Abstract
Intergroup conflict is a major evolutionary force shaping animal and human societies. Males and females should, on average, experience different costs and benefits for participating in collective action. Specifically, among mammals, male fitness is generally limited by access to mates whereas females are limited by access to food and safety. Here we analyse sex biases among 72 species of group-living mammals in two contexts: intergroup conflict and collective movements. Our comparative phylogenetic analyses show that the modal mammalian pattern is male-biased participation in intergroup conflict and female-biased leadership in collective movements. However, the probability of male-biased participation in intergroup conflicts decreased and female-biased participation increased with female-biased leadership in movements. Thus, female-biased participation in intergroup conflict only emerged in species with female-biased leadership in collective movements, such as in spotted hyenas and some lemurs. Sex differences are probably attributable to costs and benefits of participating in collective movements (e.g. towards food, water, safety) and intergroup conflict (e.g. access to mates or resources, risk of injury). Our comparative review offers new insights into the factors shaping sex bias in leadership across social mammals and is consistent with the ‘male warrior hypothesis' which posits evolved sex differences in human intergroup psychology.
This article is part of the theme issue ‘Intergroup conflict across taxa’.
Keywords: collective action, comparative social evolution, male warrior hypothesis, group travel, intergroup conflict, sex differences
1. Introduction
A striking, yet often overlooked feature of human intergroup violence is a massive gender bias in participation. Whenever humans form coalitions to injure or kill members of other such coalitions, they virtually always consist exclusively of men. Warfare among human hunter-gatherers is exclusively a male affair [1]. All historical armies lacked female members, and a strong male bias can be found among contemporary urban gangs and terrorist groups [2,3]. Only the last decades have seen deviations from this pattern, such that some contemporary armies have obligatory conscription for women (e.g. Israel, Norway), but women remain excluded from front-line combat units in all but 17 countries [4]. Looking at war across human history and cultures, it is, therefore, justified to view warfare as ‘a strategy by which coalitions of males (our emphasis) cooperate to acquire and defend resources necessary for reproduction’ [5, p. 963]. Even though warfare may have shaped key aspects of human psychology [6,7], including fundamental gender differences in social psychology [8], the underlying evolutionary origins of this gender bias in participation in intergroup violence has always been taken for granted, and comparative perspectives have rarely extended beyond chimpanzees [5,6]. Here, we offer a broader comparative perspective that examines the nature and potential drivers of sex biases in participation in intergroup conflict across diverse mammalian societies [9].
Intergroup conflict is also a major evolutionary force shaping animal societies [10–12], with the benefits of joint resource defence arguably favouring the formation of permanent social groups, as well as increased tolerance and cooperation within these groups [13–15]. However, collective intergroup aggression also poses a collective action problem: why should an individual risk being killed or injured in territorial defence when it could still reap the benefits without incurring the costs of producing them? Despite these risks [16,17], selection may favour intergroup conflicts when members of winning groups gain increased access to contested resources [14,18], such as territories and mating opportunities [19,20]. Evolutionary game theory also predicts that variation in the fitness consequences of fighting should be an important determinant of which individuals participate in fights [21]. In the extreme case when conflicts are potentially fatal, individuals are expected to participate over contested resources only when the resource value exceeds that of living into the future [22]. In some cases, the costs of losing and benefits of winning such resources are evenly distributed across group members [23,24]. In other cases, the associated costs and benefits of participation in intergroup conflicts are not equally shared among individuals [25–29]. For example, in groups with steep dominance hierarchies, high-ranking individuals may gain more benefits from intergroup conflict compared to low-ranking individuals because they have priority of access to resources [26,29–37], but this gain may be partly offset by the greater effort expended during intergroup conflicts [18]. Similar asymmetries are expected when individuals differ in motivation, strength or costs incurred [18].
The balance of the costs and benefits of participating in collective action in general, and potentially lethal intergroup conflict in particular, are also expected to vary, on average, for males and females. Because female reproduction in mammals is constrained by the energetic demands of gestation and lactation, female fitness is primarily limited by access to food and caring for young [38–40], and we might, therefore, expect females to be more involved than males in managing collective movements to locate food or safety to support their current and future reproduction [39,41,42]. Increased access to food gained from winning intergroup conflicts, however, is generally unlikely to overwhelm the risk of injury or death for females or their offspring, particularly since female fitness increases throughout the reproductive lifespan. However, females may engage in intergroup conflicts when the costs of engagement are similar for both sexes, or in female-dominated societies in which females have priority of access to defensible food resources over males (e.g. ungulate prey for spotted hyenas [30]). Moreover, in cases where highly valuable food, water or shelter is defensible, females may also incentivize male participation in intergroup conflict [43,44] to secure access to these resources.
Males, on the other hand, primarily compete for access to fertile females, which generally represent a more limited and less divisible resource than food [38–40]. Moreover, the evolutionary fitness of defence of current reproductive opportunities (mates) is expected to exceed their future reproductive value more often for males than for females owing to limited role of paternal care in most mammals. As a result, males in many species are subject to stronger intrasexual competition and have evolved traits that help them monopolize females and gain mating opportunities [32,37,45,46].
Game theory further predicts that the probability of winning conflicts and the likelihood for an individual to reap the benefits from winning should increase with the relative body (e.g. a large male versus a small female) and weapon size of an individual compared that of other contestant(s) in a fight [21]. Thus, male-biased participation in intergroup conflicts may be particularly strong when sexual size dimorphism is pronounced, a trait that often covaries with weaponry, most prominently with canine size dimorphism in primates [47–49]. Given that females are food limited, participation in intergroup conflicts by both sexes may be more equitable for mammals that feed on highly defensible food, such that both males and females may benefit from their defence (e.g. females gain access to contested food, males defend food to attract females). Thus, we expect sexual dimorphism and resource defensibility to be key predictors of sex-biased participation in intergroup conflict.
Despite these theoretical considerations, evidence for sex differences in intergroup conflict is mixed. A recent meta-analysis found moderate sex differences in intergroup aggression among primates but this was not statistically significant after correcting for evolutionary history [36]. In some primates, such as bonnet macaques (Macaca radiata) [32] or black and white colobus (Colobus guereza) [50], males tend to be more aggressive than females during intergroup conflicts [23]. In other primates, such as Verreaux's sifakas (Propithecus verreauxi) [51] and Samango monkeys (Cercopithecus mitis erythrarchus) [45], females are equally or more aggressive than males. Moreover, in vervet monkeys (Chlorocebus pygerythrus), females initiate intergroup conflicts because doing so provides them with immediate access to limited food; in these groups, females harass low-ranking males to participate in intergroup conflicts [44,52]. Thus, sex differences in behaviour during intergroup conflict may not generalize across primates [36] and such differences have yet to be quantified across social mammals more generally.
Our aim was to investigate sex-biased participation in intergroup conflict and sex-biased leadership in collective movements across mammals, controlling for phylogeny. Given the considerations about differential incentives above, we also tested whether sexual size dimorphism and food defensibility predict sex bias and whether primates differ from non-primates since most primate females carry their infants which poses an additional cost to female participation in intergroup conflict [41]. Importantly, both contexts (intergroup conflict and collective movement) require coordination among multiple group members [36,53–55] and the evolution of sex-biased influence in one context may, therefore, facilitate it in the other; hence we also tested whether sex bias in collective movements predicted sex bias in intergroup conflict. Biased influence by some individuals on the decision-making process is commonly referred to as leadership in the context of collective movements (also called group travel) [56–58]. As we discuss in the next section, while some have extended the leadership concept to explain patterns of collective action during intergroup conflicts [59,60], it is much less studied in this context.
2. Methods
(a) . Operational definitions for comparisons of sex differences in collective action
In the collective movement context, we recorded sex-bias in leadership. Leaders are generally defined as individuals that have a disproportional influence on collective behaviours of a group, regardless of how influence is achieved [49,51,61]. In the context of collective movements, we operationalized leaders as individuals that successfully initiate group movements, that is, those that move first in a direction and disproportionally influence other group members (e.g. by recruiting followers). Importantly, leadership is distinct from dominance; the latter is defined as the ability to win dyadic fights and results in priority of access to resources whereas the former focuses on influence in a decision-making hierarchy [62,63]. In the context of group movements, leadership is rarely based only upon physical force [58] but rather on followership (e.g. ability to recruit followers) [64,65]. In some cases, high-ranking individuals also lead [66,67] but leadership in animals aligns more closely with the concepts of power in sociology [68] and prestige (or status) in psychology [69,70].
Within the context of intergroup conflicts, we were limited to quantifying sex bias in participation (see also [36]). Although influential individuals are often the ones at the front lines of intergroup encounters and those that direct significantly more aggression towards individuals from other groups, intergroup conflicts involving multiple individuals joining forces are often difficult to track, particularly for primate groups with large numbers of participants or occurring within forests with low visibility in three-dimensional space; hence we could not consistently record leadership.
