Abstract
In many group-living mammals, philopatric females form the stable core of the group and defend food or shelter against other groups of females. Where males are larger, their participation could give their female group the edge. How can females secure the contribution of males that are neither the father of current infants, nor the dominant male expecting to sire the next generation of infants? It has been proposed that females recruit these males as ‘hired guns’, receiving social support and copulations in exchange for fighting, against the interests of the dominant male. We first develop the logic of this hypothesis in unprecedented detail by considering the potential pay-off consequences for females and males. We then provide empirical evidence for the existence of hired guns in this context in several primate species. The game-theoretical aspects of the phenomenon remain to be studied, as is the distribution across contexts (e.g. predation avoidance) and species of the hired gun phenomenon.
This article is part of the theme issue ‘Intergroup conflict across taxa’.
Keywords: primates, between-group competition, game theory, reputation-based partner choice, paternal investment, hired gun
1. Introduction
In humans, between-group contest competition (further called between-group conflict or BGC) has become frequent since the Neolithic revolution [1]. Warfare, which we define as repeated hostile acts by an alliance with the aim of killing or excluding opponents, is hypothesized to be the main reason for patrilocality and the natal dispersal of women [2]. Among other primates, only chimpanzees share with us the combination of male philopatry and warfare [3]. Sublethal BGC is also widespread in primates. However, unlike in humans and chimpanzees, both sexes tend to participate. Indeed, BGC in most primates may primarily reflect female interests. Among mammals, given internal fertilization, gestation and lactation are limited to females, making females the demographically dominant sex. Hence, their associations and relationships are expected to respond to the distribution of resources and risks, whereas male associations and relationships are expected to map onto them [4,5]. This framework has successfully been applied to nonhuman primates. Group living, and thus female gregariousness, is largely understood as an adaptation to reduce predation risk, whereas female relationships are understood to respond to the nature of competition, within or between groups [6–9]. For example, female philopatry may evolve if a group of related females gains inclusive fitness benefits by cooperatively defending territories or food patches against neighbouring groups and/or allospecific competitors [10]. Thus, female primates are typically seen as the driving force behind between-group aggression, though usually without escalation to the point of seeking to kill outgroup individuals. Nonetheless, among social mammals, including primates, males often participate in these BGCs [11,12], raising the question of what role males might play in BGC in these species, and under which circumstances.
No matter how many males there are in a group, average male fitness will always be affected by the female group members' fertility, and hence by the females’ access to food. Thus, one should expect that males join BGCs to secure resources for their females. On a most basic level, they should benefit from excluding other males from the group to prevent mating, thereby also reducing harassment levels for females and the number of foraging competitors to females as a by-product of self-serving actions. Rubenstein coined the term ‘hired gun’ for these by-product benefits [13,14]. He aimed to explain why zebras live in year-round stable single-male groups while other ungulates live in sexual segregation outside the mating season. Indeed, the hired gun concept warrants year-round association between males and females. However, the very term ‘hired gun’ implies that the females are employers who compensate males for services, or less anthropomorphically, that female behaviour and physiology changed evolutionarily to produce additional fitness benefits for the group's male. In a single-male system, it is not clear whether females ever need to ‘compensate’ the male. In single-male groups, the male is the obvious and sole beneficiary of the foraging success of his females as he will sire all their offspring. Thus, one can expect such males to help self-servingly in territory or food patch defence as long as the benefits of increased female fecundity outweigh the costs of participation. Even if this inequality does not hold and females would have to compensate the single male for his investments, it is unclear how they could, as they already give the highest possible pay-off (i.e. paternity) anyway. Thus, we conclude that males in single-male groups do not need to be ‘hired’; they provide services because they benefit directly without having to share paternity.
The hired gun hypothesis becomes more plausible if female behaviour and reproductive physiology are moulded so as to enable the presence of additional males, in most cases against the fitness interests of the dominant male. If females do not evolve counterstrategies against dominant male attempts to monopolize mating, single-male systems may evolve. Andelman [15] first drew attention to the fact that groups of up to ca six females could contain either a single male or multiple males, whereas above that, multi-male groups are the norm. Diana monkeys may provide an estimate on how many females a male monkey may maximally monopolize as long as females evolved to consent: a group usually has a single adult male, but regularly about 10 females [16]. The small testes size of males indicates the absence of any sperm competition [17], suggesting that the single male monopolizes mating. Diana monkeys dwell in tropical rainforests, a habitat that would facilitate hidden extra group mating if females were seeking them. This example implies that females in other species that live in multi-male groups of similar or smaller overall sizes ‘hire’ extra males as guns for fitness benefits, potentially including fighting neighbouring groups [9,18]. In fact, females in many primate species have evolved numerous derived sexual features serving to break the top-dominant male‘s monopoly on mating and paternity [19,20].
The aims of this paper are to explore the conditions under which the hired gun hypothesis could theoretically work and to summarize the current evidence. Completing these tasks will allow us to identify avenues for future theoretical and empirical research. As we see it, male services such as the hired gun phenomenon provide ample opportunities to study cooperation between unrelated individuals, with important implications for our understanding of primate social organization and BGC as a function of resource distribution.
2. Conceptual considerations
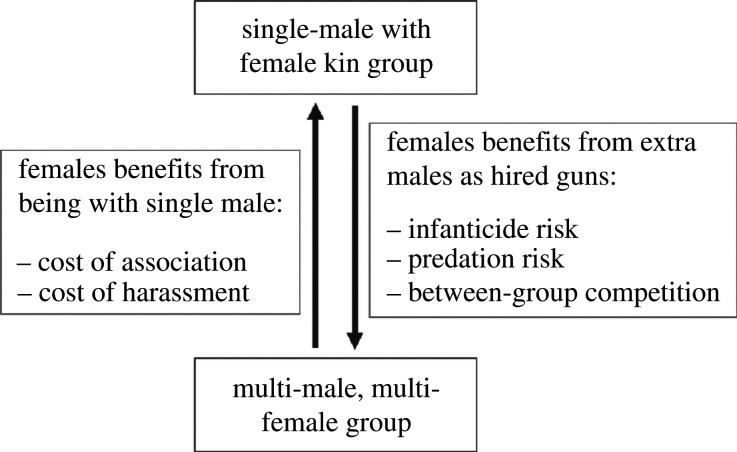
The features of female sexuality that enabled females to break the top male's monopoly on mating almost certainly evolved as a counter-strategy against infanticide by non-sire males [21]. However, they opened the door towards the broader phenomenon of male services that benefit unrelated females or their immature offspring yet do not reflect parental care or investment in secure future fitness benefits. For instance, primate females can also recruit non-sire males to assist in the defence against predators [22]. Here, our focus is on BGC. The absolute and relative importance of these three benefits is likely to vary between species and between populations of the same species. For example, infanticide risk is often lower in seasonal annual breeders, given that male infanticide would rarely shorten the time window until the mother enters into oestrus again. Predation risk varies between species according to habitat (savannah versus forest) and within species typically owing to variation in human disturbances of the ecosystem (often driving predators to local extinction outside strictly protected areas). The importance of BGC will vary as a function of food distribution (clumped versus spread) and territory/home range defensibility (the larger the home range, the more difficult it becomes to prevent intrusions); and the benefit for females of the male contribution in this context will depend on sexual size dimorphism. The general scenario for the implementation of hired guns and the resulting multi-male multi-female groups is summarized in figure 1. Figure 1 primarily refers to evolved species differences, acknowledging that transitions may happen in both directions [23,24]. Nevertheless, we acknowledge that there may be sufficient behavioural and physiological plasticity among females for intraspecific variation as well, as suggested by the literature on primate sexuality.
