Abstract
Although flow cytometry has been used to study antibiotic effects on bacterial membrane potential (MP) and membrane permeability, flow cytometric results are not always well correlated to changes in bacterial counts. Using new, precise techniques, we simultaneously measured MP, membrane permeability, and particle counts of antibiotic-treated and untreated Staphylococcus aureus and Micrococcus luteus cells. MP was calculated from the ratio of red and green fluorescence of diethyloxacarbocyanine [DiOC2(3)]. A normalized permeability parameter was calculated from the ratio of far red fluorescence of the nucleic acid dye TO-PRO-3 and green DiOC2(3) fluorescence. Bacterial counts were calculated by the addition of polystyrene beads to the sample at a known concentration. Amoxicillin increased permeability within 45 min. At concentrations of <1 μg/ml, some organisms showed increased permeability but normal MP; this population disappeared after 4 h, while bacterial counts increased. At amoxicillin concentrations above 1 μg/ml, MP decreased irreversibly and the particle counts did not increase. Tetracycline and erythromycin caused smaller, dose- and time-dependent decreases in MP. Tetracycline concentrations of <1 μg/ml did not change permeability, while a tetracycline concentration of 4 μg/ml permeabilized 50% of the bacteria; 4 μg of erythromycin per ml permeabilized 20% of the bacteria. Streptomycin decreased MP substantially, with no effect on permeability; chloramphenicol did not change either permeability or MP. Erythromycin pretreatment of bacteria prevented streptomycin and amoxicillin effects. Flow cytometry provides a sensitive means of monitoring the dynamic cellular events that occur in bacteria exposed to antibacterial agents; however, it is probably simplistic to expect that changes in a single cellular parameter will suffice to determine the sensitivities of all species to all drugs.
Flow cytometry allows measurements of several physical or chemical characteristics to be made for an individual cell in a matter of microseconds, providing an indication of the heterogeneity of a population of thousands of cells or bacteria within minutes (3, 21). Although a number of investigators have used the method to study the interactions of antibacterial agents and bacteria, most of the literature on this topic has focused on the development of rapid assays for determination of antibacterial susceptibility (5, 12, 18, 19, 26, 29) rather than on analysis of the modes of action of drugs.
Classical techniques for assessing antibacterial effects rely on the detection of changes in the number of bacteria or colonies in a sample over time; a CFU may be either a single viable bacterial cell or a clump containing one or more viable cells. While a flow cytometer can count the number of bacteria and/or clumps of bacteria present in a given volume of sample, it is harder to determine cell viability. In much of the previous work in the field, membrane potential (MP), on the one hand, and impermeability of the membrane to certain classes of fluorescent dyes, on the other, have been used as the main indicators of the physiologic state of the cytoplasmic membrane and the viability of the organism.
Although cells of different species may exhibit differences in membrane permeability, it is widely believed that certain classes of compounds, including organic compounds that bear at least two positive charges and most negatively charged organic compounds, are excluded by the intact cell membranes of both bacteria and eukaryotes. The demonstration, by microscopy or flow cytometry, of uptake of such impermeant compounds by cells is generally considered to indicate cell death. Among the compounds used as indicators of cell death are fluorescent nucleic acid binding dyes with two or more positive charges, such as propidium iodide (PI), TO-PRO-3, and Sytox Green.
In metabolically active bacteria with intact cytoplasmic membranes, there is typically a difference of electrical potential across the membrane, with the interior negative by between 100 and 200 mV with respect to the exterior. A reduction in the magnitude of MP is referred to as electrical depolarization; an increase in the magnitude of MP is referred to as electrical hyperpolarization. MP is reduced to zero if the membrane ruptures, i.e., develops holes large enough to permit inorganic ions to cross freely, as may occur when cells are killed by heating, freezing, or beta-lactam antibiotics. Under these circumstances, the membrane becomes permeable to dyes such as PI, and the change is irreversible. Several classes of chemical compounds, notably, ionophores, can also alter MP without affecting permeability to PI and similar dyes; in bacteria, administration of a proton ionophore such as carbonyl cyanide m-chlorophenylhydrazone (CCCP) will reduce the MP to zero.
A number of fluorescent dyes may be used to estimate the MP of bacteria. Cyanine dyes and other lipophilic compounds that bear a single delocalized positive charge, such as rhodamine 123, and that are added to cell suspensions are concentrated in bacteria in response to the interior negative MP gradient; the fluorescence of organisms exposed to low concentrations of these dyes increases with hyperpolarization and decreases with depolarization. Oxonol dyes, which bear a negative charge, are largely excluded from cells by the MP gradient; the fluorescence of organisms exposed to them increases with depolarization. However, although flow cytometry with either cyanine or oxonol dyes can usually discriminate apparently viable bacteria from organisms killed by heat or ethanol, it has not, until recently, been possible to obtain reliable quantitative measurements of MP in individual organisms.
While recent studies have attempted, using flow cytometry, to examine the effects of antibacterial agents on several different cellular characteristics or parameters, including membrane permeability, MP, and respiratory activity (15, 30), in those studies, only one of these parameters was measured in any given aliquot of sample, and it was thus impossible to detect and correlate changes in two or more parameters on a cell-by-cell basis.