(b) . Literature search and data collection
Our current study builds on recent reviews of collective decisions in group-living mammals [29,60,71–73], papers citing these reviews, and other papers identified via Google Scholar searches to identify species that engage in collective action during intergroup conflict, movements or both. We focused on species living in mixed-sex groups, excluding those for which adults are sexually segregated [74,75]. This yielded studies for 72 species (see figure 1) that spanned five biological orders within the Class Mammalia, including Artiodactyla (three species of even-toed ungulates), Cetacea (four species of whales and dolphins), Carnivora (10 species of carnivorans), Perissodactyla (two species of odd-toed ungulates) and Primata (53 species of primates). We scored each species and context as female-biased, no sex bias (sex differences not statistically significant), or male-biased (figure 1; electronic supplementary material, table S1) based on the analyses presented in the original studies. Sexual dimorphism was expressed as the ratio of the mean male to female body mass for each species (see the electronic supplementary material, table S1 for references). Carnivorans (e.g. eat mostly meat), frugivores (e.g. eat mostly fruit) and gummivores (e.g. eat mostly gums and saps from trees) were in contrast to grazers, browsers, piscivores, omnivores, insectivores, herbivores and folivores (diet may also include fruits) scored as having defensible foods (see the electronic supplementary material, table S1). We downloaded a sample of 100 phylogenetic trees from vertlife.org [76] to represent the evolutionary history of these species and its uncertainty.
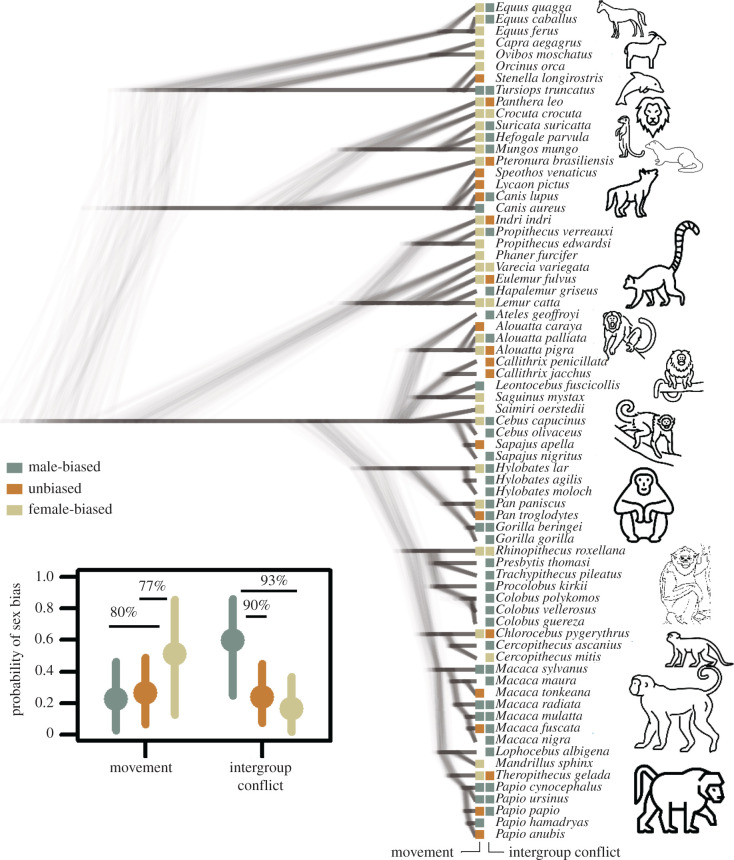
Figure 1.
Phylogeny of the studied species, with observed sex bias in movement and intergroup conflict indicated by the coloured boxes at the tips. The inserted graph shows the predicted probabilities (means ± 95% credible intervals) of different types of sex bias for both contexts. The horizontal bars and percentages inside the graph indicate the degree of support for a difference between two probabilities, e.g. the model is 93% confident that male bias in between-group conflict participation is more likely than female bias. Species icons were downloaded from thenounproject.com. (Online version in colour.)
(c) . Statistical analyses
We modelled the probability of a species having male-biased, unbiased or female-biased leadership in both contexts using multinomial models [72,77], setting ‘unbiased’ as the reference level (see the electronic supplementary material for more details). For the purpose of these analyses, we treated subspecies in our database as the same species, assigning them to the same tip of the phylogeny, and, if necessary, using a species-level random effect to account for multiple observations of the same species. For further adjustments to species names to match the available phylogeny see the accompanying R code. For each context, we ran an intercept-only model (model 1), as well as a model with the predictor variables food defensibility and sexual dimorphism (model 2). Food defensibility was coded as absent (0) or present (1), and sexual dimorphism was centred on 1 (e.g. monomorphic species with males and females of the same size) and re-scaled such that units represent standard deviations. We also fitted a model including only a dummy variable for primates versus non-primates (model 3). Finally, we included sex bias in movements as a predictor of sex bias in intergroup conflicts (model 4).
We used the Bayesian regression models using the ‘Stan’ package v. 2.14.4 [78] in R v. 4.0.4 [79] for all analyses, as well as some functions of the phytools [80], rethinking [81], ape [82] and wesanderson [83] packages. We looped all models over the sample of 100 trees and pooled the parameter estimates to account for phylogenetic uncertainty. In addition, we ran all models on a consensus tree, generated with the consensus.edges() function in phytools; as there were virtually no differences between these approaches, we report only the looped models representing the full phylogenetic uncertainty.
From each model, we present the mean probabilities of each type of sex bias with 95% credible intervals and calculate the degree of support for the most likely type of sex bias to have higher probability than (i) each of the other types (i.e. the probability of the difference being greater than 0) and (ii) expected by chance (i.e. the probability of a sex-bias being greater than 0.33). Rather than representing arbitrary cut-offs like conventional statistical significance, these probabilities can directly be interpreted as the quantitative support for a given hypothesis; we invite readers to judge for themselves the degree of support they find convincing. Lastly, for each model, we also calculated its phylogenetic signal, Pagel's λ (i.e. proportion of variance explained by the phylogenetic random effects [84]), though note that we had to use highly regularizing priors on the phylogenetic variance components to achieve good model convergence, which reduced λ by about half.
3. Results
Overall, we found that male-biased participation was most likely in intergroup conflict across mammals (figure 1; model 1; see also the electronic supplementary material). Specifically, participation in intergroup conflicts was more likely to be male-biased (probability of male bias = 0.60, 95% credible interval (CI) = 0.25–0.86) than female-biased (0.16, 0.02–0.37) or unbiased (0.24, 0.08–0.45), with 93%, 90% and 92% confidence that the probability of male bias was higher than that of female bias, unbiased and chance, respectively. By contrast, leadership in collective movement was more likely to be female-biased (0.51, 0.13–0.85), as opposed to male-biased (0.23, 0.03–0.46) or unbiased (0.27, 0.06–0.49), with the difference in probabilities supported with 80% and 77% confidence respectively, and female bias was higher than expected by chance with 79% confidence. There was weak phylogenetic signal in both models (mean Pagel's λ and 95% CI for intergroup conflict = 0.08, 0.00–0.27); for movement = 0.18, 0.01–0.53), somewhat reduced by strong priors on variance components, implying that this typical mammalian pattern of male-biased participation in intergroup conflict and female-biased leadership in movement also applies to some extent to ancestral species.
These results did not change fundamentally when adding sexual dimorphism and resource defensibility as predictor variables (model 2; see the electronic supplementary material for full summary). For instance, male-biased participation in intergroup conflict was predicted to be somewhat less likely when food was defendable versus non-defendable, but with high uncertainty (mean odds ratio (OR) = 0.79, 95% CI = 0.25–1.49, probability OR less than 1 = 78%); other associations were even more uncertain. Results did also not differ qualitatively between primates and non-primates (model 3; see the electronic supplementary material). In terms of the link between sex bias across contexts (model 4), the probability of male-biased participation in intergroup conflict was reduced in species with female-biased leadership during movement (mean OR = 0.66, 95% CI = 0.18–1.29; probability OR less than 1 = 87%), though other associations were much more uncertain (e.g. greater female bias in intergroup conflict when there is female bias in movement, OR = 1.30, 95% CI = 0.33–2.60; probability OR greater than 1 = 62%; see the electronic supplementary material; figure 2).
Figure 2.
