Abstract
Background
The sarcopenia index (SI), calculated as the ratio of serum creatinine to cystatin C levels, reflects skeletal muscle mass and strength. Patients with hip fracture (HF) and sarcopenia have poor functional outcomes, and many require long‐term care after surgery. We hypothesized that the SI can predict preoperative and early postoperative functional outcomes.
Methods
Preoperative serum creatinine and cystatin C were measured to calculate the SI for patients with surgically treated HF (n = 130, mean age: 87.8 ± 6.9 years). Walking ability before and 2 weeks after surgery was assessed, and patients were dichotomized into independent and assistance groups. To assess the validity of the SI, we examined its correlation with the quality [computed tomography (CT) value] and quantity (cross‐sectional area) of the muscles around the hip on the non‐operated side, which were preoperatively measured using CT. Receiver operating characteristic (ROC) analysis was performed to evaluate the prognostic value of the SI.
Results
The SI of the preoperative independent (n = 77) and assistance groups (n = 53) significantly differed (70.2 ± 12.4 and 60.1 ± 9.8, respectively, P < 0.000001). At 2 weeks after surgery, the SI was significantly higher in the independent group (n = 31, 73.0 ± 14.9) than in the assistance group (n = 99, 64.0 ± 10.7, P = 0.0003). In the preoperative independent group, 28 could walk independently after surgery (SI: 74.8 ± 14.0) while 49 required assistance (SI: 67.7 ± 10.6, P = 0.01). For patients with femoral neck fracture (FNF), the SIs were significantly higher in the postoperative independent group (78.6 ± 15.7) than in the postoperative assistance group (63.2 ± 10.9, P = 0.002). Logistic regression analysis showed that the odds ratio (95% confidence interval) of the SI for postoperative walking ability was 0.95 (0.91–0.99, P = 0.03). The correlations of SIs with CT values and cross‐sectional areas were as follows: iliopsoas at the apex of the femoral head, r = 0.40, P < 0.001 and r = 0.49, P < 0.001, respectively; rectus femoris at the level of the lessor trochanter, r = 0.26, P = 0.007 and r = 0.37, P < 0.001, respectively. ROC analysis for predicting postoperative walking ability in preoperative independent patients with HF and FNF revealed areas under the curve (95% confidence interval) of 0.63 (0.50–0.76) and 0.80 (0.65–0.96), respectively.
Conclusions
In patients with HF, the SI correlated with preoperative walking ability and could predict postoperative walking ability. Among patients who could walk independently before surgery, those with high SIs could walk independently early in the postoperative period. The SI is beneficial for estimating walking ability in patients with HF.
Keywords: Hip fracture, Sarcopenia, Cystatin C, Sarcopenia index, Walking ability, Functional outcome
Introduction
Hip fracture (HF) is common in older patients who sustain low‐energy trauma, such as when falling from standing height or lower. Approximately 1.6 million HFs are sustained worldwide each year—this number is increasing due to ageing populations and is expected to reach 4.5–6.3 million by 2050. 1 , 2 The 1‐year mortality rate of patients with HF is reported to be more than 20%, which is three‐fold that of the age‐matched general population. 3 , 4 Regarding functional outcome, the walking recovery rate in older patients at 1 year after HF is less than 60%, resulting in the need for long‐term care. 5 , 6 Predicting the need for long‐term care as early as possible is important because the care services are smoothly provided. The preoperative factors associated with poor functional outcomes after HF include older age, not living at home, and extracapsular fracture type (trochanteric fracture vs. femoral neck fracture). 7
Sarcopenia, characterized by the loss of skeletal muscle mass, reduced skeletal muscle function, and/or poor physical performance, 8 can lead to reduced quality of life, disability, and increased risk of mortality. 9 Some studies have reported that sarcopenia is an important predictor of functional limitations after sustaining a HF. 7 , 10 For the measurement of skeletal muscle mass, dual‐energy X‐ray absorptiometry or bioelectrical impedance analysis (BIA) is recommended 11 ; however, these methods may be challenging in the case of HF owing to patients experiencing pain during measurement.
The sarcopenia index (SI), which is calculated as serum creatinine (mg/dL)/cystatin C (mg/dL) × 100, is positively correlated with skeletal muscle mass and strength. 12 , 16 Both serum creatinine and cystatin C are well‐known biomarkers of renal function. Serum creatinine is affected not only by renal function but also by skeletal muscle mass, because creatinine is produced in skeletal muscle cells. In contrast, serum cystatin C is largely unaffected by reduced muscle mass, because cystatin C is produced in all nucleated cells. Therefore, the ratio of serum creatinine to cystatin C reflects skeletal muscle mass. 14 However, there are few studies on the use of the SI in patients with HF. 17 , 18 In particular, there are no studies on functional outcome prediction using the SI in these patients. The aim of this study was to evaluate whether the SI of patients with HF accurately reflects preoperative and early postoperative functional outcomes.
Methods
Patients and treatment
This retrospective study was conducted at the North Medical Center, Kyoto Prefectural University of Medicine. This hospital is located in the northern area of Kyoto Prefecture in the midwestern part of Japan. Japan has one of the most rapidly ageing societies globally; in this area, the ratio of individuals aged 65 years and over exceeds 35%, while the ratio of centenarians is 2.5 times the average in Japan. This study was approved by the ethics committee of the Kyoto Prefectural University of Medicine.
Eligible patients were those who were diagnosed with an HF using radiography, computed tomography (CT), and/or magnetic resonance imaging and who were surgically treated at our hospital from January 2020 to May 2021. The exclusion criteria were as follows: age < 65 years, receiving dialysis, treated with conservative therapy, presence of subtrochanteric or pathological fractures, and missing data.
The types of fracture were dichotomized into femoral neck fractures (FNFs) and trochanteric fractures (TFs). Basal fractures of the femoral neck were included in the TF group due to the surgical methods used. FNFs classified as Garden stage I or II were treated with internal fixation using the Prima Hip Screw (Japan Medical Dynamic Marketing; Tokyo, Japan), FNFs classified as Garden stage III or IV were treated with cemented femoral head replacement (FHR) using CMK stems (Zimmer Biomet; Warsaw, IN), and TFs were treated with ORIF using Japanese Proximal Femoral Nail Antirotation implants (PFNA; Depuy Synthes; Raynham, MA).
