Abstract
Background
Age‐related loss in skeletal muscle mass, quality, and strength, known as sarcopenia, is a well‐known phenomenon of aging and is determined clinically using methods such as dual‐energy X‐ray absorptiometry (DXA). However, these clinical methods to measure sarcopenia are not practical for population‐based studies, and a five‐question screening tool known as SARC‐F has been validated to screen for sarcopenia.
Methods
We investigated the relationship between appendicular skeletal lean mass/height2 (ALM/HT2) (kg/m2) assessed by DXA and SARC‐F in a subset of 1538 (778 men and 760 women) participants in the Multiethnic Cohort (MEC) Study after adjustment for race/ethnicity, age, and body mass index (BMI) at the time of DXA measurement. We then investigated the association between SARC‐F and mortality among 71 283 (41 757 women and 29 526 men) participants in the MEC, who responded to the five SARC‐F questions on a mailed questionnaire as part of the MEC follow‐up in 2012–2016.
Results
In women, SARC‐F score was significantly inversely associated with ALM/HT2 after adjusting for race/ethnicity, and age and BMI at DXA (r = −0.167, P < 0.001); the result was similar in men although it did not reach statistical significance (r = −0.056, P = 0.12). Among the 71 000+ MEC participants, SARC‐F score ≥ 4, as an indicator of sarcopenia, was higher in women (20.9%) than in men (11.2%) (P < 0.0001) and increased steadily with increasing age (6.3% in <70 vs. 41.3% in 90+ years old) (P < 0.0001). SARC‐F score ≥ 4 was highest among Latinos (30.8% in women and 16.1% in men) and lowest in Native Hawaiian women (15.6%) and Japanese American men (8.9%). During an average of 6.8 years of follow‐up, compared with men with SARC‐F score of 0–1 (indicator of no sarcopenia), men with SARC‐F 2–3 (indicator of pre‐sarcopenia) and SARC‐F ≥ 4 had significantly increased risk of all‐cause mortality [hazard ratio (HR) = 1.00, 1.77, 3.73, P < 0.001], cardiovascular disease (CVD) mortality (HR = 1.00, 1.85, 3.98, P < 0.001), and cancer mortality (HR = 1.00, 1.46, 1.96, P < 0.001) after covariate adjustment. Comparable risk association patterns with SARC‐F scores were observed in women (all‐cause mortality: HR = 1.00, 1.47, 3.10, P < 0.001; CVD mortality: HR = 1.00, 1.59, 3.54, P < 0.001; cancer mortality: HR = 1.00, 1.30, 1.77, P < 0.001). These significant risk patterns between SARC‐F and all‐cause mortality were found across all sex–race/ethnic groups considered (12 in total).
Conclusions
An indicator of sarcopenia, determined using SARC‐F, showed internal validity against DXA and displayed racial/ethnic and sex differences in distribution. SARC‐F was associated with all‐cause mortality as well as cause‐specific mortality.
Keywords: Sarcopenia, SARC‐F, Multiethnic, All‐cause mortality, Appendicular skeletal lean mass
Introduction
Age‐related loss in skeletal muscle mass, quality, and strength, known as sarcopenia, is a well‐known phenomenon of aging, chronic diseases, and physical inactivity. 1 , 2 , 3 There is tremendous recent interest in sarcopenia for a range of aging‐related conditions (e.g. cancer, metabolic syndrome, and Alzheimer's disease) as it has been found to contribute to subsequent disability and mortality. 4 Sarcopenia is assessed clinically by determining muscle mass using methods such as computed tomography, bioimpedance analysis, or dual‐energy X‐ray absorptiometry (DXA) and to measure muscle strength using various physical performance tests (e.g. grip strength, gait speed, chair rises, timed‐up‐and‐go test, and short physical performance battery). Since 2010, several entities in the USA, Europe, and Asia have developed different definitions and thresholds to assess muscle strength using grip strength and gait speed and to determine clinically low muscle mass based on sex‐specific cut‐off points of appendicular lean mass and height (ALM/height2). The specific cut‐off points varied depending on the measurement technique and on the availability of reference studies and populations. 3 , 5 , 6 , 7 , 8 For example, the respective ALM/height2 cut‐off points in men and women are <7.0 and <5.4 kg/m2 (based on DXA) according to the Asian Working Group on Sarcopenia (AWGS), 8 ≤7.23 and ≤5.67 kg/m2 according to the International Working Group on Sarcopenia (IWGS), 7 and <7.0 and <5.0 kg/m2 according to the revised European Working Group on Sarcopenia in Older People (EWGSOP2). 6 The respective AWGS, IWGS, and EWGSOP2 guidelines on gait speed are <0.8, <1, and ≤0.8 m/s, while the handgrip strength recommendations are <26 kg in men and <18 kg in women from AWGS, and <20 kg in men and <15 kg in women from EWGSOP2. Not surprisingly, population/study estimates of sarcopenia varied substantially depending on the approaches and definitions that were used. These clinical methods to assess muscle mass and strength are also costly and typically not practical for large‐scale population‐based studies. 6 Importantly, a recent position statement from the Sarcopenia Definition and Outcomes Consortium (SDOC) and several accompanying papers found that lean mass measured by DXA was not associated with adverse outcomes. 9 , 10 , 11
To facilitate screening of sarcopenia in population‐based studies, Malmstrom et al. developed and validated a five‐item questionnaire to screen for sarcopenia (SARC‐F) among Whites and African Americans in three US study populations. 12 Responses to these five questions are based on the subject's assessment of his or her limitation in strength (lifting), walking ability, rising from a chair, climbing stairs, and recent history of falls. 13 , 14 The total SARC‐F score ranges from 0 to 10, with a score of 4 or greater as predictive of sarcopenia. Organizations such as the International Clinical Practice Guidelines for Sarcopenia have recommended screening for sarcopenia using the SARC‐F or gait speed. 15 The SARC‐F has been translated into multiple languages and has been investigated in at least 28 studies in relation to various health outcomes that were conducted in Asia (n = 15), Europe (n = 7), South America (n = 4), and the USA (n = 2) (see, review 16 ). In a meta‐analysis of five studies (one USA, one Spain, and three Asia) that tested SARC‐F in relation to mortality, a significant two‐fold increased risk was found. 17 However, two of the five studies had little information on covariates, 12 , 18 and the two studies showing the strongest association had only 1 year of follow‐up. 18 , 19
We posited that the SARC‐F may be a useful tool to study sarcopenia across sex–racial/ethnic groups in the USA. As such, we investigated indicators of sarcopenia based on the SARC‐F 13 in the Multiethnic Cohort (MEC) Study, an ongoing, long‐term prospective study of older adults in Hawaii and California (CA). We first investigated among 1538 MEC participants the correlation of SARC‐F with DXA measurements on appendicular skeletal lean mass/height2 (ALM/HT2, kg/m2) that was conducted as part of the MEC Adiposity Phenotype Study (APS). 20 We then determined among 41 757 women and 29 526 men the SARC‐F scores by sex across 6 racial/ethnic groups and age categories. During an average of 6.8 years of follow‐up, we investigated the association of SARC‐F with all‐cause mortality, and cardiovascular disease (CVD)‐specific and cancer‐specific mortality by sex–race/ethnicity with adjustment for relevant lifestyle factors.
