Abstract
Purpose
Survival for patients with extensive-stage small cell lung cancer (ES-SCLC) remains poor. Consolidative thoracic radiation therapy (cTRT) and upfront immunotherapy with chemotherapy have each incrementally improved patient outcomes, but have not yet been combined in clinical trials. We sought to characterize outcomes and toxicities after first-line chemotherapy and immunotherapy followed by cTRT.
Methods and Materials
Patients with ES-SCLC who were treated with first-line chemotherapy and immunotherapy followed by cTRT were identified at 2 institutions. Patient outcomes including overall survival (OS), progression-free survival, local progression-free survival, distant progression free-survival, and toxicity were assessed.
Results
Twenty patients were included in our data set treated from 2018 to 2021 with a median follow-up of 12 months. Median OS in this cohort was 16 months with 6-month OS of 94.7% and 12-month OS of 77.5% (comparable to historical controls). There were also low rates of toxicity, including 0% grade 3+ toxicity, 0% grade 2 pneumonitis, and 5% grade 2 esophagitis.
Conclusions
Treatment of ES-SCLC with first-line chemoimmunotherapy followed by cTRT appears to be safe and to have outcomes comparable to published modern clinical trials. Further studies are warranted to determine the therapeutic effect of cTRT after chemoimmunotherapy.
Introduction
Nearly two-thirds of patients with small cell lung cancer (SCLC) present with extensive stage small cell lung cancer (ES-SCLC).1,2 Survival for these patients is poor, and despite advances in both systemic therapy and radiation therapy, overall survival (OS) has improved incrementally to approximately 12 months with the use of first-line chemoimmunotherapy.3, 4, 5
For patients with ES-SCLC, systemic therapy with a platinum-based chemotherapy and etoposide is the cornerstone of treatment.1,6 Consolidative thoracic radiation therapy (cTRT) has been shown to improve OS in selected patients who respond to initial chemotherapy.7, 8, 9 Consolidative radiation therapy to distant sites has also been shown to delay progression but was not found to improve OS.10 Prophylactic cranial irradiation (PCI) was initially shown to improve OS; however, a contradictory trial has emerged in the setting of routine magnetic resonance imaging staging and surveillance imaging.1,11, 12, 13 More recently, the phase III IMpower133 trial found that the addition of atezolizumab to first-line chemotherapy improved OS for patients with ES-SCLC.3 Similarly, the phase III CASPIAN trial showed that the addition of durvalumab to first-line chemotherapy improved OS for patients with ES-SCLC.4,14
Notably, cTRT was not permitted in either the IMpower133 or CASPIAN studies.3,4 Given that cTRT is well-tolerated and has previously demonstrated a survival advantage after chemotherapy, it may be reasonable to consider whether adding this to chemoimmunotherapy may lead to an even more substantial benefit. To our knowledge, no data exist regarding the safety and effectiveness of cTRT in patients with ES-SCLC treated with platinum-based chemotherapy and atezolizumab. We performed a multi-institutional retrospective analysis to characterize the outcomes and toxicities after first-line atezolizumab with chemotherapy followed by cTRT for patients with ES-SCLC.
Methods and Materials
This retrospective database study was approved by the institutional review boards of Rhode Island Hospital and Yale School of Medicine. A total of 20 consecutive patients with ES-SCLC from 2018 to 2021 were identified. Patients ≥18 years of age with a histologically proven diagnosis of ES-SCLC who received both first-line combination platinum (carboplatin or cisplatin) and etoposide chemotherapy as well as atezolizumab who were treated with cTRT were included in our patient cohort. All patients were restaged with intracranial and extracranial imaging with either positron emission tomography/computed tomography or computed tomography and brain magnetic resonance imaging before initiation of cTRT.
Target delineation and radiation therapy dosing
Treatment planning technique, target delineation, planning target volume (PTV) margin expansion, dose prescription, and fractionation were at the discretion of the treating radiation oncologist. All patients were treated with consolidative intent as determined by the treating radiation oncologist. Both 3-dimensional conformal and intensity modulated radiation therapy were permitted, and 4-dimensional planning was used for all patients. All intrathoracic disease was treated and extrathoracic disease was treated at the discretion of the treating radiation oncologist. The biological effect of radiation treatment among the patients in our cohort was calculated using the biologically effective dose (BED) equation, which accounts for total dose and dose per fraction to calculate a BED.15 BED10 was calculated using the linear quadratic equation, with alpha/beta set to 10, d equal to the dose per fraction in Gray units (Gy10), and n equal to the number of fractions delivered. Treatment planning software was used to calculate PTV and doses to relevant organs at risk including the heart, lungs, and esophagus.
