Abstract
Background
The diversity between the muscle cellular interactome of dependent and independent elderly people is based on the interrelationships established between different cellular mechanisms, and alteration of this balance modulates cellular activity in muscle tissue with important functional implications.
Methods
Thirty patients (85 ± 8 years old, 23% female) scheduled to undergo hip fracture surgery participated in this study. During the surgical procedures, skeletal muscle tissue was obtained from the Vastus lateralis. Two groups of participants were studied based on their Barthel index: 15 functional‐independent individuals (100‐90) and 15 severely functional‐dependent individuals (40‐0). The expression of proteins from the most important cellular mechanisms was studied by western blot.
Results
Compared with independent elderly patients, dependent elderly showed an abrupt decrease in the capacity of protein synthesis; this decrease was only partially compensated for at the response to unfolded or misfolded proteins (UPR) level due to the increase in IRE1 (P < 0.001) and ATF6 (P < 0.05), which block autophagy, an essential mechanism for cell survival, by decreasing the expression of Beclin‐1, LC3, and p62 (P < 0.001) and the antioxidant response. This lead to increased oxidative damage to lipids (P < 0.001) and that damage was directly associated with the mitochondrial impairment induced by the significant decreases in the I, III, IV, and V mitochondrial complexes (P < 0.01), which drastically reduced the energy capacity of the cell. The essential cellular mechanisms were generally impaired and the triggering of apoptosis was induced, as shown by the significantly elevated levels of most proapoptotic proteins (P < 0.05) and caspase‐3/7 (P < 0.001) in dependents. The death of highly damaged cells is not detrimental to organs as long as the regenerative capacity remains unaltered, but in the dependent patients, this ability was also significantly altered, which was revealed by the reduction in the myogenic regulatory factors and satellite cell marker (P < 0.001), and the increase in myostatin (P < 0.01). Due to the severely disturbed cell interactome, the muscle contractile capacity showed significant damage.
Conclusions
Functionally dependent patients exhibited severe alterations in their cellular interactome at the muscle level. Cell apoptosis was caused by a decrease in successful protein synthesis, to which the cellular control systems did not respond adequately; autophagy was simultaneously blocked, the mitochondrion malfunctioned, and as the essential recovery mechanisms failed, these cells could not be replaced, resulting in the muscle being condemned to a loss of mass and functionality.
Keywords: Sarcopenia, Elderly, Oxidative stress, Unfolded protein response, Mitochondria, Myogenic regulatory factors
Introduction
Sarcopenia is one of the main causes of age‐related dependency. Sarcopenia is a geriatric syndrome marked by a decline in skeletal muscle mass and function, and it occurs with increasing prevalence and diverse causalities. 1 Currently, the cellular alterations that cause sarcopenia are merely explained by a rapid increase in the breakdown of muscle protein accompanied by a reduction in muscle protein synthesis, presumably induced by a disruption of the homeostatic metabolic response. 2 However, substantial clinical information implies that a deeper molecular understanding would aid in the prevention of sarcopenia and the disability caused by it; thus, studies on the interactome underlying sarcopenia are greatly needed.
The interactome is the set of physiological processes in the cell that occur in a patterned, simultaneous, or linked way, and it allows the cell to live under normal conditions, overcome adverse events or, in the face of failure, to induce its death (reference S1 in the Supporting Information). In regard to extreme sarcopenia, drastic alterations at the cellular level in skeletal muscle have only been partially discovered in animal models 3 and scarcely in humans, 4 , 5 but based on the consequences at the clinical and muscular level, several mechanisms seem to be involved.
Classically, the loss of autonomy and aging are correlated with oxidative stress (reference S2 in the Supporting Information). The imbalance between the reactive oxygen species (ROS) produced in the body and antioxidant mechanisms promotes catabolism of muscle fibres, leading to the loss of muscle mass. 6 Disruption of endoplasmic reticulum (ER) homeostasis and the induction of ER stress have been recently described in aged muscles, resulting in a decrease in protein synthesis. 2 This reduction is accompanied by the accumulation of unfolded or misfolded proteins that raise ER stress, activating the response to unfolded or misfolded proteins (UPR), and muscle mass is subsequently decreased. 7 This multidimensional response is initiated from the ER through three transmembrane proteins that can promote cascades of action: inositol‐requiring protein 1 (IRE1), activating transcription factor 6 (ATF6) and RNA‐dependent protein kinase‐like ER eukaryotic translation initiation factor 2 alpha kinase (PERK) or eukaryotic initiation factor 2 alpha (eIF2α). Each of them activates multiple mechanisms aimed at recovering homeostasis and achieving cell survival. Therefore, one of the main mechanisms triggered is autophagy. Autophagy is a highly conserved cell survival mechanism in which cells degrade dysfunctional cellular components and protein aggregates to restore cell balance. 8 Three forms of autophagy have been identified: microautophagy, chaperone‐mediated autophagy (CMA), and macroautophagy. The last one is the most researched and, hereafter, will simply be called autophagy. Decreased autophagic flow in skeletal muscle seems to contribute significantly to degeneration, dysfunction and muscle weakness throughout adult life by reducing the elimination of organelles and toxic products in most neuromuscular disorders. 9 During aging, inefficient autophagy and damaged mitochondria have usually been described, and the association of both processes is recurrent in the literature. 8 In aged subjects, mitochondria are characterized by impaired functions, including reduced oxidative phosphorylation, lowered oxidative capacity, decreased adenosine 5′‐triphophate (ATP) production, and a significant increase in ROS. 10 Oxidative damage to mitochondrial components and altered mitochondrial turnover have been proposed as possible triggers of apoptotic events, 8 which could be the cause of the reduction in muscle mass seen in sarcopenia whenever the muscle regenerative capacity is impaired.
The regenerative power of skeletal muscle is directly dependent on two elements: myogenic regulatory factors (MRFs) and satellite cells. MRFs are mainly constituted by Myf5, MyoD, myogenin and MRF4, transcription factors sensitive to aging, 5 which are in charge of controlling postnatal myogenesis through satellite cell activation and differentiation. 11 In addition, myostatin, which plays an inhibitory role in skeletal muscle growth, contributes to muscle breakdown in elderly people. 12 Skeletal muscle satellite cells are a heterogeneous population of stem cells necessary for proper muscle development, repair, homeostasis, and muscle senescence. A correlation between the loss of satellite cell activity and reduction of muscle regeneration capacity has been described during aging, 13 giving satellite cells an essential role in the development of sarcopenia.
Treating sarcopenia requires a knowledge of the interaction between all these mechanisms and muscle loss as well as the interrelationships between them. In this article, impairments in the sarcopenic interactome of dependent vs. independent elderly people are shown. The important differences at the cellular, molecular, and tissue levels show the close relationship between sarcopenia and dependence, and they indicate the molecules and mechanisms responsible for sarcopenic dependence.
