chronic ingestion of cocoa flavonoids in young adults, better cognitive performance was found along with increased levels of neurotrophins. The study also supports the beneficial effect of cocoa flavonoids on cognitive function and neuroplasticity and indicates that such benefits are possible in early adulthood [136].
Polyphenols like catechins have also been shown to protect neurons against mitochondrial toxins and HIV proteins. A study carried out in a medium-yield assay for the screening of neuroprotective compounds using primary mixed neuronal cells and mitochondrial toxin 3-nitroprionic acid pointed out that, from the 256 compounds that showed varying degrees of neuroprotection, nine were related to epicatechin, a monomeric flavonoid found in cocoa and green tea leaves that easily crosses the blood-brain barrier. Therefore, catechin, epicatechin, and the related compound, epigallocatechin gallate (EGCG) were subsequently tested for their neuroprotective properties against HIV Tat and gp120 proteins, and compared with resveratrol [137]. The results showed that epicatechin and EGCG target BDNF and its pro-BDNF precursor signaling pathways, normalizing both Tat-mediated increases in pro-apoptotic pro-BDNF and concomitant Tat-mediated decreases in BDNF protein in neurons of the hippocampus. Besides, the study also emphasized that epicatechin may be the best therapeutic candidate for neurodegenerative diseases, involving neurocognitive disorders associated with HIV, where oxidative stress is an important mechanism [137].
Further, a review study also highlighted that a better understanding of the effects of polyphenols on neurotrophins and their receptors could generate interest for drug discovery and also for the potential food prevention of several neurological and cardiometabolic diseases [138-142].
CONCLUSION
The EOs investigated in this review, especially those that present terpenoids as major components, were able to markedly improve the natural antioxidant system and, therefore, reduced the neuronal losses which resulted in significant improvements in behavioral tests.
Limitations were faced during the bibliographic search due to the limited number of studies on the subject of this review. In addition, considerable research deficits that elucidated the mechanisms involved in the neuroprotective activity of the EOs were observed. Since neurological disorders compromise the quality of life of the affected individuals, the elucidation of the mechanisms of action of the EOs or their main secondary metabolites would increase the chances of success of such products on the treatment of neurological disorders. Besides the antioxidant activities of these essential oils, other advantages seem to be their fewer side effects and lower costs when compared to synthetic drugs. Therefore, additional studies are needed in order to clarify the neuroprotective effects of the EOs as well as their long-term safety in patients with neurological disorders.
SUMMARY POINTS
EOs from different species of medicinal plants have shown positive responses in neurological disorders such as anxiety, dementia, oxidative stress, cerebral ischemia, and oxidative toxicity.
The EOs extracted from bergamot attenuated anxiety-like behavior in rats.
Treatment with Lavandula angustifolia at doses of 200 and 400 mg/kg significantly reduced infarct size, cerebral edema, and improved functional performance after cerebral ischemia.
Cellular oxidative degradation has been hindered by EOs from Rosa damascena Mill and Lavandula angustifolia, whose antioxidant activity has been attributed to their high content of different terpenes.
Aloysia citrodora EO in the presence of 0.01 and 0.001 mg/mL provides complete and partial protection from oxidative stress in a H2O2-induced experimental model and β-amyloid-induced neurotoxicity using neuroblastoma cells.
ACKNOWLEDGEMENTS
This study was supported by the National Counsel of Technological and Scientific Development (CNPq) and the Coordination for Improvement of High Level Staff (CAPES), Brazil. LCBF, FIN, and MAMF were recipients of CNPq doctoral fellowships.
LIST OF ABBREVIATIONS
- ACTH
Adrenocorticotropic hormone
- AD
Alzheimer’s disease
- BBB
Blood brain barrier
- BCCAO
Bilateral common carotid artery occlusion
- BEO
Bergamot essential oil
- CAT
Catalase
- CORT
Cortistatin
- CPC
Carbonylated protein content
- DPPH
2,2-difenil-1-picril-hidrazil.
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- EcBs
Endocannabinoids
- EOs
Essential oils
- GABA
Gamma-AminoButyric Acid
- GPx
Glutathione peroxidase
- GR
Glutathione reductase
- GSH
Glutathione
- GSHPx
Glutathione peroxidase
- GSSH
Oxidized glutathione
- H2O2
Hydrogen peroxide
- HO-1
Heme-oxigenase
- HPA
Hypothalamic-pituitary-adrenocortical
- L-DOPA
Levodopa
- MCAO
Middle cerebral artery occlusion
- MDA
Malondialdehyde
- MK 801
Dizocilpine
- MNDA
Myeloid cell nuclear differentiation antigen
- MTT
3-4,5-Dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide
- NMDA
N-methyl-D-aspartate
- NO
Nitric oxide
- NRF2
Nuclear factor erythroid 2-related factor 2
- PC12
Cells pheochromocytoma cells
- PCC
Pheochromocytoma
- PD
Parkinson’s disease
- PEO
Propolis essential oil
- PGE2/COX-2
Prostaglandin E2/ Cyclooxygenase 2
- PN
Partenolide
- PTN
Pleiotrophin protein
- ROS
Reactive Oxygen Species
- Sf
Salvia fruticosa
- SH-SY5Y
Human neuroblastoma cells
- SOD
Superoxide dismutase
- TBPS
Tert-Butylbicyclophosphorothionate
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halliwell B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012;70(5):257–265. doi: 10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 3.WHO . Neurological disorders: Public health challenges. World Health Organization; 2006. [Google Scholar]
- 4.Shaw G. The economic burden of neurologic disease—$800 Billion annually in the US. Neurol. Today. 2017;17(12):1–14. doi: 10.1097/01.NT.0000521169.52982.7f. [DOI] [Google Scholar]
- 5.Ayaz M., Sadiq A., Junaid M., Ullah F., Subhan F., Ahmed J. Neuroprotective and anti-aging po-tentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 2017;9:168. doi: 10.3389/fnagi.2017.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abuhamdah S., Abuhamdah R., Howes M-J.R., Al-Olimat S., Ennaceur A., Chazot P.L. Pharmaco-logical and neuroprotective profile of an essential oil derived from leaves of Aloysia citrodora Palau. J. Pharm. Pharmacol. 2015;67(9):1306–1315. doi: 10.1111/jphp.12424. [DOI] [PubMed] [Google Scholar]
- 7.Misharina T.A., Polshkov A.N. Antioxidant properties of essential oils: Autoxidation of essential oils from laurel and fennel and effects of mixing with essential oil from coriander. Prikl. Biokhim. Mikrobiol. 2005;41(6):693–702. [PubMed] [Google Scholar]
- 8.Hassan W., Noreen H., Rehman S., Gul S., Amjad K.M., Paul K.J. Oxidative stress and antioxi-dant potential of one hundred medicinal plants. Curr. Top. Med. Chem. 2017;17(12):1336–1370. doi: 10.2174/1568026617666170102125648. [DOI] [PubMed] [Google Scholar]
- 9.Santos J.R., Gois A.M., Mendonça D.M., Freire M.A. Nutritional status, oxidative stress and de-mentia: The role of selenium in Alzheimer’s disease. Front. Aging Neurosci. 2014;6:206. doi: 10.3389/fnagi.2014.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D., Liberati A., Tetzlaff J., Altman D.G. 2009.
