Abstract
Sensory impairments and sarcopenia are both highly prevalent age‐related conditions, with the former having been postulated to contribute to the pathogenesis of the latter condition. Confirming this hypothesis may therefore help to better inform strategies for early treatment and intervention of sarcopenia. We performed a systematic review of the current literature examining the relationships between four major sensory impairments [vision (VI), hearing (HI), smell (SI), and taste (TI)] with (i) sarcopenia; and (ii) its associated components (low handgrip strength, slow gait speed, and low muscle mass). PubMed, EMBASE, CINAHL, and Cochrane Library databases were searched for observational studies investigating the relationship of VI, HI, SI, and TI with sarcopenia, low handgrip strength, slow gait speed, and low muscle mass, in adults aged 50 years or older, from inception until 24 May 2021. The risk of bias of the included studies was assessed using the Newcastle‐Ottawa Scale. This study was registered with PROSPERO, reference CRD42021247967. Ten cross‐sectional and three longitudinal population‐based studies of community‐dwelling adults (N = 68 235) were included, with five studies investigating more than one sensory impairment. In total, 8, 6, 3, and 1 studies investigated the relationship between VI, HI, SI, and TI and sarcopenia and its related components, respectively. Follow‐up duration for the longitudinal studies ranged from 4 to 11 years. All studies had a low or moderate risk of bias. We found that the presence of VI and SI, but not TI, independently increased the odds of sarcopenia. In addition, VI and SI were each independently associated with low muscle mass; and VI, HI, and SI were each independently associated with slow gait speed. However, we found inconclusive evidence for the associations between VI, HI and SI, and low handgrip strength. Our systematic review suggests a potential association between the presence of single or multiple sensory impairments and a greater likelihood of sarcopenia and/or deficits in its associated components, especially for VI, HI, and SI. Prospective studies are needed to untangle the relationship between sensory impairment and sarcopenia to better inform clinical guidelines for disease prevention and management.
Keywords: Sarcopenia, Sensory impairment, Visual impairment, Hearing impairment, Taste impairment, Smell impairment
Introduction
The world is undergoing an ageing demographic shift, with 1 in 6 people anticipated to be aged ≥65 years by 2050, up from 1 in 11 in 2019. 1 As such, there is growing demand for the development and implementation of interventions to prevent or delay the detrimental consequences of age‐related health conditions contributing to disability and dependence in older adults. 2 Research into sarcopenia, a skeletal muscle disorder that produces a progressive decline in skeletal muscle mass and function, is increasingly gaining traction, with the World Health Organization classifying it as a geriatric syndrome in 2016. 3 , 4 The global prevalence of sarcopenia in community‐dwelling older adults ranged between 10% and 40% depending on the definition used. 5 The most widely accepted one requires clinical findings of low muscle mass, plus low muscle strength, and/or low physical performance. 6 , 7 The adverse consequences of sarcopenia on the individual and society include physical disabilities, frailty, falls, cognitive impairment, depression, decreased quality of life (QoL), nursing home admission, and even death. 8 , 9 , 10 , 11 Although a recent systematic literature review of RCTs has shown potential improvements in the component measures of sarcopenia, that is, muscle mass, strength, and/or physical function via exercise and nutritional interventions in sarcopenic older adults, 12 it remains unclear whether sarcopenia itself can be prevented or reversed by early detection and interventions. Therefore, a better understanding of other potential modifiable risk factors for sarcopenia and its component measures is vital for informing novel alternating strategies to prevent, delay, or even reverse it.
Age is an established risk factor for declines in the visual, auditory, olfactory, and gustatory systems. 13 The global prevalence of these sensory losses are high, with up to 40%, 37%, and 41% of adults aged ≥60 years estimated to have visual impairment (VI), hearing impairment (HI) and smell impairment (SI), respectively. 14 , 15 , 16 Although there are no global data on taste impairment (TI), a US study measuring the full spectrum of age‐related sensory impairments reported that TI (74%) was the most prevalent, suggesting a correspondingly high global prevalence. 17 Individuals with sensory impairments are at high risk of profound consequences including cognitive impairment, frailty, falls, reduced QoL, poor nutrition, mobility dysfunction, and mortality. 18 , 19 , 20 , 21 Given the high prevalence rates of sarcopenia and sensory impairment in older adults, their associations with other geriatric syndromes and the presence of several shared health‐related risk factors. 5 , 14 , 15 , 16 , 17 A direct relationship between the two has been hypothesized. 22 , 23 Establishing this relationship is important, as sensory impairments can be managed effectively with sensory interventions such as cataract surgery, provision of glasses and hearing aids, and olfactory management. 24 , 25 , 26 , 27 Specifically, cross‐sectional 22 , 28 , 29 , 30 , 31 and longitudinal 32 , 33 , 34 studies have documented the association between sensory impairments and the associated components of sarcopenia. As such, sensory impairments may represent an early indicator and modifiable risk factor of sarcopenia. However, there is no comprehensive review on the relationship between sensory impairments and the presence of sarcopenia and its components.
To address this gap, we performed a systematic review of observational studies evaluating the associations between sensory impairments (VI, HI, SI, and TI) with sarcopenia and associated components, including handgrip strength, muscle mass, and gait speed. We hypothesize that sensory impairment is an independent risk factor of sarcopenia and its associated components. We also identify key knowledge gaps and suggest future research directions.
Methods
Search strategy
The PubMed, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Cochrane Library databases were searched for English language research articles published from inception until 24 May 2021. Searches were conducted systematically by using pre‐determined controlled vocabulary terms [e.g. Medical Subject Headings (MeSH) term used in PubMed] and keywords based on the two health conditions: sensory impairment (i.e. VI, HI, SI, and TI) and sarcopenia and its component measures (low muscle mass, weak muscle strength, and reduced physical performance). We chose handgrip strength and gait speed as proxy measures for muscle strength and physical performance, respectively, because they are strongly recommended by the International Clinical Practice Guidelines for Sarcopenia as feasible and valid measurements of these two components. 35 Relevant references identified from the bibliographies of pertinent articles were also retrieved. Our full search strategy and Preferred Reporting Items for Systematic Review and Meta‐Analyses (PRISMA) checklist are reported in the Supporting Information, Data S1 and S2. The review protocol is available on PROSPERO (http://www.crd.york.ac.uk/prospero/, reference CRD42021247967).
Eligibility criteria
Our inclusion criteria were (i) study design: observational studies (cross‐sectional, longitudinal, and case–control); (ii) population: adults aged ≥50 years within well‐defined populations (community‐dwelling, hospital, and nursing home/geriatric settings); (iii) exposure: sensory impairment (VI, HI, SI, or TI) measured objectively (e.g. Snellen chart, pure‐tone audiometry, sniffing sticks, and taste solutions of varying concentrations) as well as self‐report (e.g. answering a question about whether they have VI); (iv) outcomes: sarcopenia and its component measures, including low muscle mass, weak handgrip strength and slow gait speed. Studies that included continuous measures of muscle mass [e.g. appendicular skeletal muscle mass (ASM), ASM/height2], handgrip strength or gait speed were also included, and no other restrictions were placed on the definition of sarcopenia or the methods used to measure its defining components for this review; (v) studies published in English and; (vi) studies that included human participants.