Figure 1.
Various selective forces may cause a shift from a single-male group structure to a multi-male multi-female structure, owing to females actively working against the dominant male, or back when the costs for females of extra males are too high.
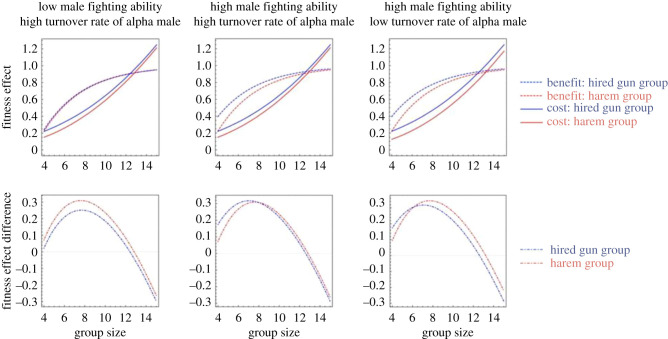
To evaluate the potential benefits and costs of adding a male to the group, we developed a simple mathematical model, where we focus on the benefits and costs of group living from the perspective of females in primate species of female philopatry. We assume that it is always more beneficial for a male to join a social group than not to do so. The model is presented in detail in the electronic supplementary material. Here, we only present the logic and a few key results. One potential benefit of group living comes from increased fighting ability of a group as a whole as the number of group members increases. Because the benefit of group fighting depends on the number of competing groups that remain unbeaten, the benefit functions are featured with diminishing return as group size increases. Because we assume that males have higher fighting abilities than females owing to their larger sizes, a hired gun group always has higher fighting abilities than a harem group of the same size, although the relative benefit contributed by a single extra male diminishes as group size increases. The cost of group living contains two additive components—the coordination cost and the relatedness cost. For simplicity, we assume that the former only depends on group size and thus is the same for harem and hired gun groups (see the electronic supplementary material for a detailed discussion). The latter differs between group types, because unlike in harem groups, paternity is shared between two males in hired gun groups, leading to lower relatedness between individuals, and also the relatedness cost of including an extra male. We introduced two parameters that control for the relatedness cost difference between harem and hired gun groups. The first parameter controls the shape of relatedness curve as a function of group size in harem groups. Larger values correspond to higher turnover rates of harem ownership. The second parameter controls the weight of relatedness cost in the total cost of group living. Increasing enlarges the difference of relatedness cost between hired gun and harem groups, and thus can be used as a proxy for the degree of reproductive skew among males in hired gun groups. Everything else being equal, the smaller is, the smaller the relatedness cost difference is between a hired gun and a harem group, and the stronger the ability of the alpha male in a hired gun group to monopolize paternity.
Depending on the relative importance of benefits versus costs, females may be under selection to hire male(s) as guns (figure 2). Interestingly, when it is beneficial for females to hire a male gun, the resulting optimal group size is smaller with an extra male than with an extra female (figure 2, lower panels). This is apparently because of groups with an extra male reaching high fighting potential with smaller group size, avoiding the extra costs of within-group competition in a larger group. Our model just focusses on the important transition from single male to more than one male. It does not allow us to answer the question of whether for any given condition females should prefer to have two or even more males. Intuitively, we expect that the larger the group, the more likely it becomes that females would benefit from including multiple males for two reasons. First, the benefit contributed by the fighting ability of one extra male diminishes as group size increases, and therefore, large groups may only have an evident fighting advantage over harem groups of similar sizes by including multiple males. Second, including multiple males in a large group would not significantly increase the relatedness cost because the relatedness among individuals is anyway low in large groups [25].
Figure 2.
Effect of varying the fighting ability of males and the turnover rate of alpha male in harem groups, which affects the relatedness between philopatric group members at any given group size. Panels in the first row show the benefit and cost separately as a function of the group size. Panels in the second row show the difference between benefit and cost for each type of groups. Male fighting ability is controlled by the parameter , which was set to 1.1 and 2.0 for low and high fighting abilities, respectively. Alpha male turnover rate in harem groups is controlled by the parameter , which was set to 0.05 and 0.1 for low and high turnover rates, respectively. The other parameter values are a = 1, b = 4, , and . For equations and further results, see the electronic supplementary material. (Online version in colour.)
While hiring males as guns against conspecific and allospecific competitors may potentially benefit females in species with a strong sexual dimorphism, the question arises why these hired males should help in BGCs. After all, fighting requires energy and may cause injury. One possibility is that joining in fights increases a male's fitness in the absence of any explicit payments. However, this can only happen if: (i) males may secure some sneaky copulations without female consent, or if becoming a hired gun automatically increases chances to replace the current alpha male and thereby gaining mating; and (ii) these mating benefits outweigh the costs of fighting. If these conditions are not fulfilled, males may benefit from withholding contributions to fighting. If that were the case, females would need to become proper ‘employers’ who pay their ‘employees’ and must monitor that these also deliver the work, or else take action.
