Abstract
The overarching logos of mammalian memory B cells (MBCs) is to cache the potential for enhanced antibody production upon secondary exposure to cognate antigenic determinants. However, substantial phenotypic diversity has been identified across MBCs, hinting at the existence of unique origins or subfunctions within this compartment. Herein, we discuss recent advancements in human circulatory MBC subphenotyping as driven by high-throughput cell surface marker analysis and other approaches, as well as speculated and substantiated subfunctions. With this in mind, we hypothesize that the relative induction of specific circulatory MBC subsets might be used as a biomarker for optimally durable vaccines and inform vaccination strategies to subvert antigenic imprinting in the context of highly mutable pathogens such as influenza virus or SARS-CoV-2.
B cell memory: context-dependent contributions and compartmental heterogeneity
Through the secretion of antibodies that can limit pathogen acquisition and infectious disease, B cells and their progeny are often integral contributors to vaccination strategies and host defense against microbial agents, such as influenza A virus (IAV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1,2]. Broadly, the landscape of B cell immunity can be described as a function of two components: the primary production of antibodies that can directly mediate protection, and the cached potential to produce more antibodies in a rapid and augmented fashion upon secondary exposure, stored in the form of memory B cells (MBCs) (see Glossary). Under ideal conditions, a single exposure to pathogen-derived antigenic determinants, whether through infection or vaccination, can elicit life-long protection via the net contribution of these and other (e.g., T cell-related) factors. However, this frequently is not the case, with protection diminishing over time to some degree, in a context-specific manner [3]. Although the immunological determinants of sustained versus depreciating immunity have not been fully resolved, it can be reasonably assumed that the optimized induction of MBC immunity should represent a high-priority objective in the development of novel vaccine candidates against antibody-susceptible pathogens.
At the same time however, MBCs may also sometimes act as a detriment to sustained immunity, specifically in the context of highly mutable pathogens, such as IAV. For instance, it is reasonably well established that early-life exposure to dominant circulating IAV strains can elicit an antigenic ‘imprint’ within individuals, wherein memory responses to these strains are continually back-boosted by subsequent exposure to antigenically drifted epidemic strains [4]. While this may be innocuous or beneficial in some instances [5], this ‘antigenic seniority’ is also thought to sometimes occur at the expense of efficacious antibody induction following exposure to heterosubtypic strains [6] and may narrowly focus antibodies generated in homosubtypic contexts against drift-prone epitopes [7]. This concept, in its detrimental form, is known as original antigenic sin (OAS) [8]. Further complicating this interplay is the phenomenon of epitope immunodominance [9,10]. For example, within hemagglutinin (HA), IAV’s receptor-binding glycoprotein and primary antigenic determinant, epitopes in the membrane-distal ‘head’ domain are far more efficient at inducing immune activation compared with the more proximal ‘stalk’ domain [9]. This is a particular issue given that evolutionary pressures from natural selection and human interventions (e.g., vaccines) drive continuous permissive mutation and rapid evolution of the head domain [11], enabling evasion of pre-existing neutralizing antibody immunity. In comparison, the stalk domain is more evolutionarily conserved and less permissive to mutation [11,12]; consequently, antibody responses against this region often neutralize a broader diversity of IAV strains. However, it is often noted that such stalk-specific responses are poorly activated relative to head-specific responses by immunization [13,14], in some cases as a result of pre-existing head-specific memory [15]. Therefore, understanding and overcoming this MBC-mediated barrier is important for the development of broadly protective universal influenza vaccines.
Which variables dictate the integrity of MBC-mediated immunity? One notable factor is the engagement of T cell help, by means of T follicular helper (T FH ) and B2 cell activation within lymphoid tissues in mice and humans [16]. This process results in the cycling of activated B cells through germinal centers (GCs) to promote affinity maturation of their B cell receptors (BCRs) for cognate antigen, while also facilitating enhanced MBC production relative to T cell-independent activation pathways [17]. Notably, while MBCs are often referred to (and assessed) as an aggregate population, it is known that, even outside of BCR-intrinsic differences such as isotype, germline affinity, and affinity maturation, these cells are not homogenous, with a number of subsets having been identified in various contexts (Box 1 ). Although some work has been conducted to investigate the relative contribution of MBC subpopulations to different functions [18], the full scope of this system remains to be fully elucidated and is a burgeoning area of active research. Given this diversity, we hypothesize that if specific circulating MBC subsets or distributions can be observed to correlate with vaccine efficacy, or be unambiguously implicated in participating in protective outcomes against infection through experimentation, these subsets might serve as biomarkers for efficacy and/or durability of candidate vaccine-mediated immune memory (Figure 1 , Key figure). In addition, we posit that a better understanding of MBC subphenotypes may enhance our understanding of antigenic imprinting, informing the development of OAS-subversive vaccination strategies. In support of these positions, we discuss: (i) subfunctions that might be performed across a heterogenous MBC population, (ii) recent subphenotyping efforts highlighting CD19hiCD11c+ nonclassical and CD45RB+CD27+CD73+CD95+ circulating MBCs as potential subsets of interest for biomarker development, and (iii) the potential utility of human seasonal coronavirus (hCoV) spike-specific MBC subphenotyping in optimizing coronaviral vaccines.
Box 1. (Why) Is MBC diversity necessary?
The overarching purpose of B cell memory is to enhance the quality and quantity of secondary antibody responses against a given antigen, thereby optimizing protection against subsequent homologous infections. However, phenotypic heterogeneity of cell surface markers within the MBC compartment raises questions about further specialization of function and purpose. On one hand, circulating MBC subphenotypes may arise as collateral byproducts of their specific activational environment (e.g., germinal center, extrafollicular cortex [19,20]) or timeframe (e.g., new versus mature/persistent GCs [21,22,77]); in such a case, these subsets might possess relatively homogenous functional capabilities outside of those dependent on BCR-intrinsic factors (respective isotype, affinity, mutational load). On the other hand, it is reasonably speculated that MBC subphenotypes may exhibit somewhat distinct operational programming and fulfill different functional or purpose-based roles. Ultimately, we argue that both scenarios likely occur at some balance.
Outside of ‘typical’ functions, what operational attributes might a diverse circulatory MBC population theoretically need to possess? One might be that of a tissue-resident fate; in addition to MBCs that circulate throughout the blood and secondary lymphoid organs, in the case of mucosal infection it would be pertinent to provide additional protection at sites of exposure [23., 24., 25., 26.]. Requirements for tissue homing and specific function likely further differ between specific tissues (such as the brain, gut, lung) given the disparate microbial and immunological conditions at different sites. Similarly, it might be advantageous to store MBCs long-term in the bone marrow [27., 28., 29.], along with other durable immune subsets such as long-lived plasma cells (LLPCs) [30]; these might constitute chronic reservoirs of protection against systemic inflammatory agents. Collaterally, MBCs might require regulatory capabilities in some contexts, such as suppressing IFNγ-producing T cell responses and preventing chronic graft-versus host disease [31,32], or express microbe-specific functional programs such as those relating to combatting bacterial pathogens versus constraining bacterial commensals. Do transient MBC phenotypes exist which undergo further selection against pathogen-derived antigens and autoantigens in circulation and in other peripheral sites? Do specific transcriptional programs limit GC re-entry of newly formed MBCs during a primary response to promote continuous clonal engagement and diversification? Conversely, are specific subsets phenotypically prioritized for re-entry into secondary GCs [18,33]? Do MBC phenotypes correlate with the immunodominant status of epitopes or antigens [34] in potential evolutionary expectation of distinct reactivation or functional requirements for highly versus lowly visible immune determinants? Are certain subsets of MBCs more secluded or refractory to reactivation than others to reduce metabolic shift and energy consumption in mild re-infection contexts, while being capable of responding to substantial re-infections? These are but a few possibilities; notably, whether operational imprints overlap to produce combinatorial MBC subsets also remains unanswered. Although some of these functions have been established, further research is required to substantiate the relationship between phenotype and function in the circulatory MBC compartment.