Material and methods
Experimental design
The population (N = 30; 23 women and 7 men; mean age 85 ± 8 years) of the present study belonged to the HYPA cohort and included a northwest Spanish cohort of older (≥70 years) individuals who were undergoing hip fracture surgery at Monte Naranco Hospital (Oviedo, Spain). 14 Barthel (BI) and Charlson (CI) indexes were used to detect disabilities in the performing of basic daily living activities and as indicators of co‐morbidity, respectively (Table S1).
Skeletal muscle biopsies and blood samples were extracted from 30 healthy patients (CI was close to 0): 15 functional‐independent patients (IP, BI: 100‐90) and 15 severely functional‐dependent patients (DP, BI: 40‐0) because the muscle loss that goes together with the physical disability indicated by low BI levels ensures extreme sarcopenia (reference S3 in the Supporting Information). The exclusion criteria were malnutrition, cognitive decline, active neoplastic diseases, and recent infections. The study was conducted according to the Declaration of Helsinki and was approved by all local and national ethical committees. All patients provided written informed consent.
Biochemical blood analysis
Blood samples were obtained by venipuncture in the morning after an overnight fast and a 15 min rest. Biochemical blood analysis was performed using an automated FALCOR 560 analyser (Menarini Diagnostics) in a well‐standardized haematology and biochemical laboratory at Monte Naranco Hospital.
Muscle collection and homogenization
Skeletal muscle aliquots were obtained during hip surgery from the Vastus lateralis and were immediately stored at −80°C. Small pieces of tissue (approximately 0.2 g) were homogenized using an Ultra‐turrax T25 homogenizer (Staufen, Germany) in 500 μL of lysis buffer (50 mM phosphate buffer pH 7.5, 1 mM NaF, 1 mM Na3VO4, 1 mM PMSF). Then, the tissue homogenates were centrifuged for 6 min at 1500 g and 4°C, and the supernatants were collected.
Muscular oxidative stress studies
Lipid peroxidation (LPO) was determined by measuring the reactive aldehyde malondialdehyde (MDA) and 4‐hydroxy‐2‐(E)‐nonenal (4‐HNE) levels using the LPO assay kit from Calbiochem (437634, CA, USA). Total antioxidant activity (TAA) was determined using the 2,2′‐azino‐bis (3‐ethylbenzothiazolin‐6‐sulfonic acid) radical cation method (ABTS). 15 Total superoxide dismutase activity (SOD; EC 1.15.1.1) and catalase activity (CAT; EC 1.11.1.6) were measured as described by Potes et al. 5
Enzyme‐linked immunosorbent assay
Tumour necrosis factor α (TNF‐α) (KMC3011, Life Technologies, MA, USA) and interleukin‐6 (IL‐6) (KMC0061, Life Technologies) ELISA kits were used to assess the inflammatory response in skeletal muscle tissue. All assays were performed following manufacturers' protocols.
Caspase activity measurement
Caspase‐3/7 activity was measured in tissue samples using Caspase‐Glo® 3/7 Assay (Promega, Southampton, UK). A 1:1 ratio of caspase reagent to sample supernatant was used for the assay, and this mixture was incubated at room temperature for 2 h. The end‐point luminescence was measured on a SIRIUS luminometer (Berthold, Pforzheim, Germany).
Adenosine 5′‐triphophate measurement
The determination of ATP levels was assessed in homogenized muscle tissue using the ATP Bioluminescent Assay Kit (FLAA, Sigma‐Aldrich). This assay is based on the consumption of ATP when firefly luciferase catalyses the oxidation of D‐luciferin. The ATP content was determined by the measure of the light emission with a luminometer.
Western blotting
The homogenized tissue (90 μg protein per sample) was mixed with Laemmli sample buffer (Bio‐Rad Laboratories, Inc., CA, USA) and denatured by boiling at 100°C for 5 min. Samples of all patients were loaded in duplicate. The samples were fractionated using SDS‐PAGE at 200 V, and then protein was transferred to polyvinylidene fluoride membranes (Immobilon TM‐P; Millipore Corp., MA, USA) at 350 mA. Once the membranes were blocked for 1 h with 10% (w/v) skim milk dissolved in Tris‐buffered saline (TBS) (50 mM Tris–HCl and 150 mM NaCl, pH 7.5), they were incubated at 4°C overnight with the corresponding primary antibodies (Table S2). All antibodies were prediluted in TBS buffer containing 5% (w/v) skim milk or albumin (BSA), as appropriate. Then, the membranes were incubated with the corresponding horseradish peroxidase‐conjugated secondary antibody (Sigma‐Aldrich, Missouri, USA) and diluted in TBS buffer with skim milk or BSA 2% (w/v) for 1 h at room temperature. The immunoconjugates were detected using a chemiluminescent horseradish peroxidase substrate (WBKLS0500, Millipore Corp., Darmstadt, Germany) according to the manufacturer's instructions. The Image Studio Lite 5.2.5 program (LI‐COR Biosciences, Nebraska, USA) allowed us to quantify the optical density of the bands. Due to variations in the typical constitutive protein levels (GAPDH, β‐actin and α‐tubulin) (reference S4 in the Supporting Information), the results were normalized to Ponceau as loading control.
Statistical analysis
Data are represented as the mean ± standard error of the mean (SEM). The normality of the data was verified using the Kolmogórov–Smirnov test. Variables between two experimental groups were compared using a t‐test of independent samples. Differences were considered statistically significant with a P < 0.05. Statistical and graphical analyses were performed with GraphPad Prism 6.0.
Results
Plasma biochemistry
Comparative biochemistry between DP and IP showed several parameters with important differences and trends (Table S3). Thus, the total protein concentration was reduced in DP compared with IP, with important differences that were close to significance. These data were confirmed in DP by the abrupt decrease in albumin levels (P < 0.01), which is normally a marker of the protein level. Lower plasma protein levels are indicators of important and severe disorders: malabsorption of nutrients and malnutrition, and the decrease in albumin levels only support the possibility of these disorders. A significant decrease in sodium levels was observed in DP (P < 0.05) compared with IP, and lower blood sodium is common in older and dependent adults, which induces symptoms of lethargy and confusion. Likewise, important but not significant reductions were observed in glucose and triglycerides, metabolites that are essential for obtaining energy and are new indicators of malabsorption. Creatinine showed a significant increase in DP (P < 0.05). Although creatinine is a classic marker of impaired kidney function or kidney disease, the role of creatinine is wider than just that, and its increase could also be related to greater muscle breakdown in the dependent population. 16
Endoplasmic reticulum stress
Due to the essential role that proteins play in skeletal muscle maintenance, three branches of UPR were evaluated to study possible disturbances in protein synthesis in the endoplasmic reticulum (Figure 1). Although significant alterations in the phosphorylation pathway of eIF2α were not found (Figure 1A), UPR was triggered in response to ER stress, and it corresponded with increased levels of IRE1α (P < 0.001) and ATF6α (P < 0.05) in DP (Figure 1B,C). Interestingly, IRE1α and ATF6α branches are associated with facilitating the cellular tolerance to chronic stress. 17
Figure 1.
