Abstract
The conflict between social groups is widespread, often imposing significant costs across multiple groups. The social insects make an ideal system for investigating inter-group relationships, because their interaction types span the full harming–helping continuum, from aggressive conflict, to mutual tolerance, to cooperation between spatially separate groups. Here we review inter-group conflict in the social insects and the various means by which they reduce the costs of conflict, including individual or colony-level avoidance, ritualistic behaviours and even group fusion. At the opposite extreme of the harming–helping continuum, social insect groups may peacefully exchange resources and thus cooperate between groups in a manner rare outside human societies. We discuss the role of population viscosity in favouring inter-group cooperation. We present a model encompassing intra- and inter-group interactions, and local and long-distance dispersal. We show that in this multi-level population structure, the increased likelihood of cooperative partners being kin is balanced by increased kin competition, such that neither cooperation (helping) nor conflict (harming) is favoured. This model provides a baseline context in which other intra- and inter-group processes act, tipping the balance toward or away from conflict. We discuss future directions for research into the ecological factors shaping the evolution of inter-group interactions.
This article is part of the theme issue ‘Intergroup conflict across taxa’.
Keywords: class-structure, inclusive fitness, intergroup conflict, intergroup cooperation, population viscosity, social insects
1. Introduction
Relationships between separate social groups are typically structured around interactions that are competitive or actively hostile, and this pattern holds across a wide range of taxa, from bacteria to humans [1–3]. In inter-group conflicts, costs are imposed by one group on one or more other groups of conspecifics [4]; these costs can be substantial and incurred by both parties, favouring behavioural and physiological adaptations that reduce the likelihood of conflict escalation, such as changes in space use, or context-dependent affiliation with members of other groups (out-groups) [5,6]. In rare cases, inter-group relationships actually can switch from negative to positive, with groups engaging in active cooperation, for example by exchanging resources [4]. The main groups in which inter-group cooperation is seen, primates and ants, are also known for extreme inter-group conflict, under certain circumstances. Here we review inter-group relationships in the social insects, covering inter-group conflict, strategies that promote inter-group tolerance, and the emergence of inter-group cooperation. We discuss the role of population viscosity in shaping intergroup relationships, from helping (cooperation) to harming (conflict), and introduce a model that provides a framework in which these relationships and their consequences can be explored. Finally, we suggest directions of future research, highlighting areas where a tight interplay between empirical and theoretical work can help clarify the nature of intergroup conflict and cooperation.
2. Inter-group conflict
Highly social animals by definition exhibit high levels of within-group cooperation, and as a result, can accumulate or generate valuable resources such as stores of food [7,8]. Their very success in doing so increases the potential for inter-group conflict: large groups need to continually acquire resources for maintenance and growth, and also the resources they hold make them attractive targets to other groups, meaning that resource-driven conflict between successful groups is likely [9]. When groups are in conflict, increased within-group cooperation is favoured, which can enable groups to increase their resources, and fuel further inter-group conflict [10,11]. Just as in humans, conflicts between conspecific social insect colonies can involve the deaths of large numbers of participants [12–14] and, as is frequently the case in human conflicts, when social insect colonies fight, the individuals that stand to gain most from the conflict are not those engaged in front line combat. Unlike in humans, in social insects, it is the colony's reproductive individuals (often a queen or queens) who directly benefit from aggressive colony defence or the acquisition of additional resources to fuel their brood production. The workers fighting on behalf of the colony are usually daughters or sisters of the reproductive/s, and thus reap inclusive fitness benefits by promoting the interests of the colony, even if they die in the process [15]. This applies particularly to workers who have little scope for direct fitness gains if they survive, i.e. in species where workers are sterile, or where fertility declines with age. In the latter case, we would expect colony defence to be the province of physiologically senescent workers, and this is indeed the case across social insect taxa [16–21]. When workers are entirely sterile, their interests align strongly with those of their reproductively active relatives: in these cases, fighting insect workers are better likened to somatic tissue of a ‘superorganism’ than to individual combatants.
It is appealing to draw parallels between social insect workers and human soldiers, and the impressive weaponry of many workers makes it easy to view an individual worker as a warrior. Social insect workers have stings, jaws and chemical sprays with which to repel intruders, and specialist defenders are often referred to as ‘soldiers’ as a result. The pitfalls of equating social insect workers and human soldiers are illustrated by attempts to apply Lanchester's laws of human warfare strategy (relating mortality to aspects of relative strength of opposing forces) to ant conflicts: across several species, outcomes do not follow or even run counter to the Lanchester predictions [22–24]. Indeed, the above examples notwithstanding, group-level combat among conspecifics is relatively rare in the social insects. The weaponry borne by social insect workers is primarily used to defend their resources (stored food and vulnerable protein-rich brood) from heterospecific predators and kleptoparasites, rather than from conspecifics [22,25,26]. Many conspicuous colony-level conflicts are in fact attempts to withstand heterospecific robbing [22,27,28].
3. Inter-group tolerance
The contrast between the fervent aggression with which social insect colonies will defend their nests from heterospecific intruders, and the rarity of all-out conflicts with conspecifics, indicate that social insects have evolved strategies to evade costly inter-group conflicts. Groups are mutually tolerant when their members neither incur a net cost nor receive a net benefit as a result of interacting with other groups [4]. The simplest of such tolerance strategies is avoidance. This can sometimes be achieved at the colony level. Army ants, for example, that live nomadically, actively avoid encounters with conspecific colonies [29], despite their warlike name and their voracious attacks on other ant species [30]. Other ant species relocate the colony in response to local competition, but this is usually heterospecific, not conspecific competition [31]. For most social insects, colony-level avoidance of conspecifics is no simple matter: depending on the level of investment in the nest and their ability to transport their brood, once established a colony may effectively be fixed in place. The consequence of this is seen in the patterns of regular spatial distribution (overdispersion) common among ants: new nests cannot thrive close to existing nests [32–35].
When avoidance at the colony level is impossible, individual-level avoidance can be employed. The most familiar implementation of this approach is through the establishment of territories: static colonies cannot entirely avoid their neighbours, but can reduce the likelihood of individual members of different colonies encountering each other. A territorial strategy is particularly beneficial when the costs of fighting are high [36], as is likely for stinging and biting insects, and so territories are used by many ant species [37,38]. Territorial boundaries may be aggressively protected to prevent encroachment by neighbouring colonies, as seen in arboreal ants Azteca trigona and Oecophylla smaragdina [39,40], or once established, may be maintained with little aggression, through mutual avoidance of the boundary zone as in wood ants Formica polyctena [12]. Alternatively to maintaining discrete territories, ants have evolved multiple ways to coexist within apparently overlapping space. They may avoid clashes by temporally partitioning active foraging periods [37,41] or by avoiding each other's foraging trails [32,42,43]. Other species show context-dependent aggression, where they actively defend their nest [44] and/or valuable resources [33] but are non-aggressive if they encounter conspecifics elsewhere in their foraging range.
