Abstract
Background
Studies show that 25 (OH) D status appears to have beneficial influence on the incidence and severity of some types of infections. However, studies with vitamin D supplementation on young children produced conflicting results. This study was conducted to assess and compare the pooled prevalence of vitamin D deficiency among healthy and sick children in sub-Saharan Africa.
Method
A systematic review of PubMed, CINAHL, Web of science, global health and Google scholar electronic databases was conducted. Both published and unpublished observational studies conducted among under-five children in the year 2010–2020 were included. STATA Version 14 was used for analysis. Heterogeneity of studies was assessed using I2 test. A random-effects model was used to estimate the pooled prevalence among both healthy and sick children.
Result
A total of 1212 articles were retrieved from data bases, of which 10 papers were included. The pooled prevalence of vitamin D deficiency among healthy children was 50.06% with mean serum vitamin D level of 41.06 nm/L. The pooled prevalence among the sick children was 39.36% with 66.96 nm/L of mean concentration. The pooled prevalence among healthy children was significantly higher compared to those who have common medical illnesses and the pooled mean concentration among the sick was also much higher than the mean concentration among healthy children.
Conclusion
The pooled prevalence among both groups of population was significantly high and a concerning public health problem. The prevalence among healthy children was much higher as compared to sick children.
Keywords: Vitamin D, Under five, Healthy, Sick, Sub-saharan
Highlights
-
•
Vitamin D status appears to have some beneficial influence on the incidence and severity of some types of infections.
-
•
This study assessed and compared the pooled prevalence of vitamin D deficiency among healthy and sick children in sub-Saharan Africa.
-
•
The pooled prevalence among the healthy children was found to be higher than the prevalence among the sick children.
-
•
The mean serum vitamin D level among the sick children was higher than among the healthy children.
-
•
The pooled prevalence among both group of population was significantly of public health concern.
1. Introduction
Micronutrients deficiencies in children can have multi-dimensional health impact on growth, immune system, cognitive development and physical growth and development [1]. Its deficiencies are also widely found in low income countries where micronutrient deficiencies account for approximately 7% of the global disease burden [2].
Globally, an estimated two billion people suffer from a chronic deficiency of essential vitamins and minerals (micronutrients), a condition known as hidden hunger [2] with Vitamin D deficiency being among the common micronutrient deficiencies worldwide [3]. According to the recent estimates, greater than 50% population is at risk in the globe [4].
There are two different precursor molecules of vitamin D; Cholecalciferol(vitamin D3) and ergocalciferol(vitamin D2). Both are biologically inactive and must be converted to the metabolically active form 1, 25-dihydroxyvitamin D. In this process vitamin D is first hydroxylated to 25-hydroxyvitamin D in hepatocytes, then a second hydroxylation in the kidney converts 25-hydroxyvitamin D to the active form, 1α,25-dihydroxyvitamin D (calcitriol) [5].
Vitamin D is a fat-soluble vitamin, and it can be stored in adipose tissue for use when necessary. This ability allows vitamin D to extend its total half-life in the body for approximately 2 months [6].
As general consensus, serum 25-hydroxyvitamin D (25(OH)D) level below 50 nmol/L considered as a cutoff point for vitamin D deficiency and if its serum level is less than 25 nmol/L, severe deficiency is diagnosed [3]. Vitamin D deficiency is associated with infection and increased autoimmunity [7]. Different studies among under five children shows that decreased level of 25(OH)D were more prevalent among under five children with respiratory tract infections (RTIs) and its deficiency was associated with increased risk of RTIs [[8], [9], [10]]. Furthermore, studies also shows that, normal to high serum 25(OH)D status appears to have some beneficial influence on the incidence and severity of some, though not all, types of infections [[10], [11], [12]].
However, studies with vitamin D supplementation on young children produced conflicting results, and it still remains unclear whether supplementation may be of benefit and at what doses [8].
An observational study conducted to assess the serum concentration of 25(OH)D among children with active tuberculosis (TB) using non-TB pneumonia and healthy controls revealed that larger number of vitamin D deficient children were from active TB group [9]. Similarly, many studies conducted elsewhere [[10], [11], [12], [13], [14]] delineated similar findings including Dabla's study conducted among pediatric osteoarticular tuberculosis patients which revealed that 56% of active TB cases were vitamin D deficient and had lower levels of serum 25(OH)D compared to healthy controls [11]. However, significant number of studies found no significant difference in vitamin D deficiency status TB patients and healthy children [[15], [16], [17]].