Laboratory data
Blood samples were obtained prior to surgery, mainly upon admission. In cases where blood tests were performed more than once, results from the first test were used to limit the effect of fracture. The following blood parameters were assessed: serum creatinine, serum cystatin C, serum albumin, lymphocyte count, and haemoglobin concentration. Serum cystatin C was measured using the gold colloid method (SRL Inc.; Tokyo, Japan), while serum creatinine was measured using enzymatic methods.
Assessment of walking ability
Walking ability was dichotomized into an independent and an assistance group. The independent group included patients who walked independently or with a T‐cane, while the assistance group included patients who required wheeled walkers, handrails, or the assistance of others. The patients' preoperative walking ability, either self‐reported or reported by family members and/or facility staff, was divided into a pre‐independent and pre‐assistance group. All postoperative walking data were collected by physiotherapists with ≥5 years of experience who were blinded to their SI. During postoperative rehabilitation, walking practice was performed with parallel bars, then walkers, and then canes, based on patients' stability and lower limb muscle strength as evaluated by the physiotherapists. The criterion of the postoperative walking ability was the ability to walk 20 m with and without a T‐cane at 2 weeks after surgery.
Computed tomography measurements
Computed tomography examinations were performed using a 320‐slice multidetector CT scanner (Siemens Healthineers, Erlangen, Germany) prior to surgery, mainly upon admission. According to previously established methodology, axial images of the iliopsoas at the apex of the femoral head and rectus femoris at the level of the lesser trochanter on the non‐operated side were evaluated if the contralateral fracture had not been surgically treated. 19 The outer edges of the muscles were manually traced, and the CT value (HU) (as an index of muscle quality) and cross‐sectional area (mm2) (as an index of muscle quantity) were calculated using PACS software. To assess the validity of the SI, we examined the correlations of SIs with the CT values and cross‐sectional areas of the measured iliopsoas and rectus femoris muscles. This method was adopted based on the correlation between these muscles and the psoas muscle at the L3 level, which is widely examined to diagnose sarcopenia. 19 , 20
Statistical analysis
Student's t‐test or χ2 test was used to compare the groups. Differences were considered significant at P < 0.05. Pearson's correlation coefficient was used to examine correlations. Logistic regression analysis was used to identify the factors of postoperative walking ability. Receiver operating characteristic (ROC) curves were constructed, and the area under the curve (AUC) was calculated using EZR software (Saitama Medical Center, Jichi Medical University; Saitama, Japan). 21
Results
During the data acquisition period, 150 patients with HF were surgically treated at our hospital. Among these, 20 patients were excluded: 5 were under 65 years of age, 2 were undergoing dialysis, and 13 had missing data. The demographic data of the 130 included patients are presented in Table 1. The mean age (±standard deviation) was 87.8 ± 6.9 years, and 28 patients (21.5%) were male. There were 50 patients with FNFs and 80 with TFs. Thirty‐five patients underwent cemented FHR and 95 underwent internal fixation. Seventy‐seven patients walked independently prior to admission and 53 required assistance.
Table 1.
Demographic data of the patients included in this study
| Variables | Mean ± SD/number (percentage) |
|---|---|
| Age (years) | 87.8 ± 6.9 |
| Sex | |
| Male | 28 (21.5%) |
| Female | 102 (78.5%) |
| Height (cm) | 147.8 ± 9.4 |
| Weight (kg) | 45.3 ± 9.0 |
| Body mass index (kg/m2) | 20.7 ± 3.2 |
| Residence | |
| Home | 91 (70.0%) |
| Other | 39 (30.0%) |
| Fracture type | |
| Femoral neck fracture | 50 (38.5%) |
| Trochanteric fracture | 80 (61.5%) |
| Surgery | |
| Femoral head replacement | 35 (26.9%) |
| Internal fixation | 95 (73.1%) |
| Surgery from admission (days) | 1.3 ± 1.2 |
| Within 2 days from admission | 114 (87.7%) |
| Preoperative walking ability | |
| Independent group | 77 (59.3%) |
| Assistance group | 53 (40.7%) |
| Laboratory data | |
| Serum creatinine (mg/dL) | 0.89 ± 0.47 |
| Serum cystatin C (mg/L) | 1.33 ± 0.57 |
| Serum albumin (g/dL) | 3.5 ± 0.5 |
| Lymphocyte count (102/μL) | 10.1 ± 0.5 |
| Haemoglobin (g/dL) | 11.2 ± 1.8 |
| Sarcopenia index | 66.1 ± 12.4 |
A strong positive correlation was found between serum creatinine and cystatin C in all 130 patients (r = 0.915, P < 0.0001; Figure S1). After dividing preoperative walking ability into a pre‐independent and pre‐assistance group, the correlation between these markers strengthened (pre‐independent group: r = 0.944, P < 0.0001; pre‐assistance group: r = 0.912, P < 0.0001; Figure 1).
Figure 1.
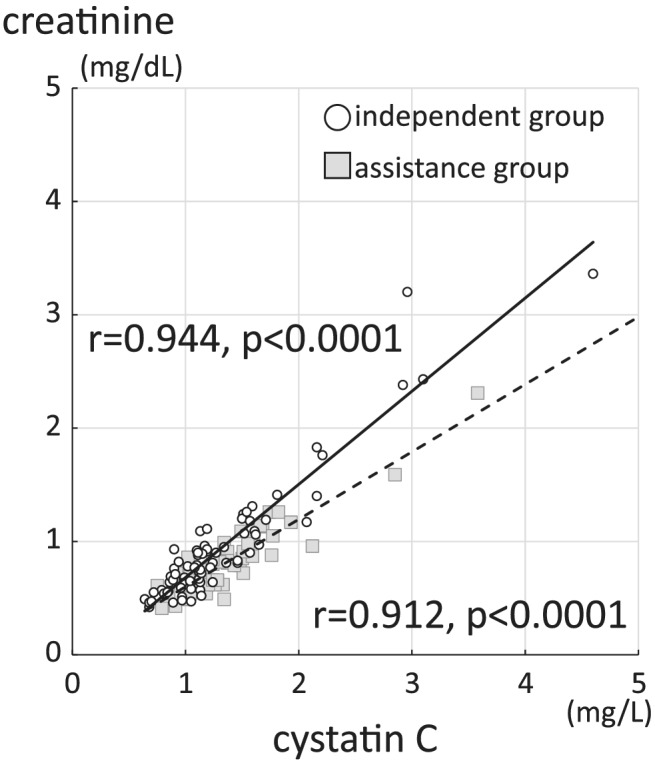
Correlation between serum creatinine and cystatin C in the pre‐independent and pre‐assistance group. The solid line and circle represent the pre‐independent group, while the dotted line and square represent the pre‐assistance group.