In many group-living territorial species, a ‘dear enemy’ pattern can be observed, where encounters with familiar neighbours are less aggressive than those with unknown intruders [45]. This pattern is rarely seen in ants, indeed, the opposite is more frequent. This is likely because in these central-place foragers with a relatively stable home base, encounters with members of distant colonies are rare and unlikely to represent a significant threat, whereas workers from nearby colonies are competitors who may attack [1,40,46]. An advantage of the ‘dear enemy’ behaviour is that it avoids costly contests where the outcome is predictable, but there are other ways to avoid contests without using familiarity as a heuristic. These include signalling fighting ability before engaging [47], and many social insect species employ such behaviours to avoid encounters escalating to fights. Just as in many other animals, pre-conflict posturing is common in social insects, and intruders will frequently retreat without engaging in a fight, especially if not in their home territory [13,14,48]. The most conspicuous example of signalling group strength is seen in ants that form lines of workers along disputed territorial boundaries, as in Tetramorium pavement ants and Myrmecocystus honeypot ants [42,49,50]. In the honeypot ants, these ‘fighting’ lines are ritualistic, involving aggressive postures; in the pavement ants, pushing and fighting do occur, but few fights escalate to actual injury or death. In these and other species, the number of ants available to line the contested territory border is an honest signal of colony strength, and the smaller colony may cede territory as a result [39,42,50].
An approach to inter-group tolerance that falls at the opposite extreme to avoidance, is colony fusion. The fusion of genetically distinct mature social insect colonies is a rare phenomenon, but one that is seen in various termite genera [51,52]. If two similar-sized colonies encounter each other, such that one cannot simply annihilate the other, they may fuse. This is not an entirely peaceful option: usually one or more reproductives is killed, but nevertheless members of both original colonies may benefit: their increased group size makes them a superior competitive force, and workers from both colonies have the potential to develop into reproductives later [52]. While there are still within-group conflicts of interest, a fused colony is now in effect a single group and may contain reproductives from one or both original groups. Army ant colonies may also fuse if one colony becomes queenless [53]. Here, the queenless workers do not gain reproductive potential by fusing. Instead, this fusion is hypothesized to be driven by the low probability of success of worker reproduction in a queenless fragment being outweighed by likely inclusive fitness gains of fusing with a neighbour, who, due to population viscosity, is likely to be related [53].
4. Inter-group cooperation
Population viscosity (local dispersal) is a common feature of social insect societies, and plays a role in the progression of some species beyond inter-group tolerance, to actively positive interactions between spatially separate stable groups. Such inter-group cooperation is characterized by the transfer of benefits from one group to one or more other groups, resulting in net benefits shared by members of the groups involved [4]. How can such a state arise? When independent nest foundation is high risk, which it frequently is in social insects, it can be adaptive for mated queens to return to their natal nest rather than strike out alone, resulting in secondary polygyny: multiple closely related queens reproducing within a single nest [54]. Colony reproduction in such cases is often by budding, a local dispersal strategy in which a queen or queens found a new nest accompanied by workers. The combination of reduced within-nest relatedness due to multiple reproductives, and high population viscosity due to reproduction by budding, together reduces the relatedness differential between one's own and neighbouring colonies, providing conditions that favour reduction in inter-group aggression [54–56].
For some ant species, these conditions result in the establishment of cooperative social connections between the occupants of spatially distinct nests. These nests form a network connected by non-aggressive mutual exchange of workers, a phenomenon termed ‘polydomy’ [57,58]. Within this network, inter-group cooperation in the form of resource exchange is possible, with workers, brood and food being peacefully transferred between nests [59,60]. In wood ants, sharing resources between groups subsidises nest establishment and can rebalance resource heterogeneity [61–63]. In extreme cases, polydomous colonies become ‘unicolonial’, lacking colony boundaries within a whole population, for example as seen in the Argentine ant, Linepithema humile [64]. This status is most common in invasive species and may result in part from reduced genetic diversity in a population arising from a single foundation event. Such huge cooperative units should be vulnerable to exploitation by cheats, for example, nests that produce only reproductives and rely on the wider workforce for support. As such, they are predicted to be evolutionarily unstable [56]. At more modest network sizes, however, this form of cooperation between groups in social insects appears to be a stable and successful strategy [58].
5. Modelling inter-group relationships
Polydomous social insect colonies pose a challenge to many traditional models of social organization and cooperation, because they comprise three levels of organization: individuals interact within nests (their ‘group’) but members of these groups also interact locally with other groups through their social connections. This means that our understanding of an individual's social relationships is complete only if we look beyond what is happening in the nest and include inter-group relationships. A key influencer of these inter-group relationships is the local relatedness environment: as we have seen above, population viscosity caused by colonies reproducing through budding can play an important role in the evolution of conflict, tolerance and cooperation in social insects.
Hamilton [55]—in his seminal work on inclusive fitness theory—was the first to suggest that population viscosity could be a key mechanism promoting the evolution of cooperation (helping). In viscous populations, a random neighbour is more related to the focal individual than a random individual in the population, and therefore population viscosity can even drive the evolution of indiscriminate cooperation. Because of its simplicity—unlike other mechanisms, such as kin discrimination and green-beard effects [55,65]—this mechanism has the potential to drive the evolution of cooperation across a wide range of taxa. However, population viscosity can also inflate competition for resources among related individuals, a factor that works against cooperation and instead promotes conflict (harming). In a theoretical model, Taylor [66] showed that in the simplest case population viscosity generates relatedness among social partners—as suggested by Hamilton—but it also enhances competition among kin in such a way that population viscosity has no net effect on the evolution of cooperation (box 1). This cancellation result has motivated a large body of work seeking to understand what ecological factors can break down the cancellation result and drive the evolution of intra-group cooperation (e.g. [73–76]). However, the role of different population viscosity processes in the genetic structure of multi-level societies and its consequences for the evolution of inter-group behaviour remain unclear.
Box 1. Population viscosity and intra-group conflict and cooperation.
