Abstract
Group territory defence poses a collective action problem: individuals can free-ride, benefiting without paying the costs. Individual heterogeneity has been proposed to solve such problems, as individuals high in reproductive success, rank, fighting ability or motivation may benefit from defending territories even if others free-ride. To test this hypothesis, we analysed 30 years of data from chimpanzees (Pan troglodytes) in the Kasekela community, Gombe National Park, Tanzania (1978–2007). We examined the extent to which individual participation in patrols varied according to correlates of reproductive success (mating rate, rank, age), fighting ability (hunting), motivation (scores from personality ratings), costs of defecting (the number of adult males in the community) and gregariousness (sighting frequency). By contrast to expectations from collective action theory, males participated in patrols at consistently high rates (mean ± s.d. = 74.5 ± 11.1% of patrols, n = 23 males). The best predictors of patrol participation were sighting frequency, age and hunting participation. Current and former alpha males did not participate at a higher rate than males that never achieved alpha status. These findings suggest that the temptation to free-ride is low, and that a mutualistic mechanism such as group augmentation may better explain individual participation in group territorial behaviour.
This article is part of the theme issue ‘Intergroup conflict across taxa’.
Keywords: intergroup aggression, territorial behaviour, collection action problems, boundary patrols, hunting, Pan troglodytes
1. Introduction
Many group-living mammals defend group territories (e.g. meerkats, Suricata suricatta [1]; free-ranging dogs, Canis lupus familiarus [2]; lions, Panthera leo [3]; ring-tailed lemurs, Lemur catta [4]; white-faced capuchins, Cebus capucinus [5]; vervet monkeys Chlorocebus pygerythrus [6]; red-tailed monkeys, Cercopithecus ascanius [7]; chimpanzees, Pan troglodytes [8,9]; other primates [10,11]). These territories provide benefits to group members, including resources such as food, water and shelter [12–15], and safety from intergroup attacks [16]. These benefits are public goods, because group members can obtain these benefits regardless of whether they have paid the costs of production [17]. This poses a collective action problem [18]: insofar as territorial effort is costly, individuals will be tempted to free-ride, benefiting from the public goods without paying the costs. But if all individuals free-ride, the good will not be produced. A group composed entirely of free-riders eventually will have no territory to defend. What motivates individuals to participate in territorial behaviour, despite the temptation to defect?
Heterogeneity among individuals has been proposed as a solution to this collective action problem [19]. Although assuming homogeneity among group members can make mathematical models of behaviour easier to construct, in the real world, individuals are unlikely to be identical to one another [20]. Individuals may vary in their fighting ability or tolerance of risk, and thus experience differing costs from participation in territorial behaviour. Additionally, despite assumptions of earlier models, public goods are not necessarily shared equally [19–21]. Individuals with high dominance rank may produce a disproportionate share of offspring, which then benefit from the food resources and safety provided by their parent's territorial effort. Thus, apparently altruistic territorial behaviour could instead result from heterogeneity in individual costs, propensities or benefits of participation in collective action [19,20].
Findings from field studies of chimpanzees (Pan troglodytes) have supported this theoretical expectation. In the Ngogo community of Kibale National Park, Uganda, individual participation in boundary patrols by male chimpanzees correlated with measures of reproductive success, rank and hunting success [22]. These findings have since been partially supported by a follow-up study examining 20 years of data from Ngogo [23], which found that males participated more in patrolling if they had more offspring and were high in dominance rank. Additionally, they found that males patrolled more when the group had fewer males, suggesting that males increased participation when the costs of defecting were higher [23]. However, this study also found results contrary to some predictions of collective action theory. In particular, males had generally high rates of participation, with each male participating in a mean of 33% of patrols. Additionally, despite the expectation that patrolling should be based on reproductive success, some males that had no offspring in the group participated frequently in patrols. Based on these and other considerations, Langergraber et al. [23] proposed that a group augmentation model was a better predictor of individual participation in patrols.
Group augmentation theory, which posits that individuals benefit directly from living in larger groups and thus behave in seemingly altruistic ways to increase reproductive success of group members, was developed to explain why helpers in cooperatively breeding species delay or even forgo their own reproduction to help others raise their young [24]. If individuals survive or reproduce better in larger groups, then they may benefit from the increased production of new group members, even if they are unrelated. In one extreme example, groups of pied babblers (Turdoides bicolor) that fail to raise their own young, may even ‘kidnap’ young birds from neighbouring groups [25]. Research suggests that larger group size may be the benefit driving this peculiar behaviour. Applying this logic to chimpanzees, Langergraber et al. [23] argued that because reproductive skew is generally low among male chimpanzees, and all males depend on successful defence of a territory to ensure safety and sufficient food for themselves, their mates and their offspring, individuals have lower incentives for free-riding than predicted by a collective action framework.
Chimpanzees are an excellent study system to investigate collective action problems, as they have been studied intensively at multiple long-term field sites [26–29]. Chimpanzees live in groups (‘communities’ [8] or ‘unit-groups’ [30]) that exhibit fission-fusion dynamics, in which individuals travel in subgroups (‘parties’) that vary in size and composition [8,31]. Male chimpanzees defend group territories and maintain hostile relations with neighbours [8,32,33], sometimes killing members of other groups [34,35]. Chimpanzees conduct boundary patrols, during which parties, often mainly of males, travel to the periphery of their home range, behaving as if they are searching for signs of chimpanzees from other communities [8,22,32]. Males can adjust their participation in boundary patrols by associating with or avoiding parties with many males, which are more likely to visit boundaries [36], or by going with or staying behind if a party travels toward the periphery [23,37]. Intergroup encounters occur most frequently when seasonally abundant fruit attracts members of rival communities to borderlands [37]. Participating in a patrol also likely increases the chances of encountering neighbours. Most intergroup encounters involve only auditory contact, in which individuals hear vocalizations from distant chimpanzees, and may respond with vocalizations of their own [37,38], and/or approach or retreat, depending on their apparent relative numerical strength [37,39,40]. If chimpanzees come within visual range, they may chase, attack and sometimes kill members of rival groups [33,38,41]. Intergroup killings occur mainly when attackers greatly outnumber victims (median ratio of attackers to victims = 8 : 1 [35]).
Winners of intergroup contests may increase their territory size [8,41], which can result in community-wide benefits, particularly increased access to food resources, as indicated by heavier body mass [13] and larger parties [12] in years with larger territory size. Increased food provides fitness benefits: in years with larger territory size, females have shorter interbirth intervals [12,15]. Thus, group territorial effort creates the public good of increased food supply. Additionally, communities with larger territories suffer lower rates of intergroup mortality, perhaps as a consequence of reduction in the relative size of the periphery as the territory increases in area [16]. Participating in patrols appears to be costly for individuals, including energetic costs from travelling further [42], and physiological costs, including higher levels of cortisol [43] and testosterone [44]. Insofar as patrolling borders increases the likelihood of meeting and fighting with members of rival communities, participating in patrols potentially increases risks of injury or death.
While the collective action framework focuses on how these costs may promote free-riding, two factors may mitigate these costs. First, as Langergraber et al. [23] argue, the benefits of territorial effort may be distributed more evenly than would appear from a focus on individual differences. In a world divided among competing social groups, individual survival and reproduction may depend so critically on intergroup effort that all individuals or all individuals of a particular sex are motivated to participate, such that participation in territorial behaviour evolves through mutualistic benefits, as in lions [45]. Second, while patrolling incurs energetic and physiological costs, these may be offset by various direct benefits to patrollers. Travelling with many males reduces the risks of being injured or killed [33,46,47], and travelling to the periphery may provide opportunities to find and exploit new food resources [7,37], monitor females with peripheral home ranges [48], recruit new females [49] and participate in other activities that benefit from larger numbers of males, such as hunting [50].