Statistical analysis
Descriptive statistics were used to characterize the patient cohort. The Kaplan-Meier method was used to estimate progression-free survival (PFS), local PFS (LPFS), distant PFS (DPFS), and OS. Data analysis was performed using SPSS version 22.
Outcomes
Patient outcome data were analyzed retrospectively in a deidentified manner. Tumor progression was assessed using the response evaluation criteria in solid tumors (RECIST) 1.1 criteria with complete response (CR) defined as disappearance of all target lesions, partial response (PR) defined as ≥30% decrease in the sum of the longest diameter of target lesions, progressive disease (PD) defined as ≥20% increase of at least 5 mm in the sum of the longest diameter of the target lesions or appearance of new lesions, and stable disease defined as neither CR, PR, nor PD.16 OS was defined as time from initiation of chemotherapy to death. PFS was defined as time from initiation of chemotherapy to progression either within or outside of the cTRT treatment field. LPFS was defined as time from initiation of chemotherapy to progression within the cTRT treatment field. DPFS was defined as time from initiation of chemotherapy to progression outside of the cTRT treatment field. Toxicities were graded in accordance with Common Terminology Criteria for Adverse Events version 5.0.17
Results
Patient demographics and clinical variables
Baseline characteristics for patients with ES-SCLC treated with chemoimmunotherapy with atezolizumab followed by cTRT are summarized in Table 1. In total, 20 patients were identified. The median age for patients was 66 (interquartile range [IQR], 58-74). All patients had Eastern Cooperative Oncology Group (ECOG) performance statuses of ≤2, with 15% of patients ECOG 0, 65% of patients ECOG 1, and 20% of patients ECOG 2. The median number of chemotherapy cycles and range was 4 (3-7). The chemotherapy agents used were carboplatin and etoposide in 90% of cases with cisplatin and etoposide used in 10% of cases. The median time from chemotherapy completion to initiation of cTRT was 45.5 days (IQR, 35.5-51). cTRT was administered before the start of maintenance atezolizumab, and atezolizumab was not administered concurrently. Median follow-up was 12 months. No patients in our cohort were treated with PCI. Intrathoracic disease was treated in all patients in our cohort and extrathoracic disease was also treated in 2 patients in our cohort. Patient treatment and clinical outcomes are listed in Table 2.
Table 1.
Baseline characteristics of patients treated with chemoimmunotherapy followed by cTRT
| Age, y (median, IQR) | 66 | 58-74 |
| Gender (n, %) | ||
| Male | 7 | 35% |
| Race (n, %) | ||
| White | 18 | 90% |
| Nonwhite | 2 | 10% |
| ECOG (n, %) | ||
| 0 | 3 | 15% |
| 1 | 13 | 65% |
| 2 | 4 | 20% |
| Brain mets at presentation (n, %) | 2 | 10% |
| Cycles of chemo (median, range) | 4 | 3-7 |
| Chemo agents (n, %) | ||
| Carboplatin/etoposide | 18 | 90% |
| Cisplatin/etoposide | 2 | 10% |
| Immunotherapy | ||
| Atezolizumab (n, %) | 20 | 100% |
| Cycle number of chemotherapy when atezolizumab was started (median, range) | 1 | 1-3 |
| Time from chemo start to RT in days (median, IQR) | 45.5 | 35.5-51 |
| PCI (n, %) | 0 | 0% |
| Distant mets at TRT (n, %) | 17 | 85% |
Abbreviations: cTRT = consolidative thoracic radiation therapy; ECOG = Eastern Cooperative Oncology Group; IQR = interquartile range; PCI = prophylactic cranial irradiation; RT = radiation therapy; TRT = thoracic radiation therapy.
Table 2.