Here, it's important to note the important role of active form of vitamin D(1,25-D) in granuloma formation.[18]Moreover, the effects of anti-TB drugs on vitamin D level are diverse. For one, studies have shown that rifampin treatment selectively induced CYP3A4, which catalyzes 25OHD3 metabolism into its inactive form 4β,25(OH)2D3. [19] Isoniazid also affects the 25-hydroxylation leading to impaired Vit D action.[20] The hepatotoxic effects of rifampicin, pyrazinamide and isoniazid [21] together with the nephrotoxic effects of isoniazid, ethambutol and rifampicin[22] might contribute to the lower levels of vitamin D seen in these patients.
For growing children, deficiency of Vitamin D is among the issues of significant concern while there are limited information on its association with malnutrition[23,24]. Studies shows that, linear growth could be affected by vitamin D status of children even in the absence of rickets clinical signs [25]. From the evidences currently available in literature world, a strong evidence base of the contributions of vitamin D deficiency for malnutrition, both regionally and globally, has been lacking. Even though the role of macronutrients in physical growth is well known, the roles of micronutrients including vitamin D in physical growth is not well established. Particularly, evidences with respect to the role of vitamin D on stunting among children are not conclusive, and sometimes conflicting [[23], [24], [25], [26], [27], [28]].
This study was conducted to assess and compare the pooled prevalence of vitamin D deficiency among healthy and sick children in sub-Saharan Africa. Hence, these review was done to avail better data on the relation between common medical illnesses and micronutrient deficiency particularly vitamin D, to guide intervention programs and to prioritize global focus on addressing vitamin D deficiency.
2. Materials and methods
2.1. Reporting and registration
This review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) 2020 statement: an updated guideline for reporting systematic reviews (Additional file-1) [29]. Quality of this systematic review was assessed using AMSTAR 2 criteria and it was found to be a high quality review [30]. The study was registered on
www.researchregistry.org with a unique identification number of research registry1266.
2.2. Study sources and search strategy
A systematic review and meta-analysis study was conducted to determine and compare the pooled prevalence of vitamin D deficiency among apparently healthy and sick under five children in sub-Saharan Africa. The major databases of PubMed, Cochrane library, CINAHL and Google scholar were used for systematic search of literatures with Boolean operators like “AND”, “NOT” and “OR”. Using the Boolean operator we searched as: ((vitamin d"[MeSH Terms] OR "ergocalciferols"[MeSH Terms] OR vitamin D [Text Word])) AND ("child"[MeSH Terms] OR CHILDREN[Text Word])) AND "Africa South of the Sahara"[Mesh])). A search output from different data bases were shown below (Table 1).
Table 1.
Summary of search results for the PubMed, Google Scholar and other databases to assess the prevalence of vitamin D deficiency among under five children in Sub-Saharan Africa.
| Databases | Searching terms | Number of studies |
|---|---|---|
| Google scholar | All in title: vitamin D OR ergocalciferols AND Children | 852 |
| PubMed | Search ((("vitamin d"[MeSH Terms] OR "ergocalciferols"[MeSH Terms] OR vitamin D[Text Word])) AND ("child"[MeSH Terms] OR CHILDREN[Text Word])) AND "Africa South of the Sahara"[Mesh] | 331 |
| Web of science, CINAHL and global health | “prevalence” or “magnitude” and “Vitamin D” and “Deficiency” or “level” and “children” or “Child” or “ergocalciferols” and “sub-Saharan Africa” | 29 |
| Total retrieved articles | 1212 | |
| Full text available papers appropriate to our review | 10 |
2.3. Selection of study and eligibility criteria
All studies that were conducted on vitamin D deficiency among under five children and those fulfilled the inclusion criteria according to the interest of the study were included. All types of article types published in English were included. Initially, availability of full text titles, and abstracts of the articles were assessed. Then the full papers of relevant articles were reviewed. We excluded articles with inaccessible full paper.
2.4. Inclusion criteria
Study area: studies conducted in Sub-Saharan Africa only.
2.5. Study design: observational studies (cross-sectional, case-control and cohort studies)
Outcome measures: Studies that measured 25(OH)D in blood.
Language: studies published/written in the English language only were included.
Population: studies conducted among under five children.
Publication issue: both published and unpublished articles were searched.
Study period: 2010–2020
2.6. Data extraction
Data extraction for all the necessary data was done by RH and MF independently using a standardized data extraction format prepared in Microsoft Excel. Any disagreements between the authors during data extractions was discussed and reached on consensus. The data extraction format includes first author, publication year, name of country, sample size, included and number of event and prevalence with 95% CI. The main outcome of interest for this study is prevalence of vitamin D deficiency among healthy and sick children in sub-Saharan Africa. The main types of illnesses used in this study were TB, malnutrition and HIV/AIDS.