We recently developed a flow cytometric method for the measurement of MP in bacteria (17). The method is substantially more accurate and more precise than methods previously used for this purpose. We now describe the extension of this technique, used in conjunction with a new, multibeam flow cytometer optimized for examination of bacteria, to provide simultaneous measurements of MP, membrane permeability, and particle counts of bacteria in suspension. This methodology is applied to the analysis of the effects of several antibacterial agents on Staphylococcus aureus and Micrococcus luteus.
MATERIALS AND METHODS
Membrane potential-sensitive dyes.
Diethyloxacarbocyanine iodide [DiOC2(3)] was obtained from Accurate Chemical (Hicksville, N.Y.) (it is now also available from Molecular Probes, Eugene, Oreg.); bis-(1,3-dibutlylbarbituric acid) trimethine oxonol [DiBAC4(3)] was obtained from Molecular Probes.
Membrane permeability indicators.
PI and TO-PRO-3, both impermeant compounds that markedly increase their fluorescence on binding to double-stranded nucleic acid, were purchased from Molecular Probes.
Ionophores.
The proton ionophore CCCP and the potassium ionophore valinomycin were from Sigma (St. Louis, Mo.).
All dyes and ionophores were made up as stock solutions at 1 mM in dimethyl sulfoxide (DMSO; Sigma) and were diluted in DMSO prior to addition to cultures; the final DMSO concentration in the cultures was below 1% (vol/vol).
Bacteria, antibacterial agents, and culture conditions.
S. aureus ATCC 29213 was obtained from Difco Laboratories (Detroit, Mich.); M. luteus was obtained from Carolina Biological Supply Company (Burlington, N.C.). Amoxicillin, chloramphenicol, erythromycin, streptomycin, and tetracycline were obtained from Sigma. Organisms were grown overnight in Trypticase soy broth (Difco) and were subcultured in the morning. Stock solutions of antibacterial agents were made by standard protocols (1). Agents were diluted to the appropriate concentration in 1 ml of Mueller-Hinton broth (Gibco, Gaithersburg, Md.) that had been filtered though a 0.22-μm-pore-size filter (Gelman Sciences, Ann Arbor, Mich.). The MIC was defined as the lowest concentration of agent at which no growth occurred after 24 h in Mueller-Hinton broth.
Subcultured bacteria grown to a concentration of approximately 107/ml were diluted into a solution of antibacterial agent to a concentration of 5.5 × 105/ml, and the mixture was incubated for the appropriate amount of time at 37°C while shaking. At each time point, a 500-μl aliquot was removed and MP-sensitive dyes and permeability indicators (described below) were added simultaneously. The bacteria were allowed to incubate with the dyes for 4 min prior to flow cytometric analysis. A second 500-μl aliquot was treated identically, except that the proton ionophore CCCP (final concentration, 15 μM; Sigma) was added to reduce the MP to zero.
Flow cytometry.
Flow cytometric studies were performed with a laboratory-built dual-laser instrument (22) optimized for bacterial analysis. A 25-mW, 488-nm beam from an argon ion laser and a 20-mW, 633-nm beam from a helium-neon laser were focused to elliptical spots that were 15 μm high and 75 μm wide and that intersected a 150-μm sample stream in air. Light scattered at small angles to the 488-nm beam (forward scatter) was used as the trigger signal. DiOC2(3) was excited at 488 nm; its green fluorescence was detected through a 530-nm, 20-nm-bandwidth band-pass filter (Omega, Brattleboro, Vt.), and its red fluorescence was detected through a 610-nm, 19-nm band-pass filter (Omega). TO-PRO-3 was excited by the helium-neon laser, and its far red fluorescence was detected through a 695-nm long-pass filter (RG695; Schott, Duryea, Pa.). All detectors were Hamamatsu (Bridgewater, N.J.) R1477 photomultiplier tubes. The sheath flow rate was 10 ml/min; the sample analysis rate was kept below 1,000 events/s. The signal pulse amplitudes were captured by high-precision peak detectors and were digitized with an Analogic (Peabody, Mass.) HSDAS-16 16-bit data acquisition system in a Pentium-class personal computer. Software was used to perform logarithmic conversion on all of the parameters, which were represented on a 256-channel, 4-decade logarithmic scale. Data were analyzed by using the FCS Express software package (De Novo Software, Toronto, Ontario, Canada).
Flow cytometric determination of bacterial particle counts.
Counts were determined by the addition of 4 μM sky blue beads (Spherotech, Libertyville, Ill.) to samples at a known concentration. A working solution of 107 beads/ml (determined by counting on a hemocytometer) in water containing 0.5% Triton X-100 was made daily. Beads were diluted 1:100 into the bacterial sample and could be identified and counted on the basis of their intense fluorescence (excitation at 633 nm; emission above 695 nm). The volume of sample analyzed in any given aliquot was calculated from the number of beads measured. The threshold of the flow cytometer was set to exclude particles with forward light-scattering signals substantially smaller than those normally obtained for bacteria. The total number of particles detected during the analysis of an aliquot of sample therefore represented the sum of the number of beads in the aliquot and the number of particles representing single bacteria or clumps of bacteria. Since the beads could readily be distinguished from the bacterial particles by their light-scattering and fluorescence characteristics, the number of bacterial particles in the aliquot could be calculated by subtraction. Dividing this number by the measured volume of the sample, calculated as described above, yielded the concentration of bacterial particles in the aliquot. The bacterial particle count per se cannot be directly related to the number of CFU per unit volume because it does not distinguish between viable and nonviable organisms. However, since the sensitivity of the measurements is sufficient to include all particles in and above the bacterial size range, the bacterial particle count cannot be less than the number of CFU.