Studies were excluded if they (i) were literature reviews, qualitative studies, letters, editorials, commentaries, economic evaluations, guidelines, protocols, and book chapters, (ii) focused on specific subpopulations/risk groups (e.g. individuals with cancer or cardiovascular disease), (iii) investigated disease‐specific instead of sensory‐specific associations (e.g. cataract or glaucoma instead of VI).
Selection process
Studies were retrieved by one author (K. C. H.) and independently verified by a second co‐author (P. G.). All discrepancies were resolved by consensus, and in cases where no consensus could be reached, the senior author (E. L.) was consulted.
Data extraction and data synthesis
Using a pre‐identified form, data extraction and risk of bias assessment were performed by one author (K. C. H.) and vetted by a co‐author (P. G.). Information extracted included author, year of publication, journal of publication, country, study design, number of study participants, age range, type of population, sex distribution, definitions of sensory impairment and sarcopenia and its components (including measurement methods and cut‐points), prevalence of sensory impairment/sarcopenia in sample (percentage/number of participants), and reported risk estimates [e.g. odds ratio (OR)] between sensory impairment and sarcopenia and its components). Authors' conclusions were also extracted.
Risk of bias assessment and data analysis
Risk of bias of the included studies was assessed by one author (K. C. H.) and vetted by co‐author (P. G.) using the Newcastle‐Ottawa Scale (NOS). 36 Studies were categorized as high (<5 stars), moderate (5–7 stars), or low risk of bias (≥8 stars) on a scale of 0 to 10 for cross‐sectional studies and 0 to 9 for longitudinal studies. 37 Where necessary, authors of included studies were contacted for clarification of study design to inform risk of bias. Authors were allowed 2 weeks to respond with a follow‐up email for another 2 weeks, after which the best available information was used if no reply was obtained. Due to the heterogeneity of included studies such as population characteristics, definitions and measurements of exposures and outcomes, analysis methods and effect estimates, it was not possible to pool the results statistically, and therefore a meta‐analysis was not undertaken.
Results
Overview of included studies
After all citations were merged and duplicates were removed, our search produced 3175 unique records, of which, 21 full‐text reports were assessed (PRISMA flow diagram; Figure 1). In total, 10 cross‐sectional and three longitudinal population‐based studies of community‐dwelling adults (N = 68 235) were included. The characteristics of these studies are summarized in Table 1. Briefly, with some studies (n = 5) investigating more than one sensory impairment, 8, 6, 3, and 1 studies investigated the relationship between VI, HI, SI, and TI and sarcopenia and its related components, respectively. Follow‐up duration for the longitudinal studies ranged from 4 to 11 years. Sample sizes ranged between 141 and 34 129. All were population‐based studies of community dwelling adults. Four studies drew from Asian populations, seven from Caucasian populations and two from a combination of multiple ethnicities from six low‐ and middle‐income countries (LMIC). Two studies 30 , 38 had a moderate (NOS 5–7) and the remaining 22 , 23 , 28 , 29 , 31 , 32 , 33 , 34 , 39 , 40 , 41 (n = 11) had a low (NOS ≥ 8) risk of bias (Table 2 for longitudinal studies; Table 3 for cross‐sectional studies).
Figure 1.
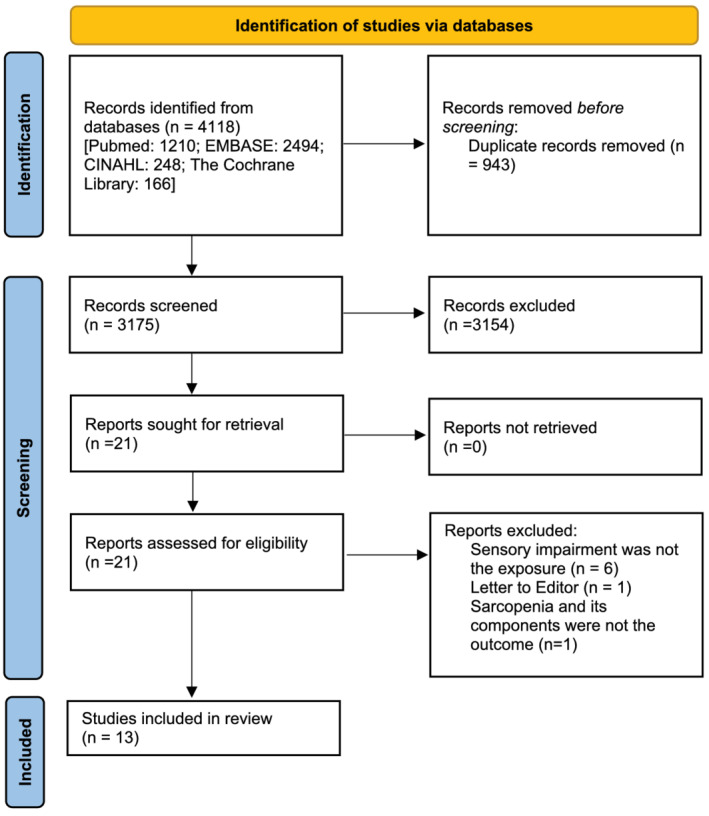
Preferred reporting items for systematic review and Meta‐analyses flow diagram showing the study selection process.
Table 1.