As it stands, females may offer compensation in three ways. The most basic form involves simply tolerating the male in the group, since being a group member reduces harassment and provides various anti-predation benefits. A more active compensation would be to provide socio-positive interactions, like grooming. Grooming in primates has both hygienic and stress-reducing functions. Finally, the most valuable form of payment would involve the permission to copulate, against the interest of the alpha male. According to our model analyses, the resulting mix in paternity would indeed impose costs on females owing to the resulting reduced relatedness among them (see the electronic supplementary material). While bird females have the behavioural freedom to provide such opportunities owing to their high mobility, mammalian females are more constrained because of male mate guarding [25]. However, mammalian females have evolved various alternative mechanisms to evade monopolization by a single male, including longer follicular phases with long periods of receptivity and multiple cycles for each conception [18], synchronization of oestrus [26], concealed ovulation (bonobos [27]) and low fertilization probability (lions [28]). Species with such female traits and larger males than females are hence logical candidates to test specific predictions based on the hired gun hypothesis.
To prevent males from withholding their expected contributions to BGCs, females must apply so-called partner control mechanisms, i.e. they must have responses to such cheating that lower male fitness [29]. Appropriate responses by females require monitoring of male behaviour. Responses to males that do not carry their load can range from withholding grooming interactions to trying to avoid or actively withholding copulation opportunities to evicting a male from the group. This latter option may not warrant active aggression by females but mere withholding of protection against the alpha male, who will then carry out the eviction. Before taking any extreme actions, females may give feedback to males regarding their performance.
An example comes from vervet monkeys (see also [30]). In this species, between-group encounters consist of waves of attack and counterattack [31]. Between such waves, it has been observed that females groom males that participated in the last wave but attack males that had not participated [32]. In return, males may counteract female goals with their own aggression, leading to a negotiation process [33]: males may prefer a de-escalation of a BGC for various reasons, including the presence of probable own vulnerable offspring, the possibility to prospect (i.e. exploring the possibility to join the opponent group in the future), or own vulnerability because of recent wounding [33].
A very interesting but underexplored aspect of females hiring males as year-round guns concerns the time dynamics of female payment and male work. Females cannot withhold group membership, the most basic payment, without losing the male services. This exchange thus fits the ‘pay-to-stay’ concept developed to explain helping in cooperatively breeding species: subordinate individuals must help to rear offspring or else breeders would benefit from evicting them [34]. Breeders must monitor the helpers' contributions but they cannot exploit the helper. If breeders demand too much, helpers will leave and no more help is provided. Females integrating males into the grooming network is a potential additional payment that can be spontaneously adjusted to any changes in male performance. A specific male service, like participation in a BGC or an alarm call, can immediately be rewarded with grooming. Thus, grooming could potentially be used as a direct payment. Finally, payment in the form of copulations has a very unusual time dynamic, at least in species with seasonal reproduction or even longer intervals between reproductive events (figure 3).
Figure 3.
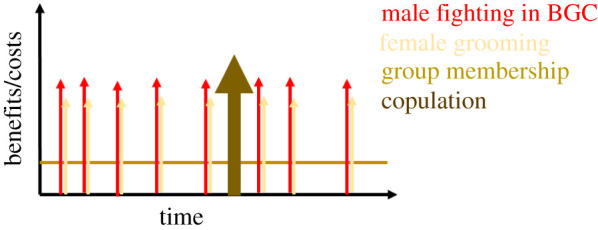
Time dynamics of male services and received payment. Males pay each time they participate in an between-group conflict (BGC; red arrows). They receive the benefits of group membership constantly (horizontal line). In addition, they may be rewarded with grooming each time they participate in a BGC (yellow arrows). Finally, males may also achieve copulations in return for their services, which would be rare events and linked to female fertility/a mating season rather than a specific own service.
First, males have to provide a series of services over a period of months, raising the question of whether females could potentially withhold compensation at the end. This is a quantitative question, as females will tend to mate with several males in order to reduce infanticide risk [2,35]. In the resulting lottery, females may bias fertilization probability by influencing the mating frequency and/or time of the mating around ovulation. Thus, how can male service providers ensure that they will be compensated by females through biased paternity probability? Also, once copulations took place, how can females be sure that males will provide future services?
To start with the latter question, one possible scenario is that copulations induce males to provide services as a form of parental investment, i.e. male services become self-serving independently of how the female mating partners behave in the future. If this were the case, females would not have to worry about males stopping to do their work. In return, males may already provide initial services to demonstrate their capability to provide sufficient paternal investment, thereby enticing females to accept/seek copulations. The complete scenario would hence be as follows: initial male services/investments must be sufficient to make it a self-serving decision for females to offer an increased share of copulations (against the interests of the dominant male), which make it a self-serving decision for males to stay and provide further services. In other words, males provide services to show their genetic quality, which females assess and base their mating decisions upon. In this scenario, the highest quality males can afford to provide more services than other males (handicap principle; [36]; for subtleties of terminology and logic see [37]). Thus, males compete among each other over access to females, partly based on their position in the hierarchy and partly based on the services they provide, and females choose accordingly. This is the concept of reputation-based partner choice (formerly called ‘competitive altruism’; [38,39]) within a biological market [40]. The competition between males regarding their reputation with females introduces a new dynamic regarding the amount of services provided. Under reputation-based partner choice, it is not good enough to provide services above a certain pay-off threshold (like ‘provide more to between-group fighting than a female could have done’) but each male has to perform better than the competitors. Therefore, an increase in the number of males may lead to a disproportional increase in male services (green region in figure 3) because of females exerting partner choice.
A point that deserves further exploration is whether under such conditions, males of a low quality/in a temporary bad state simply opt out of the competition and try to obtain the benefits of group living without contributing to the public good. As females need to monitor male behaviour anyway in order to identify the best one(s), they should be able to identify such non-contributors and keep them out of the social network. Another important point to make is that market effects can work both ways: the best guns may demand many mating or else they would switch to another group. In the worst case, they would join a neighbouring group and hence become a direct opponent. Clearly, the hired gun concept provides ample opportunities to explore and advance both reputation-based partner choice concepts and biological market theory.