Taylor [66] developed a formal model to study the impact of population viscosity on the evolution of cooperation, in which a focal actor pays a cost c to provide a benefit b to social partners. The model is based on Wright's [67] infinite island model, which assumes a large population subdivided into patches connected through ‘long-distance’ dispersal. Generations are non-overlapping and each patch contains exactly nT asexually reproducing individuals. Taylor [66] originally used the inclusive-fitness method to analyse his model. Here, we revisit his model using the neighbour-modulated method ([68], see electronic supplementary material for details). Each individual produces a very large number f(x, y) of offspring, where x represents the focal individual's investment in helping (or harming), and y is the average investment in the local patch (excluding the focal individual), in a population with an average investment z. A fraction 1 − d of the offspring remain in the local patch, where they compete for the nT breeding sites, while a fraction d disperse to a random patch. Dispersal carries a cost k, such that only a fraction 1 − k of the offspring survive dispersal. The neighbour-modulated fitness [68] of a focal mother is then given by
| 1 |
where Y is the average phenotype in the focal patch (including the focal individual), and where the first term represents the fitness accrued from philopatric offspring, and the second from offspring that disperse. The selection gradient is the derivative of fitness ω with respect to breeding value g (the heritable component of the phenotype), dω(x, y, Y, z)/dg, evaluated at x = y = Y = z [68]. We can express the selection gradient in terms of Hamilton's rule–which adopts the inclusive-fitness perspective [69]. This is given by
| 2 |
where: φT = (1 − d)/(1 − kd) is the probability of philopatry; rT is the ‘other-only’ relatedness between social partners, which excludes the focal individual; and RT is the ‘whole-group’ relatedness between social partners, which includes the focal individual [70]. The inclusive-fitness effect identifies three selective pressures acting on helping: (1) the fertility cost c to the actor; (2) the benefit b provided to the actor's social partners and (3) the kin competition cost due to the additional number of offspring produced in the local patch, b − c, that remain in the local patch and displace other related offspring. The behaviour evolves when c/b < AT, where AT = (rT − φ2TRT)/(1 − φ2TRT) is the potential for helping [71,72]. At equilibrium, relatedness is such that the potential for helping is zero, i.e. rT = φ2TRT and AT = 0. This recovers Taylor's cancellation result: the positive effects of population viscosity on helping, through increased relatedness, are fully offset by its negative effects, through increased kin competition.
Taylor's [66] model assumes intra-group social interactions and a single group per patch, in which each of the groups is equally spatially distant from any other group in the population, such that individuals in different groups are unrelated (box 1). These assumptions fail to capture the genetic, ecological and demographic context of inter-group interactions in social insects. Consider for instance the case of polydomous ants, in which colonies are composed of different nests with variable numbers of reproductives, have variable movement between nests, variable relatedness both within and between neighbouring nests, and range in size from pairs of nests to vast unicolonial populations [56–58]. Here, we extend Taylor's [66] viscous population model to study the evolution of inter-group interactions among neighbouring groups in a multi-level society, such as those seen in polydomous ants and other multi-level social systems [77].
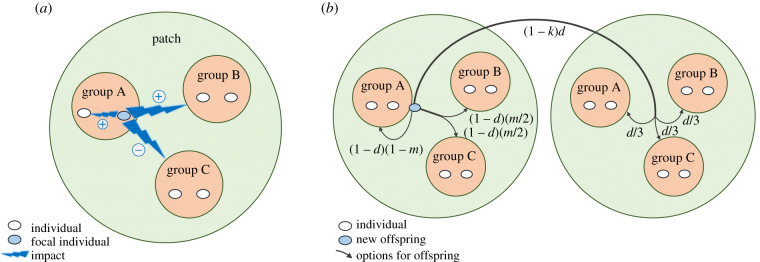
As in Taylor's model, we assume that the population is subdivided into patches connected by long-distance dispersal (figure 1 and box 1). However, rather than assuming a single group per patch, we consider a scenario in which patches are further subdivided into different groups connected by movement of individuals among groups (i.e. short-distance dispersal), (figure 1 and box 2 for details). Thus any focal group in the population now has close neighbouring groups—i.e. groups in the same patch—in addition to distant groups—i.e. groups located in other patches. In addition, two key processes now contribute to the genetic structure of the population: (1) long-distance dispersal, d, which is defined as the fraction of offspring that leave the local patch; and (2) short-distance dispersal, m, which is defined as the movement of offspring between groups within the same patch, such that the total fraction of offspring that remain in their native group is (1 – d)(1 – m). We then perform a kin selection analyses of the evolution of intra-group behaviour (cf. [66]), and of the evolution of inter-group behaviour, in which individuals in one group may help or harm individuals in other groups (see electronic supplementary material for details).
Figure 1.
Conceptual schematic of model of intra- and inter-group relationships. We assume a large population composed of patches, each of which contains a fixed and equal number N of groups (here depicted as three). A group comprises two identical individuals (n = 2). (a) Each individual can have an impact on the members of its own group and on other groups within the patch. Impacts can be positive (cooperation = helping), neutral (tolerance) or negative (conflict = harming); as an example, here the individual has a positive impact on the other member of its own group and on Group B, but a negative impact on Group C. (b) Offspring can stay in their own group, move (m) to another group in the same patch, or disperse (d) with long-distance dispersal-related mortality risk k, to a random group in a new patch (see box 2 for more details). (Online version in colour.)
Box 2. Population viscosity and inter-group conflict and cooperation.
Taylor's [66] model assumes that each patch is occupied by a single group equally distant from every other group in the population, such that individuals in different groups are unrelated. Here, we extend Taylor's model by considering multiple groups per patch connected by movement of individuals among groups, where individuals in different groups, and within the same patch, may be related (figure 1). We use the concept of ‘class’ to model an arbitrary number of groups within each patch, with variable distance between groups and variable relatedness within and between groups. Typically, classes have been considered in relation to age [78,79] and sex [78,80]. More generally, classes are any features of individuals—including social and natural environment—that influence their fitness, other than gene action [81]. Here, we define groups within a patch as classes, such that an individual belongs to a single group, and each group is a separate class. Each patch contains N groups and each group contains nj breeding females, where the subscript j denotes the group (cf. [71]). The fertility of females in group j is fj, which may vary across groups (cf. [82]). As in Taylor [66], we consider long-distance dispersal, d, between patches. However, we also consider movement between groups, such that mj→l represents the fraction of offspring born in group j and that move to group l for offspring that remain in the local patch. Dispersed offspring compete for resources in a random group of their new patch. We consider both intra- and inter-group helping and harming. Intra-group social behaviour occurs among individuals that belong to the same group. Inter-group social behaviour occurs between individuals in different groups within the same patch. We assume that social behaviour carries a fertility cost c to the actor and a fertility benefit b to the recipients, in which the behaviour can be either helping (b > 0) or harming (b < 0). We find that when groups are homogeneous, i.e. nj = n and fj = f, and the movement of offspring to other groups is random, i.e. mj→k = m (j ≠ k), the inclusive-fitness effect of the behaviour is zero, for both intra-group and inter-group behaviour (see electronic supplementary material for details). Thus, Taylor's cancellation result extends to cases in which patches contain more than one homogeneous group per patch and random movement between groups, for both intra- and inter-group social behaviours.