Methods
Study population
The MEC was established between 1993 and 1996, enrolling 96 810 men and 118 441 women, aged 45–75 years from primarily six racial/ethnic groups (African Americans and Latinos from California, mainly from Los Angeles County, and Japanese, Native Hawaiians, Whites, and other Asian Americans including Filipinos, Chinese, and Koreans, mainly from Hawaii). 21 At cohort entry, participants completed a 26‐page mailed questionnaire that assessed demographics, anthropometry, smoking, alcohol use, medical history, diet, physical activity, and reproductive history (among women).
Ascertainment of sarcopenia by questionnaire
Follow‐up questionnaires were mailed to all MEC participants about every 5 years to update select exposures or assess new exposures (Supporting Information, Figure S1). In the follow‐up questionnaire (Q×5) that was administered in 2012–2016, a geriatric assessment was conducted, which asked 21 items related to physical function, including 6 questions on activity of daily living (ADL) (using the toilet including getting up and down, eating including cutting your own food/feeding yourself, dressing, bathing/showering, getting in or out of bed, and walking across a room), 22 6 questions on instrumental activities of daily living (IADL) (using a map, shopping for grocery, preparing a hot meal, managing money, taking medicine, and using a phone), 23 and 9 questions on other parameters of physical functions including mobility (walking 1 block and climbing flight of stairs), large muscle function (sitting for ~2 h, stooping/kneeling/crouching, and getting up from chair after sitting for long periods), arm function (reaching/extending arms above shoulder, pulling/pushing large objects, and lifting or carrying weights ≥10 pounds), and fine motor functions (picking up a dime from table). In addition, questions on social network and isolation, 24 depressive symptoms based on the eight‐item Center for Epidemiological Studies Depression scale, 25 and history of chronic conditions were asked. Following the algorithm that was developed and validated by Malmstrom and Morley to assess sarcopenia, 14 we constructed a sarcopenia score (SARC‐F) based on responses to five questions. Four questions were identical to those asked by Malmstrom: (1) walking across room, (2) climbing flights of stairs, (3) getting up from a chair, and (4) lifting or carrying weights more than 10 pounds (referred hereafter as lifting), and we assigned a score of 0, 1, and 2, respectively, to responses of no difficulty, some difficulty, and cannot and will not do (i.e. a total score of 8 if participant did not do all 4 tasks). For the fifth question, Malmstrom asked number of falls, 0, 1–3, and ≥4 falls, and assigned a score of 0, 1, and 2, respectively. In the MEC, we first asked if subjects had falls (no/yes), and among those who had falls, if they received medical treatment (no/yes). We assigned a score of 0, 1, and 2, respectively, to responses of no falls, had falls that did not require medical treatment, and had falls that required medical treatment. Thus, our scoring of falls was based on severity and not the number of falls. A SARC‐F score was created (totalling 10 points) where a score of 0–1 designated no sarcopenia, a score of 2–3 as pre‐sarcopenic, and a score of ≥4 as sarcopenic. In total, 71 303 MEC participants responded to Q×5 and were included in the analysis on lifestyle determinants and mortality analysis in relation to SARC‐F.
Dual‐energy X‐ray absorptiometry assessment and dual‐energy X‐ray absorptiometry‐based sarcopenia
In 2013–2016, the MEC APS was conducted to determine image‐based body composition among a subset of MEC participants. 20 The MEC APS study excluded participants with reported body mass index (BMI) outside the range of 18.5–40 kg/m2; smoking in the past 2 years; soft or metal implants other than knee or hip replacement; insulin or thyroid medications; serious medical conditions; and likely claustrophobia to withstand the magnetic resonance imaging (MRI) bore. During the clinic visit at the University of Hawaii (UH) or University of Southern California (USC), participants provided a blood sample after an overnight fast, completed questionnaires, and underwent anthropometric measurements, an abdominal MRI, and a whole‐body DXA scan for total and regional body composition measurements. 20 Of the 1801 MEC APS participants (915 women and 886 men) with valid DXA measured ALM/HT2 (kg/m2), 1538 (778 men and 760 women) also responded to the SARC‐F assessed at Q×5.
Mortality outcome ascertainment
Deaths were identified by using state death records and the National Death Index. All‐cause mortality included deaths from CVD, cancer, as well as deaths from other causes, including accidents and suicides. All death files were current as of December 2019 for participants in Hawaii and CA. Participants with no recorded deaths as of this date were censored. All deaths were identified by using International Classification of Diseases, Ninth Revision codes 140–208 or International Classification of Disease, Tenth Revision codes C00–C97. CVD deaths included acute myocardial infarction (410, 121), other heart diseases (411, 413–414, 425–429, 120, 122–124, 142–152), and stroke (430–438, 160–169). During an average of 6.8 ± 2.2 years of follow‐up of Q×5 respondents (41 757 women and 29 526 men), there were 10 998 deaths (5605 women and 5393 men).
Statistical analyses
Among 1538 participants with both DXA measured ALM/HT2 and SARC‐F information, we computed the Pearson's correlation coefficients (r) and partial correlation coefficients between SARC‐F and DXA by sex–race/ethnicity with adjustment for age (continuous), and BMI at the time of DXA measurement, and calculated sensitivity and specificity using the IWGS's muscle mass definition for sarcopenia (≤7.23 kg/m2 for men and ≤5.67 kg/m2 for women) 7 and for the SARC‐F cut‐off point of ≥4 for men and women. Second, we classified 71 283 MEC participants by SARC‐F score (0–1, 2–3, and ≥4 as indicator of no sarcopenia, pre‐sarcopenic, and sarcopenic) and then assessed the SARC‐F score by sex–race/ethnicity (African American, Native Hawaiian, Japanese American, Latino, White, and other Asian Americans) and age categories (<70, 70–74, 75–79, 80–84, 85–89, 90+). We then used Cox proportional hazard regression to estimate the multivariable hazard ratios (HRs) and 95% confidence intervals (CIs) for all‐cause mortality, and CVD‐specific and cancer‐specific mortality in relation to SARC‐F. We also investigated each the five SARC‐F components and risk of overall, CVD‐specific, and cancer‐specific mortality. We included potential risk factors for covariate adjustment based on Q×5 data on BMI and history of chronic conditions [high blood pressure, congestive heart failure, angina, heart attack, stroke, diabetes, skin cancer (not melanoma), Alzheimer's disease, other dementia, polyps of intestines, Crohn's disease, ulcerative colitis, osteoporosis, gallbladder removal, ulcer, chronic heartburn, cataract surgery, glaucoma, asthma, chronic lung disease, Parkinson's disease, and enlarged prostate (men only)] as well as baseline variables including BMI (<25, 25–29.9, ≥30 kg/m2, missing), smoking (non‐smoker, former smoker by pack years <20, 20+, don't know; current smokers by pack years <20, 20+, don't know), alcohol consumption (0 ethanol, 1 < 12, 12–<24, ≥24 g/day), physical activity (hours spent in moderate/vigorous activity), history of chronic conditions (0–1, 2–3, 4+), and Mediterranean diet energy adjusted total score (0–9). 26 For women, the models were additionally adjusted for baseline menopausal status and menopausal hormonal therapy (MHT) use (pre‐menopause, post‐menopause: never, past, current MHT use), age at menarche (≤12, 13–14, >14), and parity (nulliparous, 1, 2–3, 4+). In addition, we conducted subgroup analyses by BMI and number of chronic conditions assessed at Q×5 and Mediterranean diet score and smoking status assessed at baseline. All P values are two‐sided with a significance level of 0.05. Analyses were performed using SAS 9.4 statistical software (SAS Institute, Cary, NC).