Upon examination of the differences between the pre‐independent and pre‐assistance group (Table 2), it was found that age (86.2 ± 7.3 and 90.0 ± 5.8 years, respectively, P = 0.002), serum albumin (3.7 ± 0.4 and 3.4 ± 0.5 g/dL, respectively, P = 0.0006) and haemoglobin concentration (11.6 ± 1.7 and 10.6 ± 1.9 g/dL, respectively, P = 0.001) significantly differed. Although no significant differences between the pre‐independent and pre‐assistance group were found for serum creatinine (0.93 ± 0.55 and 0.83 ± 0.31 mg/dL, respectively, P = 0.23) or serum cystatin C concentration (1.30 ± 0.63 and 1.38 ± 0.48 mg/L, respectively, P = 0.42), the SI, which is their ratio, significantly differed (70.2 ± 12.4 and 60.1 ± 9.8, respectively, P < 0.000001).
Table 2.
Differences between pre‐independent and pre‐assistance group
| Pre‐independent (n = 77) | Pre‐assistance (n = 53) | P value | |
|---|---|---|---|
| Sex | (M: 20, F: 57) | (M: 8, F: 45) | P = 0.21 |
| Age | 86.2 ± 7.3 | 90.0 ± 5.8 | P = 0.002 |
| Residence | (home: 68, other: 9) | (home: 23, other: 30) | P < 0.01 |
| Fracture type | (N: 35, T: 42) | (N: 15, T: 38) | P = 0.07 |
| Surgery | (FHR: 27, IF: 50) | (FHR: 8, IF: 45) | P = 0.02 |
| Surgery from admission (days) | 1.2 ± 1.1 | 1.3 ± 1.4 | P = 0.69 |
| Laboratory data | |||
| Serum creatinine (mg/dL) | 0.93 ± 0.55 | 0.83 ± 0.31 | P = 0.23 |
| Serum cystatin C (mg/L) | 1.30 ± 0.63 | 1.38 ± 0.48 | P = 0.42 |
| Serum albumin (g/dL) | 3.7 ± 0.4 | 3.4 ± 0.5 | P = 0.0006 |
| Lymphocyte count (102/μL) | 10.3 ± 0.5 | 9.7 ± 0.6 | P = 0.50 |
| Haemoglobin (g/dL) | 11.6 ± 1.7 | 10.6 ± 1.9 | P = 0.001 |
| Sarcopenia index | 70.2 ± 12.4 | 60.1 ± 9.8 | P < 0.000001 |
F, female; M, male; N, femoral neck fracture; T, trochanteric fracture.
Examination of the preoperative differences revealed that SIs were significantly, negatively correlated with age but not significantly associated with body mass index (BMI) (r = −0.18, P = 0.04 and r = 0.16, P = 0.07, respectively; Figure S2). The SI was significantly higher for men (75.1 ± 14.4) than for women (63.7 ± 10.6, P < 0.00001) (Table 3). The SI of patients who resided at home prior to admission (67.6 ± 13.2) was significantly higher than that of patients who resided elsewhere (62.7 ± 9.5, P = 0.04). There were no significant differences in the SI based on fracture type or surgical method. Even when sex differences were considered, the SI of women in the pre‐independent group was higher than that of women in the pre‐assistance group (66.8 ± 10.0 and 59.7 ± 10.1, respectively, P = 0.0006). For men, the SI was significantly higher in the pre‐independent group (80.0 ± 13.3) than in the pre‐assistance group (62.7 ± 8.2, P = 0.002).
Table 3.
Differences in the sarcopenia index score based on preoperative characteristics
| Variables | Sarcopenia index, mean ± SD | P value |
|---|---|---|
| Sex | ||
| Male | 75.1 ± 14.4 | |
| Female | 63.7 ± 10.6 | P < 0.00001 |
| Residence | ||
| Home | 67.6 ± 13.2 | |
| Others | 62.7 ± 9.5 | P = 0.04 |
| Fracture type | ||
| Femoral neck fracture | 66.1 ± 15.1 | |
| Trochanteric fracture | 66.2 ± 10.5 | P = 0.97 |
| Surgery | ||
| Femoral head replacement | 66.8 ± 16.4 | |
| Internal fixation | 65.9 ± 10.7 | P = 0.72 |
| Preoperative walking ability | ||
| Pre‐independent group | 70.2 ± 12.4 | |
| Pre‐assistance group | 60.1 ± 9.8 | P < 0.000001 |
| Male, pre‐independent | 80.0 ± 13.3 | |
| Male, pre‐assistance | 62.7 ± 8.2 | P = 0.002 |
| Female, pre‐independent | 66.8 ± 10.0 | |
| Female, pre‐assistance | 59.7 ± 10.1 | P = 0.0006 |
Boldface P values indicate a significant difference (P < 0.05).