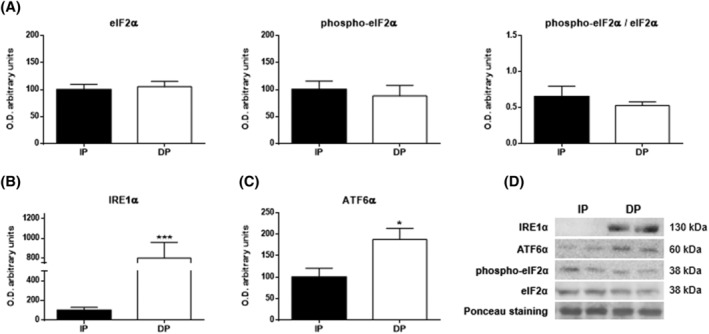
Endoplasmic reticulum stress in the muscle tissues of functional‐independent patients (IP) and functional‐dependent patients (DP). Bar chart shows the quantification of the optical densities (O.D.) of blot bands of (A) eukaryotic initiation factor 2α (eIF2α), phospho‐eIF2α, phospho‐eIF2α/eIF2α, (B) inositol‐requiring enzyme 1α (IRE1α), and (C) activating transcription factor 6α (ATF6α). (D) Representative immunoblots. Ponceau staining was used as a loading control. Data are represented as the mean ± SEM. *P < 0.05; ***P < 0.001.
Autophagy
Active UPR provokes the activation of the general quality control mechanisms, such as autophagy, to preserve protein homeostasis in muscle tissue. For this reason, we evaluated the activity of the main autophagic pathways by measuring the levels of Beclin‐1 and microtubule‐associated light‐chain protein 3 (LC3), macroautophagy markers, p62 protein as an autophagosome cargo protein and LAMP2A levels as the main marker of CMA.
Western blot analysis showed a significant increase in all macroautophagic markers in IP in comparison to DP. Thus, the expression of Beclin‐1 (P < 0.001; Figure 2A), which plays an essential role in the initiation of autophagy, in the immunoblot analysis against LC3‐I (P < 0.001) and LC3‐II (P < 0.001), resulted in higher unfolded protein concentrations than the autophagy was able to degrade in IP, and this was also seen with the quotient of both the LC3‐II/LC3‐I ratio (P < 0.05) (Figure 2B) and even in the p62 expression (P < 0.001; Figure 2C). In contrast, a significant increase in the expression of LAMP2A was observed in DP compared with IP, showing that CMA was independent from macroautophagy (P < 0.001; Figure 2D).
Figure 2.
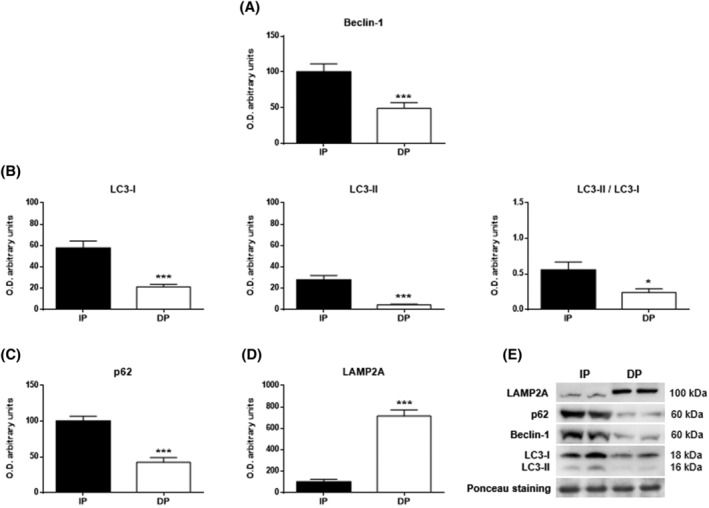
Autophagy in the muscle tissues of functional‐independent patients (IP) and functional‐dependent patients (DP). Bar chart shows the quantification of the optical densities (O.D.) of blot bands for (A) Beclin‐1, (B) LC3‐I, LC3‐II, LC3‐II/LC3‐I, (C) p62 and (D) LAMP2A. (E) Immunoblots of autophagy markers. Ponceau staining was used as a loading control. Data are represented as the mean ± SEM. *P < 0.05; ***P < 0.001.
Mitochondrial oxidative phosphorylation
The mitochondrion is an organelle that is mainly involved in cellular oxidative disruption due to its role in the electron transport chain, which produces free radicals. Alteration in the expression of its mitochondrial complexes weakens the chain coordination and worsens its energy production. 4 A virtually unanimous significant decrease was observed in all DP mitochondrial complexes (complexes I, III, and IV with P < 0.001; complex V with P < 0.01), with the exception of complex II, which showed no difference (Figure 3). When complex I is impaired, it induces complex II activation, so this discrepancy in complex II supports the idea that there is impairment of the electron transport chain in DP muscles. The intracellular ATP levels were significantly decreased in DP patients (P < 0.001; Figure 3C).
Figure 3.
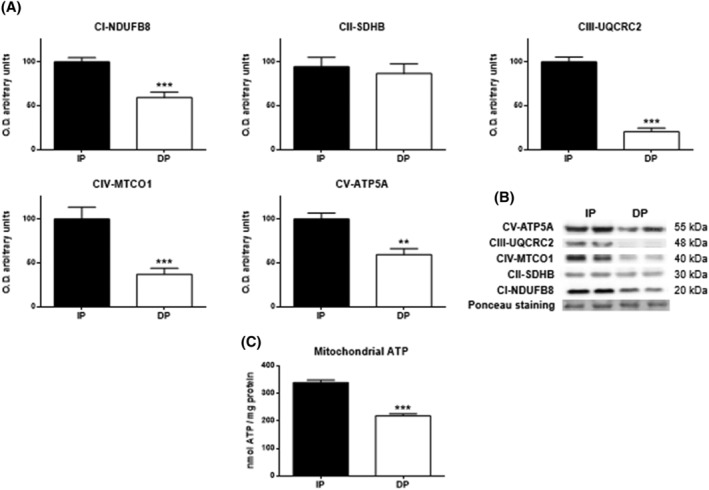
Western blot analysis for studying the oxidative phosphorylation profile in muscle tissues of functional‐independent patients (IP) and functional‐dependent patients (DP). Bar chart shows the quantification of the optical densities (O.D.) of blot bands for (A) subunits from the protein complexes of the mitochondrial electron transport chain (NADH dehydrogenase (ubiquitone) 1b subcomplex 8 (NDUFB8) from complex I (CI), iron sulfur subunit (SDHB) from complex II (CII), ubiquinol‐cytochrome c reductase core protein II (UQCRC2) subunit from complex III (CIII), cytochrome c oxidase subunit I (MTCO1) from complex IV (CIV) and ATP synthase subunit α (ATP5A) from complex V (CV). (B) Representative immunoblots. Ponceau staining was used as a loading control. (C) ATP content was evaluated by bioluminescence (expressed as nmol ATP/mg protein. Data are represented as the mean ± SEM. **P < 0.01; ***P < 0.001.