Study characteristics included in the literature review (n = 13)
| Study, design | Participants' characteristics | Measure of sensory impairment (exposure) | Measure of sarcopenia/muscle mass/grip strength/gait speed (outcome) | Covariates | Key findings |
|---|---|---|---|---|---|
| Outcome: Sarcopenia (n = 2) | |||||
| Harita et al., 23 cross‐sectional | 141 community‐dwelling adults aged ≥65 years in Japan |
SI was defined as ≤7 times correctly identify the 12 odours in the Open Essence test TI was defined as the first concentrations required to recognize salty and sweet tastes in the 1‐mL whole‐mouth gustatory test |
Sarcopenia was defined as both (i) ASM index measured by BIA divided by height squared with cut‐off values <7.0 kg/m2 for men and <5.7 kg/m2 for women; and (ii) grip strength measured by a Smedley's hand dynamometer with cut‐off values <26 kg for men and <18 kg for women | Age, sex, heart disease, digestive disease and bone/joint disease, BMI, body fat mass index and body mineral index |
Individuals with SI had higher odds of having sarcopenia (OR 47.8; 95% CI: 1.13 to 2016; P = 0.04). After stratification by sex, this independent association was only significant in women (OR 36.2; 95% CI: 1.01 to 1406; P = 0.048). No significant association was found between TI and sarcopenia |
| Smith et al., 41 cross‐sectional | 14 585 community dwelling adults aged ≥65 years in six low‐ and middle‐income countries a | VI was defined as presenting visual acuity of the better eye worse than 6/12 using the tumbling E LogMAR chart. Level of severity: mild (<6/12 and ≥6/18); moderate (<6/18 and ≥6/60); severe (<6/60) | Sarcopenia was defined as having (i) lowest quintile skeletal muscle mass (calculated as the ASM divided by BMI, where ASM was calculated based on weight, height, sex, age and race); and (ii) either a slow gait speed of a 4 m walk (lowest quintile based on height, age, and sex‐stratified values) or a weak handgrip strength (<30 kg for men and <20 kg for women) | Age, wealth, education, smoking, physical activity, obesity, chronic conditions, and country of residence | Compared with those with no VI, individuals with moderate (OR 1.69; 95% CI: 1.25 to 2.27; P < 0.001) and severe VI (OR 3.38; 95% CI: 1.69 to 6.77; P < 0.001) had higher odds of having sarcopenia, but the association was not observed in those with mild VI (OR 1.10; 95% CI: 0.87–1.40; P > 0.05) |
| Outcome: Muscle mass (n = 2) | |||||
| Purdy et al., 32 longitudinal study (7‐year follow‐up) | 2390 community‐dwelling adults aged ≥70 years in the USA | SI was defined as ≤8 times correctly identifying the 12 odours in the Brief Smell Identification Test | Decline in total lean mass was measured by DXA over a 7‐year period | Age, sex, race, height, education, clinic site, family income, smoking status, physical activity, self‐reported health status, cancer, depression, dementia, cardiovascular diseases, diabetes, and hypertension | Individuals with SI had a greater annual decline in total lean mass (−139 g, 95% CI: −236 g to −43 g; P < 0.05) compared with those without SI |
| Moon et al., 22 cross‐sectional | 1733 community‐dwelling adults aged ≥65 years in Korea | VI was defined as best‐corrected visual acuity of the better eye <6/12 at a 4 m distance using Jin's vision chart | Low muscle mass was defined as ASM index measured by DXA divided by height squared with cut‐off values of <6.43 kg/m2 | Age, smoking status, alcohol consumption, frequency of physical activity, educational level, the status of basic livelihood security recipient and history of stroke | Individuals with VI had higher odds of low muscle mass (OR 1.60; 95% CI: 1.02 to 2.50; P = 0.04), compared with those without VI |
| Outcome: Handgrip strength (n = 3) | |||||
| Gopinath et al., 39 cross‐sectional | 947 community‐dwelling adults aged ≥65 years in Australia |
VI was defined as best‐corrected visual acuity of the better eye <6/12 using a retro‐illuminated chart HI was defined as average pure‐tone air conduction threshold at 500; 1000; 2000; and 4000 Hz in the better ear >25 dB HL SI was defined as <6 times correctly identifying the eight odours in the San Diego Odour Identification Test |
Handgrip strength of the dominant hand was measured with a Jamar hand dynamometer | Age, living alone, admission to hospitals, and walking disability |
Women with two or three sensory impairments had 1.1 kg lower mean handgrip strength compared with those with no sensory impairment (17.47 ± 0.5 kg vs. 18.59 ± 0.3; P = 0.05), but the association was not observed in men No associations were observed between individuals with one sensory impairment and handgrip strength in both men and women |
| Ho et al., 40 cross‐sectional | 780 community‐dwelling adults aged ≥65 years in Singapore | VI was defined as presenting visual acuity of the better eye <6/12 at a 3 m distance using the tumbling E LogMAR chart | Weak handgrip strength of the dominant hand was measured by the Smedley's hand dynamometer with cut‐off value of <26 kg for men and <18 kg for women | Age, sex, ethnicity, and cognition | No significant association was found between VI and weak grip strength (OR −0.5; 95% CI −1.4 to 0.3; P > 0.05) |
| Vancampfort et al., 38 cross‐sectional | 34 129 community dwelling adults aged ≥50 years in 6 low‐and middle‐income countries a |
VI was defined as having ‘extreme difficulty’ in seeing and recognizing a person that the participant knows from about 20 metres. Responses included none, mild, moderate, severe, or extreme HI was defined based on an interviewer's observation during the survey |
Weak handgrip strength of the dominant hand was measured by the Smedley's hand dynamometer, with cut‐off value of <30 kg for men and <20 kg for women | Age, sex, wealth, education, marital status, BMI, physical activity, smoking, depression, other chronic conditions, and country of residence |
No significant association was found between VI and weak handgrip strength (OR 1.33; 95% CI: 0.83 to 2.13; P > 0.05). Individuals with HI had a higher odd of weak handgrip strength (OR 1.40; 95% CI: 1.16 to 1.70; P < 0.001), compared with those without HI |
| Outcome: Gait speed (n = 6) | |||||
| Verghese et al., 34 longitudinal study (4‐year follow‐up) | 2306 community‐dwelling adults aged ≥65 years in the USA | VI was defined as self‐reported ‘fair’ or ‘poor’ ability to see objects at far or near distances. Responses included excellent, very good, good, fair, or poor. | Slow gait speed of a 2.5 m walk was defined as 0.57 m/s, 0.49 m/s and 0.38 m/s in women aged <70, 70–79 and ≥80 years, respectively. In men, it was defined as 0.62, 0.56, and 0.45 m/s for those aged <70, 70–79, and ≥80 years, respectively | Age, sex, education, muscle weakness, cognitive impairment, alcohol consumption, pain, falls, poor sleep quality, physical inactivity, obesity, arthritis, stroke, diabetes, hypertension, heart condition and depression | Individuals with VI had a higher risk of incident slow gait speed (RR 1.36 95% CI: 1.03 to 1.80; P < 0.05), compared with those without VI |