To provide an additional test of factors affecting individual participation in territorial behaviour, we examined data from the Kasekela community of chimpanzees in Gombe National Park, Tanzania. The Ngogo community is exceptionally large, with up to 206 members [23]. Additionally, this community fissioned into two mutually hostile communities in 2017 [51]. Given the expectation that larger group size exacerbates collective action problems, and the possibility that substructuring within Ngogo affected patterns of cooperation in that community, we sought to test whether similar patterns held in a community that is more typical in size and not undergoing a fission. With a median population of 50 individuals (range 39–61, 1978–2007), the Kasekela community is close to the median size for chimpanzee communities in long-term studies (median = 42.3 members; range: 6.7–144; including a median 8.2 males (range: 1.9–37) and 14.4 females (range: 2.1–51) at least 12 years old; N = 18 communities [35]). Additionally, by using 30 years of data, we sought to explore how individual participation in boundary patrols varies across the male lifespan.
In the present study, we tested four sets of variables predicted to promote individual participation in boundary patrols. First, according to the collective action problem framework, individuals should participate according to their expected returns [18,19]. Insofar as males defend a feeding territory for themselves, their mates and offspring, males with more mating opportunities and more existing offspring stand to benefit more. Because we do not have genetic paternity data for the early decades of the study, we considered three main proxies of reproductive success: mating rate, age and dominance rank. In the short term, males that mate more frequently with fertile females should have a higher chance of siring offspring. In the long term, if males continue to sire offspring, older males should have more offspring. High-ranking males sire more offspring [52], although recent analysis indicates that only the highest-ranking males (alphas) sire a disproportionate share [53].
Second, collective action theory predicts that individuals may contribute territorial effort if the costs of doing so are low, because they are strong fighters and/or highly motivated [19,54]. In male chimpanzees, dominance rank typically peaks in early adulthood [55], and thus likely reflects peak physical condition and competitive ability, in addition to being correlated with reproductive success as discussed above. Individuals may also differ in motivation due to differences in propensity for boldness, exploration or aggression [54]. Inter-group aggression may be a driving force behind such behavioural variation [56]. We considered two additional factors associated with individual fighting ability and motivation: hunting and personality ratings. Chimpanzees engage in group-level hunts of monkeys, which like intergroup contests involve collective efforts to attack and kill victims [50]. Watts & Mitani [22] found that at Ngogo, males who hunted more often and were more successful at hunting also participated in patrols more often. For measures of motivation, we considered personality scores derived from a modified version of the Hominoid Personality Questionaire wherein researchers scored individuals on 24 items [57]. Weiss et al. [57] used these scores to calculate six personality factors identified in a previous personality study of captive chimpanzees [58]: Dominance, Extraversion, Agreeableness, Conscientiousness, Neuroticism, and Openness. We considered scores for two personality dimensions expected to correlate with willingness to patrol boundaries: Dominance and Conscientiousness. These personality dimensions are derived differently and independently from behavioural measures such as dominance rank. Based on studies of personality in captive chimpanzees [58,59], we predict that individuals with high Dominance scores should patrol more often, due to being bolder, and that individuals with low Conscientiousness scores should patrol more often, due to reduced concern for the associated risks.
Third, the ‘group size paradox’ contends that collective action should break down more readily in larger groups due to increased opportunity for free-riders and decreased benefits for individuals [18]. Studies of individual species [2,5,23] and comparative analysis [11] indicate that cooperative investment decreases with increase in group size. Because chimpanzees are more likely to initiate boundary patrols when travelling in larger parties [47], in communities with fewer males, a larger proportion of males may be required to mount an effective patrol. Furthermore, individuals may be more sensitive to the costs of defecting when in smaller groups, because each individual's contribution matters more to the success of the collective effort. In this case, individual participation in boundary patrols should increase when the number of males in the community is low.
Fourth, variation in patrolling frequency potentially reflects differences in gregariousness, or an individual's propensity to travel in larger groups. Because patrols typically involve parties with many males, an individual who rarely spends time travelling in large parties will likely participate in patrols less frequently. To control for this possibility, we included a measure of sighting frequency, the proportion of days on which an individual was observed.
2. Methods
(a) . Study site
The site of long-term chimpanzee research since 1960 [8], Gombe National Park (4°40′ S 29°38′ E) covers a rugged landscape with a land area of 35.69 km2 along the shore of Lake Tanganyika as well as 20.72 km2 of the lake itself [60]. During our study period, three chimpanzee communities lived in Gombe. We analyze data from the Kasekela community, which occupied a large range at the centre of Gombe during our study period (1978–2007; median = 12.17 km2, range 5.30–19.16 km2), with a median population of 50 individuals (1978–2007, range 39–61, n = 30 years). To the north, the Mitumba community (1994–2007; median = 4.63 km2, range 3.19–5.91 km2) had a median population of 22 individuals (range 20–25, 1996–2007). To the south, the Kalande community [61] remained unhabituated for most of this study period. Observational monitoring and non-invasive genetic sampling indicated that Kalande had a median population of 14.5 individuals (range 12–19, 2001–2007). Both communities were likely larger and more formidable earlier in the study period [61,62].
In Kasekela, researchers conducted all-day focal follows of individual chimpanzees on a nearly daily basis [63]. Chimpanzees use branches to make simple sleeping platforms in trees each night. Ideally, follows started at the focal target's sleeping site before dawn, and continued until the target nested that night. Observers recorded party location at 15-minute intervals while maintaining a continuous record of party composition and focal feeding behaviour. Researchers collected continuous data on the behaviour of a single focal individual, while also recording an ad libitum narrative of the day's events. Behaviors collected in the ad libitum narrative included aggression, mating, boundary patrols and intercommunity interactions. Observers documented the reproductive state of all adult females seen each day, based on the size of anogenital sexual swellings [64], and recorded the dates of births, deaths and changes in community membership.
(b) . Study subjects
We analysed 30 years of behavioural observations of male chimpanzees of the Kasekela community, starting with the first year for which daily dominance hierarchy data have been calculated (1978 [55]) and ending with the most recent year for which boundary patrol data have been extracted (2007 [50]). To include all potential sires, we included males starting from 12 years old, the age of the youngest known sire from Gombe [52]. We refer to these males as ‘adult males’, though we recognize that most males do not reach full adult size (median = 39 kg [13]) and behaviour until later. Our sample included 23 males that were ≥12 years old during the study period, excluding from analysis one male (PX) who was effectively castrated by a scrotal injury at a young age [8]. We calculated annual records for each male, which resulted in 283 male years with complete data, with a median of 12 years per male (range = 2–22 years), during which males had a median age of 22.8 years (range = 12.1–40.6 years). Because some males reached 12 years of age and/or died part way through the year, observation days per year ranged from 20 to 366 days (median = 365 days). We calculated the mean number of adult males present in the community each year based on the number of days each male was known to be alive and ≥12 years old. Over these years, Kasekela had a median of 11.1 males (range = 7.6–13.0 males).
(c) . Boundary patrols
We extracted boundary patrols from long-term records, based on explicit statements that chimpanzees were patrolling, and descriptions of cautious travel where individuals appeared to be looking and/or listening for chimpanzees from neighbouring communities [50]. We considered all adult males present in the party at the start of the boundary patrol to be participants. We used records of party composition to calculate the proportion of time each participant stayed for each patrol.
(d) . Periphery visits
To capture any visits to the far periphery missed by our method of extracting boundary patrols, we used map location data to identify all occasions on which parties travelled ≥ 3 standard deviations north or south of the annual north-south range centre. We chose this benchmark as it identified periphery visits on approximately the same order as boundary patrols. Kasekela faced intergroup threats to the north (Mitumba) and south (Kalande), but not from Lake Tanganyika to the west, or the human-dominated village lands to the east. Any boundary patrols that also met the criteria for periphery visits were considered solely as boundary patrols for purposes of analysis. We identified all adult Kasekela males present during a periphery visit as participants. We considered all adult Kasekela males alive at the time, but absent from the visit, to be non-participants.
