Abstract
Background
Iron deficiency (ID) is a common co‐morbidity in patients with cardiovascular disease and contributes to impaired functional capacity. The relevance of ID in patients in recovery after acute stroke is not known. We assessed the prevalence of ID and anaemia in relation to functional capacity and to recovery during early rehabilitation after stroke.
Methods
This observational study enrolled consecutively 746 patients with ischaemic or haemorrhagic stroke at in‐patient early rehabilitation (age 68 ± 13 years, female 47%, ischaemic stroke 87%). Functional capacity was assessed before and after rehabilitation using Barthel index (reha‐BI), motricity index (MI), trunk control test (TCT), and functional ambulatory category (FAC). ID was defined as ferritin <100 μg/L or as transferrin saturation (TSAT) < 20% if ferritin was 100‐ < 300 μg/L or if CrP > 5 mg/L. Anaemia was defined as Hb < 12 g/dL (women) and <13 g/dL (men).
Results
The prevalence of ID and anaemia before rehabilitation were 45% and 46%, respectively, and remained high at discharge (after 27 ± 17 days) at 40% and 48%, respectively. Patients with ID had lower functional capacity compared with patients without ID (reha‐BI 20 [±86] vs. 40 [±80], MI 64 [±66] vs. 77 [±41], TCT 61 [±76] vs. 100 [±39], FAC 1 [±4] vs. 4 [±4]; median [IQR], all P < 0.001). ID was related to inflammation (OR 2.68 [95% CI 1.98–3.63], P < 0.001), female sex (OR 2.13 [95% CI 1.59–2.85], P < 0.001), haemorrhagic stroke (OR 1.70 [95% CI 1.11–2.61], P = 0.015), initial treatment on stroke unit (OR 3.59 [95% CI 1.08–11.89], P < 0.001), and anaemia (OR 2.94 [95% CI 2.18–3.96], P < 0.001), while age, BMI, and renal function were not related to ID. In adjusted analysis, ID was associated with low functional capacity in all functional scores: reha‐BI (OR 1.66 [95% CI 1.08–2.54], P = 0.02), motricity index (OR 1.94 [95% CI 1.36–2.76], P < 0.001), trunk control test (OR 2.34 [95% CI] 1.64–3.32, P < 0.001) and functional ambulatory category (OR 1.77 [95% CI 1.2–2.63], P < 0.02). Functional capacity improved during rehabilitation regardless of presence of ID, but functional outcome remained significantly lower in patients with ID at the end of rehabilitation (rehab BI and MI, both P < 0.001).
Conclusions
Iron deficiency and anaemia are common and persistent findings in patients after acute stroke. ID and anaemia are independently related to lower functional capacity after acute stroke and to poor functional outcome after rehabilitation. Regular assessment of iron status may identify patients at risk of low functional recovery.
Keywords: Ischaemic stroke, Haemorrhagic stroke, Muscle, Iron deficiency, Rehabilitation, Outcome
Introduction
Stroke is a leading cause of mortality and the single greatest cause of disability in the adult population in modern society. 1 Even with optimal therapy, up to 50% of the patients suffer from relevant physical disability. 2 While mortality trends are decreasing in western society, this is not matched by changes in incidence of stroke. 3 , 4 Therefore, rehabilitation efforts to regain physical capacity after stroke are of increasing importance. The disability after stroke is typically attributed to brain injury itself and lost neuronal control. Accordingly, partial re‐innervation, cerebral restructuration, and re‐learning are important adaptive processes that contribute to functional recovery after stroke. Rehabilitation efforts are predominantly focused on these processes of limited neuronal adaptive plasticity. Less attention is paid, however, to the metabolic and functional capacity of the skeletal muscle, the main effector organ of physical capacity. Muscle weakness and fatigue and early exhaustion are common in patients after stroke and are limiting factors during rehabilitation. Factors that limit functional capacity of skeletal muscle beside the paralytic impairment should be identified as potential targets to boost functional recovery and to improve rehabilitation outcome.
Iron deficiency and anaemia are common co‐morbidities in elderly patients with cardiovascular disease 5 and have a strong impact on muscle function and physical performance. Iron deficiency and anaemia result in impaired strength, early fatigue, poor quality of life, prolonged hospitalization, and impaired survival. 6 Notably, iron deficiency and anaemia have been shown to exert independent and additive detrimental effects on functional capacity in patients with various diseases such as heart failure, 7 , 8 COPD, 9 multiple sclerosis, 10 metabolic diseases, 11 adults, 12 or elderly hospitalized patients. 13 Iron deficiency was shown to account for poor functional recovery after hospitalization. 13 The prevalence of iron deficiency as a potentially treatable condition in patients in rehabilitation after acute stroke and its relation to functional capacity after stroke are not known. In this observational study, we aimed to investigate iron deficiency with or without anaemia and functional outcome in patients at rehabilitation after stroke.
Subjects and methods
This prospective longitudinal observational study was conducted at the rehabilitation centre Brandenburgklinik, Bernau, Germany. All patients consecutively admitted for in‐patient rehabilitation with ischaemic or haemorrhagic stroke for a 12 month period (May 2010–April 2011) were included in the study. According to national standards for early rehabilitation after stroke, patients were admitted within 21 days after stroke by direct transfer from primary care hospital to the rehabilitation centre. Minimum standard duration of hospitalized rehabilitation is 3 weeks, but duration may be extended depending on clinical needs and on the prospects of continued improvement. Patients were evaluated for functional independence at admission to the rehabilitation centre and at discharge. Acute ischaemic stroke was classified according to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria. 14 The study had been approved by the ethics committee of medical association of Brandenburg.