Computed tomography measurements were performed on the contralateral side in 105 patients without HF. The correlation of the SI with the CT value and cross‐sectional area of the iliopsoas at the apex of the femoral head was positive and significant (r = 0.40, P < 0.001 and r = 0.49, P < 0.001, respectively; Figure 2A,B), while the correlation of the SI with the CT value and cross‐sectional area of the rectus femoris at the level of the lesser trochanter was weak but significant (r = 0.26, P = 0.007 and r = 0.37, P < 0.001, respectively; Figure 2C,D). The SI/BMI ratio had a stronger correlation with the CT value (iliopsoas: r = 0.49, P < 0.001; rectus femoris: r = 0.40, P < 0.001) than did the SI alone (Figure S3).
Figure 2.
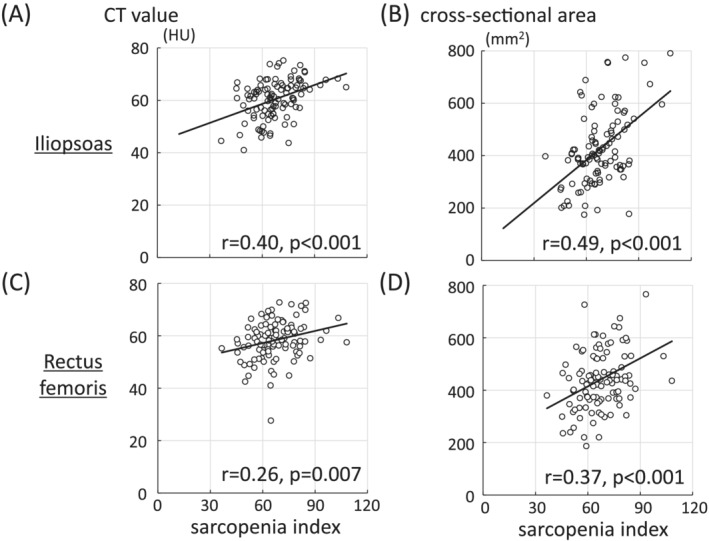
Correlation of the sarcopenia index with the (A) computed tomography (CT) value and (B) cross‐sectional area of the iliopsoas at the apex of the femoral head, and the (C) CT value and cross‐sectional area of the rectus femoris at the level of the lesser trochanter (D).
We also investigated walking ability 2 weeks after surgery. Thirty‐one patients were included in the post‐independence group and 99 in the post‐assistance group (Table 4). In the post‐independent group, 10 patients were male and 21 were female; all patients resided at home prior to admission. Serum albumin levels prior to surgery were significantly different between the post‐independent and post‐assistance groups (3.7 ± 0.5 and 3.5 ± 0.5 g/L, respectively, P = 0.04). Any other blood parameter data, including haemoglobin concentration prior to surgery, were not significantly different between the post‐independent and post‐assistance groups. However, the SI in the post‐independence group (73.0 ± 14.9) was significantly higher than that in the post‐assistance group (64.0 ± 10.7, P = 0.0003). For men, the SIs in the post‐independence and post‐assistance groups were 83.9 ± 16.6 and 70.2 ± 10.5 (P = 0.01), respectively. For women, the SI in the post‐independence and post‐assistance groups was 67.8 ± 11.0 and 62.6 ± 10.4 (P = 0.04). Logistic regression analysis showed that the SI [odds ratio (OR), 95% confidence interval (CI): 0.94, 0.91–0.98; P = 0.003], age (OR, 95% CI: 1.09, 1.02–1.17; P = 0.008), and TF (OR, 95% CI: 3.32, 1.31–8.43: P = 0.01) were predictors of postoperative walking ability (Table 5). The ROC analysis of the preoperative SI and postoperative walking ability for all patients and for the patients diagnosed with FNF is illustrated in Figure 3. The AUC (95% CI) for all patients and the patients diagnosed with FNF was 0.68 (0.57–0.80) and 0.77 (0.62–0.93), respectively (Figure 3A,B). The cut‐off value of the SI, determined by Youden's index, for all patients and for the patients diagnosed with FNF were 68.7 (sensitivity: 0.72, specificity: 0.68) and 70.0 (sensitivity: 0.90, specificity: 0.68), respectively.
Table 4.
Differences between the post‐independent and post‐assistance group
| Post‐independent, mean ± SD | Post‐assistance, mean ± SD | P value | |
|---|---|---|---|
| Sex | (M:10, F:21) | (M:18, F:81) | P = 0.16 |
| Age | 84.1 ± 7.8 | 88.9 ± 6.2 | P = 0.0005 |
| Residence | (home: 31, other: 0) | (home: 60, other: 39) | |
| Laboratory data | |||
| Serum creatinine (mg/dL) | 0.87 ± 0.52 | 0.89 ± 0.45 | P = 0.84 |
| Serum cystatin C (mg/L) | 1.16 ± 0.48 | 1.38 ± 0.59 | P = 0.07 |
| Serum albumin (g/dL) | 3.7 ± 0.5 | 3.5 ± 0.5 | P = 0.04 |
| Lymphocyte count (102/μL) | 10.7 ± 4.0 | 9.9 ± 5.7 | P = 0.44 |
| Haemoglobin (g/dL) | 11.7 ± 1.7 | 11.0 ± 1.8 | P = 0.08 |
| Sarcopenia index | |||
| Total | 73.0 ± 14.9 | 64.0 ± 10.7 | P = 0.0003 |
| Male | 83.9 ± 16.6 | 70.2 ± 10.5 | P = 0.01 |
| Female | 67.8 ± 11.0 | 62.6 ± 10.4 | P = 0.04 |
Boldface P values indicate a significant difference (P < 0.05).
Table 5.
Logistic regression analysis of the relationship between postoperative walking ability and the sarcopenia index, age, and fracture type
| OR | 95%CI | P value | |
|---|---|---|---|
| Sarcopenia index | 0.94 | 0.91–0.98 | P = 0.003 |
| Age | 1.09 | 1.02–1.17 | P = 0.008 |
| Fracture type (TF) | 3.32 | 1.31–8.43 | P = 0.01 |
95%CI, 95% confidence interval; OR, odds ratio; TF, trochanteric fracture.
Boldface P values indicate a significant difference (P < 0.05).
Figure 3.