(e) . Comparison of boundary patrols and periphery visits
Visits to the periphery could serve other purposes, such as searching for food or females. We, therefore, compared party composition and feeding behaviour during each of these two categories of event, using a set of boundary patrols (N = 180) and periphery visits (N = 147) for which we had complete party composition and feeding data. We used Mann-Whitney U-tests with corrections for the false discovery rate [65] to test for differences in party size, number of adult males and number of adult females. We used Poisson regression to compare time spent feeding during boundary patrols and periphery visits. To model rates with a Poisson regression, we use the log of the denominator as an offset. The regression modelled the count variable (minutes spent feeding) as the dependent variable with duration of boundary patrols as an offset to model the rate of time spent feeding. Model diagnostics indicated overdispersion for this model, hence we used the quasi-Poisson family to account for this overdispersion [65].
(f) . Mating frequency
To obtain unbiased estimates of mating frequency, we estimated each male's rate of mating on days when he was the focal follow target. We limited analysis to matings with females that had given birth (parous females) because they are more likely to conceive than females who had not given birth (nulliparous females), and males compete more intensively over access to parous females [66,67]. Researchers observed each male as a focal target for a median of 137.8 h annually (range 1.57–941.9 h per male per year). Males mated with parous females a median of 2 times during focal follows per year (range: 0–60 mating events per year).
(g) . Dominance rank
Chimpanzees give pant-grunt vocalizations to indicate submission towards higher-ranking individuals [8,68,69]. To determine relative dominance rank for each individual in each year of observation, we used rank data from Foerster et al. [55], who calculated mean daily Elo scores based on pant-grunt records, using a modified method that employed maximum-likelihood fitting to optimize starting parameters, using the EloOptimized package in R [70]. From these daily scores, they calculated cardinalized Elo scores, which take into account the Elo scores of all males in the community and specify the probability that an individual will succeed in a given contest [55,70]. Males had a median annual cardinalized daily Elo score of 0.536 (range 0.014–1.000).
(h) . Hunts
We extracted hunts from narrative notes of focal follows [50], counting hunts only if at least one individual climbed in pursuit of prey. For each hunt, we extracted the identities of all individuals that observers named as participants. Each male had the opportunity to join a median of 38 hunts (range: 2–64 hunts) annually and participated in a median of 8 hunts (range: 0–39 hunts) annually.
(i) . Personality
For measures of personality traits, we use scores from Weiss et al. [50]. For this study, long-term Tanzanian field researchers rated chimpanzees using a 24-item version of the Hominoid Personality Questionnaire [57]. For each item, raters scored each individual on a 7-point scale, based on the extent to which that individual's behaviour and interactions with others corresponded to a particular adjective (e.g. ‘Fearful’, ‘Dominant’, ‘Persistent’). Each adjective was followed by a brief explanation of how that trait may be manifested in chimpanzees. For example, researchers scored the personality dimension Dominance according to their assessment that the individual ‘is able to displace, threaten, or take food from other chimpanzees’ or that the subject ‘may express high status by decisively intervening in social interactions’ [57]. These ratings were then used to estimate each chimpanzee's standing on six personality factors identified in captive studies of chimpanzees [58]: Dominance, Extraversion, Agreeableness, Conscientiousness, Neuroticism and Openness. For this study, we used scores for two dimensions that had high inter-observer reliability: Dominance and Conscientiousness. Dominance is similar to, but not synonymous with, dominance rank. Conscientiousness can best be described as the degree to which individuals are predictable and careful. Multiple researchers rated each chimpanzee. Males from our sample had median scores of 4.2 for Dominance (range: 2.8–5.7, N = 23 males) and 4.0 for Conscientiousness (range: 2.4–5.2, N = 23 males).
(j) . Sighting frequency
To control for variation in observation time for each individual, we calculated the proportion of days on which each male was recorded as present during a focal follow within each observation period.
(k) . Statistical analyses
All statistical analyses were performed using R v. 4.1.2 [71]. We performed model diagnostics using the DHARMa package [72]. We used the DAGitty [73], lme4 [74] and AICcmodavg [75] R packages to conduct analysis.
(l) . Causal inference
To minimize the chances of discovering spurious or misleading relationships among the variables of interest, we employed a causal inference framework to inform our modelling [76,77]. We incorporated our prior understanding of causal relationships among the variables using a directed acyclic graph (DAG; electronic supplementary material figure S1). DAGs facilitate causal inference by allowing researchers to identify causal relationships that result in (i) spurious statistical correlations between variables and (ii) masking of real statistical correlations between variables. This knowledge allows researchers to make causally informed decisions about which variables should and should not be controlled for in the statistical analysis. Using the R-package DAGitty [73], we specified the DAG and identified the minimally sufficient adjustment sets of confounding variables to be controlled for when testing for relationships of interest in our multiple regressions using the adjustmentSets function. A minimally sufficient adjustment set is a set of variables that is sufficient to control for to obtain an unbiased estimate of the effect of an independent variable on a dependent variable [77]. We summarize these sets for the relationships of interest in table 1.
Table 1.
Minimally sufficient adjustment sets (i.e. confounding variables to control for) for the tests of relationships of interest.
| model ID | relationship of interest | dependent variable (DV) | confounding variables to control for |
| independent variable (IV) | |||
| 1 | patrolling/periphery visits <— age | DV: patrolling or periphery visits | none |
| IV: age | |||
| 2 | patrolling/periphery visits <— rank | DV: patrolling or periphery visits | (1) personality, if model 1 relationship does not hold |
| IV: rank | (2) personality & age, if model 1 relationship holds | ||
| 3 | patrolling/periphery visits <— mating | DV: patrolling or periphery visits | (1) personality, if neither model 1 or 2 relationships hold |
| IV: mating | (2) personality & age, if model 1 relationship holds AND model 2 relationship does not hold | ||
| (3) personality & rank, if model 2 relationship holds | |||
| 4 | patrolling/periphery visits <— no. of adult males | DV: patrolling or periphery visits | none |
| IV: no. of adult males | |||
| 5 | patrolling/periphery visits <— hunting | DV: patrolling or periphery visits | personality & sighting frequency |
| IV: hunting | |||
| 6 | patrolling/periphery visits <— personality | DV: patrolling or periphery visits | none |
| IV: personality | |||
| 7 | patrolling/periphery visits <— sighting frequency | DV: patrolling or periphery visits | none |
| IV: sighting frequency |
(m) . Model fitting and model selection
We used binomial Generalized Linear Mixed Models to model the relationships in table 1. The dependent variable in each model was a binary variable for either patrols, representing whether an individual participated in a given boundary patrol (N = 1945 opportunities to patrol), or periphery visits, representing whether an individual participated in a given periphery visit (N = 1511 opportunities to participate in a periphery visit). These models examined the effect of variables associated with reproductive success (mating rate, age, dominance rank), costs of participation (hunting), motivation (personality scores), costs of defecting (number of males ≥12 years old) and a measure of gregariousness (sighting frequency) on patrolling and periphery visit behaviours. All models included individual identity as a random intercept to control for individual variation. Models 2, 3 and 5 (table 1) included additional independent variables to account for their confounding effects.