Assessment of functional capacity
Functional capacity of patients was assessed using the early rehabilitation Barthel index (reha‐BI), the motoric capacity was assessed using the motricity index (MI), trunk control test (TCT), and functional ambulatory category (FAC). The reha‐BI is an ordinal score to assess independence in activities of daily living (ADL) related to self‐care and mobility. The reha‐BI includes 10 items of the Barthel Index 15 and additional scores for specific requirements of patient care in early rehabilitation after stroke, ranging from −300 to 100. 16 Maximum scores of the reha‐BI of 100 indicates full independence with lower levels indicating increasing impairment in ADL. The MI is an ordinal weighted scale ranging from 0 (no motoric movement) to 100 (normal movement and strength) to assess motor functional capacity and strength of the upper and lower extremity after stroke. 17 The TCT is a score ranging from 0 to 100 points to assess items of trunk control (rolling, sitting, and maintaining balance in the sitting position) with a higher score indicating a better performance. 18
FAC is a common assessment scale of gait that assesses ambulation status ranging from 0 (non‐functional ambulation) to 5 (independent). The reliability, predictive validity, and responsiveness of FAC have been proven to be excellent in patients with stroke. 19
Laboratory measurements and definitions
Routine venous blood samples were taken at admission to the rehabilitation centre and at discharge. Full blood count including erythrocyte indices mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), reticulocyte count and reticulocyte production index (RPI), and standard biochemical variables were assessed through the routine system of the certified standard laboratory. The following parameters on iron metabolism were assessed: serum concentrations of ferritin, transferrin, iron, and total iron binding capacity (TIBC), and soluble transferrin receptor. Transferrin saturation (TSAT) was calculated as a ratio serum iron and TIBC, multiplied by 100 and expressed in percentage. Haemoglobin was assessed using CELL‐DYN 3700 (Abbot), ferritin was assessed by immunoassay and transferrin, and C‐reactive protein (CrP) were assessed by immune turbimetric tests [all (cobas e601, Roche Diagnostics GmbH, Mannheim, Germany)]. Renal function was assessed by creatinine levels and by estimated glomerular filtration rate (eGFR) calculated according to the MDRD formula. Anaemia was defined as Hb < 12 g/dL (women) and <13 g/dL (men). Iron deficiency is defined as described previously at ferritin <100 μg/L or ferritin 100‐ < 300 μg/L when TSAT <20%. 20 Further, the characteristic of ferritin as acute‐phase protein was taken into account as elevated ferritin levels in the presence of inflammatory activation may not reflect the true iron load of the apoferritin. 21 Therefore, iron deficiency was considered solely on the basis of TSAT<20% if elevated levels CrP (>5 mg/L) indicated presence of inflammatory activation.
Statistics
All data are presented as percentage for categorical variables, as mean with standard deviation (SD), or as medians with inter quartile range (IQR) for continuous variables. Variables with change over time are presented as mean with standard error (SEM). Unpaired and paired t‐test and Mann–Whitney U‐test were applied as appropriate. Chi‐square test was used to compare categorical variables and analysis of variance and Kruskal–Wallis test were used for comparison of continuous variables. Multivariable models for associations of risk factors with ID were applied (logistic regression analysis) including all factors showing significant association with ID univariable analysis plus age and odds ratios with 95% confidence intervals (OR [95% CI]) are reported. To assess the independent effect of ID and anaemia on the variance of functional capacity, two‐way analysis of variance was performed. The relation of ID to functional outcomes was further assessed for the lowest tertiles of functional variables vs. the upper two tertiles as the lowest tertiles was considered a clinical meaningful degree of functional impairment. Multivariate models adjusted for age, sex, stroke aetiology, and stroke unit treatment. Logistic regression was used for assessment. A two‐sided P‐value <0.05 was considered significant. StatView 4.5 (Abacus Concepts Inc., Berkeley, CA, USA) was used for statistical analyses.
Results
The study included 746 consecutive patients admitted to rehabilitation after cerebral stroke (mean age 68 ± 13 years, 47% female). Of these, 98 patients (13%) had haemorrhagic insults, while the majority (87%) had an ischaemic stroke. In 78% of the patients, the event was the first stroke, and 22% of patients had a recurrent stroke. Admission to the rehabilitation centre was on average 21 ± 14 days after the stroke, and duration of hospitalized rehabilitation was on average 27 ± 17 days. Clinical characteristics of the patients on admission to rehabilitation are shown in Table 1.
Table 1.
