Abstract
Introduction
The reduction of the risk of asthma attacks is a major goal of guidelines. The fact that type-2 inflammatory biomarkers identify a higher risk, anti-inflammatory responsive phenotype is potentially relevant to this goal. We aim to quantify the relation between blood eosinophils, exhaled nitric oxide (FeNO) and the risk of severe asthma attacks.
Methods and analysis
A systematic review of randomised controlled trials (RCTs) will be conducted by searching MEDLINE from January 1993 to April 2021. We will include RCTs that investigated the effect of fixed treatment(s) regimen(s) on severe asthma exacerbation rates over at least 24 weeks and reported a baseline value for blood eosinophils and FeNO. Study selection will follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, and the methodological appraisal of the studies will be assessed by the Cochrane Risk-of-Bias Tool for RCTs. Study authors will be contacted to request anonymised individual participant data (IPD) for patients randomised to the trial’s control arm. An IPD meta-analysis will be performed for multivariable prognostic modelling with performance assessment (calibration plots and the c-statistic) in a cross-validation by study procedure. The outcome to predict is the absolute number of severe asthma attacks to occur in the following 12 months if anti-inflammatory therapy is not changed (ie, annualised number of attacks requiring ≥3 days of systemic corticosteroids and/or hospitalisation if the patient was randomised to the control arm of an RCT). A summary prognostic equation and risk stratification chart will be reported as a basis for further analyses of individualised treatment benefit.
Ethics and dissemination
The protocol has been reviewed by the relevant Oxford academic ethics committee and found to comprise fully anonymised data not requiring further ethical approbation. Results will be communicated in an international meeting and submitted to a peer-reviewed journal.
PROSPERO registration number
CRD42021245337.
Keywords: asthma, immunology, epidemiology, thoracic medicine, preventive medicine
Strengths and limitations of this study.
The prognostic (ie, predicting adverse outcomes) and theragnostic (ie, predicting treatment responsiveness) values of type-2 inflammatory biomarkers are established; we thus speculate that a clinical prediction model centred on blood eosinophils and exhaled nitric oxide will provide a useful framework for a preventive, treatable trait-based management.
This systematic review and individual patient data (IPD) level meta-analysis of randomised controlled trials (RCTs) across the spectrum of asthma severities will support clinical decision-making based on type-2 inflammatory biomarkers and other clinical prognostic factors.
We aim to include data from a substantial number of RCTs (N>10) for a large number of patients in total (n>5000), which allows for reliable statistical modelling (internal validity) and assessment of transportability across settings (external validity).
The participating studies’ authors and sponsors will form an international, collaborative and not-for-profit consortium to allow efficient use of high-quality IPD.
Potential weaknesses are the low number of events reported in RCTs enrolling mild asthmatics and the absence of active arm IPD.
Introduction
Reduction of the risk of severe asthma attacks is a major goal of asthma management.1 The current recommendation is to perform risk assessment based on a history of a asthma attack and a list of clinical risk factors (table 1).1 However, many of these prognostic factors are unmodifiable or difficult to modify and a key risk factor (treatment adherence) is difficult to identify and quantify before starting treatment. In contrast, some risk factors are modifiable, such as symptoms and lung function, while they are not necessarily on the causal pathway of asthma attacks. As a result of these deficiencies, risk quantification in asthma is an inexact art and the impact of treatment is difficult to predict.2–13
Table 1.
Clinical risk factors for asthma exacerbations with their traditional categorisations
| Risk factors | Value (if pertinent) |
| Poor control of asthma symptoms | Mean ACQ score ≥1.5 |
| Limited lung function | |
| Low FEV1 | <60%–80% predicted |
| High postbronchodilator reversibility | >12% change in FEV1 |
| Adherence poor (inadequate technique or inhaler use) | |
| Reliever use excessive | >One 200-dose canister/month |
| Intubation or ICU admission for asthma on history | |
| Comorbidities | |
| Chronic rhinosinusitis | |
| Obesity | Body mass index ≥35 kg/m² |
| Psychiatric disease | Psychosis, substance abuse |
| Environmental exposure | |
| Smoking | |
| Allergen exposure in sensitised patient | |
| Air pollution | Especially high O3 and/or NO3 |
PoLAR ICE: mnemonic (see bold characters in table). Adapted from Global Initiative for Asthma Guidelines.1 Where possible, risk factors will also be analysed in continuous versions with restricted cubic splines to allow for non-linear associations.