Alt-text: Box 1
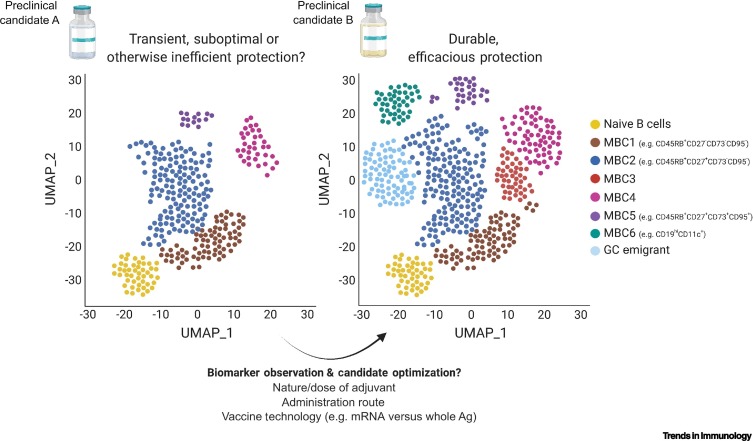
Figure 1.
Key figure. Prospective utility of antigen-specific MBC subphenotyping as a possible vaccine durability biomarker strategy.
MBC induction is thought to be a crucial component of vaccine durability. We propose that correlating particular distributions of circulating antigen-specific MBCs with existing vaccination strategies of known high- or low-efficacy/durability can inform the optimal distribution of MBC subphenotypes for novel vaccine candidates. For instance, if during testing, vaccine candidate B (right) induces a broad distribution of MBC subsets (as measured by single-cell analyses such as RNA sequencing or mass cytometry), particularly including established naïve-distal ‘advanced’ clusters (e.g., potentially CD19hiCD11c+, CD95+) and/or recent germinal center (GC) emigrants, and this distribution is known to be elicited by successful vaccines of a similar immunogenic profile, then such distributions may be useful in predicting long-term candidate efficacy. However, if another candidate (A; left) elicits a more restricted or skewed MBC distribution (e.g., perhaps constituting more naïve-proximal, ‘early’, CD73–CD95– phenotypes), this might be an useful indicator of inefficient immune engagement or skewed immunogenic profile, therein providing early insights into deficits in long-term efficacy and indicating the potential need to modify the vaccination strategy in terms of the nature/dose of adjuvant, administration route, or even vaccine technology. Ultimately, whether particular MBC subphenotypes or distributions possess positive or negative predictive value remains to be experimentally substantiated. Created with BioRender.com. Abbreviations: Ag, antigen; MBC, memory B cell; UMAP, uniform manifold approximation and projection [36].
Can circulating MBC subsets serve as candidate biomarkers for optimized vaccine immunity?
Recent work using unbiased high-throughput screening technologies to assess B lymphocyte populations has greatly improved our understanding of MBC diversity (Figure 2 ). For instance, mass cytometry has been valuable in deeply interrogating cell surface marker-based subphenotypes within human peripheral blood B cell populations during homeostasis [27]. Using this highly multiplexed approach, six distinct circulating MBC phenotypes were found that were definable based on the combinatorial expression of CD27, CD73, CD95, CD19, CD11c, and the CD45 isoform CD45RB. With the exception of CD11c (a typically innate cell-restricted integrin), this set of markers (along with a comprehensive list of over 63 others) was independently validated as positively identifying human splenic MBCs from naïve B cells in other recent flow cytometry-based surface screening efforts [28]. Notably, among these circulating subtypes was a CD45RB+CD27–CD73– cluster: among MBCs, these cells were the most proximal to naïve B cells and presented as the root to an apparent continuum of MBC subsets characterized by the iterative acquisition of CD27, CD73, and CD95 expression [27]. The localization of this cluster, along with its largely unswitched repertoire (referring to isotype switching), low metabolic/transcriptional activity, moderate mutational burden, and relative unresponsiveness to BCR engagement/CD40L stimulation suggested that it represented an early MBC subpopulation. Although circulating CD45RB+CD27– MBCs have been described in the past [35], the relative position of this population as an early memory phenotype is newly described here [27]. Potentially in line with this population, other work has newly defined CD11a and CD200 as potential positive and negative identifiers of isotype-switched CD27– MBCs, respectively [28]. If this is indeed an ‘early’ MBC population [27], a relative bottleneck of antigen-specific MBCs in this compartment may be a useful potential indicator of inefficient immune activation by vaccine candidates, given that one would likely expect a more diversified or ‘advanced’ MBC response to an optimal vaccine.
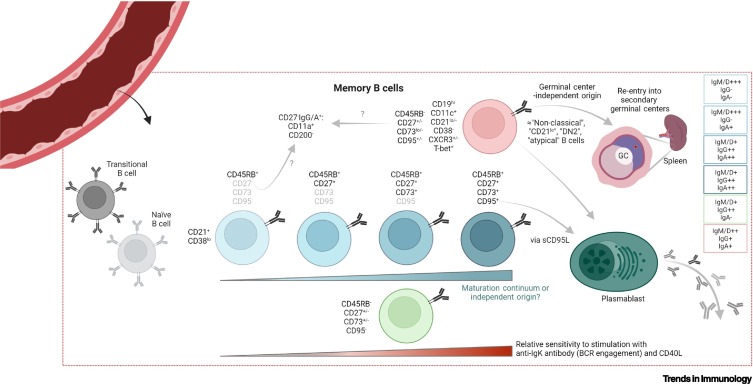
Figure 2.
Recent advances in human circulatory memory B cell (MBC) subphenotyping.
The diagram indicates the broad MBC populations, as recently defined in the peripheral blood of healthy donors [27]. Circulating MBCs can be segregated into six isotype-agnostic populations with different isotype distributions (displayed at right) based on the expression of CD45RB, CD27, CD73, CD95, CD11c, and CD19. Beginning with a naïve-proximal CD45RB+CD27–CD73–CD95– ‘early’ memory phenotype, three phenotypically similar subpopulations (middle) iteratively gain expression of CD27, CD73, and CD95. It is unclear whether this represents a maturation continuum or independent cell lineages. Two transcriptionally distinct CD45RB– subsets are also detected, including a naïve-distal (seemingly ‘advanced’) CD19hiCD11c+ group reminiscent of ‘atypical’, CD21lo, and other nonclassical B cells that have been elsewhere described [19,20,52,58,68] (top), and an IgG-dominant cluster that also demonstrates little to no expression of CD27, CD73, or CD95 (bottom). Subsets are positioned left-to-right in terms of signaling capacity following B cell receptor (BCR) ligation/CD40L stimulation but, coincidentally, also in approximate reference to their phenotypic similarity (based on cell surface marker expression) with naïve cells [27]. CD11a positivity and CD200 negativity may be used to further identify isotype-switched CD27– MBC subsets [28]. Soluble (s)CD95L may promote plasmablast differentiation via CD95 ligation [51]; CD11c+ MBCs have been identified as developing in a germinal center (GC)-independent manner, while possessing the ability to seed secondary GCs and differentiate into antibody-secreting cells [19,20,68]. Created with BioRender.com.