We used information-theoretic model selection [78] to assess the relative importance of different variables hypothesized to predict participation in patrols and other periphery visits. We used the AICcmodavg package [75] in R to calculate each model's weight (w). Model weight represents the probability that each model is the best model of a set of models. We used these model weights to identify the best predictors of patrolling and periphery visits. Furthermore, we report the unbiased estimates of the causal effects of each independent variable on patrolling and periphery visits and its 95% confidence interval. Finally, we calculated marginal and conditional R2 values for each candidate model using the piecewiseSEM package in R [79].
3. Results
(a) . Boundary patrols and periphery visits
During the study period, we documented 180 patrols and 147 other periphery visits. Thirty of the 180 identified boundary patrols also met the criteria to be considered periphery visits. The number of such events varied from year to year (patrols: median = 4.5, range = 0–19; periphery visits: median = 4, range = 0–11). Boundary patrols (median = 88.5 min, range = 3–595) and periphery visits (median = 90 min, range = 15–735) did not differ significantly in duration (Mann-Whitney U test, U = 13080, p = 0.86, BH correction p = 0.86). Boundary patrols contained more individuals (patrols: median = 14 independently travelling individuals, range = 1–32; periphery visits: median = 12 individuals, range = 1–47; Mann-Whitney U test, U = 14922, p = 0.05, BH correction p = 0.06), and also contained more adult males than other periphery visits (patrols: median = 8 males, range = 1–13; periphery visits: median = 7 males, range = 0–13; Mann-Whitney U, U = 16214, p < 0.01, BH correction p < 0.01). After correction for multiple testing, boundary patrols and periphery visits did not differ significantly in the number of adult females present (patrols: median = 3 females, range = 0–20; other periphery visits: median = 2 females, range = 0–15; Mann-Whitney U, U = 15022, p = 0.03, BH correction p = 0.06) or the number of adult females with sexual swellings (patrols: median = 1 swollen female, range = 0–6; other periphery visits: median = 1 swollen female, range = 0–4; Mann-Whitney U, U = 14956, p = 0.03, BH correction p = 0.06). Chimpanzees on boundary patrols spent less time feeding than those on other periphery visits (patrols: median = 6.51 min per hour, range = 0–60; other periphery visits: median = 17.2 min per hour, range = 0–60; quasi-Poisson regression, β = 0.30, 95% CI = [0.13–0.46], n1 = 180 patrols, n2 = 147 periphery visits, z = 18.3, p < 0.01).
(b) . Individual variation in participation
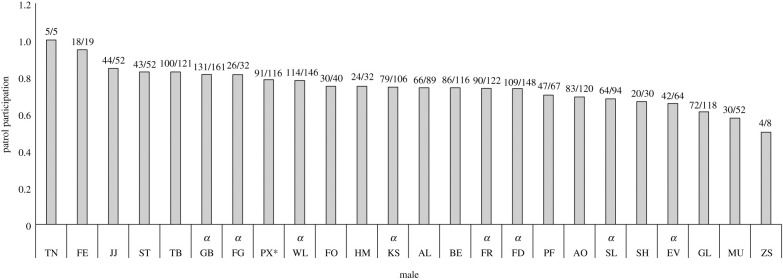
Over the entire study period, individuals participated in a mean of 74.5 ± 11% of boundary patrols (figure 1). The few males with extremely low or high rates of participation were young males who reached age 12 only during the last years of the study, and therefore had small sample sizes (50%: ZS, b. 1993, N = 4 patrols; 100%: TN, b. 1994, N = 5 patrols; 95%: FE, b. 1992, N = 18 patrols). All other males exhibited strikingly similar patrol participation rates, between 58 and 85%. Moreover, males identified to be present at the start of the patrol stayed for a median 96% of the duration of the patrol (range = 92–100%, N = 23 males). We, therefore, considered each male present at the start of a patrol to have participated in that patrol.
Figure 1.
Boundary patrol participation for 24 Kasekela males. Column height indicates the percentage of patrols that each individual participated in while greater than 12 years old. Numbers above the columns indicate the total number of patrols that each individual joined (numerator), and the number of patrols recorded while that individual was alive and ≥12 years old (denominator). All current and former alpha males are labelled with an alpha (α). Here, we include PX, who suffered a scrotal wound at a young age and was effectively castrated. We excluded PX from analyses.
For both boundary patrols and periphery visits, information-theoretic model selection identified the hunting model, which also controlled for sighting frequency and personality dimensions, to be the most-likely candidate model (boundary patrols, table 2: w = 0.99, periphery visits, table 3: w = 0.80), followed by the model with sighting frequency alone. Males varied considerably in the percentage of days they were recorded by observers (mean = 0.52 ± 0.13% of days per year; N = 283 male-years). Males who were observed more frequently participated in more patrols (figure 2, β = 3.37, 95% CI = [2.42–4.35]), as did males who participated in more hunts (figure 3, β = 1.83, 95% CI = [0.79–2.92]).
Table 2.
Information-Theoretic Model selection results: boundary patrols. Values in italics indicate variables with non-zero model averaged parameter estimates. Parameters include: the intercept; Elo rank; operational sex ratio; age; age2; patrol participation, periphery visit participation, hunting participation; Dominance, Conscientiousness; the number of free parameters (K); the marginal pseudo R2 for fixed effects; the conditional R2 for fixed and random effects; the difference in Akaike information criterion between the ith model and the best model (Δi); and model weight (wi). Models are arranged in order from best (lowest ΔAICc) to worst (highest ΔAICc). The weight of the model (wi) is the probability that a given model is the best model in a given set of models. Model-averaged parameter estimates (MAP) with upper (97.5%) and lower (2.5%) bounds of the 95% confidence intervals are given in the bottom rows.
| model | intercept | age | mating | rank | adult males in the community | hunting participation | Dominance | Conscientious-ness | sighting frequency | K | marginal R2 | conditional R2 | Δi | wi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hunting | −1.52 | 1.83 | 0.21 | −0.08 | 2.82 | 6 | 0.05 | 0.08 | 0.00 | 0.99 | ||||
| observation frequency | −0.73 | 3.37 | 3 | 0.03 | 0.05 | 9.18 | 0.01 | |||||||
| total males | 0.20 | 0.08 | 3 | 0.03 | 0.03 | 54.97 | 0.00 | |||||||
| rank | 0.97 | 0.38 | 0.13 | −0.18 | 5 | 0.01 | 0.02 | 56.76 | 0.00 | |||||
| personality | 1.28 | 0.14 | −0.22 | 4 | 0.00 | 0.02 | 56.93 | 0.00 | ||||||
| mating frequency | 1.26 | 2.45 | 0.13 | −0.22 | 5 | 0.01 | 0.02 | 58.31 | 0.00 | |||||
| age | 1.03 | 0.00 | 3 | 0.00 | 0.02 | 58.69 | 0.00 | |||||||
| model-averaged parameter | −1.51 | 0.00 | 2.45 | 0.38 | 0.08 | 1.83 | 0.21 | −0.08 | 2.82 | |||||
| 2.5% | −3.51 | −0.02 | −3.34 | −0.11 | 0.00 | 0.77 | −0.08 | −0.38 | 1.81 | |||||
| 97.5% | 0.5 | 0.02 | 8.23 | 0.86 | 0.16 | 2.89 | 0.50 | 0.23 | 3.84 |
Table 3.
