Abstract
Objectives:
No-reflow is a complication that frequently occurs after stenting during primary percutaneous coronary intervention. In this study, we focused on angiographic results and clinical outcomes after no-reflow in the left anterior descending (LAD) artery versus non–left anterior descending artery ST-elevation myocardial infarction (STEMI).
Methods:
In this prospective study, a total of 201 patients who had developed no-reflow during primary percutaneous coronary intervention were enrolled. The patients were divided into left anterior descending artery culprit and non-left anterior descending artery culprit groups. The primary endpoints were final thrombolysis in myocardial infarction flow, corrected thrombolysis in myocardial infarction frame count and final myocardial blush grade. Secondary endpoints were major adverse cardiovascular events in-hospital and at 1 month.
Results:
Out of the 201 patients, 60.19% had culprit left anterior descending artery. Pulse rate, baseline systolic and diastolic blood pressure, single-vessel disease, left ventricular ejection fraction <30%, baseline thrombolysis in myocardial infarction I flow and final thrombolysis in myocardial infarction II flow (24.8% vs 11.3%, p = .017), and thrombolysis in myocardial infarction frame count (28.17 ± 11.86 vs 24.38 ± 9.05, p = .016) were significantly higher in the left anterior descending artery group. In contrast, baseline Killip Class I, three-vessel disease, baseline thrombolysis in myocardial infarction II flow, final thrombolysis in myocardial infarction III flow (74.4% vs 87.5%, p = .024) and left ventricular ejection fraction >40% were significantly greater in the non–left anterior descending artery group. However, for both in-hospital and at 30 days, overall major adverse cardiovascular event was similar in the two groups. The demographics, clinical and medication profiles and the routes used to treat no-reflow were all comparable in both groups.
Conclusions:
No-reflow in left anterior descending artery ST-elevation myocardial infarction is associated with lower final thrombolysis in myocardial infarction III flow, higher thrombolysis in myocardial infarction frame count and relatively lower Grade III myocardial blush than non-left anterior descending artery ST-elevation myocardial infarction with subsequent lower left ventricular ejection fraction and a higher frequency of in-hospital heart failure and hospitalisation due to heart failure.
Keywords: No-reflow, primary PCI, stenting, LAD-culprit STEMI
Introduction
No-reflow is defined as thrombolysis in myocardial infarction (TIMI) flow grade of <3 with patent coronary artery with an absence of dissection or spasm. 1 It is a serious complication and accounts for 11%−41% of cases of ST-elevation myocardial infarction (STEMI) during primary percutaneous coronary intervention (PCI). 2 Numerous clinical and angiographic factors have been shown to be associated with no-reflow, including advanced age, a reperfusion time > 6 h, Killip Class ⩾ 3, long lesion length, high thrombus burden (grade ⩾ 3), a high admission glucose to estimated average glucose ratio and PRECISE-DAPT score.3–6 Moreover, there is evidence of a correlation between no-reflow and reduced left ventricular function, worse clinical outcome and higher mortality. 7
Of particular importance, Nair et al. have reported a significant association of anterior wall myocardial infarction (AWMI) with the development of no-reflow (71.4% vs 43.2%, p ⩽ .001). 8 Other studies have shown that left anterior descending artery (LAD)-related STEMI leads to significantly lower post-myocardial infarction (MI) left ventricular ejection fraction (LVEF) compared with non-LAD-related MI. 9 Several studies have been performed to address the predictors and use of medication for treatment. In the main, adenosine, verapamil, nitroprusside or nicardipine have resulted in improved coronary flow and better prognoses.10–12 However, literature related to immediate treatment outcomes in terms of TIMI flow, final corrected TIMI frame count (cTFC) and myocardial blush grade (MBG), and lately overall clinical outcomes comparing LAD versus non-LAD STEMI is sparse. Therefore, we aimed to conduct this study of our population where the proportion of young patients with STEMI is on the rise, and the majority of cases were found to have LAD territory STEMI, as has been reported recently. 13 In this study, we focused on a comparison of angiographic results and clinical outcomes after no-reflow in the LAD versus non-LAD STEMI irrespective of the age.