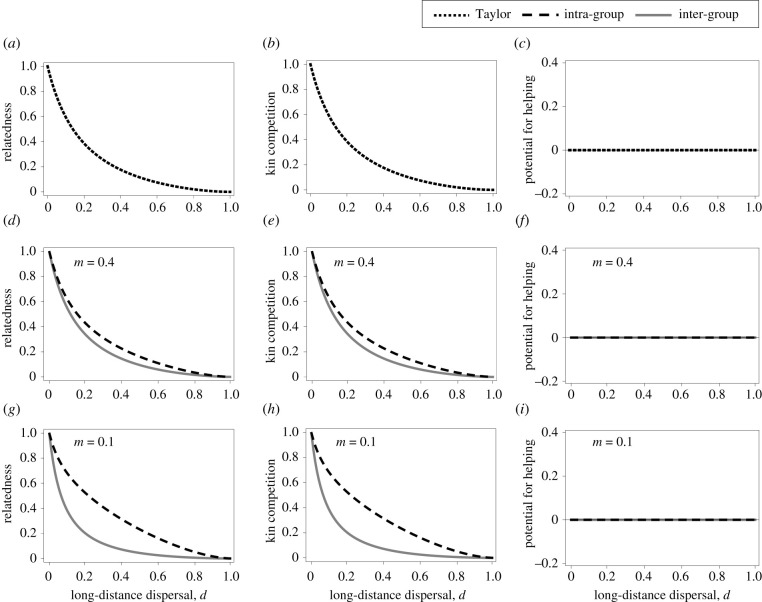
The first important result of our model shows that Taylor's cancellation result for intra-group behaviour extends to the evolution of inter-group social behaviour (figure 2a–c). Further, the cancellation result emerges independently of the level at which we consider population viscosity. That is, the intensity of selection for inter-group social behaviour remains invariant to the degree of both long-distance dispersal, d, and short-distance dispersal, m. First, we find that reduced long-distance dispersal of offspring, i.e. lower d, increases average relatedness within a patch (figure 2d), which aligns the interests of the different groups within a patch and which favours the evolution of inter-group helping behaviour. However, reduced long-distance dispersal also increases the number of related offspring that compete locally for the same resources, which disfavours the evolution of inter-group helping. These two opposing forces cancel each other out such that reduced long-distance dispersal has no net effect on the evolution of inter-group helping and harming. Second, we find that limited movement among groups (i.e. reduced short-distance dispersal or lower m) within a patch leads to increased relatedness within groups but decreased relatedness among groups (figure 2d), which reduces the costs of inter-group harming. However, limited movement among groups also reduces the fraction of offspring that obtain resources in neighbouring groups, which decreases the benefits of inter-group harming. These two opposing forces cancel each other out such that limited movement between groups within a patch does not influence the evolution of inter-group helping and harming.
Figure 2.
Relatedness, kin competition, and the potential for helping (i.e. cooperation) as a function of long-distance dispersal, d. (a–c) Taylor's cancellation result: limited dispersal increases relatedness among group members, but it also increases the intensity of competition among related individuals; these two forces cancel each other out, such that population viscosity has no net effect on the evolution of helping or harming (negative interactions, i.e. conflict). (d–i) Taylor's cancellation result extends to higher levels of biological organization when individuals form groups within patches, for both intra- and inter-group helping and harming, irrespective of the amount of movement, m, between groups within the focal patch, where m is the fraction of offspring that move to a different group among those offspring that remain in the local patch, i.e. 1 – d. This cancellation result holds irrespective of the number of individuals within each group, n, the number of groups within a patch, N, and the long-distance dispersal mortality risk, k (see electronic supplementary material for details). Parameter values: (a–c) k = 0.5, nT = 6; (d–i) k = 0.5, n = 2, N = 3; (d–f) m = 0.4; (g–i) m = 0.1. (See figure 1, box 1 and box 2 for definitions of parameters.)
Thus, while population viscosity at the patch level (i.e. lower d) may align the interests of all groups within a patch, population viscosity at the group level (i.e. lower m) may align the interest of individuals within a group, but not among groups. Both mechanisms however have no net effect on the evolution of inter-group helping and harming. On the one hand, reduced long-distance dispersal (i.e. lower d) increases the intensity of competition among related individuals, irrespective of the amount of short-distance dispersal, m. On the other hand, reduced short-distance dispersal (i.e. lower m) decreases between-group relatedness but it also decreases the intensity of competition among groups. These multiple opposing fitness-effects of population viscosity operating at different levels of biological organization (both at the patch and group levels) are such that they have no net effect on the evolution of helping and harming between groups.
The second key result of our model shows that population viscosity (both reduced long-distance dispersal, i.e. lower d, and reduced short-distance dispersal, i.e. lower m) has no net effect on the evolution of intra-group helping and harming in a multi-level society, and, therefore, we extend Taylor's cancellation result for cases in which patches contain an arbitrary number of groups. Intra-group behaviour affects both the intensity of competition for resources within the focal group and in neighbouring groups. Reduced long-distance dispersal (i.e. lower d) inflates relatedness within a group, but it also increases the intensity of kin competition both within the focal group and between the focal group and neighbouring groups. These two opposing forces cancel each other out, such that long-distance dispersal does not impact the evolution of intra-group helping and harming. Similarly, reduced short-distance dispersal (i.e. lower m) increases relatedness within a group. However, it also increases the intensity of kin competition with the group. As in the previous cases, these two forces cancel each other out, such that short-distance dispersal does not mediate the evolution of intra-group helping and harming in a multi-level society.
6. Discussion and future directions
Our review of the literature suggests that inter-group relationships in the social insects are highly diverse, including inter-group conflict, multiple strategies that promote inter-group tolerance, and cases of inter-group cooperation. We have highlighted the role of population viscosity in shaping inter-group relationships, from helping (cooperation) to neutral (tolerance) to harming (conflict), and its interaction with patterns of dispersal, and relatedness both within and between groups. While the theoretical underpinnings of the role of population viscosity in the evolution of intra-group relationships are well understood, how population viscosity mediates inter-group relationship is still relatively unclear. Here, we have introduced a model that provides a framework in which inter-group relationships and their consequences can be explored.