At the other end of the spectrum, two naïve-distal MBC clusters were noted in this study; the first expressed high amounts of CD95 along with CD45RB, CD27, and CD73, while the second was enriched for CD19 and CD11c while displaying relatively low amounts of CD45RB, CD27, CD73, and CD95 [27]. These clusters were of interest given that they clearly segregated from one another, and quite far from naïve cells, within the uniform manifold approximation and projection (UMAP) [36] landscape, but yet were both highly responsive to anti-kappa light chain antibody/CD40L stimulation – indicative of two quite phenotypically different ‘advanced’ memory phenotypes with similar BCR sensitivity. Notably, CD19hiCD11c+ MBCs were also uniquely enriched for oxidative phosphorylative metabolism and general transcriptional activity, as determined by mass cytometric analysis of key metabolic enzymes (e.g., IDH1 and ATPA5) and 5-bromouridine (BRU)-labeled RNA incorporation, respectively [27,37].
It is essential to understand the relationship between different MBC clusters, as this information has potential implications for vaccine biomarker development. For instance, does an enriched induction of CD19hiCD11c+ and/or CD45RB+CD27+CD73+CD95+/– cells versus comparatively ‘early’ CD45RB+CD27+/–CD73– MBCs indicate a more robust or efficacious response to immunization? The apparent dissimilarity in cell surface phenotype between the former groups and naïve B cells, perhaps indicating a mature or advanced phenotype [27], suggests that optimizing their activation might be an important aim in eliciting robust and durable memory-mediated protection. The situation may alternatively be more complex, however. For example, given the known function of CD73 in catalyzing anti-inflammatory adenosine production [38., 39., 40.], it might be speculated that the CD45RB+ MBC activation spectrum might be bimodal, with CD45RB+CD27+CD73– cells being optimized responders relative to CD45RB+CD27–CD73– cells, and CD73 acquisition representing an autocrine/paracrine autoregulatory mechanism to limit self- or cognate TFH cell activation in a similar manner to regulatory T cells [41]. In this case, although CD73 acquisition might represent a normal part of the CD45RB+ memory activation cascade, a vaccine candidate that elicits a CD73+ bias too early, or in too great an extent, might be unfavorable, resulting in a self-suppressing MBC compartment that does not respond effectively to secondary stimulation. However, while CD73+ MBCs trend towards being less metabolically active than CD73– cells (as per the expression of enzymes relating to glycolysis, oxidative phosphorylation, and fatty acid oxidation) [27], they appear to be similar, or more responsive to BCR crosslinking/CD40L stimulation, as reflected in the degree of downstream p38, PLCγ2, and Syk phosphorylation [27]. Furthermore, CD73+ MBCs are also proportionally more isotype-switched than CD73– MBCs [27], allowing them to produce IgG and IgA antibodies to facilitate a more diverse set of effector functions [42]. With this in mind, if CD73 is serving an autoregulatory role, it appears not to be one that substantially limits inherent cellular responsiveness in this context; in this case, modifying vaccines candidates to drive the development/differentiation of CD73+ cells in the memory compartment would again represent an uncomplicated priority for optimizing vaccine-associated immunity.
The same logic may apply to the even more ‘advanced’ (naïve-distal) CD73+CD95+ population. CD95 (FAS) is classically thought of as a proapoptotic signaling receptor, which, in response to ligation by CD95L on TFH cells, constrains B cell proliferation, clonal expansion of low affinity cells, and autoreactivity in GCs [43., 44., 45.]. This is evidenced by work investigating the effect of ex vivo CD95 ligation on the proliferation/survival of human GC B cells, assessments of GC B cell proliferation, survival, and heavy-chain variable region (V H ) gene diversity in mice with complete (Fas lpr/lpr) or B cell-conditional (Cr2-cre Fas fl/fl) CD95 deficiency subject to experimental lymphoma, as well as associations of human FAS gene alterations with GC-derived diffuse large B cell lymphoma, among other studies [43., 44., 45.]. On the one hand, we speculate that this may suggest that CD95 on circulating MBCs might compromise the longevity of a memory response by facilitating the susceptibility of these cells to deletion by T cells and other peripheral CD95L+ cells (e.g., natural killer and endothelial cells [46,47]). On the other hand, in this context it is possible that CD95 might permit the continued homeostatic selection of MBC clones, perhaps as part of a programed contraction/refinement of the maturing memory compartment. In addition, it is known that CD95L can be cleaved by proteases and enter the circulation in a soluble form [48] where it might also ligate MBCs. Indeed, while sCD95L amounts have been reported to correlate with lymphopenia in malarial patients [49], with an inferred potential effect on circulating MBCs, recent work suggests that sCD95L elicits non- apoptotic responses upon receptor ligation potentiating inflammation in various contexts [50]. Furthermore, it can specifically trigger plasmablast/antibody-secreting cell (ASC) differentiation, as evidenced by screening soluble molecules eliciting this transition in ex vivo-differentiated human CD27+ MBCs [51]. Thus, in addition to its well-known apoptotic function, CD95 may have other non-cell death-related roles in promoting memory reactivation, although in vivo confirmation of this phenomenon is warranted. In summary, the relative function of CD95 within the circulating MBC population has yet to be fully elucidated in the context of antigen-specific MBCs. However, if we did assume that an MBC clone of interest was protective, the potent responsiveness and highly isotype-switched antibody repertoire of this population, along with the well-known expression of CD95 on affinity-matured GC-origin B cells, would render CD73+CD95+ MBCs a population of considerable interest for achieving optimized immunity. If, for instance, the objective of a novel universal influenza vaccine candidate is to overcome immunodominance of the HA head versus stalk domains and promote stalk-specific antibody responses, then the activation of stalk-specific GC B cells may likely be of benefit for achieving robust immunity given that the magnitude of antigen-specific GC B cell induction correlates with cognate serum antibody titers [14]. Consequently, we argue that the relative activation of CD95+ MBCs, or any subset that has a GC-like phenotypic signature, might serve as a tangible biomarker for this desired outcome, an attractive hypothesis that merits further investigation.