ACQ, Asthma Control Questionnaire; FEV1, forced expiratory volume in 1 s; ICU, intensive care unit.
One approach to targeted risk reduction is to use a scale centred on readily available prognostic factors that quantify the risk of the adverse outcome of interest in a manner which also predicts the benefits of preventative treatment. This approach has been successful in cardiovascular disease risk reduction where charts14 15 focus on modifiable factors such as blood pressure and cholesterol with age and gender as key prognostic demographic factors. We speculate that a similar framework can be applied to predict asthma attacks in patients with asthma.
Type-2 airway inflammation is important in the pathogenesis of many asthma attacks16 where this immune response characterised by interleukin (IL)-4, IL-5, IL-13 and eosinophilic airway infiltration forms a distinct clinical phenotype.16 In clinic, the actions of type-2 immunity are readily identified by two independent, complementary and accessible biomarkers: the peripheral blood eosinophil count and fractional exhaled nitric oxide (FeNO).17–24 Importantly, the excess risk conferred by raised type-2 biomarkers can be removed with appropriate treatment,24 be it low-dose inhaled corticosteroids (ICSs) in mild asthma,19 25 a higher dose of ICS in moderate asthma21 26 or biological agents targeting type-2 cytokines in moderate and severe asthma.18 27–29 In effect, blood eosinophils and FeNO have emerged as ‘treatable traits’.30
We have previously established a proof-of-concept biomarker-stratified asthma attack scale using publication-level data which is promising and potentially useful to support clinical decision-making.23 24 The prototype lacked detailed and statistically robust assessment of multivariable prognostic relations and systematic assessment of external validity, which is possible with an individual participant data (IPD) meta-analysis (MA).
Review question
In people ≥12 years old diagnosed with asthma of any severity randomised to the control arm of a clinical trial, what is the annualised rate of severe asthma attacks (defined as acute asthma requiring ≥3 days of systemic corticosteroids and/or hospitalisation)31 to occur in relation to their peripheral blood eosinophil count, FeNO and other prognostic factors at baseline?
Objectives
Specific aims of this systematic review are
To systematically identify randomised controlled trials (RCTs) in people ≥12 years old diagnosed with asthma of any severity which measured (i) the peripheral blood eosinophil count and FeNO at baseline and (ii) assessed the incident severe asthma attacks over ≥24 weeks of follow-up.
To perform an IPD MA for the participants randomised to the control arms (defined as no ICS, lowest dose ICS or placebo) of the RCTs identified in aim 1.
To assess the multivariable prognostic relations of the peripheral blood eosinophil count, FeNO and other risk factors assessed at baseline.
To develop and validate a clinical prediction model for the absolute number of severe asthma attacks to occur in the following 12 months in relation to the peripheral blood eosinophil count, FeNO and other risk factors at baseline.
Methods and analysis
Eligibility
Types of studies
In keeping with the objectives of the systematic review, we will include RCTs completed between 1 January 1993 and 1 April 2021 that investigated the effect of fixed treatment(s) regimen(s) on severe asthma attack rates over at least 6 months, also reporting a baseline value for blood eosinophils and FeNO.
Types of participants
We will include studies on participants ages 12 and over diagnosed with asthma of any severity according to objective criteria. We will exclude patients if both the baseline blood eosinophil count and FeNO are missing. We will also exclude patients with missing follow-up duration while on the allocated therapy, or missing number of severe asthma attacks during follow-up.
Types of interventions
We will request IPD for the control arm(s) of each trial. We define the ‘control arm’ as patients with the lowest anti-inflammatory therapy intensity after randomisation (ie, group with no ICS, lowest dose ICS or placebo).
Types of comparison conditions
Not applicable, as this is a prognostic IPD MA.