Baseline characteristics in 746 patients admitted to inpatient early rehabilitation after acute stroke
| Variable (unit) | All subjects (n = 746) | Patients without ID (N = 412) | Patients with ID (N = 334) | P‐value ID vs. no ID |
|---|---|---|---|---|
| Age (years) | 68 ± 13 | 68 ± 12 | 69 ± 13 | 0.8 |
| Male/n (%) | 395 (53%) | 251 (61) | 144 (43) | <0.0001 |
| Weight (kg) | 77.1 ± 16 | 78.5 ± 16.0 | 75.2 ± 15.6 | 0.01 |
| Body mass index (kg/m2) | 27.5 ± 6 | 27.6 ± 5.0 | 27.4 ± 6.9 | 0.6 |
| Systolic blood pressure (mmHg) | 136 ± 22 | 136.2 ± 21.5 | 135.0 ± 21.7 | 0.5 |
| Diastolic blood pressure (mmHg) | 80 ± 14 | 80.4 ± 13.7 | 78.5 ± 14.1 | 0.09 |
| Stroke subtype (%) | 0.014 | |||
| Ischaemic | 648 (87%) | 370 (90%) | 278 (83%) | |
| Haemorrhagic | 98 (13%) | 43 (10%) | 55 (16%) | |
| TOAST criteria for ischaemic stroke aetiology a (%) | 0.6 | |||
| Cardioembolic | 216 (29) | 115 (28) | 101 (30) | |
| Macroangiopathic | 223 (30) | 125 (30) | 98 (29) | |
| Microangiopathic | 143 (19) | 91 (22) | 52 (16) | |
| Unknown | 157 (21) | 78 (19) | 79 (24) | |
| Rare causes | 5 (<1) | 3 (<1) | 2 (<1) | |
| Treated on stroke unit, n (%) | 393 (53) | 195 (47) | 198 (59) | 0.003 |
| Thrombolytic therapy, n (%) | 92 (12) | 45 (11) | 47 (14) | 0.3 |
| Co‐morbidities (%) | ||||
| Anaemia | 343 (46) | 140 (34) | 203 (61) | <0.0001 |
| Arterial hypertension | 502 (67) | 282 (68) | 220 (66) | 0.8 |
| Ischaemic heart disease | 106 (14) | 57 (14) | 49 (15) | 0.8 |
| Atrial fibrillation | 159 (21) | 77 (19) | 82 (25) | 0.3 |
| Diabetes mellitus | 186 (25) | 99 (24) | 87 (26) | 0.1 |
| Adipositas | 84 (11) | 52 (13) | 32 (10) | 0.3 |
| Dyslipidaemia | 218 (29) | 126 (31) | 92 (28) | 0.6 |
| Stroke severity at baseline, median [IQR] | ||||
| Reha‐Barthel index | 30 [85] | 40 [80] | 20 [86] | <0.001 |
| Motricity index | 74 [55] | 77 [41] | 64 [66] | <0.0001 |
| Trunk control test | 87 [52] | 100 [39] | 61 [76] | <0.0001 |
| Functional ambulatory category | 3.0 [5.0] | 4.0 [4.0] | 1.0 [4.0] | <0.0001 |
| Creatinine (μmol/L) | 87 ± 62 | 89 ± 67 | 85 ± 56 | 0.3 |
| Glomerular filtration rate, MDRD (mL/min/1.73 m2) | 88 ± 39 | 85 ± 34 | 90 ± 45 | 0.09 |
| C‐reactive protein, CrP (mg/L) | 17 ± 32 | 10.8 ± 22.1 | 25.0 ± 41.5 | <0.0001 |
| Uric acid (μmol/L) | 338 ± 123 | 349 ± 120 | 324 ± 126 | 0.01 |
| Leucocytes (/nL) | 8.3 ± 3.2 | 7.84 ± 3.01 | 8.92 ± 3.31 | <0.0001 |
| Glutamate oxaloacetate transaminase, GOT (U/L) | 0.53 ± 0.27 | 0.56 ± 0.27 | 0.51 ± 0.28 | 0.02 |
| Glutamate pyruvate transaminase, GPT (U/L) | 0.66 ± 0.57 | 0.70 ± 0.58 | 0.61 ± 0.55 | 0.10 |
| Haemoglobin (g/dL) | 12.6 ± 1.6 | 13.2 ± 1.6 | 12.1 ± 1.6 | <0.0001 |
| Haematocrit | 0.37 ± 0.05 | 0.39 ± 0.05 | 0.36 ± 0.05 | <0.0001 |
| Mean corpuscular haemoglobin, MCH (fmol) | 1.89 ± 0.2 | 1.94 ± 0.24 | 1.84 ± 0.13 | <0.0001 |
| Mean corpuscular haemoglobin concentration, MCHC (mmol/L) | 21.1 ± 0.49 | 21.2 ± 0.44 | 20.9 ± 0.51 | <0.0001 |
| Mean corpuscular volume, MCV (fL) | 89.5 ± 4.8 | 90.7 ± 4.31 | 87.8 ± 4.96 | <0.0001 |
| Retikulocyte count (‰) | 15.8 ± 8.5 | 14.61 ± 7.82 | 17.39 ± 8.96 | <0.0001 |
| Reticulocyte production index, RPI (%) | 0.95 ± .04 | 0.95 ± 0.38 | 0.94 ± 0.43 | <0.0001 |
| Ferritin (μg/L) | 322 ± 294 | 388 ± 297 | 240 ± 271 | |
| Transferrin saturation, TSAT (%) | 25.0 ± 11.7 | 31.6 ± 10.2 | 16.9 ± 7.5 | |
| Soluble transferrin receptor, sol TfR (mg/L) | 3.1 ± 1.3 | 2.72 ± 0.91 | 3.64 ± 1.55 | <0.0001 |
TOAST, the Trial of ORG 10172 in Acute Stroke Treatment—classifies ischaemic stroke aetiology.