Results
Comparison between SARC‐F defined sarcopenia and ALM/HT2
Table 1 shows results in 1538 MEC participants with DXA measured ALM/HT2 (kg/m2) and SARC‐F. In men, the respective average age ± SD at DXA measurement and Q×5 response was 69.2 ± 2.7 and 69.5 ± 2.7, and in women, the corresponding ages were 69.0 ± 2.7 and 69.2 ± 2.7. Men compared with women had a lower SARC‐F ≥ 4 (2.2% vs. 5.9%), lower SARC‐F 2–3 (9.3% vs. 19.1%), and higher ALM/HT2 (8.68 ± 1.11 vs. 6.83 ± 1.08 kg/m2). After adjusting for race/ethnicity, age, and BMI at DXA assessment, in women, SARC‐F score was significantly inversely associated with ALM/HT2 (r = −0.167, P < 0.0001). This inverse association was observed in each racial/ethnic group, was borderline statistically significant in Latino women (r = −0.146, P = 0.07), and reached statistical significance in African American women (r = −0.17, P = 0.04). In all men, SARC‐F score was inversely associated with ALM/HT2 (r = −0.056, P = 0.12); this was borderline statistically significant in Latino men (r = −0.133, P = 0.08). Using IWGS definition of sarcopenia, that is, ALM/HT2 (≤7.23 kg/m2 for men and ≤5.67 kg/m2 for women), 7 7.8% (61 of 778) of men and 13.4% (102 of 760) of women in this sub‐study would be classified as sarcopenic. Using SARC‐F ≥ 4 as the cut‐off point, the respective sensitivity and specificity were 3.3% (95% CI 0.4, 11.4), and 97.9% (95% CI 96.6, 98.8) in men, and 6.9% (95% CI 2.8, 13.6), and 94.2% (95% CI 92.2, 95.9) in women. Using SARC‐F ≥ 2 as the cut‐off point, the sensitivity increased to 9.8% (95% CI 3.7, 20.2) in men and 23.5% (95% CI 15.7, 33.0) in women while the specificity dropped to 88.4% (95% CI 85.9, 90.7) in men and 74.8% (95% CI 71.3%, 78.1) in women.
Table 1.
Mean age and body mass index (BMI) of Multiethnic Cohort (MEC) participants with information on appendicular lean mass/height2 (ALM/Ht2) from dual‐energy X‐ray absorptiometry (DXA) examination and self‐reported sarcopenia based on SARC‐F, in 1538 men and women, and by race/ethnicity (2013–2016)
| Men | Women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | African American | Native Hawaiian | Latino | Japanese American | White | All | African American | Native Hawaiian | Latino | Japanese American | White | |
| n | 778 | 97 | 115 | 179 | 208 | 179 | 760 | 146 | 124 | 162 | 171 | 157 |
| Age (SD) a | 69.2 (2.7) | 70.4 (3.1) | 69.0 (3.1) | 69.9 (2.7) | 68.8 (2.4) | 68.6 (2.4) | 69.0 (2.7) | 69.5 (2.4) | 67.8 (3.4) | 69.5 (2.7) | 68.8 (2.5) | 68.9 (2.3) |
| BMI (SD) a | 27.79 (4.41) | 28.45 (4.33) | 28.95 (4.94) | 29.00 (4.27) | 26.40 (3.77) | 27.04 (4.35) | 27.98 (5.22) | 29.86 (5.63) | 28.67 (5.14) | 28.97 (5.12) | 25.93 (4.31) | 26.89 (4.91) |
| ALM/HT2 (kg/m2) b | ||||||||||||
| Mean (SD) | 8.68 (1.11) | 9.16 (1.21) | 9.27 (1.18) | 8.55 (0.96) | 8.29 (0.95) | 8.64 (0.95) | 6.83 (1.08) | 7.40 (1.10) | 7.23 (1.12) | 6.60 (0.92) | 6.37 (0.96) | 6.75 (0.95) |
| Pearson correlations | ||||||||||||
| r | 0.113 | 0.048 | 0.337 | 0.050 | 0.124 | 0.044 | 0.105 | 0.067 | 0.190 | 0.180 | 0.127 | 0.173 |
| P value | 0.002 | 0.64 | 0.0002 | 0.51 | 0.08 | 0.55 | 0.004 | 0.42 | 0.03 | 0.02 | 0.10 | 0.03 |
| Partial correlations c | ||||||||||||
| r | −0.056 | −0.007 | −0.027 | −0.133 | 0.058 | −0.037 | −0.167 | −0.170 | −0.033 | −0.146 | −0.112 | −0.014 |
| P value | 0.12 | 0.94 | 0.78 | 0.08 | 0.41 | 0.62 | <0.0001 | 0.04 | 0.72 | 0.07 | 0.15 | 0.86 |
| SARC‐F (0–1 no sarcopenia, 2–3 pre‐sarcopenia, ≥4 sarcopenia) d | ||||||||||||
| 0–1 | 689 (88.6%) | 74 (76.3%) | 106 (92.9%) | 143 (80.0%) | 194 (93.3%) | 172 (96.1%) | 570 (75.0%) | 105 (71.9%) | 103 (83.1%) | 101 (62.4%) | 127 (74.3%) | 134 (85.4%) |
| 2–3 | 72 (9.3%) | 17 (17.5%) | 8 (7.0%) | 28 (15.6%) | 13 (6.3%) | 6 (3.4%) | 145 (19.1%) | 34 (23.3%) | 18 (14.5%) | 36 (22.2%) | 35 (20.5%) | 22 (14.0%) |
| ≥4 | 17 (2.2%) | 6 (6.2%) | 1 (0.9%) | 8 (4.5%) | 1 (0.5%) | 1 (0.6%) | 45 (5.9%) | 7 (4.8%) | 3 (2.4%) | 25 (15.4%) | 9 (5.3%) | 1 (0.6%) |
Mean (standard deviation) of age and body mass index at time of DXA measurements.
Mean (standard deviation) appendicular lean mass/height2 (ALM/HT2) adjusted for age at DXA measurement in race/ethnic specific analyses and also adjusted for race/ethnicity in analyses for all subjects combined. We applied IWCS definition of sarcopenia as defined by low ALM/HT2 (≤7.23 kg/m2 for men and ≤5.67 kg/m2 for women); 61 of 778 (7.8%) men and 102 of 760 women (13.4%) would be classified to have low ALM/HT2.
Also adjusted for BMI at DXA measurement.
Five questions to compute SARC‐F were asked at the MEC fifth follow‐up questionnaire (Q×5).