2.7. Risk of bias
Two authors (RH and MF) independently assessed the risk of bias for each article using the Newcastle-Ottawa Scale quality assessment tool [31]. Using an assessment tool as a guideline, two authors independently evaluated the qualities of the original articles. Quality of each study was evaluated using these parameters; those with medium (fulfilling 50% of quality assessment criteria) and high quality (≥ 6 out of 10 scales) were included for analysis. Disagreements between assessors were solved by taking the mean score of their assessment results.
2.8. Data processing and analysis
After extraction, the data were imported to STATA Version 14.0 statistical software for analysis. The standard error for each original study was calculated using the binomial distribution formula. Heterogeneity among reported prevalence was assessed by computing p-value I2 test static [32]. As the test statistic showed there is significant heterogeneity among the studies (I2 = 98.3%, p = 0.001) and as a result a random effect meta-analysis model was used to estimate the pooled effect. To reduce the random effect variations between the point estimates of the primary studies, a subgroup analysis was done based on sample size. Funnel plot and Egger's test at 5% significance level was used to assess the publication bias. From the result, the articles were symmetrically distributed and Egger's test at 5% significant level was not significant for the publication bias. Pooled prevalence with 95% confidence intervals was presented in the forest plot format for both healthy and sick children.
3. Results
3.1. General characteristics of studies
In the first step screening, we got a total number of 1112 papers and 478 papers were excluded due to repetition. According to the titles and abstract 621 papers were excluded. Thirteen papers were found to be suitable. After assessing the full text 3 papers were excluded due to failure to report outcome of interest/exposure. Ten papers were finally included in the study (Fig. 1) [[33], [34], [35], [36], [37], [38], [39], [40], [41], [42]]. After identification of the available papers, the papers were categorized in to two (healthy and sick) based on the type of population used to study. If the individual study used both types of population we used the included population of each population to do the pooled measurement for the current study.
Fig. 1.
Diagrammatic flow of data extraction process for systematic review and meta-analysis of the prevalence of vitamin D deficiency among children in sub-Saharan Africa.
3.2. Prevalence of vitamin D deficiency among healthy children
The finding from the seven studies that included 2736 participants resulted in pooled prevalence of vitamin D deficiency among healthy children to be50.06% (CI ranges from 33.46% to 66.67%) (Fig. 2) [[34], [35], [36], [37], [38], [39], [40]]. The lowest prevalence was 17% from Botswana and the highest prevalence was 90% from Ethiopia.
Fig. 2.
Pooled prevalence of vitamin D deficiency among healthy children in sub-Saharan Africa.
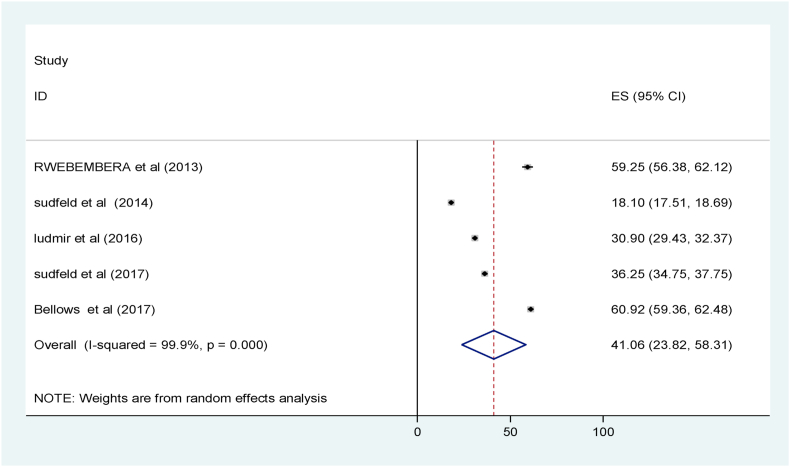
The pooled mean serum vitamin D level among the healthy children was 41.06 nm/L with CI of 23.82 nm/L to 58.31 nm/L (Fig. 4) [[35], [36], [37], [38], [39]].
Fig. 4.
Mean serum vitamin D level among healthy children in sub-Saharan Africa, 2020.