Determination of MP.
MP was determined as described previously (17). Briefly, the bacteria were incubated with 30 μM DiOC2(3) for 4 mins, and MP was estimated from the ratio of red to green DiOC2(3) fluorescence. At the concentration of dye used, the green fluorescence, due primarily to emission from single dye molecules, varies with the size of the bacterial cell or clump but is largely independent of MP, while the red fluorescence, due to emission from dye aggregates, is dependent on both size and MP. The ratio therefore provides a cell size-independent measure of MP. The red fluorescence/green fluorescence ratio was calibrated by depolarizing the bacteria to various extents by addition of 5 μM valinomycin in the presence of different known external concentrations of potassium ion. For comparison purposes, in some experiments, 1 μM DiBAC4(3) instead of DiOC2(3) was used as a putative MP indicator; DiBAC4(3) was excited at 488 nm, and its fluorescence was measured through a 530-nm-band-pass filter.
Membrane permeability assessment.
Membrane permeability was determined with 100 nM TO-PRO-3 when MP was measured with DiOC2(3) or 75 μM PI when MP was measured with DiBAC4(3). Both TO-PRO-3 and PI exhibit substantially increased fluorescences on binding to intracellular nucleic acids; both dyes normally bear two positive charges and are excluded from cells with intact membranes, while they stain nucleic acids in cells with damaged membranes (10). Cells killed by heat exposure (100°C for 10 min) were used as controls for TO-PRO-3 or PI staining. PI fluorescence was excited at 488 nm and was measured above 600 nm.
When the DiBAC4(3)–PI and DiOC2(3)–TO-PRO-3 combinations were compared, aliquots were taken from a single culture, stained appropriately, and analyzed on two different flow cytometers; a cytometer with a single 488-nm argon laser source was used for analysis of organisms stained with DiBAC4(3) and PI. This was done primarily to eliminate the possibility that residual DiBAC4(3) in the instrument tubing and flow cell would interfere with DiOC2(3) measurements and vice versa; it also prevented similar interference from the other dyes used.
Since the TO-PRO-3 fluorescence of a bacterial cell or clump is size dependent, we derived a normalized permeability parameter from the ratio of TO-PRO-3 fluorescence to green DiOC2(3) fluorescence.
RESULTS
The effects of the antibacterial-agents on MP and the permeability of S. aureus are summarized in Table 1.
TABLE 1.
Effects of antimicrobial agents on MP and permeability of S. aureus
| Drug | MIC (μg/ml) | Dose (μg/ml) | Drug effect
|
|
|---|---|---|---|---|
| MP | Permeability | |||
| Amoxicillin | 1.0 | 0.25, 0.5 | Little or no change | At 2 h, 33% permeabilized; at 3 to 4 h, <8% permeabilized |
| 1.0 | At 3 h, >99% depolarized | At 3 h, 69% permeabilized | ||
| 4.0 | At 3 h, >99% depolarized | At 3 h, 29% permeabilized | ||
| Chloramphenicol | NDa | 4.0b | No change after 4 h | No change after 4 h |
| Erythromycin | 4.0 | <1.0 | No change after 4 h | No change after 4 h |
| 4.0 | Depolarization by 4 h | 20% permeabilized at 4 h | ||
| Streptomycin | ND | 5.0b | Depolarization by 1 h | No change after 4 h |
| Tetracycline | 0.25 | <1.0 | No change after 4 h | No change after 4 h |
| 4.0 | Depolarization by 4 h | 50% permeabilized at 4 h | ||
ND, not determined.
The dose completely inhibited bacterial growth.
Tetracycline and erythromycin.
Tetracycline (MIC, 0.25 μg/ml) and erythromycin (MIC, 4 μg/ml) had similar effects. Both produced concentration- and time-dependent depolarizations; 4 μg of either drug per ml reduced MP to −50 mV after 4 h. Concentrations of these drugs below 1 μg/ml did not cause any permeability changes; however, after 4 h of exposure to 4 μg/ml, 50 and 20% of tetracycline- and erythromycin-treated bacteria, respectively, were stained by TO-PRO-3.
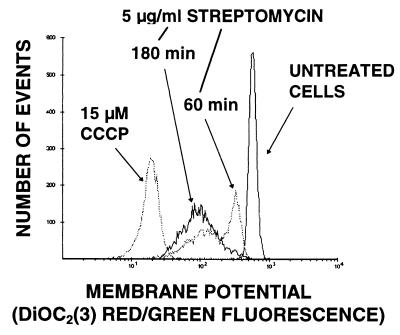
Streptomycin.
Streptomycin (5 μg/ml) produced depolarization by 1 h (the earliest time point) (Fig. 1), and MP remained reduced at 105 min and 4 h. No permeability changes were seen. The effect of streptomycin on MP was abolished if the organisms were preincubated with 1 μg of erythromycin per ml for 30 min.
FIG. 1.
Effect of 5 μg of streptomycin per ml on MP of S. aureus. Higher negative MP values appear to the right along the x axis.
Chloramphenicol.
Although 4 μg of chloramphenicol per ml completely prevented bacterial growth, no changes in either MP or permeability were observed after 4 h.
Amoxicillin.