- 11.Downs S.H., Black N. The feasibility of creating a checklist for the assessment of the methodologi-cal quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ainge H., Thompson C., Ozanne S.E., Rooney K.B. A systematic review on animal models of ma-ternal high fat feeding and offspring glycaemic control. Intern. J. Obes. 2011;35(3):325–335. doi: 10.1038/ijo.2010.149. [DOI] [PubMed] [Google Scholar]
- 13.Li Y.J., Xuan H.Z., Shou Q.Y., Zhan Z.G., Lu X., Hu F.L. Therapeutic effects of propolis essen-tial oil on anxiety of restraint-stressed mice. Hum. Exp. Toxicol. 2012;31(2):157–165. doi: 10.1177/0960327111412805. [DOI] [PubMed] [Google Scholar]
- 14.Hancianu M., Cioanca O., Mihasan M., Hritcu L. Neuroprotective effects of inhaled lavender oil on scopolamine-induced dementia via anti-oxidative activities in rats. Phytomedicine. 2013;20(5):446–452. doi: 10.1016/j.phymed.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Wang D., Yuan X., Liu T., Liu L., Hu Y., Wang Z., Zheng Q. Neuroprotective activity of laven-der oil on transient focal cerebral ischemia in mice. Molecules. 2012;17(8):9803–9817. doi: 10.3390/molecules17089803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikolova G., Karamalakova Y., Kovacheva N., Stanev S., Zheleva A., Gadjeva V. Protective ef-fect of two essential oils isolated from Rosa damascena Mill. and Lavandula angustifolia Mill, and two classic antioxidants against L-dopa oxidative toxicity induced in healthy mice. Regul. Toxicol. Pharmacol. 2016;81:1–7. doi: 10.1016/j.yrtph.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Quartu M., Serra M.P., Boi M., Pillolla G., Melis T., Poddighe L., Del Fiacco M., Falconieri D., Carta G., Murru E., Cordeddu L., Piras A., Collu M., Banni S. Effect of acute administration of Pistacia lentiscus L. essential oil on rat cerebral cortex following transient bilateral common carotid artery occlusion. Lipids Health Dis. 2012;11(1):8. doi: 10.1186/1476-511X-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porres-Martínez M., González-Burgos E., Carretero M.E., Gómez-Serranillos M.P. Protective prop-erties of Salvia lavandulifolia Vahl. essential oil against oxidative stress-induced neuronal injury. Food Chem. Toxicol. 2015;80:154–162. doi: 10.1016/j.fct.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Rombolà L., Tridico L., Scuteri D., Sakurada T., Sakurada S., Mizoguchi H., Avato P., Corasa-niti M.T., Bagetta G., Morrone L.A. Bergamot essential oil attenuates anxiety-like behaviour in rats. Molecules. 2017;22(4):614. doi: 10.3390/molecules22040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vakili A., Sharifat S., Akhavan M.M., Bandegi A.R. Effect of lavender oil (Lavandula angustifolia) on cerebral edema and its possible mechanisms in an experimental model of stroke. Brain Res. 2014;1548:56–62. doi: 10.1016/j.brainres.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Borkovec T.D., Ruscio A.M. 2001.