Information-Theoretic Model selection results: periphery visits. Values in italics indicate variables with non-zero model averaged parameter estimates. Parameters include: the intercept; Elo rank; operational sex ratio; age; age2; patrol participation, periphery visit participation, hunting participation; Dominance, Conscientiousness; the number of free parameters (K); the marginal pseudo R2 for fixed effects; the conditional R2 for fixed and random effects; the difference in Akaike information criterion between the ith model and the best model (Δi); and model weight (wi). Models are arranged in order from best (lowest ΔAICc) to worst (highest ΔAICc). The weight of the model (wi) is the probability that a given model is the best model in a given set of models. Model-averaged parameter estimates (MAP) with upper (97.5%) and lower (2.5%) bounds of the 95% confidence intervals are given in the bottom rows.
| model | intercept | age | mating | rank | adult males in the community | hunting participation | Dominance | Conscientious-ness | sighting frequency | K | marginal R2 | conditional R2 | Δi | wi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hunting | −1.82 | 1.27 | 0.02 | 0.00 | 3.73 | 6 | 0.07 | 0.07 | 0.00 | 0.80 | ||||
| sighting frequency | −1.74 | 4.41 | 3 | 0.07 | 0.07 | 0.73 | 0.20 | |||||||
| mating frequency | 0.78 | 9.16 | 0.01 | −0.11 | 5 | 0.01 | 0.02 | 64.63 | 0.00 | |||||
| age | 0.97 | −0.02 | 3 | 0.00 | 0.03 | 69.55 | 0.00 | |||||||
| total males | 0.41 | 0.01 | 3 | 0.00 | 0.02 | 72.89 | 0.00 | |||||||
| personality | 0.98 | −0.28 | −0.01 | −0.10 | 4 | 0.00 | 0.02 | 74.61 | 0.00 | |||||
| rank | 1.17 | −0.30 | 0.00 | −0.12 | 5 | 0.00 | 0.02 | 75.61 | 0.00 | |||||
| model-averaged parameter | −1.80 | −0.02 | 9.16 | −0.30 | 0.01 | 1.27 | 0.00 | −0.10 | 3.87 | |||||
| 2.5% | −3.06 | −0.04 | 3.77 | −0.87 | −0.06 | 0.39 | −0.21 | −0.38 | 2.65 | |||||
| 97.5% | −0.54 | 0.00 | 14.55 | 0.27 | 0.09 | 2.14 | 0.20 | 0.18 | 5.09 |
Figure 2.
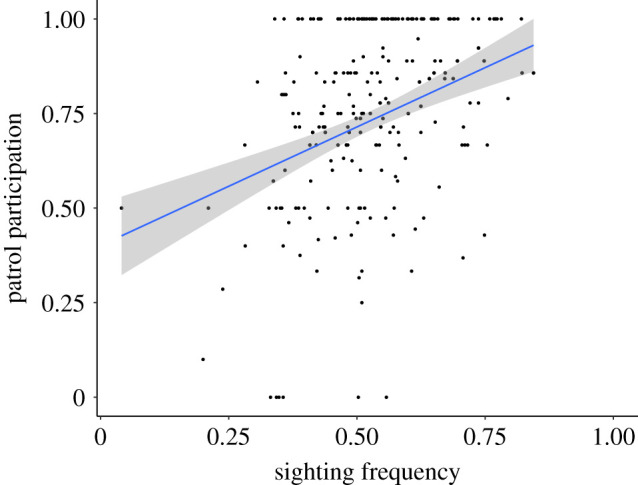
Boundary patrol participation versus sighting frequency. Each point represents the percentage of patrols in which a male participated for a given year. Plotted regression line represents the probability of a male participating in a given patrol relative to annual sighting frequency. The grey area represents the 95% confidence interval for the regression line. (Online version in colour.)
Figure 3.
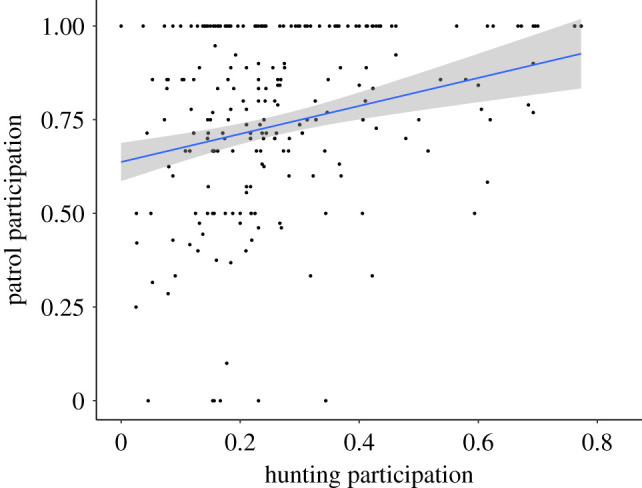
Boundary patrol participation versus hunting participation. Each point represents the percentage of patrols in which a male participated for a given year. Plotted regression line represents the probability of a male participating in a given patrol relative to annual hunting participation. The grey area represents the 95% confidence interval for the regression line. (Online version in colour.)
For the remaining variables tested, the 95% confidence interval of the parameter estimate included zero. Though we therefore lack confidence in the effect of these parameters, some variables had effects in the direction predicted by collective action theory: higher rank, greater mating success, high scores for Dominance, and low scores for Conscientiousness correlated with higher patrol participation. The estimates for periphery visits closely resembled those for boundary patrols (table 3), with the exception that males who mated more often during focal follows were more likely to participate in periphery visits (β = 9.16, 95% CI = [3.90–14.69]).
Mating frequency is admittedly an imperfect measure of reproductive success, given that chimpanzees mate promiscuously. Given that only alpha males obtain a disproportionate share of paternities [53], we compared patrol participation rates for current and former alpha males with those of males that never reached top rank. However, the patrol participation rate for current and former alpha males (mean = 75 ± 3%, N = 8) did not differ significantly from that of males who had never reached alpha status (70 ± 3%, N = 15; binomial regression, β = 0.11, z = 1.00, p = 0.32).
4. Discussion
Collective action theory predicts that individuals in group-territorial species should be strongly tempted to free-ride [11,17,18]. As a result, territorial effort should be undertaken disproportionately by individuals that expect to gain a greater share of the spoils, or that can afford the costs because they are high-ranking, strong, or highly motivated [17,19]. By contrast to these expectations, we found that male chimpanzees in the Kasekela community participated in patrols at an exceptionally high rate (mean = 74.5%). Kasekela males participated at more than double the rate of Ngogo males (mean = 33%), which Langergraber et al. [23] considered to be higher than expected from collective action theory. Moreover, we found little evidence that individual traits, such as rank, mating rate or age explained variation in patrolling. Instead, all Kasekela males participated in at least half of all boundary patrols. Recent paternity analysis at Gombe indicate that the alpha male obtains a disproportionate share of paternities in Kasekela [53]. Despite these reproductive benefits, current and former alpha males did not patrol at significantly higher rates than other males. Indeed, one male that we excluded from our analysis because he was not a potential father (PX) nonetheless participated in patrols at a high rate (78.4%; figure 1; electronic supplementary material figure S1D); higher, indeed, than some males (e.g. FR, SL) that attained alpha status and sired many offspring.
Why did Kasekela males join patrols at such high rates, regardless of their rank or mating rate? Potential motivating factors include (a) a broader than expected distribution of benefits; (b) lower than expected costs of participation; (c) effects of group size; and (d) benefits of gregariousness.
(a) . Distribution of benefits
If males participate in patrols mainly to benefit their own current or future offspring, then correlates of reproductive success, such as rank, mating rate and age should predict patrol participation. However, we did not find strong effects of rank, mating rate or age on patrol participation. One possible explanation for this is that the benefits of territorial behaviour are distributed broadly enough that each male has a strong incentive to participate. This is the perspective of group augmentation theory: if individual survival and reproduction depend strongly on group size, then each individual can be motivated to invest in increasing group size, even if reproductive skew is high [23,24]. All males participate because they all benefit from raising more future fighters to defend their community.