The effects of amoxicillin (MIC, 1 μg/ml) were strongly concentration dependent. Bacterial particle counts increased for the first 2 h of exposure at 1 μg/ml and for the first 1 h of exposure at 4 μg/ml and decreased thereafter, with the decrease presumably reflecting cell lysis. At 0.25 and 0.5 μg/ml, the counts increased slowly between 0 and 2 h and increased more markedly at 3 to 4 h, as growth resumed.
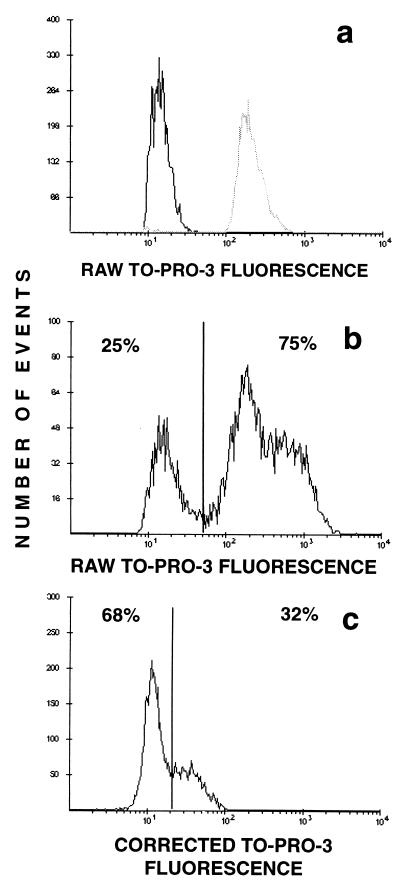
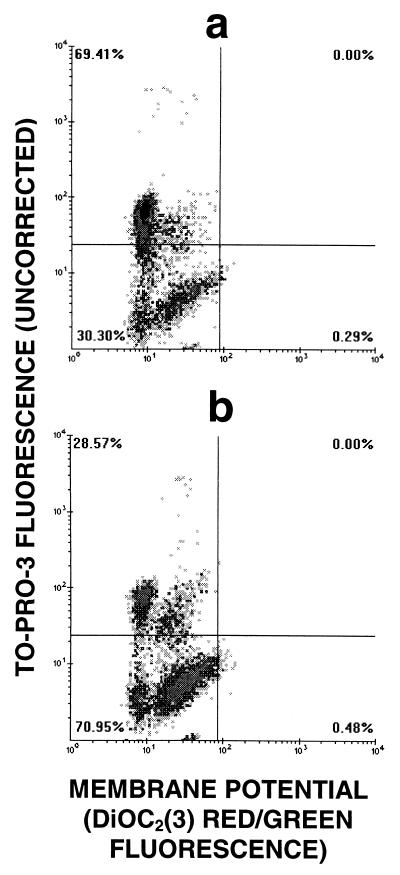
If concentrations below the MIC (0.25 and 0.5 μg/ml) were used, the bacteria drastically increased in size, as seen by microscopy and as indicated by large increases in the forward-scatter signal (data not shown). Changes in permeability could be observed 45 min after antibiotic addition; however, the variance of the TO-PRO-3 fluorescence distribution was quite large (Fig. 2), due primarily to the large variance in size resulting from drug administration. We corrected the raw TO-PRO-3 fluorescence data for size variation by calculating a quantity proportional to the logarithm of the ratio of the TO-PRO-3 fluorescence to the green DiOC2(3) fluorescence, which is known to be proportional to size; the logarithm of green fluorescence was subtracted from the logarithm of TO-PRO-3 fluorescence, and a constant, 96, was added to keep the values on scale. This correction (Fig. 2c) demonstrated that the brightly staining population in Fig. 2b was an artifact and that the majority of cells were not permeable to TO-PRO-3.
FIG. 2.
Correction of TO-PRO-3 fluorescence for variation in bacterial size. (a) Black line, S. aureus control cells; gray line, control cells permeabilized by heat treatment (10 min, 100°C). (b) S. aureus treated with 0.5 μg of amoxicillin per ml for 180 min. TO-PRO-3 fluorescence is uncorrected. (c) Same data as in panel b, but with TO-PRO-3 fluorescence corrected as described in the Results section.