| Chen et al., 33 longitudinal study (11‐year follow‐up) | 2190 community‐dwelling adults aged ≥70 years in the USA | HI was defined as average pure‐tone air conduction threshold at 500, 1000, 2000, and 4000 Hz in the better ear >25 dB HL. Level of severity: mild (>25 and ≤40 dB HL); moderate or great (>40 dB HL) | Gait speed of a 3, 4, or 6 m walk was measured. Mean gait speed was compared between groups | Age, sex, race, education, study site, smoking status, hypertension, diabetes, and stroke |
Individuals with moderate or worse HI had slower gait speed than participants with no HI at Visit 1 (1.18 m/s 95% CI: 1.16 to 1.21 vs. 1.22 m/s 95% CI: 1.20 to 1.23; P < 0.05), Visit 5 (1.08 m/s 95% CI: 1.06 to 1.10 vs. 1.11 m/s 95% CI: 1.09 to 1.13; P < 0.05), and Visit 11 (0.80 m/s 95% CI: 0.77 to 0.84 vs. 0.88 m/s 95% CI: 0.86 to 0.91; P < 0.01) No significant association was found between mild HI and slow gait speed in any visit. |
| Li et al., 29 cross‐sectional | 1180 community‐dwelling adults aged ≥50 years in the USA | HI was defined as average pure‐tone air conduction threshold at 500, 1000, 2000, and 4000 Hz in the better ear >25 dB HL | Slow gait speed of a 6.1 m walk was defined as <1.0 m/s. | Age, sex, race, and education. Cardiovascular risk factors, including smoking status, hypertension, diabetes mellitus, and stroke | Individuals with HI had higher odds of slow gait speed (OR 2.0; 95% CI: 1.2 to 3.2; P < 0.01), compared with those without HI. |
| Mikkola et al., 30 cross‐sectional | 848 community‐dwelling adults aged ≥75 years in Finland | HI was defined as self‐reported ‘major difficulty’ when conversing with another person in a noisy environment. Responses included no difficulty, sometimes, some difficulty, major difficulty | Gait speed of a 2.4 m walk was measured. Mean gait speed was compared between groups | Age, years of education, cognitive functioning, cardiac, circulatory, locomotor diseases | Individuals with major HI had significantly slower walking speed than those with no HI (0.80 m/s vs. 0.88 m/s; P = 0.008) |
| Huang et al., 28 cross‐sectional | 4197 community‐dwelling adults aged ≥50 years in the UK |
VI was defined as self‐reported ‘fair’ or ‘poor’ for eyesight. Responses included excellent, very good, good, fair, poor, or registered blind HI was measured by 2 pure pure‐tone air conduction at 3000 Hz with 3 thresholds: 75, 55, and 35 dB HL and at 1000 Hz with 3 thresholds: 55, 35, and 20 dB HL for each ear. Level of severity: moderate (heard 3 to 5 tones at either ear), and severe (heard 0 to 2 tones at either ear). DSI was defined as having both VI and severe HI |
Gait speed of a 2.4 m walk was measured. Mean gait speed was compared between groups | Age, sex, education level, cigarette smoking, alcohol drinking, physical activity, hearing aid, depressive symptoms, heart attack, hypertension, diabetes, arthritis, and osteoporosis |
Compared with those who self‐reported ‘excellent’ or ‘very good’ vision, individuals with VI had a slower gait speed (−0.03 m/s 95% CI: −0.06 to −0.01; P = 0.03) Compared with those with no HI, individuals with HI had a slower gait speed with a mean difference as −0.03 m/s (95% CI: −0.05 to −0.02; P < 0.001) and −0.07 m/s (95% CI: −0.11 to −0.04, P < 0.001) for moderate and severe HI, respectively Compared with those with no DSI, individuals with DSI had a slower gait speed with a mean difference as–0.08 m/s (95% CI: −0.14 to −0.02, P = 0.01) for DSI |
| Miyata et al., 31 cross‐sectional | 2809 community‐dwelling adults aged ≥70 years in the UK | VI was defined as best‐corrected visual acuity of the better eye <6/12 at a 5 m distance using a Landolt ring chart | Slow gait speed of a 10 m walk was defined as <1.0 m/s and an additional cut‐off at ≤0.8 m/s was also analysed | Age, gender, BMI, current smoking, and number of other health conditions | Individuals with VI had higher odds of having slow gait speed <1.0 m/s (OR 4.50; 95% CI: 1.87 to 10.85; P = 0.001) and ≤0.8 m/s (OR 3.51; 95% CI: 1.21 to 10.15; P = 0.02) |
ASM, appendicular skeletal muscle mass; BIA, bioelectrical impedance analysis; BMI, body mass index; CI, confidence interval; dB HL, decibel hearing loss; DSI, dual sensory impairment; DXA, dual‐energy X‐ray absorptiometry; HI, hearing impairment; LogMAR, logarithm of the minimum angle of resolution; OR, odds ratio; RR, relative risk; SI, smell impairment; TI, taste impairment; VI, vision impairment.
Six low‐ and middle‐income countries include China, Ghana, India, Mexico, Russia, and South Africa.
Table 2.
NOS for risk of bias assessment of included longitudinal studies
| Study | Domains a | Results b | |||
|---|---|---|---|---|---|
| Selection (4) | Comparability (2) | Outcome (3) | Score | Risk | |
| Purdy et al. 32 | 4 | 3 | 2 | 9 | Low |
| Verghese et al. 34 | 3 | 3 | 2 | 8 | Low |
| Chen et al. 33 | 4 | 3 | 1 | 8 | Low |
Domains of Newcastle‐Ottawa Scale (NOS): Selection (representativeness of the exposed cohort; selection of the non‐exposed cohort; ascertainment of exposure and demonstration that outcome of interest was not present at start of study); Comparability (principal factor and any additional factor); and Outcome (assessment of outcome; if the follow‐up was long enough for outcomes to outcome occurs; and adequacy of follow‐up of cohorts).
Studies were categorized as high (<5), moderate (5–7), or low risk of bias (≥8) on the scale of 0 to 9 for longitudinal studies.
Table 3.
NOS for risk of bias assessment of included cross‐sectional studies
| Study | Domains a | Results b | |||
|---|---|---|---|---|---|
| Selection (5) | Comparability (2) | Outcome (3) | Score (10) | Risk | |
| Harita et al. 23 | 3 | 2 | 3 | 8 | Low |
| Smith et al. 41 | 3 | 2 | 2 | 7 | Low |
| Moon et al. 22 | 3 | 2 | 3 | 8 | Low |
| Gopinath et al. 39 | 3 | 2 | 3 | 8 | Low |
| Ho et al. 40 | 3 | 2 | 3 | 8 | Low |
| Vancampfort et al. 38 | 1 | 2 | 3 | 6 | Moderate |
| Li et al. 29 | 3 | 2 | 3 | 8 | Low |
| Mikkola et al. 30 | 1 | 2 | 3 | 6 | Moderate |
| Huang et al. 28 | 2 | 2 | 3 | 7 | Low |
| Miyata et al. 31 | 3 | 2 | 3 | 8 | Low |
Domains of Newcastle‐Ottawa Scale (NOS): Selection (representativeness of the sample; sample size; non‐respondents; and ascertainment of the exposure); Comparability (confounding factors are controlled); and Outcome (assessment of outcome; and statistical test).
Studies were categorized as high (<5), moderate (5–7), or low risk of bias (≥8) on the scale of 0 to 10 for cross‐sectional studies.