A final point we discuss concerns the potential interaction structures between females and hired guns. More specifically, one has to ask whether females act as a community or as individuals. The answer to this question is important to understand how males get paid for their services. At first sight, males provide a public good to all females when participating in BGCs. Therefore, all females should be willing to compensate for the service, with immediate grooming after the conflict ends and/or with copulations later. However, this scenario might lead to complications. To avoid overcompensation in terms of grooming, females would have to monitor each other's behaviour. The compensation would also become a public good, and hence females might be tempted to shirk their own contributions. While there are potential solutions to ensure female contributions to the public, a simpler alternative would be that specific females may develop frequent interactions with specific males. In other words, females and males develop specific social bonds, also called ‘friendships’. Bonds could change the dynamics of the exchange of male services for payment. A participating male would be groomed by his bonded female rather than by any female group member, and increase his chances of mating with her. As a consequence, males should try to adjust their services such that the bonded female benefits most. Spatial proximity would be a simple mechanism to achieve more directed services in the context of protecting the female's offspring against infanticide and/or predatory attacks. In BGCs, males may join a fight next to their bonded female to specifically protect her from harm. The resulting asymmetries in how the benefits of male services are distributed among female group members would render the system more stable against cheating [41]. The interaction pattern would become more like a two-player game, where an initial Prisoner's Dilemma-type interaction gets eventually transformed to mutually beneficial interactions because of the developing bond/interdependence [42,43].
3. Empirical evaluation
Our conceptual considerations allow us to make various predictions that can in principle be tested empirically. First, if females hire males as guns at least in part to improve chances to win BGCs, we predict that the number of males will affect chances of winning and perhaps also home range size. To assess these predictions, we need to exclude cases of male participation in BGCs that are purely owing to mate defence. Where males alone are involved in BGCs, this is a parsimonious explanation of the phenomenon, and no underlying male–female interactions need to be invoked. This is in fact the situation pertaining to many southeast Asian forest langurs [44], where young males attract females to form a group and retain their females until their ability to defend them against younger rivals breaks down [45]. A similar pattern may pertain in various African colobines [46–48]: males are the main participants and group dominance, if present, may primarily reflect the benefits for the participating male, who invests in his reproductive future [47]. Female secondary dispersal is also common [49,50], suggesting a mix of male take-overs of existing groups and a cycle of group formation and disbanding in which females are often not close relatives. Overall, the general lack of female participation indicates that female groups do not benefit enough from resource defence to make female participation adaptive, since their participation would otherwise easily tip the balance. Hence, it is not warranted to call these male behaviours a service to the females.
In order to identify male services in the BGC context, then, it is best to concentrate on species in which philopatric, gregarious females have a stake in defending their range and its resources. Joint participation of males and females is in fact reported for most primate species with female philopatry [51,52]. There are two experimental studies of such species that attempted to evoke male defence of resources for females. One experiment by Scarry [53] showed that tufted capuchin males approached rich contestable food patches, regardless of female fertility status, suggesting they are truly defending access to resources for their females. The other experiment [31], in which vervet monkey males were given the opportunity to defend food patches that were made only accessible to females, yielded no evidence of males spontaneously providing a resource defence service to their females (although they did participate when females led the group to the contested location). In fact, in this same population, females do reward males with grooming when they participate, or punish them by attacking them if they do not [32]. Females also mate more with participating males [54], although this pattern could potentially be confounded by other factors, like male rank. Unfortunately, no data exist whether (i) an individual male is more likely to join if his socially closest female participates, and (ii) whether this female is most likely to reward and punish that male for his actions. Without such information, we cannot assess how personalized male services and female assessments are in vervet monkeys, and hence how the underlying game structure is best described. Nevertheless, the data reveal several important building blocks of the game: females monitor and respond to what they observe both on short- and long-time scales (indicating reputation-based partner choice), and males show evidence of providing services quite freely after mating success (indicating self-serving paternal investment).
The non-experimental approach to test this prediction is to find cases where between-group dominance exists and is determined by the number of sexually mature males in the group and not by the overall group size or the number of adult females. We found several of these: wedge-capped capuchins [55], white-faced capuchins [56], tufted capuchins [53], black and gold howler monkeys [57], chacma baboons [58], savannah baboons [59], Sulawesi black macaques [60], Japanese macaques [61] and perhaps Nicobar long-tailed macaques [62]. There were also cases where such an effect was not observed, even though it was examined (e.g. bonnet macaques [63]; crested mangabeys [64]). However, these cases cannot serve as evidence of the absence of male services, because they may reflect conditions with lower density and lower frequencies of BGC, when groups generally avoid each other.
The second prediction was that the number of sexually mature or adult males in a group affects the group's home range size, beyond the influence of group size and thus energetic needs alone. Few monkey studies report on this question, but positive evidence exists. Richter et al. [65] showed it for Assamese macaques. Anderson [66] found that baboon groups with more adult males occupied more productive and larger home ranges. C. van Schaik 1985 (unpublished observation) found for Sumatran long-tailed macaques that a small group's home range expanded dramatically after the single adult male was replaced by a number of other males. While more data are clearly needed, it is interesting to see that male services can lead to similar patterns as to what is expected and well established in primate species that have a male resource defence polygyny mating system in which alliances of philopatric males defend a joint territory: among chimpanzees, male lethal between-community aggression determines territory size [67]; which in turn affects female reproductive success [68]. Among spider monkeys, which have a similar social system, the number of males predicts the length of ‘risky boundary’, the length of territory perimeter shared with other groups [69], thus indirectly indicating that more males are needed to defend resources where groups are surrounded on all sides by other groups.
4. Discussion
We had asked whether females may have evolved strategies to ensure the presence of multiple males as a means (as ‘guns’) to increase resource holding potential in BGCs. Note that our definition of a ‘hired gun’ hence differs from the original one by Rubenstein [13], who used the term to explain the evolution of year-round single-male groups. Defendable resources selecting for female philopatry provide the basic conditions. Males being stronger than females and gaining net benefits from participating in BGCs are additional requirements. We used game-theoretic considerations to identify conditions under which females would benefit from hiring males as ‘guns’. We recommend reading of the electronic supplementary material, for those readers interested in the modelling part. The rich number of potential games requires future detailed investigations in order to separate by-product benefits owing to group augmentation and/or paternal investment from pay-to-stay concepts and from reputation-based partner choice models. As it stands, we identified some primate species where males apparently act as hired guns (as defined in this paper): species in which the number of males in a group predicts winning BGCs and/or home range size, independently of the number of females. Thus, the importance of winning BGCs appears to be strong enough to select for female strategies to ensure the presence of multiple males in their group. In other words, BGCs can apparently affect the evolution of primate social systems.