Patient characteristics, treatment summary, and clinical outcomes
| Number | Age | Sex | Race | ECOG | AJCCTNM 8th | Systemic therapy response | cTRT dose and fractionation | cTRT response | Time to local progression (months) | Time to distant progression (months) | Treatment at progression | Follow- up time (mo) | OS(mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | M | White | 0 | T4N3M1c | PR | 30 Gy in 10 Fx | SD | - | 6.5 | Lurbinectedin | 12.9 | 12.9 |
| 2 | 73 | F | White | 0 | T1bN3M1c | PR | 30 Gy in 10 Fx | SD | 11.2 | 6.3 | WBRT, lurbinectedin | 14.8 | 14.8 |
| 3 | 78 | M | White | 1 | T3N3M1c | PR | 30 Gy in 10 Fx | CR | - | - | 18.3 | - | |
| 4 | 74 | F | Hispanic | 1 | T2aN2M1b | PR | 30 Gy in 10 Fx | SD | - | 7.6 | WBRT, lurbinectedin | 7.9 | - |
| 5 | 72 | M | White | 1 | T3N3M1c | PR | 54 Gy in 30 Fx | PR | 12.2 | 6.5 | Subcarinal RT, irinotecan | 16.1 | 16.1 |
| 6 | 57 | M | White | 1 | T4N3M1c | PR | 30 Gy in 10 Fx | SD | - | 6.6 | Docetaxel | 11.2 | - |
| 7 | 68 | F | White | 1 | T3N3M1c | PR | 30 Gy in 10 Fx | SD | 9.2 | 5.9 | Irinotecan | 12.8 | 12.8 |
| 8 | 77 | F | White | 1 | T1cN3M1c | SD | 30 Gy in 10 Fx | PR | - | - | 10 | 10 | |
| 9 | 64 | F | White | 1 | T4N3M1a | PR | 60 Gy in 30 Fx | SD | 18.3 | 18.3 | 31.5 | 31.5 | |
| 10 | 78 | F | White | 1 | T3NxM1b | PR | 30 Gy in 10 Fx | PD | 6.2 | 6.2 | 7.1 | 7.1 | |
| 11 | 56 | M | White | 1 | T2N3M1c | PR | 60 Gy in 30 Fx | PR | - | 6.9 | Ipilimumab/nivolumab | 31 | - |
| 12 | 66 | F | African American | 1 | T4N2M1b | PR | 30 Gy in 10 Fx | PR | - | 5.6 | 5.9 | 5.9 | |
| 13 | 62 | M | White | 2 | T4N3M1b | PR | 30 Gy in 10 Fx | Not assessed | - | - | 3.4 | - | |
| 14 | 55 | F | White | 2 | T4N3M1b | PD | 30 Gy in 10 Fx | PR | 6.8 | 3.5 | Ipilimumab/nivolumab | 8.7 | - |
| 15 | 60 | F | White | 0 | T1N1M1b | PR | 30 Gy in 10 Fx | SD | - | 10 | Temozolomide | 17.2 | 17.2 |
| 16 | 74 | M | White | 1 | T2N3M1c | PR | 30 Gy in 10 Fx | PD | - | 7 | Topotecan | 9.6 | 9.6 |
| 17 | 58 | F | White | 1 | T4N2M1b | PR | 30 Gy in 10 Fx | PR | - | - | Ipilimumab/nivolumab | 10.1 | - |
| 18 | 66 | F | White | 2 | T4N3M01a | PR | 30 Gy in 10 Fx | Not assessed | 9.3 | 9.3 | Ipilimumab/nivolumab | 16 | 16 |
| 19 | 87 | F | White | 1 | T4NxM1b | PR | 30 Gy in 10 Fx | SD | - | 7.7 | Paclitaxel | 10.2 | - |
| 20 | 55 | F | White | 2 | T2aN2M1b | SD | 30 Gy in 10 Fx | PR | 13.7 | 12.4 | Topotecan | 16.4 | 16.4 |
Abbreviations: AJCC = American Joint Committee on Cancer; CR = complete response; cTRT = consolidative thoracic radiation therapy; ECOG = Eastern Cooperative Oncology Group; F = female; M = male; OS = overall survival; PD = progressive disease; PR = partial response; RT = radiation therapy; SD = stable disease; TNM = tumor, nodes, metastases; WBRT = whole brain radiation therapy.