- 22.O’Connor P.J., Raglin J.S., Martinsen E.W. Physical activity, anxiety and anxiety disorders. Int. J. Sport Psychol. 2000;31(2):136–155. [Google Scholar]
- 23.Jafarzadeh M., Arman S., Pour F.F. Effect of aromatherapy with orange essential oil on salivary cor-tisol and pulse rate in children during dental treatment: A randomized controlled clinical trial. Adv. Biomed. Res. 2013;2:10. doi: 10.4103/2277-9175.107968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasper S., Volz H-P., Dienel A., Schläfke S. Efficacy of Silexan in mixed anxiety-depression--A randomized, placebo-controlled trial. Eur. Neuropsychopharmacol. 2016;26(2):331–340. doi: 10.1016/j.euroneuro.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Komiya M., Takeuchi T., Harada E. Lemon oil vapor causes an anti-stress effect via modulating the 5-HT and DA activities in mice. Behav. Brain Res. 2006;172(2):240–249. doi: 10.1016/j.bbr.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Pultrini A. M.; Galindo, L.A.; Costa, M. Effects of the essential oil from Citrus aurantium L. in ex-perimental anxiety models in mice. Life Sci. 2006;78(15):1720–1725. doi: 10.1016/j.lfs.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Navarra M., Mannucci C., Delbò M., Calapai G. Citrus bergamia essential oil: From basic research to clinical application. Front. Pharmacol. 2015;6:36. doi: 10.3389/fphar.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saiyudthong S., Marsden C.A. Acute effects of bergamot oil on anxiety-related behaviour and corti-costerone level in rats. Phytother. Res. 2011;25(6):858–862. doi: 10.1002/ptr.3325. [DOI] [PubMed] [Google Scholar]
- 29.Peng S-M., Koo M., Yu Z-R. Effects of music and essential oil inhalation on cardiac autonomic bal-ance in healthy individuals. J. Altern. Complement. Med. 2009;15(1):53–57. doi: 10.1089/acm.2008.0243. [DOI] [PubMed] [Google Scholar]
- 30.de Almeida A.A.C., Costa J.P., de Carvalho R.B.F., de Sousa D.P., de Freitas R.M. Evaluation of acute toxicity of a natural compound (+)-limonene epoxide and its anxiolytic-like action. Brain Res. 2012;1448:56–62. doi: 10.1016/j.brainres.2012.01.070. [DOI] [PubMed] [Google Scholar]
- 31.Zhou W., Yoshioka M., Yokogoshi H. Sub-chronic effects of s-limonene on brain neurotransmitter levels and behavior of rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2009;55(4):367–373. doi: 10.3177/jnsv.55.367. [DOI] [PubMed] [Google Scholar]
- 32.Linck V.M., da Silva A.L., Figueiró M., Caramão E.B., Moreno P.R.H., Elisabetsky E. Effects of inhaled linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine. 2010;17(8-9):679–683. doi: 10.1016/j.phymed.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Kuroda K., Inoue N., Ito Y., Kubota K., Sugimoto A., Kakuda T. Sedative effects of the jasmine tea odor and (R)-(-)-linalool, one of its major odor components, on autonomic nerve activity and mood states. Eur. J. Appl. Physiol. 2005;95(2–3):107–114. doi: 10.1007/s00421-005-1402-8. [DOI] [PubMed] [Google Scholar]
- 34.Lehrner J., Eckersberger C., Walla P., Pötsch G., Deecke L. Ambient odor of orange in a dental office reduces anxiety and improves mood in female patients. Physiol. Behav. 2000;71(1-2):83–86. doi: 10.1016/S0031-9384(00)00308-5. [DOI] [PubMed] [Google Scholar]
- 35.Carvalho-Freitas M.I.R., Costa M. Anxiolytic and sedative effects of extracts and essential oil from Citrus aurantium L. Biol. Pharm. Bull. 2002;25(12):1629–1633. doi: 10.1248/bpb.25.1629. [DOI] [PubMed] [Google Scholar]
- 36.Gargano A.C., Costa C., Costa M. Essential oils from citrus latifolia and citrus reticulate reduces anxiety and prolong ether sleeping time in mice. Tree of Sci. Biotech. 2008;2(Suppl. 1):121–124. [Google Scholar]
- 37.Faturi C.B., Leite J.R., Alves P.B., Canton A.C., Teixeira-Silva F. Anxiolytic-like effect of sweet orange aroma in Wistar rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(4):605–609. doi: 10.1016/j.pnpbp.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 38.Park H.M., Lee J.H., Yaoyao J., Jun H.J., Lee S.J. Limonene, a natural cyclic terpene, is an agonis-tic ligand for adenosine A(2A) receptors. Biochem. Biophys. Res. Commun. 2011;404(1):345–348. doi: 10.1016/j.bbrc.2010.11.121. [DOI] [PubMed] [Google Scholar]
- 39.Viuda-Martos M., Ruiz-Navajas Y., Fernández-López J., Pérez-Alvarez J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008;73(9):R117–R124. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 40.Nakajima Y., Shimazawa M., Mishima S., Hara H. Neuroprotective effects of Brazilian green propo-lis and its main constituents against oxygen-glucose deprivation Stress, with a gene-expression anal-ysis. Phyto Res. 2009;23(10):1431–1438. doi: 10.1002/ptr.2797. [DOI] [PubMed] [Google Scholar]
- 41.Association A.P., Association A.P. 2000. [Google Scholar]
- 42.Finkel T., Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 43.Freire M.A.M. Pathophysiology of neurodegeneration following traumatic brain injury. West Indian Med. J. 2012;61(7):751–755. [PubMed] [Google Scholar]
- 44.Mattson M.P. Contributions of mitochondrial alterations, resulting from bad genes and a hostile envi-ronment, to the pathogenesis of Alzheimer’s disease. Int. Rev. Neurobiol. 2002;53:387–409. doi: 10.1016/S0074-7742(02)53014-2. [DOI] [PubMed] [Google Scholar]