Method
This was a prospective study conducted in a tertiary care cardiac hospital in an urban area in Pakistan from December 2020 to May 2021. Out of all STEMI patients (n = 1862) presented to the National Institute of Cardiovascular Diseases (NICVD), 201 were enrolled in the study, having fulfilled the inclusion criteria. The inclusion criteria for the study were patients aged ⩾18 years, who had been diagnosed with STEMI, were undergoing primary PCI and had developed TIMI flow grade ⩽2 post stenting. Patients with cardiogenic shock at the time of presentation, valvular or congenital heart disease, cardiomyopathy, myocarditis, pericarditis, contraindication to the use of antiplatelet or anticoagulation and those who did not give their consent to participate in the study were excluded.
The recruited patients were divided into two groups: LAD-culprit STEMI and non-LAD culprit STEMI. Data pertaining to sex, age, body mass index (BMI), hypertension, diabetes mellitus, smoking, prior coronary artery diseases (CADs), a family history of premature CAD, dyslipidaemia, haemodynamics and Killip Class on presentation were recorded after written and informed consent had been given in the emergency room. LVEF was assessed via echocardiogram on discharge and during the 30-day follow-up. Angiographic parameters, including vessel size (diameter), heparin dose, use of glycoprotein (GP) IIb/IIIa inhibitor (as indicated by American College of Cardiology and American Heart Association (ACC-AHA) guidelines), intracoronary use of medication, like nitrate, adenosine or adrenaline, and drug delivery route, such as proximal via guide catheter or distal via a device, were documented and compared.
Improvement in TIMI flow grade, reduction in cTFC (the number of cine-frames needed for contrast to get to the distal coronary landmark, corrected for the vessel length) and final cTFC, and improvement in MBG after the intracoronary administration of medicine were assessed by at least two experienced interventional cardiologists blinded to the groups and then compared as the primary endpoint. Major adverse cardiovascular events, including all-cause mortality, re-infarction, repeat revascularisation and cerebrovascular accidents (CVAs) in-hospital and at 1 month, were compared as a secondary endpoint.
Statistical analyses
The sample size for the study was the number of consecutive patients that fulfilled the inclusion criteria out of the total STEMI patients presented during the study period. A power analysis showed the power of 0.657 for the detection of difference in the proportion of final TIMI III flow between the LAD-culprit STEMI and non-LAD culprit STEMI groups, at .05 level of significance. IBM SPSS Version 21 was used for the analysis of the data. Descriptive statistics such as mean ± SD or median (interquartile range (IQR)) and frequency and percentages were calculated for quantitative and qualitative variables, respectively. The outcomes were compared between the two groups with the help of an appropriate chi-square test and independent sample t test or Mann–Whitney U test. A two-sided p value of ⩽.05 was taken as the criteria for significance.
Results
Out of the 201 consecutively enrolled patients with no-reflow after stenting, the proportion of patients in the LAD-culprit STEMI group was 60.19% (121) and the non-LAD culprit STEMI group was 39.8% (80) of which 82.5% (66/80) had culprit right coronary artery (RCA) and the remaining 17.5% (14/80) had culprit left circumflex artery. Among the patients with culprit LAD, 73.6% (89/121) had disease in the proximal segment, 34.8% (21/121) in the mid-distal segment, and one patient (3.2%) had disease in the diagonal. Baseline demographic and clinical profiles, including sex, mean age, hypertension, diabetes mellitus, smoking, dyslipidaemia, family history of premature CAD and history of prior CAD, were comparable in the two groups, as shown in Table 1. Moreover, procedural medication profiles, including dose of heparin, use of GP IIb/IIIa inhibitor (as per ACC-AHA indications), doses of intracoronary medications used to manage no-reflow, such as nitrate, adenosine and adrenaline and the proportion of patients that received intracoronary medication proximally via guide catheter and distally via a device were also comparable in both groups, as shown in Table 2.
Table 1.
Baseline demographic and clinical characteristics.