More segregated from the central cluster, CD19hiCD11c+ MBCs represent another enigmatic yet interesting subset. By virtue of their naïve-distal phenotypic signature, lack of CD21, high expression of CD19, positivity for CD11c, T-bet, and CD183, as well as variable CD27 expression [27], this MBC population seems to exhibit significant overlap with a subset of CD21lo B cells that were previously described by our group [52], as well as those described by others as ‘double negative (DN)2’ (IgD-CD27-CD11c+), ‘atypical’, and nonclassical B cells [53., 54., 55., 56., 57., 58., 59.]. This phenotype has been previously associated, at elevated frequency, with chronic conditions such as rheumatoid arthritis and common variable immunodeficiency (autoreactive clones) [53], human immunodeficiency virus (HIV-1) infection [54,55], hepatitis C virus-associated mixed cryoglobulinemia (HCV-MC) [56], and Plasmodium sp. infection (malaria) [57,58]. Moreover, it has been suggested to represent an exhausted or anergic MBC phenotype in these contexts [55,56,59] based on, for example, evidence of decreased calcium flux and plasmablast differentiation in ex vivo IL-2/10 + CD40L-stimulated CD27+CD21lo versus CD27+CD21hi cells from HCV-MC patients [56] and a reduced proliferation ability in IL-2/10 + CD40L + anti-Ig-stimulated CD27-CD21lo versus CD27+ MBCs isolated from the blood of viremic HIV-1+ individuals [55]. In addition, we [52] and others [58] have shown that IAV-specific CD21lo/atypical memory-like B cells also expand following seasonal influenza vaccination; other recent work suggests a similar induction of this population following severe coronavirus disease 2019 (COVID-19) presentation [60]. We found that human CD21lo cells did not secrete antibody and exhibited high expression of genes relating to negative regulation of BCR signaling (e.g., FCRL5, SIGLEC6, SIGLEC10) [52], which might be potentially in line with the anergic phenotype suggested by other groups [55,56,59], although this remains to be further assessed. Of note, these cells were equally if not slightly more responsive to ionomycin and anti-IgG/IgK antibody stimulation, as evidenced by an increased intracellular calcium flux compared with classical MBCs; this suggested that they are capable of effectively responding to BCR ligation by antigen surrogates [52]. A recent study similarly found that human CD19hiCD11c+ MBCs were the most responsive among all MBC subsets to anti-IgK antibody/CD40L stimulation [27]. This difference in phenotype (anergic versus responsive) might be attributable to differences in context; the sensitivity of these cells to antigen may be more limited during chronic antigen exposure as a result of acquiring a stimulation-refractory phenotype [56], while those induced by acute antigenic exposure or circulating in the absence of antigen might retain higher responsiveness, although this remains conjectural.
Nonetheless, the unique phenotype of these cells may be functionally informative. For instance, CXCR3 (CD183) is a chemokine receptor shown to be expressed on GC B cells to facilitate GC dark zone migration [61], as well as to mediate the homing of other lymphocytes to the lungs under conditions such as IAV infection and tuberculosis [62,63]. Similarly, CD11c is an integrin known to bind fibrinogen [64] (a molecule highly expressed by follicular dendritic cells within GCs [65]), as well as facilitate cellular adherence to inflamed endothelial cells [66]. Consequently, the presence of CXCR3 and CD11c suggests that CD19hiCD11c+ MBCs might have recently undergone affinity maturation and/or are cells in the process of tissue migration, potentially to establish themselves as resident memory B cells (in some contexts reported to be CXCR3+) [23,24]. Furthermore, based on their limited expression of CXCR4 and CXCR5 (known to regulate GC dark zone/light zone chemotaxis, respectively [67]), and detectable amounts of CD95 [27,52], we speculate that: (i) CD21lo/CD19hiCD11c+ MBCs might be unable to re-enter ongoing primary GC reactions, perhaps to prevent competition between early and late clones, thereby promoting clonal diversification; and (ii) that they may retain susceptibility to cell-associated CD95L-mediated cell death, potentially representing a mechanism for circulatory post-GC clonal selection or contraction (as similarly posited in [52]). Other recent work suggests that, despite displaying evidence of affinity maturation [52,59], these nonclassical B cells are GC-independent: one study showed that isotype-switched CD27–CD11c+ ‘DN2’ B cells of extrafollicular origin, expanded in systemic lupus erythematosus patients, were primed to differentiate into autoantibody-producing ASCs and positively correlated with disease activity indices [19]. Another study demonstrated that murine Tbet+CD11c+ MBCs elicited following IAV or lymphocytic choriomeningitis virus-Armstrong acute infection were initially GC-independent (as evidenced by anatomical localization, S1pr2-TdTomato lineage tracing, and bone marrow chimeras), but participated in secondary GC reactions, with speculated CXCR3 function to facilitate splenic marginal zone migration [20]. These findings identify a somewhat clear anatomical origin for this subphenotype. Finally, because of transcription factor BLIMP1 expression and lower BACH2 transcription relative to classical CD21+ MBCs [52], as well as the described ASC fate predisposition [68], it is possible that a subset of these nonclassical B cells might represent precursors to long-lived plasma cells (LLPCs), a cell type that can live and secrete antibody for long periods of time to provide sustained host protection. Indeed, CD19hiCD11c+ MBCs are detectable in the bone marrow of healthy individuals (a well-known site of LLPC residence), although they represent a smaller fraction compared with CD45RB+ MBC subsets [27]. If MBCs in the bone marrow provide a direct reservoir for LLPC differentiation, this might suggest that CD19hiCD11c+ cells contribute a minor fraction to this population. Overall, while the origin and surface phenotype of these cells has been relatively well substantiated, their functional contributions remain incompletely resolved and will require further assessment. However, their ASC-fate disposition and potential relationship to resident MBCs suggests that these cells, similar to CD95+ MBCs, might be preferable targets for optimized vaccine-associated immunity.
Overall, our understanding of circulating MBC cell subset diversity is improving, although many gaps remain, and further rigorous research is required to link MBC subphenotypes with functional outcomes. We hypothesize that inducing a more (as opposed to less) diverse mixture of circulating MBC subsets might be preferable for electing an optimized response to a given vaccine; however, this begs the question as to what MBC distribution is optimal. With this in mind, a subsequent question of interest for vaccine design is whether MBC subsets can be independently manipulated (e.g., by modifying the nature/dose of adjuvant, administration route, or vaccine technology), or whether they represent a co-dependent, developmentally related constellation of cells that can only be engaged to a greater or lesser degree. Does this outcome vary on a subtype-by-subtype basis? Clonal analysis demonstrates a dominant restriction of clones within specific CD45RB+/–CD27+/– quadrants [27], seemingly indicating that MBC clones arise independently within these phenotypic groups and that they may be independently manipulated. However, the relationship within these broader clusters remains to be determined. Understanding the biology underlying these subsets is relevant as it may inform on particular strategies to exploit B cell memory and achieve improved immune outcomes after vaccination.
Do MBC subsets compartmentalize antigenic imprinting/OAS?
In some instances, the idea of manipulating vaccine formulations to induce specific MBC subsets may be complicated by pre-existing, crossreactive MBCs against a pathogen of interest. Along these lines, a study from our group used single-cell RNA sequencing to identify four transcriptionally distinct subsets of coronavirus-specific MBCs among 15 total clusters of antigen-specific peripheral blood B cells from SARS-CoV-2-infected individuals [69]. MBC identity was assigned based on the expression of genes, including CD27, CD86, RASSF6, TRERF1, TRPV3, POU2AF1, RORA, TNFRSF13B, CD80, FCRL5, GDPD5, BAIAP3, TGM2, and MUC16, as well as the relative absence of genes corresponding to ASCs and other B cell subsets. Of note, the classical definition of B cell memory is an antigen-experienced and long-lived population; thus, while these four clusters expressed memory-associated gene expression profiles, a number of other B cell clusters could also have fit the category of MBCs.