SARC‐F scores as an indicator of pre‐sarcopenia and sarcopenia in the Multiethnic Cohort
Indicators of sarcopenia (SARC‐F ≥ 4) and pre‐sarcopenia (SARC‐F 2–3) among 71 283 MEC participants by sex–race/ethnicity are presented in Table 2. Crude and age‐standardized percentages of SARC‐F scores were similar and we reported the latter. SARC‐F ≥ 4 was higher in women (20.9%) than in men (11.2%) and varied by ~2‐fold across race/ethnicity. In men, SARC‐F ≥ 4 was highest in Latinos (16.1%) and African Americans (15.4%), intermediate in Native Hawaiians (10.5%) and other Asian Americans (10.9%), and lowest in Japanese Americans (8.9%) and Whites (8.8%). In women, SARC‐F ≥ 4 was also highest in Latinos (30.8%) and African Americans (27.2%), intermediate in Whites (17.4%) and other Asian Americans (16.9%), and lowest in Japanese Americans (16.3%) and Native Hawaiians (15.6%). The distribution of SARC‐F in each sex–race/ethnic group (except for Japanese Americans men) differed significantly from that of White men or White women. SARC‐F ≥ 4 rose from 3.8% in the youngest (age < 70) to 31.4% in the oldest (age 90+) men and from 7.6% to 46.8% in women. The above patterns in men and women were largely observed in each of the 5 year age groups (<70, 70–74, 75–79, 80–84, and 85+) by race/ethnicity (Figure 1). Deficits of IADL and ADL, average number of chronic conditions, and history of diabetes tended to be highest among those with SARC‐F ≥ 4, intermediate among those with SARC‐F 2–3, and lowest among participants with SARC‐F 0–1 (Table 2).
Table 2.
Prevalence (%) of sarcopenia (SARC‐F ≥ 4), pre‐sarcopenia (SARC‐F 2–3), and no sarcopenia (SARC‐F 0–1) and characteristics by SARC‐F in 71 283 men and women, by race/ethnicity, Multiethnic Cohort (2012–2016)
| SARC‐F | African American men | Native Hawaiian men | Latino men | Japanese American men | White men | Other Asian Am men | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–1 | 2–3 | 4+ | 0–1 | 2–3 | 4+ | 0–1 | 2–3 | 4+ | 0–1 | 2–3 | 4+ | 0–1 | 2–3 | 4+ | 0–1 | 2–3 | 4+ | |
| N | 1570 | 549 | 367 | 1408 | 386 | 199 | 3829 | 1446 | 967 | 7070 | 1509 | 762 | 6197 | 1312 | 668 | 839 | 315 | 133 |
| Crude % | 63.2 | 22.1 | 14.8 | 70.6 | 19.4 | 10.0 | 61.3 | 23.2 | 15.5 | 75.7 | 16.2 | 8.1 | 75.8 | 16.0 | 8.2 | 65.2 | 24.5 | 10.3 |
| Age adj % (std err of %) | 62.4 (1.0) | 22.3 (0.8) | 15.4 (0.7) | 69.7 (1.0) | 19.8 (0.9) | 10.5 (0.7) | 60.3 (0.6) | 23.5 (0.5) | 16.1 (0.5) | 74.3 (0.5) | 16.8 (0.4) | 8.9 (0.3) | 74.5 (0.5) | 16.7 (0.4) | 8.8 (0.3) | 64.1 (1.4) | 24.9 (1.2) | 10.9 (0.9) |
| Mean IADL a | 0.3 | 1.1 | 3.0 | 0.3 | 1.1 | 2.7 | 0.4 | 1.1 | 2.8 | 0.4 | 1.2 | 3.0 | 0.2 | 0.8 | 2.6 | 0.4 | 1.2 | 3.3 |
| % IADL ≥ 2 | 23.7 | 26.9 | 49.4 | 29.1 | 32.2 | 38.7 | 22.1 | 30.4 | 47.4 | 35.7 | 30.6 | 33.7 | 28.8 | 26.8 | 44.4 | 25.1 | 37.1 | 37.8 |
| Mean ADL a | 0.3 | 1.6 | 3.6 | 0.4 | 1.7 | 3.8 | 0.3 | 1.4 | 3.5 | 0.3 | 1.5 | 3.5 | 0.3 | 1.5 | 3.5 | 0.3 | 1.7 | 3.7 |
| %ADL ≥ 2 | 15.5 | 36.3 | 48.3 | 23.8 | 40 | 36.3 | 13.5 | 36.6 | 49.9 | 24.8 | 38.1 | 37.1 | 26.5 | 35.4 | 38.2 | 14.2 | 48.6 | 37.2 |
| Conditions b | 2.8 | 3.6 | 4.4 | 2.6 | 3.7 | 4.4 | 2.7 | 3.5 | 4.4 | 2.8 | 3.8 | 4.5 | 2.8 | 4.0 | 4.8 | 2.6 | 3.8 | 4.9 |
| % Diabetes b | 5.2 | 8.2 | 13.9 | 6.0 | 12.7 | 20.6 | 6.4 | 11.1 | 16.9 | 5.8 | 10.5 | 15.0 | 1.7 | 4.2 | 7.0 | 8.1 | 11.7 | 18.8 |
| % Cancer c | 10.6 | 14.9 | 13.4 | 9.9 | 13.5 | 11.1 | 7.6 | 9.1 | 11.9 | 8.1 | 11.5 | 16.0 | 13.2 | 18.1 | 19.3 | 5.6 | 9.5 | 6.0 |
| BMI (kg/m2) b | 26.5 | 27.7 | 27.0 | 28.1 | 29.8 | 29.7 | 26.8 | 27.5 | 27.2 | 25.1 | 25.4 | 24.1 | 26.2 | 27.0 | 26.6 | 25.5 | 26.1 | 26.2 |
| Cigarette/day d | 8.3 | 9.6 | 10.1 | 10.1 | 11.3 | 11.4 | 6.9 | 7.6 | 7.7 | 11.0 | 12.7 | 12.7 | 10.3 | 12.2 | 12.9 | 8.5 | 9.9 | 9.6 |
| Medit diet d | 4.4 | 4.1 | 4.1 | 4.0 | 3.9 | 4.0 | 3.9 | 3.9 | 3.8 | 4.2 | 4.3 | 4.4 | 4.5 | 4.2 | 4.3 | 4.1 | 3.9 | 4.0 |
| Activity (h/day) d | 1.3 | 1.3 | 1.0 | 1.9 | 1.8 | 1.5 | 1.4 | 1.4 | 1.1 | 1.4 | 1.4 | 1.4 | 1.7 | 1.6 | 1.4 | 1.4 | 1.3 | 1.2 |
| SARC‐F | African American women | Native Hawaiian women | Latino women | Japanese American women | White women | Other Asian Am women | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–1 | 2–3 | 4+ | 0–1 | 2–3 | 4+ | 0–1 | 2–3 | 4+ | 0–1 | 2–3 | 4+ | 0–1 | 2–3 | 4+ | 0–1 | 2–3 | 4+ | |