- 45.Hritcu L., Cioanca O., Hancianu M. Effects of lavender oil inhalation on improving scopolamine-induced spatial memory impairment in laboratory rats. Phytomedicine. 2012;19(6):529–534. doi: 10.1016/j.phymed.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Koulivand P.H., Khaleghi G.M., Gorji A. Lavender and the nervous system. Evid. Based Complement. Alternat. Med. 2013;2013:681304. doi: 10.1155/2013/681304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi M., Yoshino A., Yamanaka A., Asanuma C., Satou T., Hayashi S., Masuo Y., Sadamoto K., Koike K. Effects of inhaled lavender essential oil on stress-loaded animals: Changes in anxiety-related behavior and expression levels of selected mRNAs and proteins. Nat. Prod. Commun. 2012;7(11):1539–1544. doi: 10.1177/1934578X1200701132. [DOI] [PubMed] [Google Scholar]
- 48.Da Porto C., Decorti D. Analysis of the volatile compounds of flowers and essential oils from La-vandula angustifolia cultivated in Northeastern Italy by headspace solid-phase microextraction cou-pled to gas chromatography-mass spectrometry. Planta Med. 2008;74(02):182–187. doi: 10.1055/s-2008-1034295. [DOI] [PubMed] [Google Scholar]
- 49.Sabogal-Guáqueta A.M., Osorio E., Cardona-Gómez G.P. Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer’s mice. Neuropharmacology. 2016;102:111–120. doi: 10.1016/j.neuropharm.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu P., Wang K., Lu C., Dong L., Gao L., Yan M. The protective effect of lavender essential oil and its main component linalool against the cognitive deficits induced by D-galactose and aluminum trichloride in mice. Evid. Based Complement. Alternat. Med. 2017;2017:7426538. doi: 10.1155/2017/7426538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emerit J., Edeas M., Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother. 2004;58(1):39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Dasuri K., Zhang L., Keller J.N. Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free Radic. Biol. Med. 2013;62:170–185. doi: 10.1016/j.freeradbiomed.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 53.González-Burgos E., Gómez-Serranillos M.P. Terpene compounds in nature: A review of their poten-tial antioxidant activity. Curr. Med. Chem. 2012;19(31):5319–5341. doi: 10.2174/092986712803833335. [DOI] [PubMed] [Google Scholar]
- 54.Guerra-Araiza C., Álvarez-Mejía A.L., Sánchez-Torres S., Farfan-García E., Mondragón-Lozano R., Pinto-Almazán R., Salgado-Ceballos H. Effect of natural exogenous antioxidants on aging and on neurodegenerative diseases. Free Radic. Res. 2013;47(6-7):451–462. doi: 10.3109/10715762.2013.795649. [DOI] [PubMed] [Google Scholar]
- 55.Bak M-J., Jun M., Jeong W-S. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H(2)O(2)-treated hepG2 cells and CCl(4)-treated mice. Int. J. Mol. Sci. 2012;13(2):2314–2330. doi: 10.3390/ijms13022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ray G, Husain SA. Oxidants, antioxidants and carcinogenesis. 2002. [PubMed]
- 57.Elmann A., Mordechay S., Rindner M., Larkov O., Elkabetz M., Ravid U. Protective effects of the essential oil of Salvia fruticosa and its constituents on astrocytic susceptibility to hydrogen per-oxide-induced cell death. J. Agric. Food Chem. 2009;57(15):6636–6641. doi: 10.1021/jf901162f. [DOI] [PubMed] [Google Scholar]
- 58.da Costa I.M., Cavalcanti J.R.L.P., de Queiroz D.B., de Azevedo E.P., do Rêgo A.C.M., Araújo F.I., Parente P., Botelho M.A., Guzen F.P. Supplementation with herbal extracts to promote be-havioral and neuroprotective effects in experimental models of Parkinson’s disease: A systematic re-view. Phytother. Res. 2017;31(7):959–970. doi: 10.1002/ptr.5813. [DOI] [PubMed] [Google Scholar]
- 59.Neta F.I., Da Costa I.M., Lima F.O.V., Fernandes L.C.B., Cavalcanti J.R.L.D.P., Freire M.A.D.M. Effects of Mucuna pruriens (L.) supplementation on experimental models of Parkinson’s disease: A systematic review. Pharmacogn. Rev. 2018;12(23):78. doi: 10.4103/phrev.phrev_46_17. [DOI] [Google Scholar]
- 60.Small D.L., Buchan A.M. Mechanisms of cerebral ischemia: Intracellular cascades and therapeutic interventions. J. Cardiothorac. Vasc. Anesth. 1996;10(1):139–146. doi: 10.1016/S1053-0770(96)80189-3. [DOI] [PubMed] [Google Scholar]
- 61.Valdez M.C., Freeborn D., Valdez J.M., Johnstone A.F.M., Snow S.J., Tennant A.H., Kodavanti U.P., Kodavanti P.R.S. Mitochondrial bioenergetics in brain following ozone exposure in rats main-tained on coconut, fish and olive oil-rich diets. Int. J. Mol. Sci. 2019;20(24):6303. doi: 10.3390/ijms20246303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tresserra-Rimbau A., Lamuela-Raventos R.M. 22 Olives and olive oil: A Mediterranean source of polyphenols. Olives and olive oil as functional foods. Bioactive. Chem. Proces. 2017;417 doi: 10.1002/9781119135340.ch22. [DOI] [Google Scholar]
- 63.Ramírez-Anaya J.D.P., Castañeda-Saucedo M.C., Olalla-Herrera M., Villalón-Mir M., Serrana H.L., Samaniego-Sánchez C., Serrana H. L-G de la.; Samaniego-Sánchez, C. Changes in the antiox-idant properties of extra virgin olive oil after cooking typical mediterranean vegetables. Antioxidants. 2019;8(8):246. doi: 10.3390/antiox8080246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Francioso A., Federico R., Maggiore A., Fontana M., Boffi A., D’Erme M., Mosca L. Green route for the isolation and purification of hyrdoxytyrosol, tyrosol, oleacein and oleocanthal from ex-tra virgin olive oil. Molecules. 2020;25(16):3654. doi: 10.3390/molecules25163654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lozano-Castellón J., López-Yerena A. Rinaldi, de Alvarenga, J.F.; Romero, del Castillo-Alba, J.; Vallverdú-Queralt, A.; Escribano-Ferrer, E. Health-promoting properties of oleocanthal and oleacein: Two secoiridoids from extra-virgin olive oil. Crit. Rev. Food Sci. Nutr. 2020;60(15):2532–2548. doi: 10.1080/10408398.2019.1650715. [DOI] [PubMed] [Google Scholar]