Types of outcome measures
The outcome is the occurrence of severe asthma attacks, defined as the number of acute asthma episodes requiring ≥3 days of systemic corticosteroids and/or hospitalisation. This was the primary outcome in many RCTs. Severe asthma attacks are important for patients, physicians and health insurance providers due to the high morbidity and financial burden.31 The severe asthma attack rate is known to be modifiable following appropriate anti-inflammatory therapy in patients with high type-2 biomarkers.18 19 21 24 26 The minimal clinically important difference for the annualised severe asthma attack rates in RCTs has not been determined, although it has been estimated to be 20%–40% in a recent expert consensus document.32
Search strategy
We will search MEDLINE (PubMed interface) for RCTs from 1 January 1993 to 1 April 2021 that fit the eligibility criteria.
Our search will use the term ‘asthma exacerbations’ ((“asthma”[MeSH Terms] OR “asthma”[All Fields] OR “asthmas”[All Fields] OR “asthma s”[All Fields]) AND (“exacerbate”[All Fields] OR “exacerbated”[All Fields] OR “exacerbates”[All Fields] OR “exacerbating”[All Fields] OR “exacerbation”[All Fields] OR “exacerbations”[All Fields] OR “exacerbator”[All Fields] OR “exacerbators”[All Fields])), filtered for ‘randomised controlled trials’ ‘humans’ ‘ages 12 and over’ and languages English and French. The details of the PubMed query are listed in online supplemental material. Literature search results will be uploaded to Microsoft EndNote. Titles and abstracts of all records returned by the literature search will be screened to identify potentially relevant publications which include the word ‘eosinophil’ OR ‘FeNO’ OR ‘nitric oxide’ OR ‘exhaled NO’. Manual reference searching will be performed for completed clinical trials that are in press at the time of the systematic review. Two reviewers (SC and IP) will independently review the retained publications to select trials for inclusion. We will resolve disagreement through discussion. We will record the reasons for excluding trials. Neither of the authors will be blind to the journal titles or to the study authors or institutions.
bmjopen-2021-058215supp001.pdf (538.8KB, pdf)
Data collection
Request for IPD
The authors of the retained studies will be contacted to obtain IPD. The corresponding author of each publication, and the representative(s) of the trial sponsor when applicable, will be sent an invitation letter and a skeleton Microsoft Excel spreadsheet containing the relevant fields for data extraction.
Data items
Anonymised individual patient data (IPD) to be requested includes demographics (age, body mass index); baseline lung function with postbronchodilator reversibility; treatment step according to anti-inflammatory components (table 2); ICS daily dosage; other asthma controller or reliever medications; presence of any Global Initiative for Asthma (GINA) defined risk factors (table 1) at baseline, when available; severe asthma attack history in the year prior to trial enrolment; the intervention the patient was randomised to; the peripheral blood eosinophil count, total IgE, specific airborne sensitisation and FeNO at baseline; duration of follow-up under controlled therapy; and the outcome of interest, that is, the number of severe asthma attacks during follow-up.
Table 2.
Treatment step definitions
| Treatment step | Definition |
| Step 1 | As-needed short-acting beta2-agonist |
| Step 2 | Daily low dose ICS or As-needed low dose ICS-formoterol Daily leukotriene receptor agonist |
| Step 3 | Daily low dose ICS+an additional controller therapy |
| Step 4 | Any medium dose ICS-containing regimen |
| Step 5 | Any high dose ICS-containing regimen or Any maintenance systemic corticosteroid use (defined as use of systemic corticosteroids for ≥50% of the previous year) |
Modified from Global Initiative for Asthma 2017 and 20211 guidelines.
ICS, inhaled corticosteroid.
Risk of bias in individual studies
To facilitate the assessment of possible bias for each study, we will collect information using the Cochrane Collaboration tool for assessing the risk of bias,33 which covers: sequence generation, allocation concealment, blinding, incomplete outcome data (eg, dropouts and withdrawals) and selective outcome reporting. For each domain in the tool, we will detail the procedures undertaken for each study, including verbatim quotes. A judgement as to the risk of bias on each of the six domains will be made from the extracted information, rated as ‘high risk’ or ‘low risk’. If there is insufficient detail reported in the study, we will judge the risk of bias as ‘unclear’ and the original study investigators will be contacted for more information. These judgements will be made independently by two authors based on the criteria for judging the risk of bias.33 Disagreements will be resolved first by discussion and then by consulting a third author for arbitration. We will compute graphic representations of potential bias within and across studies. We will consider each item in the risk of bias assessment independently without an attempt to collate and assign an overall score.