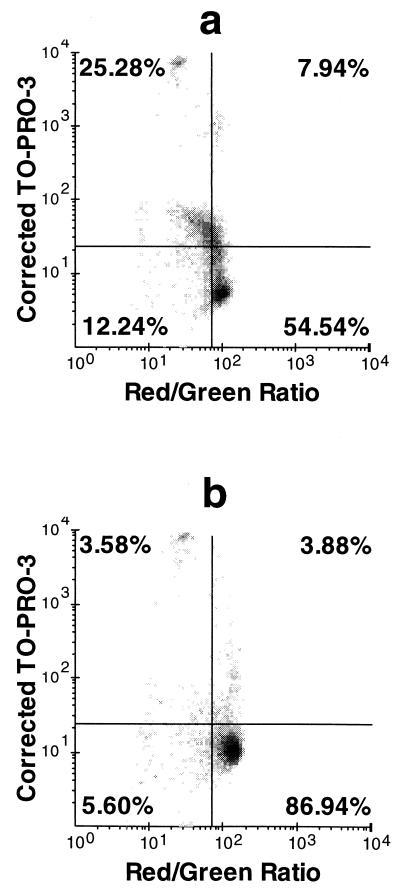
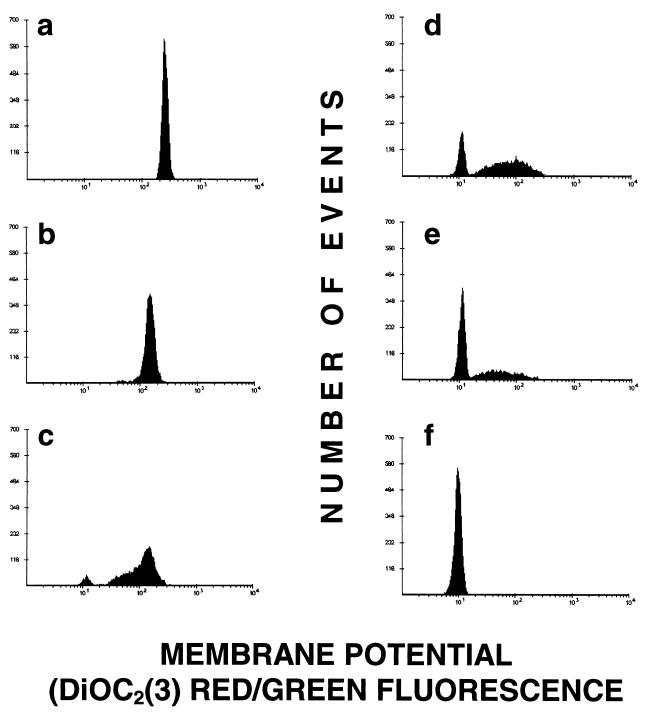
After 2 h of treatment with amoxicillin at either 0.25 or 0.5 μg/ml, a population that was permeable to TO-PRO-3 but that maintained a near-normal MP, which could be reduced to zero by addition of 15 μM CCCP, was detected (Fig. 3a). After 4 h, the majority of the cells were not permeable (Fig. 3b). Resumption of bacterial growth, as detected flow cytometrically by an increase in bacterial counts, was coincident with the decrease in the fraction of permeable cells, which occurred after 3 h in the presence of 0.25 μg of amoxicillin per ml and after 4 h in the presence of 0.5 μg of amoxicillin per ml. In contrast to what was observed with amoxicillin concentrations below the MIC, the dominant characteristic of cells treated with concentrations above the MIC was a decrease in MP (Fig. 4). In fact, bacteria exposed to 4 μg/ml consistently had a lower percentage of cells permeable to TO-PRO-3 than did those exposed to 1 μg/ml (Fig. 5). Treatment of the bacteria with 1 μg of erythromycin per ml for 30 min prior to amoxicillin exposure completely prevented any changes that would otherwise have been induced by the latter drug.
FIG. 3.
Effects of 0.25 μg of amoxicillin per ml on S. aureus after 2 h (a) and 4 h (b). Quadrants were set such that untreated controls are contained completely in the lower right quadrant. Thus, any bacteria above the horizontal line are considered permeable and any bacteria to the left of the vertical line are depolarized in comparison to the controls. TO-PRO-3 fluorescence is corrected as explained in the Results section.
FIG. 4.
Effects of 1 μg of amoxicillin per ml on the membrane potential of S. aureus at 0 min (a), 50 min (b), 75 min (c), 105 min (d), and 135 min (e) after antibiotic treatment. (f) CCCP-treated cells.
FIG. 5.
Effects of a 1 μg (a) and 4 μg (b) of amoxicillin per ml on S. aureus after 3 hs. Quadrants were set as described in the legend to Fig. 3. TO-PRO-3 fluorescence is uncorrected.
Drug effects were qualitatively very similar for S. aureus and M. luteus; we determined the MICs of the drugs that we used, and they were the same for both species. Frequency distributions of measured parameters (scatter and fluorescence) showed less variance for M. luteus than for S. aureus; this would be expected since the former organism tends to grow in tetrads, while the latter forms clumps with various numbers of cells.
Comparison of DiBAC4(3)–PI with DiOC2(3)–TO-PRO-3.
Others (6, 12) have used the oxonol dye DiBAC4(3) as an indicator of MP and the nucleic acid stain PI to demonstrate membrane permeability. We compared the combination of DiBAC4(3) and PI with that of DiOC2(3) and TO-PRO-3 to see if the two were equivalent in demonstrating the effects of amoxicillin. Membrane permeability measurements with PI and TO-PRO-3 were comparable; at any given time point, the percentages of cells permeable to one dye or the other were essentially equal (data not shown). However, there were large discrepancies between the results obtained with DiBAC4(3) and DiOC2(3) for measurement of MP. DiBAC4(3) staining was strongly influenced by cell size. In organisms exposed to concentrations of amoxicillin below the MIC, when the forward-scatter intensity had increased by at least a factor of 10, DiBAC4(3) staining also increased by the same factor, while the DiOC2(3) ratiometric measurement, which largely corrects for the effects of size variation, indicated that MP was unchanged (Fig. 3). An attempt was made to correct the DiBAC4(3) measurement for size variation by computing the log of the ratio of DiBAC4(3) fluorescence to scatter intensity; however, discrepancies between the data obtained with DiBAC4(3) and DiOC2(3) remained (data not shown).