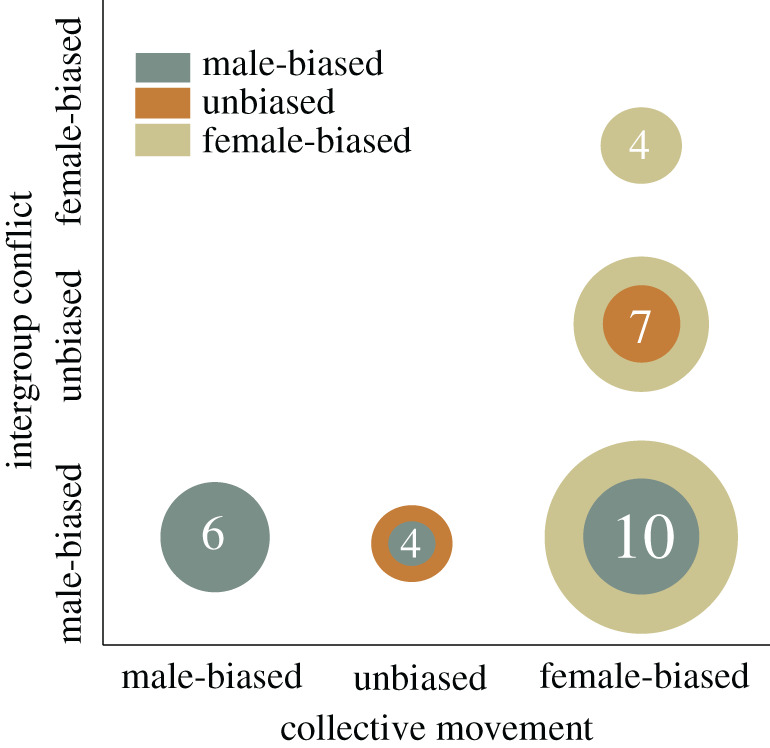
Distribution of sex-biased movements versus intergroup conflicts for the subset of social mammals for which data on the sexual distributions in collective action is available for both contexts. The size of each circle (and numbers inside each) reflect the numbers of species represented in each of the nine possible categories. Female-biased movements and male-biased intergroup conflict was the most common pattern (n= 10 species). Intergroup conflict was male-biased for all species with male-biased (n = 6) or unbiased (n = 4) leadership in movements. Female-bias in intergroup conflict (n = 4) or unbiased (n = 7) only emerged in species with a female bias in movements. (Online version in colour.)
4. Discussion
(a) . The general pattern: male warriors in intergroup conflict
We found that male bias in intergroup conflicts was the modal pattern across social mammals, accounting for phylogeny and several covariates. This pattern seems to reflect the differential costs and benefits of participation developed in the introduction, e.g. the possibility for males to gain mating opportunities (with in-group or out-group females) or defending in-group females, outweighing whatever resource benefits females may gain, or the costs of participation being substantially higher for females. This general pattern is typified by some of the best-studied non-human primates such as chimpanzees (Pan troglodytes) [85,86] and mountain gorillas (Gorilla gorilla gorilla) [87–90] in which males are considerably larger than females and, at least in the case of gorillas, the resources that males compete over (harems of females) are much more defensible than the resources females compete over (foliage). However, it is important to note that even for these species there is some evidence for female participation in intergroup conflicts; for instance, female gorillas direct aggression towards solitary males to avoid takeovers and the subsequent risk of infanticide [87]. More generally, sexual dimorphism in our study failed to predict male bias in intergroup conflicts. In fact, adult males and females are similarly sized in many species with male bias in intergroup conflict, including meerkats (Suricata suricatta) [91,92], Geoffroy's spider monkeys (Ateles geoffroyi) [61], Zanzibar red colobus monkeys (Piliocolobus kirkii) [93], and Thomas's langurs (Presbytis thomasi) [94]. Our analyses also suggest that food defensibility plays a minor role in shaping sex bias in intergroup conflict. In fact, species with male bias in intergroup conflicts spanned all diet types, ranging from herbivores (e.g. feral horses; Equus caballus) [95] to omnivores (e.g. white-faced capuchins; Cebus capucinus) [96,97] and carnivorans (e.g. grey wolves; Canis lupus) [98]. Thus, perhaps these sex differences are attributed more strongly to the differences in the costs of participation because female mammals are energetically limited by the demands of gestation, lactation and, in some species such as primates, the carrying of infants [51]. However, there was virtually no difference between primates and non-primates, hence the costs of carrying infants [51,74,99] do not seem to be a general explanation for this sex bias.
In summary, male-biased participation in intergroup conflict is the mammalian norm and most likely the ancestral pattern, which makes sense in light of differential costs and benefits. However, these costs and benefits are not well captured by our predictor variables sexual dimorphism, food defensibility or infant-carrying, hence future studies should test more nuanced predictions.
(b) . The general pattern: female guides in collective movements
Our analysis demonstrated that female mammals generally serve as 'guides' in collective movements. That is, on average, females recruit followers more often than males in the context of collective group movements, accounting for phylogeny and covariates. In most mammals, females are philopatric and may have increased knowledge about local resources, resulting in more frequent initiations or leadership of group movements [58,65]. Indeed, some of the best-studied examples of collective movements include adult female zebras (Equus burchellii) leading groups to water holes [100] and elder (post-menopausal) female orca whales (Orcinus orca) leading groups to limited food resources [101]. Food defensibility, but not sexual dimorphism, further affected collective movement in that male-biased leadership was less likely when food is defendable. Our analysis also uncovered associations between sex bias across the two contexts of collective action. Specifically, male-biased participation in intergroup conflict was less likely and female-biased participation more likely (though with much greater uncertainty) in species with female leadership during collective movement.
In summary, female leadership in movements was the norm and is consistent with female philopatry and the fact that female fitness is limited by access to food. Female leadership in the movement context also seems to constrain sex-biased participation in intergroup conflict, making male bias less and female bias more likely. We discuss exceptions to the general patterns in the next section by focusing on those species with female bias during intergroup conflicts.
(c) . Lessons from exceptions to the rule: the female warriors
A handful of species deviate from the modal pattern of male-biased participation in intergroup conflict which could shed additional light on the factors contributing to sex differences in this context. These ‘female warriors’ span four biological orders, including carnivorans and primates, suggesting independent evolutionary origins (see figure 3 for details). Whereas factors such as direct benefits, female dominance, intense female–female competition and female-biased sex ratios within groups may facilitate or permit female-biased leadership, there appear to be many paths favouring female bias during intergroup conflicts across social mammals.
Figure 3.
Female warriors among social mammals include a handful of species for which females participate in intergroup conflicts more often than do males, such as in the (b) spotted hyena, (c) ringtailed lemur, (d) golden snub-nosed monkey, (e) blue monkey and (f) black-and-white ruffed lemur. The (a) banded mongoose is also of particular note; male warriors participate in intergroup conflicts more often than females, but female banded mongooses instigate most intergroup conflicts and, therefore, disproportionally influence the initial group decision to engage in this form of collective action. Photos by Axel Tschentscher, Jack Grady and Charles J. Sharp with permission or part of the public domain. (Online version in colour.)
First, two plural breeding social carnivorans, banded mongooses (Mungos mungo) [43,102,103] and spotted hyenas (Crocuta crocuta) [30], experience intense female–female competition [104,105], infanticide [106,107] and territoriality [108–110], presumably owing to highly defensible food [30,111]. For both species, females instigate intergroup conflicts more than males, but only female spotted hyenas participate in conflicts at higher rates than do males. Thus, although banded mongooses fail to meet our definition of female warriors, they are still of particular note because they are exploitative leaders that instigate intergroup conflicts more than males [43]. The banded mongoose case is an important example for underscoring the critical importance of using precise definitions and consistent data collection when defining leadership (influence) versus participation (aggression) in intergroup conflicts. Interestingly, female mongooses gain direct incentives by mating with males from rival groups [43,102,103,112] and female hyenas gain more direct benefits (ephemeral, contested ungulate prey) than males from winning intergroup conflicts over territories [109,113]. High-ranking hyenas in female-dominated societies are also most often the ones charging in the front lines at intruders [30,114,115]. Black-and-white ruffed lemurs (Varecia variegata) [116] and ring-tailed lemurs (Lemur catta) [117–121] also live in female-dominated societies [122–124]; in these species females participate more and are more aggressive than males in defending fruits from intruders [116–121].
Second, female warriors also emerge in several species for which adult females are more numerically frequent than males, which may contribute to this pattern. For example, in golden snub-nosed (Rhinopithecus roxellana) [125], Samango (Cercopithecus albogularis) [126] and blue (Cercopithecus mitis stuhlmanni) [127] monkeys, one adult male is associated with multiple females. However, note that one-male multi-female groups are also common in gorillas and some langurs, who do not show female-biased participation in intergroup conflict. Female sexual competition is unusually intense among snub-nosed monkeys [128]; females initiate roughly 96% of courtship attempts [129,130] and join forces to protect against infanticidal males [131]. Female–female intergroup competition for access to fruit trees is also particularly intense for blue monkeys [132–134]. Thus, intense female competition may affect sex roles in collective action.
(d) . Open questions and a path forward for understanding collective action in animals
Overall, our analyses revealed that for a typical mammal, males participate more often in intergroup conflicts, and females lead more often in group movements. The patterns are generally consistent with sex-specific incentives for participation, though these are not well captured by our predictor variables of sexual dimorphism, food defensibility and infant carrying. We also found that female leadership in movements is associated with a reduced male bias and perhaps increased female bias in intergroup conflicts, suggesting some carry-over effects or constraints on leadership across these two contexts.
Our synthesis also raises many unanswered questions and there are few—if any—explanations that fit all of the species in our sample. Moreover, the available data cannot offer insights regarding whether intergroup encounters are fundamentally motivated by attacks (expanding access to resources) or defence (protecting current resources). Distinctions between these categories could provide further insights into sex differences in the motivations to attack, presumably to expand access to resources, versus simply defending existing ones. Thus, we propose that research should inquire about this distinction to the extent possible in field observations.