This modelling approach is applicable to a wide range of animal taxa but fits particularly well with some aspects of social insect ecology. If we view a ‘group’ in the model as the occupants of a social insect nest, then the modelled ‘individuals’ represent the reproductives (usually queens). The presence of multiple reproductives is widespread among social insects and can result from ‘primary polygyny’ whereby two or more mated females cooperate to establish a nest, or from ‘secondary polygyny’ where one or more mated females join a nest that already has a reproductive present [54]. Interactions between these individuals can be negative, where one suppresses reproduction of the other (common in the later stages of primary polygyny), neutral, where reproduction is tolerated, or positive, where the resources produced by one reproductive (workers) are available to help rear the offspring of the other reproductive. This latter process is a major advantage of secondary polygyny to the newly joining reproductives. Relationships between the groups, i.e. nests, within a particular habitat patch can also cover the full range of interaction types, as discussed in the review above, from aggressive conflict, to tolerance, to active cooperation through resource sharing.
The options available to new reproductives in social insects also match well to the model options—for example in the wood ants, newly mated queens may return to their natal nest, move to a nearby nest of the same cooperative network, or disperse to a new area by flying [83]. Clearly, real movement (m) and dispersal (d) processes are much more spatially heterogeneous than the specific case presented above. In cooperative networks, active trails along which local movement is possible are more likely between closer neighbours, but their nature is also shaped by the resource environment [63]. Thus, while we assumed random movement between groups, exploring cases in which movement between some groups is more frequent than others, and how these heterogeneous patterns impact inter-group relationships deserves future analyses.
In our model, long-distance dispersal between patches is random. For many flying social insects, wind-aided dispersal is somewhat undirected and can cover long distances [84,85], and human-mediated jump dispersal commonly occurs in invasive ant species [64] so the random dispersal model used here is not entirely unrealistic. In many cases, however, long-distance dispersal is more likely between nearby patches. Further model extensions will be required to analyse more complex patterns of dispersal and how these mediate inter-group interactions.
Our model assumes an individual mode of dispersal, a factor that underlies the cancellation between the kin-selected benefits and kin competition costs of inter-group helping and harming. As described above, insect societies often adopt a budding mode of dispersal, in which one or more reproductives disperse accompanied by workers to establish a new colony [54,56]. Theoretical and empirical studies of viscous populations show that budding dispersal can uncouple within-group relatedness from the intensity of kin competition, such that intra-group cooperation, in single-group patches, is favoured [75,86,87]. We therefore expect budding dispersal to affect patterns of inter-group conflict and cooperation. For instance, if the different groups within a patch are established through competition between unrelated buds—following multiple long-distance budding dispersal events—we expect high within-group relatedness, low between-group relatedness, and low kin competition, a combination of factors that may drive the evolution of conflict. However, if each group emerges from a single large bud after competition for patch ownership, then we expect high within- and between-group relatedness, and low kin competition, a combination of factors that may favour the evolution of tolerance and cooperation.
In natural populations, inter-group conflict is characterized by high cohesion, coordination and some degree of unity of purpose among group members [10,11,88]. High relatedness within a group is a key factor aligning the interests across group members. Our model shows that population viscosity can increase within-group relatedness, but is in itself insufficient to promote the evolution of inter-group conflict (or cooperation). More generally, our model shows that in the simplest scenario, population viscosity processes that contribute to the genetic structure of multi-level societies do not modulate the evolution of inter-group social behaviour. From this perspective, our model can be seen as a null-model that provides a benchmark that facilitates the development of future empirical and theoretical work. What additional ecological and demographic conditions are needed to drive the evolution of within-group cooperation and between-group conflict (cooperation) is still, to some degree, unknown. Exploring how these and other factors influence the evolution of inter-group helping and harming can bring new insights into the nature of inter-group conflict.
7. Conclusion
Our model demonstrates the benefits of a multilevel approach for investigating between- and within-group relationships. Multilevel social organization is widespread among animals [77], but among multi-level societies, organizational systems where groups interact with other groups without fusing into a single larger group are relatively rare. Such networks of interacting groups provide ideal conditions for investigating the ecology and evolution of inter-group processes. The social insects thus make an ideal study system for addressing these relationships, because they exhibit such a wide range of interaction types, both within and between spatially separate groups.
Contributor Information
António M. M. Rodrigues, Email: antonio.rodrigues@yale.edu.
Elva J. H. Robinson, Email: elva.robinson@york.ac.uk.
Data accessibility
The equations necessary to reproduce the model are provided in the electronic supplementary material [89].
Authors' contributions