| Characteristics | Total | Group | p | |
|---|---|---|---|---|
| LAD | Non-LAD | |||
| Total (N) | 201 | 121 | 80 | – |
| Gender | ||||
| Male | 72.6% (146) | 69.4% (84) | 77.5% (62) | .209 |
| Female | 27.4% (55) | 30.6% (37) | 22.5% (18) | |
| Age (years) | 57.15 ± 11.39 | 57.85 ± 11.46 | 56.1 ± 11.27 | .287 |
| Body mass index (BMI) | 27.3 ± 4.05 | 27.14 ± 3.72 | 27.56 ± 4.52 | .475 |
| Admission blood sugar (mg/dL) | 161 (130–207) | 164 (129–204) | 160 (133.5–217) | .247 |
| Admission serum creatinine (mg/dL) | 1 (0.8–1.2) | 1 (0.8–1.2) | 0.9 (0.8–1.1) | .518 |
| Total ischemic time (minutes) | 440 (320–710) | 440 (320–720) | 420 (320–645) | .053 |
| Co-morbid conditions | ||||
| Hypertension | 71.6% (144) | 68.6% (83) | 76.3% (61) | .239 |
| Diabetes mellitus | 45.3% (91) | 46.3% (56) | 43.8% (35) | .724 |
| Smoking | 32.8% (66) | 29.8% (36) | 37.5% (30) | .252 |
| Family history of IHD | 2.5% (5) | 0.8% (1) | 5% (4) | .063 |
| Prior CAD | 8.5% (17) | 9.1% (11) | 7.5% (6) | .692 |
| Dyslipidaemia | 10% (20) | 12.4% (15) | 6.3% (5) | .154 |
LAD: left anterior descending artery; IHD: ischemic heart disease; CAD: coronary artery disease.
Table 2.
Procedural medication profile.
| Characteristics | Total | Group | p | |
|---|---|---|---|---|
| LAD | Non-LAD | |||
| Total (N) | 201 | 121 | 80 | – |
| Heparin dose (IU) | 8039.8 ± 1398.54 | 7925.62 ± 1397.41 | 8212.5 ± 1391.15 | .155 |
| Use of GP IIb/IIIa inhibitor | 21.9% (44) | 24% (29) | 18.8% (15) | .381 |
| Use of nitrates | 81.6% (164) | 84.3% (102) | 77.5% (62) | .224 |
| Dose (mcg) | 351.83 ± 267.57 | 374.51 ± 273.84 | 314.52 ± 254.69 | .120 |
| Use of adrenaline | 50.2% (101) | 47.9% (58) | 53.8% (43) | .42 |
| Dose (mcg) | 240.1 ± 131.53 | 243.97 ± 133.14 | 234.88 ± 130.72 | .634 |
| Use of adenosine | 49.8% (100) | 52.1% (63) | 46.3% (37) | .42 |
| Dose (mcg) | 257.5 ± 132.55 | 254.44 ± 133.64 | 262.7 ± 132.34 | .667 |
| Administration | ||||
| Distal via device | 11.9% (24) | 12.4% (15) | 11.3% (9) | .806 |
| Proximal via guide | 88.1% (177) | 87.6% (106) | 88.8% (71) | |
LAD: left anterior descending artery; GP: glycoprotein.
Considering the hemodynamic profiles of the LAD-culprit and non-LAD culprit STEMI groups, pulse rate was 90.24 ± 12.68 versus 72.3 ± 17.73, p ⩽ .001, baseline systolic blood pressure was 133.01 ± 19.5 versus 127.2 ± 15.61, p = .027, diastolic blood pressure was 83.26 ± 12.51 versus 78.83 ± 11.15, p = .011 and baseline Killip Class I was 69.4% versus 82.5%, p = .037, respectively. Angiographic profiling showed single-vessel disease as 44.6% versus 21.3%, p ⩽ .001, three-vessel disease at 28.1% versus 42.5%, p = .035 and the culprit vessel diameter at 3.41 mm ± 0.3 mm versus 3.44 mm ± 0.37 mm, p = .536, respectively, in the LAD and non-LAD groups, as shown in Table 3.
Table 3.
Haemodynamics and angiographic profile.
| Characteristics | Total | Group | p | |
|---|---|---|---|---|
| LAD | Non-LAD | |||
| Total (N) | 201 | 121 | 80 | – |
| Killip Class | ||||
| I | 74.6% (150) | 69.4% (84) | 82.5% (66) | .037* |
| II | 14.9% (30) | 18.2% (22) | 10% (8) | .111 |
| III | 10.4% (21) | 12.4% (15) | 7.5% (6) | .267 |
| Pulse rate (bpm) | 83.1 ± 17.27 | 90.24 ± 12.68 | 72.3 ± 17.73 | <.001* |
| Systolic blood pressure (mmHg) | 130.7 ± 18.24 | 133.01 ± 19.5 | 127.2 ± 15.61 | .027* |
| Diastolic blood pressure (mmHg) | 81.49 ± 12.15 | 83.26 ± 12.51 | 78.83 ± 11.15 | .011* |
| Number of diseased vessels | ||||
| Single-vessel disease | 35.3% (71) | 44.6% (54) | 21.3% (17) | <.001* |
| Two-vessel disease | 30.8% (62) | 27.3% (33) | 36.3% (29) | .177 |
| Three-vessel disease | 33.8% (68) | 28.1% (34) | 42.5% (34) | .035* |
| Pre-procedure thrombus grade | ||||
| Low thrombus grade (1 to 3) | 61.2% (123) | 56.2% (68) | 68.8% (55) | .074 |
| High thrombus grade (4 and 5) | 38.8% (78) | 43.8% (53) | 31.3% (25) | |
| Vessel size | 3.42 ± 0.33 | 3.41 ± 0.3 | 3.44 ± 0.37 | .536 |
LAD: left anterior descending artery.