Anaemia and iron deficiency prevalence and change during rehabilitation
ID was present on admission to rehabilitation in 334 patients (44.8%). Patients with and without ID were similar for age, BMI, blood pressure, and renal function, but patients with ID were more likely to be women and had more often a haemorrhagic stroke. No difference between groups was observed in distribution of ischaemic stroke aetiology and for the use of thrombolytic therapy, but ID patients were more often treated in stroke units.
Anaemia was present on admission in 342 patients (45.8%). The prevalence of anaemia was similar in male (47.1%) and in female (44.4%) patients (Figure 1A; P = 0.5). Comparing age groups (<60 years, 60 to <75 years and ≥75 years), an increasing prevalence of anaemia was observed in male subjects with higher age (P < 0.0001) that was not seen in female patients (Figure 1B).
Figure 1.
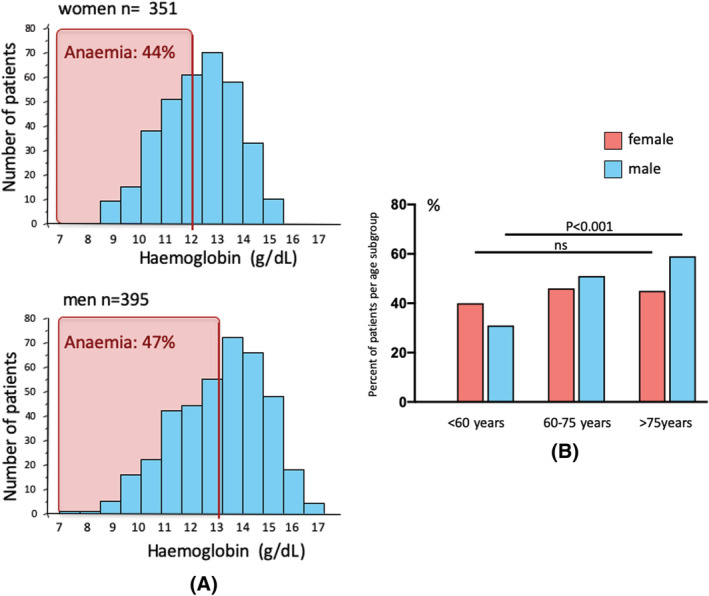
(A) Frequencies of haemoglobin concentration in female and male patients with stroke indicating the prevalence of anaemia on admission to in‐patient rehabilitation after acute stroke. (B) Prevalence of anaemia in male and female patients in age groups (<60 years, 60 to 75 years and >75 years).
As expected, an overlap but no congruency was observed between ID and anaemia (Figure 2A). Of those patients with ID, 202 patients (60.5%) presented ID combined with anaemia while 132 patients (39.5%) had ID without anaemia. In turn, anaemia without iron deficiency was seen in 140 patients (40.9% of anaemic patients). A normal profile of haemoglobin levels and iron status (TSAT ≥20% and Ferritin ≥100 mg/dL and haemoglobin levels ≥12 g/dL in female and ≥13 g/dL in male patients) was observed in 272 patients (36.5%). Repeated assessment of anaemia and iron status at discharge from the hospital was available in 183 Patients. The prevalence of anaemia or ID did not improve during the rehabilitation period. After the rehabilitation, anaemia was present in 48% and ID was present in 40% of the patients (Figure 2B).
Figure 2.
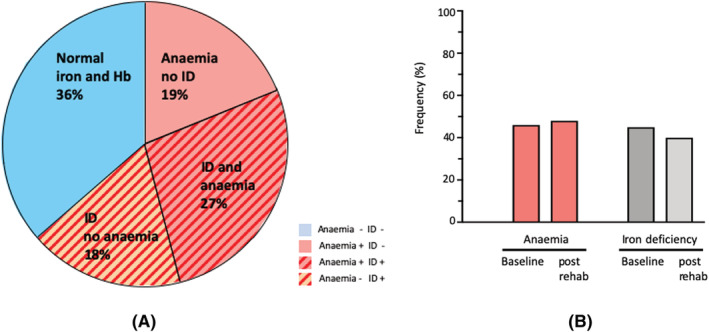
(A) Prevalence and overlap of iron deficiency and anaemia in patients with stroke. Percentage are given based on the entire study population. (B) Frequency of anaemia (left) and iron deficiency (right) on admission and at discharge from in‐patients rehabilitation after acute stroke.
Inflammatory activation (CrP > 5 mg/L) was observed in 395 (53%) patients. Patients with inflammation had lower Hb levels, lower TSAT levels, but higher ferritin levels (Supporting Information, Table 1). ID was observed in 56% of patients with inflammation vs. 32% in patients without inflammation (P < 0.0001). Inflammation was a risk factor for ID (OR 2.68 [95% CI 1.98–3.63, 0 < 0.0001]). Further risk factors of ID were female sex, haemorrhagic stroke, prior treatment at a stroke unit and presence of anaemia (Table 2). In multivariate analysis, only age, female sex, inflammation, and anaemia were independently associated with ID, whereas stroke subtype, stroke unit treatment, or impaired renal function did not contribute to the model.
Table 2.