Patients still living at last clinical follow-up are denoted with a blank cell in the OS column.
Dosimetry
Target volume, planning technique, and dosimetry data are summarized in Table 3. The median dose was 30 Gy (IQR, 30-30 Gy) and the median fraction number was 10 (IQR, 10-10). A total of 17 (85%) of the patients received this dose of 30 Gy in 10 fractions, with the remaining 3 (15%) receiving 54 to 60 Gy in 30 fractions. The median BED10 was 39 Gy10 (IQR, 39-39 Gy10). Intensity modulated radiation therapy was used in 70% of patients. Median doses to critical organs at risk included a median mean heart dose of 4.34 Gy (IQR, 2.1-10.39 Gy), a median mean esophagus dose of 13.65 Gy (IQR, 9.74-16.04 Gy), and a median mean lung dose of 7.75 Gy (IQR, 6.82-10.39 Gy). The median lung volume receiving 20 Gy (V20) was 16.86% (IQR, 12.61%-21.97%). The median PTV size was 337.89 cc (IQR, 206.93-487.8 cc).
Table 3.
Target volumes, treatment planning technique, and dosimetry
| Median | IQR | Range | |
|---|---|---|---|
| Dose | 30 | 30-30 | 30-60 |
| BED10 | 39 | 39-39 | 39-72 |
| PTV size (cc) | 337.89 | 206.93-487.8 | 71.29-1362.6 |
| IMRT (n, %) | 14 (70%) | ||
| Mean heart (Gy) | 4.34 | 2.1-10.39 | 0.58-32.13 |
| Mean esophagus (Gy) | 13.65 | 9.74-16.04 | 1.6-40.27 |
| Mean lung (Gy) | 7.75 | 6.82-10.39 | 5.86-24 |
| Lung V20 (%) | 16.86% | 12.61%-21.97% | 3.64%-42.03% |
Abbreviations: BED10 = biologically effective dose (α/β = 10); IMRT = intensity modulated radiation therapy; IQR = interquartile range; PTV = planning target volume.
Patient outcomes and toxicity
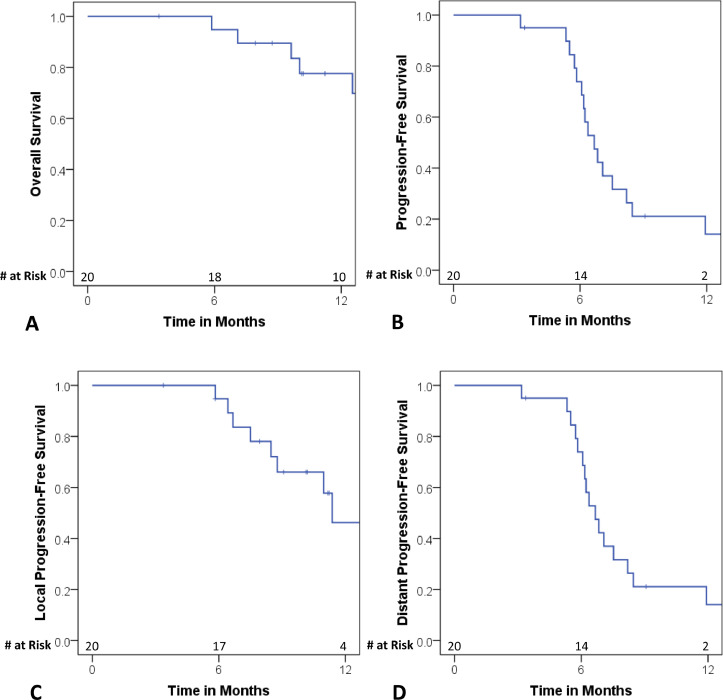
Based on RECIST criteria, 17 patients (85%) had a PR, 2 patients (10%) had stable disease, and 1 patient (5%) had PD after systemic treatment. After cTRT, using RECIST criteria, 1 patient (5%) had a CR, 7 patients (35%) had a PR, 8 patients (40%) had stable disease, and 2 patients (10%) had PD. At a median follow-up of 12 months, the median OS of our patient cohort was 16 months (95% confidence interval [CI], 12.4-19.7) (Table 4). The estimated 6-month OS was 94.7% and the estimated 12-month OS was 77.5% (Fig. 1A). The median PFS was 6.7 months (95% CI, 5.8-7.5). The estimated 6-month PFS was 73.9% and the estimated 12-month PFS was 14.1%. The median LPFS was 11.4 months (95% CI, 8.5-14.2) (Table 4). The estimated 6-month LPFS was 94.7% and the estimated 12-month LPFS was 46.2% (Fig. 1C). The median DPFS was 6.7 months (95% CI, 5.8-7.5) (Table 4). The estimated 6-month DPFS was 73.9% and the estimated 12-month DPFS was 14.1% (Fig. 1D). Despite no patients receiving PCI, only 5 patients (20%) failed intracranially and no patients experienced neurologic death (Table 4). In our cohort, we observed remarkably low rates of toxicity, including 0% grade ≥3 toxicities, 0% grade 2 radiation pneumonitis, and 5% grade 2 esophagitis (Table 4). The 1 case of grade 2 esophagitis occurred in a patient with a mean esophagus dose of 14.22 Gy and resolved within 1 month of completion of cTRT.