Sensory impairments and sarcopenia
Two studies investigated the association between objectively measured sensory impairment measurements and sarcopenia (Table 1). In a cross‐sectional study of adults aged ≥65 years in six LMIC, Smith and associates 41 reported that subjects with moderate (OR 1.69; 95% CI: 1.25 to 2.27; P < 0.001), and severe (OR 3.38; 95% CI: 1.69 to 6.77; P < 0.001), but not mild VI (OR 1.10; 95% CI: 0.87–1.40; P > 0.05), had higher odds of having sarcopenia compared with those with no VI in multivariate analyses. Results were similar in sensitivity analyses using different ways of defining sarcopenia [e.g. weak handgrip vs. (weak handgrip + low muscle mass) vs. (weak handgrip + low muscle mass + slow gait)]. Similarly, in a cross‐sectional study of Japanese adults aged ≥65 years, Harita and co‐workers 23 found that individuals with SI had higher odds of sarcopenia (OR 47.8; 95% CI: 1.13–2016; P = 0.04) after adjusting for potential confounders. Interestingly, sex‐stratified analyses revealed that this association was only significant in women (OR 36.2; 95% CI: 1.01–1406; P = 0.048), and no significant association was found between TI and sarcopenia.
Sensory impairments and muscle mass
Two studies showed evidence of significant associations between objectively measured sensory impairment parameters and low muscle mass. In a cross‐sectional study of Koreans aged ≥65 years, Moon and colleagues demonstrated that VI was associated with a 1.6‐fold higher odds of low muscle mass (OR 1.60; 95% CI 1.02 to 2.50; P = 0.04). 22 Similarly, in a longitudinal study of US adults ≥70 years by Purdy and associates, individuals with SI at baseline experienced a greater annual decline in total lean mass (−139 g/year, 95% CI: −236 to −43; P < 0.05) compared with those without SI at baseline. 32
Sensory impairments and handgrip strength
The association between sensory impairments and handgrip strength was equivocal. In a cross‐sectional study of Australians aged ≥65 years, Gopinath and colleagues 39 reported that women with two to three concomitant sensory impairments (VI, HI, and/or SI) had 1.1 kg lower mean handgrip strength compared with those with no sensory loss (P = 0.05). However, these associations were not observed in men. Interestingly, no significant difference in handgrip strength was observed in individuals with a single sensory impairment compared with no impairment. Two other studies 38 , 40 also found no association between VI (both self‐reported and objectively measured) and weak handgrip strength; however, Vancampfort and associates 38 reported that individuals with HI (based on an interviewer's observation during the survey) had higher odds of weak handgrip strength (OR 1.40; 95% CI: 1.16 to 1.70; P < 0.001).
Sensory impairments and gait speed
We found strong evidence of an independent association between sensory impairment parameters, particularly, HI 28 , 29 , 30 , 33 and VI, 28 , 31 , 34 and slow gait speed. For instance, Chen and co‐workers 33 in an 11‐year longitudinal study of US adults aged ≥70 years found that subjects with HI had slower slow gait speed than those with no HI. Individuals with moderate or greater HI had significantly slower gait speeds (P < 0.05) at all visits where gait speed was measured (Table 1). Similarly, Verghese and colleagues 34 reported a significantly higher risk of incident slow gait speed (RR 1.36 95% CI: 1.03 to 1.80; P < 0.05) in US adults aged ≥65 years with self‐reported VI than those without over the 4‐year follow up periods. Corroborating these findings, Miyata and associates 31 observed independent associations between VI and slow gait speed based on two commonly used gait speed cut‐offs: <1.0 m/s (OR 4.50; 95% CI: 1.87 to 10.85; P = 0.001) and ≤0.8 m/s (OR 3.51; 95% CI: 1.21 to 10.15; P = 0.02). Critically, presence of dual sensory impairment (DSI), which refers to the co‐occurrence of VI and HI, was also reported as a risk factor for slow gait speed. For instance, Huang and associates 28 reported that, compared with those with no DSI, individuals with DSI had a slower gait speed, with a mean difference of −0.08 m/s (95% CI: −0.14 m/s to −0.02 m/s, P = 0.01) for DSI.
Discussion
In our systematic review, we found 13 studies investigating the association between sensory impairment parameters and sarcopenia (n = 2), muscle mass (n = 2), handgrip strength (n = 3) and gait speed (n = 6). Overall, we found that the presence of VI and SI (only in women), but not TI, independently increased the odds of having sarcopenia; the association between VI, HI, and SI, and low handgrip strength was inconsistent across studies; VI and SI were each independently associated with low muscle mass; and VI, HI, and DSI were all associated with slow gait speed. Early detection and intervention to prevent the onset of sensory impairment may lower the risk of the development and progression of sarcopenia and its related components. However, given the small number of studies on the sensory impairment‐sarcopenia relationship, coupled with the cross‐sectional nature of most of these studies, there is currently insufficient evidence to indicate a reliable and valid independent association between sensory impairment and sarcopenia and its related components. More longitudinal investigations are warranted to validate current consensus and confirm causality.
Current hypotheses suggest that sensory impairment contributes to decreased physical activity 29 , 42 , 43 , 44 , 45 , 46 and poor nutrition 19 , 47 , 48 leading to aggregate physiological dysfunction, in turn, leading to decreased muscle mass, strength and/or gait speed as well as sarcopenia. 4 , 7 For instance, VI and HI can lead to difficulty in balance control and mobility, 29 , 42 , 43 , 44 , 45 , 46 resulting in decreased levels of physical activity and slower gait speed. Similarly, VI,SI and/or TI can alter older adults' long‐term food consumption patterns including a decline in appetite and food intake, 19 leading to suboptimal diet and poor nutritional status. 49 , 50 Similarly, physical activity and nutrition are critical to maintaining muscle strength and mass in the elderly, with exercise and nutritional interventions demonstrating efficacy in improving muscle mass gain, strength, and gait speed in patients with sarcopenia. 12
The association between sensory impairment and sarcopenia in older adults may be explained by several psychosocial factors. 47 , 48 , 51 For instance, individuals with DSI have markedly worse psychosocial outcomes, such as depressive symptoms, low self‐efficacy, social isolation, and loneliness, compared with those with no sensory impairment. 51 Fear of falling is also commonly reported in individuals with VI and/or HI. 47 , 48 All these psychosocial factors have been linked, either directly or indirectly, with a greater risk of sarcopenia in older adults. 52 , 53 , 54 , 55 As such, it is surprising that only two studies assessing the relationship between VI, SI, TI, and sarcopenia were found, with no studies evaluating the associations between HI and sarcopenia or the associated underpinning mechanisms of action.
Evidence for an association between the four sensory impairments and the components defining sarcopenia were equally sparse and, in the case of handgrip strength, findings were equivocal. Specifically, Gopinath and colleagues did not find any association between single sensory impairment and low handgrip strength, but women who had two to three sensory impairments had lower mean handgrip strength compared with those who had one or no sensory impairment. 39 This finding may suggest a sex‐based disparity in the relationship between sensory impairment and weak handgrip strength. Previous studies have also shown that predictors of handgrip strength are different for men and women. 56 For example, occupation was a predictor of handgrip strength in men, but not women, while the opposite was observed that body weight was a predictor of handgrip strength in women, but not men. 56 Further investigations are needed to confirm whether sensory impairment is associated with low handgrip strength and there is a sex‐based disparity.