Several other key questions remain. Among these are whether intergroup conflicts in animals are comparable with those of human raids or warfare in size [36,135], motivation [27], or intensity [136]. With respect to motivation and intensity, intergroup conflicts by non-human animals may be based on individual incentives rather than on the understanding of a territory as a common good [27]; yet, individuals incentives may also contribute to human warfare to some extent [137]. Moreover, factors such as the adult sex ratio or risk of infanticide, which we were unable to explicitly include in our analysis, may further contribute to sex bias in intergroup behaviours. For example, in Indo-Pacific bottlenose dolphins with intense mating competition, a skewed adult sex ratio is associated with the formation of male alliances, pronounced male bias during inter-alliance conflicts, and infanticide [138–140]. More generally, future studies should compare observed and expected frequencies of participation in intergroup conflict by both sexes based on the adult sex ratio.
We focused on participation rather than leadership in intergroup conflict owing to limited data on the latter (see §2a). Studies identifying who initiated conflicts or showing that participation of specific individuals results in the recruitment of more actively participating individuals may help us to understand whether a leadership role can also be assigned to intergroup conflicts in a practical and meaningful way. Being able to quantify collective behaviours in these ways will also be able to contribute more broadly to understanding leadership within a comparative perspective. These efforts could also help to provide more concrete information on how the fitness consequences associated with each context vary by sex and intraspecifcally among groups; robust measures of reproductive outcomes [141] that allow for comparative analysis within and across species could offer more insights.
(e) . Possible implications for understanding the psychology of human collective behaviour
Our findings are consistent with the notion that male bias in participation in intergroup conflict (as seen in human warfare) has deep evolutionary roots within the mammalian lineage. Men are generally the main participants in warfare in both small-scale societies and large, industrialized societies [5,142] and the ‘male warrior hypothesis' [8,143,162] was thus proposed to explain male-dominated initiation, planning and participation in intergroup aggression and its effect on psychology [40,54]. On average, men are more prejudiced against and openly hostile towards members of outgroups, for example, immigrant groups in society, and outgroup men are more likely to be the target of intergroup prejudice [8]. Men have a stronger preference than women for inequalities between groups in society (i.e. social dominance orientation [144]), and this tendency is exacerbated when they are experimentally primed with an outgroup [145]. Men cooperate more with their group than women do in economic games when their group is in competition with other groups [143], and there is some evidence that this effect is stronger for physically more formidable men [146]. Moreover, men tend to, on average, take more risks and be more overconfident in risky situations than women [147–149], a finding consistent with evolutionary models [150]. While there is much debate about the antiquity of warfare in human history (e.g. with some arguing for a recent origin with complex foragers or the domestication of plants and animals [151,152]), our results suggest that male bias in intergroup conflict—whether considered ‘warfare’ or not—has deep roots in the mammalian lineage, giving it ample time to shape sex differences in intergroup psychology. Nevertheless, it is likely that certain aspects of women's psychology have also been shaped by intergroup conflict, but possibly in different ways (e.g. avoiding sexual coercion by men) and these await investigation [8].
Direct information about gender-biased leadership during collective movements in humans is limited. However, anthropological accounts of hunter-gatherer decision-making (e.g. about residential moves) generally emphasize consensus building [153,154], and models assuming gender equality in decisions regarding camp moves are most consistent with observed patterns [155]. Moreover, studies focused on leadership in human crowds in industrialized societies also usually involve mixed-gender groups and focus on how group size and uninformed individuals influence collective movements rather than gender differences in leadership [156].
Although leadership in collective movements in humans is less well studied, our findings that the sex bias among mammals is reversed between contexts are consistent with previous work on humans showing that gender-biased influence in human societies is often situational, varying across society type and organizational context [157]; for example, preferences for leaders with masculine traits are strongest during times of conflict [158,159] and conservative voters more strongly prefer masculine-looking political candidates than do more liberal voters [160]. Thus, more human studies focused on the extent of gender-biased leadership for various contexts and whether it covaries among contexts are needed. Together with our current findings, these studies could offer transdisciplinary insights [161] into the general patterns giving rise to sex differences in collective action across mammals, including humans.
Acknowledgements
We are grateful to the guest editors, Carsten K W De Dreu and Zegni Triki, for organizing this special issue and to Maddie Buhbe and Chelsea Ortiz-Jimenez for their contributions in the early phases of data extraction for this study. We thank Chris von Rueden, Michael Cant and two anonymous referees for their useful comments on an earlier version of the manuscript as well as the Institute for Advanced Study in Berlin (Wiko) for hosting a workshop (convened by P.M.K. and J.E.S.) that contributed to the ideas presented here.
Data accessibility
All data and R code to reproduce the results are available at https://github.com/adrianjaeggi/sexbiasmammals.
Authors' contributions
J.E.S.: conceptualization, data curation, investigation, methodology, project administration, resources, supervision, visualization, writing—original draft, writing—review and editing; C.F.: data curation, methodology, writing—review and editing; R.K.H.: data curation, writing—review and editing; P.M.K.: methodology, writing—review and editing; M.v.V.: methodology, writing—review and editing; A.V.J.: formal analysis, methodology, visualization, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
The authors have no competing interests.
Funding
We received no funding for this study.
References
- 1.Gat A. 1999. The pattern of fighting in simple, small-scale, prestate societies. J. Anthropol. Res. 55, 563-583. ( 10.1086/jar.55.4.3631615) [DOI] [Google Scholar]
- 2.Eagly AH, Johnson BT. 1990. Gender and leadership style: a meta-analysis. Psychol. Bull. 108, 233-256. ( 10.1037/0033-2909.108.2.233) [DOI] [Google Scholar]
- 3.Banks C. 2019. Introduction: women, gender, and terrorism: gendering terrorism. Women Crim. Justice 29, 181-187. ( 10.1080/08974454.2019.1633612) [DOI] [Google Scholar]
- 4.Wikipedia. 2021. Women in combat. See https://en.wikipedia.org/wiki/Women_in_combat.
- 5.Glowacki L, Wilson ML, Wrangham RW. 2020. The evolutionary anthropology of war. J. Econ. Behav. Organ. 178, 963-982. ( 10.1016/j.jebo.2017.09.014) [DOI] [Google Scholar]
- 6.Wrangham RW. 1999. Evolution of coalitionary killing. Yearb. Phys. Anthropol. 42, 1-30. () [DOI] [PubMed] [Google Scholar]
- 7.Bowles S. 2009. Did warfare among ancestral hunter-gatherers affect the evolution of human social behaviors? Science 324, 1293-1298. ( 10.1126/science.1168112) [DOI] [PubMed] [Google Scholar]
- 8.Mcdonald MM, Navarrete CD, Van Vugt M. 2012. Evolution and the psychology of intergroup conflict: the male warrior hypothesis. Phil. Trans. R. Soc. B 367, 670-679. ( 10.1098/rstb.2011.0301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fry DP, Söderberg P. 2013. Lethal aggression in mobile forager bands and implications for the origins of war. Science 341, 270-273. ( 10.1126/SCIENCE.1235675) [DOI] [PubMed] [Google Scholar]