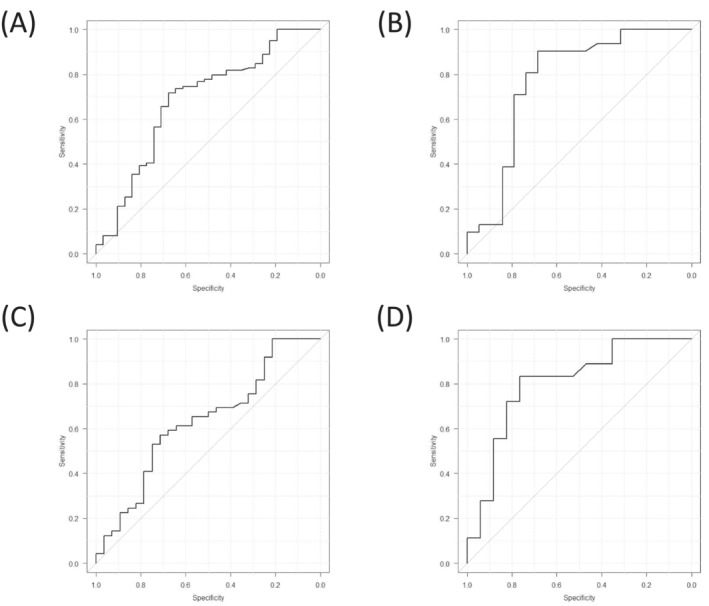
Receiver operating characteristic analysis of the preoperative sarcopenia index and the postoperative walking ability in (A) all patients, (B) femoral neck fracture patients, (C) all patients in the pre‐independent group and patients with femoral neck fracture in the pre‐independent group (D).
In the pre‐independent group, 28 patients (36%) walked independently at 2 weeks after surgery (post‐independent group) and 49 (64%) walked with assistance (post‐assistance group; Table 6). The SIs of the post‐independence and post‐assistance groups were 74.8 ± 14.0 and 67.7 ± 10.6 (P = 0.01), respectively, although, when considering sex differences, the significance disappeared. For patients diagnosed with FNF, the SI was significantly higher in the post‐independent group (78.6 ± 15.7) than in the post‐assistance group (63.2 ± 10.9, P = 0.002). For women diagnosed with FNFs, the SI was significantly higher in the post‐independent group (72.7 ± 10.8) than in the post‐assistance group (61.6 ± 10.7, P = 0.03); for all patients and women diagnosed with TF, there were no significant differences between groups. Logistic regression analysis showed that the SI (OR, 95% CI: 0.95, 0.91–0.99; P = 0.03) and age (OR, 95% CI: 1.09, 1.02–1.18; P = 0.02) were predictors of postoperative walking ability (Table 7). ROC analysis revealed that the AUCs (95% CI) for all patients and patients diagnosed with FNF in the pre‐independent group were 0.63 (0.50–0.76) and 0.80 (0.65–0.96), respectively (Figure 3C,D). The cut‐off values for the SI, determined by Youden's index, for all patients and patients diagnosed with FNF in the pre‐independent group were 67.7 (sensitivity: 0.57, specificity: 0.71) and 69.6 (sensitivity: 0.83, specificity: 0.77), respectively.
Table 6.
Differences in the sarcopenia index between the post‐independent and post‐assistance group (pre‐independent group)
| Post‐independent, mean ± SD (number) | Post‐assistance, mean ± SD (number) | P value | |
|---|---|---|---|
| Pre‐independent | |||
| Total | 74.8 ± 14.0 (28) | 67.7 ± 10.6 (49) | P = 0.01 |
| Male | 82.8 ± 16.4 (11) | 75.4 ± 7.4 (13) | P = 0.16 |
| Female | 69.5 ± 9.4 (17) | 64.9 ± 10.3 (36) | P = 0.12 |
| Fracture type | |||
| Total, FNF | 78.6 ± 15.7 (17) | 63.2 ± 10.9 (18) | P = 0.002 |
| Total, TF | 68.9 ± 8.4 (11) | 70.2 ± 9.8 (31) | P = 0.12 |
| Female, FNF | 72.7 ± 10.8 (8) | 61.6 ± 10.7 (15) | P = 0.03 |
| Female, TF | 66.7 ± 7.5 (9) | 67.1 ± 9.6(21) | P = 0.89 |
FNF, femoral neck fracture, TF, trochanteric fracture.
Numbers in parentheses indicate the number of patients. Boldface values indicate a significant difference (P < 0.05).
Table 7.
Logistic regression analysis of the relationship between postoperative walking ability and the sarcopenia index, age, and fracture type (pre‐independent group)
| OR | 95%CI | P value | |
|---|---|---|---|
| Sarcopenia index | 0.95 | 0.91–0.99 | P = 0.03 |
| Age | 1.09 | 1.02–1.18 | P = 0.02 |
| Fracture type (TF) | 2.57 | 0.91–7.3 | P = 0.08 |
95%CI, 95% confidence interval; OR, odds ratio; TF, trochanteric fracture.
Boldface values indicate a significant difference (P < 0.05).
Discussion
In this study, we determined the SI of patients with HF prior to surgery and found that it not only reflected preoperative but also postoperative walking ability. It should be noted that even though the patients with HF were able to walk independently prior to surgery, the SI could predict their walking ability 2 weeks after surgery. To the best of our knowledge, this is the first study to assess the relationship between the SI and preoperative and early postoperative functional outcomes in patients with HF.
Sarcopenia is diagnosed by low muscle mass, low muscle strength, and/or poor physical performance. The SI was originally designed to estimate skeletal muscle mass in patients admitted to the intensive care unit. 14 It has been shown to correlate with mortality, 22 , 23 short‐term postoperative complications, 24 skeletal muscle mass, 16 , 25 , 26 and skeletal muscle strength including hand grip strength and gait speed. 15 , 16 , 22 , 25 Consistent with these studies showing the correlation with muscle function, the SI of patients with HF in this study correlated with preoperative walking ability. The SI correlated significantly with postoperative walking ability, although this correlation was weaker than that with preoperative walking ability, partly because of postoperative pain. FNFs are intra‐articular fractures that involve less bleeding than do extra‐articular fractures; TFs and cemented FHRs are associated with less postoperative pain owing to their high initial fixation strength. 27 , 28 A stronger correlation between SIs and postoperative walking ability was confirmed in cases of FNF. These results reflect the usefulness of the SI for predicting walking ability.