Data extraction
Data providers contacted following the systematic review will be provided sufficient time and support to confirm their consent for data extraction through data sharing contracts. Data sharing will be free of charge, financial contributions, and/or barriers to the dissemination of the results.
Data management and sharing
Secure digital transfer and storage solutions are provided by the University of Oxford. Under the terms of the data sharing agreements, access to the complete dataset is restricted to the named authors on the current study protocol who are bound by contract to the University of Oxford. Future third-party data sharing requests will need to be submitted to the original study authors.
Data analysis and synthesis
In relation with the objectives of this study, the data will be analysed and presented according to the following formats:
Results of the systematic review will be reported as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.34 All identified studies will be enumerated and detailed, irrespective of the provision of IPD.
Results of the multivariable prognostic analysis will report on univariate and multivariable coefficients from negative binomial regression on the annualised severe asthma attack rates. Important predictors to be assessed are the baseline blood eosinophil count and baseline FeNO values. Reporting will be in categories according to commonly accepted cut-offs (blood eosinophils, 0.15–<0.30, ≥0.30×109 cells/L; FeNO, <25, 25–<50, ≥50 ppb), with more detailed modelling as continuous variables. Non-linearity will be explored with rcs functions, with the number of knots guided by the Akaike Information Criterion (AIC). Relations will be plotted with 95% CIs. Other important prognostic factors include treatment steps (as per table 2), asthma attack history, postbronchodilator forced expiratory volume in 1 s percentage predicted, mean score on the 5-item Asthma Control Questionnaire, and body mass index; potential predictors are listed fully in the statistical analysis plan version 1.1, section 4.4 (online supplemental material). Interactions between blood eosinophil and FeNO values will be assessed according to AIC. If relevant, combined effects will be summarised in a 3×3 matrix stratified by the blood eosinophil count (<0.15, 0.15–<0.30, ≥0.30×109 cells/L) and FeNO (<25, 25–<50, ≥50 ppb), and plotted in interaction plots with 95% CI. Heterogeneity in estimates between studies will be quantified by I2 statistics.
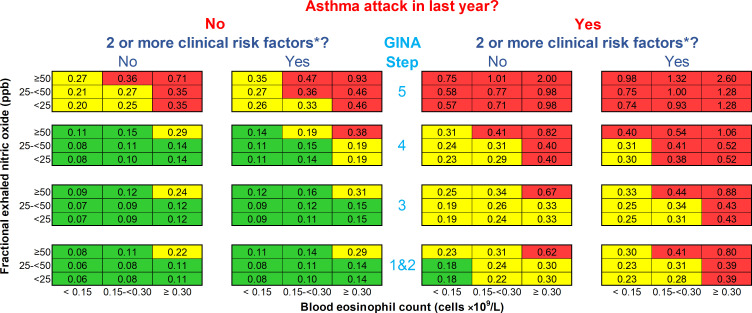
Clinical prediction modelling will be based on the statistical analysis plan (version 1.1) presented in online supplemental material. Briefly, we will use the study population as a derivation cohort, with stratification by study. Validation will be according to an internal – external cross-validation procedure, where each study is left out once.35 The selection of predictors will be based on the results of the multivariable prognostic analyses. A summary prognostic equation will be produced, assessed by the principal investigators and adapted to GINA treatment step reference attack rates (eg, Suruki et al36) to allow for a user-friendly prediction summary table similar to the reported prototype (figure 1). Performance of the predictive equation and table will be assessed separately with calibration plots, c-statistic and decision-analytic measures as outlined in the statistical analysis plan (see online supplemental material).
Figure 1.
The prototype OxfoRd Asthma attaCk risk scaLE (ORACLE). Numbers in each cell are predicted annual asthma attack rates for patients over the age of 12 if treatment is not changed. An asthma attack is an episode of acute asthma requiring treatment with systemic steroids ≥3 days and/or hospitalisation. The blood eosinophil count is contemporaneous or the highest result in last 12 months; fractional exhaled nitric oxide level (FeNO) is contemporaneous. *Risk factors are defined by the Global Initiative for Asthma (GINA) guidelines1: poor symptom control (Asthma Control Questionnaire score ≥1.5), low lung function (forced expiratory volume in 1 s <80% predicted), adherence issues, reliever over-use (>200 dose salbutamol cannister/month), intubation or intensive care unit admission for asthma previously, comorbidities (one of: chronic rhinosinusitis, obesity, psychiatric disease), environmental exposures (one of: smoking, allergen, pollution). Reproduced from Couillard et al23 with permission under the original CC BY public copyright license.
