Abstract
Background: Coronavirus disease 2019 (COVID-19) has been associated with many neurological complications affecting the central nervous system. Purpose: Our aim was to describe a case of COVID-19 associated with a probable variant of acute necrotizing encephalopathy (ANE). Results: A 60-year-old man who presented with a 3-day history of dyspnea, fever, and cough tested positive for severe acute respiratory syndrome–coronavirus 2 (SARS-CoV-2). Five days following his admission, the patient was intubated secondary to respiratory failure. Following his extubation 16 days later, he was found to have a left-sided weakness. Magnetic resonance imaging (MRI) of the brain showed hemorrhagic rim-enhancing lesions involving the right thalamus, left hippocampus, and left parahippocampal gyrus. These lesions showed decreased relative cerebral blood flow on MR perfusion and restricted on diffusion-weighted imaging. These neuroimaging findings were consistent with ANE. The left-sided weakness gradually improved over the subsequent weeks. Conclusions: We concluded that COVID-19 can be associated with ANE, a condition believed to be the result of an immune-mediated process with activation of the innate immune system. Future studies must address whether biological drugs targeting the pro-inflammatory cytokines could prevent the development of this condition.
Keywords: COVID-19, acute necrotizing encephalopathy, magnetic resonance imaging, case report
Introduction
In addition to the predominant pulmonary and cardiovascular manifestations of coronavirus disease 2019 (COVID-19), this virus is also associated with several central nervous system (CNS) manifestations including headaches, impaired consciousness, meningoencephalitis, intracerebral hemorrhage, and ischemic stroke. 1 Another rare CNS complication of this virus is acute necrotizing encephalopathy (ANE), a condition characterized by symmetric, usually hemorrhagic lesions lesions invariably involving the thalami and other brain regions including the brainstem and cerebellum.1,2 In this letter, we are reporting a variant of this condition in an adult with COVID-19 whose brain magnetic resonance imaging (MRI) was characterized by asymmetrical hemorrhagic and ring-enhancing lesions.
Case Report
A 60-year-old man with no previous medical history presented to the emergency department with a 3-day history of dyspnea, fever, and non-productive cough. He was diagnosed with COVID-19 by reverse transcription polymerase chain reaction assay on a nasopharyngeal swab. His neurological examination was normal at that time with no evidence of focal deficits.
A chest computed tomography (CT) scan revealed bilateral patchy ground-glass interstitial opacities. Interleukin-6 level was elevated at 21.5 pg/mL (normal range: < 7.0 pg/mL).
On day 5 of his admission, the patient was intubated because of respiratory failure. He received dexamethasone, remdesivir, convalescent plasma, and antibiotic treatment. His hematological profile was indicative of a hypercoagulable state with elevated D-dimer reaching up to 6386 ng/mL (normal range: < 255 ng/mL), elevated fibrinogen level of 4.91 g/L (normal range: 1.7–4.0 g/L), and a prolonged PT at 16.1 seconds (normal range: 10.0–13.0) for which he was maintained on subcutaneous enoxaparin at a dose of 40 mg twice daily. He also had evidence of an inflammatory process with an elevated level of C-reactive protein (CRP) reaching up to 219.7 mg/L (normal range: 0–2.5 mg/L).