A systematic review and meta‐analysis revealed that patients with HF and sarcopenia have a high risk of mortality and poor functional outcomes. 7 , 29 The Sarcopenia Working Group recommends measuring skeletal muscle mass using dual‐energy X‐ray absorptiometry or BIA; however, in patients presenting with HF prior to surgery, the use of these methods is challenging. To address this issue, grip strength has been used to estimate muscle function and was found to predict functional outcomes. 30 Previous reports have shown that preoperative hypoalbuminemia is related to limited functioning prior to surgery and predicts poor functional outcomes 31 , 32 ; we found an albumin concentration within the normal range to positively correlate with preoperative and early postoperative walking ability. However, the SI strongly correlated with preoperative and early postoperative walking ability. Given that blood tests are essential prior to surgery, the SI is the simplest and least invasive method to estimate muscle function.
In our study, the SI was a good predictor of postoperative walking ability, even when patients were able to walk independently. Moreover, a recent study reported that the glomerular filtration rate estimated from serum creatinine was overestimated in patients with HF and decreased skeletal muscle mass. 17 The estimated glomerular filtration rate is important for determining the required antibiotic dose and dosage schedule. Considering the high prevalence of sarcopenia in patients with HF, 33 we concluded that the preoperative serum cystatin C concentration should be measured in patients with HF.
Computed tomography measurements of the lower limb muscles are a well‐established marker of physical function, such as walking ability. 34 , 35 In this study, skeletal muscle loss was determined as the loss of muscle quality (CT value) and quantity (cross‐sectional area) on CT. Because the CT value of fat is lower than that of skeletal muscle, the difference in CT value is often used in studies on sarcopenia. Some papers reported that psoas muscle attenuation in the lumbar area in patients with HF was useful for predicting mortality. 36 , 38 Although fatty degeneration of the mid‐thigh muscle measured by CT in patients with HF correlates with the bone mineral density of the entire hip, 39 CT measurement in patients with HF is currently limited to predicting functional outcomes. In this study, there was a weak correlation between the SI and skeletal muscle loss around the hip on CT. Consistent with our data, the correlations between the SI and skeletal muscle mass in previous reports were not high. The correlation coefficients in a study by Yang et al., who evaluated the correlations of the SI with the CT value and cross‐sectional areas of muscles at the L3 level, were r = 0.378 and r = 0.375, 40 while that in a study by Hirai et al., who reported the correlations between the SI and the skeletal muscle mass using BIA, was r = 0.44. 41 Although we showed that the SI was a good predictor of postoperative functional outcome, given that there are not always correlations between the skeletal muscle mass and physical performance in older individuals, 42 the SI should be cautiously used as a surrogate maker for the evaluation of sarcopenia. Nishida et al. reported that the SI/body weight ratio has a stronger correlation with the skeletal muscle index than does the SI alone. 13 Therefore, we also examined the correlation between the SI/BMI ratio and the CT value—the BMI‐adjusted SI had a stronger positive correlation with the CT value than did the SI alone. Thus, further research on CT and the SI is needed.
A clear sex difference in SIs was observed in our study, consistent with several previous studies. 12 , 16 Creatinine is produced in the skeletal muscle cells, and the serum concentration in men is generally higher than that in women; this indicates that the skeletal muscle mass is greater in men than in women. Diagnostic criteria for sarcopenia are also different for men and women. 11 Given these points, we considered that a sex difference in SIs was observed.
This study had several limitations. First, it had a retrospective design, a relatively small sample size, and was conducted at a single hospital. Second, we did not examine the criteria for a sarcopenia diagnosis, such as handgrip strength or skeletal muscle mass. However, the results related to the SI were consistent with those from previous reports and muscle mass properties. To confirm the validity of our results, a prospective study that measures muscle strength and/or muscle mass in a larger number of patients should be conducted. Although we did not examine bone mineral density, sarcopenia and osteoporosis are correlated with each other. HFs occurred in osteoporotic patients. Future studies of the relationship between SIs and bone mineral density are needed. Third, we exclusively assessed short‐term functional outcomes—because medical records were followed during the course of hospitalization only, further follow‐up was not possible. However, short‐term functional outcomes correlate with 1‐year mortality and walking ability, 43 , 44 and therefore, the primary goal of rehabilitation is to walk independently as early as possible. The strength of our study was that all postoperative mobility evaluations were performed by skilled physiotherapists; the mean hospitalization period in Japan is reportedly more than 30 days, which is longer than that in other countries. 45
In conclusion, we evaluated the correlation of the SI with preoperative blood tests and walking ability in 130 patients with HF. The SI strongly correlated with preoperative walking ability and was able to predict postoperative walking ability. Among patients who could walk independently prior to surgery, those with a high SI score were able to walk independently early in the postoperative recovery period. The SI was beneficial for estimating walking ability in patients with HF.
Supporting information
Figure S1. The correlation between serum creatinine and cystatin C
Figure S2. The correlation of the sarcopenia index with (A) age and (B) body mass index
Figure S3. The correlation between the sarcopenia index/body mass index ratio and the computed tomography values of the (A) iliopsoas at the apex of the femoral head and the (B) rectus femoris at the level of the lesser trochanter
Acknowledgements
This study received no external funding. Naoki Okubo, Takashi Yoshida, Kazuya Tanaka, Naoya Okada, Kunihiko Hosoi, Masato Ohara, and Kenji Takahashi declare that they have no conflict of interest. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval was obtained from the ethics committee of the Kyoto Prefectural University of Medicine. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 46 NOkubo, TY, and KT contributed to the conception and design of the study. NOkubo, KT, NOkada, KH, MO, and TY participated in data collection. NOkubo, MO, and TY performed the statistical analysis. NOkubo, TY, and KT drafted the manuscript. All authors read and approved the final manuscript. We would like to thank Editage (www.editage.com) for English language editing.