- 66.Karković M.A.; Torić J.; Barbarić M.; Jakobušić B.C. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules. 2019;24(10):2001. doi: 10.3390/molecules24102001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kucukgul A., Isgor M.M., Duzguner V., Atabay M.N., Kucukgul A. Antioxidant effects of oleuropein on hydrogen peroxide-Induced neuronal stress-an in vitro study. Antiinflamm. Antiallergy Agents Med. Chem. 2020;19(1):74–84. doi: 10.2174/1871523018666190201145824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.D’Andrea G., Ceccarelli M., Bernini R., Clemente M., Santi L., Caruso C., Micheli L., Tirone F. Hydroxytyrosol stimulates neurogenesis in aged dentate gyrus by enhancing stem and progenitor cell proliferation and neuron survival. FASEB J. 2020;34(3):4512–4526. doi: 10.1096/fj.201902643R. [DOI] [PubMed] [Google Scholar]
- 69.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamber-lain A.M., Chang A.R., Cheng S., Das S.R., Delling F.N., Djousse L., Elkind M.S.V., Ferguson J.F., Fornage M., Jordan L.C., Khan S.S., Kissela B.M., Knutson K.L., Kwan T.W., Lackland D.T., Lewis T.T., Lichtman J.H., Longenecker C.T., Loop M.S., Lutsey P.L., Martin S.S., Matsushita K., Moran A.E., Mussolino M.E., O’Flaherty M., Pandey A., Perak A.M., Rosa-mond W.D., Roth G.A., Sampson U.K.A., Satou G.M., Schroeder E.B., Shah S.H., Spartano N.L., Stokes A., Tirschwell D.L., Tsao C.W., Turakhia M.P., VanWagner L.B., Wilkins J.T., Wong S.S., Virani S.S. Heart disease and stroke statistics-2019 update: A report from the Ameri-can Heart Association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 70.Lopes R.S., Cardoso M.M., Sampaio A.O., Barbosa M.S., Jr, Souza C.C., Silva D.A. M.C.; Fer-reira, E.M.; Freire, M.A.; Lima, R.R.; Gomes-Leal, W. Indomethacin treatment reduces microglia ac-tivation and increases numbers of neuroblasts in the subventricular zone and ischaemic striatum after focal ischaemia. J. Biosci. 2016;41(3):381–394. doi: 10.1007/s12038-016-9621-1. [DOI] [PubMed] [Google Scholar]
- 71.Liang S., Zhang J., Zhang Q., Li L., Zhang Y., Jin T., Zhang B., He X., Chen L., Tao J., Li Z., Liu W., Chen L. Longitudinal tracing of white matter integrity on diffusion tensor imaging in the chronic cerebral ischemia and acute cerebral ischemia. Brain Res. Bull. 2020;154:135–141. doi: 10.1016/j.brainresbull.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 72.Woodruff T.M., Thundyil J., Tang S-C., Sobey C.G., Taylor S.M., Arumugam T.V. Pathophysiol-ogy, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011;6(1):11. doi: 10.1186/1750-1326-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jivad N., Rabiei Z. Review on herbal medicine on brain ischemia and reperfusion. Asian Pac. J. Trop. Biomed. 2015;5(10):789–795. doi: 10.1016/j.apjtb.2015.07.015. [DOI] [Google Scholar]
- 74.Perazzo F.F., Lima L.M., Maistro E.L., Carvalho J.E., Rehder V.L. Carvalho, JC Effect of Artemisia annua L. leaves essential oil and ethanol extract on behavioral assays. Rev. Bras. Farmacogn. 2008;18:686–689. [Google Scholar]
- 75.Sharma E., Anand G., Kapoor R. Terpenoids in plant and arbuscular mycorrhiza-reinforced defence against herbivorous insects. Ann. Bot. 2017;119(5):791–801. doi: 10.1093/aob/mcw263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Batish D.R., Singh H.P., Setia N., Kaur S., Kohli R.K. Chemical composition and inhibitory activi-ty of essential oil from decaying leaves of Eucalyptus citriodora. Z. Natforsch. C J. Biosci. 2006;61(1-2):52–56. doi: 10.1515/znc-2006-1-210. [DOI] [PubMed] [Google Scholar]
- 77.Devi S.L., Kannappan S., Anuradha C.V. Evaluation of in vitro antioxidant activity of Indian bay leaf, Cinnamomum tamala (Buch. -Ham.) T. Nees & Eberm using rat brain synaptosomes as model system. Indian J. Exp. Biol. 2007;45(9):778–784. [PubMed] [Google Scholar]
- 78.Silva Brum L.F., Emanuelli T., Souza D.O., Elisabetsky E. Effects of linalool on glutamate release and uptake in mouse cortical synaptosomes. Neurochem. Res. 2001;26(3):191–194. doi: 10.1023/A:1010904214482. [DOI] [PubMed] [Google Scholar]
- 79.Coelho V.R., Gianesini J., Von B.R., Mazzardo-Martins L., Martins D.F., Picada J.N., Santos A.R., Brum L.F., Pereira P. (-)-Linalool, A naturally occurring monoterpene compound, impairs memory acquisition in the object recognition task, inhibitory avoidance test and habituation to a novel environment in rats. Phytomedicine. 2011;18(10):896–901. doi: 10.1016/j.phymed.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 80.Chang H-J., Kim J-M., Lee J-C., Kim W-K., Chun H.S. Protective effect of β-caryophyllene, a natural bicyclic sesquiterpene, against cerebral ischemic injury. J. Med. Food. 2013;16(6):471–480. doi: 10.1089/jmf.2012.2283. [DOI] [PubMed] [Google Scholar]
- 81.Chang H-J., Kim H.J., Chun H.S. Quantitative structure-activity relationship (QSAR) for neuropro-tective activity of terpenoids. Life Sci. 2007;80(9):835–841. doi: 10.1016/j.lfs.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 82.Pandey D.K., Rajkumar R., Mahesh R., Radha R. Depressant-like effects of parthenolide in a ro-dent behavioural antidepressant test battery. J. Pharm. Pharmacol. 2008;60(12):1643–1650. doi: 10.1211/jpp.60.12.0010. [DOI] [PubMed] [Google Scholar]