Table 4.
Summarized data of clinical response and toxicity to chemoimmunotherapy and cTRT
| Response to systemic therapy (n, %) | ||
|---|---|---|
| PD | 1 | 5.0% |
| SD | 2 | 10.0% |
| PR | 17 | 85.0% |
| CR | 0 | 0.0% |
| Not assessed | 0 | 0.0% |
| Local response to TRT (n, %) | ||
| PD | 2 | 10.0% |
| SD | 8 | 40.0% |
| PR | 7 | 35.0% |
| CR | 1 | 5.0% |
| Not assessed | 2 | 10.0% |
| Progression (n,%) | 17 | 85% |
| PFS in months (median, IQR) | 6.7 | 5.8-7.5 |
| Local progression (n, %) | 10 | 50.0% |
| Local PFS in months (median, IQR) | 11.4 | 8.4-14.2 |
| Distant progression (n, %) | 17 | 85.0% |
| Time to distant progression (median, IQR) | 6.7 | 5.8-7.5 |
| Intracranial progression | 5 | 20% |
| Neurologic death (n, %) | 0 | 0% |
| Median FU in months (median, IQR) | 12 | 9.4-16.2 |
| Median OS in months (median, 95% CI) | 16.0 | 12.4-19.7 |
| Toxicity (n, %) | ||
| Grade 2 + esophagitis | 1 | 5.00% |
| Grade 2 + radiation pneumonitis | 0 | 0.00% |
Abbreviations: CI = confidence interval; CR = complete response; cTRT = consolidative thoracic radiation therapy; FU = follow up time; IQR = interquartile range; OS = overall survival; PD = progressive disease; PFS = progression-free survival; PR = partial response; SD = stable disease; TRT = thoracic radiation therapy.
Fig. 1.
Kaplan-Meier curves showing (A) overall survival, (B) progression-free survival (C) local progression-free survival, and (D) distant progression-free survival. # = number.
Discussion
In this multi-institutional case series of patients with ES-SCLC treated with chemoimmunotherapy followed by cTRT, we found a favorable safety profile with very low rates of toxicity. Our observed median OS of 16 months is comparable to modern clinical trials, including the CREST trial (8 months), IMpower133 (12.3 months), and CASPIAN (12.9 months).3,4,7,14 We also found a median PFS of 6.7 months with a median LPFS of 11.4 months and a median DPFS of 6.7 months, which are comparable to the PFS reported in modern clinical trials, including the CREST trial (4 months), IMpower133 (5.2 months), and CASPIAN (5.1 months). To our knowledge, our investigation is the first case series of patients with ES-SCLC treated with chemoimmunotherapy with atezolizumab and cTRT.