In cells exposed to amoxicillin at concentrations above the MIC, DiOC2(3) staining revealed a fully depolarized population and a population with an MP intermediate between those observed for intact and CCCP-treated cells; after a longer period of time, the population with the intermediate MP decreased in size, apparently being replaced by a population of fully depolarized cells. This progressive loss of MP was never detectable on the basis of DiBAC4(3) staining; fluorescence distributions always showed only a single peak. Although we have previously suggested that DiBAC4(3) staining is highly dependent on membrane permeability (17), the intensity of DiBAC4(3) fluorescence did not give any clear indication that there were separate populations of permeable and impermeable cells, even in samples in which PI staining clearly showed two populations.
DISCUSSION
We hypothesized a priori that our technique should yield a predictable pattern of results depending on the mechanism of action of the antibacterial agent being tested. Since most bacteriostatic agents work by interfering with protein synthesis, we did not expect that they would cause changes in either permeability or MP in the short term following drug exposure. We further predicted that agents known to damage the cell wall and membrane would cause changes in permeability as well as MP. We expected that cells that were permeable to TO-PRO-3 would always show a concomitant depolarization, assuming that the associated membrane damage would be sufficient to abolish the ion gradients needed to maintain a normal MP. Our studies have shown that these assumptions were too simplistic and indicate that the mechanisms of action of some of the antibacterial agents examined may be more complex than commonly perceived.
Chloramphenicol was the only agent that behaved as we expected, causing neither depolarization nor increased permeability. Mortimer et al. (16) have also shown that chloramphenicol does not cause changes in permeability, even at high concentrations. Since chloramphenicol works by binding to the 50S subunit of the ribosome and inhibiting the interaction between the aminoacyl-tRNA and the ribosome (7), we can conclude that de novo protein synthesis is not needed for the maintenance of MP or permeability, at least for a 4-h period.
Streptomycin is the prototype of the aminoglycosides, a class of antibiotics for which the mechanism of action is incompletely understood (4). The major site of action of the aminoglycosides is the ribosome, where the drugs block the initiation of protein synthesis and also cause misreading in protein translation (4). However, a secondary effect of the aminoglycosides is membrane damage (4). Some investigators have reported that membrane damage depends on de novo protein synthesis (9), noting that it is prevented by pretreatment with chloramphenicol. Others claim that membrane damage is not dependent on active protein synthesis, as damage is evident within 1 min of gentamicin treatment and occurs in the presence of metabolic inhibitors (14). Irrespective of the exact mechanism of membrane damage, our studies did not show any changes in membrane permeability, as seen by TO-PRO-3 staining. Other investigators also show no (23) or very small (16) changes in permeability to nucleic acid stains, as detected by flow cytometry, induced by gentamicin in gram-negative organisms, even at concentrations up to 10 times the MIC. Since aminoglycosides are known to increase membrane permeability to a variety of small molecules including nucleotides, citrate, and amino acids (4), the lack of staining by nucleic acid dyes suggests that a different pathway of membrane damage might be involved in making cells permeable to the dyes (a greater degree or a different type of permeability is needed to permit entry of these dyes).
Streptomycin did reduce MP, as others, using DiBAC4(3) as an indicator, have reported previously (16). Streptomycin is known to cause K+ efflux and inhibit respiration (8), both of which should produce electrical depolarization. Chloramphenicol has been shown to prevent streptomycin-induced cell death (4), and chloramphenicol, tetracycline, and erythromycin all prevent streptomycin-induced membrane blebbing and damage (9, 11). In our hands, erythromycin prevented streptomycin-induced depolarization, indicating that active protein synthesis is a prerequisite for this phenomenon or that erythromycin antagonizes streptomycin binding to the cellular constituent responsible for this effect. The fact that the level of depolarization stayed constant from 105 min to 4 h is significant, as it shows that the damage is not cumulative and that the organism still maintains a partial ability to regulate its MP.
In our assay, tetracycline and erythromycin produced qualitatively similar responses. Tetracycline and erythromycin inhibit protein synthesis by binding to the ribosome on the 30S and 50S subunits, respectively (24, 25). Since studies with chloramphenicol showed that protein synthesis is not necessary for the maintenance of MP or permeability, it is possible that tetracycline and erythromycin may have more direct effects on the cell membrane. While it is known that subinhibitory concentrations of erythromycin can induce alterations in the outer membrane (2, 27), it is unlikely that this occurred in our experiments, as permeability changes were induced only by concentrations above the MIC. Although tetracycline itself acts primarily on protein synthesis, tetracyclines that act primarily on the membrane are known (20), and it is conceivable to us that tetracycline itself could perturb membranes at concentrations well above the MIC.
The increase in membrane permeability to nucleic acid dyes in cell populations exposed to β-lactam antibiotics is a well-known phenomenon (6, 19). It is also well known that concentrations of β-lactams below the MIC cause the formation of giant, multicellular structures as a consequence of incomplete bacterial fission (13). Figure 2 highlights the importance of correcting for this phenomenon when making permeability measurements. In uncorrected samples (Fig. 2b) it appears as if there are two populations, with the majority of cells being permeable to roughly the same extent as heat-treated cells. The difficulties in interpreting permeability data when there is a wide variation in size were noted by Gant et al. (6). Once size is corrected for by using the DiOC2(3) fluorescence measurement value (17), it becomes evident that the majority of cells have maintained their membrane integrity (Fig. 2c) and that those that are permeable to TO-PRO-3 are permeable to only a small degree. When correction of the raw TO-PRO-3 fluorescence measurements was attempted by using forward-scatter measurement values, the corrected distribution still exhibited a large variance (data not shown). The superiority of the DiOC2(3) correction probably depends on the fact that DiOC2(3) staining reflects cell volume, while forward scatter is more directly representative of cell cross-sectional area. We also noted a strong size dependence of the DiBAC4(3) fluorescence distribution when this dye was used to assess MP in bacteria treated with sub-MICs of β-lactams. In these cases, DiBAC4(3) staining was increased over control values by a factor of 10 (data not shown), even when the DiOC2(3) ratiometric method, which largely eliminates the effects of size variation (17), indicated minimal changes in MP.