Notwithstanding the current lack of evidence and a need for more research in this area, our results suggest a potential relationship between sensory impairment and sarcopenia component measures, which may warrant screening for early detection of sarcopenia in those with sensory impairment. However, rather than using dual‐energy X‐ray absorptiometry (DXA), which is the gold standard for measurement of muscle mass but is seldom available or used in clinics, screening questionnaires could be used. For example, the SARC‐F questionnaire, 57 which include five components: Strength, Assistance with walking, Rise from a chair, Climb stairs, and Falls, has been developed as a possible rapid diagnostic test for sarcopenia. The questionnaire has been validated against several sets of diagnostic criteria (e.g. diagnostic criteria of EWGSOP2) in different populations. 58 , 59 , 60 , 61 The use of such brief and self‐complete tools may facilitate early detection and intervention for sarcopenia in those with sensory impairment.
Importantly, several aspects of the sensory impairment‐sarcopenia relationship remain unknown due to limited number of studies investigating this association, the general paucity of longitudinal studies and the wide variation in definitions and measurement techniques of both sensory impairment and sarcopenia adopted across studies. Moreover, it is still unclear whether there are race‐ or sex‐based disparities in the relationships between sensory impairment and sarcopenia. Moreover, the temporality and direction of the sensory impairment‐sarcopenia relationship is uncertain: rather than a causal relationship, the link between sensory impairment and sarcopenia could be due to a common factor, that is, a common cause hypothesis. For example, oxidative stress, an imbalance between the production of reactive oxygen species and the detoxification of their reactive intermediates, 62 has been reported as a risk factor of VI, 63 HI, 64 SI, 65 and sarcopenia. 66 Other factors such as age‐related chronic metabolic disorders include obesity, type 2 diabetes mellitus, cardiovascular disease and neurodegenerative diseases have been linked to VI, 67 HI, 68 SI, 69 TI, 70 and sarcopenia. 71 Even once causality has been established, more research to examine the factors involved in mediating the sensory impairment‐sarcopenia relationship is needed. Last, a consistent definition and standardized measurement techniques for sensory impairment, sarcopenia, and sarcopenia components should also be used to allow for the comparison across studies and aggregation of data from multiple studies to perform meta‐analyses.
Strengths of our study include a rigorous search strategy with a comprehensive list of search term among multiple electronic databases and involvement of two experienced researchers to assess the risk of bias of the included studies. Nonetheless, our study has several limitations. First, the small number of studies, particularly those of longitudinal design, limit our ability to infer causality and demonstrate the need for more well‐designed cohort studies. Second, we excluded non‐English publications which may have resulted in some publication bias. Third, the inconsistency in measurement techniques [e.g. bioelectrical impedance analysis (BIA) vs. DXA in measuring muscle mass] and definitions [e.g. presenting visual acuity (VA) vs. best‐corrected VA in defining VI] of sensory impairment, and sarcopenia and its associated components across studies have precluded our ability to compare across studies and perform a meta‐analysis. Fourth, we have only included studies which defined weak muscle strength and low physical functions based on handgrip strength and slow gait speed. Although they are most used measures, our analyses would not apply to studies using other clinical measures for muscle strength and physical functions. Last, the general senses (touch, pressure, pain, temperature, etc.) were not considered in this review, as they are composite senses with a complex range of pathology that require separate consideration.
In conclusion, notwithstanding the limited studies on the relationship between sensory impairment and sarcopenia and its associated components, our systematic review suggests a potential association between the presence of single or multiple sensory impairments and a greater likelihood of sarcopenia and/or deficits in its associated components, especially for VI, HI, and SI. These findings suggest the need for early detection and intervention to prevent the onset of sensory impairment which, in turn, may reduce the risk of the development and progression of sarcopenia. However, further prospective studies to untangle the relationship between sensory impairment on sarcopenia and its associated components are needed to better inform clinical guidelines for disease prevention and management.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Supporting information
Data S1. Supporting Information
Data S2. Supporting Information
Acknowledgements
Professor Lamoureux is supported by the National Medical Research Council Senior Clinician Scientist Award (NMRC‐CSA‐SI #JRNMRR140601). Asst. Prof. Man is supported by the NMRC Transition Award (#MOH‐TA19may‐0002). The funding organizations had no role in the design or conduct of this research or preparation of this manuscript. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 72
Ho K. C., Gupta P., Fenwick E. K., Man R. E. K., Gan A. T. L., and Lamoureux E. L. (2022) Association between age‐related sensory impairment with sarcopenia and its related components in older adults: a systematic review, Journal of Cachexia, Sarcopenia and Muscle, 13, 811–823, 10.1002/jcsm.12930
References
- 1. United Nations DoEaSA, Population Division,. World Population Ageing 2019: Highlights (ST/ESA/SER.A/430). 2019.
- 2. United Nations Department of Economic and Social Affairs . Ageing‐related Policies and Priorities in Voluntary National Reviews (2016–2019). 2019. https://www.un.org/development/desa/ageing/wp‐content/uploads/sites/24/2019/12/Briefing‐Paper_VNRs‐and‐OPs‐2019.pdf. Accessed 25 Nov 2020.