| N | 2478 | 1750 | 1504 | 1714 | 878 | 449 | 3220 | 2474 | 2417 | 6825 | 3194 | 1770 | 6229 | 2630 | 1716 | 1419 | 690 | 400 |
| Crude % | 43.2 | 30.5 | 26.2 | 56.4 | 28.9 | 14.8 | 39.7 | 30.5 | 29.8 | 57.9 | 27.1 | 15.0 | 58.9 | 24.9 | 16.2 | 56.6 | 27.5 | 15.9 |
| Age adj% (std err of %) | 42.0 (0.7) | 30.8 (0.6) | 27.2 (0.6) | 55.3 (0.9) | 29.1 (0.8) | 15.6 (0.7) | 38.7 (0.5) | 30.5 (0.5) | 30.8 (0.5) | 56.2 (0.5) | 27.5 (0.4) | 16.3 (0.4) | 57.3 (0.5) | 25.4 (0.4) | 17.4 (0.4) | 55.0 (1.0) | 28.1 (0.9) | 16.9 (0.8) |
| Mean IADL a | 0.4 | 1.0 | 2.4 | 0.3 | 0.7 | 2.3 | 0.4 | 0.9 | 2.4 | 0.3 | 0.9 | 2.7 | 0.2 | 0.7 | 2.1 | 0.4 | 0.9 | 2.3 |
| % IADL ≥ 2 | 9.6 | 28.1 | 62.3 | 15.0 | 26.3 | 58.8 | 8.5 | 24.4 | 67.1 | 15.4 | 30.1 | 54.4 | 14.5 | 24.6 | 60.9 | 17.2 | 30.3 | 52.5 |
| Mean ADL a | 0.2 | 1.3 | 3.1 | 0.2 | 1.2 | 3.0 | 0.2 | 1.0 | 2.8 | 0.2 | 1.1 | 2.9 | 0.2 | 1.1 | 2.8 | 0.2 | 1.1 | 3.0 |
| % ADL ≥ 2 | 4.4 | 31.5 | 64.1 | 7.1 | 40.1 | 52.9 | 3.2 | 24.6 | 72.2 | 6.1 | 35.1 | 58.8 | 7.8 | 30.9 | 61.3 | 5.7 | 36.7 | 57.6 |
| Conditions b | 2.4 | 3.2 | 4.0 | 2.2 | 3.2 | 4.3 | 2.4 | 3.2 | 4.1 | 2.4 | 3.2 | 3.9 | 2.3 | 3.3 | 4.2 | 2.3 | 3.1 | 4.2 |
| % Diabetes b | 4.5 | 7.7 | 12.2 | 4.7 | 9.2 | 14.9 | 4.9 | 8.4 | 12.7 | 4.4 | 6.9 | 10.0 | 1.3 | 2.9 | 6.2 | 4.7 | 5.1 | 14.5 |
| % Cancer c | 9.4 | 12.6 | 13.1 | 10.6 | 13.7 | 16.3 | 9.2 | 11 | 12.9 | 11.3 | 11.8 | 15.1 | 12.3 | 15.2 | 16.0 | 8.9 | 12.5 | 13.3 |
| BMI (kg/m2) b | 27.3 | 28.7 | 29.0 | 27.1 | 29.3 | 29.6 | 26.2 | 27.5 | 27.9 | 23.1 | 23.9 | 23.0 | 24.8 | 26.5 | 26.9 | 23.7 | 24.7 | 25.4 |
| Cigarettes/day d | 5.1 | 5.4 | 5.8 | 6.0 | 7.4 | 7.9 | 2.6 | 3.2 | 2.8 | 3.7 | 4.0 | 3.5 | 7.2 | 8.1 | 7.8 | 2.8 | 3.3 | 2.8 |
| Medit diet d | 4.4 | 4.1 | 4.1 | 3.9 | 3.8 | 3.8 | 3.8 | 3.6 | 3.6 | 4.2 | 4.3 | 4.4 | 4.4 | 4.1 | 4.0 | 4.1 | 4.0 | 4.1 |
| Activity d (h/day) | 1.0 | 0.9 | 0.9 | 1.5 | 1.3 | 1.3 | 1.2 | 1.0 | 0.9 | 1.1 | 1.1 | 1.1 | 1.5 | 1.4 | 1.3 | 1.1 | 1.1 | 1.0 |
At the fifth follow‐up questionnaire (Q×5), six questions on activity of daily living (ADL) and six questions on instrumental activity daily living (IADL) were asked. Responses to each question were converted to a dichotomous physical function variable (score 0 for no difficulty, score 1 for some difficulty, cannot do, or don't do). The total score for ADL and IADL was 6 points each. Mean score of ADL and IADL and the per cent of subjects with scores of 2 or greater for ADL and IADL are shown.
Mean number of chronic conditions at Q×5. The specific chronic conditions asked were high blood pressure, congestive heart failure, angina, heart attack, stroke, diabetes, skin cancer (non‐melanoma), Alzheimer's disease, other dementia, polyps of intestines, Crohn's disease, ulcerative colitis, osteoporosis, gallbladder removal, ulcer, chronic heartburn, cataract surgery, glaucoma, asthma, chronic lung disease, Parkinson's disease, and enlarged prostate (men only); mean body mass index at Q×5.
Based on incident cancer diagnosis between entry into MEC cohort and Q×5 that were identified in Hawaii and California Surveillance, Epidemiology, and End Results Registry.
Responses from baseline questionnaires: mean number of cigarettes smoked per day (cigarette = 0 for never smoker); Medit = mean Mediterranean diet score calculated from baseline food frequency questionnaires (scores from 0 to 9); mean hours of moderate/vigorous activity from baseline questionnaire.
Figure 1.
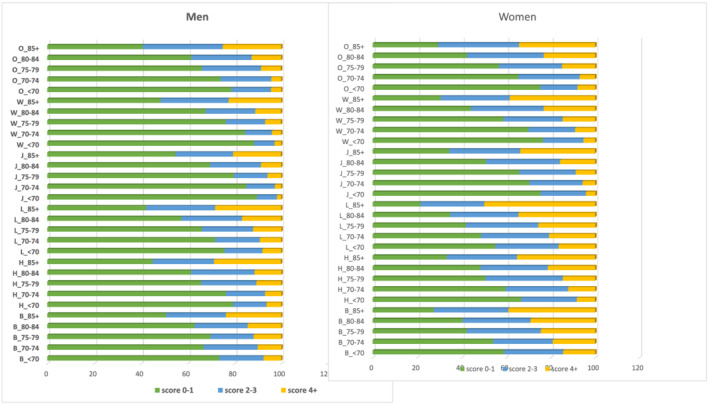
Distribution of SARC‐F in men and women, by race/ethnicity and age groups. (A) Distribution of no sarcopenia (SARC‐F score 0–1), pre‐sarcopenia (SARC‐F score 2–3), and sarcopenia (SARC‐F ≥ 4) separately in men, by race/ethnicity (B = African American, H = Native Hawaiian; L = Latino; J = Japanese American; W = Whites, O = other Asian Americans), and age categories (<70, 70–74, 75–59, 80–84, 85+). (B) Distribution of no sarcopenia (SARC‐F score 0–1), pre‐sarcopenia (SARC‐F score 2–3), and sarcopenia (SARC‐F ≥ 4) separately in women, by race/ethnicity (B = African American, H = Native Hawaiian; L = Latino; J = Japanese American; W = Whites, O = other Asian Americans), and age categories (<70, 70–74, 75–59, 80–84, 85+).