It is usually assumed that bacterial permeability to nucleic acid dyes such as TO-PRO-3, PI, and Sytox Green is associated with the presence of substantial, irreparable breaches in the membrane, in the presence of which the organisms cannot maintain MP and are therefore nonviable (19). However, the data presented in Fig. 3 show that this is not always the case. Bacteria exposed to concentrations of amoxicillin below the MIC always demonstrated a population with permeable membranes and a normal MP, and eventual increases in bacterial counts were coincident with a reversion to a single population with impermeable membranes. Several possible bases for this observation come to mind.
The first is that events in the upper right quadrant of Fig. 3a represent clumps containing both dead TO-PRO-3-permeable cells, which do not maintain MP, and viable cells, which maintain MP and which exclude TO-PRO-3. This seems unlikely; if the clump contained both depolarized and viable cells, the red fluorescence/green fluorescence ratio should report an intermediate value. The second is that the TO-PRO-3-permeable cells are dead or dying and that the TO-PRO-3-impermeable, viable population with normal MP, represented in the lower right quadrant of Fig. 3a, expands over the course of the following 2 h until it comprises the majority of the cells, as seen in Fig. 3b. The third is that viable cells, which possess a relatively normal MP, have become transiently permeable to TO-PRO-3, due to amoxicillin treatment, but can repair the damage and can continue dividing once a permeability barrier has been restored.
While we were not able to eliminate either of the last two possibilities conclusively, the kinetics of the disappearance of the permeable population suggests that viable cells do become transiently permeabilized and later revert to an impermeable state (data not shown). It has been suggested that such a transition from a permeable to an impermeable state is responsible for the lag period before growth is observed following the addition of nutrients to starved cultures of M. luteus (28). Thus, although permeability to nucleic acid dyes often is an indicator of cell death, it may be unwise to apply this criterion in all situations.
Concentrations of amoxicillin above the MIC, in contrast to those below the MIC, produced both electrical depolarization and permeability increases (Fig. 5). Although it appears from Fig. 5b that organisms exposed to higher concentrations of amoxicillin showed less intense TO-PRO-3 fluorescence, many of the datum points shown in Fig. 5b are likely to represent lysed cells and subcellular debris. Given the apparently transient nature of permeabilization, on the one hand, and the relative difficulty of interpreting TO-PRO-3 fluorescence measurements, on the other, it seems that in amoxicillin-treated cells, loss of MP is a more reliable indicator of cell death than membrane permeability is.
Our results suggest that simultaneous assessment of changes in two or more physiological characteristics by multiparameter flow cytometry can allow distinctions between the mechanisms of actions of different classes of antibacterial agents to be made and indicate that some agents have additional mechanisms of action besides those classically attributed to them. The ratiometric technique for MP measurement that we have recently described (17) and the normalized permeability measure described here correct for cell size variations, including those resulting from the actions of antibiotics, and for cell clumping and should therefore be more precise than methods used previously by us and by others. While a broadly applicable cytometric criterion for viability remains to be found, we believe that further application of multiparameter flow cytometry can provide valuable information about the pharmacology of existing and newly developed antibiotics.
REFERENCES
- 1.Anhalt J, Washington J. Preparation and storage of antimicrobial solutions. In: Balows A, Hausler W J Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C.: American Society for Microbiology; 1991. pp. 1199–1200. [Google Scholar]
- 2.Brenciaglia M, Fornara A, Scaltrito M, Dubini F. Influence of amoxicillin, erythromycin and metronidazole on adherence of Helicobacter pylori. Microbiologica. 1995;18:283–288. [PubMed] [Google Scholar]
- 3.Davey H, Kell D. Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analysis. Microbiol Rev. 1996;60:641–696. doi: 10.1128/mr.60.4.641-696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis B. Mechanism of bactericidal action of aminoglycosides. Microbiol Rev. 1987;51:341–350. doi: 10.1128/mr.51.3.341-350.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durodie J, Coleman K, Simpson I, Loughborough S, Winstanley D. Rapid detection of antimicrobial activity using flow cytometry. Cytometry. 1995;21:374–377. doi: 10.1002/cyto.990210409. [DOI] [PubMed] [Google Scholar]
- 6.Gant V A, Warnes G, Phillips I, Savidge G F. The application of flow cytometry to the study of bacterial responses to antibiotics. J Med Microbiol. 1993;39:147–154. doi: 10.1099/00222615-39-2-147. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert D. Tetracyclines and chloramphenicol. In: Mandell G, Bennet J, Dolin R, editors. Principles and practice of infectious disease. New York, N.Y: Churchill Livingstone; 1995. pp. 306–317. [Google Scholar]