Okubo N., Yoshida T., Tanaka K., Okada N., Hosoi K., Ohara M., and Takahashi K. (2022) Serum creatinine to cystatin C ratio reflects preoperative and early postoperative walking ability in older patients with hip fracture, Journal of Cachexia, Sarcopenia and Muscle, 13, 945–954, 10.1002/jcsm.12940
References
- 1. Gullberg B, Johnell O, Kanis JA. World‐wide projections for hip fracture. Osteoporos Int 1997;7:407–413. [DOI] [PubMed] [Google Scholar]
- 2. Cooper C, Campion G, Melton LJ 3rd. Hip fractures in the elderly: a world‐wide projection. Osteoporos Int 1992;2:285–289. [DOI] [PubMed] [Google Scholar]
- 3. Gundel O, Thygesen LC, Gögenur I, Ekeloef S. Postoperative mortality after a hip fracture over a 15‐year period in Denmark: a national register study. Acta Orthop 2020;91:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldacre MJ, Roberts SE, Yeates D. Mortality after admission to hospital with fractured neck of femur: database study. BMJ 2002;325:868–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kyo T, Takaoka K, Ono K. Femoral neck fracture. Factors related to ambulation and prognosis. Clin Orthop Relat Res 1993;215–222. [PubMed] [Google Scholar]
- 6. Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002;359:1761–1767. [DOI] [PubMed] [Google Scholar]
- 7. Xu BY, Yan S, Low LL, Vasanwala FF, Low SG. Predictors of poor functional outcomes and mortality in patients with hip fracture: a systematic review. BMC Musculoskelet Disord 2019;20:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenberg IH. Summary comments. Am J Clin Nutr 1989;50:1231–1233. [Google Scholar]
- 9. Woo J, Leung J, Morley JE. Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc 2015;16:247–252. [DOI] [PubMed] [Google Scholar]
- 10. González‐Montalvo JI, Alarcón T, Gotor P, Queipo R, Velasco R, Hoyos R, et al. Prevalence of sarcopenia in acute hip fracture patients and its influence on short‐term clinical outcome. Geriatr Gerontol Int 2016;16:1021–1027. [DOI] [PubMed] [Google Scholar]
- 11. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Osaka T, Hamaguchi M, Hashimoto Y, Ushigome E, Tanaka M, Yamazaki M, et al. Decreased the creatinine to cystatin C ratio is a surrogate marker of sarcopenia in patients with type 2 diabetes. Diabetes Res Clin Pract 2018;139:52–58. [DOI] [PubMed] [Google Scholar]
- 13. Nishida K, Hashimoto Y, Kaji A, Okamura T, Sakai R, Kitagawa N, et al. Creatinine/(cystatin C × body weight) ratio is associated with skeletal muscle mass index. Endocr J 2020;67:733–740. [DOI] [PubMed] [Google Scholar]
- 14. Kashani KB, Frazee EN, Kukrálová L, Sarvottam K, Herasevich V, Young PM, et al. Evaluating muscle mass by using markers of kidney function: development of the sarcopenia index. Crit Care Med 2017;45:e23–e29. [DOI] [PubMed] [Google Scholar]
- 15. Ichikawa T, Miyaaki H, Miuma S, Motoyoshi Y, Yamashima M, Yamamichi S, et al. Indices calculated by serum creatinine and cystatin C as predictors of liver damage, muscle strength and sarcopenia in liver disease. Biomed Rep 2020;12:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin YL, Chen SY, Lai YH, Wang CH, Kuo CH, Liou HH, et al. Serum creatinine to cystatin C ratio predicts skeletal muscle mass and strength in patients with non‐dialysis chronic kidney disease. Clin Nutr 2019;39:2435–2441. [DOI] [PubMed] [Google Scholar]
- 17. Iacomelli I, Giordano A, Rivasi G, Rafanelli M, Tortù V, Cartei A, et al. Low creatinine potentially overestimates glomerular filtration rate in older fracture patients: a plea for an extensive use of cystatin C? Eur J Intern Med 2020;84:74–79. [DOI] [PubMed] [Google Scholar]
- 18. M HJ, Åkesson A, Hommel A, Grubb A, Bentzer P. Markers of renal function at admission and mortality in hip fracture patients—a single center prospective observational study. Scand J Clin Lab Invest 2021;81(3):201–207. 10.1080/00365513.2021.1884892 [DOI] [PubMed] [Google Scholar]
- 19. Zannoni S, Albano D, Jannone ML, Messina C, Sconfienza LM. Correlation between muscle mass and quality around the hip and of psoas muscles at L3 level using unenhanced CT scans. Skeletal Radiol 2020;49:1649–1655. [DOI] [PubMed] [Google Scholar]
- 20. Bahat G, Turkmen BO, Aliyev S, Catikkas NM, Bakir B, Karan MA. Cut‐off values of skeletal muscle index and psoas muscle index at L3 vertebra level by computerized tomography to assess low muscle mass. Clin Nutr 2021;40:4360–4365. [DOI] [PubMed] [Google Scholar]
- 21. Kanda Y. Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang T, Zhuo Y, Xie L, Wang H, Yang M. Sarcopenia index based on serum creatinine and cystatin C is associated with 3‐year mortality in hospitalized older patients. Sci Rep 2020;10:1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ziolkowski SL, Long J, Baker JF, Chertow GM, Leonard MB. Relative sarcopenia and mortality and the modifying effects of chronic kidney disease and adiposity. J Cachexia Sarcopenia Muscle 2019;10:338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang J, Zhang T, Feng D, Dai X, Lv T, Wang X, et al. A new diagnostic index for sarcopenia and its association with short‐term postoperative complications in patients undergoing surgery for colorectal cancer. Colorectal Dis 2019;21:538–547. [DOI] [PubMed] [Google Scholar]
- 25. Ichikawa T, Miyaaki H, Miuma S, Motoyoshi Y, Yamashima M, Yamamichi S, et al. Calculated body muscle mass as a useful screening marker for low skeletal muscle mass and sarcopenia in chronic liver disease. Hepatol Res 2020;50:704–714. [DOI] [PubMed] [Google Scholar]