- 10.Smith JE, Lacey EA, Hayes LD. 2017. Sociality in non-primate mammals. In Comparative social evolution (eds Rubenstein DR, Abbot P), pp. 284-319. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 11.Silk JB, Kappeler PM. 2017. Sociality in primates. In Comparative social evolution, pp. 253-283. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 12.Bshary R, van Schaik C. 2022. Male services during intergroup conflict: the ‘hired gun’ hypothesis revisited. Phil. Trans. R. Soc. B 377, 20210150. ( 10.1098/rstb.2021.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wrangham RW. 1980. An ecological model of female-bonded primate groups. Behaviour 75, 262-300. ( 10.1163/156853980X00447) [DOI] [Google Scholar]
- 14.Sterck EHM, Watts DP, van Schaik CP. 1997. The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 41, 291-309. ( 10.1007/s002650050390) [DOI] [Google Scholar]
- 15.Radford AN, Majolo B, Aureli F. 2016. Within-group behavioural consequences of between-group conflict: a prospective review. Proc. R. Soc. B 283, 20161567. ( 10.1098/rspb.2016.1567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitani JC, Watts DP, Amsler SJ. 2010. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr. Biol. 20, R507-R508. ( 10.1016/j.cub.2010.04.021) [DOI] [PubMed] [Google Scholar]
- 17.Tórrez-Herrera LL, Davis GH, Crofoot MC. 2020. Do monkeys avoid areas of home range overlap because they are dangerous? A test of the risk hypothesis in white-faced capuchin monkeys (Cebus capucinus). Int. J. Primatol. 41, 246-264. ( 10.1007/s10764-019-00110-0) [DOI] [Google Scholar]
- 18.Gavrilets S, Fortunato L. 2014. A solution to the collective action problem in between-group conflict with within-group inequality. Nat. Commun. 5, 3526. ( 10.1038/ncomms4526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radford AN, Fawcett TW. 2014. Conflict between groups promotes later defense of a critical resource in a cooperatively breeding bird. Curr. Biol. 24, 2935-2939. ( 10.1016/j.cub.2014.10.036) [DOI] [PubMed] [Google Scholar]
- 20.Wilson ML, Wrangham RW. 2003. Intergroup relations in chimpanzees. Annu. Rev. Anthropol. 32, 363-392. ( 10.1146/annurev.anthro.32.061002.120046) [DOI] [Google Scholar]
- 21.Smith JM, Price GR. 1973. The logic of animal conflict. Nature 246, 15-18. ( 10.1038/246015a0) [DOI] [Google Scholar]
- 22.Enquist M, Leimar O. 1990. The evolution of fatal fighting. Anim. Behav. 39, 1-9. ( 10.1016/S0003-3472(05)80721-3) [DOI] [Google Scholar]
- 23.Kitchen DM, Beehner JC. 2007. Factors affecting individual participation in group-level aggression among non-human primates. Behaviour 144, 1551-1581. ( 10.1163/156853907782512074) [DOI] [Google Scholar]
- 24.Crofoot MC. 2013. The cost of defeat: capuchin groups travel further, faster and later after losing conflicts with neighbors. Am. J. Phys. Anthropol. 152, 79-85. ( 10.1002/ajpa.22330) [DOI] [PubMed] [Google Scholar]
- 25.Crofoot MC, Gilby IC. 2012. Cheating monkeys undermine group strength in enemy territory. Proc. Natl Acad. Sci. USA 109, 501-505. ( 10.1073/pnas.1115937109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nunn C, Deaner R. 2004. Patterns of participation and free riding in territorial conflicts among ringtailed lemurs (Lemur catta). Behav. Ecol. Sociobiol. 57, 50-61. ( 10.1007/s00265-004-0830-5) [DOI] [Google Scholar]
- 27.Willems EP, Hellriegel B, van Schaik CP. 2013. The collective action problem in primate territory economics. Proc. R. Soc. B 280, 20130081. ( 10.1098/rspb.2013.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunn C. 2000. Collective benefits, free-riders, and male extra-group conflict. In Primate males: causes and consequences of variation in group composition (ed. Kappeler P), pp. 192-204. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 29.Willems EP, van Schaik CP. 2015. Collective action and the intensity of between-group competition in nonhuman primates. Behav. Ecol. 26, 625-631. ( 10.1093/beheco/arv001) [DOI] [Google Scholar]
- 30.Boydston EE, Morelli TL, Holekamp KE. 2001. Sex differences in territorial behavior exhibited by the spotted hyena (Hyaenidae, Crocuta crocuta). Ethology 107, 369-385. ( 10.1046/j.1439-0310.2001.00672.x) [DOI] [Google Scholar]
- 31.Lazaro-Perea C. 2001. Intergroup interactions in wild common marmosets, Callithrix jacchus: territorial defence and assessment of neighbours. Anim. Behav. 62, 11-21. ( 10.1006/anbe.2000.1726) [DOI] [Google Scholar]
- 32.Cooper MA, Aureli F, Singh M. 2004. Between-group encounters among bonnet macaques (Macaca radiata). Behav. Ecol. Sociobiol. 56, 217-227. ( 10.1007/s00265-004-0779-4) [DOI] [Google Scholar]
- 33.Alesina A, Giuliano P, Nunn N. 2011. On the origins of gender roles: women and the plough. Inst. Study Labor Discuss. Pap. 5735, 1-43. ( 10.3386/w17098) [DOI] [Google Scholar]
- 34.Willems EP, Arseneau TJM, Schleuning X, van Schaik CP. 2015. Communal range defence in primates as a public goods dilemma. Phil. Trans. R. Soc. B 370, 20150003. ( 10.1098/rstb.2015.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arseneau-Robar TJM, Taucher AL, Schnider AB, van Schaik CP, Willems EP. 2017. Intra- and interindividual differences in the costs and benefits of intergroup aggression in female vervet monkeys. Anim. Behav. 123, 129-137. ( 10.1016/j.anbehav.2016.10.034) [DOI] [Google Scholar]
- 36.Majolo B, deBortoli Vizioli A, Martínez-Íñigo L, Lehmann J. 2020. Effect of group size and individual characteristics on intergroup encounters in primates. Int. J. Primatol. 41, 325-341. ( 10.1007/s10764-019-00119-5) [DOI] [Google Scholar]
- 37.Majolo B, Ventura R, Koyama NF. 2005. Sex, rank and age differences in the Japanese macaque (Macaca fuscata yakui) participation in inter-group encounters. Ethology 111, 455-468. ( 10.1111/j.1439-0310.2005.01087.x) [DOI] [Google Scholar]
- 38.Andersson MB. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 39.Kappeler PM, Van Schaik CP. 2002. Evolution of primate social systems. Int. J. Primatol. 23, 707-740. ( 10.1023/A:1015520830318) [DOI] [Google Scholar]
- 40.Emlen S, Oring L. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197, 215-223. ( 10.1126/science.327542) [DOI] [PubMed] [Google Scholar]
- 41.Smith JE, Ortiz CA, Buhbe MT, van Vugt M. 2020. Obstacles and opportunities for female leadership in mammalian societies: a comparative perspective. Leadersh. Q. 31, 101267. ( 10.1016/j.leaqua.2018.09.005) [DOI] [Google Scholar]
- 42.Smith JE, von Rueden CR, van Vugt M, Fichtel C, Kappeler PM. 2021. An evolutionary explanation for the female leadership paradox. Front. Ecol. Evol. 9, 468. ( 10.3389/FEVO.2021.676805) [DOI] [Google Scholar]
- 43.Johnstone RA, Cant MA, Cram D, Thompson FJ. 2020. Exploitative leaders incite intergroup warfare in a social mammal. Proc. Natl Acad. Sci. USA 117, 29 759-29 766. ( 10.1073/pnas.2003745117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arseneau-Robar TJM, Taucher AL, Müller E, Van Schaik C, Bshary R, Willems EP. 2016. Female monkeys use both the carrot and the stick to promote male participation in intergroup fights. Proc. R. Soc. B 283, 20161817. ( 10.1098/rspb.2016.1817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Payne HFP, Lawes MJ, Henzi SP. 2003. Fatal attack on an adult female Cercopithecus mitis erythrarchus : implications for female dispersal in female-bonded societies. Int. J. Primatol. 24, 1245-1250. ( 10.1023/B:IJOP.0000005990.39403.96) [DOI] [Google Scholar]
- 46.Lewis RJ, Sandel AA, Hilty S, Barnett SE. 2020. The collective action problem but not numerical superiority explains success in intergroup encounters in Verreaux's sifaka (Propithecus verreauxi): implications for individual participation and free-riding. Int. J. Primatol. 41, 305-324. ( 10.1007/s10764-020-00155-6) [DOI] [Google Scholar]
- 47.Blanckenhorn WU. 2005. Behavioral causes and consequences of sexual size dimorphism. Ethology 111, 977-1016. ( 10.1111/j.1439-0310.2005.01147.x) [DOI] [Google Scholar]
- 48.Plavcan JM. 2012. Sexual size dimorphism, canine dimorphism, and male-male competition in primates: where do humans fit in? Hum. Nat. 23, 45-67. ( 10.1007/s12110-012-9130-3) [DOI] [PubMed] [Google Scholar]
- 49.Leutenegger W, Kelly JT. 1977. Relationship of sexual dimorphism in canine size and body size to social, behavioral, and ecological correlates in anthropoid primates. Primates 18, 117-136. ( 10.1007/BF02382954) [DOI] [Google Scholar]