We also examined responses to each specific SARC‐F question to assess which components were more frequently identified as very difficult and cannot do (score 2) (Table 3). Among men, 5.8% responded it was very difficult (cannot do, score 2) to climb stairs and 4.7% could not lift (strength); these estimates were about doubled in women (10.8% climb stairs, 13.5% lifting). Women were more likely than men to have falls, required medical treatment (score 2) (9.1% vs. 5.3%) or no treatment (score 1) (10.3% vs. 8.4%). In contrast, men and women were similar in terms of having great difficulty rising from a chair (1.2% in men, 1.7% in women) or walking across a room (1.0% men, 1.5% men). A higher proportion of women than men reported they had some difficulty (score 1) with rising from a chair (43.1% vs. 35.1%), climbing stairs (27.1% vs. 18.2%), and lifting (30.2% vs. 16.4%).
Table 3.
Responses to each of the five questions of SARC‐F, in men and women, by race/ethnic group, Multiethnic Cohort (2012–2016)
| By race/ethnicity, men | By race/ethnicity, women | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All a | African American | Native Hawaiian | Latino | Japanese American | White | Other Asian Am | All a | African American | Native Hawaiian | Latino | Japanese American | White | Other Asian Am | |
| Strength b | ||||||||||||||
| Some difficulty | 16.4% | 19.5% | 15.3% | 18.7% | 15.4% | 14.3% | 21.5% | 30.2% | 32.3% | 28.1% | 30.4% | 31.3% | 27.6% | 32.3% |
| Cannot do | 4.7% | 7.2% | 4.2% | 7.4% | 3.5% | 3.1% | 5.7% | 13.5% | 18.7% | 8.0% | 21.4% | 10..5% | 10.0% | 11.2% |
| Walking b | ||||||||||||||
| Some difficulty | 5.8% | 9.9% | 5.8% | 7.3% | 4.6% | 4.5% | 5.9% | 7.8% | 13.3% | 6.6% | 9.5% | 5.9% | 6.2% | 6.3% |
| Cannot do | 1.0% | 1.8% | 1.0% | 1.3% | 0.7% | 1.0% | 1.2% | 1.5% | 1.7% | 1.0% | 2.1% | 1.0% | 1.4% | 1.4% |
| Rise from chair b | ||||||||||||||
| Some difficulty | 35.1% | 40.0% | 39.1% | 37.8% | 32.8% | 32.8% | 36.1% | 43.1% | 50.6% | 43.0% | 45.9% | 40.4% | 40.6% | 39.6% |
| Cannot do | 1.2% | 1.6% | 1.2% | 2.0% | 0.9% | 0.6% | 1.8% | 1.7% | 2.1% | 1.5% | 3.2% | 1.0% | 1.2% | 1.5% |
| Climb stairs b | ||||||||||||||
| Some difficulty | 18.2% | 22.8% | 21.1% | 23.8% | 14.9% | 14.3% | 26.2% | 27.1% | 32.2% | 30.6% | 32.0% | 24.5% | 22.5% | 26.5% |
| Cannot do | 5.8% | 8.6% | 5.2% | 9.9% | 3.7% | 4.5% | 5.5% | 10.8% | 15.1% | 7.9% | 19.0% | 6.3% | 8.9% | 7.2% |
| Falls b | ||||||||||||||
| Yes, no treatment | 8.4% | 7.8% | 6.4% | 12.1% | 7.9% | 7.1% | 5.7% | 10.3% | 10.0% | 8.5% | 15.4% | 9.3% | 8.7% | 8.5% |
| Yes, treated | 5.3% | 5.3% | 4.6% | 7.3% | 4.6% | 4.8% | 5.0% | 9.1% | 7.3% | 6.0% | 13.4% | 8.5% | 8.9% | 7.5% |
Forty‐four men (6 African Americans, 2 Native Hawaiians, 16 Latinos, 11 Japanese American, 7 Whites, and 2 other Asian Americans) and 76 women (22 African Americans, 1 Native Hawaiian, 22 Latinos, 13 Japanese Americans, 14 Whites, and 4 other Asian Americans) were excluded because of missing in all five questions.
Score 0 was not shown but it can be calculated as follows, for example, strength in men, score 0 (no difficulty) = 100% − 16.4% (score 1, some difficulty) − 4.7% (score 2, cannot do) − 0.6% (missing) = 78.3%. The %missing ranged from 0.5% to 1.5% for the five questions in men and from 0.8% to 2.5% for the five questions in women.
Association between SARC‐F and all‐cause and cause‐specific mortality
SARC‐F was statistically significantly associated with risk of all‐cause mortality in men and women (Table 4). Compared with men with SARC‐F 0–1, adjusted HR for overall mortality was 1.77 (95% CI 1.63–1.92) for SARC‐F 2–3, and 3.73 (95% CI 3.40–4.09) for SARC‐F ≥ 4. The respective covariate adjusted HRs in women were 1.47 (95% CI 1.35–1.59) and 3.10 (95% CI 2.86–3.36). For each 1 unit increase in SARC‐F score from 0 to 10, HR for overall mortality in men was 1.32 (95% CI 1.30–1.34); HRs ranged from 1.25 (95% CI 1.21–1.29) in Latino men to 1.45 (95% CI 1.38–1.48) in White men. The corresponding HR in women was 1.27 (95% CI 1.25–1.29) and the HRs ranged from 1.20 (95% CI 1.15–1.24) in African American women to 1.35 (95% CI 1.31–1.39) in Japanese American women. We also explored the associations by follow‐up period and found that SARC‐F scores ≥ 4 were associated with risk for both shorter (<6 years) and longer (≥6 years) periods of follow‐up; the respective HRs were 2.33 (95% CI 1.45–3.74) and 1.82 (95% CI 1.35–2.44) in men and 1.60 (95% CI 1.17–2.18) and 1.71 (95% 1.34–2.18) in women. The associations between SARC‐F and all‐cause mortality were observed across Q×5 BMI (<25, 25–30, >30 kg/m2) and history of chronic conditions (0–1, 2–3, 4–5, 6+) as well as baseline Mediterranean diet score (0–2, 3, 4, 5, 6–9) and smoking status (never, former, current) in both men and women (Table 5).
Table 4.