Study power
Considering a mean annualised severe asthma attack of 0.6 in the entire study population and a conservative estimate that the derivation cohort will comprise 50% of the IPD reported in our prototype scale (0.5×3051=1525),23 there should be approximately 915 events to derive a clinical prediction model. This provides for a solid basis for statistical modelling considering the limited number of potential predictors (around 10), leading a favourable event per variable (EPV) ratio (EPV=92).37 However, we concede that the EPV will be considerably lower for mild asthma populations, where trials identified less than 100 severe asthma attack events in their control arms.25 38 Conversely, the study will be more than adequately powered for moderate-to-severe asthma.
Statistical software and CIs
Data analysis will be conducted in collaboration with the study statistician (ES) using R software and the rms package. Reported outputs will present estimates and accompanying two-sided 95% CI. Bootstrap resampling will be applied to assess internal validity. Cross-validation by study will be performed to assess external validity.
Ethics and dissemination
The protocol has been reviewed by the academic ethics committee (Oxford Tropical Research Ethics Committee (OxTREC)) and found to comprise fully anonymised data not requiring further ethical approbation. The results of the systematic review, patient-level multivariable prognostic MA, and clinical prediction models will be presented in an international scientific meeting and submitted for publication.
Discussion
This protocol for a systematic review and IPD MA of RCTs across the spectrum of asthma severities coincides with a clinical prediction modelling effort centred on the peripheral blood eosinophil count and FeNO. Indeed, we speculate that these two biomarkers are the airway equivalent of high blood pressure or serum cholesterol, insofar as they identify a pathological process which relates to the risk key adverse outcomes (asthma attacks) that is modifiable by treatment (anti-inflammatory medication).
The focus on two biomarkers to predict the modifiable risk of asthma attacks is novel compared with existing clinical prediction models,2–13 where prognostic variables do not include nor adjust for blood eosinophils and FeNO. The established mechanistic, prognostic (ie, predicting adverse outcomes) and theragnostic (ie, predicting treatment responsiveness) values of these type-2 inflammatory biomarkers17–24 26 provide a strong basis for a clinical prediction model centred on these independent, additive, and, most importantly, modifiable risk factors. The current protocol extends our previous proof-of-concept23 24 work suggesting that traditional clinical risk factors can and should be adjusted for type-2 inflammatory biomarkers. Another novel aspect of our project is our intention to collaborate with a wide variety of authors and sponsors to form an international, data-driven, and not-for-profit consortium to support the development and validation of a robust clinical prediction model.
Despite the rigorous PRISMA34 and Cochrane33 methodologies which will be used to identify high-quality RCTs, there are areas of potential weaknesses in our study design which warrant discussion. First, we will limit our search strategy to MEDLINE. This approach was decided after a preliminary search in MEDLINE alone showed potential for >5000 control arm patients eligible to the IPD MA component; more than required to power our multivariable prognostic assessment and sufficient to claim that the included studies will be identified systematically rather than subjectively. Second, RCTs enrolling mild asthmatics have reported low absolute severe asthma attack rates,25 38 which may limit the model’s reliability for low-risk patients. Third, an RCT-based clinical prediction model will be difficult to subsequently validate in real-world settings where treatment intensity fluctuates in response to the perceived risk of asthma attacks. Such real-world fluctuation in treatment regimens may weaken the relation between static biomarker measurements and 12-month observed asthma attack rates. Nevertheless, we speculate that physician-patient discussions can be assisted by a clinical prediction model which estimates the risk of asthma attacks if anti-inflammatory treatment is not changed, that is: if the patient were randomised to the control arm of an RCT. Fourth, controlled trials in asthma are notorious for a strong placebo effect. This caveat may be due to improved adherence to ICS, the Hawthorne effect, regression to the mean, or a combination of factors.39 It is potentially surmountable by adapting the resultant clinical prediction model using reference asthma attack rates according to treatment intensity, as previously reported in a claims-based study36 and proposed in our statistical analysis plan. Finally, we have not planned to request active arm IPD, thus limiting our ability to assess the individual treatment benefit40 or model heterogeneity of treatment effects.41 We will not pursue the active arms’ data to promote collaboration between competing sponsors but envision a decentralised computation of individual treatment benefit and aggregate performance measures, such as the c-for-benefit statistic,40 at a later stage.