MBC clusters segregated into two groups: the first group constituted one cluster (number 8) that was proximal to naïve-like B cells, with minimal V H gene somatic mutations and almost no isotype switching [69]. In comparison, the second group comprised three clusters (numbers 4, 6, 7) that generally exhibited transcriptional signatures indicative of a isotype-switched phenotype [70] and displayed intermediately high levels of V H mutation. Within the transcriptional landscape, these latter MBCs segregated quite distally from naïve-like cells and in rather close proximity to apparent recent GC emigrants [69]. Within these three clusters there was notable variability; for instance, cluster 4 was IgG1 (IGHG1) dominant with minimal contributions from other isotypes, cluster 7 was more balanced but with a slight bias towards IgA (IGHA1/IGHA2) overall, while cluster 6 had relatively minimal isotype switching, albeit more than cluster 8. In addition, cluster 7 displayed almost double the median number of V H mutations compared with clusters 4 and 6. In terms of gene expression, cluster 4 uniquely exhibited high expression of FAS (CD95), CD80, and TGM2 (CD30) relative to other MBCs [69], potentially in line with the earlier-described CD45RB+CD27+CD73+CD95+ MBC phenotype [27].
Although our study focused primarily on investigating the activation of SARS-CoV-2-specific cells, we were also able to profile seasonal coronavirus-specific responses by probing our samples with a cocktail of oligonucleotide-conjugated hCoV 229E, NL63, HKU1, and OC43 spike (S) protein probes [69]. We noted that during the acute phase of SARS-CoV-2 infection (0–14 days post-symptom onset), the vast majority of ASCs were not specific to the spike of SARS-CoV-2, but rather, to that of seasonal hCoVs, as evidenced by higher detection of hCoV-spike+ cells. In addition, the magnitude of the hCoV spike ASC response was significantly greater than that directed against the spike of SARS-CoV-2, which was the infecting agent. This was evidence of back-boosting, which could suggest the presence of an OAS-like response. In such a scenario, the activation of crossreactive memory responses to seasonal hCoVs by SARS-CoV-2 might promote the recall of conserved, non-neutralizing [71], and/or nonprotective clones [such as those that target the virally encapsulated, antibody-inaccessible nucleoprotein or open-reading frame 8 (ORF8) proteins] [69] and/or limit de novo protective antibody induction against the primary SARS-CoV-2 agent itself [72,73]. This idea may be further supported by evidence of boosted pre-existing hCoV-specific antibodies during SARS-CoV-2 infection [74,75]. Ultimately, despite these findings, a concrete negative impact of SARS-CoV-2-mediated back-boosting of hCoV-specific MBCs has not been unambiguously demonstrated; conversely, some work has proposed that the high rates of hCoV infection/seroconversion in children/adolescents might contribute to the reduced predisposition for severe SARS-CoV-2 infection in this demographic group [76].
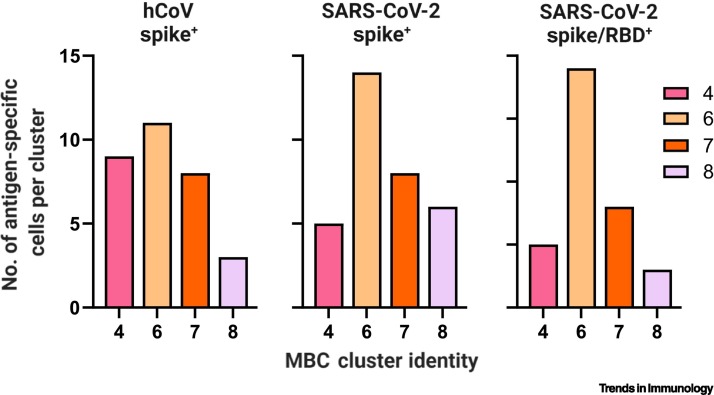
Regardless of impact, determining how hCoV spike-specific cells are distributed within MBC subsets could be important to inform the development of future vaccination strategies that aim to avoid reactivating specific MBC subsets and eliciting OAS-like responses. To this end, and for illustration purposes, we highlight a preliminary trend within our data that will require further and robust validation. According to MBC cluster identification [69], we notice that hCoV spike-binding cells are present in all four MBC clusters (4,6,7,8) at the relatively quiescent convalescent visit 2 timepoint (~4.5 months post-symptom onset). Similar numbers of hCoV-spike+ MBCs were detected in clusters 4, 6, and 7, while cluster 8 had relatively few (Figure 3 ). In comparison, SARS-CoV-2 spike-binding MBCs, in particular those specific for the glycoprotein’s receptor-binding domain, harbored an enrichment of cluster 6 MBCs relative to clusters 4, 7, and 8 (the latter clusters being themselves relatively balanced in terms of detectable cell numbers). Of note, cluster 6 appeared to be largely isotype-unswitched (~75% IgM+) [69], suggesting that this might be an ‘early’ or immature memory phenotype. This raises the question as to whether such an isotypic restriction might be expected, or whether it might be suggestive of an hCoV-OAS-restricted phenotype. This question may be resolved by comparing MBC induction profiles under naïve and imprinted experimental conditions, for instance, using flow or mass cytometry to characterize MBC subsets in mice vaccinated against SARS-CoV-2 with or without prior vaccination against hCoVs. In addition, studies assessing how B cell memory is preferentially encoded in children, who are more antigenically naïve, versus adults, would represent a useful and somewhat comparable human model system. Ultimately, these types of analyses could illustrate how potentially detrimental memory responses might be distributed within MBC subsets. By carefully assessing and understanding such MBC transcriptional profiles and subphenotypes, it may be possible to improve our approaches to vaccine design; indeed, an important goal is to develop vaccines that can optimally subvert OAS-like responses [73].
Figure 3.
Distribution of hCoV spike-specific, SARS-CoV-2 spike-specific, and SARS-CoV-2 spike/RBD-specific cells within individual MBC clusters.