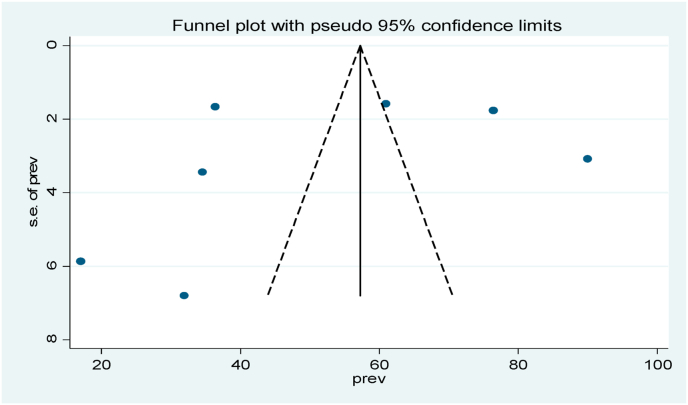
Publication bias using funnel plot for prevalence of VDD and concentration of vitamin D level among healthy children was assessed and it shows a relatively symmetrical shape.
Funnel plot and Egger's test were used to test Publication bias. From the result the articles were symmetrically distributed for prevalence (Fig. 3) and (Fig. 5) and Egger's test result for small study effects revealed that publication bias in estimating vitamin D deficiency and its concentration was not statistically significant.
Fig. 3.
Funnel plot with 95% confidence interval of the pooled prevalence of vitamin D deficiency among healthy under five children in sub-Saharan Africa, 2020.
Fig. 5.
Publication bias for mean concentration of serum vitamin D among health children in sub-Saharan Africa.
3.3. Pooled prevalence of vitamin D deficiency among sick children in sub-Saharan Africa
Analysis was done to assess the pooled prevalence of vitamin D deficiency among sick children (children having certain medical conditions). The analysis of 5 studies including 684 children (253 having HIV, 39 TB, 251 malnutrition, 47 diarrhea and 94 other) revealed that, the pooled prevalence of vitamin D deficiency among the sick children was 39.36% with CI of 20.57–57.96% (Fig. 6) [[33], [34], [35], 38 and 41]].
Fig. 6.
Pooled prevalence of vitamin D deficiency among sick children in sub-Saharan Africa.
The lowest prevalence is 21% from Botswana and the highest was 70.2% from Tanzania.
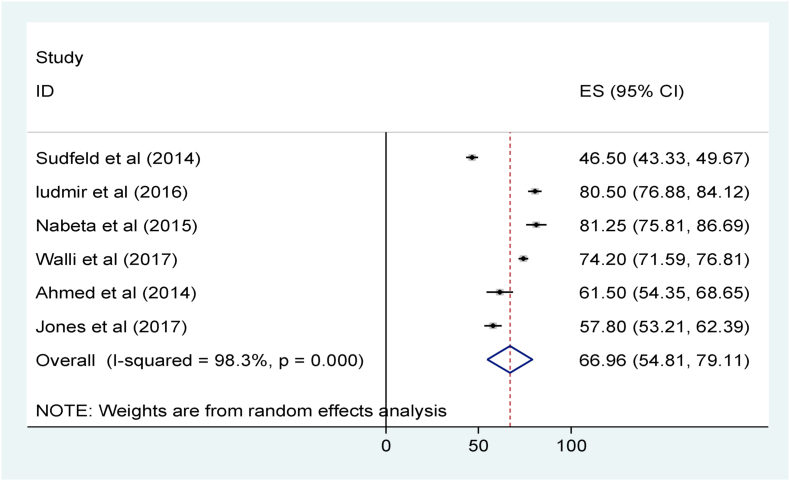
The pooled mean serum vitamin D level among the sick children was 66.96 nm/L with 95% CI of 54.81 nm/L to 79.11 nm/L (Fig. 8) [33, 35, [40], [41], [42]]. Publication bias was assessed using funnel plot and the diagram shows that, there is symmetrical distribution of articles showing no significant publication bias for prevalence of VDD (Fig. 7) and mean serum concentration of vitamin D (Fig. 9).
Fig. 8.
Mean serum vitamin D level among sick children in sub-Saharan Africa.
Fig. 7.
Funnel plot with 95% confidence interval for publication bias of prevalence of vitamin D deficiency among sick under five children in sub-Saharan Africa, 2020.
Fig. 9.
Publication bias for mean concentration of serum vitamin D among sick children in sub-Saharan Africa, August 2020.
4. Discussion
This study has tried to assess the pooled prevalence of vitamin D deficiency among under-five children in sub-Saharan Africa. Attempts were made to see the pooled prevalence among the healthy and sick children separately. The common illness conditions considered in this review were mainly malnutrition, HIV/AIDS and tuberculosis.