Oxidative stress status and inflammation
As shown in Figure 4, data analysis revealed that dependent patients showed a loss of antioxidant capacity compared with independent patients: in the first antioxidant line, at the enzymatic level, there were significant reductions in SOD (P < 0.05; Figure 4A) and CAT (P < 0.01; Figure 4B), and at the global level, there was a significant decrease in TAA (P < 0.01; Figure 4C). This antioxidant loss led to an increase in oxidative damage, as shown by a significant increase in the LPO in DP (P < 0.001; Figure 4D). In accordance with the oxidative stress status, greater inflammatory response was observed in DP patients, with significant increases of cytokine IL‐6 and TNF‐α detected in the muscle of DP patients (P < 0.001; Figure 4E,F).
Figure 4.
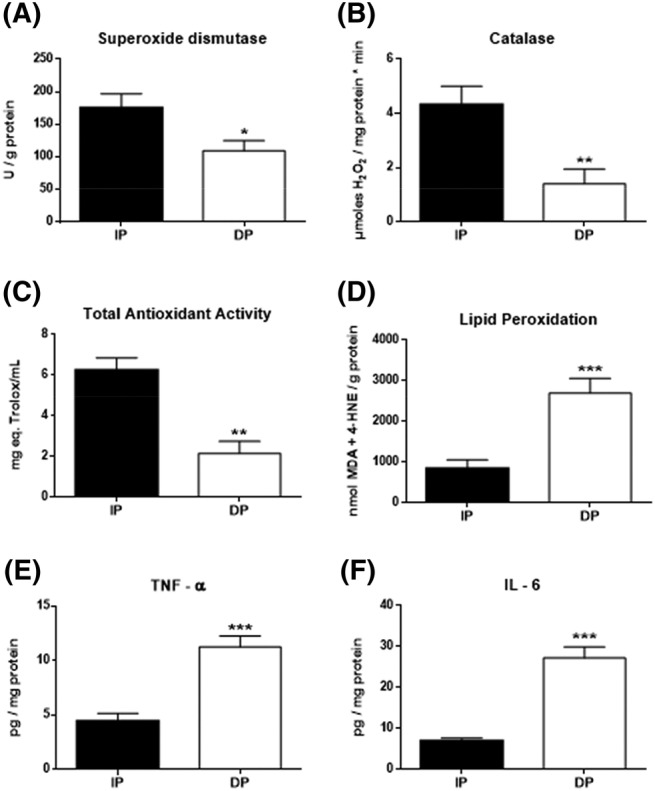
Oxidative stress status and inflammatory response in the muscle of functional‐independent patients (IP) and functional‐dependent patients (DP). (A) Superoxide dismutase (expressed as SOD units/mg of protein), (B) catalase (expressed as μmol H2O2/min mg protein), (C) total antioxidant activity (expressed as mg eq. Trolox/mL), and (E) lipid peroxidation (expressed as MDA + 4‐HNE/g protein). Inflammation levels by the determination of (E) TNF‐α and (F) IL‐6 (expressed as pg/mg protein). Data are represented as the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Excitation‐contraction coupling
Excitation–contraction coupling is the basis of muscle contraction, and a loss of coordination or damage to any of its essential elements should have a significant impact on disability. Our results support this assertion, as both phosphorylated Ryanodine receptor 1 (phospho‐RYR1) and Ca2+/calmodulin‐dependent phosphorylated protein kinase II (phospho‐CaMKII), active forms of their corresponding proteins, were detected at significantly lower levels (P < 0.001) in functionally dependent patients (Figure 5A). Likewise, the protein expression of Hsp27 (P < 0.001; Figure 5B) and dystrophin (P < 0.001; Figure 5C), with a role in providing cellular protection and structural integrity of muscle, was reduced in DP patients while, consequently, the expression of muscle‐ring finger protein‐1 (MuRF‐1), a ubiquitin ligase involved in muscle degradation, was significantly increased in DP (P < 0.001; Figure 5D).
Figure 5.
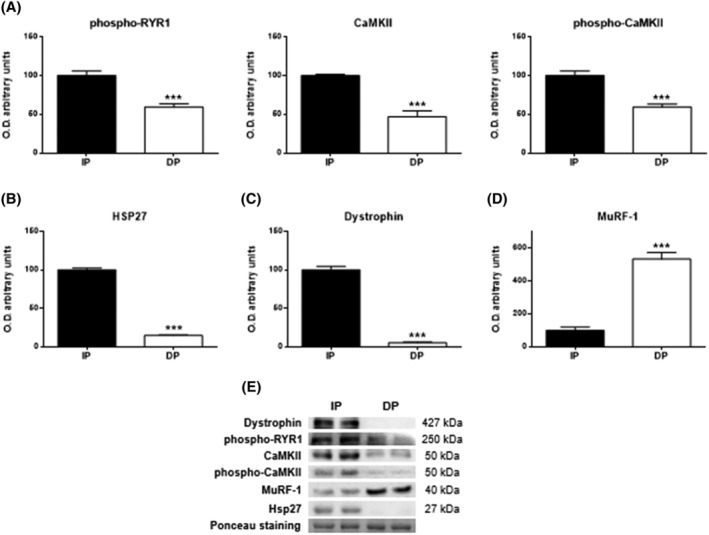
Skeletal muscle excitation‐contraction coupling mechanism in muscle tissues of functional‐independent patients (IP) and functional‐dependent patients (DP). (A) Levels of phospho‐RYR1, Ca2+/calmodulin‐dependent protein kinase II (CaMKII) and phospho‐CaMKII. (B) Levels of Hsp27, (C) Dystrophin, and (D) MuRF‐1. Bar chart shows the quantification of the optical densities (O.D.). (E) Representative immunoblots. Ponceau staining was used as a loading control. Data are represented as the mean ± SEM. ***P < 0.001.