For illustrative purposes, the dataset featured in [69] was assessed for the number of hCoV spike-specific, SARS-CoV-2 spike-specific, and SARS-CoV-2 spike/RBD-specific cells identified within individual MBC-identified clusters (4,6,7,8) at the 4.5-month convalescent timepoint. Presented are the absolute number of indicated antigen-specific cells detected, per indicated cluster. This represents a preliminary analysis that requires further experimental substantiation; however, these data suggest that MBC responses to different pathogens, or of different maturation levels (i.e., with different durations postexposure) may exhibit different subset distributions within the broader MBC compartment. This exercise illustrates the manner in which further experimental analysis of antigen-specific MBC subset distributions may be valuable; by understanding how imprinted memory is distributed across subsets and how vaccine formulations affect those subsets, it may be possible to design novel candidates and strategies that avoid activating imprinted subsets while eliciting the intended, protective de novo responses. Abbreviations: hCoV, human seasonal coronavirus; MBC, memory B cell; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Concluding remarks
Often considered as a homogenous population, recent advances have improved our resolution of MBCs to identify them as a phenotypically diverse collection of long-lived, antigen-experienced cells. Although it is easy to envision distinct roles for multiple MBC subsets, this remains to be fully experimentally substantiated. However, we hypothesize that the induction of specific populations or distributions in the systemic circulation can be used to parse out optimal versus suboptimal vaccine candidates in preclinical and clinical studies. In its simplest form, identifying these distributions might involve quantifying MBC induction profiles and distributions in response to approved vaccines of known efficacy and durability when compared with vaccines of suboptimal or unknown potency. In the interim, we present the case for CD95+ and CD19hiCD11c+ MBCs as potential candidate cell types for optimized immunity based on recent advances in the literature. This position is based in part on inferences derived from work in murine models; validating this position will require studies to establish these or other MBC subsets/distributions as correlates of vaccine efficacy in human vaccination systems, as mentioned above. Finally, we illustrate the potential utility of subphenotyping MBC subsets in improving our understanding of antigenic imprinting at the cellular level, which may inform vaccine development in the context of highly mutable viruses such as IAV and SARS-CoV-2 in which pre-existing memory may elicit back-boosting of nonprotective clones and/or limit de novo protective responses. One limitation of our viewpoint is that the practical utility relies on being able to manipulate the induction or reactivation of particular MBC subsets to some degree, which may be difficult to achieve. Ultimately, further and robust preclinical investigation is required (see Outstanding questions) to evaluate the predictive utility of MBC subphenotyping and practical utility of MBC manipulation in the design of novel vaccine candidates for highly burdensome pathogens; we argue that it remains an important and necessary area of future investigation.
Outstanding questions.
To what extent are circulating MBC subphenotypes indicative of distinct temporal/anatomical origins and/or subspecialized functional imprints? Answering this question can help explain the observed heterogeneity in the MBC compartment.
Can circulatory MBC distributions identify optimal versus suboptimal vaccination strategies among existing vaccines in terms of efficacy and durability? If so, these distributions may be able to serve as candidate biomarkers for identifying goal-optimized outcomes during preclinical/clinical testing of novel vaccine candidates.
Do circulatory MBC subsets represent a developmental/maturation spectrum, independent trajectories, or some combination of both? This is an important step in understanding whether MBC subsets can be manipulated, either independently or concomitantly, by modifying the nature/dose of antigen/adjuvant, immunization route, or other variables.
What is the developmental relationship between circulating and tissue-resident MBCs at mucosal sites (e.g., gut, lung) and the bone marrow? Resident MBCs are thought to provide an additional source of local protection against re-infection, so understanding exactly how they arise could help understand how to best elicit them.
What roles do canonically immunosuppressive/inhibitory molecules such as CD73 and CD95 play on the surface of circulating MBCs? Investigating the context-dependent function of these molecules may improve our understanding of MBC dynamics during infectious disease.
Do crossreactive and/or immuno(sub)dominant B cell clones (e.g., among influenza virus, coronaviral lineages) exhibit specific subset distributions? This information may be useful in optimizing influenza/coronaviral vaccines to subvert antigenic imprinting.
Alt-text: Outstanding questions
Acknowledgments
Acknowledgments
This work was supported in parts by NIH/NIAID grants 5P01AI097092-08, 5U01AI144616-02, and P01AI165077, as well as NIH contracts 75N93019C00051 and 75N93019R00028.
Declaration of interests
The authors have no conflicts of interest to declare regarding this work.
Glossary
- Affinity maturation
process by which B cell clones attempt to improve their binding affinity for cognate antigens, facilitated by enzymatic somatic hypermutation of the cell’s heavy- and light-chain variable regions.
- Anergic
state of unresponsiveness to antigen exposure; in reference to T or B lymphocytes.
- Antibody-secreting cell (ASC)
broad term for all B cell variants that secrete antibody.
- Antigenically drifted
process of cumulative antigenic mutation that modifies antibody-binding epitopes, often resulting in the reduced ability of existing, previously effective antibodies, to bind those epitopes. Frequently observed in the context of mutation-prone viral glycoproteins, such as influenza virus hemagglutinin.
- Autoreactive
in reference to antibodies or B cell responses, those that are directed against autoantigens.
- B2 cell
‘classical’ B cells that are activated in secondary lymphoid tissues, typically in a T cell-dependent manner; as opposed to B1 cells, which are often activated in a T cell-independent manner.
- Clonal expansion
process during which B cell ‘clones’ (single, mature, activated B cells of defined germline BCR sequence) proliferate to produce a lineage of cells that may each independently undergo further proliferation, affinity maturation, isotype switching, and/or phenotypic differentiation.
- Exhausted
refers to a similar concept of anergy/dysfunction but implies a prior state of responsiveness for the cell/population previous to the current state of unresponsiveness.
- Extrafollicular
occurring outside of B cell follicles.
- Follicular dendritic cell
specialized stromal cells within B cell follicles of secondary lymphoid tissues that capture and retain antigen for the subsequent purpose of providing a reservoir of antigen for B cell activation.
- Germinal center (GC)
organized anatomical region within B cell follicles of secondary lymphoid organs (e.g., lymph nodes, spleen) in which B cell clones undergo isotype switching, somatic hypermutation, and affinity maturation at high efficiency.
- Germline affinity
with respect to a given antigen, the affinity an unmutated (germline-configuration) BCR or antibody possesses for that antigen.
- Heavy-chain variable region (VH)
region of a BCR/antibody, which, along with the heavy-chain diversity (D) and joining (J) regions, and light-chain V/J regions, encodes specificity.
- Hepatitis C virus-associated mixed cryoglobulinemia (HCV-MC)
HCV-associated, antibody-mediated autoimmune condition that results from pathological immune complex formation in the blood.
- Heterosubtypic strain
in terms of influenza virus, a strain that is of a different subtype relative to another strain (e.g., H1N1 versus H3N2).
- Isotype
characteristic of a BCR/antibody that does not directly encode specificity but affects antibody effector capabilities. Encoded by the ‘constant’ region of the heavy chain.
- Isotype switching
or class-switch recombination; process by which B cells change their isotype; naïve B cells express immunoglobulin (Ig)D and IgM and undergo isotype switching to produce IgM, IgA, IgG, or IgE exclusively.
- Long-lived plasma cells (LLPCs)
ASCs that persist and secrete antibodies for prolonged durations; these are classically thought to reside in the bone marrow.
- Mass cytometry
high-throughput proteomic analysis technique that uses mass spectrometry to analyze profiles of molecule-targeted, metal-conjugated antibodies on a single-cell basis. Similar in principle to flow cytometry but using metals instead of fluorophores.
- Memory B cell (MBC)
antigen-experienced B cells that persist long-term, do not spontaneously secrete antibodies, and are not actively undergoing affinity maturation. If noncirculating and localized to a specific tissue, considered a tissue-resident MBC.
- Original antigenic sin (OAS)
the concept of back-boosting (activating pre-existing) crossreactive antibody responses upon exposure to a novel antigenic determinant with epitopes that are conserved relative to determinants of prior exposures, with negative consequence (e.g., when crossreactive antibodies are nonprotective). Antigenic imprinting refers to the same, but ambivalently (without reference to positive or negative consequence).
- Plasmablasts
ASCs that are non-terminally differentiated and proliferation competent. Plasma cells are terminally differentiated ASCs that no longer proliferate.
- Regulatory T cell
specific CD4+ T cell subset that possesses anti-inflammatory or immunosuppressive capabilities.