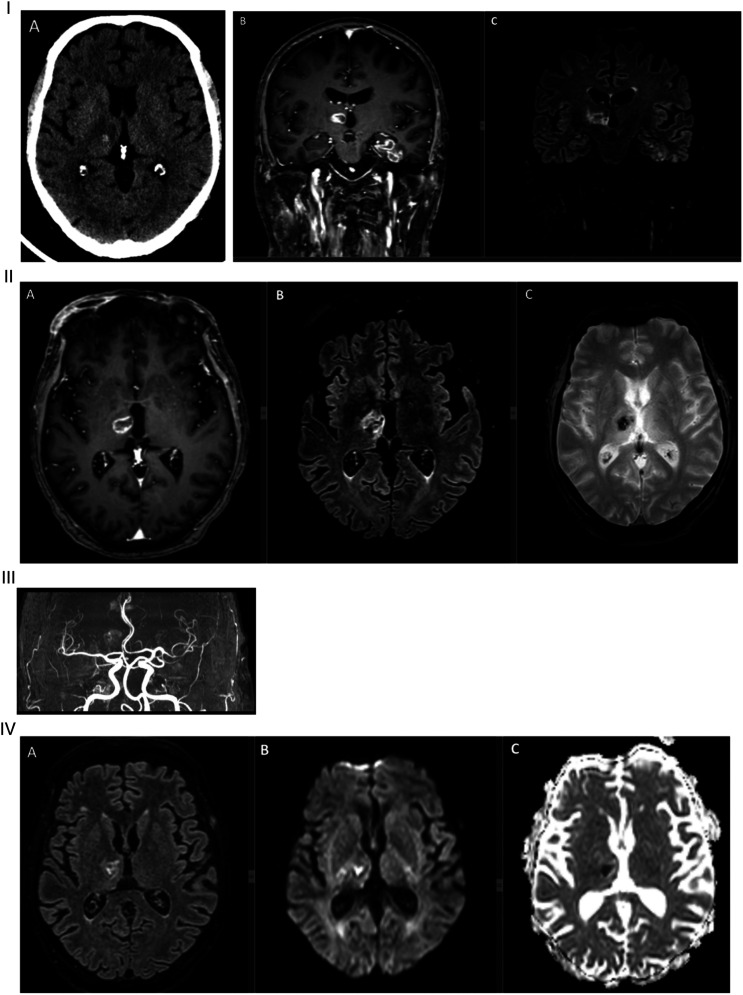
The patient was noted to have a left hemiparesis when extubated 16 days later. On examination, he was somnolent, with a left central facial paresis and a motor strength of 2/5 in his left upper and lower extremities. Head CT showed evidence of a bleed in the right thalamus and a left temporal hypodensity (Figure 1). Brain MRI performed 1 day later demonstrated hemorrhagic ring-enhancing lesions within the right thalamus, left hippocampus, and left parahippocampal gyrus, as well as an increased FLAIR signal in the right cerebellum (Figure 1). The dural sinuses were patent and MR angiography was normal (Figure 1). A lumbar puncture was normal with no white blood cells (WBC), a protein level of .27 g/L (normal range: .1–.5 g/L), an IgG index of .56 (normal range: .3–.77) and no oligoclonal band. A multiplexed cerebrospinal fluid (CSF) polymerase chain reaction (PCR) meningitis panel (herpes simplex virus 1 and 2, haemophilus influenza, Listeria monocytogenes, Neisseria meningitis, Streptococcus agalactiae and pneumonia, varicella-zoster virus, Enterovirus, Cytomegalovirus, Parechovirus, human herpesvirus 6 and Cryptococcus neoformans/gattii) and CSF cultures were negative. CSF PCR for severe acute respiratory syndrome–corona virus 2 (SARS-CoV-2) was not obtained. A transthoracic echocardiography and a CT of the abdomen and pelvis were unremarkable.
Figure 1.
I: (A) Initial head computed tomography showed a bleed in the right thalamus with a hypodensity extending to the posterior limb of the internal capsule. (B) Coronal T1 section of initial gadolinium-enhanced brain MRI showing 2 ring-enhancing lesions over the right thalamus and left mesial temporal lobe. (C) Coronal FLAIR section showing increased signal in the right thalamus, and left hippocampus, and parahippocampal gyrus. II: (A) Axial T1 section of initial gadolinium-enhanced brain MRI showing a ring-enhancing lesion involving the right thalamus. (B) Axial FLAIR section showing increased signal surrounding an area of decreased signal consistent with acute or early subacute bleed. (C) Axial T2 FFE cut showing the right thalamic hemorrhage. III: MR angiogram showing normal intracranial vasculature. IV: (A) Axial FLAIR section of follow-up brain MRI at 1 week showing partial resolution of the right thalamic hemorrhage. (B) DWI and (C) apparent diffusion coefficient showing restricted diffusion of the right thalamic lesion. MRI, magnetic resonance imaging.
A repeat brain MRI 1 week later showed partial resolution of the hemorrhagic component with definite restriction on the apparent diffusion coefficient map (Figure 1). MR perfusion showed decreased relative cerebral blood flow and cerebral blood volume in the lesions (Figure 2). On MR spectroscopy (MRS), no lactate peak was identified, and an inversed N-acetylaspartate/choline ratio was noted (Figure 3).