(b) . Costs of participation
Based on collective action theory, we expected to find evidence that males with individual traits that made patrolling less costly for them would patrol more often. Males that hunt frequently might also be expected to be good fighters; and individuals with certain behavioural tendencies or personality traits might be more strongly motivated to patrol. Males that hunted more did patrol more as well—though the possibility exists that this correlation results, at least to some extent, from the fact that both patrols and hunting occur more often when males group together and travel long distances [50]. Personality scores correlated in the expected directions with patrolling, but the model including personality scores received weak support. This may partially result from the temporal scale of these measures. The personality questionnaire produced a single value for each personality dimension for each individual, whereas hunting observations reflected the behaviour of each individual in a given year. We found substantial variation in patrol participation rates both between individuals and within individuals over their lifetime (electronic supplementary material figure S1). With a single value per individual, the personality scores do not capture such variation.
While patrols entail energetic and physiological costs [42–44], they may also provide direct benefits to patrollers, such as providing opportunities to find food and females in areas that would otherwise be unsafe to visit. While chimpanzees spent less time feeding during patrols than other visits to the far periphery, patrols nonetheless may provide opportunities to find remote food resources and assess the safety of visiting them. Travelling in large groups may also provide increased opportunities to eat meat. Hunting and patrolling are temporally correlated because both are more likely to occur when parties contain many males and travel long distances [50]. In our dataset, 26% of patrols (N = 47) took place on the same day that a hunt occurred. With all of these direct benefits from patrol participation, the temptations to free-ride may in fact be low.
Additionally, while we might expect patrols to be costly because of the increased risk of encountering neighbours, the costs of such encounters might in fact be low, provided chimpanzees travel in sufficiently large parties. Because chimpanzees prefer to attack when they greatly outnumber their opponents [35,46], attackers rarely suffer injuries in intergroup fights. Indeed, if travelling with many males in the periphery, staying with those males is likely safer than attempting to defect by dropping away. Both chimpanzees [39] and another group territorial species, lions [45], exhibit low rates of defection in response to simulated intruders.
(c) . Group size effects
Following Olson's [18] argument that collective action is more effective in smaller groups, Watts & Mitani [22] predicted that participation in boundary patrols would be more consistent among males living in smaller communities. We found mixed support for this prediction. As noted above, Kasekela males participated in a much larger percentage of patrols than males in the much larger Ngogo community. Nonetheless, in our analysis, the model including the number of males received weak support, and the parameter estimate was positive instead of negative. Perhaps the number of males in Kasekela during our study period (7–13 males) remained within a range conductive to collective action in chimpanzees.
The mean participation rate in patrols is mathematically a function of the mean number of males patrolling and the mean total number of males in the community. For Kasekela, these numbers yield an expected participation rate of 8/11 = 0.73, close to the observed mean of 74.5%. If safety requires an average of eight males to patrol, then either 73% of males will need to participate 100% of the time, or each male must participate on average 73% of the time. Similarly, at Ngogo, patrols contained a mean 37.5% of the group's males, similar to the mean rate of participation (33%) [23]. Kasekela differs strikingly from Ngogo both in the higher overall rate of participation, and in the much narrower range of participation among males (Kasekela: range = 50–100%; Ngogo: range = 2–74%).
(d) . Benefits of gregariousness
The factor that best predicted participation in patrols by Kasekela males was sighting frequency, which likely reflects gregariousness. Males that spend more time with other chimpanzees are more likely to be observed by researchers, and also more likely to be present when a patrol starts. Males likely gain many benefits from gregariousness, including opportunities for mating, grooming, coalition building and hunting. The costs of patrolling may thus be negligible compared to these benefits of socializing.
In summary, our study provides further insight into how collective action problems are solved in nature. By contrast to expectations that group territorial effort would be undertaken primarily by those males with more to gain, we found that males consistently participated in patrols at a high rate. Participation may be encouraged by an even distribution of the benefits accrued to individuals, an increased cost of defection in smaller groups, and/or direct benefits from travelling in large parties.
Ethics
Research at Gombe National Park was conducted with approval from the Tanzania Wildlife Research Institute and the Tanzania Commission for Science and Technology. This research follows guidelines set forth by the University of Minnesota IACUC, but did not require IACUC approval, because data collection involved observation of natural behaviours.
Data accessibility
Summary data and R code used in this analysis are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.z8w9ghxdb [81]. https://datadryad.org/stash/share/31Ea2gi-f0RG_ZKwTtNDr-_W308nFSYNA7bzSCo_MbQ
Authors' contributions
A.M.: conceptualization, formal analysis, funding acquisition, investigation, methodology, visualization, writing—original draft; I.C.G.: data curation, funding acquisition, investigation, writing—review and editing; N.P.D.: formal analysis, methodology, visualization, writing—review and editing; A.W.: data curation, funding acquisition, investigation, writing—review and editing; J.T.F.: data curation, writing—review and editing; A.P.: data curation, funding acquisition, project administration, resources, writing—review and editing; M.W.: conceptualization, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
Agence Nationale de la Recherche (ANR-17-EURE-0010), Arcus Foundation, Arizona State University, Carnegie Corporation of New York, Duke University, Jane Goodall Institute, Leakey Foundation, Leo S. Guthman Foundation, Margo Marsh, National Geographic Society, U.S. Department of Health and Human Services, National Institutes of Health (R00 HD057992, R01 AI050529, R01 AI120810), National Science Foundation (BCS-0452315, BCS-0648481, BCS-1743506, BCS-1753437, DBS-9021946, IOS-1052693, IOS-1457260, SBR-9319909), the Harris Steel Group, University of Minnesota, Wilkie Foundation, William T. Grant Foundation, Windibrow Foundation.
References
- 1.Mares R, Young AJ, Clutton-Brock TH. 2012. Individual contributions to territory defence in a cooperative breeder: weighing up the benefits and costs. Proc. R. Soc. B 279, 3989-3995. ( 10.1098/RSPB.2012.1071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonanni R, Cafazzo S, Valsecchi P, Natoli E. 2010. Effect of affiliative and agonistic relationships on leadership behaviour in free-ranging dogs. Anim. Behav. 79, 981-991. ( 10.1016/J.ANBEHAV.2010.02.021) [DOI] [Google Scholar]
- 3.Heinsohn R, Packer C. 1995. Complex cooperative strategies in group-territorial African lions. Science 269, 1260-1262. ( 10.1126/SCIENCE.7652573) [DOI] [PubMed] [Google Scholar]