- Somatic hypermutation
process of introducing point mutations into the variable regions of the heavy- and light-chain B cell receptor genes during the process of affinity maturation. Accumulated mutations are referred to as mutational ‘burden’ or ‘load’.
- Subphenotype
cellular distinction defined by the combinatorial expression of particular cell surface markers, intracellular proteins, mRNA signatures, functional abilities, or other attributes.
- T follicular helper(TFH)
specific CD4+ T cell subset that aids in the activation of B cell responses in secondary lymphoid tissues.
- Uniform manifold approximation and projection (UMAP)
bioinformatic dimensionality reduction technique used to visualize the relatedness of individual cells within a population based on a defined set of single-cell parameters (e.g., gene or cell surface protein expression).
- Zones
(marginal, dark, light); anatomical regions of secondary lymphoid tissues. The marginal zone is an extrafollicular region at the interface of the red and white pulps of the spleen. Dark zones are regions of GCs where B cell clones undergo proliferation/somatic hypermutation, and light zones are GC regions where clones are selected based on antigen-binding avidity. GC B cells cycle between light and dark zones.
References
- 1.Guthmiller J.J., et al. B cell responses against influenza viruses: short-lived humoral immunity against a life-long threat. Viruses. 2021;13:965. doi: 10.3390/v13060965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadarangani M., et al. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021;21:475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu X.X., et al. Waning immunity and microbial vaccines-workshop of the national institute of allergy and infectious diseases. Clin. Vaccine Immunol. 2017;24 doi: 10.1128/CVI.00034-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry C., et al. From original antigenic sin to the universal influenza virus vaccine. Trends Immunol. 2018;39:70–79. doi: 10.1016/j.it.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gostic K.M., et al. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science. 2016;354:722–726. doi: 10.1126/science.aag1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arevalo C.P., et al. Original antigenic sin priming of influenza virus hemagglutinin stalk antibodies. Proc. Natl. Acad. Sci. U. S. A. 2020;117:1–7. doi: 10.1073/pnas.1920321117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang K.Y.A., et al. Focused antibody response to influenza linked to antigenic drift. J. Clin. Invest. 2015;125:2631–2645. doi: 10.1172/JCI81104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis T. On the doctrine of original antigenic sin. Proc. Am. Philos. Soc. 1960;104:572–578. [Google Scholar]
- 9.Angeletti D., Yewdell J.W. Understanding and manipulating viral immunity: antibody immunodominance enters center stage. Trends Immunol. 2018;39:549–561. doi: 10.1016/j.it.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Knight M., et al. Imprinting, immunodominance, and other impediments to generating broad influenza immunity. Immunol. Rev. 2020;296:191–204. doi: 10.1111/imr.12900. [DOI] [PubMed] [Google Scholar]
- 11.Kirkpatrick E., et al. The influenza virus hemagglutinin head evolves faster than the stalk domain. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-28706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heaton N.S., et al. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc. Natl. Acad. Sci. U. S. A. 2013;110:20248–20253. doi: 10.1073/pnas.1320524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angeletti D., et al. Outflanking immunodominance to target subdominant broadly neutralizing epitopes. Proc. Natl. Acad. Sci. U. S. A. 2019;116:13474–13479. doi: 10.1073/pnas.1816300116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angeletti D., et al. Defining B cell immunodominance to viruses. Nat. Immunol. 2017;18:456–463. doi: 10.1038/ni.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrews S.F., et al. Immune history profoundly affects broadly protective B cell responses to influenza. Sci. Transl. Med. 2015;7:316ra192. doi: 10.1126/scitranslmed.aad0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurosaki T., et al. Memory B cells. Nat. Rev. Immunol. 2015;15:149–159. doi: 10.1038/nri3802. [DOI] [PubMed] [Google Scholar]
- 17.Victora G.D., Nussenzweig M.C. Germinal centers. Annu. Rev. Immunol. 2022;40:413–442. doi: 10.1146/annurev-immunol-120419-022408. [DOI] [PubMed] [Google Scholar]
- 18.Zuccarino-Catania G.V., et al. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat. Immunol. 2014;15:631–637. doi: 10.1038/ni.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenks S.A., et al. Distinct effector B cells induced by unregulated Toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity. 2018;49:725–739. doi: 10.1016/j.immuni.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song W., et al. Development of Tbet- and CD11c-expressing B cells in a viral infection requires T follicular helper cells outside of germinal centers. Immunity. 2022;55:1–18. doi: 10.1016/j.immuni.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner J.S., et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yewdell W.T., et al. Temporal dynamics of persistent germinal centers and memory B cell differentiation following respiratory virus infection. Cell Rep. 2021;37 doi: 10.1016/j.celrep.2021.109961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan H.-X., et al. Lung-resident memory B cells established after pulmonary influenza infection display distinct transcriptional and phenotypic profiles. Sci. Immunol. 2022;7:eabf5314. doi: 10.1126/sciimmunol.abf5314. [DOI] [PubMed] [Google Scholar]
- 24.Allie S.R., et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat. Immunol. 2019;20:97–108. doi: 10.1038/s41590-018-0260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allie S.R., Randall T.D. Resident memory B cells. Viral Immunol. 2020;33:282–293. doi: 10.1089/vim.2019.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barker K.A., et al. Lung-resident memory B cells protect against bacterial pneumonia. J. Clin. Invest. 2021;131 doi: 10.1172/JCI141810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glass D.R., et al. An integrated multi-omic single-cell atlas of human B cell identity. Immunity. 2020;53:217–232. doi: 10.1016/j.immuni.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisel N.M., et al. Surface phenotypes of naive and memory B cells in mouse and human tissues. Nat. Immunol. 2022;23:135–145. doi: 10.1038/s41590-021-01078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riedel R., et al. Discrete populations of isotype-switched memory B lymphocytes are maintained in murine spleen and bone marrow. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-020-16464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner J.S., et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021;595:421–425. doi: 10.1038/s41586-021-03647-4. [DOI] [PubMed] [Google Scholar]
- 31.Iwata Y., et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khoder A., et al. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood. 2014;124:2034–2045. doi: 10.1182/blood-2014-04-571125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mesin L., et al. Restricted clonality and limited germinal center reentry characterize memory B cell reactivation by boosting. Cell. 2020;180:92–106. doi: 10.1016/j.cell.2019.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson J.L., et al. The transcription factor T-bet resolves memory B cell subsets with distinct tissue distributions and antibody specificities in mice and humans. Immunity. 2020;52:842–855. doi: 10.1016/j.immuni.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koethe S., et al. CD45RB glycosylation is specifically regulated during human peripheral B cell differentiation. J. Leukoc. Biol. 2011;90:5–19. doi: 10.1189/jlb.0710404. [DOI] [PubMed] [Google Scholar]
- 36.McInnes L., et al. UMAP: uniform manifold approximation and projection. J. Open Source Softw. 2018;3:861. [Google Scholar]