To conclude, we propose a systematic review and IPD MA to predict severe asthma attacks based on the inflammatory and clinical risk profile. Our emphasis on the risk conferred by raised type-2 inflammatory biomarkers and the consortium approach central to our endeavour may distinguish it from existing prediction models.2–13 We speculate that a clinical prediction model centred on blood eosinophils and FeNO will provide a useful basis for a preventive, treatable trait-based asthma management.
Supplementary Material
Footnotes
Twitter: @simcouillard
Contributors: SC drafted the protocol and participated in the systematic review literature search, contacted relevant data providers and will participate in data extraction and analysis. RB provided insight on the study design and contributed to manuscript writing. ES will provide statistical expertise and support. IP is the guarantor of this publication, contributed to the writing of the protocol manuscript, participated in the systematic review literature search, and approved the final manuscript. All authors reviewed and approved the final manuscript.
Funding: This work was supported by the NIHR Oxford BRC. The funders had no role in the conduct of the study nor the writing of the manuscript. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Competing interests: SC received a non-restricted research grant from Sanofi-Genzyme for investigator-initiated type 2 innovation research and received speaker honoraria from GlaxoSmithKline, Sanofi-Regeneron and AstraZeneca; outside the submitted work. ES receives royalties from Springer for the textbook entitled Clinical Prediction Models and received speaker honoraria from GlaxoSmithKline; outside the submitted work. RB has received honoraria for presentations or consulting in Adboards from AstraZeneca, Asthma and Respiratory Foundation of New Zealand, Avillion, Cipla and Theravance; and research grants from AstraZeneca, CureKids (NZ), Genentech, and the Health Research Council of New Zealand. IP: in the last 5 years, IP has received speaker’s honoraria for speaking at sponsored meetings from AstraZeneca, Boehringer Ingelheim, Aerocrine AB, Almirall, Novartis, Teva, Chiesi, Sanofi/Regeneron, Menarini, and GSK, and payments for organising educational events from AstraZeneca, GSK, Sanofi/Regeneron, and Teva. He has received honoraria for attending advisory panels with Genentech, Sanofi/Regeneron, AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Teva, Merck, Circassia, Chiesi, and Knopp, and payments to support FDA approval meetings from GSK. He has received sponsorship to attend international scientific meetings from Boehringer Ingelheim, GSK, AstraZeneca, Teva, and Chiesi. He has received a grant from Chiesi to support a phase 2 clinical trial in Oxford. He is copatent holder of the rights to the Leicester Cough Questionnaire and has received payments for its use in clinical trials from Merck, Bayer, and Insmed. In 2014–2015 he was an expert witness for a patent dispute involving AstraZeneca and Teva.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Global Initiative for Asthma (GINA) . Global strategy for asthma management and prevention (2021 update), 2021. Available: https://ginasthma.org/
- 2.Bateman ED, Buhl R, O'Byrne PM, et al. Development and validation of a novel risk score for asthma exacerbations: the risk score for exacerbations. J Allergy Clin Immunol 2015;135:1457–64. 10.1016/j.jaci.2014.08.015 [DOI] [PubMed] [Google Scholar]
- 3.Miller MK, Lee JH, Blanc PD, et al. TENOR risk score predicts healthcare in adults with severe or difficult-to-treat asthma. Eur Respir J 2006;28:1145–55. 10.1183/09031936.06.00145105 [DOI] [PubMed] [Google Scholar]
- 4.Loymans RJB, Honkoop PJ, Termeer EH, et al. Identifying patients at risk for severe exacerbations of asthma: development and external validation of a multivariable prediction model. Thorax 2016;71:838–46. 10.1136/thoraxjnl-2015-208138 [DOI] [PubMed] [Google Scholar]