Significant at 5%.
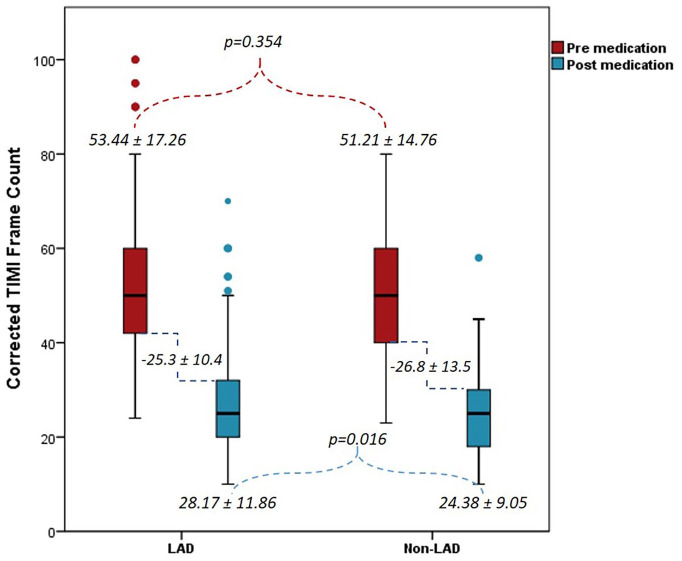
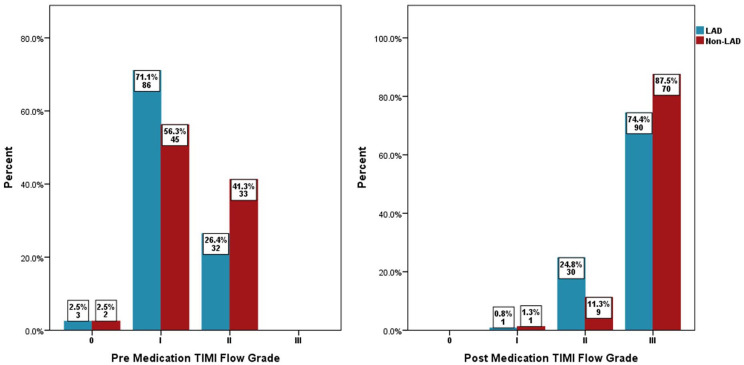
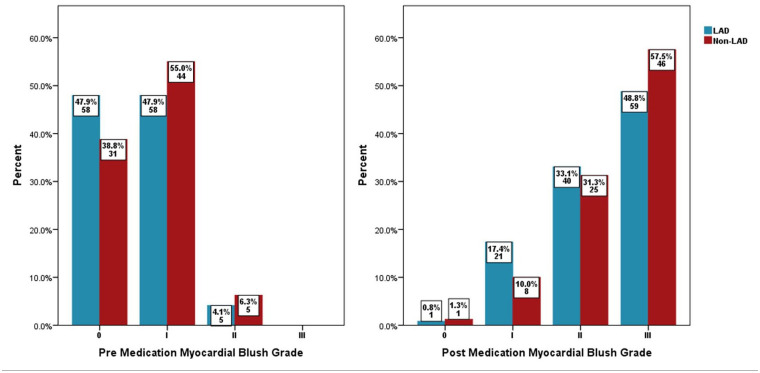
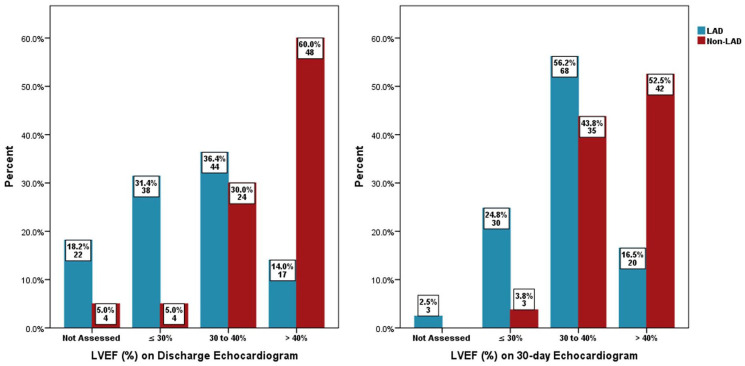
Baseline TIMI I flow in the two groups was 71.1% versus 56.3%, p = .028, the TIMI II flow was 26.4% versus 41.3%, p = .028, and the pre-PCI high thrombus grade (⩾4) was 43.8% versus 31.3%, p = .074, respectively, in the LAD versus non-LAD arm. Post-medication treatment of no-reflow in the LAD and non-LAD groups revealed a final TIMI II flow of 24.8% versus 11.3%, p = .017, a final TIMI III flow of 74.4% versus 87.5%, p = .024, a final cTFC of 28.17 ± 11.86 versus 24.38 ± 9.05, p = .016, an overall reduction in cTFC in each group at −25.27 ± 10.44 versus −26.84 ± 13.45, p = .356 and a final MBG III of 48.8% versus 57.5%, p = .225, respectively, as shown in Figures 1–3. Echocardiography showed LVEF of ⩽30% at 31.4% versus 5%, p ⩽ .001 and 24.8% versus 3.8%, p ⩽ .001 while LVEF of >40% was 14% versus 60%, p ⩽ .001 and 14% versus 60%, p ⩽ .001, at hospital discharge and at the 30-day point, respectively, in the LAD and non-LAD groups, as shown in Figure 4.
Figure 1.
Pre- and post-medication corrected thrombolysis in myocardial infarction (TIMI) frame count.
LAD: left anterior descending artery; TIMI: thrombolysis in myocardial infarction.
Figure 2.
Pre- and post-medication thrombolysis in myocardial infarction (TIMI) flow grade.
LAD: left anterior descending artery; TIMI: thrombolysis in myocardial infarction.
Figure 3.
Pre- and post-medication myocardial blush grade.
LAD: left anterior descending artery.
Figure 4.
At discharge and 30-day (echocardiogram) left ventricular ejection fraction (%).