In our data set, we found that cTRT was well-tolerated with low rates of toxicity, similar to the results of the CREST trial, which reported 1.2% grade 3 dyspnea and 1.6% grade 3 esophagitis with cTRT.7 Likewise, a combined analysis of 3 phase I/II trials in which patients with a variety of malignancies received concurrent immunotherapy with either thoracic stereotactic body radiation therapy or chemoradiation also found low rates of grade 3 or higher toxicities.18 The KEYNOTE-799 and DETERRED phase II trials investigating concurrent chemoradiation with immunotherapy also demonstrated the safety of thoracic radiation therapy and immunotherapy.19,20 Additionally, a recent phase I trial of patients with ES-SCLC treated with cTRT and subsequent immunotherapy with ipilimumab and nivolumab demonstrated a toxicity profile consistent with the known toxicity rates of ipilimumab and nivolumab, suggesting that cTRT did not increase toxicity.21 Our current data suggest a favorable safety profile of cTRT after chemoimmunotherapy with atezolizumab for patients with ES-SCLC. Given the overall low rate of toxicity, further prospective studies are warranted to characterize the efficacy of the addition of cTRT to first-line chemoimmunotherapy with atezolizumab. The ongoing RAPTOR trial (NRG-LU007) will provide further data, is currently activated, and is randomizing patients to standard chemotherapy with atezolizumab followed by atezolizumab maintenance versus atezolizumab maintenance with consolidative radiation therapy to up to 5 thoracic and/or extrathoracic sites.22 Similarly, the ongoing NRG-LU005 trial is randomizing patients with limited-stage SCLC to chemoradiation with or without concurrent atezolizumab.23 Moreover, the ADRIATIC trial has randomized patients with limited-stage SCLC to consolidative durvalumab with or without tremelimumab after chemoradiation.24 These trials will provide further data as to the safety of the combination of thoracic radiation therapy and immunotherapy.
This study is limited by the small sample size and retrospective design, which may have selected for patients with more favorable responses to systemic therapy and good performance status. Therefore, we are unable to determine whether our OS, which was comparable but slightly longer than modern clinical trials, is due to our patients receiving both immunotherapy and cTRT. We look forward to the results of phase III trials, including the RAPTOR (NRG-LU007) trial, which will provide further data as to the efficacy of chemoimmunotherapy followed by cTRT in patients with ES-SCLC. Lastly, given the overall poor prognosis of patients with ES-SCLC and the relatively short follow-up time, we primarily were able to assess acute and subacute toxicities, and our data may not be applicable to chronic toxicities of combination immunotherapy and cTRT for longer-term survivors. Nevertheless, our data suggest that cTRT after chemoimmunotherapy with atezolizumab in the management of ES-SCLC is safe and warrants further study.
Conclusion
In this retrospective, multi-institutional analysis, we found a favorable safety profile with the use of chemoimmunotherapy with cTRT in the management of ES-SCLC. We also found similar patient outcomes compared with historical controls. This suggests that chemoimmunotherapy with cTRT may be safe but should be investigated further by further studies, including the ongoing RAPTOR (NRG-LU007) trial.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: Dr Park reports a research grant from the U.S. Food and Drug Administration; consulting fees from AstraZeneca, Guidepoint, Grand Rounds Health, Healthline, and Healthcasts; honoraria from Bristol Myers Squibb, Varian Medical Systems, and Rad Onc Questions; and participation on the data safety monitoring board of Galera Therapeutics. All other authors have no disclosures to declare.