- 4.Nunn CL, Deaner RO. 2004. Patterns of participation and free riding in territorial conflicts among ringtailed lemurs (Lemur catta). Behav. Ecol. Sociobiol. 57, 50-61. ( 10.1007/s00265-004-0830-5) [DOI] [Google Scholar]
- 5.Crofoot MC, Gilby IC. 2012. Cheating monkeys undermine group strength in enemy territory. Proc. Natl Acad. Sci. USA 109, 501-505. ( 10.1073/PNAS.1115937109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gareta GM, de Guinea M, Waal E, Bshary R. 2022. Drivers and outcomes in territory disputes: from ecological characteristics to numerical asymmetry in vervet monkeys. Phil. Trans. R. Soc. B 377, 20210145. ( 10.1098/rstb.2021.0145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown M, Steinitz R, Emery TM. 2022. Wins and losses in intergroup conflicts reflect energy balance in red-tailed monkeys. Phil. Trans. R. Soc. B 377, 20210152. ( 10.1098/rstb.2021.0152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodall J. 1986. The chimpanzees of Gombe: patterns of behavior. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 9.Lemoine S, Samuni L, Crockford C, Wittig R. 2022. Parochial cooperation in wild chimpanzees: a model to explain the evolution of parochial altruism. Phil. Trans. R. Soc. B 377, 20210149. ( 10.1098/rstb.2021.0149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitchen DM, Beehner JC. 2007. Factors affecting individual participation in group-level aggression among non-human primates. Behaviour 144, 1551-1581. ( 10.1163/156853907782512074) [DOI] [Google Scholar]
- 11.Willems EP, Hellriegel B, van Schaik CP. 2013. The collective action problem in primate territory economics. Proc. R. Soc. B 280, 20130081. ( 10.1098/RSPB.2013.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams JM, Oehlert GW, Carlis JV, Pusey AE. 2004. Why do male chimpanzees defend a group range? Anim. Behav. 68, 523-532. ( 10.1016/j.anbehav.2003.09.015) [DOI] [Google Scholar]
- 13.Pusey AE, Oehlert GW, Williams JM, Goodall J. 2005. Influence of ecological and social factors on body mass of wild chimpanzees. Int. J. Primatol. 26, 3-31. ( 10.1007/s10764-005-0721-2) [DOI] [Google Scholar]
- 14.Mosser A, Packer C. 2009. Group territoriality and the benefits of sociality in the African lion, Panthera leo. Anim. Behav. 78, 359-370. ( 10.1016/j.anbehav.2009.04.024) [DOI] [Google Scholar]
- 15.Lemoine S, Preis A, Samuni L, Boesch C, Crockford C, Wittig RM. 2020. Between-group competition impacts reproductive success in wild chimpanzees. Curr. Biol. 30, 312-318. ( 10.1016/j.cub.2019.11.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crouse KN, Desai N, Cassidy KA, Stahler EE, Lehman CJ, Wilson ML. Submitted. Larger territories reduce mortality risk for chimpanzees, wolves, and agents: multiple lines of evidence in a model validation framework. Ecol. Model. [Google Scholar]
- 17.Nunn CL. 2000. Collective benefits, free-riders, and male extra-group conflict. In Primate males: causes and consequences of variation in group composition (ed. Kappeler PM), pp. 192-204. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 18.Olson M. 1965. The logic of collective action. Cambridge, MA: Harvard University Press. [Google Scholar]
- 19.Gavrilets S, Fortunato L. 2014. A solution to the collective action problem in between-group conflict with within-group inequality. Nat. Commun. 5, 1-11. ( 10.1038/ncomms4526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gavrilets S. 2015. Collective action problem in heterogeneous groups. Phil. Trans. R. Soc. B 370, 20150016. ( 10.1098/rstb.2015.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sankey DWE, Hunt K, Croft D, Franks D, Green P, Thompson F, Johnstone R, Cant M. 2022. Leaders of war: modelling the evolution of conflict among heterogeneous groups. Phil. Trans. R. Soc. B 377, 20210140. ( 10.1098/rstb.20210140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watts DP, Mitani JC. 2001. Boundary patrols and intergroup encounters in wild chimpanzees. Behaviour 138, 299-327. ( 10.1163/15685390152032488) [DOI] [Google Scholar]
- 23.Langergraber KE, Watts DP, Vigilant L, Mitani JC. 2017. Group augmentation, collective action, and territorial boundary patrols by male chimpanzees. Proc. Natl Acad. Sci. USA 114, 7337-7342. ( 10.1073/pnas.1701582114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kokko H, Johnstone RA, Clutton-Brock TH. 2001. The evolution of cooperative breeding through group augmentation. Proc. R. Soc. Lond. B 268, 187-196. ( 10.1098/RSPB.2000.1349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridley A, Nelson-Flower M, Wiley E, Humphries D, Kokko H. 2022. Kidnapping intergroup young: an alternative strategy to maintain group size in the group-living pied babbler (Turdoides bicolor). Phil. Trans. R. Soc. B 377, 20210153. ( 10.1098/rstb.20210153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura M, Hosaka K, Itoh N, Zamma K. (eds). 2015. Mahale chimpanzees: 50 years of research. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 27.Stanford C. 2018. The new chimpanzee. Cambridge, MA: Harvard University Press. [Google Scholar]
- 28.Boesch C, Wittig R, Crockford C, Vigilant L, Deschner T, Leendertz F. (eds). 2019. The chimpanzees of the Taï forest: 40 years of research. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 29.Hunt KD. 2020. Chimpanzee: lessons from our sister species. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 30.Nishida T. 1968. The social group of wild chimpanzees in the Mahali mountains. Primates 9, 167-224 ( 10.1007/BF01730971) [DOI] [Google Scholar]
- 31.Itani J, Suzuki A. 1967. The social unit of chimpanzees. Primates 8, 355-381. ( 10.1007/BF01792020) [DOI] [Google Scholar]
- 32.Boesch C, Boesch-Achermann H. 2000. The chimpanzees of the Taï forest: behavioural ecology and evolution. Cambridge, UK: Oxford University Press. [Google Scholar]
- 33.Wilson ML, Wrangham RW. 2003. Intergroup relations in chimpanzees. Annu. Rev. Anthropol. 32, 363-392. ( 10.1146/annurev.anthro.32.061002.120046) [DOI] [Google Scholar]
- 34.Goodall J, Bandora A, Bergmann A, Busse C, Matama H, Mpongo E, Pierce A, Riss D. 1979. Inter-community interactions in the chimpanzee population of the Gombe National Park. In The great apes (eds Hamburg DA, McCown ER), pp. 13-54. Menlo Park, CA: Benjamin/Cummings. [Google Scholar]
- 35.Wilson ML, et al. 2014. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature 513, 414-417. ( 10.1038/nature13727) [DOI] [PubMed] [Google Scholar]
- 36.Wilson ML, Hauser MD, Wrangham RW. 2007. Chimpanzees (Pan troglodytes) modify grouping and vocal behavior in reponse to location-specific risk. Behavior 144, 1621-1653. ( 10.1163/156853907782512137) [DOI] [Google Scholar]
- 37.Wilson ML, Kahlenberg SM, Wells M, Wrangham RW. 2012. Ecological and social factors affect the occurrence and outcomes of intergroup encounters in chimpanzees. Anim. Behav. 83, 277-291. ( 10.1016/j.anbehav.2011.11.004) [DOI] [Google Scholar]
- 38.Boesch C, Crockford C, Herbinger I, Wittig R, Moebius Y, Normand E. 2008. Intergroup conflicts among chimpanzees in Taï National Park: lethal violence and the female perspective. Am. J. Primatol. 70, 519-532. ( 10.1002/AJP.20524) [DOI] [PubMed] [Google Scholar]
- 39.Wilson ML, Hauser MD, Wrangham RW. 2001. Does participation in intergroup conflict depend on numerical assessment, range location, or rank for wild chimpanzees? Anim. Behav. 61, 1203-1216. ( 10.1006/anbe.2000.1706) [DOI] [Google Scholar]
- 40.Herbinger I, Papworth S, Boesch C, Zuberbühler K. 2009. Vocal, gestural and locomotor responses of wild chimpanzees to familiar and unfamiliar intruders: a playback study. Anim. Behav. 78, 1389-1396. ( 10.1016/j.anbehav.2009.09.010) [DOI] [Google Scholar]