Figure 2.
(A) Brain MR perfusion targeting the selected areas over the thalami showing (B) decreased cerebral blood flow and (C) decreased cerebral blood volume over the right thalamic lesion.
Figure 3.
MR spectroscopy over right thalamic lesion showing inverted N-acetylaspartate/choline ratio.
Clinically, the patient weakness gradually improved, and he was nearly back to his baseline strength when evaluated in clinic 3 weeks later. The patient was evaluated in the clinic 5 months following his discharge from the hospital. At that visit, the patient’s medical and neurological examinations were normal except for a mild short-term memory deficit. A follow-up brain MRI at that time showed near complete resolution of the right thalamic lesion and the development of a cystic encephalomalacia in the left mesial temporal lobe (Figure 4).
Figure 4.
I: (A) Axial FLAIR section of the follow-up MRI at 5 months showing resolution of the right thalamic lesion. (B) Axial DWI showing no restricted diffusion over the right thalamus. (C) Axial T2 FFE showing near complete resolution of the right thalamic hemorrhage. II: (A) Coronal gadolinium-enhanced T1 section and (B) coronal FLAIR of the follow-up brain MRI at 5 months showing resolution of the right thalamic lesion and development of a cystic encephalomalacia in the left mesial temporal lobe. MRI, magnetic resonance imaging.
Discussion
We are presenting a case of a probable variant of ANE secondary to COVID-19 infection. Before reaching this conclusion, we considered several alternative diagnoses that could explain the neuroimaging findings in our patient. The possibility of abscesses was excluded because of the hemorrhagic nature of the lesions, the lack of a lactate peak on MRS, and the clinical course. We dismissed the possibility of metastases based on the reduction of cerebral blood flow on perfusion MRI, follow-up neuroimaging studies, and clinical course. Acute hemorrhagic leukoencephalitis was considered highly improbable given the lack of confluent or tumefactive white matter lesions and the relatively selective involvement of the thalamus and hippocampus. 3
Acute necrotizing encephalopathy is a condition that predominantly affects the pediatric age group and is a rare complication of viral infections.1,4 The neuroimaging findings were initially described as consisting of symmetric multifocal lesions involving gray and white matter structures with invariable involvement of the thalami.1,2 However, cases with an asymmetrical involvement subsequently published with this pattern were believed to portend a better prognosis. 5 Hemorrhage within the lesions and ring enhancement are common but not invariable features of this condition. 2
Only few cases of COVID-19–associated probable ANE with variable MRI features were reported.6-8 Our 60-year-old patient is the oldest reported case with COVID-19–related ANE. Similar to the case described by Delamarre et al, 8 the initial neurological examination on our patient was normal and his hemiparesis was only noted after he was extubated 16 days later. This is unlike the clinical status of the other 2 reported cases who presented with altered mental status with or without seizures.6,7 The CSF finding of normal protein in our patient is an unusual finding in ANE. The previously reported cases consistently showed an albumino-cytologic dissociation with elevated CSF protein and normal white blood cell count.7,8 However, what mostly distinguishes our patient from the other published cases with COVID-19–related ANE were the radiological features. Our case is unique because his brain lesions were highly asymmetrical, with involvement of the right thalamus, left hippocampus, and right cerebellum. The previously published cases were all characterized by bilateral and symmetrical involvement of the thalami as well as other brain structures including the amygdalae, medial temporal lobes, and deep grey nuclei.6-8 In addition, we obtained additional brain imaging modalities such as MRS and MR perfusion imaging which helped in ruling out alternative causes and further supported our diagnosis.