From the result of analysis, the pooled prevalence among healthy children in sub-Saharan Africa was found to be 50.06% with 95% CI ranging from 33.46% to 66.67%. On the other hand the pooled prevalence among sick children was 39.36% with CI of 20.57–57.96%. Among the studies included for the sick children the lowest prevalence (14.6%) of vitamin D deficiency was found in Uganda among malnourished children [34] and the highest prevalence (70.2%) was from Tanzania among patients with diarrhea [35]. Regarding the healthy children the lowest prevalence (17%) was among children from Botswana [36] and the highest prevalence of 90% was from Ethiopia [40]. In this study the pooled prevalence of vitamin D deficiency among both healthy and sick children was found to be significantly of public health concern.
Regarding the comparison between the pooled prevalence among the sick and healthy children, the pooled prevalence among healthy children was found to be significantly higher. Correspondingly, the mean serum vitamin D level among healthy children (41.06 nm/L) was found to be significantly lower than the level among sick children (66.96 nm/L). The finding of the current study was in contrary to the presumed expectations and findings of other individual studies conducted elsewhere which shows vitamin D deficiency more prevalent among people with some type illnesses and health conditions [43,44,45]. In the current study the lesser prevalence of vitamin D among sick children than healthy children might be due to the fact that, most of the studies included for the sick children were conducted among malnourished children and tuberculosis children. Malnourished children were usually fed malnutrition treatment formula which have high vitamin D [46] and this might mask the usual vitamin deficiency among children. Similarly, as tuberculosis is a granulomatous disease and in granulomatous diseases, activated mononuclear cells (particularly macrophages) in the lung and lymph nodes produce calcitriol from calcidiol independent of PTH [[47], [48], [49], [50]]. This may increase the serum level of vitamin D among children with tuberculosis. Furthermore, some studies among HIV patients show that ART treatment drugs particularly, use of ritonavir or tenofovir, was associated with higher levels of 25OHD [51] and this might also be contributed for higher vitamin D among the sick groups.
From another systematic review and meta-analysis done to assess vitamin D deficiency in critically ill children in the other study, the pooled VDD event rate was 54.8 (95% CI 45.4–63.9) and another study done in developing countries comparable to the current study also showed a higher pooled VDD rate of 64% with 95% CI 51–75%) [39] which is much higher than the finding of the current study. This might be due to the nature of the participants of the studies, where the participants of the previous studies were critically ill children in ICU while those in the current studies were not ICU patients.
Finding of systematic review and meta-analysis conducted to assess vitamin D deficiency in Africa among the healthy children found that, the pooled prevalence was 49.07 with 95% CI of (24·88–73.49) among newborns and 22.99 (12.03–36.14) among older children [52]. The pooled prevalence from this study is much lower than the finding from the current study. This might be due to the fact that, most of the studies included in the earlier meta-analysis were relatively from well-off countries and the studies also used random effect meta-analysis to report the pooled prevalence.
This study has attempted to put the burden of vitamin D deficiency among under-five children in sub-Saharan Africa using different segment of population based on health status of children. It has tried to delineate the difference in the burden of the problem among healthy and sick children. However, there are certain important limitations to be considered while using the findings of this study. One of the limitation of this study was that, all most all of the studies included in this review considered different age groups of children and this resulted in difficulty of conducting subgroup analysis using the age group and other important factors.
A preprint of this manuscript has previously been published [53].
4.1. Limitation
The main limitation of this study was only English articles or reports were considered to conduct this continent based systemic-review. In addition, this meta-analysis represented only studies reported from few countries, which may reflect under-representation due to the limited number of studies included.
4.2. Implication
The findings of this study were indicative that vitamin D deficiency in children was a public health in both apparently healthy and sick childrens. Future researches better focus on prospective studies in different segments of children populations to generate a strong evidence. Routine vitamin D status assessment by public health practitioners and clinicians are highly crucial.
5. Conclusion
In general, the pooled prevalence of vitamin D deficiency among under-five children in this study was found to be among the higher public health concern and there is significant variation in the prevalence from country to country. The pooled prevalence among the healthy children was found to be higher than the prevalence among the sick children. Correspondingly, the mean serum vitamin D level among the sick children was higher than among the healthy children.
Disclosure
Preprint of this manuscript can be found at https://www.preprints.org/manuscript/202012.0187/v1.
Funding
This research received no external funding.
Availability of data and materials
Data underlying the study is readily available can be obtained from the corresponding author on reasonable request.
Code availability
No code available.
Ethics approval
The procedure used in this study respects the scientific ethical rules.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Authors’ contributions
All authors made a significant contribution to the work reported, that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Declaration of competing interest
The authors have declared that no competing interests exist.
Acknowledgement
We acknowledge the authors and participants of the included original studies in this systematic review and Meta-analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.103403.