A.M.M.R.: conceptualization, formal analysis, methodology, visualization, writing—original draft, writing—review and editing; J.L.B.: conceptualization, writing—review and editing; E.J.H.R.: conceptualization, project administration, visualization, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Christensen C, Radford AN. 2018. Dear enemies or nasty neighbors? Causes and consequences of variation in the responses of group-living species to territorial intrusions. Behav. Ecol. 29, 1004-1013. ( 10.1093/beheco/ary010) [DOI] [Google Scholar]
- 2.Granato ET, Meiller-Legrand TA, Foster KR. 2019. The evolution and ecology of bacterial warfare. Curr. Biol. 29, R521-R537. ( 10.1016/j.cub.2019.04.024) [DOI] [PubMed] [Google Scholar]
- 3.De Dreu CKW, Fariña A, Gross J, Romano A. 2021. Pro-sociality as a foundation for intergroup conflict. Curr. Opin. Psychol. 44, 112-116. ( 10.1016/j.copsyc.2021.09.002) [DOI] [PubMed] [Google Scholar]
- 4.Robinson EJH, Barker JL. 2017. Inter-group cooperation in humans and other animals. Biol. Lett. 13, 20160793. ( 10.1098/rsbl.2016.0793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aureli F, Cords M, Van Schaik CP. 2002. Conflict resolution following aggression in gregarious animals: a predictive framework. Anim. Behav. 64, 325-343. ( 10.1006/anbe.2002.3071) [DOI] [Google Scholar]
- 6.Morris-Drake A, Kennedy P, Braga Goncalves I, Radford A. 2022. Variation between species, populations, groups and individuals in the fitness consequences of out-group conflict. Phil. Trans. R. Soc. B 377, 20210148. ( 10.1098/rstb.2021.0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crane E. 1991. Honey from honeybees and other insects. Ethol. Ecol. Evol. 3, 100-105. ( 10.1080/03949370.1991.10721919) [DOI] [Google Scholar]
- 8.Karsai I, Schmickl T. 2020. Social stomach. In Encyclopedia of social insects (ed. Starr C), pp. 1-4. Cham, Switzerland: Springer International Publishing. ( 10.1007/978-3-319-90306-4_111-10) [DOI] [Google Scholar]
- 9.De Dreu CKW, Gross J, Fariña A, Ma Y. 2020. Group cooperation, carrying-capacity stress, and intergroup conflict. Trends Cogn. Sci. 24, 760-776. ( 10.1016/j.tics.2020.06.005) [DOI] [PubMed] [Google Scholar]
- 10.Reeve HK, Hölldobler B. 2007. The emergence of a superorganism through intergroup competition. Proc. Natl Acad. Sci. USA 104, 9736-9740. ( 10.1073/pnas.0703466104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korb J, Foster KR. 2010. Ecological competition favours cooperation in termite societies. Ecol. Lett. 13, 754-760. ( 10.1111/j.1461-0248.2010.01471.x) [DOI] [PubMed] [Google Scholar]
- 12.Mabelis AA. 1979. Wood ant wars. The relationship between aggression and predation in the red wood ant (Formica polyctena Först). Neth. J. Zool. 29, 451-620. ( 10.1163/002829679X00016) [DOI] [Google Scholar]
- 13.Salzemann A, Jaffe K. 1990a. On the territorial behaviour of field colonies of the leaf-cutting ant Atta laevigata (Hymenoptera: Myrmicinae). J. Insect. Physiol. 36, 133-138. ( 10.1016/0022-1910(90)90184-H) [DOI] [Google Scholar]
- 14.Grüter C, Von Zuben LG, Segers FHID, Cunningham JP. 2016. Warfare in stingless bees. Insectes Soc. 63, 223-236. ( 10.1007/s00040-016-0468-0) [DOI] [Google Scholar]
- 15.Hamilton WD. 1972. Altruism and related phenomena, mainly in social insects. Annu. Rev. Ecol. Syst. 3, 193-232. ( 10.1146/annurev.es.03.110172.001205) [DOI] [Google Scholar]
- 16.Cammaerts-Tricot MC. 1975. Ontogenesis of defence reactions in workers of Myrmica rubra L. (Hymenoptera: Formicidae). Anim. Behav. 23, 124-130. ( 10.1016/0003-3472(75)90058-5) [DOI] [Google Scholar]
- 17.Porter SD, Jorgensen CD. 1981. Foragers of the harvester ant, Pogonomyrmex owyheei: a disposable caste? Behav. Ecol. Sociobiol. 9, 247-256. ( 10.1007/BF00299879) [DOI] [Google Scholar]
- 18.Moore AJ, Breed MD, Moore MJ. 1987. The guard honey bee: ontogeny and behavioural variability of workers performing a specialized task. Anim. Behav. 35, 1159-1167. ( 10.1016/S0003-3472(87)80172-0) [DOI] [Google Scholar]
- 19.O'Donnell S. 2001. Worker age, ovary development, and temporal polyethism in the swarm-founding wasp Polybia occidentalis (Hymenoptera: Vespidae). J. Insect Behav. 14, 201-213. ( 10.1023/A:1007837727984) [DOI] [Google Scholar]
- 20.Uematsu K, Kutsukake M, Fukatsu T, Shimada M, Shibao H. 2010. Altruistic colony defense by menopausal female insects. Curr. Biol. 20, 1182-1186. ( 10.1016/j.cub.2010.04.057) [DOI] [PubMed] [Google Scholar]
- 21.Yanagihara S, Suehiro W, Mitaka Y, Matsuura K. 2018. Age-based soldier polyethism: old termite soldiers take more risks than young soldiers. Biol. Lett. 14, 20180025. ( 10.1098/rsbl.2018.0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitehouse MEA, Jaffe K. 1996. Ant wars: combat strategies, territory and nest defence in the leaf-cutting ant Atta laevigata. Anim. Behav. 51, 1207-1217. ( 10.1006/anbe.1996.0126) [DOI] [Google Scholar]
- 23.Plowes NJR, Adams ES. 2005. An empirical test of Lanchester's square law: mortality during battles of the fire ant Solenopsis invicta. Proc. R. Soc. B 272, 1809-1814. ( 10.1098/rspb.2005.3162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clifton E. 2020. A brief review on the application of Lanchester's models of combat in nonhuman animals. Ecol. Psychol. 32, 181-191. ( 10.1080/10407413.2020.1846456) [DOI] [Google Scholar]
- 25.López-Incera A, Nouvian M, Ried K, Müller T, Briegel HJ. 2021. Honeybee communication during collective defence is shaped by predation. BMC Biol. 19, 1-16. ( 10.1186/s12915-021-01028-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann K, Pinter-Wollman N. 2022. The effect of resource availability on interspecific competition between a native and an invasive ant. Phil. Trans. R. Soc. B 377, 20210146. ( 10.1098/rstb.2021.0146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell S, Clark E. 2004. Combat between large derived societies: a subterranean army ant established as a predator of mature leaf-cutting ant colonies. Insectes Soc. 51, 342-351. ( 10.1007/s00040-004-0752-2) [DOI] [Google Scholar]
- 28.Cunningham JP, Hereward JP, Heard TA, De Barro PJ, West SA. 2014. Bees at war: interspecific battles and nest usurpation in stingless bees. Am. Nat. 184, 777-786. ( 10.1086/678399) [DOI] [PubMed] [Google Scholar]