- 41.Mitani JC, Watts DP, Amsler SJ. 2010. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr. Biol. 20, 507-508. ( 10.1016/j.cub.2010.04.021) [DOI] [PubMed] [Google Scholar]
- 42.Amsler SJ. 2010. Energetic costs of territorial boundary patrols by wild chimpanzees. Am. J. Primatol. 72, 93-103. ( 10.1002/ajp.20757) [DOI] [PubMed] [Google Scholar]
- 43.Sobolewski ME, Brown JL, Mitani JC. 2012. Territoriality, tolerance and testosterone in wild chimpanzees. Anim. Behav. 84, 1469-1474. ( 10.1016/J.ANBEHAV.2012.09.018) [DOI] [Google Scholar]
- 44.Wittig RM, Crockford C, Weltring A, Langergraber KE, Deschner T, Zuberbühler K. 2016. Social support reduces stress hormone levels in wild chimpanzees across stressful events and everyday affiliations. Nat. Commun. 7, 1-8. ( 10.1038/ncomms13361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grinnell J, Packer C, Pusey AE. 1995. Cooperation in male lions: kinship, reciprocity or mutualism? Anim. Behav. 49, 95-105. ( 10.1016/0003-3472(95)80157-X) [DOI] [Google Scholar]
- 46.Wrangham RW. 1999. Evolution of coalitionary killing. Am. J. Phys. Anthropol. 29, 1-30. () [DOI] [PubMed] [Google Scholar]
- 47.Mitani JC, Watts DP. 2005. Correlates of territorial boundary patrol behaviour in wild chimpanzees. Anim. Behav. 70, 1079-1086. ( 10.1016/j.anbehav.2005.02.012) [DOI] [Google Scholar]
- 48.Williams JM, Pusey AE, Carlis JV, Farm BP, Goodall J. 2002. Female competition and male territorial behaviour influence female chimpanzees' ranging patterns. Anim. Behav. 63, 347-360. ( 10.1006/ANBE.2001.1916) [DOI] [Google Scholar]
- 49.Boesch C. 2009. The real chimpanzee: sex strategies in the forest. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 50.Gilby IC, Wilson ML, Pusey AE. 2013. Ecology rather than psychology explains co-occurrence of predation and border patrols in male chimpanzees. Anim. Behav. 86, 61-74. ( 10.1016/J.ANBEHAV.2013.04.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandel AA, Watts DP, Setchell JM. 2021. Lethal coalitionary aggression associated with a community fission in chimpanzees (Pan troglodytes) at Ngogo, Kibale National Park, Uganda. Int. J. Primatol. 42, 26-48. ( 10.1007/s10764-020-00185-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE. 2009. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim. Behav. 77, 873-885. ( 10.1016/j.anbehav.2008.12.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feldblum JT, Krupenye C, Bray J, Pusey AE, Gilby IC. 2021. Social bonds provide multiple pathways to reproductive success in wild male chimpanzees. iScience 24, 102864. ( 10.1016/j.isci.2021.102864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glowacki L, McDermott R. 2022. Key individuals catalyze intergroup violence. Phil. Trans. R. Soc. B 377, 20210141. ( 10.1098/rstb.2021.0141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foerster S, Franz M, Murray CM, Gilby IC, Feldblum JT, Walker KK, Pusey AE. 2016. Chimpanzee females queue but males compete for social status. Sci. Rep. 6, 35404. ( 10.1038/srep35404) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mullon C, Lehmann L. 2022. Evolution of warfare by resource raiding favours polymorphism in belligerence and bravery. Phil. Trans. R. Soc. B 377, 20210136. ( 10.1098/rstb.2021.0136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiss A, Wilson ML, Collins DA, Mjungu D, Kamenya S, Foerster S, Pusey AE. 2017. Personality in the chimpanzees of Gombe National Park. Sci. Data 4, 1-18. ( 10.1038/sdata.2017.146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King JE, Figueredo AJ. 1997. The five-factor model plus dominance in chimpanzee personality. J. Res. Pers. 31, 257-271. ( 10.1006/JRPE.1997.2179) [DOI] [Google Scholar]
- 59.Weiss A, King JE, Murray L. (eds). 2011. Personality and temperament in nonhuman primates. Berlin, Germany: Springer. [Google Scholar]
- 60.Wilson ML, et al. 2020. Research and conservation in the greater Gombe ecosystem: challenges and opportunities. Biol. Conserv. 252, 108853. ( 10.1016/j.biocon.2020.108853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rudicell RS, et al. 2010. Impact of simian immunodeficiency virus infection on chimpanzee population dynamics. PLoS Pathog. 6, e1001116. ( 10.1371/journal.ppat.1001116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pusey AE, Wilson ML, Collins DA. 2008. Human impacts, disease risk, and population dynamics in the chimpanzees of Gombe National Park, Tanzania. Am. J. Primatol. 70, 738-744. ( 10.1002/ajp.20567) [DOI] [PubMed] [Google Scholar]
- 63.Wilson ML. 2012. Long-term studies of the chimpanzees of Gombe National Park, Tanzania. In Long-term field studies of primates (eds Kappeler PM, Watts DP), pp. 357-384. Heidelberg, Germany: Springer. [Google Scholar]
- 64.Wallis J. 1994. A survey of reproductive parameters in the free-ranging chimpanzees of Gombe National Park. J. Reprod. Fertil. 109, 297-307. ( 10.1530/jrf.0.1090297) [DOI] [PubMed] [Google Scholar]
- 65.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289-300. ( 10.1111/j.2517-6161.1995.tb02031.x) [DOI] [Google Scholar]
- 66.Tutin CEG. 1979. Mating patterns and reproductive strategies in a community of wild chimpanzees (Pan troglodytes schweinfurthii). Behav. Ecol. Sociobiol. 6, 29-38. ( 10.1007/BF00293242) [DOI] [Google Scholar]
- 67.Muller MN, Thompson ME, Wrangham RW. 2006. Male chimpanzees prefer mating with old females. Curr. Biol. 16, 2234-2238. ( 10.1016/j.cub.2006.09.042) [DOI] [PubMed] [Google Scholar]
- 68.Bygott JD. 1979. Agonistic behavior, dominance and social structure in wild chimpanzees of the Gombe National Park. In The great apes (eds Hamburg DA, McCown ER), pp. 405-428. Menlo Park, CA: Benjamin/Cummings Pub. Co. [Google Scholar]
- 69.Newton-Fisher NE. 2017. Modeling social dominance: Elo-ratings, prior history, and the intensity of aggression. Int. J. Primatol. 38, 427-447. ( 10.1007/s10764-017-9952-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feldblum JT, Foerster S, Franz M. 2022. EloOptimized: optimized Elo rating method for obtaining dominance ranks. See https://github.com/jtfeld/EloOptimized.
- 71.R Development Core Team. 2021. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 72.Hartig F. 2022. DHARMa: Residual diagnostics for hierarchical (multi-level / mixed) regression models. R package version 0.4.5. See https://CRAN.R-project.org/package=DHARMa.
- 73.Textor J, Hardt J. 2011. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology 22, 745. ( 10.1097/EDE.0B013E318225C2BE) [DOI] [PubMed] [Google Scholar]
- 74.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 75.Mazerolle MMJ. 2020. Package ‘AICcmodavg’. See https://cran.uib.no/web/packages/AICcmodavg/AICcmodavg.pdf.
- 76.Glymour M, Pearl J, Jewell NP. 2016. Causal inference in statistics: a primer. Hoboken, NJ: John Wiley & Sons, Ltd. [Google Scholar]
- 77.McElreath R. 2018. Statistical rethinking: a Bayesian course with examples in R and Stan. London, UK: Chapman and Hall/CRC. [Google Scholar]
- 78.Burnham KP, Anderson DR, Huyvaert KP. 2011. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 65, 23-35. ( 10.1007/s00265-010-1029-6) [DOI] [Google Scholar]
- 79.Lefcheck JS. 2016. piecewiseSEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573-579. ( 10.1111/2041-210X.12512) [DOI] [Google Scholar]
- 80.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289-300. ( 10.1111/j.2517-6161.1995.tb02031.x) [DOI] [Google Scholar]
- 81.Massaro AP, et al. 2022. Data and code from: Correlates of individual participation in boundary patrols by male chimpanzees. Dryad Digital Repository. ( 10.5061/dryad.z8w9ghxdb) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Summary data and R code used in this analysis are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.z8w9ghxdb [81]. https://datadryad.org/stash/share/31Ea2gi-f0RG_ZKwTtNDr-_W308nFSYNA7bzSCo_MbQ