- 50.Fashing PJ. 2001. Male and female strategies during intergroup encounters in guerezas (Colobus guereza): evidence for resource defense mediated through males and a comparison with other primates. Behav. Ecol. Sociobiol. 50, 219-230. ( 10.1007/s002650100358) [DOI] [Google Scholar]
- 51.Koch F, Signer J, Kappeler PM, Fichtel C. 2016. Intergroup encounters in Verreaux's sifakas (Propithecus verreauxi): who fights and why? Behav. Ecol. Sociobiol. 70, 797-808. ( 10.1007/s00265-016-2105-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arseneau-Robar TJM, Müller E, Taucher AL, Van Schaik CP, Bshary R, Willems EP. 2018. Male monkeys use punishment and coercion to de-escalate costly intergroup fights. Proc. R. Soc. B 285, 20172323. ( 10.1098/rspb.2017.2323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conradt L, Roper TJ. 2005. Consensus decision making in animals. Trends Ecol. Evol. 20, 449-456. ( 10.1016/j.tree.2005.05.008) [DOI] [PubMed] [Google Scholar]
- 54.Bourjade M, Sueur C. 2010. Shared or unshared consensus for collective movement? Towards methodological concerns. Behav. Processes 84, 648-652. ( 10.1016/j.beproc.2010.02.027) [DOI] [PubMed] [Google Scholar]
- 55.Conradt L, Roper T. 2007. Democracy in animals: the evolution of shared group decisions. Proc. R. Soc. B 274, 2317-2326. ( 10.1098/rspb.2007.0186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boinski S, Garber PA. 2000. On the move: how and why animals travel in groups. Chicago, IL: University of Chicago Press. [Google Scholar]
- 57.Reynolds CW. 1987. Flocks, herds and schools: a distributed behavioral model. ACM SIGGRAPH Comput. Graph. 21, 25-34. ( 10.1145/37402.37406) [DOI] [Google Scholar]
- 58.Smith JE, Estrada JR, Richards HR, Dawes SE, Mitsos K, Holekamp KE. 2015. Collective movements, leadership and consensus costs at reunions in spotted hyaenas. Anim. Behav. 105, 187-200. ( 10.1016/j.anbehav.2015.04.023) [DOI] [Google Scholar]
- 59.Smith JE, et al. 2016. Leadership in mammalian societies: emergence, distribution, power, and payoff. Trends Ecol. Evol. 31, 54-66. ( 10.1016/j.tree.2015.09.013) [DOI] [PubMed] [Google Scholar]
- 60.Smith JE, van Vugt M. 2020. Leadership and status in mammalian societies: context matters. Trends Cogn. Sci. 24, 263-264. ( 10.1016/j.tics.2020.01.003) [DOI] [PubMed] [Google Scholar]
- 61.Fedigan LM, Baxter MJ. 1984. Sex differences and social organization in free-ranging spider monkeys (Ateles geoffroyi). Primates 25, 279-294. ( 10.1007/BF02382267) [DOI] [Google Scholar]
- 62.de Waal FBM. 1986. The integration of dominance and social bonding in primates. Q. Rev. Biol. 61, 459-479. ( 10.1086/415144) [DOI] [PubMed] [Google Scholar]
- 63.van Vugt M. 2006. Evolutionary origins of leadership and followership. Personal. Soc. Psychol. Rev. 10, 354-371. ( 10.1207/s15327957pspr1004_5) [DOI] [PubMed] [Google Scholar]
- 64.Hollander EP. 1992. Leadership, followership, self, and others. Leadersh. Q. 3, 43-54. ( 10.1016/1048-9843(92)90005-Z) [DOI] [Google Scholar]
- 65.Fichtel C, Pyritz L, Kappeler PM. 2011. Coordination of group movements in non-human primates. In Coordination in human and primate groups (eds Boos M, Kolbe M, Kappeier PM, Elwart T), pp. 37-56. Berlin, Germany: Springer-Verlag. [Google Scholar]
- 66.King AJ, Douglas CMS, Huchard E, Isaac NJB, Cowlishaw G. 2008. Dominance and affiliation mediate despotism in a social primate. Curr. Biol. 18, 1833-1838. ( 10.1016/j.cub.2008.10.048) [DOI] [PubMed] [Google Scholar]
- 67.Hemelrijk CK, Wantia J, Isler K. 2008. Female dominance over males in primates: self-organisation and sexual dimorphism. PLoS ONE 3, e2678. ( 10.1371/journal.pone.0002678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simon HA. 1953. Notes on the observation and measurement of political power. J. Polit. 15, 500-516. ( 10.2307/2126538) [DOI] [Google Scholar]
- 69.Cheng JT, Tracy JL, Foulsham T, Kingstone A, Henrich J. 2013. Two ways to the top: evidence that dominance and prestige are distinct yet viable avenues to social rank and influence. J. Pers. Soc. Psychol. 104, 103-125. ( 10.1037/a0030398) [DOI] [PubMed] [Google Scholar]
- 70.van Vugt M, Smith JE. 2019. A dual model of leadership and hierarchy: evolutionary synthesis. Trends Cogn. Sci. 23, 952-967. ( 10.1016/j.tics.2019.09.004) [DOI] [PubMed] [Google Scholar]
- 71.King AJ, Johnson DDP, van Vugt M. 2009. The origins and evolution of leadership. Curr. Biol. 19, R911-R916. ( 10.1016/j.cub.2009.07.027) [DOI] [PubMed] [Google Scholar]
- 72.Hadfield JD, Nakagawa S. 2010. General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evol. Biol. 23, 494-508. ( 10.1111/j.1420-9101.2009.01915.x) [DOI] [PubMed] [Google Scholar]
- 73.Smith JE, Van Horn RC, Powning KS, Cole AR, Graham KE, Memenis SK, Holekamp KE. 2010. Evolutionary forces favoring intragroup coalitions among spotted hyenas and other animals. Behav. Ecol. 21, 284-303. ( 10.1093/beheco/arp181) [DOI] [Google Scholar]
- 74.Van Schaik CP, Kappeler PM. 1997. Infanticide risk and the evolution of male–female association in primates. Proc. R. Soc. Lond. B 264, 1687-1694. ( 10.1098/rspb.1997.0234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruckstuhl KE, Neuhaus P. 2002. Sexual segregation in ungulates: a comparative test of three hypotheses. Biol. Rev. 77, 77-96. ( 10.1017/S1464793101005814) [DOI] [PubMed] [Google Scholar]
- 76.Upham NS, Esselstyn JA, Jetz W. 2019. Inferring the mammal tree: species-level sets of phylogenies for questions in ecology, evolution, and conservation. PLoS Biol. 17, e3000494. ( 10.1371/journal.pbio.3000494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koster J, McElreath R. 2017. Multinomial analysis of behavior: statistical methods. Behav. Ecol. Sociobiol. 71, 1-14. ( 10.1007/s00265-017-2363-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bürkner PC. 2017. Brms: an R package for Bayesian multilevel models using Stan. J. Stat. Softw. 80, 1-28. ( 10.18637/jss.v080.i01) [DOI] [Google Scholar]
- 79.R Development Core Team. 2021. R: a language and environment for statistical computing. Boston, MA: R Foundation for Statistical Computing. (https://www.r-project.org/. [Google Scholar]
- 80.Revell LJ. 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217-223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 81.McElreath R. 2020. Statistical rethinking: a Bayesian course with examples in R and stan. Boca Raton, FL: Chapman and Hall/CRC. [Google Scholar]
- 82.Paradis E. 2012. Analysis of phylogenetics and evolution with R: second edition. 2nd edn. Dordrecht, Germany: Springer New York. [Google Scholar]
- 83.Ram K, Wickham H, Richards C, Baggett A. In press. A Wes Anderson palette generator. See https://github.com/karthik/wesanderson.
- 84.Nakagawa S, Johnson PCD, Schielzeth H. 2017. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 14, 20170213. ( 10.1098/rsif.2017.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilson ML, Hauser MD, Wrangham RW. 2001. Does participation in intergroup conflict depend on numerical assessment, range location, or rank for wild chimpanzees? Anim. Behav. 61, 1203-1216. ( 10.1006/anbe.2000.1706) [DOI] [Google Scholar]
- 86.Muller MN, Mitani JC. 2005. Conflict and cooperation in wild chimpanzees. Adv. Study Behav. 35, 275-331. ( 10.1016/S0065-3454(05)35007-8) [DOI] [Google Scholar]
- 87.Mirville MO, Ridley AR, Samedi JPM, Vecellio V, Ndagijimana F, Stoinski TS, Grueter CC. 2018. Factors influencing individual participation during intergroup interactions in mountain gorillas. Anim. Behav. 144, 75-86. ( 10.1016/j.anbehav.2018.08.003) [DOI] [Google Scholar]
- 88.Mirville MO, Ridley AR, Samedi JPM, Vecellio V, Ndagijimana F, Stoinski TS, Grueter CC. 2018. Low familiarity and similar ‘group strength’ between opponents increase the intensity of intergroup interactions in mountain gorillas (Gorilla beringei beringei). Behav. Ecol. Sociobiol. 72, 1-14. ( 10.1007/s00265-018-2592-5) [DOI] [Google Scholar]
- 89.Robbins M, Sawyer S. 2007. Intergroup encounters in mountain gorillas of Bwindi Impenetrable National Park, Uganda. Behaviour 144, 1497-1519. ( 10.1163/156853907782512146) [DOI] [Google Scholar]