We found various cases, but for the non-experimental ones, this does not tell us about the behavioural mechanisms involved. In some species, males apparently spontaneously produce services in the BGC context, but in others, females may actually force males into providing them, at some cost, by provoking BGCs during times of likely fertility, simply by approaching neighbouring groups in important resource patches (or sleeping sites). Then, females force the group's males into defending access to them in savannah baboons and grey-cheeked mangabeys [70–72]. Moreover, in baboons, males guarding females with swellings are more likely to participate in BGCs than other males [71]. Less direct evidence applies where BGCs are more common during the mating season (Japanese macaques: [61]; Nicobar macaques: [62]). Thus, males are often recruited as participants rather than joining spontaneously or even initiating the conflict.
Females may not elicit male participation in BGCs unconditionally. Male participation varies across populations of the same species (Japanese monkeys [73]; baboons [66,74]; black colobus [75]; white-faced capuchins [56,76]) and perhaps even over time within the same population. This variability suggests both plasticity in decision making by males and females, as well as variation in fitness benefits, depending on conditions. Primate females are known to show plasticity in reproductive physiology and behaviour, which will affect the ability to monopolize mating [77,78]. The participation by primate males is expected to be plastic as well, because males are often unrelated and generally compete among each other for access to females, and we therefore expect they will tend to avoid BGC expected on the basis of the collective action problem [79].
Measuring the relative importance of male contribution to BGCs in the evolution of primate social systems is difficult, given that males provide two additional benefits to females: (i) reduced infanticide risk [80,81], which in fact has been suggested as a major selective influence on female sexuality in nonhuman primates [2] could also be responsible for the year-round male–female association in this lineage [82]; and (ii) multiple males may reduce the risk of predation because non-dominant males tend to be more peripheral and may protect females and their offspring through vigilance, alarms, mobbing and counterattacks [22,83]. Indeed, protection against predation shows a pronounced male bias across primates [22,84]. As we see it, sexual dimorphism leading to infanticide by new harem owners appears to be the strongest effect on female fitness, where hiding paternity through multiple mating appears to have been the prime mover leading to the presence of multiple males, consistent with the idea that infanticide avoidance selected for mixed-sex groups in the first place. While dominant males can still use their status to gain most copulations/the best-timed copulations, females may bias mating success by males through indirect (reproductive physiology) and active choice. Under female choice, males compete among each other to impress females, and this can be best done by providing services to females or the group. In other words, we view male services in multi-male groups largely as the outcome of reputation-based partner choice by females (see also [39]). The benefits of these services only arise once females have evolved promiscuity to reduce infanticide risk and hence can be considered secondary. Note, however, that only these secondary benefits warrant year-round associations between females and extra males, while promiscuous mating can also be achieved with males visiting during the reproductive season. Thus, we propose that male services, including participation in BGCs, may affect the evolution of primate social organization.
Research on male services is still in its infancy [22]. As a consequence, we found it impossible to assess what kind of games best describe the exchanges that take place between males and females. Given the large reproductive skew in males based on their rank, benefits in the form of group membership (pay to stay) and social integration, including the development of valuable bonds with females, must also be considered at least on shorter time scales. In this context, the evolution of close male–female social bonds as a means to turn a public goods kind of game into a largely dyadic game should be considered. The resulting interdependence [43] would lead to a win–win situation and offer an alternative reproductive strategy for lower-ranking young or older males [85]. We consider reputation-based partner choice by females as the main mechanism promoting services of newly immigrated males, while males with longer tenure and likely reproductive success may eventually provide services as a form of paternal investment [22]. Further studies will be necessary to test these predictions.
In conclusion, we found evidence that male primates may play an important role in BGCs. Being typically larger than females, extra males add fighting capacity to a group. Males can hence give their females access to food resources, which are considered to be the main limiting factor for female fitness. As a consequence, females may have evolved means to hire extra males as ‘guns’ against the interests of the dominant male in order to win BGCs. Currently, few studies report the detailed dynamics of male and female actions during intergroup encounters, and the long-term consequences of male services on mating. While we have focussed entirely on primates in this paper, the logic developed can be tested with any species in which multiple males could contribute to securing food resources for the female group members.
Data accessibility
The code of the model is publicly archived at https://github.com/XiangyiLi/Hired-Guns.
Authors' contributions
R.B.: conceptualization, funding acquisition and writing—original draft; X.-Y.L.R.: formal analysis and writing—original draft; C.V.S.: conceptualization and writing—original draft.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (grant nos. 180145 and 310030_197884).
References
- 1.Keeley LH. 1997. Frontier warfare in the Early Neolithic. Troubl. Times Violence Warf. Past 4, 303. [Google Scholar]
- 2.van Schaik CP. 2016. The primate origins of human nature. New York, NY: John Wiley & Sons. [Google Scholar]
- 3.Wilson ML, et al. 2014. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature 513, 414-417. ( 10.1038/nature13727) [DOI] [PubMed] [Google Scholar]
- 4.Ims RA. 1988. The potential for sexual selection in males: effect of sex ratio and spatiotemporal distribution of receptive females. Evol. Ecol. 2, 338-352. ( 10.1007/BF02207565) [DOI] [Google Scholar]
- 5.Trivers RL (ed.). 1972. Parental investment and sexual selection. In Sexual selection and the descent of Man (ed. B Campbell), pp. 136–179. Chicago, IL: Aldine. [Google Scholar]
- 6.Isbell LA. 1991. Contest and scramble competition: patterns of female aggression and ranging behavior among primates. Behav. Ecol. 2, 143-155. ( 10.1093/beheco/2.2.143) [DOI] [Google Scholar]
- 7.Schülke O, Ostner J. 2021. Ecological and social influences on sociality. In The evolution of primate societies (eds JC Mitani, J Call, PM Kappeler, RA Palombit, JB Silk), pp. 195-219. Chicago, IL: University of Chicago Press. [Google Scholar]
- 8.van Schaik CP. 1989. The ecology of social relationships amongst female primates. In Comparative socioecology: the behavioural ecology of humans and other mammals (eds V Standen, R Foley), pp. 195–218. Oxford, UK: Blackwells. [Google Scholar]
- 9.Wrangham RW. 1980. An ecological model of female-bonded primate groups. Behaviour 75, 262-300. ( 10.1163/156853980X00447) [DOI] [Google Scholar]
- 10.Port M, Hildenbrandt H, Pen I, Schülke O, Ostner J, Weissing FJ. 2020. The evolution of social philopatry in female primates. Am. J. Phys. Anthropol. 173, 397-410. ( 10.1002/ajpa.24123) [DOI] [PubMed] [Google Scholar]
- 11.Smith J, Fichtel C, Holmes R, Kappeler P, van vugt M, Jaeggi A. 2022. Sex bias in intergroup conflict and collective movements among social mammals: male warriors and female guides. Phil. Trans. R. Soc. B 377, 20210142. ( 10.1098/rstb.2021.0142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massaro A, Gilby I, Desai N, Weiss A, Feldblum J, Pusey A, Wilson M. 2022. Correlates of individual participation in boundary patrols by male chimpanzees. Phil. Trans. R. Soc. B 377, 20210151. ( 10.1098/rstb.2021.0151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubenstein D. 1986. Ecology and sociality in zebras and horses. In Ecological aspects of social evolution, birds and mammals (eds DJ Rubenstein, RW Wrangham), pp. 282–302. Princeton, NJ: Princeton Univesity Press.