Apoptosis
Comparing results between IP and DP, from the low autophagic activity to the reduction in energy production, led us to suspect the cells were inviable, so we investigated the existence of an apoptotic processes. For this purpose, we first studied the balance between pro‐ and antiapoptotic proteins of the Bcl‐2 family and the activity of the main effector caspases 3 and 7. The results showed without doubt a clear activation of apoptosis in DP. Thus, elevated levels of all proapoptotic proteins were observed in the DP group compared with the IP group: Bad (P < 0.05; Figure 6A), Bax (P < 0.01; Figure 6B), Bik (P < 0.01; Figure 6C), Bim (P < 0.01; Figure 6D), Puma (P < 0.01; Figure 6E), while significantly lower levels of the antiapoptotic protein Bcl‐2 (P < 0.001; Figure 6F). Moreover, Caspase‐3/7 activity showed a significative increase in DP vs IP (P < 0.001; Figure 6H), indicating an evident cellular loss by apoptosis in DP.
Figure 6.
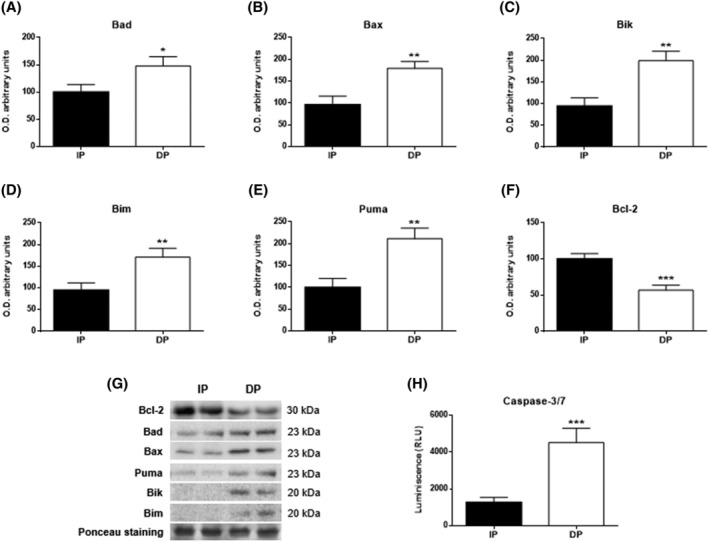
Apoptosis in muscle tissues of functional‐independent patients (IP) and functional‐dependent patients (DP). Bar chart shows the quantification of the optical densities (O.D.) of blot bands for (A) bad, (B) Bax, (C) Bik, (D) Bim, (E) Puma, and (F) Bcl‐2. (G) Representative immunoblots. (H) Caspase‐3/7 was evaluated by bioluminescence. Ponceau staining was used as a loading control. Data are represented as the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Myogenic differentiation
Myogenic regulatory factors are part of a superfamily of transcription factors involved in the differentiation of satellite cells. Growth differentiation factor 8, which is also known as myostatin, is involved in the inhibition of this pathway. Our data indicated that MyoD, Myf5, and Myf6 levels were significantly reduced in the muscle of the dependent group compared with the independent group (P < 0.001, P < 0.001, P < 0.001; Figure 7A–C). In addition, a significant increase in myostatin (P < 0.01; Figure 7D) along with a significant decrease in Pax7 (P < 0.001; Figure 7E) expression was observed in the dependent patients. These remarkable alterations in factors related to muscle degeneration and blockage of muscle stem cells lead to an inability of muscle regeneration in DP and may become the inducer of dependency.
Figure 7.
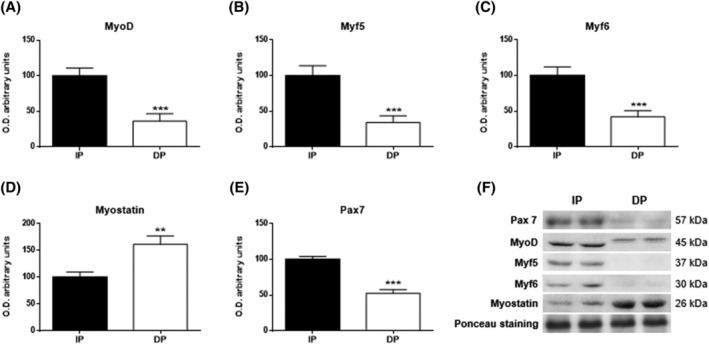
Myogenic differentiation in muscle tissues of functional‐independent patients (IP) and functional‐dependent patients (DP). Bar chart shows the quantification of the optical densities (O.D.) of blot bands for (A) MyoD, (B) Myf5, (C) Myf6, (D) myostatin, and (E) Pax7. (F) Representative immunoblots. Ponceau staining was used as a loading control. Data are represented as the mean ± SEM. **P < 0.01; ***P < 0.001.
Discussion
Disability in the elderly population is caused in a large number of cases by significant impairments of muscle function, known as severe sarcopenia, which seriously hampers movement. At the macroscopic level, this alteration is the result of the disruption of multiple mechanisms and links at the cellular level, which modulates a specific and severe sarcopenia interactome. Unravelling the differential mechanism characteristics could reveal new therapeutic targets for reversing the disability.