- 83.Hu M., Zhang X., Liu W., Cui H., Di N. Longitudinal changes of defensive and offensive factors in focal cerebral ischemia-reperfusion in rats. Brain Res. Bull. 2009;79(6):371–375. doi: 10.1016/j.brainresbull.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 84.Yang C., Zhang X., Fan H., Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expres-sion and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–141. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 85.Chen Y-F., Wang Y-W., Huang W-S., Lee M-M., Wood W.G., Leung Y-M., Tsai H.Y. Trans-cinnamaldehyde, an essential oil in cinnamon powder, ameliorates cerebral ischemia-induced brain injury via inhibition of neuroinflammation through attenuation of iNOS, COX-2 expression and NFκ-B signaling pathway. Neuromol.Med. 2016;18(3):322–333. doi: 10.1007/s12017-016-8395-9. [DOI] [PubMed] [Google Scholar]
- 86.Braidy N., Jugder B-E., Poljak A., Jayasena T., Mansour H., Nabavi S.M., Sachdev P., Grant R. Resveratrol as a potential therapeutic candidate for the treatment and management of Alzheimer’s disease. Curr. Top. Med. Chem. 2016;16(17):1951–1960. doi: 10.2174/1568026616666160204121431. [DOI] [PubMed] [Google Scholar]
- 87.Ro J-H., Liu C-C., Lin M-C. Resveratrol mitigates cerebral ischemic injury by altering levels of trace elements, toxic metal, lipid peroxidation, and antioxidant activity. Biol. Trace Elem. Res. 2020;199(10):3718–3727. doi: 10.1007/s12011-020-02497-x. [DOI] [PubMed] [Google Scholar]
- 88.Akyuva Y. Nazıroğlu, M. Resveratrol attenuates hypoxia-induced neuronal cell death, inflammation and mitochondrial oxidative stress by modulation of TRPM2 channel. Sci. Rep. 2020;10(1):6449. doi: 10.1038/s41598-020-63577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Assis P.M., Fávero A., Menegasso J.F., Meinel R.S., Marion G.M., Nunes V.S.P., Goliatt P.V.Z.C., da Silva A.D., Dutra R.C., Raposo N.R.B. In silico, in vitro and in vivo studies indicate resveratrol analogue as a potential alternative for neuroinflammatory disorders. Life Sci. 2020;249:117538. doi: 10.1016/j.lfs.2020.117538. [DOI] [PubMed] [Google Scholar]
- 90.Isaev N.K., Chetverikov N.S., Stelmashook E.V., Genrikhs E.E., Khaspekov L.G., Illarioshkin S.N. Thymoquinone as a potential neuroprotector in acute and chronic forms of cerebral pathology. Biochemistry (Mosc.) 2020;85(2):167–176. doi: 10.1134/S0006297920020042. [DOI] [PubMed] [Google Scholar]
- 91.da Costa I.M., Freire M.A.M., de Paiva Cavalcanti J.R.L., de Araújo D.P., Norrara B., Moreira Rosa I.M.M., de Azevedo E.P., do Rego A.C.M., Filho I.A., Guzen F.P. Supplementation with Curcuma longa reverses neurotoxic and behavioral damage in models of Alzheimer’s disease: A sys-tematic review. Curr. Neuropharmacol. 2019;17(5):406–421. doi: 10.2174/0929867325666180117112610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garcia-Alloza M., Borrelli L.A., Rozkalne A., Hyman B.T., Bacskai B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007;102(4):1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- 93.Gil A., Van Baren C.M., Di Leo Lira P.M., Bandoni A.L. Identification of the genotype from the content and composition of the essential oil of lemon verbena (Aloysia citriodora Palau). J. Agric. Food Chem. 2007;55(21):8664–8669. doi: 10.1021/jf0708387. [DOI] [PubMed] [Google Scholar]
- 94.Bush A.I. The metallobiology of Alzheimer’s disease. Trends Neurosci. 2003;26(4):207–214. doi: 10.1016/S0166-2236(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 95.Horai H., Arita M., Kanaya S., Nihei Y., Ikeda T., Suwa K., Ojima Y., Tanaka K., Tanaka S., Aoshima K., Oda Y., Kakazu Y., Kusano M., Tohge T., Matsuda F., Sawada Y., Hirai M.Y., Nakanishi H., Ikeda K., Akimoto N., Maoka T., Takahashi H., Ara T., Sakurai N., Suzuki H., Shibata D., Neumann S., Iida T., Tanaka K., Funatsu K., Matsuura F., Soga T., Taguchi R., Sai-to K., Nishioka T. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010;45(7):703–714. doi: 10.1002/jms.1777. [DOI] [PubMed] [Google Scholar]
- 96.Caputo L., Piccialli I., Ciccone R., de Caprariis P., Massa A., De Feo V. Lavender and coriander essential oils and their main component linalool exert a protective effect against amyloid-β neurotox-icity. Phytother. Res. 2021;35(1):486–493. doi: 10.1002/ptr.6827. [DOI] [PubMed] [Google Scholar]
- 97.Eskandari-Roozbahani N., Shomali T., Taherianfard M. Neuroprotective effect of Zataria Multiflora essential oil on rats with Alzheimer disease: A mechanistic study. Basic Clin. Neurosci. 2019;10(1):85–97. doi: 10.32598/bcn.9.10.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rao Y.L., Ganaraja B., Joy T., Pai M.M., Ullal S.D., Murlimanju B.V. Neuroprotective effects of resveratrol in Alzheimer’s disease. Front. Biosci. (Elite Ed.) 2020;12:139–149. doi: 10.2741/e863. [DOI] [PubMed] [Google Scholar]
- 99.Ma X., Sun Z., Han X., Li S., Jiang X., Chen S. Neuroprotective effect of resveratrol via activa-tion of Sirt1 signaling in a rat model of combined diabetes and Alzheimer’s disease. Front. Neurosci. 2019;13:1400. doi: 10.3389/fnins.2019.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cummings J., Lee G., Ritter A., Zhong K. Alzheimer’s disease drug development pipeline. Alzheimers Dement. 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hampel H., Williams C., Etcheto A., Goodsaid F., Parmentier F., Sallantin J., Kaufmann W.E., Missling C.U., Afshar M. A precision medicine framework using artificial intelligence for the iden-tification and confirmation of genomic biomarkers of response to an Alzheimer’s disease therapy: Analysis of the blarcamesine (ANAVEX2-73) Phase 2a clinical study. Alzheimers Dement. (N. Y.) 2020;6(1):e12013. doi: 10.1002/trc2.