- 14.Wrangham RW, Rubenstein D, Wrangham R. 1986. Ecological aspects of social evolution. Princeton, NJ: Princeton University Press.
- 15.Andelman SJ. 2014. Ecological and social determinants of cercopithecine mating patterns. In Ecological aspects of social evolution (eds DJ Rubenstein, RW Wrangham), pp. 201-216. Princeton, NJ: Princeton University Press. [Google Scholar]
- 16.Candiotti A, Zuberbühler K, Lemasson A. 2012. Convergence and divergence in Diana monkey vocalizations. Biol. Lett. 8, 382-385. ( 10.1098/rsbl.2011.1182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harcourt AH, Purvis A, Liles L. 1995. Sperm competition: mating system, not breeding season, affects testes size of primates. Funct. Ecol. 9, 468-476. ( 10.2307/2390011) [DOI] [Google Scholar]
- 18.van Schaik CP, Hörstermann M. 1994. Predation risk and the number of adult males in a primate group: a comparative test. Behav. Ecol. Sociobiol. 35, 261-272. ( 10.1007/BF00170707) [DOI] [Google Scholar]
- 19.Clarke P, Pradhan G, van Schaik C. 2009. 3 Intersexual conflict in primates: infanticide, paternity allocation, and the role of coercion. In Sexual coercion in primates and humans (eds MN Muller, RW Wrangham), pp. 42-78. Cambridge, MA: Harvard University Press. [Google Scholar]
- 20.Port M, Schülke O, Ostner J. 2018. Reproductive tolerance in male primates: old paradigms and new evidence. Evol. Anthropol. Issues News Rev. 27, 107-120. ( 10.1002/evan.21586) [DOI] [PubMed] [Google Scholar]
- 21.Sterck EHM, Watts DP, van Schaik CP. 1997. The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 41, 291-309. ( 10.1007/s002650050390) [DOI] [Google Scholar]
- 22.van Schaik CP, Bshary R, Wagner G, Cunha F. 2021. Male anti-predation services in primates as costly signalling? A comparative analysis and review. Ethology 128, 1-14. [Google Scholar]
- 23.Shultz S, Opie C, Atkinson QD. 2011. Stepwise evolution of stable sociality in primates. Nature 479, 219-222. ( 10.1038/nature10601) [DOI] [PubMed] [Google Scholar]
- 24.Kappeler PM, Pozzi L. 2019. Evolutionary transitions toward pair living in nonhuman primates as stepping stones toward more complex societies. Sci. Adv. 5, eaay1276. ( 10.1126/sciadv.aay1276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pradhan GR, Van Schaik CP. 2009. Why do females find ornaments attractive? The coercion-avoidance hypothesis. Biol. J. Linn. Soc. 96, 372-382. ( 10.1111/j.1095-8312.2008.01131.x) [DOI] [Google Scholar]
- 26.Ostner J, Nunn CL, Schülke O. 2008. Female reproductive synchrony predicts skewed paternity across primates. Behav. Ecol. 19, 1150-1158. ( 10.1093/beheco/arn093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douglas PH, Hohmann G, Murtagh R, Thiessen-Bock R, Deschner T. 2016. Mixed messages: wild female bonobos show high variability in the timing of ovulation in relation to sexual swelling patterns. BMC Evol. Biol. 16, 1-17. ( 10.1186/s12862-016-0691-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Packer C, Pusey AE. 1983. Adaptations of female lions to infanticide by incoming males. Am. Nat. 121, 716-728. ( 10.1086/284097) [DOI] [Google Scholar]
- 29.Bshary R, BergmüLler R. 2008. Distinguishing four fundamental approaches to the evolution of helping. J. Evol. Biol. 21, 405-420. ( 10.1111/j.1420-9101.2007.01482.x) [DOI] [PubMed] [Google Scholar]
- 30.Gareta GM, de Guinea M, Waal E, Bshary R. 2022. Drivers and outcomes in territory disputes: from ecological characteristics to numerical asymmetry in vervet monkeys. Phil. Trans. R. Soc. B 377, 20210145. ( 10.1098/rstb.2021.0145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arseneau-Robar TJM, Müller E, Taucher AL, van Schaik CP, Willems EP. 2016. Male food defence as a by-product of intersexual cooperation in a non-human primate. Sci. Rep. 6, 1-7. ( 10.1038/s41598-016-0001-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arseneau-Robar TJM, Taucher AL, Müller E, van Schaik C, Bshary R, Willems EP. 2016. Female monkeys use both the carrot and the stick to promote male participation in intergroup fights. Proc. R. Soc. B 283, 20161817. ( 10.1098/rspb.2016.1817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arseneau-Robar TJM, Müller E, Taucher AL, van Schaik CP, Bshary R, Willems EP. 2018. Male monkeys use punishment and coercion to de-escalate costly intergroup fights. Proc. R. Soc. B 285, 20172323. ( 10.1098/rspb.2017.2323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaston A. 1978. The evolution of group territorial behavior and cooperative breeding. Am. Nat. 112, 1091-1100. ( 10.1086/283348) [DOI] [Google Scholar]
- 35.Palombit RA. 2015. Infanticide as sexual conflict: coevolution of male strategies and female counterstrategies. Cold Spring Harb. Perspect. Biol. 7, a017640. ( 10.1101/cshperspect.a017640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zahavi A. 1975. Mate selection—a selection for a handicap. J. Theor. Biol. 53, 205-214. ( 10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 37.Penn DJ, Számadó S. 2020. The Handicap Principle: how an erroneous hypothesis became a scientific principle. Biol. Rev. 95, 267-290. ( 10.1111/brv.12563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts G. 1998. Competitive altruism: from reciprocity to the handicap principle. Proc. R. Soc. Lond. B 265, 427-431. ( 10.1098/rspb.1998.0312) [DOI] [Google Scholar]