Several markers used in standard biochemical analysis have shown significant differences and clear trends between DP and IP. The global study of these markers can provide us with important information about the muscular state of the individual, even if each marker can separately be a classic marker of another pathology. Thus, albumin and creatinine are typical markers of kidney damage. 18 Furthermore, the albumin and sodium decreases observed in DP with clear downward trends in glucose and detected total proteins are also clear indicators of malnutrition and poor intestinal absorption, 19 which have a devastating effect on muscles and promotes protein catabolism that further accelerates muscle wasting, and recent articles support their role as a warning signal in elderly people. 20 Creatinine, in turn, is filtered out of the blood by the renal glomeruli, so increased blood levels of creatinine are often used as an indicator of renal dysfunction. 18 However, creatinine is also a breakdown product of creatine phosphate in muscle, and as a waste product of muscle metabolism, 21 it is released into the blood. Therefore, the increase in its presence at the systemic level could also be a marker of muscle degradation, and it not only reflects kidney function. In this way, the increased creatinine levels observed in DP reinforce the information provided by alterations in albumin, sodium, glucose, and total proteins levels, forming a set of tools for predicting the muscular state of the patient, in which the reduction in the protein level seems to be most noticeable. The systemic decline in protein capacity observed under disability was corroborated by an impairment in the protein synthesis of the muscle cells present in DP patients. During aging, the capacity of the endoplasmic reticulum for protein synthesis and folding is compromised, mainly due to the progressive failure of chaperone systems. 22 This leads to an accumulation of unfolded or misfolded proteins inside the ER lumen, called reticulum stress, and UPR activation. In our study, two of the three pathways that make up the UPR were increased in dependent patients. PERK activation, the only UPR pathway without differences between DP and IP, is usually the first indicator of UPR activation, and it phosphorylates eIF2α to induce the down‐regulation of protein synthesis. 23 Different results have been reported regarding PERK activation, including a prevalent lack of change and even a reduction in its activation with age, which seems to indicate that the UPR pathway is the most sensitive branch to age‐induced damage. 24 Decreased PERK gene expression was observed in aged brains in rats, 25 and it remained unaltered in human muscles 24 ; eIF2α expression was also reduced in the mouse cerebral cortex, 26 and no change was observed in phospho‐eIF2α in aged human muscle. 27 The conservation of the activation capacity during aging by IRE1/ATF6 has, however, been reported in several papers. 5 , 24 Thus, the cascade of cellular responses to protein synthesis damage is maintained in DP muscle, although it is drastically diminished by blocking essential PERK‐dependent mechanisms such as antioxidant response and autophagy. 28
Autophagy is an essential mechanism in the maintenance of cellular protein quality; it has decreasing efficiency with aging 8 and leads to the accumulation of autophagosomes that significantly hinder vesicular transport in the cell. However, in DP muscle, we suggest that there is not a reduction in autophagic activity but a complete blockage of autophagic activity, as evidenced by p62. p62 is a potent marker of autophagic flux that binds to autophagic substrates in autophagosomes and is degraded when autophagy is adequately induced. Thus, an inefficient autophagic response is usually shown by p62 accumulation in aging 5 and under several pathological conditions. 29 However, in DP patients, the autophagic situation is aggravated by a total blockade from the onset that prevents the activation of p62 and keeps its concentration reduced to the level of the other macroautophagic markers. On the other hand, CMA exhibited an abrupt rise in its levels in dependent elderly patients, showing an opposite trend to macroautophagy. This dissonant evolution has been observed in other stress situations, suggesting a sequential and related action of both pathways. 30 Furthermore, CMA is up‐regulated in response to a wide variety of stressors and is a key element in the regulation of lipid and glucose metabolism, 31 which links this series of alterations to the other fundamental pillar of cell function, energy production.
Our results showed a significant reduction in the expression of the main complexes of the mitochondrial electron transport chain in DP patients: complexes I, III, IV, and V. However, complex II, which is activated as a compensatory mechanism to support mitochondrial metabolism when complex I is compromised, did not exhibit changed levels. This indicates a drastic loss in mitochondrial capacity and implies a significant drop in ATP production at the muscle level. Several articles have shown that mitochondria have an essential role in the development of sarcopenia and that their preservation would slow the progression towards severe sarcopenia and disability. 4 , 32 This mitochondrial involvement in sarcopenia evolution seems greater when the concentration of type II fibres in the muscle is higher, 33 and Vastus lateralis, the same muscle under study in the present work, has specifically shown mitochondrial impairment with important muscular effects. 33 Only the elimination of dysfunctional mitochondria could prevent muscle damage, but the blockage of autophagy displayed in the muscles of DP prevents mitophagy 28 and, thus, the ability to recover.
Muscle deterioration due to mitochondrial malfunction goes beyond the drop in ATP production. Thus, malfunctioning of the electron transport chain is the main source of oxygen free radicals 34 ; in turn, these reactive species favour the uncoupling of the respiratory chain, which causes a vicious cycle 32 that enhances the initial damage and ultimately regulates the quality of skeletal muscle.
Our data regarding cellular oxidative damage confirm that the increase in ROS were due to mitochondrial alterations, which was already identified by other authors, 10 , 34 because damage to macromolecules is significantly increased in DP patients. An interesting relationship between reticulum stress and oxidative stress has also been found previously, 5 so we hypothesize that oxidative damage must be considered multifactorial. This oxidative impairment of macromolecules is undoubtedly aggravated by the subsequent observed inflammation and the deficit in the antioxidant capacity of the disability group, which shows a significant reduction in both its main antioxidant enzymes and the total antioxidant activity together with an increase in main cytokines. The loss of antioxidant responsiveness to macromolecule damage is probably due to the significant protein loss detected, 5 and this leads us back to the blockade of PERK, which, as we had indicated, blocked not only autophagy but also the activation of antioxidants, 28 while the observed increase in inflammation supports the activation of IRE‐1 discovered in DP, already indicated in several pathologies (reference S5 in the Supporting Information).
The loss of energy production capacity at the mitochondrial level and the damage in protein production have a direct effect on muscle cell contractility, 4 and the reduction in the expression of the main molecules involved in contractility and even in the strengthening of muscle fibres and their protection, such as dystrophin, observed in DP patients confirm this asseveration. In addition, CaMKII is highly susceptible to oxidation by ROS due to its high methionine content, which has been shown to accelerate muscle degeneration in aging. 35 Antioxidant treatment may reduce the RYR1 involvement, 36 but antioxidant capacity was severely impaired in the DP group, so it appears that contractile capacity in these individuals is severely compromised.
Protein synthesis and energy generation are the two pillars on which cellular activity is based. The perturbation of at least one of them causes a cascading effect on the essential mechanisms of the cell that can lead to cell death. In our work, functionally dependent patients not only showed impaired capacity for protein synthesis and energy production, but the most important survival mechanism within the cell, autophagy, was also shown to be blocked; therefore, that the discovery of the increase in the main markers of apoptosis triggering only confirmed the predictions. It has been described the existence of apoptosis in aged muscle, 37 but our work shows that the causal relationship of muscle apoptosis is not necessarily with aging but with severe sarcopenia, because independent individuals of the same age group had drastically reduced levels of these markers, both at the adaptor protein level with a clear imbalance towards antiapoptotic proteins and at the effector level with a significant reduction of caspases activity.
The loss of muscle mass that occurs with age and is induced by apoptosis 37 can only materialize if the muscle has lost its regenerative capacity. Therefore, the significant reduction in MRFs expression and Pax7, as satellite cell marker, found in the disability group was necessary for the irreversible loss of muscle mass in that group. Moreover, DP presented a significant increase in myostatin levels and MuRF‐1, both essential regulators of degradation in skeletal muscle (reference S6 in the Supporting Information). Some studies emphasize the antiproliferative effect of myostatin on muscle stem cells. 38 Supporting our results, Suetta et al. 39 observed increased levels of myostatin in elderly people with immobility‐induced muscle atrophy and attenuated down‐regulation after rehabilitation. This reduction in MRFs expression together with an increase in myostatin implies a reduction in the satellite cell niche in dependent elderly individuals, and it also contributes to muscular degeneration and atrophy. Furthermore, recovery of skeletal muscle mass after immobility‐induced muscle atrophy is faster in young people than in elderly people due to a reduced number of satellite cells and/or their myogenic potential, which contributes to functional decline. 39
Our results confirm the hypothesis initially put forward and validate our previous division of the study population, assigning exacerbated sarcopenia to extreme disability. Thus, data show large differences in the muscular interactome between dependent and independent patients, revealing that sarcopenia is not only a result of age but also an evolutionary syndrome in which multiple cellular alterations are involved. The study of the series of processes connected in the interactome provides us with precise information for identifying the main target at the early stages of sarcopenia with the intention of slowing down its progression and, thus, delaying the induced disability (Figure 8).