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pujalté I., Passagne I., Brouillaud B., Tréguer M., Durand E., Ohayon-Courtès C., L’Azou B. Cytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cells. Part. Fibre Toxicol. 2011;8(1):10. doi: 10.1186/1743-8977-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dexter D.T., Jenner P. Parkinson disease: From pathology to molecular disease mechanisms. Free Radic. Biol. Med. 2013;62:132–144. doi: 10.1016/j.freeradbiomed.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 104.Bottiglieri T., Arning E., Wasek B., Nunbhakdi-Craig V., Sontag J-M., Sontag E. Acute admin-istration of L-DOPA induces changes in methylation metabolites, reduced protein phosphatase 2A methylation, and hyperphosphorylation of Tau protein in mouse brain. J. Neurosci. 2012;32(27):9173–9181. doi: 10.1523/JNEUROSCI.0125-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mokni M., Elkahoui S., Limam F., Amri M., Aouani E. Effect of resveratrol on antioxidant en-zyme activities in the brain of healthy rat. Neurochem. Res. 2007;32(6):981–987. doi: 10.1007/s11064-006-9255-z. [DOI] [PubMed] [Google Scholar]
- 106.Schaffer S., Eckert G.P., Schmitt-Schillig S., Müller W.E. Plant foods and brain aging: a critical appraisal. Local Mediterranean Food Plants and Nutraceuticals. Karger Publishers; 2006. pp. 86–115. [DOI] [PubMed] [Google Scholar]
- 107.Thiagarajan K., Gamit N., Mandal S., Ayyathan D.M., Chandrasekaran R. Amelioration of methylmercury induced neural damage by essential oil of Selinum vaginatum (Edgew). C. B. Clarke. Pak. J. Pharm. Sci. 2018;31(2):399–404. [PubMed] [Google Scholar]
- 108.Beserra-Filho J.I.A., de Macêdo A.M., Leão A.H.F.F., Bispo J.M.M., Santos J.R., de Oliveira-Melo A.J., Menezes P.D.P., Duarte M.C., de Souza Araújo A.A., Silva R.H., Quintans-Júnior L.J., Ribeiro A.M. Eplingiella fruticosa leaf essential oil complexed with β-cyclodextrin produces a superior neuroprotective and behavioral profile in a mice model of Parkinson’s disease. Food Chem. Toxicol. 2019;124:17–29. doi: 10.1016/j.fct.2018.11.056. [DOI] [PubMed] [Google Scholar]
- 109.Ali B., Al-Wabel N.A., Shams S., Ahamad A., Khan S.A., Anwar F. Essential oils used in aro-matherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015;5(8):601–611. doi: 10.1016/j.apjtb.2015.05.007. [DOI] [Google Scholar]
- 110.Bilsland D., Strong A. Allergic contact dermatitis from the essential oil of French marigold (Taget-es patula) in an aromatherapist. Contact Dermat. 1990;23(1):55–56. doi: 10.1111/j.1600-0536.1990.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 111.Tisserand R., Balacs T. Essential oil safety. Int. J. Aromather. 1996;7(3):28–32. doi: 10.1016/S0962-4562(96)80009-9. [DOI] [Google Scholar]
- 112.Tisserand R., Young R. Essential oil safety-e-book: A guide for health care professionals. Elsevier Health Science; 2013. [Google Scholar]
- 113.Azirak S., Rencuzogullari E. The in vivo genotoxic effects of carvacrol and thymol in rat bone mar-row cells. Environ. Toxicol. 2008;23(6):728–735. doi: 10.1002/tox.20380. [DOI] [PubMed] [Google Scholar]
- 114.Maistro E.L., Mota S.F., Lima E.B., Bernardes B.M., Goulart F.C. Genotoxicity and mutagenici-ty of Rosmarinus officinalis (Labiatae) essential oil in mammalian cells in vivo. Gen Mol Res; 2010. pp. 2113–2122. [DOI] [PubMed] [Google Scholar]
- 115.Di Sotto A., Mazzanti G., Carbone F., Hrelia P., Maffei F. Genotoxicity of lavender oil, linalyl acetate, and linalool on human lymphocytes in vitro. Environ. Mol. Mutagen. 2011;52(1):69–71. doi: 10.1002/em.20587. [DOI] [PubMed] [Google Scholar]
- 116.Chao M.V., Rajagopal R., Lee F.S. Neurotrophin signalling in health and disease. Clin. Sci. (Lond.) 2006;110(2):167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- 117.Barnabé-Heider F., Miller F.D. Endogenously produced neurotrophins regulate survival and differ-entiation of cortical progenitors via distinct signaling pathways. J. Neurosci. 2003;23(12):5149–5160. doi: 10.1523/JNEUROSCI.23-12-05149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nguyen N., Lee S.B., Lee Y.S., Lee K-H., Ahn J-Y. Neuroprotection by NGF and BDNF against neurotoxin-exerted apoptotic death in neural stem cells are mediated through Trk receptors, activating PI3-kinase and MAPK pathways. Neurochem. Res. 2009;34(5):942–951. doi: 10.1007/s11064-008-9848-9. [DOI] [PubMed] [Google Scholar]
- 119.Fiore M., Chaldakov G.N., Aloe L. Nerve growth factor as a signaling molecule for nerve cells and also for the neuroendocrine-immune systems. Rev. Neurosci. 2009;20(2):133–145. doi: 10.1515/REVNEURO.2009.20.2.133. [DOI] [PubMed] [Google Scholar]
- 120.Allen S.J., Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin. Sci. (Lond.) 2006;110(2):175–191. doi: 10.1042/CS20050161. [DOI] [PubMed] [Google Scholar]
- 121.Aloe L., Alleva E., Fiore M. Stress and nerve growth factor: Findings in animal models and hu-mans. Pharmacol. Biochem. Behav. 2002;73(1):159–166. doi: 10.1016/S0091-3057(02)00757-8. [DOI] [PubMed] [Google Scholar]