Association between SARC‐F scores and risk of all‐cause mortality by sex and race/ethnicity, Multiethnic Cohort (2012–2019)
| By race–ethnicity | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All men | African American | Native Hawaiian | Latino | Japanese American | White | Other Asian American | ||||||||
| SARC‐F | #case (C)/noncase (NC) | HR (95% CI) a | #C/NC | HR (95% CI) a | #C/NC | HR (95% CI) a | #C/NC | HR (95% CI) a | #C/NC | HR (95% CI) a | #C/NC | HR (95% CI) a | #C/NC | HR (95% CI) a |
| 0–1 | 2476/18 437 | 1.00 | 302/1268 | 1.00 | 181/1227 | 1.00 | 455/3374 | 1.00 | 771/6299 | 1.00 | 679/5518 | 1.00 | 88/751 | 1.00 |
| 2–3 | 1444/4073 | 1.77 (1.63–1.92) | 166/383 | 1.56 (1.23–1.99) | 106/280 | 1.66 (1.23–2.25) | 316/1130 | 1.59 (1.34–1.88) | 400/1109 | 1.79 (1.54–2.08) | 377/935 | 1.97 (1.68–2.31) | 79/236 | 2.26 (1.57–3.25) |
| 4+ | 1473/1623 | 3.73 (3.40–4.09) | 184/182 | 3.33 (2.54–4.35) | 87/112 | 2.87 (1.99–4.13) | 363/604 | 2.94 (2.46–3.52) | 410/352 | 4.30 (3.60–5.14) | 371/297 | 5.12 (4.22–6.22) | 57/76 | 3.65 (2.31–5.74) |
| P trend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| P (per 1 unit SARC‐F) a | 1.32 (1.30–1.34) | 1.27 (1.21–1.31) | 1.29 (1.21–1.39) | 1.25 (1.21–1.29) | 1.35 (1.31–1.40) | 1.45 (1.38–1.48) | 1.33 (1.23–1.45) | |||||||
| All women | African American | Native Hawaiian | Latino | Japanese American | White | Other Asian American | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–1 | 1476/20 409 | 1.00 | 252/2226 | 1.00 | 114/1600 | 1.00 | 225/2995 | 1.00 | 384/6441 | 1.00 | 418/5811 | 1.00 | 83/1336 | 1.00 |
| 2–3 | 1579/10 034 | 1.47 (1.35–1.59) | 275/1475 | 1.14 (0.94–1.39) | 129/749 | 1.84 (1.38–2.45) | 293/2181 | 1.41 (1.17–1.71) | 401/2793 | 1.57 (1.34–1.83) | 426/2204 | 1.65 (1.41–1.93) | 57/633 | 0.93 (0.64–1.36) |
| 4+ | 2546/5706 | 3.10 (2.86–3.36) | 460/1044 | 2.20 (1.82–2.67) | 152/287 | 3.90 (2.87–5.30) | 579/1838 | 2.51 (2.09–3.00) | 644/1126 | 4.17 (3.56–4.89) | 612/1104 | 3.41 (2.90–4.01) | 101/299 | 2.89 (2.01–4.16) |
| P trend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| P (per 1 unit SARC‐F) b | 1.27 (1.25–1.29) | 1.20 (1.15–1.24) | 1.33 (1.26–1.41) | 1.21 (1.17–1.24) | 1.35 (1.31–1.39) | 1.29 (1.25–1.33) | 1.26 (1.18–1.35) | |||||||
Adjusted for age at Q×5, and race/ethnicity, neighbourhood socio‐economic status, education, baseline variables including BMI (<25, 25–29.9, ≥30 kg/m2, missing), smoking (non‐smoker, former smoker by pack years <20, 20+, don't know; current smokers by pack years <20, 20+, don't know), alcohol consumption (0 ethanol, 1 < 12, 12–<24, ≥24 g/day), physical activity (hours spent in moderate/vigorous activity), Mediterranean diet score (0–9) and history of chronic conditions, as well as BMI and chronic conditions and previous cancer at Q×5. In race/ethnic‐specific analyses, race/ethnicity was not included in the model.
As above and also parity, age at menarche, menopausal status, and menopausal hormone therapy.
Table 5.
Association between self‐reported sarcopenia (SARC‐F score) and sex‐specific risk of all‐cause mortality, stratified by various baseline and Q×5 variables, Multiethnic Cohort (2012–2019)
| HR (95% CI) in men a | |||||
|---|---|---|---|---|---|
| Q×5 BMI | <25 kg/m2 | 25–30 kg/m2 | >30 kg/m2 | Don't know BMI | |
| SARC‐F score 2–3 vs. score 0–1 | 1.92 (1.71–2.16) | 1.67 (1.46–1.90) | 1.82 (1.48–2.25) | 1.53 (0.44–5.27) | |
| SARC‐F score 4+ vs. score 0–1 | 4.45 (3.87–5.11) | 3.05 (2.61–3.58) | 3.58 (2.84–4.51) | 7.28 (2.03–26.08) | |
| Q×5 chronic conditions | 0–1 | 2–3 | 4–5 | 6+ | |
| SARC‐F score 2–3 vs. score 0–1 | 1.76 (1.41–2.19) | 1.57 (1.37–1.80) | 1.84 (1.60—2.13) | 1.80 (1.48–2.20) | |
| SARC‐F score 4+ vs. score 0–1 | 2.85 (2.09–3.88) | 3.44 (2.89–4.09) | 3.89 (3.30–4.59) | 3.83 (3.13–4.70) | |
| Baseline Mediterranean score | 0–2 | 3 | 4 | 5 | 6–9 |
| SARC‐F score 2–3 vs. score 0–1 | 1.68 (1.37–2.06) | 1.53 (1.26–1.87) | 1.84 (1.52–2.22) | 1.87 (1.56–2.25) | 1.77 (1.50–2.10) |
| SARC‐F score 4+ vs. score 0–1 | 3.49 (2.74–4.43) | 3.35 (2.66–4.22) | 4.06 (3.29–5.00) | 3.80 (3.05–4.74) | 3.29 (2.68–4.04) |
| Baseline smoking status | Never smoker | Former smoker | Current smoker | ||
| SARC‐F score 2–3 vs. score 0–1 | 1.83 (1.58–2.12) | 1.71 (1.53–1.91) | 1.78 (1.45–2.17) | ||
| SARC‐F score 4+ vs. score 0–1 | 3.75 (3.16–4.44) | 3.76 (3.31–4.27) | 3.60 (2.85–4.54) | ||
| HR (95% CI) in women b | |||||
|---|---|---|---|---|---|
| Q×5 BMI | <25 kg/m2 | 25–30 kg/m2 | >30 kg/m2 | DK BMI | |
| SARC‐F score 2–3 vs. score 0–1 | 1.54 (1.38–1.71) | 1.45 (1.23–1.71) | 1.30 (1.04–1.61) | 2.47 (1.30–4.69) | |
| SARC‐F score 4+ vs. score 0–1 | 3.51 (3.15–3.90) | 2.88 (2.44–3.39) | 2.67 (2.17–3.30) | 3.48 (1.87–6.47) | |
| Q×5 chronic conditions | 0–1 | 2–3 | 4–5 | 6+ | |
| SARC‐F score 2–3 vs. score 0–1 | 1.38 (1.14–1.66) | 1.42 (1.25–1.61) | 1.57 (1.34–1.84) | 1.26 (0.97–1.64) | |
| SARC‐F score 4+ vs. score 0–1 | 2.12 (1.70–2.65) | 3.19 (2.79–3.65) | 2.86 (2.46–3.34) | 3.19 (2.51–4.04) | |
| Baseline Mediterranean score | 0–2 | 3 | 4 | 5 | 6–9 |
| SARC‐F score 2–3 vs. score 0–1 | 1.59 (1.28–1.96) | 1.80 (1.48–2.18) | 1.35 (1.12–1.62) | 1.23 (1.03–1.47) | 1.51 (1.27–1.79) |
| SARC‐F score 4+ vs. score 0–1 | 3.15 (2.54–3.90) | 2.77 (2.26–3.39) | 3.45 (2.87–4.14) | 2.76 (2.29–3.33) | 3.17 (2.65–3.78) |
| Baseline smoking status | Never smoker | Former smoker | Current smoker | ||
| SARC‐F score 2–3 vs. score 0–1 | 1.44 (1.28–1.60) | 1.47 (1.26–1.70) | 1.57 (1.27–1.94) | ||
| SARC‐F score 4+ vs. score 0–1 | 3.11 (2.79–3.46) | 3.24 (2.78–3.77) | 3.04 (2.44–3.79) | ||
Adjusted for age at Q×5, and race/ethnicity, neighbourhood socio‐economic status, education, baseline variables including BMI (<25, 25–29.9, ≥30 kg/m2, missing), smoking (non‐smoker, former smoker by pack years <20, 20+, don't know; current smokers by pack years <20, 20+, don't know), alcohol consumption (0 ethanol, 1 < 12, 12–<24, ≥24 g/day), physical activity (hours spent in moderate/vigorous activity), Mediterranean diet score (0–9), history of chronic conditions, as well as BMI and chronic conditions and previous cancer at Q×5. In the stratified analyses of Q×5 BMI and Q×5 chronic conditions, the respective variables were not included in the model. In the stratified analysis of baseline Mediterranean diet score and baseline smoking status, the respective variables were not included in the model.