Figure 8.
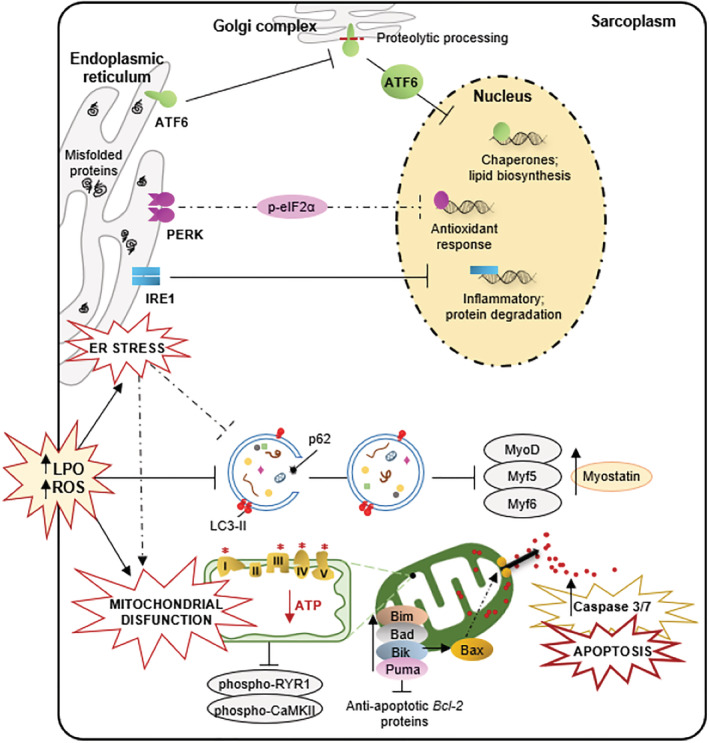
Global effect of dependence in the skeletal muscle of elderly people. In the muscle of dependent patients, a concatenation of cellular alterations ends up inducing their death by apoptosis and reducing their regenerative capacity, which could be the main causes of the muscle reduction evident in these subjects. Thus, the abrupt decrease in protein synthesis triggers reticular stress and activation of the response to non‐folded proteins (mainly IRE‐1, ATF‐6) which induces an increase in oxidative damage (LPO) and blockage of autophagy (LC3‐II, p62) which prevents the degradation of damaged proteins while mitochondrial injury reduces cellular energy capacity. This has a negative effect on muscle contraction (phospho‐RYR1, phospho‐CaMKII) and cell proliferation by reducing myogenic regulatory factors (MyoD, Myf5, Myf6) and Pax7, while increasing myostatin. These last alterations will be the cause of the inhibition of muscle regeneration. Simultaneously, the accumulation of cellular damage causes an apoptotic imbalance with an increase in proapoptotic proteins (Bad, Bax, Bik, Bim, Puma) and a decrease in antiapoptotic proteins (Bcl‐2) leading to the death by apoptosis of muscle cells in extreme sarcopenia.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Table S1 Participant characteristics
Table S2 Primary antibodies used in the western blot
Table S3 Biochemical evaluation
Data S1. Supporting Information
Acknowledgements
We are members of the Cellular Response to Oxidative Stress (cROS) research group, Instituto de Investigación Sanitaria del Principado de Asturias (ISPA), the INPROTEOLYS and Instituto de Neurociencias del Principado de Asturias (INEUROPA) networks. This work was supported by FISS‐14‐PI13/02741 and PI21/01596 [Instituto de Salud Carlos III (ISCIII), Spanish Ministry of Science, Innovation and Universities (MCIU)] and by Regional Ministry of Science of Asturias (IDI/2021/000033). Laura González‐Blanco acknowledges her contract to the MCIU (PRE2019‐091053). The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 40
González‐Blanco L., Bermúdez M., Bermejo‐Millo J. C., Gutiérrez‐Rodríguez J., Solano J. J., Antuña E., Menéndez‐Valle I., Caballero B., Vega‐Naredo I., Potes Y., and Coto‐Montes A. (2022) Cell interactome in sarcopenia during aging, Journal of Cachexia, Sarcopenia and Muscle, 13, 919–931, 10.1002/jcsm.12937
Contributor Information
Laura González‐Blanco, Email: lgblanco@serida.org.
Ana Coto‐Montes, Email: acoto@uniovi.es.