- 29.Franks NR, Fletcher CR. 1983. Spatial patterns in army ant foraging and migration: Eciton burchelli on Barro Colorado Island, Panama. Behav. Ecol. Sociobiol. 12, 261-270. ( 10.1007/BF00302894) [DOI] [Google Scholar]
- 30.Hoenle PO, Blüthgen N, Brückner A, Kronauer DJC, Fiala B, Donoso DA, Smith MA, Ospina Jara B, von Beeren C. 2019. Species-level predation network uncovers high prey specificity in a neotropical army ant community. Mol. Ecol. 28, 2423-2440. ( 10.1111/mec.15078) [DOI] [PubMed] [Google Scholar]
- 31.McGlynn TP. 2012. The ecology of nest movement in social insects. Annu. Rev. Entomol. 57, 291-308. ( 10.1146/annurev-ento-120710-100708) [DOI] [PubMed] [Google Scholar]
- 32.Ryti RT, Case TJ. 1986. Overdispersion of ant colonies: a test of hypotheses. Oecologia 69, 446-453. ( 10.1007/BF00377067) [DOI] [PubMed] [Google Scholar]
- 33.Boulay R, Cerdá X, Simon T, Roldan M, Hefetz A. 2007. Intraspecific competition in the ant Camponotus cruentatus: should we expect the ‘dear enemy’ effect? Anim. Behav. 74, 985-993. ( 10.1016/j.anbehav.2007.02.013) [DOI] [Google Scholar]
- 34.Franks NR, Dornhaus A, Hitchcock G, Guillem R, Hooper J, Webb C. 2007. Avoidance of conspecific colonies during nest choice by ants. Anim. Behav. 73, 525-534. ( 10.1016/j.anbehav.2006.05.020) [DOI] [Google Scholar]
- 35.Eyer P-A, Espinoza EM, Blumenfeld AJ, Vargo EL. 2019. The underdog invader: breeding system and colony genetic structure of the dark rover ant (Brachymyrmex patagonicus Mayr). Ecol. Evol. 10, 493-505. ( 10.1002/ece3.5917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrell LJ, Kokko H. 2005. Bridging the gap between mechanistic and adaptive explanations of territory formation. Behav. Ecol. Sociobiol. 57, 381-390. ( 10.1007/s00265-004-0859-5) [DOI] [Google Scholar]
- 37.Hölldobler B, Lumsden CJ. 1980. Territorial strategies in ants. Science 210, 732-739. ( 10.1126/science.210.4471.732) [DOI] [PubMed] [Google Scholar]
- 38.Adams ES. 2016. Territoriality in ants (Hymenoptera: Formicidae): a review. Myrmecol. News 23, 101-118. [Google Scholar]
- 39.Adams ES. 1990. Boundary disputes in the territorial ant Azteca trigona: effects of asymmetries in colony size. Anim. Behav. 39, 321-328. ( 10.1016/S0003-3472(05)80877-2) [DOI] [Google Scholar]
- 40.Newey PS, Robson SKA, Crozier RH. 2010. Weaver ants Oecophylla smaragdina encounter nasty neighbors rather than dear enemies. Ecology 91, 2366-2372. ( 10.1890/09-0561.1) [DOI] [PubMed] [Google Scholar]
- 41.Salzemann A, Jaffe K. 1990b. Territorial ecology of the leaf-cutting ant, Atta laevigata (Fr. Smith) Hymenoptera: myrmicinae. Boulder, Colorado: Westview Press. [Google Scholar]
- 42.Hölldobler B. 1981. Foraging and spatiotemporal territories in the honey ant Myrmecocystus mimicus Wheeler (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 9, 301-314. ( 10.1007/BF00299887) [DOI] [Google Scholar]
- 43.Gordon D. 1992. How colony growth affects forager intrusion in neighbouring harvester ant colonies. Behav. Ecol. Sociobiol. 31, 417-427. ( 10.1007/BF00170609) [DOI] [Google Scholar]
- 44.Uematsu J, Hayashi M, Shimoji H, Laurent Salazar M-O, Tsuji K. 2019. Context-dependent aggression toward non-nestmates in the ant Diacamma sp. from Japan. J. Ethol. 37, 259-264. ( 10.1007/s10164-019-00611-8) [DOI] [Google Scholar]
- 45.Temeles EJ. 1994. The role of neighbours in territorial systems: when are they ‘dear enemies’? Anim. Behav. 47, 339-350. ( 10.1006/anbe.1994.1047) [DOI] [Google Scholar]
- 46.Gordon DM. 1989. Ants distinguish neighbors from strangers. Oecologia 81, 198-200. ( 10.1007/BF00379806) [DOI] [PubMed] [Google Scholar]
- 47.Parker GA. 1974. Assessment strategy and the evolution of fighting behaviour. J. Theor. Biol. 47, 223-243. ( 10.1016/0022-5193(74)90111-8) [DOI] [PubMed] [Google Scholar]
- 48.Bell WJ, Hawkins WA. 1974. Patterns of intraspecific agonistic interactions involved in nest defense of a primitively eusocial halictine bee. J. Comp. Physiol. 93, 183-193. ( 10.1007/BF00606998) [DOI] [Google Scholar]
- 49.Hoover KM, Bubak AN, Law IJ, Yaeger JDW, Renner KJ, Swallow JG, Greene MJ. 2016. The organization of societal conflicts by pavement ants Tetramorium caespitum: an agent-based model of amine-mediated decision making. Cur. Zool. 62, 277-284. ( 10.1093/cz/zow041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams ES, Plowes NJR. 2019. Self-organizing conflicts: group assessment and the spatio-temporal dynamics of ant territory battles. Behav. Processes 162, 119-129. ( 10.1016/j.beproc.2019.01.009) [DOI] [PubMed] [Google Scholar]
- 51.Korb J, Roux EA. 2012. Why join a neighbour: fitness consequences of colony fusions in termites. J. Evol. Biol. 25, 2161-2170. ( 10.1111/j.1420-9101.2012.02617.x) [DOI] [PubMed] [Google Scholar]
- 52.Howard KJ, Johns PM, Breisch NL, Thorne BL. 2013. Frequent colony fusions provide opportunities for helpers to become reproductives in the termite Zootermopsis nevadensis. Behav. Ecol. Sociobiol. 67, 1575-1585. ( 10.1007/s00265-013-1569-7) [DOI] [Google Scholar]
- 53.Kronauer DJC, Schöning C, d'Ettorre P, Boomsma JJ. 2010. Colony fusion and worker reproduction after queen loss in army ants. Proc. R. Soc. B 277, 755-763. ( 10.1098/rspb.2009.1591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hölldobler B, Wilson EO. 1977. The number of queens: an important trait in ant evolution. Naturwissenschaften 64, 8-15. ( 10.1007/BF00439886) [DOI] [Google Scholar]
- 55.Hamilton WD. 1964. The genetical evolution of social behaviour. J. Theor. Biol. 7, 17-52. ( 10.1016/0022-5193(64)90039-6) [DOI] [PubMed] [Google Scholar]
- 56.Helanterä H, Strassman JE, Carrillo J, Queller DC. 2009. Unicolonial ants: where do they come from, what are they and where are they going? Trends Ecol. Evol. 24, 341-349. ( 10.1016/j.tree.2009.01.013) [DOI] [PubMed] [Google Scholar]
- 57.Debout G, Schatz B, Elias M, McKey D. 2007. Polydomy in ants: what we know, what we think we know, and what remains to be done. Biol. J. Linnean Soc. 90, 319-348. ( 10.1111/j.1095-8312.2007.00728.x) [DOI] [Google Scholar]
- 58.Robinson EJH. 2014. Polydomy: the organisation and adaptive function of complex nest systems in ants. Curr. Opin. Insect Sci. 5, 37-43. ( 10.1016/j.cois.2014.09.002) [DOI] [PubMed] [Google Scholar]