LAD: left anterior descending artery; LVEF: left ventricular ejection fraction.
Major adverse cardiovascular event (MACE) in-hospital and at 30 days revealed all-cause mortality at 3.3% versus 1.3%, p = .36 and 4.5% versus 2.5%, p = .484, re-infarction was 0.8% versus 1.3% and 1.8% versus 3.8%, p = .391, repeat revascularisation was 2.5% versus 1.3%, p = .541 and 2.7% versus 2.5%, p = .95, CVA was 1.7% versus 1.3%, p = .818 and 0.9% versus 0%, p = .4, and heart failure stood at 25.6% versus 10%, p = .006 and 3.6% versus 1.3%, p = .326, respectively, in the LAD and non-LAD culprit STEMI groups, as shown in Table 4.
Table 4.
In-hospital and 30-day follow-up outcomes.
| Characteristics | Total | Group | p | |
|---|---|---|---|---|
| LAD | Non-LAD | |||
| Total (N) | 201 | 121 | 80 | – |
| In-hospital outcomes | ||||
| Mortality | 2.5% (5) | 3.3% (4) | 1.3% (1) | .36 |
| Arrhythmia | 5% (10) | 5% (6) | 5% (4) | .989 |
| Re-infarction | 1% (2) | 0.8% (1) | 1.3% (1) | .767 |
| Repeat revascularisation | 2% (4) | 2.5% (3) | 1.3% (1) | .541 |
| Cerebrovascular accident | 1.5% (3) | 1.7% (2) | 1.3% (1) | .818 |
| Heart failure | 19.4% (39) | 25.6% (31) | 10% (8) | .006* |
| Follow-up | 95% (191) | 92.6% (112) | 98.8% (79) | .048* |
| Duration of follow-up | 29.77 ± 1.5 | 29.68 ± 1.53 | 29.91 ± 1.44 | .291 |
| Mortality | 3.7% (7) | 4.5% (5) | 2.5% (2) | .484 |
| Re-infarction | 2.6% (5) | 1.8% (2) | 3.8% (3) | .391 |
| Repeat revascularisation | 2.6% (5) | 2.7% (3) | 2.5% (2) | .95 |
| Cerebrovascular accident | 0.5% (1) | 0.9% (1) | 0% (0) | .4 |
| Hospitalisation due to heart failure | 2.6% (5) | 3.6% (4) | 1.3% (1) | .326 |
| Cumulative mortality | 6% (12) | 7.4% (9) | 3.8% (3) | .235 |
LAD: left anterior descending artery.