- 3. Anker SD, Morley JE, von Haehling S. Welcome to the ICD‐10 code for sarcopenia. J Cachexia Sarcopenia Muscle 2016;7:512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta‐ analysis of general population studies. J Diabetes Metab Disord 2017;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020;21:300–7 e2. [DOI] [PubMed] [Google Scholar]
- 7. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beaudart C, Rizzoli R, Bruyere O, Reginster JY, Biver E. Sarcopenia: burden and challenges for public health. Arch Public Health 2014;72:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyere O. Health Outcomes of Sarcopenia: A Systematic Review and Meta‐Analysis. PLoS ONE 2017;12:e0169548. 10.1371/journal.pone.0169548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Waite SJ, Maitland S, Thomas A, Yarnall AJ. Sarcopenia and frailty in individuals with dementia: A systematic review. Arch Gerontol Geriatr 2020;92:104268. 10.1016/j.archger.2020.104268 [DOI] [PubMed] [Google Scholar]
- 11. Fischer ME, Cruickshanks KJ, Schubert CR, Pinto AA, Carlsson CM, Klein BE, et al. Age‐Related Sensory Impairments and Risk of Cognitive Impairment. J Am Geriatr Soc 2016;64:1981–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshimura Y, Wakabayashi H, Yamada M, Kim H, Harada A, Arai H. Interventions for Treating Sarcopenia: A Systematic Review and Meta‐Analysis of Randomized Controlled Studies. J Am Med Dir Assoc 2017;18:e1–e16. [DOI] [PubMed] [Google Scholar]
- 13. Nusbaum NJ. Aging and sensory senescence. South Med J 1999;92:267–275. [DOI] [PubMed] [Google Scholar]
- 14. Bourne RRA, Flaxman SR, Braithwaite T, Cicinelli MV, Das A, Jonas JB, et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta‐analysis. Lancet Glob Health 2017;5:e888–e897. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization . Addressing the rising prevalence of hearing loss. Geneva; 2018. [Google Scholar]
- 16. Desiato VM, Levy DA, Byun YJ, Nguyen SA, Soler ZM, Schlosser RJ. The Prevalence of Olfactory Dysfunction in the General Population: A Systematic Review and Meta‐analysis. Am J Rhinol Allergy 2020;35:195–205. 10.1177/1945892420946254 [DOI] [PubMed] [Google Scholar]
- 17. Correia C, Lopez KJ, Wroblewski KE, Huisingh‐Scheetz M, Kern DW, Chen RC, et al. Global Sensory Impairment in Older Adults in the United States. J Am Geriatr Soc 2016;64:306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harithasan D, Mukari SZS, Ishak WS, Shahar S, Yeong WL. The impact of sensory impairment on cognitive performance, quality of life, depression, and loneliness in older adults. Int J Geriatr Psychiatry 2020;35:358–364. [DOI] [PubMed] [Google Scholar]
- 19. Kershaw JC, Mattes RD. Nutrition and taste and smell dysfunction. World J Otorhinolaryngol Head Neck Surg 2018;4:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tan BKJ, Man REK, Gan ATL, Fenwick EK, Varadaraj V, Swenor BK, et al. Is Sensory Loss an Understudied Risk Factor for Frailty? A Systematic Review and Meta‐analysis. J Gerontol A Biol Sci Med Sci 2020;75:2461–2470. [DOI] [PubMed] [Google Scholar]
- 21. Siantar RG, Cheng CY, Gemmy Cheung CM, Lamoureux EL, Ong PG, Chow KY, et al. Impact of Visual Impairment and Eye diseases on Mortality: the Singapore Malay Eye Study (SiMES). Sci Rep 2015;5:16304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moon JH, Oh YH, Kong MH, Kim HJ. Relationship between visual acuity and muscle mass in the Korean older population: A cross‐sectional study using Korean National Health and Nutrition Examination Survey. BMJ Open 2019;9:e033846. 10.1136/bmjopen-2019-033846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harita M, Miwa T, Shiga H, Yamada K, Sugiyama E, Okabe Y, et al. Association of olfactory impairment with indexes of sarcopenia and frailty in community‐dwelling older adults. Geriatr Gerontol Int 2019;19:384–391. [DOI] [PubMed] [Google Scholar]
- 24. Lansingh VC, Carter MJ, Martens M. Global cost‐effectiveness of cataract surgery. Ophthalmology 2007;114:1670–1678. [DOI] [PubMed] [Google Scholar]
- 25. Taylor RS, Paisley S, Davis A. Systematic review of the clinical and cost effectiveness of digital hearing aids. Br J Audiol 2001;35:271–288. [DOI] [PubMed] [Google Scholar]
- 26. Oleszkiewicz A, Hanf S, Whitcroft KL, Haehner A, Hummel T. Examination of olfactory training effectiveness in relation to its complexity and the cause of olfactory loss. Laryngoscope 2018;128:1518–1522. [DOI] [PubMed] [Google Scholar]
- 27. Hummel T, Rissom K, Reden J, Hahner A, Weidenbecher M, Huttenbrink KB. Effects of olfactory training in patients with olfactory loss. Laryngoscope 2009;119:496–499. [DOI] [PubMed] [Google Scholar]
- 28. Huang C, Sun S, Wang W, Li Y, Feng W, Wu Y. Cognition Mediates the Relationship Between Sensory Function and Gait Speed in Older Adults: Evidence from the English Longitudinal Study of Ageing. J Alzheimers Dis 2019;70:1153–1161. [DOI] [PubMed] [Google Scholar]
- 29. Li L, Simonsick EM, Ferrucci L, Lin FR. Hearing loss and gait speed among older adults in the United States. Gait Posture 2013;38:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mikkola TM, Polku H, Portegijs E, Rantakokko M, Rantanen T, Viljanen A. Self‐Reported Hearing Status Is Associated with Lower Limb Physical Performance, Perceived Mobility, and Activities of Daily Living in Older Community‐Dwelling Men and Women. J Am Geriatr Soc 2015;63:1164–1169. [DOI] [PubMed] [Google Scholar]
- 31. Miyata K, Yoshikawa T, Harano A, Ueda T, Ogata N. Effects of visual impairment on mobility functions in elderly: Results of Fujiwara‐kyo Eye Study. PLoS ONE 2021;16:e0244997. 10.1371/journal.pone.0244997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Purdy F, Luo Z, Gardiner JC, Pinto JM, Shiroma EJ, Simonsick EM, et al. Olfaction and changes in body composition in a large cohort of older US adults. J Gerontol A Biol Sci Med Sci 2020;75:2434–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen DS, Betz J, Yaffe K, Ayonayon HN, Kritchevsky S, Martin KR, et al. Association of hearing impairment with declines in physical functioning and the risk of disability in older adults. J Gerontol A Biol Sci Med Sci 2015;70:654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Verghese J, Wang C, Allali G, Holtzer R, Ayers E. Modifiable Risk Factors for New‐Onset Slow Gait in Older Adults. J Am Med Dir Assoc 2016;17:421–425. [DOI] [PubMed] [Google Scholar]
- 35. Dent E, Morley JE, Cruz‐Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J Nutr Health Aging 2018;22:1148–1161. [DOI] [PubMed] [Google Scholar]
- 36. Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non‐randomised intervention studies. Health Technol Assess 2003;7:1–173. [DOI] [PubMed] [Google Scholar]
- 37. Vetrano DL, Palmer KM, Galluzzo L, Giampaoli S, Marengoni A, Bernabei R, et al. Hypertension and frailty: a systematic review and meta‐analysis. BMJ Open 2018;8:e024406. 10.1136/bmjopen-2018-024406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vancampfort D, Stubbs B, Firth J, Koyanagi A. Handgrip strength, chronic physical conditions and physical multimorbidity in middle‐aged and older adults in six low‐ and middle income countries. Eur J Intern Med 2019;61:96–102. [DOI] [PubMed] [Google Scholar]