References
- 1. Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short‐term disuse: implications for age‐related sarcopenia. Ageing Res Rev 2013;12:898–906. [DOI] [PubMed] [Google Scholar]
- 2. Lee SM, Lee SH, Jung Y, Lee Y, Yoon JH, Choi JY, et al. FABP3‐mediated membrane lipid saturation alters fluidity and induces ER stress in skeletal muscle with aging. Nat Commun 2020;11:5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Christian CJ , Benian GM. Animal models of sarcopenia. Aging Cell 2020;19:e13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Potes Y, Pérez‐Martinez Z, Bermejo‐Millo JC, Rubio‐Gonzalez A, Fernandez‐Fernández M, Bermudez M, et al. Overweight in the elderly induces a switch in energy metabolism that undermines muscle integrity. Aging Dis 2019;10:217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Potes Y, de Luxán‐Delgado B, Rodriguez‐González S, Guimarães MRM, Solano JJ, Fernández‐Fernández M, et al. Overweight in elderly people induces impaired autophagy in skeletal muscle. Free Radic Biol Med 2017;110:31–41. [DOI] [PubMed] [Google Scholar]
- 6. Bowen TS, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle 2015;6:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deldicque L. Endoplasmic reticulum stress in human skeletal muscle: any contribution to sarcopenia? Front Physiol 2013;4:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life‐long exercise. Exp Gerontol 2010;45:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castets P, Frank S, Sinnreich M, Rüegg MA. "Get the Balance Right": pathological significance of autophagy perturbation in neuromuscular disorders. J Neuromuscul Dis 2016;3:127–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sreedhar A, Aguilera‐Aguirre L, Singh KK. Mitochondria in skin health, aging, and disease. Cell Death Dis 2020;11:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zammit PS. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin Cell Dev Biol 2017;72:19–32. [DOI] [PubMed] [Google Scholar]
- 12. Bagheri R, Rashidlamir A, Motevalli MS, Elliott BT, Mehrabani J, Wong A. Effects of upper‐body, lower‐body, or combined resistance training on the ratio of follistatin and myostatin in middle‐aged men. Eur J Appl Physiol 2019;119:1921–1931. [DOI] [PubMed] [Google Scholar]
- 13. Sousa‐Victor P, Muñoz‐Cánoves P. Regenerative decline of stem cells in sarcopenia. Mol Aspects Med 2016;50:109–117. [DOI] [PubMed] [Google Scholar]
- 14. Bermúdez M, Caballero B, de Luxán‐Delgado B, Potes Y, Fernández‐Fernández M, Coto‐Montes A, et al. Physical Performance Drops after Hip Fracture Surgery: HIPA Study. J Gerontol Aging Res 2017;1:105. [Google Scholar]
- 15. Arnao MB, Cano A, Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem 2001;73 (2):239–244. [Google Scholar]
- 16. Roshanravan B, Patel KV, Robinson‐Cohen C, de Boer IH, O'Hare AM, Ferrucci L, et al. Creatinine clearance, walking speed, and muscle atrophy: a cohort study. Am J Kidney Dis 2015;65:737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, et al. ATF6α optimizes long‐term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell 2007;13(3):351–364. [DOI] [PubMed] [Google Scholar]
- 18. Obert LA, Elmore SA, Ennulat D, Frazier KS. A review of specific biomarkers of chronic renal injury and their potential application in nonclinical safety assessment studies. Toxicol Pathol 2021;49:996–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Souweine JS, Pasquier G, Kuster N, Rodriguez A, Patrier L, Morena M, et al. Dynapaenia and sarcopaenia in chronic haemodialysis patients: do muscle weakness and atrophy similarly influence poor outcome? Nephrol Dial Transplant 2021;36(10):1908–1918. [DOI] [PubMed] [Google Scholar]
- 20. Schiller LR. Maldigestion versus malabsorption in the elderly. Curr Gastroenterol Rep 2020;22:33. [DOI] [PubMed] [Google Scholar]
- 21. Pasala S, Carmody JB. How to use… serum creatinine, cystatin C and GFR. Arch Dis Childhood‐Educ Pract 2017;102:37–43. [DOI] [PubMed] [Google Scholar]
- 22. Estébanez B, de Paz JA, Cuevas MJ, González‐Gallego J. Endoplasmic reticulum unfolded protein response, aging and exercise: an update. Front Physiol 2018;9:1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown MK, Chan MT, Zimmerman JE, Pack AI, Jackson NE, Naidoo N. Aging induced endoplasmic reticulum stress alters sleep and sleep homeostasis. Neurobiol Aging 2014;35:1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ogborn DI, McKay BR, Crane JD, Parise G, Tarnopolsky MA. The unfolded protein response is triggered following a single, unaccustomed resistance‐exercise bout. Am J Physiol Regul Integr Comp Physiol 2014;307:R664–R669. [DOI] [PubMed] [Google Scholar]
- 25. Paz Gavilán M, Vela J, Castaño A, Ramos B, del Río JC, Vitorica J, et al. Cellular environment facilitates protein accumulation in aged rat hippocampus. Neurobiol Aging 2006;27:973–982. [DOI] [PubMed] [Google Scholar]
- 26. Naidoo N, Ferber M, Master M, Zhu Y, Pack AI. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci 2008;28:6539–6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, et al. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol 2011;111:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu CY, Kaufman RJ. The unfolded protein response. J Cell Sci 2003;116:1861–1862. [DOI] [PubMed] [Google Scholar]
- 29. Zhang S, Shao Z, Liu X, Hou M, Cheng F, Lei D, et al. The E50K optineurin mutation impacts autophagy‐mediated degradation of TDP‐43 and leads to RGC apoptosis in vivo and in vitro. Cell Death Dis 2021;7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaushik S, Massey AC, Mizushima N, Cuervo AM. Constitutive activation of chaperone‐mediated autophagy in cells with impaired macroautophagy. Mol Biol Cell 2008;19:2179–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liao Z, Wang B, Liu W, Xu Q, Hou L, Song J, et al. Dysfunction of chaperone‐mediated autophagy in human diseases. Mol Cell Biochem 2021;476:1439–1454. [DOI] [PubMed] [Google Scholar]
- 32. Harper C, Gopalan V, Goh J. Exercise rescues mitochondrial coupling in aged skeletal muscle: a comparison of different modalities in preventing sarcopenia. J Transl Med 2021;19:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Conley KE, Amara CE, Jubrias SA, Marcinek DJ. Mitochondrial function, fibre types and ageing: new insights from human muscle in vivo. Exp Physiol 2007;92:333–339. [DOI] [PubMed] [Google Scholar]
- 34. Nakamura S, Takamura T, Matsuzawa‐Nagata N, Takayama H, Misu H, Noda H, et al. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem 2009;284:14809–14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nelson SED, Weber DK, Rebbeck RT, Cornea RL, Veglia G, Thomas DD. Met125 is essential for maintaining the structural integrity of calmodulin's C‐terminal domain. Sci Rep 2020;10:21320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wyckelsma VL, Venckunas T, Brazaitis M, Gastaldello S, Snieckus A, Eimantas N, et al. Vitamin C and E treatment blunts sprint interval training‐induced changes in inflammatory mediator‐, calcium‐, and mitochondria‐related signaling in recreationally active elderly humans. Antioxidants (Basel) 2020;9:879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Song W, Kwak HB, Lawler JM. Exercise training attenuates age‐induced changes in apoptotic signaling in rat skeletal muscle. Antioxid Redox Signal 2006;8:517–528. [DOI] [PubMed] [Google Scholar]
- 38. Amthor H, Otto A, Vulin A, Rochat A, Dumonceaux J, Garcia L, et al. Muscle hypertrophy driven by myostatin blockade does not require stem/precursor‐cell activity. Proc Natl Acad Sci U S A 2009;106:7479–7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suetta C, Frandsen U, Mackey AL, Jensen L, Hvid LG, Bayer ML, et al. Ageing is associated with diminished muscle re‐growth and myogenic precursor cell expansion early after immobility‐induced atrophy in human skeletal muscle. J Physiol 2013;591:3789–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2021. J Cachexia Sarcopenia Muscle 2021;12:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Participant characteristics
Table S2 Primary antibodies used in the western blot
Table S3 Biochemical evaluation
Data S1. Supporting Information