The pathophysiology of the hemorrhagic and ring-enhancing lesions in our patient remains unclear. Various explanations were proposed to explain the CNS complications of COVID-19 infection including tissue hypoxia, dysregulated immune reactions, endothelial injury caused by direct viral invasion, molecular mimicry between viral and neuronal antigens, cell-induced neurotoxicity, and a hypercoagulable state.1,9,10 It is possible that different mechanisms may be at play in separate patients or that the etiology is multifactorial. Previous studies of ANE in children suggested that this condition was not due to a direct viral infection1,2,9,10 but rather due to an immune-mediated process involving pro-inflammatory cytokines. 4 This is a plausible explanation following COVID-19 since the acute respiratory distress syndrome associated with this virus is considered to be caused by a cytokine storm or a hyperinflammatory response. 11 The cytokine storm could then lead to a hypercoagulable state, resulting in thrombosis of terminal blood vessels. 12 In our case, we were unable to confirm the absence of viral particles in the CNS by performing a CSF PCR for SARS-CoV-2. This is in contrast to the cases described by Dixon et al 7 and Delamarre et al, 8 which showed a negative CSF PCR test for COVID-19. Our patient had evidence of a pro-inflammatory and hypercoagulable state, which supports this hypothesis. The possibility of a molecular mimicry between the virus and neuronal antigen was suggested in a patient diagnosed with COVID-19–associated ANE based on indirect immunofluorescence staining of the patient’s IgG on rat hippocampal and monkey cerebellar slices. 8 However, the normal CSF findings in conjunction with the normal IgG index and absence of oligoclonal band in our patient makes it highly unlikely that an auto-antibody–mediated response against neuronal antigens played a role in the pathophysiology of his illness.
In conclusion, our case depicts a probable variant of COVID-19–related ANE in a patient with evidence of a hypercoagulable state and with activation of the innate immune system. The neuroimaging findings of our case further expand our knowledge of the spectrum of MRI abnormalities seen in patients with CNS complications of COVID-19. Whether treatment with biologic drugs targeting the pro-inflammatory response in patients with COVID-19 infections could prevent this condition will need to be addressed in future studies.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Informed consent was obtained from patient’s legal guardian (wife).
Data Availability Statement: The data that support the findings of this letter are available from the corresponding author upon reasonable request.
ORCID iDs
Najo Jomaa https://orcid.org/0000-0002-5710-9926
Ahmad Beydoun https://orcid.org/0000-0002-9047-1185
References
- 1.Mizuguchi M, Abe J, Mikkaichi K, et al. Acute necrotising encephalopathy of childhood: A new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatr. 1995;58:555-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong A, Simon E, Zimmerman R, et al. Acute necrotizing encephalopathy of childhood: Correlation of MR findings and clinical outcome. American journal of neuroradiology. 2006;27:1919-1923. [PMC free article] [PubMed] [Google Scholar]
- 3.Grzonka P, Scholz MC, De Marchis GM, et al. Acute Hemorrhagic Leukoencephalitis: A Case and Systematic Review of the Literature. Front Neurol 2020;11:899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito Y, Ichiyama T, Kimura H, et al. Detection of influenza virus RNA by reverse transcription‐PCR and proinflammatory cytokines in influenza‐virus‐associated encephalopathy. J Med Virol. 1999;58:420-425. [DOI] [PubMed] [Google Scholar]
- 5.Yoshikawa H, Watanabe T, Abe T, et al. Clinical diversity in acute necrotizing encephalopathy. J Child Neurol. 1999;14:249-255. [DOI] [PubMed] [Google Scholar]
- 6.Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features. Radiology. 2020;296:E119-E120. 2020/04/02. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon L, Varley J, Gontsarova A, et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm. 2020;7:e789. 2020/05/28. doi: 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delamarre L, Gollion C, Grouteau G, et al. COVID-19–associated acute necrotising encephalopathy successfully treated with steroids and polyvalent immunoglobulin with unusual IgG targeting the cerebral fibre network. J Neurol Neurosurg Psychiatr. 2020;91:1004-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mastroyianni SD, Gionnis D, Voudris K, et al. Acute necrotizing encephalopathy of childhood in non-Asian patients: Report of three cases and literature review. J Child Neurol. 2006;21:872-879. [DOI] [PubMed] [Google Scholar]
- 10.Offiah C, Hall E. Acute necrotizing encephalopathy associated with novel influenza H1N1 (pdm09) infection: MRI and correlation with brain necropsy. J Pediatr Neuroradiol. 2013;2:319-324. [Google Scholar]
- 11.Gonaglea D, Sharifa K, O’Regand A. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi X, Keith KA, Huang JH. COVID-19 and stroke: A review. Brain hemorrhages 2020;2:76-83. [DOI] [PMC free article] [PubMed] [Google Scholar]