Contributor Information
Mohammed Feyisso Shaka, Email: mamfys8@gmail.com.
Robel Hussen kabthymer, Email: robelk@du.edu.et.
Meiraf Daniel Meshesha, Email: meirafdanielm@gmail.com.
Moges Tadesse Borde, Email: moges125@yahoo.com.
Abbreviation
- CINAHL
Cumulated Index in Nursing and Allied Health Literature
- HIV/AIDS
Human Immunovirus/Acquired Immunodeficiency syndrome
- ICU
Intensive care unit
- MeSH
Medical Subject Heading
- Nmol/L
Nanomole per liter
- TB
Tuberculosis
- VDD
Vitamin D deficiency
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Viteri F.E., Gonzalez H. Adverse outcomes of poor micronutrient in childhood and adolescence. Nutr. Rev. 2002;60:S77–S83. doi: 10.1301/00296640260130795n.d. [DOI] [PubMed] [Google Scholar]
- 2.Sight and life Executive report, the global hidden hunger indices and maps: an advocacy tool for action. Vitam. Motion. 2017:1–4. [Google Scholar]
- 3.Bosomworth N.J. Mitigating epidemic vitamin D deficiency: the agony of evidence. Can. Fam. Physician. 2011;57:16–20. n.d. [PMC free article] [PubMed] [Google Scholar]
- 4.Norman A.W., Bouillon R. Vitamin D nutritional policy needs a vision for the future. Exp. Biol. Med. 2010 Sep;235(9) doi: 10.1258/ebm.2010.010014. 1034-45 n.d. [DOI] [PubMed] [Google Scholar]
- 5.Bikle D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014;21(3):319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acar S., Özkan B. In: Vitamin D [Internet] Özdemir Ö., editor. IntechOpen; London: 2021. Vitamin D metabolism. [Google Scholar]
- 7.Omar N., Mosaad Y. Vitamin D and immune system. Vitam. Miner. 2017;6:151. n.d) [Google Scholar]
- 8.Zisi D., Challa A., Makis A. The association between vitamin D status and infectious diseases of the respiratory system in infancy and childhood. Hormones (Basel) 2019;18:353–363. doi: 10.1007/s42000-019-00155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buonsenso D., et al. Vitamin D levels in active TB, latent TB, non-TB pneumonia and healthy children: a prospective observational study. Fetal Pediatr. Pathol. 2018:1–11. doi: 10.1080/15513815.2018.1509407. n.d) [DOI] [PubMed] [Google Scholar]
- 10.Agarwal A., et al. Vitamin D status in pediatric osteoarticular tuberculosis. J. Clin. Orthop. Trauma. 2015;6(4):227–229. doi: 10.1016/j.jcot.2015.05.001. n.d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dabla P.K., et al. Vitamin D deficiency among pediatric osteoarticular tuberculosis patients. J. Clin. Orthop. Trauma. 2016;7(Suppl 2):147–149. doi: 10.1016/j.jcot.2016.10.006. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams B., Williams A.J., Anderson S.T. Vitamin D deficiency and insufficiency in children with tuberculosis. Pediatr. Infect. Dis. J. 2008;27(10):941–942. doi: 10.1097/INF.0b013e31817525df. n.d. [DOI] [PubMed] [Google Scholar]
- 13.Gray K., et al. Vitamin d and tuberculosis status in refugee children. Pediatr. Infect. Dis. J. 2012;31(5):521–523. doi: 10.1097/INF.0b013e3182456c55. n.d. [DOI] [PubMed] [Google Scholar]
- 14.Venturini E., et al. Vitamin D and tuberculosis: a multicenter study in children. BMC Infect. Dis. 2014;14:652. doi: 10.1186/s12879-014-0652-7. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO W.H.O. 2013. Global Tuberculosis Report 2013.https://apps.who.int/iris/handle/10665/91355 n.d. [Google Scholar]
- 16.Ludmir J., et al. Vitamin D status in Botswana children under 2 years old with and without active tuberculosis. Am. J. Trop. Med. Hyg. 2016;94(5):971–974. doi: 10.4269/ajtmh.15-0864. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jubulis J., et al. Modifiable risk factors associated with tuberculosis disease in children in Pune. India Int. J. Tuberc. Lung. Dis. 2014;18(2):198–204. doi: 10.5588/ijtld.13.0314. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skodrić-Trifunović V., Vucinić-Mihailović V. [The role of vitamin D in the formation of granuloma and in calcium metabolism] Med. Pregl. 2005;58(Suppl 1):17–20. Serbian. PMID: 16526260. [PubMed] [Google Scholar]