Risk factors of ID in patients after acute stroke (univariable and multivariable analysis)
| Variable | OR | (95% CI) | P value |
|---|---|---|---|
| Univariable analysis | |||
| Age (per 1 year) | 1.0 | 0.99–1.01 | 0.8 |
| Sex (female) | 2.13 | 1.59–2.85 | <0.0001 |
| Body mass index (per kg/m2) | 1.01 | 0.98–1.04 | 0.6 |
| eGFR (per mL/min/1.73 m2) | 1.00 | 0.99–1.001 | 0.090 |
| Haemorrhagic stroke (yes) | 1.70 | 1.11–2.61 | 0.015 |
| Thrombolysis (yes) | 2.05 | 0.57–7.37 | 0.3 |
| Stroke unit (yes) | 3.59 | 1.08–11.89 | 0.036 |
| Anaemia (yes) | 2.94 | 2.18–3.96 | <0.0001 |
| Inflammation CrP > 5 g/dL (yes) | 2.68 | 1.98–3.63 | <0.0001 |
| Multivariable analysis | |||
| Age (per 1 year) | 0.98 | 0.97–1.0 | 0.038 |
| Sex (female) | 2.36 | 1.66–3.36 | <0.0001 |
| eGFR (per mL/min/1.73 m2) | 1.0 | 0.99–1.01 | 0.98 |
| Haemorrhagic stroke (yes) | 1.01 | 0.59–1.74 | 0.97 |
| Stroke unit (yes) | 0.80 | 0.56–1.15 | 0.24 |
| Anaemia (yes) | 2.70 | 1.88–3.87 | <0.0001 |
| Inflammation CrP > 5 g/dL (yes) | 2.69 | 1.89–3.82 | <0.0001 |
eGFR, estimated glomerular filtration rate; CrP, C‐reactive protein.
Relation of iron deficiency, functional capacity after stroke, and functional recovery during rehabilitation
All measures of functional capacity at baseline (reha‐BI, MI, TCT, and FAC) showed a more severe physical disability in patients with ID compared to patients without ID (Table 1). The presence of ID was related to lower functional capacity (lowest tertiles) in all functional tests in univariable and multivariable adjusted analyses (Table 3:).
Table 3.
Association of iron deficiency and functional outcome in patients after acute stroke in unadjusted and adjusted analysis
| Functional score | Unadjusted OR | Adjusted OR | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P‐value | OR | 95% CI | P‐value | |
| Reha‐Barthex Index | 1.68 | 1.15–2.46 | 0.007 | 1.66 | 1.08–2‐54 | 0.020 |
| Motricity index | 2.06 | 1.50–2.84 | <0.0001 | 1.94 | 1.36–2.76 | 0.0002 |
| Trunk control test | 2.33 | 1.70–3.21 | <0.0001 | 2.34 | 1.64–3.32 | <0.0001 |
| Functional ambulatory scale | 2.24 | 1.64–3.06 | <0.0001 | 1.77 | 1.20–2.63 | 0.004 |
Reha‐Barthel Index, Motricity Index and Trunk Control Test are grouped in tertiles, assessing lowest tertiles vs. upper two tertiles. FAC as categorical variable with six categories was analysed grouping category 0 + 1 vs. categories 2–5. Multivariate model adjusted for age, sex, stroke aetiology, and stroke unit treatment.
The presence of ID (F = 5.19, P < 0.05) and of anaemia (F = 29.0, P < 0.001) had an independent and additive effect to differentiate functional capacity as assessed by reha‐BI (interaction: P = 0.76, Figure 3A). A similar stepwise independent additive effect of ID and of anaemia on lower functional capacity was observed for all scores assessing functional capacity (MI, TCT, and FAC, Figure 3B–D).
Figure 3.
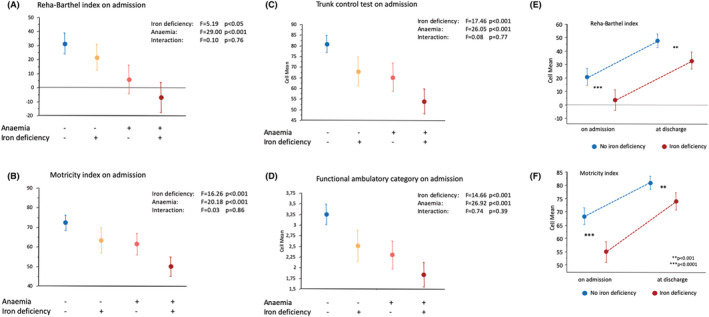
(A–D) Functional capacity assessed by reha‐Barthel index (A), motricity index (B), trunk control test (C) and functional ambulatory category (D) in patients after acute stroke (mean values ± 95% CI) with vs. without anaemia and with vs. without iron deficiency. (E–F) Change of functional capacity assessed by reha‐Barthel index (E) and motricity index (D) in patients during rehabilitation after acute stroke with vs. without iron deficiency (mean values ± 95% CI). P‐values between groups at each time point.
Functional capacity improved during the rehabilitation period, and the degree of improvement was not significantly different between patients with and without ID (Figure 3E,F ). Functional capacity remained, however, lower in patients with ID throughout the rehabilitation period. At discharge from the rehabilitation centre, patients with ID remained at significantly lower functional capacity than patients without ID.
Discussion
The main findings of this study are that (i) ID and anaemia are common co‐morbidities in patients at rehabilitation after stroke. (ii) Patients with ID showed lower functional capacity independent of anaemia, while the combination with anaemia had an additive effect on impaired functional capacity as assessed by a range of functional scores. (iii) Despite general improvement of functional capacity during rehabilitation, patients with ID remained at significantly lower functional capacity at discharge from rehabilitation compared with patients without ID.