- 26. Yanishi M, Kinoshita H, Tsukaguchi H, Kimura Y, Koito Y, Sugi M, et al. The creatinine/cystatin C ratio provides effective evaluation of muscle mass in kidney transplant recipients. Int Urol Nephrol 2019;51:79–83. [DOI] [PubMed] [Google Scholar]
- 27. Schmitz P, Gueorguiev B, Zderic I, Pfeifer C, Nerlich M, Grechenig S. Primary stability in total hip replacement: a biomechanical investigation. Medicine (Baltimore) 2017;96:e8278, 10.1097/MD.0000000000008278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lo WH, Chen WM, Huang CK, Chen TH, Chiu FY, Chen CM. Bateman bipolar hemiarthroplasty for displaced intracapsular femoral neck fractures. Uncemented versus cemented. Clin Orthop Relat Res 1994;75–82, 10.1097/00003086-199405000-00014 [DOI] [PubMed] [Google Scholar]
- 29. Smith T, Pelpola K, Ball M, Ong A, Myint PK. Pre‐operative indicators for mortality following hip fracture surgery: a systematic review and meta‐analysis. Age Ageing 2014;43:464–471. [DOI] [PubMed] [Google Scholar]
- 30. Selakovic I, Dubljanin‐Raspopovic E, Markovic‐Denic L, Marusic V, Cirkovic A, Kadija M, et al. Can early assessment of hand grip strength in older hip fracture patients predict functional outcome? PLoS ONE 2019;14:e0213223, 10.1371/journal.pone.0213223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sim SD, Sim YE, Tay K, Howe TS, Png MA, Chang CCP, et al. Preoperative hypoalbuminemia: poor functional outcomes and quality of life after hip fracture surgery. Bone 2021;143:115567, 10.1016/j.bone.2020.115567 [DOI] [PubMed] [Google Scholar]
- 32. Ryan S, Politzer C, Fletcher A, Bolognesi M, Seyler T. Preoperative hypoalbuminemia predicts poor short‐term outcomes for hip fracture surgery. Orthopedics 2018;41:e789–e796. [DOI] [PubMed] [Google Scholar]
- 33. Chen YP, Wong PK, Tsai MJ, Chang WC, Hsieh TS, Leu TH, et al. The high prevalence of sarcopenia and its associated outcomes following hip surgery in Taiwanese geriatric patients with a hip fracture. J Formos Med Assoc 2020;119:1807–1816. [DOI] [PubMed] [Google Scholar]
- 34. Harris‐Love MO, Avila NA, Adams B, Zhou J, Seamon B, Ismail C, et al. The comparative associations of ultrasound and computed tomography estimates of muscle quality with physical performance and metabolic parameters in older men. J Clin Med 2018;7: 10.3390/jcm7100340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berg HE, Truong D, Skoglund E, Gustafsson T, Lundberg TR. Threshold‐automated CT measurements of muscle size and radiological attenuation in multiple lower‐extremity muscles of older individuals. Clin Physiol Funct Imaging 2020;40:165–172. [DOI] [PubMed] [Google Scholar]
- 36. Bae SJ, Lee SH. Computed tomographic measurements of the psoas muscle as a predictor of mortality in hip fracture patients: muscle attenuation helps predict mortality in hip fracture patients. Injury 2020;52:1456–1461. [DOI] [PubMed] [Google Scholar]
- 37. Boutin RD, Bamrungchart S, Bateni CP, Beavers DP, Beavers KM, Meehan JP, et al. CT of patients with hip fracture: muscle size and attenuation help predict mortality. AJR Am J Roentgenol 2017;208:W208–w15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Byun SE, Kim S, Kim KH, Ha YC. Psoas cross‐sectional area as a predictor of mortality and a diagnostic tool for sarcopenia in hip fracture patients. J Bone Miner Metab 2019;37:871–879. [DOI] [PubMed] [Google Scholar]
- 39. Hahn MH, Won YY. Bone mineral density and fatty degeneration of thigh muscles measured by computed tomography in hip fracture patients. J Bone Metab 2016;23:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang Q, Zhang M, Sun P, Li Y, Xu H, Wang K, et al. Cre/CysC ratio may predict muscle composition and is associated with glucose disposal ability and macrovascular disease in patients with type 2 diabetes. BMJ Open Diabetes Res Care 2021;9:e002430, 10.1136/bmjdrc-2021-002430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hirai K, Tanaka A, Homma T, Goto Y, Akimoto K, Uno T, et al. Serum creatinine/cystatin C ratio as a surrogate marker for sarcopenia in patients with chronic obstructive pulmonary disease. Clin Nutr 2021;40:1274–1280. [DOI] [PubMed] [Google Scholar]
- 42. Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev 2013;35:51–65. [DOI] [PubMed] [Google Scholar]
- 43. Dubljanin‐Raspopović E, Marković‐Denić L, Marinković J, Nedeljković U, Bumbaširević M. Does early functional outcome predict 1‐year mortality in elderly patients with hip fracture? Clin Orthop Relat Res 2013;471:2703–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fukui N, Watanabe Y, Nakano T, Sawaguchi T, Matsushita T. Predictors for ambulatory ability and the change in ADL after hip fracture in patients with different levels of mobility before injury: a 1‐year prospective cohort study. J Orthop Trauma 2012;26:163–171. [DOI] [PubMed] [Google Scholar]
- 45. Hagino H, Endo N, Harada A, Iwamoto J, Mashiba T, Mori S, et al. Survey of hip fractures in Japan: recent trends in prevalence and treatment. J Orthop Sci 2017;22:909–914. [DOI] [PubMed] [Google Scholar]
- 46. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the journal of cachexia, sarcopenia and muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The correlation between serum creatinine and cystatin C
Figure S2. The correlation of the sarcopenia index with (A) age and (B) body mass index
Figure S3. The correlation between the sarcopenia index/body mass index ratio and the computed tomography values of the (A) iliopsoas at the apex of the femoral head and the (B) rectus femoris at the level of the lesser trochanter