- 19.Wang Z., Lin Y.S., Zheng X.E., Senn T., Hashizume T., Scian M., et al. An inducible cytochrome P4503A4-dependent vitamin D catabolic pathway. Mol. Pharmacol. 2012;81(4):498–509. doi: 10.1124/mol.111.076356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ralph A.P., Lucas R.M., Norval M. Vitamin D and solar ultraviolet radiation in the risk and treatment of tuberculosis. Lancet Infect. Dis. 2013;13(1):77–88. doi: 10.1016/S1473-3099(12)70275-X. [DOI] [PubMed] [Google Scholar]
- 21.Hassen Ali A., Belachew T., Yami A., Ayen W.Y. Anti-tuberculosis drug induced hepatotoxicity among TB/HIV co-infected patients at Jimma University Hospital, Ethiopia: nested case-control study. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0064622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang C.H., Chen Y.F., Wu V.C., Shu C.C., Lee C.H., Wang J.Y., et al. Acute kidney injury due to anti-tuberculosis drugs: a five-year experience in an aging population. BMC Infect. Dis. 2014;14:23. doi: 10.1186/1471-2334-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachrach L.K. Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol. Metabol. 2001;12:22–28. doi: 10.1016/s1043-2760(00)00336-2. [DOI] [PubMed] [Google Scholar]
- 24.Rauch F. Bone accrual in children: adding substance to surfaces. Pediatrics. 2007;119(2):S137–S140. doi: 10.1542/peds.2006-2023E. [DOI] [PubMed] [Google Scholar]
- 25.Mokhtar R.R., Holick M.F., Sempértegui F., Griffiths J.K., Estrella B., Moore L.L., et al. Vitamin D status is associated with underweight and stunting in children aged 6-36 months residing in the Ecuadorian Andes. Publ. Health Nutr. 2018;21 doi: 10.1017/S1368980017002816. 1974–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.umar G.T., Sachdev H.S., Het al Chellani. Effect of weekly vitamin D supplements on mortality, morbidity, and growth of low birthweight term infants in India up to age 6 months: randomised controlled trial. BMJ. 2011;342:d2975. doi: 10.1136/bmj.d2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert-Diamond D., Baylin A., Mora-Plazas M., Marin C., Arsenault J.E., Hughes M.D., et al. Vitamin D deficiency and anthropometric indicators of adiposity in school-age children: a prospective study. Am. J. Clin. Nutr. 2010;92:1446–1451. doi: 10.3945/ajcn.2010.29746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson S.L., Ramirez-Zea M., Roman AVet al. Cor-relates and family aggregation of vitamin D concentrations in school-aged children and their parents in nine Mesoamerican countries. Publ. Health Nutr. 2017;20:2754–2765. doi: 10.1017/S1368980017001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 30.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., Moher D., Tugwell P., Welch V., Kristjansson E., Henry D.A. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wells G., Shea B., OConnell D. Ottawa Hospital Research Institute; Ottawa, Canada: 2009. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [Google Scholar]
- 32.Rücker G., Schwarzer G., Carpenter J.R., Schumacher M. Undue reliance on I2 in assessing heterogeneity may mislead. BMC Med. Res. Methodol. 2008;8:79. doi: 10.1186/1471-2288-8-79. PMID: 19036172 n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nabeta H.W., Kasolo J., Kiggundu R.K., Kiragga A.N., Kiguli S. Serum vitamin D status in children with protein - energy malnutrition admitted to a national referral hospital in Uganda. BMC Res. Notes. 2015;8:1–8. doi: 10.1186/s13104-015-1395-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassam I., Kisenge R., Aboud S., Manji K. Association of vitamin D and diarrhoea in children aged less than five years at Muhimbili national hospital , Dar es Salaam : an unmatched case control study. BMC Pediatr. 2019;19:1–9. doi: 10.1186/s12887-019-1614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ludmir J., Mazhani L., Cary M.S., Chakalisa U.A., Pettifor J.M., Molefi M., et al. Vitamin D status in Botswana children under 2 Years old with and without active tuberculosis. Am. J. Trop. Med. Hyg. 2016;94:971–974. doi: 10.4269/ajtmh.15-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sudfeld C.R., Manji K.P., Smith E.R., Aboud S., Kisenge R., Fawzi W.W., et al. Vitamin D deficiency is not associated with growth. JPGN. 2017;65:467–474. doi: 10.1097/MPG.0000000000001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rwebembera Anath, Sudfeld Christopher R., Manji Karim P., et al. Prevalence and risk factors for vitamin D deficiency among Tanzanian HIV-exposed uninfected infants. J. Trop. Pediatr. 2013;59(5):426–429. doi: 10.1093/tropej/fmt028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christopher R Sudfeld, Christopher Duggan, Said Aboud et al. Vitamin D Status Is Associated with Mortality, Morbidity, and Growth Failure Among a Prospective Cohort of HIV-. [DOI] [PMC free article] [PubMed]