Data sharing statement: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Farago AF, Keane FK. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. 2018;7:69–79. doi: 10.21037/tlcr.2018.01.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stahel R, Thatcher N, Früh M, et al. 1st ESMO consensus conference in lung cancer; Lugano 2010: Small-cell lung cancer. Ann Oncol. 2011;22:1973–1980. doi: 10.1093/annonc/mdr313. [DOI] [PubMed] [Google Scholar]
- 3.Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 4.Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 5.Huang C, Huang C, Gan GN, Gan GN, Zhang J, IMpower Zhang J. CASPIAN, and more: Exploring the optimal first-line immunotherapy for extensive-stage small cell lung cancer. J Hematol Oncol. 2020;13:69. doi: 10.1186/s13045-020-00898-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Socinski MA, Smit EF, Lorigan P, et al. Phase III study of pemetrexed plus carboplatin compared with etoposide plus carboplatin in chemotherapy-naive patients with extensive-stage small-cell lung cancer. J Clin Oncol. 2009;27:4787–4792. doi: 10.1200/JCO.2009.23.1548. [DOI] [PubMed] [Google Scholar]
- 7.Slotman BJ, Van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: A phase 3 randomised controlled trial. Lancet. 2015;385:36–42. doi: 10.1016/S0140-6736(14)61085-0. [DOI] [PubMed] [Google Scholar]
- 8.Higgins KA, Slotman BJ. What is the role of consolidative thoracic radiotherapy in the era of chemo-immunotherapy for extensive stage small cell lung cancer? J Thorac Dis. 2020;12:6308–6310. doi: 10.21037/jtd.2020.03.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: A randomized study. J Clin Oncol. 1999;17:2092–2099. doi: 10.1200/JCO.1999.17.7.2092. [DOI] [PubMed] [Google Scholar]
- 10.Gore EM, Hu C, Sun AY, et al. Randomized phase II study comparing prophylactic cranial irradiation alone to prophylactic cranial irradiation and consolidative extra-cranial irradiation for extensive disease small cell lung cancer (ED-SCLC): NRG Oncology RTOG 0937. J Thorac Oncol. 2017;12:1561–1570. doi: 10.1016/j.jtho.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664–672. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:663–671. doi: 10.1016/S1470-2045(17)30230-9. [DOI] [PubMed] [Google Scholar]
- 13.Maeng CH, Song JU, Shim SR, Lee J. The role of prophylactic cranial irradiation in patients with extensive stage small cell lung cancer: A systematic review and meta-analysis. J Thorac Oncol. 2018;13:840–848. doi: 10.1016/j.jtho.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 14.Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): Updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22:51–65. doi: 10.1016/S1470-2045(20)30539-8. [DOI] [PubMed] [Google Scholar]
- 15.Fowler JF. 21 Years of biologically effective dose. Br J Radiol. 2010;83:554–568. doi: 10.1259/bjr/31372149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz LH, Litière S, De Vries E, et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer. 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr. 2021;112:90–92. doi: 10.1016/j.ad.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Verma V, Cushman TR, Selek U, Tang C, Welsh JW. Safety of combined immunotherapy and thoracic radiation therapy: Analysis of 3 single-institutional phase I/II trials. Int J Radiat Oncol Biol Phys. 2018;101:1141–1148. doi: 10.1016/j.ijrobp.2018.04.054. [DOI] [PubMed] [Google Scholar]
- 19.Jabbour SK, Lee KH, Frost N, et al. Pembrolizumab plus concurrent chemoradiation therapy in patients with unresectable, locally advanced, stage III non-small cell lung cancer: The phase 2 KEYNOTE-799 nonrandomized trial. JAMA Oncol. 2021;7(9):1–9. doi: 10.1001/jamaoncol.2021.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin SH, Lin Y, Yao L, et al. Phase II trial of concurrent atezolizumab with chemoradiation for unresectable NSCLC. J Thorac Oncol. 2020;15:248–257. doi: 10.1016/j.jtho.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Perez BA, Kim S, Wang M, et al. Prospective single-arm phase 1 and 2 study: Ipilimumab and nivolumab with thoracic radiation therapy after platinum chemotherapy in extensive-stage small cell lung cancer. Int J Radiat Oncol Biol Phys. 2021;109:425–435. doi: 10.1016/j.ijrobp.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NCT04402788. Testing the addition of radiation therapy to the usual immune therapy treatment (atezolizumab) for extensive stage small cell lung cancer, RAPTOR trial. Available at: https://clinicaltrials.gov/show/NCT04402788. Accessed February 1, 2022.
- 23.Higgins K, Hu C, Ross H, et al. P2.12-20 NRG Oncology/Alliance LU005: A phase II/III randomized study of chemoradiation versus chemoradiation plus atezolizumab in LS-SCLC. J Thorac Oncol. 2019;14(suppl):S821. [Google Scholar]
- 24.Senan S, Okamoto I, won Lee G, et al. Design and rationale for a phase III, randomized, placebo-controlled trial of durvalumab with or without tremelimumab after concurrent chemoradiotherapy for patients with limited-stage small-cell lung cancer: The ADRIATIC study. Clin Lung Cancer. 2020;21:e84–e88. doi: 10.1016/j.cllc.2019.12.006. [DOI] [PubMed] [Google Scholar]