This is the first study to investigate the prevalence of iron deficiency and anaemia in relation to functional outcome in a large study cohort of patients at rehabilitation after acute stroke. A high prevalence of ID (45%) and of anaemia (46% of patients) was observed and almost two third (63%) of patients had either ID or anaemia or a combination or both. Only 37% of patients had normal values of both iron balance and haemoglobin. The patient group with ID was not characterized by higher mean age, more sever impairment of renal function, more pronounced co‐morbidities or a different pattern of ischaemic stroke aetiology compared with the patients with no ID. However, other patient characteristics such as female gender or higher proportion of haemorrhagic stroke may contribute to the ID seen in these patients. Importantly, a higher level of inflammatory activation was observed in patients with ID which may be one underlying mechanisms to explain the ID in these patients. 22
The prevalence of ID and anaemia did not improve during the hospitalized rehabilitation period. This is an important finding as it underscores the lack of awareness of this co‐morbidity in both diagnostic workup and adequate therapeutic measures. Our study showing the high prevalence of ID and the association of ID with functional capacity and with outcome after rehabilitation underscores the need for a more regular assessment of iron status and for the initiation of respective therapeutic measured upon the detection of ID in patients after stroke.
Physical impairment after stroke is classically considered as the consequence of lateralized paralytic injury due to innervation failure after a stroke. Beside this focal denervation injury, however, it has been shown that structural and metabolic changes occur on a systemic level after stroke 23 and are related to impaired functional capacity after stroke. 24 , 25 Optimized metabolic integrity of muscle tissue on a systemic level (i.e. including affected and non‐affected limbs) should therefore be a desired therapeutic target in order to achieve best possible rehabilitation results. 26 While physical exercise training is a well‐established component in rehabilitation programmes, 27 , 28 randomized controlled trials in stroke could not show a benefit on functional capacity by early physical exercise programmes. 29 , 30 In this context, the high prevalence of ID in patients after stroke and the observed relation of ID with impaired functional capacity throughout the course of in‐patient rehabilitation is a hypothesis generating finding. ID has been reported to predict impaired functional capacity in a range of diseases including heart failure, COPD, pulmonary hypertension, or liver disease 7 , 9 , 11 , 31 and to be related to poor recovery after hospitalization. 13 ID may therefore be discussed as a relevant factor of the functional recovery in stroke patients and may contribute to explain the lacking efficacy of exercise programmes in patients after stroke. In turn, replenishment of depleted iron stores with iv iron supplementation has been shown to improve muscle energy reserves and functional capacity in vivo 32 and in patients with ID and heart failure. 33 , 34 It is therefore intriguing to hypothesize if replenishment of endogenous iron stores by iv iron supplementation may be a supportive therapy to improve exercise capacity and rehabilitation outcome after stroke. Improved functional capacity may result in better functional independence, shorter rehabilitation periods, and improved long‐term outcome of the patients.
In our study, the functional status and the recovery of patients were assessed using standard scoring systems for evaluation of detailed motoric function and of global functional outcome at rehabilitation after stroke. The Barthel Index is suggested by the European Medicines Agency (EMA) for functional assessment for use in stroke trials 35 and is considered particularly suitable in stroke rehabilitation settings. 36 The reha‐BI has been validated to assess functional independence in ADL with a strong inter‐rater reliability. 16 The motricity index has been validated for the application in stroke as a robust and reliable tool to assess functional motoric capacity and ambulatory recovery after stroke. 37 The motricity index has emerged as a routine tool for functional assessment of patients with stroke due to the inclusion of all extremities and the recording of the full functional spectrum from complete paralysis to normal muscle strength. 17 Thus, in our study, we evaluated the ambulatory capacity in a spectrum ranging from basic motoric functions to activities of daily life.
There are several limitations to the study that need to be addressed. The measurement of clinical severity of the acute stroke was not included in this analysis such as by assessment of the National Institute of Health stroke scale (NIHSS) or modified Rankin scale. These scores are widely used to assess stroke severity in the acute setting of stroke; however, acute impairment often improves within hours and days after the acute event. The patients in the present study were investigated on admission at an inpatient rehabilitation facility on average 21 ± 14 days after the acute event. Hence, the NIHSS score at this time point is not applicable to evaluate the clinical severity of the acute event and was therefore not obtained on admission to the rehabilitation facility. In turn, the rehabilitation Barthel index is a validated standard score to assess functional capacity in rehabilitation after stroke and was therefore routinely used on admission to the rehabilitation facility after stroke. The ID status prior to the acute cerebral event is unknown, and it cannot be excluded that prevalent ID may have been be a contributing factor to cause a stroke or to relate to stroke severity. A large population based study including over 200 000 subjects (over 50 000 with stroke) showed that the presence of ID increased the risk of stroke (OR 1.45, 95% CI [1.34–1.58]). 38 Other co‐morbidities such as malignancy or heart failure may have contributed to the prevalent ID that were not included in the analysis. Regardless of such underlying co‐morbidities, the presence of ID is related to functional outcome of stroke as observed in our study. We assessed ID in this cohort of stroke patients in relation to functional capacity; however, association to cognitive function and recovery may be another relevant factor related to ID. ID is known to have a negative impact on cognitive function in geriatric patients and may as well have an impact on cognitive impairment and recovery in patients with stroke. 39 Further studies are needed to investigate the importance of ID for cognitive status and recovery after stroke.