Significant at 5%.
Discussion
No-reflow has always been a critical problem during primary PCI for all STEMI, but earlier studies, by Ito et al. 14 in 1996 and Pantea-Roșan et al. 15 in 2020, have reported that cases of LAD-culprit MI are more prone to having this complication and consequently experiencing more severe myocardial damage with an increased risk of future cardiovascular events and death.7,16 The present study also supported this fact, as 60.19% (121) of patients who had developed no-reflow were found to have LAD-culprit STEMI, in contrast to 39.8% (80) with non-LAD culprit STEMI. In a recent study, Refaat et al. also reported no-reflow in 53.6% of anterior STEMI, in contrast to 43.6% of non-anterior STEMI. 17 In the present study, no significant differences were observed in the patients’ demographics and clinical profiles, as depicted in Table 1, between LAD-culprit and non-LAD culprit STEMI. However, the frequency of patients with baseline Killip Class I was significantly lower in LAD-culprit STEMI compared to the non-LAD group, with a concomitant higher baseline heart rate and both systolic and diastolic blood pressure manifesting a slightly less stable presentation to start with in this group. A study by Vicent et al. reported an odd ratio of 2.4 (1.2–2.7) for anterior STEMI and 1.8 (1.2–2.7) for TIMI flow grade of <3 in patients with higher Killip Class ⩾ 2. 18 Considering the angiographic profile, there was no significant difference in the vessel diameter of the culprit vessel in the study groups; however, most of the LAD-culprit STEMI patients were found to have single-vessel involvement, in contrast to the non-LAD culprit patients. Niccoli at al. also reported similar findings in their study where 62% of patients with no-reflow exhibited single-vessel disease and LAD was culprit in 48% of them. 19
Regarding the severity of no-reflow, the majority of patients in the LAD-culprit STEMI group had TIMI I flow in contrast to the no-reflow severity in the non-LAD culprit group, whose proportion of baseline TIMI II flow was more than that of the LAD group, suggesting the more extensive nature of myocardial involvement with LAD-culprit STEMI. This was also shown by Elakabawi et al.’s study where, despite medical treatment, 22.2% (out of 1104) anterior STEMI patients continued to have suboptimal flow (TIMI ⩽ 2).16,20 This finding supports the earlier observation that anterior STEMI is an independent predictor of procedural no-reflow. 21
As shown in Table 2, patients in both groups were given similar medical treatment pre and post no-reflow with no significant differences in doses of heparin, utilisation of GP IIb/IIIa inhibitor and intracoronary drugs. Nonetheless, in view of primary outcomes, there was a significantly lower frequency of final TIMI III flow with a concomitant higher frequency of final TIMI II flow and higher final cTFC post intracoronary medication in the LAD group, compared with the non-LAD group. However, there was no significant difference in individual reduction of frame count in each group and the only downwards trend was observed in the achievement of final Grade III myocardial blush in the LAD-culprit STEMI group, as shown in Figures 1–3. These findings pointed towards a more refractory nature of no-reflow in LAD-related STEMI, as shown in earlier studies by Lee et al. where 67% of patients with refractory no-reflow were from anterior STEMI. Iwakura et al. also reported a significant association between no-reflow and severe myocardial damage and worse outcome in LAD-culprit STEMI.20,22 Furthermore, our evaluation of LVEF via echocardiography, both on discharge and at 30 days, revealed the proportion of LVEF ⩽30% as significantly higher in LAD-culprit STEMI after no-reflow, in contrast to the non-LAD culprit group, suggesting the more extensive nature of anterior STEMI and associated no-reflow. Sjögren et al. have also reported significantly impaired longitudinal strain in LAD infarction and, subsequently, more heart failure symptoms in their study. 23 This further highlights the more devastating nature of LAD-culprit STEMI and no-reflow, as compared to the non-LAD group.