- 37.Kimmey S.C., et al. Parallel analysis of tri-molecular biosynthesis with cell identity and function in single cells. Nat. Commun. 2019;10:1–11. doi: 10.1038/s41467-019-09128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou S.N., et al. Skewed CD39/CD73/adenosine pathway contributes to B-cell hyperactivation and disease progression in patients with chronic hepatitis B. Gastroenterol. Rep. 2021;9:49–58. doi: 10.1093/gastro/goaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colgan S.P., et al. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic Signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang W.-X., et al. Skewed CD39/CD73/adenosine pathway in B cells is associated with innate immune hyperactivation in chronic HIV-1 infection. Transl. Med. Commun. 2019;4:1–11. [Google Scholar]
- 41.Deaglio S., et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu L.L., et al. Beyond binding: antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2018;18:46–61. doi: 10.1038/nri.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Razzaghi R., et al. Compromised counterselection by FAS creates an aggressive subtype of germinal center lymphoma. J. Exp. Med. 2021;218 doi: 10.1084/jem.20201173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koncz G., Hueber A.O. The Fas/CD95 receptor regulates the death of autoreactive B cells and the selection of antigen-specific B cells. Front. Immunol. 2012;3:207. doi: 10.3389/fimmu.2012.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lagresle C., et al. Regulation of germinal center B cell differentiation: role of the human APO-1/Fas (CD95) molecule. J. Immunol. 1995;154:5746–5756. [PubMed] [Google Scholar]
- 46.Montel A.H., et al. Fas involvement in cytotoxicity mediated by human NK cells. Cell. Immunol. 1995;166:236–246. doi: 10.1006/cimm.1995.9974. [DOI] [PubMed] [Google Scholar]
- 47.Gao L., et al. Endothelial cell-derived CD95 ligand serves as a chemokine in induction of neutrophil slow rolling and adhesion. eLife. 2016;5 doi: 10.7554/eLife.18542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levoin N., et al. CD95 structure, aggregation and cell signaling. Front. Cell Dev. Biol. 2020;8:314. doi: 10.3389/fcell.2020.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kern P., et al. Increased levels of soluble Fas ligand in serum in Plasmodium falciparum malaria. Infect. Immun. 2000;68:3061–3063. doi: 10.1128/iai.68.5.3061-3063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Gallo M., et al. CD95/Fas, non-apoptotic signaling pathways, and kinases. Front. Immunol. 2017;8:1216. doi: 10.3389/fimmu.2017.01216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Asten S.D., et al. Soluble FAS ligand enhances suboptimal CD40L/IL-21-mediated human memory B cell differentiation into antibody-secreting cells. J. Immunol. 2021;207:449–458. doi: 10.4049/jimmunol.2001390. [DOI] [PubMed] [Google Scholar]
- 52.Lau D., et al. Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation. Sci. Immunol. 2017;2:eaai8153. doi: 10.1126/sciimmunol.aai8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Isnardi I., et al. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood. 2010;115:5026–5036. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kardava L., et al. Abnormal B cell memory subsets dominate HIV-specific responses in infected individuals. J. Clin. Invest. 2014;124:3252–3262. doi: 10.1172/JCI74351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moir S., et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Charles E.D., et al. Clonal B cells in patients with hepatitis C virus-associated mixed cryoglobulinemia contain an expanded anergic CD21 low B-cell subset. Blood. 2011;117:5425–5437. doi: 10.1182/blood-2010-10-312942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aye R., et al. Malaria exposure drives both cognate and bystander human B cells to adopt an atypical phenotype. Eur. J. Immunol. 2020;50:1187–1194. doi: 10.1002/eji.201948473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sutton H.J., et al. Atypical B cells are part of an alternative lineage of B cells that participates in responses to vaccination and infection in humans. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2020.108684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Portugal S., et al. Malaria-associated atypical memory B cells exhibit markedly reduced B cell receptor signaling and effector function. eLife. 2015;4 doi: 10.7554/eLife.07218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oliviero B., et al. Expansion of atypical memory B cells is a prominent feature of COVID-19. Cell. Mol. Immunol. 2020;17:1101–1103. doi: 10.1038/s41423-020-00542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ly A., et al. Transcription factor T-bet in B cells modulates germinal center polarization and antibody affinity maturation in response to malaria. Cell Rep. 2019;29:2257–2269. doi: 10.1016/j.celrep.2019.10.087. [DOI] [PubMed] [Google Scholar]
- 62.Carlin L.E., et al. Natural killer cell recruitment to the lung during influenza A virus infection is dependent on CXCR3, CCR5, and virus exposure dose. Front. Immunol. 2018;9:781. doi: 10.3389/fimmu.2018.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeyanathan M., et al. CXCR3 signaling is required for restricted homing of parenteral tuberculosis vaccine–induced T cells to both the lung parenchyma and airway. J. Immunol. 2017;199:2555–2569. doi: 10.4049/jimmunol.1700382. [DOI] [PubMed] [Google Scholar]
- 64.Nagy-Baló Z., et al. Activated human memory B lymphocytes use CR4 (CD11c/CD18) for adhesion, migration, and proliferation. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.565458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lefevre E.A., et al. Fibrinogen is localized on dark zone follicular dendritic cells in vivo and enhances the proliferation and survival of a centroblastic cell line in vitro. J. Leukoc. Biol. 2007;82:666–677. doi: 10.1189/jlb.0107050. [DOI] [PubMed] [Google Scholar]
- 66.Sadhu C., et al. CD11c/CD18: novel ligands and a role in delayed-type hypersensitivity. J. Leukoc. Biol. 2007;81:1395–1403. doi: 10.1189/jlb.1106680. [DOI] [PubMed] [Google Scholar]
- 67.Allen C.D.C., et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat. Immunol. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 68.Golinski M.L., et al. CD11c+ B cells are mainly memory cells, precursors of antibody secreting cells in healthy donors. Front. Immunol. 2020;11:32. doi: 10.3389/fimmu.2020.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dugan H.L., et al. Profiling B cell immunodominance after SARS-CoV-2 infection reveals antibody evolution to non-neutralizing viral targets. Immunity. 2021;54:1290–1303. doi: 10.1016/j.immuni.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moroney J.B., et al. Integrative transcriptome and chromatin landscape analysis reveals distinct epigenetic regulations in human memory B cells. Nat. Commun. 2020;11:1–18. doi: 10.1038/s41467-020-19242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Amanat F., et al. The plasmablast response to SARS-CoV-2 mRNA vaccination is dominated by non-neutralizing antibodies that target both the NTD and the RBD. medRxiv. 2021 doi: 10.1101/2021.03.07.21253098. Published online May 1, 2021. [DOI] [Google Scholar]
- 72.Guthmiller J.J., Wilson P.C. Remembering seasonal coronaviruses. Science. 2020;370:1272–1273. doi: 10.1126/science.abf4860. [DOI] [PubMed] [Google Scholar]
- 73.Wheatley A.K., et al. Immune imprinting and SARS-CoV-2 vaccine design. Trends Immunol. 2021;42:956–959. doi: 10.1016/j.it.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin C.-Y., et al. Pre-existing humoral immunity to human common cold coronaviruses negatively impacts the protective SARS-CoV-2 antibody response. Cell Host Microbe. 2022;30:83–96. doi: 10.1016/j.chom.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shrock E., et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science. 2020;370:eabd4250. doi: 10.1126/science.abd4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ng K.W., et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science. 2020;370:1339–1343. doi: 10.1126/science.abe1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weisel F.J., et al. A temporal switch in the germinal center determines differential output of memory B and plasma cells. Immunity. 2016;44:116–130. doi: 10.1016/j.immuni.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]