Conclusions
Iron deficiency and anaemia are common co‐morbidities in patients at rehabilitation after acute stroke. No improvement of the high prevalence of ID or anaemia is observed during the rehabilitation period. ID with and without anaemia is associated with lower functional capacity after acute stroke and with worse functional outcome after rehabilitation. This study suggests that iron status should be assessed regularly in patients during rehabilitation after stroke to identify patients at higher risk of poor functional outcome. Whether iron supplementation can improve functional capacity and efficacy of exercise based rehabilitation programmes requires further studies.
Funding
W.D. received financial support from the German Federal Ministry of Education and Research (BMBF Grant No. 01 EO 0801).
Conflict of interest
W.D. reports speaker fees and advisory honoraria from Aimediq, Bayer, Boehringer Ingelheim, Lilly, Medtronic, Pfizer, Sanofi‐Aventis, Sphingotec, Vifor Pharma outside the submitted work and research support Vifor Pharma, ZS Pharma outside the submitted work. E.A.J. reports speaker advisory honoraria from Vifor Pharma outside of the submitted work and received an unrestricted grant from Vifor Pharma for Wroclaw Medical University outside of the submitted work. S.v.H. received consultancy and speaker honoraria from Bayer, Boehringer Ingelheim, BRAHMS, Chugai, Grünenthal, Helsinn, Hexal, Novartis, Respicardia, Roche, Sorin, and Vifor. T.S., J.F.S., N.S., and M.J. report no conflict of interest.
Supporting information
Table S1: Comparison of patients with and without inflammatory activation (CrP >5mg/L) on admission to rehabilitation after acute stroke
Doehner W., Scherbakov N., Schellenberg T., Jankowska E. A., Scheitz J. F., von Haehling S., and Joebges M. (2022) Iron deficiency is related to low functional outcome in patients at early rehabilitation after acute stroke, Journal of Cachexia, Sarcopenia and Muscle, 13, 1036–1044, 10.1002/jcsm.12927
References
- 1. Martin J, Meltzer H, Elliot D, eds. The prevalence of disability among adults. London: HMSO; 1998. [Google Scholar]
- 2. Kelly‐Hayes M, Beiser A, Kase CS, Scaramucci A, D'Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis 2003;12:119–126. [DOI] [PubMed] [Google Scholar]
- 3. Vaartjes I, O'Flaherty M, Capewell S, Kappelle J, Bots M. Remarkable decline in ischemic stroke mortality is not matched by changes in incidence. Stroke 2013;44:591–597. [DOI] [PubMed] [Google Scholar]
- 4. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013;380:2197–2223. [DOI] [PubMed] [Google Scholar]
- 5. Beattie JM, Khatib R, Phillips CJ, Williams SG. Iron deficiency in 78 805 people admitted with heart failure across England: a retrospective cohort study. Open Heart 2020;7: 10.1136/openhrt-2019-001153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ebner N, Jankowska EA, Ponikowski P, Lainscak M, Elsner S, Sliziuk V, et al. The impact of iron deficiency and anaemia on exercise capacity and outcomes in patients with chronic heart failure. Results from the studies investigating co‐morbidities aggravating heart failure. Int J Cardiol 2016;205:6–12. [DOI] [PubMed] [Google Scholar]
- 7. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail 2011;17:899–906. [DOI] [PubMed] [Google Scholar]
- 8. von Haehling S, Garfias Macedo T, Valentova M, Anker MS, Ebner N, Bekfani T, et al. Muscle wasting as an independent predictor of survival in patients with chronic heart failure. J Cachexia Sarcopenia Muscle 2020;11:1242–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pizzini A, Aichner M, Sonnweber T, Tancevski I, Weiss G, Löffler‐Ragg J. The significance of iron deficiency and anemia in a real‐life COPD cohort. Int J Med Sci 2020;17:2232–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knyszyńska A, Radecka A, Zabielska P, Łuczak J, Karakiewicz B, Lubkowska A. The role of iron metabolism in fatigue, depression, and quality of life in multiple sclerosis patients. Int J Environ Res Public Health 2020;17:6818, 10.3390/ijerph17186818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitchell T, McKinnon E, Ayonrinde O, Adams LA, Trinder D, Chua ACG, et al. Decreased physical working capacity in adolescents with nonalcoholic fatty liver disease associates with reduced iron availability. Clin Gastroenterol Hepatol 2020;18:1584–1591. [DOI] [PubMed] [Google Scholar]
- 12. Houston BL, Hurrie D, Graham J, Perija B, Rimmer E, Rabbani R, et al. Efficacy of iron supplementation on fatigue and physical capacity in non‐anaemic iron‐deficient adults: a systematic review of randomised controlled trials. BMJ Open 2018;8:e019240, 10.1136/bmjopen-2017-019240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neidlein S, Wirth R, Pourhassan M. Iron deficiency, fatigue and muscle strength and function in older hospitalized patients. Eur J Clin Nutr 2020;75:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 15. Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol 2006;5:603–612. [DOI] [PubMed] [Google Scholar]
- 16. Rollnik JD. The early rehabilitation Barthel Index (ERBI). Rehabilitation (Stuttg) 2011;50:408–411. [DOI] [PubMed] [Google Scholar]