- 5.Eisner MD, Yegin A, Trzaskoma B. Severity of asthma score predicts clinical outcomes in patients with moderate to severe persistent asthma. Chest 2012;141:58–65. 10.1378/chest.11-0020 [DOI] [PubMed] [Google Scholar]
- 6.Sato R, Tomita K, Sano H, et al. The strategy for predicting future exacerbation of asthma using a combination of the asthma control test and lung function test. J Asthma 2009;46:677–82. 10.1080/02770900902972160 [DOI] [PubMed] [Google Scholar]
- 7.Peters D, Chen C, Markson LE, et al. Using an asthma control questionnaire and administrative data to predict health-care utilization. Chest 2006;129:918–24. 10.1378/chest.129.4.918 [DOI] [PubMed] [Google Scholar]
- 8.Yurk RA, Diette GB, Skinner EA, et al. Predicting patient-reported asthma outcomes for adults in managed care. Am J Manag Care 2004;10:321–8. [PubMed] [Google Scholar]
- 9.Schatz M, Cook EF, Joshua A, et al. Risk factors for asthma hospitalizations in a managed care organization: development of a clinical prediction rule. Am J Manag Care 2003;9:538–47. [PubMed] [Google Scholar]
- 10.Lieu TA, Capra AM, Quesenberry CP, et al. Computer-Based models to identify high-risk adults with asthma: is the glass half empty or half full? Journal of Asthma 1999;36:359–70. 10.3109/02770909909068229 [DOI] [PubMed] [Google Scholar]
- 11.Ellman MS, Viscoli CM, Sears MR, et al. A new index of prognostic severity for chronic asthma. Chest 1997;112:582–90. 10.1378/chest.112.3.582 [DOI] [PubMed] [Google Scholar]
- 12.Grana J, Preston S, McDermott PD, et al. The use of administrative data to risk-stratify asthmatic patients. Am J Med Qual 1997;12:113–9. 10.1177/0885713X9701200205 [DOI] [PubMed] [Google Scholar]
- 13.Osborne ML, Pedula KL, O'Hollaren M, et al. Assessing future need for acute care in adult asthmatics: the profile of asthma risk study: a prospective health maintenance organization-based study. Chest 2007;132:1151–61. 10.1378/chest.05-3084 [DOI] [PubMed] [Google Scholar]
- 14.Jackson R, Barham P, Bills J, et al. Management of raised blood pressure in New Zealand: a discussion document. BMJ 1993;307:107–10. 10.1136/bmj.307.6896.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the score project. Eur Heart J 2003;24:987–1003. 10.1016/S0195-668X(03)00114-3 [DOI] [PubMed] [Google Scholar]
- 16.Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet 2018;391:350–400. 10.1016/S0140-6736(17)30879-6 [DOI] [PubMed] [Google Scholar]
- 17.Busse WW, Wenzel SE, Casale TB, et al. Baseline FeNO as a prognostic biomarker for subsequent severe asthma exacerbations in patients with uncontrolled, moderate-to-severe asthma receiving placebo in the liberty asthma quest study: a post-hoc analysis. Lancet Respir Med 2021;9:1165–73. 10.1016/S2213-2600(21)00124-7 [DOI] [PubMed] [Google Scholar]
- 18.Shrimanker R, Keene O, Hynes G, et al. Prognostic and Predictive Value of Blood Eosinophil Count, Fractional Exhaled Nitric Oxide, and Their Combination in Severe Asthma: A Post Hoc Analysis. Am J Respir Crit Care Med 2019;200:1308–12. 10.1164/rccm.201903-0599LE [DOI] [PubMed] [Google Scholar]
- 19.Pavord ID, Holliday M, Reddel HK, et al. Predictive value of blood eosinophils and exhaled nitric oxide in adults with mild asthma: a prespecified subgroup analysis of an open-label, parallel-group, randomised controlled trial. Lancet Respir Med 2020;8:671–80. 10.1016/S2213-2600(20)30053-9 [DOI] [PubMed] [Google Scholar]
- 20.Kraft M, Brusselle G, FitzGerald JM, et al. Patient characteristics, biomarkers and exacerbation risk in severe, uncontrolled asthma. Eur Respir J 2021;58. 10.1183/13993003.00413-2021. [Epub ahead of print: 16 12 2021]. [DOI] [PubMed] [Google Scholar]
- 21.Lee LA, Bailes Z, Barnes N, et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respir Med 2021;9:69–84. 10.1016/S2213-2600(20)30389-1 [DOI] [PubMed] [Google Scholar]