- 39.Roberts G, Raihani N, Bshary R, Manrique HM, Farina A, Samu F, Barclay P. 2021. The benefits of being seen to help others: indirect reciprocity and reputation-based partner choice. Phil. Trans. R. Soc. B 376, 20200290. ( 10.1098/rstb.2020.0290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noë R, Hammerstein P. 1995. Biological markets. Trends Ecol. Evol. 10, 336-339. ( 10.1016/S0169-5347(00)89123-5) [DOI] [PubMed] [Google Scholar]
- 41.Sherratt T, Roberts G, Kassen R. 2009. Evolutionary stable investment in products that confer both an individual benefit and a public good. Front. Biosci. J. Virtual Libr. 14, 4557-4564. ( 10.2741/3548) [DOI] [PubMed] [Google Scholar]
- 42.Bshary R, Zuberbühler K, van Schaik CP. 2016. Why mutual helping in most natural systems is neither conflict-free nor based on maximal conflict. Phil. Trans. R. Soc. B 371, 20150091. ( 10.1098/rstb.2015.0091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts G. 2005. Cooperation through interdependence. Anim. Behav. 70, 901-908. ( 10.1016/j.anbehav.2005.02.006) [DOI] [Google Scholar]
- 44.van Schaik CP, Assink PR, Salafsky N. 1992. Territorial behavior in Southeast Asian langurs: resource defense or mate defense? Am. J. Primatol. 26, 233-242. ( 10.1002/ajp.1350260402) [DOI] [PubMed] [Google Scholar]
- 45.Steenbeek R. 1999. Tenure related changes in wild Thomas's langurs I: between-group interactions. Behaviour 136, 595-625. ( 10.1163/156853999501487) [DOI] [Google Scholar]
- 46.Fashing PJ. 2001. Male and female strategies during intergroup encounters in guerezas (Colobus guereza): evidence for resource defense mediated through males and a comparison with other primates. Behav. Ecol. Sociobiol. 50, 219-230. ( 10.1007/s002650100358) [DOI] [Google Scholar]
- 47.Harris TR. 2006. Between-group contest competition for food in a highly folivorous population of black and white colobus monkeys (Colobus guereza). Behav. Ecol. Sociobiol. 61, 317-329. ( 10.1007/s00265-006-0261-6) [DOI] [Google Scholar]
- 48.Sicotte P, MacIntosh AJ. 2004. Inter-group encounters and male incursions in Colobus vellerosus in Central Ghana. Behaviour 141, 533-553. ( 10.1163/1568539041166717) [DOI] [Google Scholar]
- 49.Harris TR, Caillaud D, Chapman CA, Vigilant L. 2009. Neither genetic nor observational data alone are sufficient for understanding sex-biased dispersal in a social-group-living species. Mol. Ecol. 18, 1777-1790. ( 10.1111/j.1365-294X.2009.04139.x) [DOI] [PubMed] [Google Scholar]
- 50.Teichroeb JA, Wikberg EC, Sicotte P. 2009. Female dispersal patterns in six groups of ursine colobus (Colobus vellerosus): infanticide avoidance is important. Behaviour 146, 551-582. ( 10.1163/156853909X426363) [DOI] [Google Scholar]
- 51.Beehner J, Kitchen D. 2007. Factors affecting individual participation in group-level aggression among non-human primates. Behaviour 144, 1551-1581. ( 10.1163/156853907782512074) [DOI] [Google Scholar]
- 52.Cheney DL. 2008. Interactions and relationships between groups. In Primate societies (eds BB Smuts, DL Cheney, RM Seyfarth, RW Wrangham), pp. 267–281. Chicago, IL: University of Chicago Press. [Google Scholar]
- 53.Scarry CJ. 2013. Between-group contest competition among tufted capuchin monkeys, Sapajus nigritus, and the role of male resource defence. Anim. Behav. 85, 931-939. ( 10.1016/j.anbehav.2013.02.013) [DOI] [Google Scholar]
- 54.Arseneau TJM, Taucher AL, van Schaik CP, Willems EP. 2015. Male monkeys fight in between-group conflicts as protective parents and reluctant recruits. Anim. Behav. 110, 39-50. ( 10.1016/j.anbehav.2015.09.006) [DOI] [Google Scholar]
- 55.Robinson JG. 1988. Group size in wedge-capped capuchin monkeys Cebus olivaceus and the reproductive success of males and females. Behav. Ecol. Sociobiol. 23, 187-197. ( 10.1007/BF00300353) [DOI] [Google Scholar]
- 56.Crofoot MC, Gilby IC, Wikelski MC, Kays RW. 2008. Interaction location outweighs the competitive advantage of numerical superiority in Cebus capucinus intergroup contests. Proc. Natl Acad. Sci. USA 105, 577-581. ( 10.1073/pnas.0707749105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kowalewski MM, Garber PA. 2015. Solving the collective action problem during intergroup encounters: the case of black and gold howler monkeys (Alouatta caraya). In Howler monkeys (eds MM Kowalewski, PA Garber, L Cortés-Ortiz, B Urbani, D Youlatos), pp. 165-189. Berlin, Germany: Springer. [Google Scholar]
- 58.Hamilton WJ III, Buskirk RE, Buskirk WH. 1975. Chacma baboon tactics during intertroop encounters. J. Mammal. 56, 857-870. ( 10.2307/1379657) [DOI] [PubMed] [Google Scholar]
- 59.Markham AC, Alberts SC, Altmann J. 2012. Intergroup conflict: ecological predictors of winning and consequences of defeat in a wild primate population. Anim. Behav. 84, 399-403. ( 10.1016/j.anbehav.2012.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okamoto K, Matsumura S. 2002. Intergroup encounters in wild moor macaques (Macaca maurus). Primates 43, 119-125. ( 10.1007/BF02629671) [DOI] [PubMed] [Google Scholar]
- 61.Saito C, et al. 1998. Aggressive intergroup encounters in two populations of Japanese macaques (Macaca fuscata). Primates 39, 303-312. ( 10.1007/BF02573079) [DOI] [Google Scholar]
- 62.Pal A, Kumara HN, Mishra PS, Velankar AD, Singh M. 2018. Between-group encounters in Nicobar long-tailed macaque (Macaca fascicularis umbrosus). Ethol. Ecol. Evol. 30, 582-599. ( 10.1080/03949370.2018.1459866) [DOI] [Google Scholar]