- 17. Demeurisse G, Demol O, Robaye E. Motor evaluation in vascular hemiplegia. Eur Neurol 1980;19:382–389. [DOI] [PubMed] [Google Scholar]
- 18. Parlak Demir Y, Yıldırım SA. Reliability and validity of trunk control test in patients with neuromuscular diseases. Physiother Theory Pract 2015;31:39–44. [DOI] [PubMed] [Google Scholar]
- 19. Mehrholz J, Wagner K, Rutte K, Meissner D, Pohl M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch Phys Med Rehabil 2007;88:1314–1319. [DOI] [PubMed] [Google Scholar]
- 20. von Haehling S, Jankowska EA, van Veldhuisen DJ, Ponikowski P, Anker SD. Iron deficiency and cardiovascular disease. Nat Rev Cardiol 2015;12:659–669. [DOI] [PubMed] [Google Scholar]
- 21. Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol 2006;1:S4–S8. [DOI] [PubMed] [Google Scholar]
- 22. Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood 2019;133:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Springer J, Schust S, Peske K, Tschirner A, Rex A, Engel O, et al. Catabolic signaling and muscle wasting after acute ischemic stroke in mice: indication for a stroke‐specific sarcopenia. Stroke 2014;45:3675–3683. [DOI] [PubMed] [Google Scholar]
- 24. Scherbakov N, Pietrock C, Sandek A, Ebner N, Valentova M, Springer J, et al. Body weight changes and incidence of cachexia after stroke. J Cachexia Sarcopenia Muscle 2019;10:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Veltkamp R, Uhlmann S, Marinescu M, Sticht C, Finke D, Gretz N, et al. Experimental ischaemic stroke induces transient cardiac atrophy and dysfunction. J Cachexia Sarcopenia Muscle 2019;10:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scherbakov N, Sandek A, Doehner W. Stroke‐related sarcopenia: specific characteristics. J Am Med Dir Assoc 2015;16:272–276. [DOI] [PubMed] [Google Scholar]
- 27. Uchiyama K, Adachi K, Muraoka K, Nakayama T, Oshida T, Yasuda M, et al. Home‐based aerobic exercise and resistance training for severe chronic kidney disease: a randomized controlled trial. J Cachexia Sarcopenia Muscle 2021.Online ahead of print;12:1789–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sáez de Asteasu ML, Martínez‐Velilla N, Zambom‐Ferraresi F, Ramírez‐Vélez R, García‐Hermoso A, Cadore EL, et al. Changes in muscle power after usual care or early structured exercise intervention in acutely hospitalized older adults. J Cachexia Sarcopenia Muscle 2020;11:997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. LEAPS Investigative Team. Body‐weight‐supported treadmill rehabilitation after stroke. N Engl J Med 2011;364:2026–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nave AH, Rackoll T, Grittner U, Bläsing H, Gorsler A, Nabavi DG, et al. Physical fitness training in patients with subacute stroke (PHYS‐STROKE): multicentre, randomised controlled, endpoint blinded trial. BMJ 2019;366:l5101, 10.1136/bmj.l5101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kramer T, Wissmüller M, Natsina K, Gerhardt F, Ten Freyhaus H, Dumitrescu D, et al. Ferric carboxymaltose in patients with pulmonary arterial hypertension and iron deficiency: a long‐term study. J Cachexia Sarcopenia Muscle 2021.Online ahead of print;12:1501–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haddad S, Wang Y, Galy B, Korf‐Klingebiel M, Hirsch V, Baru AM, et al. Iron‐regulatory proteins secure iron availability in cardiomyocytes to prevent heart failure. Eur Heart J 2017;38:362–372. [DOI] [PubMed] [Google Scholar]
- 33. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009;361:2436–2448. [DOI] [PubMed] [Google Scholar]
- 34. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin‐Colet J, Ruschitzka F, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron‐deficient heart failure patients: an individual patient data meta‐analysis. Eur J Heart Fail 2018;20:125–133. [DOI] [PubMed] [Google Scholar]
- 35. European Agency for the Evaluation of Medicinal Products . Points to consider on clinical investigation of medicinal products for the treatment of acute stroke. CPMP/EWP/560/98. Sept 20, 2001.
- 36. Taylor‐Rowan M, Wilson A, Dawson J, Quinn TJ. Functional assessment for acute stroke trials: properties, analysis, and application. Front Neurol 2018;9:191, 10.3389/fneur.2018.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Masiero S, Avesani R, Armani M, Verena P, Ermani M. Predictive factors for ambulation in stroke patients in the rehabilitation setting: a multivariate analysis. Clin Neurol Neurosurg 2007;109:763–769. [DOI] [PubMed] [Google Scholar]
- 38. Chang YL, Hung SH, Ling W, Lin HC, Li HC, Chung SD. Association between ischemic stroke and iron‐deficiency anemia: a population‐based study. PLoS ONE 2013;8:e82952, 10.1371/journal.pone.0082952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yavuz BB, Cankurtaran M, Haznedaroglu IC, Halil M, Ulger Z, Altun B, et al. Iron deficiency can cause cognitive impairment in geriatric patients. J Nutr Health Aging 2012;16:220–224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Comparison of patients with and without inflammatory activation (CrP >5mg/L) on admission to rehabilitation after acute stroke