- 8.Hancock R. Aminoglycoside uptake and mode of action—with special reference to streptomycin and gentamicin. II. Effects of aminoglycosides on cells. J Antimicrob Chemother. 1981;8:429–445. doi: 10.1093/jac/8.6.429. [DOI] [PubMed] [Google Scholar]
- 9.Hancock R, Raffle V, Nicas T. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1981;19:777–785. doi: 10.1128/aac.19.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haugland R. Nucleic acid detection. In: Haugland R, editor. Handbook of fluorescent probes and research chemicals. Eugene, Oreg: Molecular Probes Inc.; 1996. pp. 144–178. [Google Scholar]
- 11.Iida K, Koike M. Cell wall alteration in gram negative bacteria by aminoglycoside antibiotics. Antimicrob Agents Chemother. 1974;5:95–97. doi: 10.1128/aac.5.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jepras R, Paul F, Pearson S, Wilkinson M. Rapid assessment of antibiotic effects on Escherichia coli by bis-(1,3-dibutylbarbituric acid) trimethine oxonol and flow cytometry. Antimicrob Agents Chemother. 1998;41:2001–2005. doi: 10.1128/aac.41.9.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorian V, Gemmel C. Effect of low antibiotic concentrations on bacteria: effects on ultrastructure, virulence, and susceptibility to immunodefenses. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 493–455. [Google Scholar]
- 14.Martin N, Beveridge T. Gentamicin interaction with Pseudomonas aeruginosa cell envelope. Antimicrob Agents Chemother. 1986;29:1079–1087. doi: 10.1128/aac.29.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason D, Power G, Talsania H, Phillips I, Gant V. Antibacterial action of ciprofloxacin. Antimicrob Agents Chemother. 1995;39:2752–2758. doi: 10.1128/aac.39.12.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortimer F, Mason D, Gant V. The use of cell impermeant fluorescent probes to assess antibiotic induced injury. Cytometry Suppl. 1998;9:127. [Google Scholar]
- 17.Novo D, Perlmutter N G, Hunt R H, Shapiro H M. Accurate flow cytometric membrane potential measurement in bacteria using diethylcarbocyanine and a ratiometric technique. Cytometry. 1999;35:55–63. doi: 10.1002/(sici)1097-0320(19990101)35:1<55::aid-cyto8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Ordonez J, Wehman N. Rapid flow cytometric antibiotic susceptibility assay for Staphylococcus aureus. Cytometry. 1993;14:811–818. doi: 10.1002/cyto.990140714. [DOI] [PubMed] [Google Scholar]
- 19.Roth B, Poot M, Yue S, Millard P J. Bacterial viability and antibiotic susceptibility testing with Sytox Green nucleic acid stain. Appl Environ Microbiol. 1997;63:2421–2431. doi: 10.1128/aem.63.6.2421-2431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnappinger D, Hillen W. Tetracyclines: antibiotic action, uptake and resistance mechanisms. Arch Microbiol. 1996;165:359–369. doi: 10.1007/s002030050339. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro H M. Practical flow cytometry. 3rd ed. New York, N.Y: Wiley-Liss; 1995. [Google Scholar]
- 22.Shapiro H M. Practical flow cytometry. 2nd ed. New York, N.Y: Wiley-Liss; 1988. [Google Scholar]
- 23.Siegel S, Evans M, Pollack M, Leone A, Kinney C, Tam S, Daddona P. Antibiotics enhance binding by human lipid A-reactive monoclonal antibody HA-1A to smooth gram-negative bacteria. Infect Immun. 1993;61:512–519. doi: 10.1128/iai.61.2.512-519.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Standiford H. Tetracyclines and chloramphenicol. In: Mandell G, Bennet J, Dolin R, editors. Principles and practice of infectious disease. New York, N.Y: Churchill Livingstone; 1995. pp. 306–317. [Google Scholar]
- 25.Steigbigel N. Macrolides and clindamycin. In: Mandell G, Bennet J, Dolin R, editors. Principles and practice of infectious disease. New York, N.Y: Churchill Livingstone; 1995. pp. 334–346. [Google Scholar]
- 26.Suller M, Stark J, Lloyd D. A flow cytometric study of antibiotic induced damage and evaluation as a rapid susceptibility test for methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1997;40:77–83. doi: 10.1093/jac/40.1.77. [DOI] [PubMed] [Google Scholar]
- 27.Tateda K, Ishii Y, Hirakata Y, Matsumoto T, Ohno A, Yamaguchi K. Profiles of outer membrane proteins and lipopolysaccharide of Pseudomonas aeruginosa grown in the presence of sub-MICs of macrolide antibiotics and their relation to enhanced serum sensitivity. J Antimicrob Chemother. 1994;34:931–942. doi: 10.1093/jac/34.6.931. [DOI] [PubMed] [Google Scholar]
- 28.Tatyana V, Kaprelyants A, Kell D. Influence of viable cells on the resuscitation of dormant cells in Micrococcus luteus held in an extended stationary phase: the population effect. Appl Environ Microbiol. 1994;60:3284–3291. doi: 10.1128/aem.60.9.3284-3291.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walberg M, Gaustad P, Steen H. Rapid flow cytometric assessment of mecillinam and ampicillin bacteria susceptibility. J Antimicrobial Chemother. 1996;37:1063–1075. doi: 10.1093/jac/37.6.1063. [DOI] [PubMed] [Google Scholar]
- 30.Yeaman M, Bayer A, Koo S, Foss W, Sullam P. Platelet microbicidal proteins and neutrophil defensins disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J Clin Invest. 1998;101:178–187. doi: 10.1172/JCI562. [DOI] [PMC free article] [PubMed] [Google Scholar]