Finally, there was a significantly greater frequency of heart failure in LAD-culprit STEMI after no-reflow than non-LAD culprit patients, and this finding was consistent with several earlier studies.24–26 However, there was no significant difference found in overall MACE in-hospital and at the 30-day point, except for an increasing trend in mortality in the LAD-culprit group.
In summary, despite optimal treatment of no-reflow with widely used medications in both groups, the final angiographic results, in terms of TIMI III flow, remained less impressive in the LAD group. This is likely due to the extensive nature of myocardial involvement as a result of large territorial distribution, and conversely it was reflected in less reduction of cTFC and lower levels of improvement in LVEF at 30 days in this group. Nonetheless, overall MACE, both in-hospital and at 30 days, failed to reach statistical significance between the two groups, except a significantly higher frequency of in-hospital heart failure in the LAD group.
Our study has certain limitations, such as the exclusion of haemodynamically unstable patients, a small sample size (low power of test) and single-centred recruitment, which can become a source of selection bias. Second, due to the low-event rate of suboptimal final TIMI III flow, the sample size was not sufficient to conduct multivariable analysis. Hence, large-scale multicentre studies are needed to further validate the findings of our study.
Conclusion
No-reflow in LAD-culprit STEMI is associated with lower final TIMI III flow, higher final cTFC and relatively lower Grade III myocardial blush than non-LAD STEMI. Subsequently, there is lower LVEF and a higher frequency of in-hospital heart failure and hospitalisation due to heart failure in this group. There is an increasing trend in mortality with no-reflow in LAD-culprit group but overall, in-hospital and at 3 months MACE is similar to that of non-LAD culprit group.
Acknowledgments
The authors wish to acknowledge the support of the staff members of the Clinical Research Department of the National Institute of Cardiovascular Diseases (NICVD), Karachi, Pakistan.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from institutional ethical review board with approval number 76/2020.
Informed consent: Written informed consent was obtained from all subjects before the study.
ORCID iD: Kamran Ahmed Khan  https://orcid.org/0000-0003-2665-1410
https://orcid.org/0000-0003-2665-1410
References
- 1. Mazhar J, Mashicharan M, Farshid A. Predictors and outcome of no-reflow post primary percutaneous coronary intervention for ST elevation myocardial infarction. Int J Cardiol Heart Vasc 2016; 10: 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harrison RW, Aggarwal A, Ou FS, et al. Incidence and outcomes of no-reflow phenomenon during percutaneous coronary intervention among patients with acute myocardial infarction. Am J Cardiol 2013; 111(2): 178–184. [DOI] [PubMed] [Google Scholar]
- 3. Schwartz BG, Kloner RA. Coronary no reflow. J Mol Cell Cardiol 2012; 52(4): 873–882. [DOI] [PubMed] [Google Scholar]
- 4. Aggarwal P, Rekwal L, Sinha SK, et al. Predictors of no-reflow phenomenon following percutaneous coronary intervention for ST-segment elevation myocardial infarction. Ann Cardiol Angeiol 2021; 70(3): 136–142. [DOI] [PubMed] [Google Scholar]
- 5. Şimşek B, Çınar T, Ozan V, et al. The association of acute-to-chronic glycemic ratio with no-reflow in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Kardiol Pol 2021; 79(2): 170–178. [DOI] [PubMed] [Google Scholar]
- 6. Selçuk M, Çınar T, Şaylık F, et al. The association of a PRECISE-DAPT score with no-reflow in patients with ST-segment elevation myocardial infarction. Angiology 2022; 73(1): 68–72. [DOI] [PubMed] [Google Scholar]
- 7. Ndrepepa G, Tiroch K, Fusaro M, et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol 2010; 55(21): 2383–2389. [DOI] [PubMed] [Google Scholar]