- 90.Caillaud D, Levréro F, Gatti S, Ménard N, Raymond M. 2008. Influence of male morphology on male mating status and behavior during interunit encounters in western lowland gorillas. Am. J. Phys. Anthropol. 135, 379-388. ( 10.1002/ajpa.20754) [DOI] [PubMed] [Google Scholar]
- 91.Jordan NR, Cherry MI, Manser MB. 2007. Latrine distribution and patterns of use by wild meerkats: implications for territory and mate defence. Anim. Behav. 73, 613-622. ( 10.1016/j.anbehav.2006.06.010) [DOI] [Google Scholar]
- 92.Drewe JA, Madden JR, Pearce GP. 2009. The social network structure of a wild meerkat population: 1. Inter-group interactions. Behav. Ecol. Sociobiol. 63, 1295-1306. ( 10.1007/s00265-009-0782-x) [DOI] [Google Scholar]
- 93.Siex KS. 2003. Effects of population compression on the demography, ecology, and behavior of the zanzibar red colobus monkey (procolobus kirkii). Durham, NC: Duke University. [Google Scholar]
- 94.Steenbeek R. 1999. Tenure related changes in wild Thomas langurs. I: between-group interactions. Behaviour 136, 595-625. ( 10.1163/156853999501487) [DOI] [Google Scholar]
- 95.Berger J. 1977. Organizational systems and dominance in feral horses in the Grand Canyon. Behav. Ecol. Sociobiol. 2, 131-146. ( 10.1007/BF00361898) [DOI] [Google Scholar]
- 96.Perry S. 1996. Intergroup encounters in wild white-faced capuchins (Cebus capucinus). Int. J. Primatol. 17, 309-330. ( 10.1007/BF02736624) [DOI] [Google Scholar]
- 97.Vogel ER, Munch SB, Janson CH. 2007. Understanding escalated aggression over food resources in white-faced capuchin monkeys. Anim. Behav. 74, 71-80. ( 10.1016/j.anbehav.2007.02.003) [DOI] [Google Scholar]
- 98.Cassidy KA, Mech LD, MacNulty DR, Stahler DR, Smith DW. 2017. Sexually dimorphic aggression indicates male gray wolves specialize in pack defense against conspecific groups. Behav. Processes 136, 64-72. ( 10.1016/j.beproc.2017.01.011) [DOI] [PubMed] [Google Scholar]
- 99.Koch F, Signer J, Kappeler PM, Fichtel C. 2016. The role of the residence-effect on the outcome of intergroup encounters in Verreaux's sifakas. Sci. Rep. 6, 28457. ( 10.1038/srep28457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fischhoff IR, Sundaresan SR, Cordingley J, Larkin HM, Sellier MJ, Rubenstein DI. 2007. Social relationships and reproductive state influence leadership roles in movements of plains zebra, Equus burchellii. Anim. Behav. 73, 825-831. ( 10.1016/j.anbehav.2006.10.012) [DOI] [Google Scholar]
- 101.Brent LJN, Franks DW, Foster EA, Balcomb KC, Cant MA, Croft DP. 2015. Ecological knowledge, leadership, and the evolution of menopause in killer whales. Curr. Biol. 25, 746-750. ( 10.1016/j.cub.2015.01.037) [DOI] [PubMed] [Google Scholar]
- 102.Cant MA, Vitikainen E, Nichols HJ. 2013. Demography and social evolution of banded mongooses. Amsterdam, The Netherlands: Elsevier Inc. [Google Scholar]
- 103.Furrer RD, Kyabulima S, Willems EP, Cant MA, Manser MB. 2011. Location and group size influence decisions in simulated intergroup encounters in banded mongooses. Behav. Ecol. 22, 493-500. ( 10.1093/beheco/arr010) [DOI] [Google Scholar]
- 104.Cant MA, Otali E, Mwanguhya F. 2001. Eviction and dispersal in co-operatively breeding banded mongooses (Mungos mungo). J. Zool. 254, 155-162. ( 10.1017/S0952836901000668) [DOI] [Google Scholar]
- 105.Frank LG. 1986. Social organization of the spotted hyaena Crocuta crocuta. II. Dominance and reproduction. Anim. Behav. 34, 1510-1527. ( 10.1016/S0003-3472(86)80221-4) [DOI] [Google Scholar]
- 106.East ML, Burke T, Wilhelm K, Greig C, Hofer H. 2003. Sexual conflicts in spotted hyenas: male and female mating tactics and their reproductive outcome with respect to age, social status and tenure. Proc. R. Soc. Lond. B 270, 1247-1254. ( 10.1098/rspb.2003.2363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gilchrist JS. 2006. Female eviction, abortion, and infanticide in banded mongooses (Mungos mungo): implications for social control of reproduction and synchronized parturition. Behav. Ecol. 17, 664-669. ( 10.1093/beheco/ark012) [DOI] [Google Scholar]
- 108.Cant MA. 2000. Social control of reproduction in banded mongooses. Anim. Behav. 59, 147-158. ( 10.1006/anbe.1999.1279) [DOI] [PubMed] [Google Scholar]
- 109.Van Horn RC, Engh AL, Scribner KT, Funk SM, Holekamp KE. 2004. Behavioural structuring of relatedness in the spotted hyena (Crocuta crocuta) suggests direct fitness benefits of clan-level cooperation. Mol. Ecol. 13, 449-458. ( 10.1046/j.1365-294X.2003.02071.x) [DOI] [PubMed] [Google Scholar]
- 110.Smith JE, Kolowski JM, Graham KE, Dawes SE, Holekamp KE. 2008. Social and ecological determinants of fission-fusion dynamics in the spotted hyaena. Anim. Behav. 76, 619-636. ( 10.1016/j.anbehav.2008.05.001) [DOI] [Google Scholar]
- 111.Thompson F, Marshall H, Vitikainen E, Behaviour MC. 2017. Causes and consequences of intergroup conflict in cooperative banded mongooses. Anim. Behav. 126, 31-40. ( 10.1016/j.anbehav.2017.01.017) [DOI] [Google Scholar]
- 112.Cant MA, Otali E, Mwanguhya F. 2002. Fighting and mating between groups in a cooperatively breeding mammal, the banded mongoose. Ethology 108, 541-555. ( 10.1046/j.1439-0310.2002.00795.x) [DOI] [Google Scholar]
- 113.Smith JE, Holekamp KE. In press. Behavioral strategies and morphological adaptations contributing to hunting success in the spotted hyena. In Social strategies of carnivorous mammalian predators (eds Srinivasan LB, Würsig B). Cham, Switzerland: Springer Nature Publishers. [Google Scholar]
- 114.Kruuk H. 1972. The spotted hyena: a study of predation and social behavior. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 115.Holekamp KE, Ogutu JO, Dublin HT, Frank LG, Smale L. 1993. Fission of a spotted hyena clan: consequences of prolonged female absenteeism and causes of female emigration. Ethology 93, 285-299. ( 10.1111/j.1439-0310.1993.tb01210.x) [DOI] [Google Scholar]
- 116.Morland HS. 1991. Preliminary report on the social organization of ruffed lemurs (Varecia variegata variegata) in a northeast Madagascar rain forest. Folia Primatol. 56, 157-161. ( 10.1159/000156540) [DOI] [Google Scholar]
- 117.Pereira ME, Kappeler PM. 1997. Divergent systems of agonistic behaviour in lemurid primates. Behaviour 134, 225-274. ( 10.1163/156853997X00467) [DOI] [Google Scholar]
- 118.Sauther ML, Sussman RW, Gould L. 1999. The socioecology of the ringtailed lemur: thirty-five years of research. Evol. Anthropol. 8, 120-132. () [DOI] [Google Scholar]
- 119.Nakamichi M, Koyama N. 1997. Social relationships among ring-tailed lemurs (Lemur catta) in two free-ranging troops at Berenty Reserve, Madagascar. Int. J. Primatol. 18, 73-93. ( 10.1023/A:1026393223883) [DOI] [Google Scholar]
- 120.Jolly A. 1998. Behaviour: reviewed article pair-bonding, female aggression and the keynote address. Folia Primatol. 69, 1-13. ( 10.1159/000052693) [DOI] [PubMed] [Google Scholar]
- 121.Hood LC, Jolly A. 1995. Troop fission in female Lemur catta at Berenty Reserve, Madagascar. Int. J. Primatol. 16, 997-1015. ( 10.1007/BF02696113) [DOI] [Google Scholar]
- 122.Kappeler PM. 1990. Female dominance in Lemur catta: more than just female feeding priority? Folia Primatol. 55, 92-95. ( 10.1159/000156504) [DOI] [PubMed] [Google Scholar]
- 123.Raps S, White FJ. 1995. Female social dominance in semi-free-ranging ruffed lemurs (Varecia variegata). Folia Primatol. 65, 163-168. ( 10.1159/000156883) [DOI] [PubMed] [Google Scholar]
- 124.Kappeler PM, Fichtel C. 2015. Eco-evo-devo of the lemur syndrome: did adaptive behavioral plasticity get canalized in a large primate radiation? Front. Zool. 12, 1-16. ( 10.1186/1742-9994-12-S1-S15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xiang ZF, Yang BH, Yu Y, Yao H, Grueter CC, Garber PA, Li M. 2014. Males collectively defend their one-male units against bachelor males in a multi-level primate society. Am. J. Primatol. 76, 609-617. ( 10.1002/ajp.22254) [DOI] [PubMed] [Google Scholar]
- 126.Payne HFP, Lawes MJ, Henzi SP. 2003. Competition and the exchange of grooming among female samango monkeys (Cercopithecus mitis erythrarchus). Behaviour 140, 453-471. ( 10.1163/156853903322127931) [DOI] [Google Scholar]
- 127.Roth AM, Cords M. 2016. Effects of group size and contest location on the outcome and intensity of intergroup contests in wild blue monkeys. Anim. Behav. 113, 49-58. ( 10.1016/j.anbehav.2015.11.011) [DOI] [Google Scholar]