- 63.Cooper MA, Aureli F, Singh M. 2004. Between-group encounters among bonnet macaques (Macaca radiata). Behav. Ecol. Sociobiol. 56, 217-227. ( 10.1007/s00265-004-0779-4) [DOI] [Google Scholar]
- 64.Kinnaird MF. 1992. Variable resource defense by the Tana River crested mangabey. Behav. Ecol. Sociobiol. 31, 115-122. ( 10.1007/BF00166344) [DOI] [Google Scholar]
- 65.Richter C, Heesen M, Nenadić O, Ostner J, Schülke O. 2016. Males matter: increased home range size is associated with the number of resident males after controlling for ecological factors in wild Assamese macaques. Am. J. Phys. Anthropol. 159, 52-62. ( 10.1002/ajpa.22834) [DOI] [PubMed] [Google Scholar]
- 66.Anderson CM. 1981. Intertroop relations of chacma baboon (Papio ursinus). Int. J. Primatol. 2, 285-310. ( 10.1007/BF02693480) [DOI] [Google Scholar]
- 67.Mitani JC, Watts DP, Amsler SJ. 2010. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr. Biol. 20, R507-R508. ( 10.1016/j.cub.2010.04.021) [DOI] [PubMed] [Google Scholar]
- 68.Williams JM, Oehlert GW, Carlis JV, Pusey AE. 2004. Why do male chimpanzees defend a group range? Anim. Behav. 68, 523-532. ( 10.1016/j.anbehav.2003.09.015) [DOI] [Google Scholar]
- 69.Wallace RB. 2008. Towing the party line: territoriality, risky boundaries and male group size in spider monkey fission–fusion societies. Am. J. Primatol. 70, 271-281. ( 10.1002/ajp.20484) [DOI] [PubMed] [Google Scholar]
- 70.Brown M, Waser PM. 2018. Group movements in response to competitors’ calls indicate conflicts of interest between male and female grey-cheeked mangabeys. Am. J. Primatol. 80, e22918. ( 10.1002/ajp.22918) [DOI] [PubMed] [Google Scholar]
- 71.Kitchen DM, Cheney DL, Seyfarth RM. 2005. Contextual factors meditating contests between male chacma baboons in Botswana: effects of food, friends and females. Int. J. Primatol. 26, 105-125. ( 10.1007/s10764-005-0725-y) [DOI] [Google Scholar]
- 72.Rasmussen DR. 1979. Correlates of patterns of range use of a troop of yellow baboons (Papio cynocephalus). I. Sleeping sites, impregnable females, births, and male emigrations and immigrations. Anim. Behav. 27, 1098-1112. ( 10.1016/0003-3472(79)90058-7) [DOI] [Google Scholar]
- 73.Sugiura H, Saito C, Sato S, Agetsuma N, Takahashi H, Tanaka T, Furuichi T, Takahata Y. 2000. Variation in intergroup encounters in two populations of Japanese macaques. Int. J. Primatol. 21, 519-535. ( 10.1023/A:1005448120967) [DOI] [Google Scholar]
- 74.Cowlishaw G. 1995. Behavioural patterns in baboon group encounters: the role of resource competition and male reproductive strategies. Behaviour 132, 75-86. ( 10.1163/156853995X00298) [DOI] [Google Scholar]
- 75.Korstjens A, Bergmann K, Deffernez C, Krebs M, Nijssen E, van Oirschot B, Paukert C, Schippers EP. 2007. How small-scale differences in food competition lead to different social systems in three closely related sympatric colobines. Camb. Stud. Biol. Evol. Anthropol. 51, 72. [Google Scholar]
- 76.Perry S. 1996. Intergroup encounters in wild white-faced capuchins (Cebus capucinus). Int. J. Primatol. 17, 309-330. ( 10.1007/BF02736624) [DOI] [Google Scholar]
- 77.Borries C, Koenig A, Winkler P. 2001. Variation of life history traits and mating patterns in female langur monkeys (Semnopithecus entellus). Behav. Ecol. Sociobiol. 50, 391-402. ( 10.1007/s002650100391) [DOI] [Google Scholar]
- 78.van Schaik CP, van Noordwijk MA, Nunn CL. 1999. Sex and social evolution in primates. In Comparative primate socioecology (ed. PC Lee), pp. 204–231. Cambridge, UK: Cambridge University Press.
- 79.Willems EP, van Schaik CP. 2015. Collective action and the intensity of between-group competition in nonhuman primates. Behav. Ecol. 26, 625-631. ( 10.1093/beheco/arv001) [DOI] [Google Scholar]
- 80.Borries C, Launhardt K, Epplen C, Epplen JT, Winkler P. 1999. Males as infant protectors in Hanuman langurs (Presbytis entellus) living in multimale groups–defence pattern, paternity and sexual behaviour. Behav. Ecol. Sociobiol. 46, 350-356. ( 10.1007/s002650050629) [DOI] [Google Scholar]
- 81.Pradhan GR, van Schaik C. 2008. Infanticide-driven intersexual conflict over matings in primates and its effects on social organization. Behaviour 145, 251-275. ( 10.1163/156853907783244710) [DOI] [Google Scholar]
- 82.van Schaik CP, Kappeler PM. 1997. Infanticide risk and the evolution of male–female association in primates. Proc. R. Soc. Lond. B 264, 1687-1694. ( 10.1098/rspb.1997.0234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rhine R. 1986. The socio-spatial organization of baboon (Papio cynocephalus) progressions at Mikumi National Park, Tanzania. Misc. Zool. 10, 359-370. [Google Scholar]
- 84.Zuberbühler K. 2009. Survivor signals: the biology and psychology of animal alarm calling. In Advances in the study of behavior (eds M Naguib, K Zuberbühler, NS Clayton, VM Janik), pp. 277-322. New York, NY: Academic Press. [Google Scholar]
- 85.Silk JB, Städele V, Roberts EK, Vigilant L, Strum SC. 2020. Shifts in male reproductive tactics over the life course in a polygynandrous mammal. Curr. Biol. 30, 1716-1720. ( 10.1016/j.cub.2020.02.013) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The code of the model is publicly archived at https://github.com/XiangyiLi/Hired-Guns.