- 122.Ceccanti M., Mancinelli R., Tirassa P., Laviola G., Rossi S., Romeo M., Fiore M. Early exposure to ethanol or red wine and long-lasting effects in aged mice. A study on nerve growth factor, brain-derived neurotrophic factor, hepatocyte growth factor, and vascular endothelial growth factor. Neurobiol. Aging. 2012;33(2):359–367. doi: 10.1016/j.neurobiolaging.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 123.Mitre M., Mariga A., Chao M.V. Neurotrophin signalling: Novel insights into mechanisms and pathophysiology. Clin. Sci. (Lond.) 2017;131(1):13–23. doi: 10.1042/CS20160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Egan M.F., Kojima M., Callicott J.H., Goldberg T.E., Kolachana B.S., Bertolino A., Zaitsev E., Gold B., Goldman D., Dean M., Lu B., Weinberger D.R. The BDNF val66met polymorphism af-fects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/S0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 125.Chen Z-Y., Jing D., Bath K.G., Ieraci A., Khan T., Siao C-J., Herrera D.G., Toth M., Yang C., McEwen B.S., Hempstead B.L., Lee F.S. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.De Nicoló S., Tarani L., Ceccanti M., Maldini M., Natella F., Vania A., Chaldakov G.N., Fiore M. Effects of olive polyphenols administration on nerve growth factor and brain-derived neu-rotrophic factor in the mouse brain. Nutrition. 2013;29(4):681–687. doi: 10.1016/j.nut.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 127.Liu D., Zhang Q., Gu J., Wang X., Xie K., Xian X., Wang J., Jiang H., Wang Z. Resveratrol prevents impaired cognition induced by chronic unpredictable mild stress in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;49:21–29. doi: 10.1016/j.pnpbp.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 128.Radecki D.T., Brown L.M., Martinez J., Teyler T.J. BDNF protects against stress-induced im-pairments in spatial learning and memory and LTP. Hippocampus. 2005;15(2):246–253. doi: 10.1002/hipo.20048. [DOI] [PubMed] [Google Scholar]
- 129.Tang X., Zhao Y., Zhou Z., Yan J., Zhou B., Chi X., Luo A., Li S. Resveratrol mitigates sevoflurane-induced neurotoxicity by the SIRT1-dependent regulation of BDNF expression in de-veloping mice. Oxid. Med. Cell. Longev. 2020;2020:9018624. doi: 10.1155/2020/9018624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hassanzadeh P., Arbabi E., Atyabi F., Dinarvand R. The endocannabinoid system and NGF are involved in the mechanism of action of resveratrol: A multi-target nutraceutical with therapeutic po-tential in neuropsychiatric disorders. Psychopharmacology (Berl.) 2016;233(6):1087–1096. doi: 10.1007/s00213-015-4188-3. [DOI] [PubMed] [Google Scholar]
- 131.Ding M.L., Ma H., Man Y.G., Lv H.Y. Protective effects of a green tea polyphenol, epigallocate-chin-3-gallate, against sevoflurane-induced neuronal apoptosis involve regulation of CREB/BDNF/TrkB and PI3K/Akt/mTOR signalling pathways in neonatal mice. Can. J. Physiol. Pharmacol. 2017;95(12):1396–1405. doi: 10.1139/cjpp-2016-0333. [DOI] [PubMed] [Google Scholar]
- 132.Lee B., Shim I., Lee H., Hahm D-H. Effects of epigallocatechin gallate on behavioral and cogni-tive impairments, hypothalamic–pituitary–adrenal Axis dysfunction, and alternations in hippocampal BDNF expression under single prolonged stress. J. Med. Food. 2018;21(10):979–989. doi: 10.1089/jmf.2017.4161. [DOI] [PubMed] [Google Scholar]
- 133.Yang J-R., Ren T-T., Lan R., Qin X-Y. Tea polyphenols attenuate staurosporine-induced cytotox-icity and apoptosis by modulating BDNF-TrkB/Akt and Erk1/2 signaling axis in hippocampal neu-rons. IBRO Rep. 2020;8:115–121. doi: 10.1016/j.ibror.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Song Y., Li X., Gong X., Zhao X., Ma Z., Xia T., Gu X. Green tea polyphenols improve isoflu-rane-induced cognitive impairment via modulating oxidative stress. J. Nutr. Biochem. 2019;73:108213. doi: 10.1016/j.jnutbio.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 135.Cimini A., Gentile R., D’Angelo B., Benedetti E., Cristiano L., Avantaggiati M.L., Giordano A., Ferri C., Desideri G. Cocoa powder triggers neuroprotective and preventive effects in a human Alzheimer’s disease model by modulating BDNF signaling pathway. J. Cell. Biochem. 2013;114(10):2209–2220. doi: 10.1002/jcb.24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Martín M.A., Goya L., de Pascual-Teresa S. Effect of cocoa and cocoa products on cognitive per-formance in young adults. Nutrients. 2020;12(12):3691. doi: 10.3390/nu12123691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nath S., Bachani M., Harshavardhana D., Steiner J.P. Catechins protect neurons against mito-chondrial toxins and HIV proteins via activation of the BDNF pathway. J. Neurovirol. 2012;18(6):445–455. doi: 10.1007/s13365-012-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Carito V., Ceccanti M., Tarani L., Ferraguti G., Chaldakov G.N., Fiore M.N., Chaldakov G., Fiore M. Neurotrophins’ modulation by olive polyphenols. Curr. Med. Chem. 2016;23(28):3189–3197. doi: 10.2174/0929867323666160627104022. [DOI] [PubMed] [Google Scholar]
- 139.Costa I.M., Lima F.O.V., Fernandes L.C.B., Norrara B., Neta F.I., Alves R.D., Cavalcanti J.R.L.P., Lucena E.E.S., Cavalcante J.S., Rego A.C.M., Filho I.A., Queiroz D.B., Freire M.A.M., Guzen F.P. Astragaloside, I.V supplementation promotes a neuroprotective effect in ex-perimental models of neurological disorders: A systematic review. Curr. Neuropharmacol. 2019;17(7):648–665. doi: 10.2174/1570159X16666180911123341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Freire M.A.M., Falcao D. Inflammatory response, excitotoxicity and oxidative stress following traumatic brain injury. Recent Developments in Neurodegeneration. New York: Nova Science Publishers; 2020. [Google Scholar]
- 141.Yadav A.P., Fuentes R., Zhang H., Vinholo T., Wang C.H., Freire M.A., Nicolelis M.A. Chron-ic spinal cord electrical stimulation protects against 6-hydroxydopamine lesions. Sci. Rep. 2014;4:3839. doi: 10.1038/srep03839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barde Y-A. Neurotrophins: A family of proteins supporting the survival of neurons. Prog. Clin. Biol. Res. 1994;390:45–56. [PubMed] [Google Scholar]