- 22.Couillard S, Shrimanker R, Chaudhuri R, et al. Fractional exhaled nitric oxide nonsuppression identifies corticosteroid-resistant type 2 signaling in severe asthma. Am J Respir Crit Care Med 2021;204:731–4. 10.1164/rccm.202104-1040LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couillard S, Laugerud A, Jabeen M, et al. Derivation of a prototype asthma attack risk scale centred on blood eosinophils and exhaled nitric oxide. Thorax 2022;77:199–202. 10.1136/thoraxjnl-2021-217325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couillard S, Do WIH, Beasley R, et al. Predicting the benefits of type-2 targeted anti-inflammatory treatment with the prototype Oxford asthma attack risk scale (ORACLE). ERJ Open Res 2022;8. 10.1183/23120541.00570-2021. [Epub ahead of print: 07 02 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beasley R, Holliday M, Reddel HK, et al. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med 2019;380:2020–30. 10.1056/NEJMoa1901963 [DOI] [PubMed] [Google Scholar]
- 26.Couillard S, Pavord ID. Fluticasone furoate: CAPTAIN of fluticasones in type 2 inflammatory asthma. Respirology 2022;27:184–6. 10.1111/resp.14213 [DOI] [PubMed] [Google Scholar]
- 27.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012;380:651–9. 10.1016/S0140-6736(12)60988-X [DOI] [PubMed] [Google Scholar]
- 28.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med 2018;378:2486–96. 10.1056/NEJMoa1804092 [DOI] [PubMed] [Google Scholar]
- 29.Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. New England Journal of Medicine 2021;384:1800–9. 10.1056/NEJMoa2034975 [DOI] [PubMed] [Google Scholar]
- 30.Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J 2016;47:410–9. 10.1183/13993003.01359-2015 [DOI] [PubMed] [Google Scholar]
- 31.Reddel HK, Taylor DR, Bateman ED, et al. An official American thoracic Society/European respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009;180:59–99. 10.1164/rccm.200801-060ST [DOI] [PubMed] [Google Scholar]
- 32.Bonini M, Di Paolo M, Bagnasco D, et al. Minimal clinically important difference for asthma endpoints: an expert consensus report. European Respiratory Review 2020;29:190137–14. 10.1183/16000617.0137-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins J, Thomas J, Chandler J. Cochrane Handbook for systematic reviews of interventions (version 6.2). Cochrane, 2021. Available: www.training.cochrane.org/handbook [Accessed 15 Sep 2021].
- 34.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349:g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 35.Steyerberg EW, Harrell FE. Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol 2016;69:245–7. 10.1016/j.jclinepi.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suruki RY, Daugherty JB, Boudiaf N, et al. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med 2017;17:74. 10.1186/s12890-017-0409-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steyerberg EW. Clinical prediction models. Cham: Springer International Publishing, 2019. [Google Scholar]
- 38.Hardy J, Baggott C, Fingleton J, et al. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (practical): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet 2019;394:919–28. 10.1016/S0140-6736(19)31948-8 [DOI] [PubMed] [Google Scholar]
- 39.Luc F, Prieur E, Whitmore GA, et al. Placebo effects in clinical trials evaluating patients with uncontrolled persistent asthma. Ann Am Thorac Soc 2019;16:1124–30. 10.1513/AnnalsATS.201901-071OC [DOI] [PubMed] [Google Scholar]
- 40.van Klaveren D, Steyerberg EW, Serruys PW, et al. The proposed 'concordance-statistic for benefit' provided a useful metric when modeling heterogeneous treatment effects. J Clin Epidemiol 2018;94:59–68. 10.1016/j.jclinepi.2017.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kent DM, Paulus JK, van Klaveren D, et al. The predictive approaches to treatment effect heterogeneity (path) statement. Ann Intern Med 2020;172:35–45. 10.7326/M18-3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-058215supp001.pdf (538.8KB, pdf)