- 39.Bellows Alexandra L., Smith Emily R. Alfa Muhihi et al. Micronutrient Deficiencies among Breastfeeding Infants in Tanzania. Nutrients. 2017;9:1258. doi: 10.3390/nu9111258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bodin Johanna, Mihret Adane, Holm-Hansen Carol, et al. Vitamin D deficiency is associated with increased use of antimicrobials among preschool girls in Ethiopia. Nutrients. 2019;11:575. doi: 10.3390/nu11030575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walli Nahida Z., Munubhi Emmanuel K., Aboud Said, et al. Vitamin D levels in malnourished children under 5 Years in a tertiary care center at muhimbili national hospital, dar es salaam, Tanzania—a cross-sectional study. J. Trop. Pediatr. 2017;63:203–209. doi: 10.1093/tropej/fmw081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed Patience, Babaniyi I.B., Yusuf K.K., et al. Vitamin D status and hospitalisation for childhood acute lower respiratory tract infections in Nigeria. Paediatr. Int. Child Health. 2014;35(2):151–156. doi: 10.1179/2046905514Y.0000000148. [DOI] [PubMed] [Google Scholar]
- 43.Naik A.L., Rajan M.G., Manjrekar P.A., Shenoy M.T., Shreelata S., Srikantiah R.M., et al. Effect of DOTS treatment on vitamin D levels in pulmonary tuberculosis. J. Clin. Diagn. Res. 2017;11 doi: 10.7860/JCDR/2017/24501.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prentice A., Schoenmakers I., Jones K.S., Jarjou L.M.A., Goldberg G.R. Vitamin D deficiency and its health consequences in Africa. Clin. Rev. Bone Miner. Metabol. 2009;7:94–106. doi: 10.1007/s12018-009-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.WHO . 2009. Guidelines for an Integrated Approach to the Nutritional Care of HIV-Infected Children (6 Months-14 Years) [PubMed] [Google Scholar]
- 46.Insogna K.L., Dreyer B.E., Mitnick M., et al. Enhanced production rate of 1,25-dihydroxyvitamin D in sarcoidosis. J. Clin. Endocrinol. Metab. 1988;66:72. doi: 10.1210/jcem-66-1-72. n.d) [DOI] [PubMed] [Google Scholar]
- 47.Mason R.S., Frankel T., Chan Y.L., et al. Vitamin D conversion by sarcoid lymph node homogenate. Ann. Intern. Med. 1984;100:59. doi: 10.7326/0003-4819-100-1-59. n.d) [DOI] [PubMed] [Google Scholar]
- 48.Adams J.S., Singer F.R., Gacad M.A., et al. Isolation and structural identification of 1,25-dihydroxyvitamin D3 produced by cultured alveolar macrophages in sarcoidosis. J. Clin. Endocrinol. Metab. 1985;60:960. doi: 10.1210/jcem-60-5-960. n.d. [DOI] [PubMed] [Google Scholar]
- 49.Adams J.S., Sharma O.P., Gacad M.A., Singer F.R. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J. Clin. Invest. 1983;72:1856. doi: 10.1172/JCI111147. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin M., Stein E. The effect of antiretrovirals on vitamin D. Clin. Infect. Dis. 2011;52:406–408. doi: 10.1093/cid/ciq169. [DOI] [PubMed] [Google Scholar]
- 51.McNally J.D., Nama N., O'Hearn K., Sampson M., Amrein K., Iliriani K., et al. Vitamin D deficiency in critically ill children: a systematic review and meta-analysis. Crit. Care. 2017;21:1–13. doi: 10.1186/s13054-017-1875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mogire R.M., Mutua A., Kimita W., Kamau A., Bejon P., Pettifor J.M., et al. Prevalence of vitamin D deficiency in Africa: a systematic review and meta-analysis. Lancet Global Health. 2020;8:e134–e142. doi: 10.1016/S2214-109X(19)30457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaka, M.; Kabthymer, R.; Meshesha, M. Vitamin D deficiency is less prevalent among children with common medical illnesses than apparently healthy children in sub-saharan Africa: a systematic review and meta-analysis. Preprints 2020, 2020120187 (doi: 10.20944/preprints202012.0187.v1).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the study is readily available can be obtained from the corresponding author on reasonable request.