- 128.Peng Z, Baoguo L, Wada K, Watanabe K. 2003. Social structure of a group of Sichuan snub nosed monkeys (Rhinopithecus roxellana) in the Qinling Mountains of China. Acta Zool. Sin. 49, 727-735. [Google Scholar]
- 129.Li B, Zhao D. 2007. Copulation behavior within one-male groups of wild Rhinopithecus roxellana in the Qinling Mountains of China. Primates 48, 190-196. ( 10.1007/s10329-006-0029-7) [DOI] [PubMed] [Google Scholar]
- 130.Smuts B. 1987. Sexual competition and mate choice. In Primate societies (eds Smuts BB, Cheney DL, Wrangham RW, Seyfarth RM), pp. 385-399. Chicago, IL: Universtiy of Chicago Press. [Google Scholar]
- 131.Yao H, Yu H, Yang B, Yang W, Xu H, Grueter CC, Li M, Xiang Z. 2016. Male infanticide in the golden snub-nosed monkey (Rhinopithecus roxellana), a seasonally breeding primate. Int. J. Primatol. 37, 175-184. ( 10.1007/s10764-016-9892-2) [DOI] [Google Scholar]
- 132.Cords M. 1986. Interspecific and intraspecific variation in diet of two forest guenons, Cercopithecus ascanius and C. mitis. J. Anim. Ecol. 55, 811. ( 10.2307/4418) [DOI] [Google Scholar]
- 133.Förster S, Cords M. 2005. Socialization of infant blue monkeys (Cercopithecus mitis stuhlmanni): allomaternal interactions and sex differences. Behaviour 142, 869-896. ( 10.1163/1568539055010138) [DOI] [Google Scholar]
- 134.Cords M. 2002. Friendship among adult female blue monkeys (Cercopithecus mitis). Behaviour 139, 291-314. ( 10.1163/156853902760102681) [DOI] [Google Scholar]
- 135.Mathew S, Boyd R. 2011. Punishment sustains large-scale cooperation in prestate warfare. Proc. Natl Acad. Sci. USA 108, 11 375-11 380. ( 10.1073/pnas.1105604108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Aureli F, Schaffner CM, Verpooten J, Slater K, Ramos-Fernandez G. 2006. Raiding parties of male spider monkeys: insights into human warfare? Am. J. Phys. Anthropol. 131, 486-497. ( 10.1002/ajpa.20451) [DOI] [PubMed] [Google Scholar]
- 137.Glowacki L, Wrangham RW. 2013. The role of rewards in motivating participation in simple warfare. Hum. Nat. 24, 444-460. ( 10.1007/s12110-013-9178-8) [DOI] [PubMed] [Google Scholar]
- 138.Scott EM, Mann J, Watson-Capps JJ, Sargeant BL, Connor RC. 2005. Aggression in bottlenose dolphins: evidence for sexual coercion, male-male competition, and female tolerance through analysis of tooth-rake marks and behaviour. Behaviour 142, 21-44. ( 10.1163/1568539053627712) [DOI] [Google Scholar]
- 139.Randic S, Connor RC, Sherwin WB, Krutzen M. 2012. A novel mammalian social structure in Indo-Pacific bottlenose dolphins (Tursiops sp.): complex male alliances in an open social network. Proc. R. Soc. B 279, 3083-3090. ( 10.1098/rspb.2012.0264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Connor RC, Watson-Capps JJ, Sherwin WB, Krutzen M. 2011. A new level of complexity in the male alliance networks of Indian Ocean bottlenose dolphins (Tursiops sp .). Biol. Lett. 7, 623-626. ( 10.1098/rsbl.2010.0852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ross CT, Jaeggi AV, Mulder MB, Smith JE, Smith EA, Gavrilets S, Hooper PL. 2020. The multinomial index: a robust measure of reproductive skew. Proc. R. Soc. B 287, 20202025. ( 10.1098/rspb.2020.2025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goldstein JS. 2003. Gender and war: how gender shapes the war system and vice versa. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 143.Van Vugt M, De Cremer D, Janssen DP. 2007. Gender differences in cooperation and competition: the male-warrior hypothesis. Psychol. Sci. 18, 19-23. ( 10.1111/j.1467-9280.2007.01842.x) [DOI] [PubMed] [Google Scholar]
- 144.Ho AK, Sidanius J, Kteily N, Sheehy-Skeffington J, Pratto F, Henkel KE, Foels R, Stewart AL. 2015. The nature of social dominance orientation: theorizing and measuring preferences for intergroup inequality using the new SDO7 scale. J. Pers. Soc. Psychol. 109, 1003-1028. ( 10.1037/pspi0000033) [DOI] [PubMed] [Google Scholar]
- 145.Sugiura H, Mifune N, Tsuboi S, Yokota K. 2017. Gender differences in intergroup conflict: the effect of outgroup threat priming on social dominance orientation. Pers. Individ. Dif. 104, 262-265. ( 10.1016/j.paid.2016.08.013) [DOI] [Google Scholar]
- 146.Muñoz-Reyes JA, Polo P, Valenzuela N, Pavez P, Ramírez-Herrera O, Figueroa O, Rodriguez-Sickert C, Díaz D, Pita M. 2020. The male warrior hypothesis: testosterone-related cooperation and aggression in the context of intergroup conflict. Sci. Rep. 10, 1-12. ( 10.1038/s41598-019-57259-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Starkweather KE, Shenk MK, McElreath R. 2020. Biological constraints and socioecological influences on women's pursuit of risk and the sexual division of labour. Evol. Hum. Sci. 2, 1-17. ( 10.1017/ehs.2020.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Byrnes JP, Miller DC, Schafer WD. 1999. Gender differences in risk taking: a meta-analysis. Psychol. Bull. 125, 367-383. ( 10.1037/0033-2909.125.3.367) [DOI] [Google Scholar]
- 149.Johnson DD., McDermott R, Barrett ES, Cowden J, Wrangham R, McIntyre MH, Rosen SP. 2006. Overconfidence in wargames: experimental evidence on expectations, aggression, gender and testosterone. Proc. R. Soc. B 273, 2513-2520. ( 10.1098/rspb.2006.3606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Johnson DDP, Fowler JH. 2011. The evolution of overconfidence. Nature 477, 317-320. ( 10.1038/nature10384) [DOI] [PubMed] [Google Scholar]
- 151.Kaplan HS, Hooper PL, Gurven M. 2009. The evolutionary and ecological roots of human social organization. Phil. Trans. R. Soc. B 364, 3289-3299. ( 10.1098/rstb.2009.0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fry DP, Keith CA, Söderberg P. 2020. Social complexity, inequality and war before farming: congruence of comparative forager and archaeological data. In Social inequality before farming? Multidisciplinary approaches to the study of social organization in prehistoric and ethnographic hunter-gatherer-fisher societies (ed. Moreau L), pp. 303-320. Cambridge, UK: McDonald Institute for Archaeological Research University of Cambridge. [Google Scholar]
- 153.Wiessner PW. 2014. Embers of society: Firelight talk among the Ju/’hoansi Bushmen. Proc. Natl Acad. Sci. USA 111, 14 027-14 035. ( 10.1073/pnas.1404212111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Venkataraman VV, Kraft TS, Dominy NJ, Endicott KM. 2017. Hunter-gatherer residential mobility and the marginal value of rainforest patches. Proc. Natl Acad. Sci. USA 114, 3097-3102. ( 10.1073/pnas.1617542114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dyble M, Salali G, Chaudhary A, Page A, Smith D, Thompson J, Vinicius L, Mace R, Migliano A. 2015. Sex equality can explain the unique social structure of hunter-gatherer bands. Science 348, 796-798. ( 10.1126/science.aaa5139) [DOI] [PubMed] [Google Scholar]
- 156.Dyer JRG, Johansson A, Helbing D, Couzin ID, Krause J. 2009. Leadership, consensus decision making and collective behaviour in humans. Phil. Trans. R. Soc. B 364, 781-789. ( 10.1098/rstb.2008.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ayman R, Korabik K. 2010. Leadership: why gender and culture matter. Am. Psychol. 65, 157-170. ( 10.1037/a0018806) [DOI] [PubMed] [Google Scholar]
- 158.Little AC, Burriss RP, Jones BC, Roberts SC. 2007. Facial appearance affects voting decisions. Evol. Hum. Behav. 28, 18-27. ( 10.1016/j.evolhumbehav.2006.09.002) [DOI] [Google Scholar]
- 159.Spisak BR, Homan AC, Grabo A, van Vugt M. 2012. Facing the situation: testing a biosocial contingency model of leadership in intergroup relations using masculine and feminine faces. Leadersh. Q. 23, 273-280. ( 10.1016/j.leaqua.2011.08.006) [DOI] [Google Scholar]
- 160.Laustsen L, Petersen MB. 2020. Why are right-wing voters attracted to dominant leaders? Assessing competing theories of psychological mechanisms. Leadersh. Q. 31, 101301. ( 10.1016/j.leaqua.2019.06.002) [DOI] [Google Scholar]
- 161.Kappeler PM, Fichtel C, van Vugt M, Smith JE. 2019. Female leadership: a transdisciplinary perspective. Evol. Anthropol. 28, 160-163. ( 10.1002/evan.21783) [DOI] [PubMed] [Google Scholar]
- 162.van Vugt M. 2009. Sex differences in intergroup competition, aggression, and warfare: the male warrior hypothesis. Ann. N.Y. Acad. Sci. 1167, 124-134. ( 10.1111/j.1749-6632.2009.04539.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and R code to reproduce the results are available at https://github.com/adrianjaeggi/sexbiasmammals.