- 59.Ellis S, Franks DW, Robinson EJH. 2014. Resource redistribution in polydomous ant nest networks: local or global? Behav. Ecol. 25, 1183-1191. ( 10.1093/beheco/aru108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellis S, Robinson EJH. 2016. Inter-nest food sharing in wood ant colonies: resource redistribution behavior in a complex system. Behav. Ecol. 27, 660-668. ( 10.1093/beheco/arv205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ellis S, Robinson EJH. 2015. The role of non-foraging nests in polydomous wood ant colonies. PLoS ONE 10, e0138321. ( 10.1371/journal.pone.0138321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burns DDR, Franks DW, Parr CL, Robinson EJH. 2020. Ant colony nest networks adapt to resource disruption. J. Anim. Ecol. 90, 143-152. ( 10.1111/1365-2656.13198) [DOI] [PubMed] [Google Scholar]
- 63.Lecheval V, Larson H, Burns DD, Ellis S, Powell S, Donaldson-Matasci MC, Robinson EJH. 2021. From foraging trails to transport networks: how the quality-distance trade-off shapes network structure. Proc. R. Soc. B 288, 1949. ( 10.1098/rspb.2021.0430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suarez AV, Holway DA, Case TJ. 2001. Patterns of spread in biological invasions dominated by long-distance jump dispersal: insights from Argentine ants. Proc. Natl Acad. Sci. USA 98, 1095-1100. ( 10.1073/pnas.98.3.1095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gardner A, West SA. 2010. Greenbeards. Evolution 64, 25-38. ( 10.1111/j.1558-5646.2009.00842.x) [DOI] [PubMed] [Google Scholar]
- 66.Taylor PD. 1992. Altruism in viscous populations — an inclusive fitness model. Evol. Ecol. 6, 352-356. ( 10.1007/BF02270971) [DOI] [Google Scholar]
- 67.Wright S. 1931. Evolution in Mendelian populations. Genetics 16, 97. ( 10.1093/genetics/16.2.97) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor PD, Frank SA. 1996. How to make a kin selection model. J. Theor. Biol. 180, 27-37. ( 10.1006/jtbi.1996.0075) [DOI] [PubMed] [Google Scholar]
- 69.Gardner A, West SA, Wild G. 2011. The genetical theory of kin selection. J. Evol. Biol. 24, 1020-1043. ( 10.1111/j.1420-9101.2011.02236.x) [DOI] [PubMed] [Google Scholar]
- 70.Pepper JW. 2000. Relatedness in trait group models of social evolution. J. Theor. Biol. 206, 355-368. ( 10.1006/jtbi.2000.2132) [DOI] [PubMed] [Google Scholar]
- 71.Rodrigues AMM, Gardner A. 2013. Evolution of helping and harming in heterogeneous groups. Evolution 67, 2284-2298. ( 10.1111/evo.12110) [DOI] [PubMed] [Google Scholar]
- 72.Rodrigues AMM, Gardner A. 2013. Evolution of helping and harming in viscous populations when group size varies. Am. Nat. 181, 609-622. ( 10.1086/670031) [DOI] [PubMed] [Google Scholar]
- 73.Taylor PD , Irwin AJ. 2000. Overlapping generations can promote altruistic behavior. Evolution 54(4), 1135-1141. ( 10.1554/0014-3820(2000)054[1135:OGCPAB]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 74.Lehmann L, Bargum K, Reuter M. 2006. An evolutionary analysis of the relationship between spite and altruism. J Evol Biol 19(5), 1507-1516. ( 10.1111/j.1420-9101.2006.01128.x) [DOI] [PubMed] [Google Scholar]
- 75.Gardner A, West SA. 2006. Demography, altruism, and the benefits of budding. J. Evol. Biol. 19, 1707-1716. ( 10.1111/j.1420-9101.2006.01104.x) [DOI] [PubMed] [Google Scholar]
- 76.Wild G, Fernandes A. 2009. Investment in the public good through conditional phenotypes of large effect. J. Evol Biol 22(5), 927-941. ( 10.1111/j.1420-9101.2009.01711.x) [DOI] [PubMed] [Google Scholar]
- 77.Grueter CC, et al. 2020. Multilevel organisation of animal sociality. Trends Ecol. Evol. 35, 834-847. ( 10.1016/j.tree.2020.05.003) [DOI] [PubMed] [Google Scholar]
- 78.Fisher RA. 1930. The genetical theory of natural selection. Oxford, UK: Clarendon Press. [Google Scholar]
- 79.Grafen A. 2006. A theory of Fisher's reproductive value. J. Math. Biol. 53, 15-60. ( 10.1007/s00285-006-0376-4) [DOI] [PubMed] [Google Scholar]
- 80.Price GR. 1970. Selection and covariance. Nature 227, 520-521. ( 10.1038/227520a0) [DOI] [PubMed] [Google Scholar]
- 81.Rodrigues AMM, Gardner A. 2021. Reproductive value and the evolution of altruism. Trends Ecol. Evol. ( 10.1016/j.tree.2021.11.007) [DOI] [PubMed] [Google Scholar]
- 82.Rodrigues AMM, Gardner A. 2012. Evolution of helping and harming in heterogeneous populations. Evolution 66(7), 2065-2079. ( 10.1111/j.1558-5646.2012.01594.x) [DOI] [PubMed] [Google Scholar]
- 83.Sundström L, Seppä P, Pamilo P. 2005. Genetic population structure and dispersal patterns in Formica ants—a review. Annales Zoologici Fennici 42, 163-177. [Google Scholar]
- 84.Markin GP, Dillier JH, Hill SO, Blum MS, Hermann HR. 1971. Nuptial flight and flight ranges of the imported fire ant, Solenopsis saevissima richteri (Hymenoptera: Formicidae). J. Ga. Entomol. Soc. 6, 145-156. [Google Scholar]
- 85.Messenger MT, Mullins AJ. 2005. New flight distance recorded for Coptotermes formosanus (Isoptera: Rhinotermitidae). Fla. Entomol. 88, 99-100. ( 10.1653/0015-4040(2005)088[0099:NFDRFC]2.0.CO;2) [DOI] [Google Scholar]
- 86.Kümmerli R, Gardner A, West SA, Griffin AS. 2009. Limited dispersal, budding dispersal, and cooperation: an experimental study. Evolution 63, 939-949. ( 10.1111/j.1558-5646.2008.00548.x) [DOI] [PubMed] [Google Scholar]
- 87.Rodrigues AMM, Taylor TB. 2018. Ecological and demographic correlates of cooperation from individual to budding dispersal. J. Evol. Biol. 31, 1058-1070. ( 10.1111/jeb.13286) [DOI] [PubMed] [Google Scholar]
- 88.Shen S-F, Reeve HK. 2010. Reproductive skew theory unified: the general bordered tug-of-war model. J. Theor. Biol. 263, 1-12. ( 10.1016/j.jtbi.2009.11.009) [DOI] [PubMed] [Google Scholar]
- 89.Rodrigues AMM, Barker JL, Robinson EJH. 2022. From inter-group conflict to inter-group cooperation: insights from social insects. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Rodrigues AMM, Barker JL, Robinson EJH. 2022. From inter-group conflict to inter-group cooperation: insights from social insects. Figshare. [DOI] [PMC free article] [PubMed]
Data Availability Statement
The equations necessary to reproduce the model are provided in the electronic supplementary material [89].