- 8. Nair Rajesh G, Jayaprasad N, Madhavan S, et al. Predictors and prognosis of no-reflow during primary percutaneous coronary intervention. Proc (Bayl Univ Med Cent) 2019; 32(1): 30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen ZW, Yu ZQ, Yang HB, et al. Rapid predictors for the occurrence of reduced left ventricular ejection fraction between LAD and non-LAD related ST-elevation myocardial infarction. BMC Cardiovasc Disord 2016; 16(1): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Micari A, Belcik TA, Balcells EA, et al. Improvement in microvascular reflow and reduction of infarct size with adenosine in patients undergoing primary coronary stenting. Am J Cardiol 2005; 96(10): 1410–1415. [DOI] [PubMed] [Google Scholar]
- 11. Marzilli M, Orsini E, Marraccini P, et al. Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction. Circulation 2000; 101(18): 2154–2159. [DOI] [PubMed] [Google Scholar]
- 12. Claeys MJ, Bosmans J, De Ceuninck M, et al. Effect of intracoronary adenosine infusion during coronary 6 intervention on myocardial reperfusion injury in patients with acute myocardial infarction. Am J Cardiol 2004; 94(1): 9–13. [DOI] [PubMed] [Google Scholar]
- 13. Khan KA, Khan MN, Kumar R, et al. A surge in prevalence and factors affecting early onset acute coronary syndrome. Signa Vitae 2022; 18: 63–70. [Google Scholar]
- 14. Ito H, Maruyama A, Iwakura K, et al. Clinical implications of the ‘no reflow’ phenomenon: a predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation 1996; 93(2): 223–228. [DOI] [PubMed] [Google Scholar]
- 15. Pantea-Roșan LR, Pantea VA, Bungau S, et al. No-reflow after PPCI – a predictor of short-term outcomes in STEMI patients. J Clin Med 2020; 9(9): 2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iwakura K, Ito H, Kawano S, et al. Predictive factors for development of the no-reflow phenomenon in patients with reperfused anterior wall acute myocardial infarction. J Am Coll Cardiol 2001; 38(2): 472–477. [DOI] [PubMed] [Google Scholar]
- 17. Refaat H, Tantawy A, Gamal AS, et al. Novel predictors and adverse long-term outcomes of No-reflow phenomenon in patients with acute ST elevation myocardial infarction undergoing primary percutaneous coronary intervention. Indian Heart J 2021; 73(1): 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vicent L, Velásquez-Rodríguez J, Valero-Masa MJ, et al. Predictors of high Killip class after ST segment elevation myocardial infarction in the era of primary reperfusion. Int J Cardiol 2017; 248: 46–50. [DOI] [PubMed] [Google Scholar]
- 19. Niccoli G, Lanza GA, Spaziani C, et al. Baseline systemic inflammatory status and no-reflow phenomenon after percutaneous coronary angioplasty for acute myocardial infarction. Int J Cardiol 2007; 117(3): 306–311. [DOI] [PubMed] [Google Scholar]
- 20. Elakabawi K, Huang X, Shah SA, et al. Predictors of suboptimal coronary blood flow after primary angioplasty and its implications on short-term outcomes in patients with acute anterior STEMI. BMC Cardiovasc Disord 2020; 20(1): 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayıroğlu Mİ, Uzun AO, Keskin M, et al. Which admission electrocardiographic parameter is more powerful predictor of no-reflow in patients with acute anterior myocardial infarction who underwent primary percutaneous intervention? J Electrocardiol 2018; 51(2): 203–209. [DOI] [PubMed] [Google Scholar]
- 22. Lee CH, Wong HB, Tan HC, et al. Impact of reversibility of no reflow phenomenon on 30-day mortality following percutaneous revascularization for acute myocardial infarction – insights from a 1,328 patient registry. J Interv Cardiol 2005; 18(4): 261–266. [DOI] [PubMed] [Google Scholar]
- 23. Sjögren H, Pahlm U, Engblom H, et al. Anterior STEMI associated with decreased strain in remote cardiac myocardium. Int J Cardiovasc Imaging 2022; 38(2): 375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lamblin N, Fertin M, De Groote P, et al. Cardiac remodeling and heart failure after a first anterior myocardial infarction in patients with diabetes mellitus. J Cardiovasc Med 2012; 13(6): 353–359. [DOI] [PubMed] [Google Scholar]
- 25. Kapur NK, Alkhouli MA, DeMartini TJ, et al. Unloading the left ventricle before reperfusion in patients with anterior ST-segment–elevation myocardial infarction: a pilot study using the Impella CP. Circulation 2019; 139(3): 337–346. [DOI] [PubMed] [Google Scholar]
- 26. Gho JM, Postema PG, Conijn M, et al. Heart failure following STEMI: a contemporary cohort study of incidence and prognostic factors. Open Heart 2017; 4(2): e000551. [DOI] [PMC free article] [PubMed] [Google Scholar]