- 39. Gopinath B, Liew G, Burlutsky G, Mitchell P. Associations Between Vision, Hearing, and Olfactory Impairment With Handgrip Strength. J Aging Health 2020;32:654–659. [DOI] [PubMed] [Google Scholar]
- 40. Ho VWT, Chen C, Merchant RA. Cumulative Effect of Visual Impairment, Multimorbidity, and Frailty on Intrinsic Capacity in Community‐Dwelling Older Adults. J Aging Health 2020;32:670–676. [DOI] [PubMed] [Google Scholar]
- 41. Smith L, Allen P, Pardhan S, Gorely T, Grabovac I, Smith A, et al. Self‐rated eyesight and handgrip strength in older adults. Wien Klin Wochenschr 2020;132:132–138. [DOI] [PubMed] [Google Scholar]
- 42. Woollacott MH, Shumway‐Cook A, Nashner LM. Aging and posture control: changes in sensory organization and muscular coordination. Int J Aging Hum Dev 1986;23:97–114. [DOI] [PubMed] [Google Scholar]
- 43. Menant JC, Schoene D, Sarofim M, Lord SR. Single and dual task tests of gait speed are equivalent in the prediction of falls in older people: a systematic review and meta‐analysis. Ageing Res Rev 2014;16:83–104. [DOI] [PubMed] [Google Scholar]
- 44. Weih LM, Hassell JB, Keeffe J. Assessment of the impact of vision impairment. Invest Ophthalmol Vis Sci 2002;43:927–935. [PubMed] [Google Scholar]
- 45. Lubetzky AV. Balance, Falls, and Hearing Loss: Is It Time for a Paradigm Shift? JAMA Otolaryngol Head Neck Surg 2020;146:535–536. [DOI] [PubMed] [Google Scholar]
- 46. Jiam NT, Li C, Agrawal Y. Hearing loss and falls: A systematic review and meta‐analysis. Laryngoscope 2016;126:2587–2596. [DOI] [PubMed] [Google Scholar]
- 47. White UE, Black AA, Wood JM, Delbaere K. Fear of falling in vision impairment. Optom Vis Sci 2015;92:730–735. [DOI] [PubMed] [Google Scholar]
- 48. Malini FM, Lourenco RA, Lopes CS. Prevalence of fear of falling in older adults, and its associations with clinical, functional and psychosocial factors: the Frailty in Brazilian Older People‐Rio de Janeiro study. Geriatr Gerontol Int 2016;16:336–344. [DOI] [PubMed] [Google Scholar]
- 49. Ganapathy A, Nieves JW. Nutrition and Sarcopenia‐What Do We Know? Nutrients 2020;12:1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hong T, Flood V, Rochtchina E, Mitchell P, Russell J, Wang JJ. Adherence to dietary guidelines and the 10‐year cumulative incidence of visual impairment: the Blue Mountains Eye Study. Am J Ophthalmol 2014;158:302–308. [DOI] [PubMed] [Google Scholar]
- 51. Hajek A, Konig HH. Dual sensory impairment and psychosocial factors. Findings based on a nationally representative sample. Arch Gerontol Geriatr 2020;91:104234. 10.1016/j.archger.2020.104234 [DOI] [PubMed] [Google Scholar]
- 52. Jefferis BJ, Iliffe S, Kendrick D, Kerse N, Trost S, Lennon LT, et al. How are falls and fear of falling associated with objectively measured physical activity in a cohort of community‐dwelling older men? BMC Geriatr 2014;14:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Johnson KE, Taliaferro LA. Relationships between physical activity and depressive symptoms among middle and older adolescents: a review of the research literature. J Spec Pediatr Nurs 2011;16:235–251. [DOI] [PubMed] [Google Scholar]
- 54. Robins LM, Hill KD, Finch CF, Clemson L, Haines T. The association between physical activity and social isolation in community‐dwelling older adults. Aging Ment Health 2018;22:175–182. [DOI] [PubMed] [Google Scholar]
- 55. Pels F, Kleinert J. Loneliness and physical activity: A systematic review. Int Rev Sport Exerc Psychol 2016;9:231–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moy FM, Darus A, Hairi NN. Predictors of handgrip strength among adults of a rural community in Malaysia. Asia Pac J Public Health 2015;27:176–184. [DOI] [PubMed] [Google Scholar]
- 57. Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC‐F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016;7:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ida S, Kaneko R, Imataka K, Okubo K, Shirakura Y, Azuma K, et al. Verification of the predictive validity for mortality of the SARC‐F questionnaire based on a meta‐analysis. Aging Clin Exp Res 2021;33:835–842. [DOI] [PubMed] [Google Scholar]
- 59. Nguyen TN, Nguyen AT, Khuong LQ, Nguyen TX, Nguyen HTT, Nguyen TTH, et al. Reliability and Validity of SARC‐F Questionnaire to Assess Sarcopenia Among Vietnamese Geriatric Patients. Clin Interv Aging 2020;15:879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim S, Kim M, Won CW. Validation of the Korean Version of the SARC‐F Questionnaire to Assess Sarcopenia: Korean Frailty and Aging Cohort Study. J Am Med Dir Assoc 2018;19:40–5 e1. [DOI] [PubMed] [Google Scholar]
- 61. Krzyminska‐Siemaszko R, Deskur‐Smielecka E, Kaluzniak‐Szymanowska A, Styszynski A, Wieczorowska‐Tobis K. Polish version of SARC‐F to assess sarcopenia in older adults: An examination of reliability and validity. PLoS ONE 2020;15:e0244001. 10.1371/journal.pone.0244001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000;408:239–247. [DOI] [PubMed] [Google Scholar]
- 63. Jarrett SG, Boulton ME. Consequences of oxidative stress in age‐related macular degeneration. Mol Aspects Med 2012;33:399–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fujimoto C, Yamasoba T. Oxidative stresses and mitochondrial dysfunction in age‐related hearing loss. Oxid Med Cell Longev 2014;2014:582849. 10.1155/2014/582849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vaishnav RA, Getchell ML, Poon HF, Barnett KR, Hunter SA, Pierce WM, et al. Oxidative stress in the aging murine olfactory bulb: redox proteomics and cellular localization. J Neurosci Res 2007;85:373–385. [DOI] [PubMed] [Google Scholar]
- 66. Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci 2010;11:1509–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Poh S, Mohamed Abdul RB, Lamoureux EL, Wong TY, Sabanayagam C. Metabolic syndrome and eye diseases. Diabetes Res Clin Pract 2016;113:86–100. [DOI] [PubMed] [Google Scholar]
- 68. Kim TS, Kim EH, Chung JW. The Association Between Age‐Related Hearing Impairment and Metabolic Syndrome in Korean Women: 5‐Year Follow‐Up Observational Study. Metab Syndr Relat Disord 2017;15:240–245. [DOI] [PubMed] [Google Scholar]
- 69. Palouzier‐Paulignan B, Lacroix MC, Aime P, Baly C, Caillol M, Congar P, et al. Olfaction under metabolic influences. Chem Senses 2012;37:769–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vecek NN, Mucalo L, Dragun R, Milicevic T, Pribisalic A, Patarcic I, et al. The Association between Salt Taste Perception, Mediterranean Diet and Metabolic Syndrome: A Cross‐Sectional Study. Nutrients 2020;12:1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rubio‐Ruiz ME, Guarner‐Lans V, Perez‐Torres I, Soto ME. Mechanisms Underlying Metabolic Syndrome‐Related Sarcopenia and Possible Therapeutic Measures. Int J Mol Sci 2019;20:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information
Data S2. Supporting Information