As above and also parity, age at menarche, menopausal status, and menopausal hormone therapy.
Cardiovascular disease and cancer accounted for ~60% of all deaths in men (1897 of 5391, 35.2% heart disease; 1437 of 5391, 26.7% cancer) and women (2018 of 5601, 36.0% heart disease; 1411 of 5601, 25.2% cancer) (Table 6). CVD mortality was associated with SARC‐F score. Compared with men with SARC‐F 0–1, the respective covariate adjusted HRs for CVD were 1.85 (95% CI 1.64–2.09) for SARC‐F 2–3 and 3.98 (95% CI 3.49–4.55) for SARC‐F ≥ 4; the corresponding HRs in women were 1.59 (95% CI 1.39–1.81) and 3.54 (95% CI 3.12–4.02). In men, the HRs for CVD per 1 unit increase in SARC‐F score were highest in Whites and Japanese Americans and lowest in Latinos (all P values < 0.0001) while in women, the HRs were highest in Japanese Americans and Native Hawaiians and lowest in African Americans (all P values < 0.0001). Although the SARC‐F score was also associated with cancer‐specific mortality, the covariate adjusted HRs were considerably lower. Compared with SARC‐F 0–1, the respective HRs for cancer‐specific mortality among those with SARC‐F 2–3 and SARC‐F ≥ 4 were 1.46 (95% 1.28–1.67) and 1.96 (95% CI 1.66–2.31) in men and 1.30 (95% CI 1.14–1.49) and 1.77 (95% CI 1.53–2.05) in women.
Table 6.
Association between SARC‐F scores and risk of cardiovascular (CVD) and cancer (CA)‐specific mortality by sex and race/ethnicity, Multiethnic Cohort (2012–2019)
| All | By race–ethnicity | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| African American | Native Hawaiian | Latino | Japanese American | White | Other Asian American | |||||||||
| CVD‐specific mortality | ||||||||||||||
| SARC‐F | #CVD | HR (95% CI) | #CVD | HR (95% CI) | #CVD | HR (95% CI) | #CVD | HR (95% CI) | #CVD | HR (95% CI) | #CVD | HR (95% CI) a | #CVD | HR (95% CI) a |
| All men | ||||||||||||||
| 0–1 | 798 | 1.00 | 97 | 1.00 | 66 | 1.00 | 159 | 1.00 | 232 | 1.00 | 210 | 1.00 | 34 | 1.00 |
| 2–3 | 533 | 1.85 (1.64–2.09) | 77 | 2.29 (1.63–3.23) | 39 | 1.68 (1.06–2.66) | 106 | 1.48 (1.13–1.93) | 137 | 1.91 (1.51–2.42) | 137 | 1.90 (1.48–2.44) | 37 | 2.91 (1.71–4.94) |
| 4+ | 566 | 3.98 (3.49–4.55) | 80 | 4.16 (2.88–6.02) | 42 | 3.38 (2.06–5.55) | 136 | 3.06 (2.35–4.00) | 146 | 4.64 (3.59–6.01) | 139 | 4.73 (3.58–6.26) | 23 | 3.51 (1.82–6.67) |
| P trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
| P (per 1 SARC‐F) a | 1.33 (1.30–1.36) | 1.32 (1.23–1.42) | 1.35 (1.22–1.48) | 1.25 (1.19–1.32) | 1.36 (1.29–1.43) | 1.41 (1.34–1.49) | 1.32 (1.17–1.47) | |||||||
| All women | ||||||||||||||
| 0–1 | 449 | 1.00 | 91 | 1.00 | 32 | 1.00 | 64 | 1.00 | 121 | 1.00 | 118 | 1.00 | 23 | 1.00 |
| 2–3 | 567 | 1.59 (1.39–1.81) | 105 | 1.12 (0.83–1.53) | 49 | 2.22 (1.37–3.60) | 87 | 1.40 (1.00–1.97) | 140 | 1.59 (1.23–2.06) | 160 | 1.94 (1.50–2.52) | 26 | 1.39 (0.75–2.59) |
| 4+ | 1002 | 3.54 (3.12–4.02) | 186 | 2.33 (1.75–3.12) | 60 | 5.13 (3.15–8.38) | 228 | 3.13 (2.30–4.24) | 257 | 4.43 (3.45–5.67) | 236 | 3.89 (3.00–5.05) | 35 | 1.29 (1.15–1.44) |
| P trend | <0.001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
| P (per 1 SARC‐F) b | 1.30 (1.27–1.33) | 1.21 (1.15–1.28) | 1.37 (1.26–1.50) | 1.26 (1.20–1.32) | 1.38 (1.32–1.44) | 1.32 (1.26–1.38) | 1.29 (1.15–1.44) | |||||||
| Cancer (CA)‐specific mortality | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #CA | HR (95% CI) | #CA | HR (95% CI) | #CA | HR (95% CI) | #CA | HR (95% CI) | #CA | HR (95% CI) b | #CA | HR (95% CI) | #CA | HR (95% CI) | |
| All men | ||||||||||||||
| 0–1 | 829 | 1.00 | 107 | 1.00 | 61 | 1.00 | 157 | 1.00 | 244 | 1.00 | 235 | 1.00 | 25 | 1.00 |
| 2–3 | 373 | 1.46 (1.28–1.67) | 46 | 1.14 (0.77–1.69) | 27 | 1.21 (0.72–2.03) | 91 | 1.41 (1.07–1.86) | 97 | 1.55 (1.20–2.00) | 95 | 1.57 (1.21–2.05) | 17 | 1.83 (0.93–3.61) |
| 4+ | 235 | 1.96 (1.66–2.31) | 30 | 1.40 (0.87–2.24) | 15 | 1.82 (0.94–3.50) | 67 | 1.78 (1.29–2.44) | 47 | 1.81 (1.28–2.57) | 70 | 3.15 (2.28–4.34) | 6 | 1.67 (0.62–4.53) |
| P trend | <0.001 | 0.36 | 0.20 | <0.001 | <0.001 | <0.001 | 0.19 | |||||||
| P (per 1 SARC‐F) a | 1.17 (1.14–1.21) | 1.09 (1.00–1.19) | 1.13 (1.00–1.28) | 1.13 (1.07–1.20) | 1.18 (1.11–1.25) | 1.30 (1.22–1.38) | 1.13 (0.95–1.33) | |||||||
| All women | ||||||||||||||
| 0–1 | 535 | 1.00 | 77 | 1.00 | 49 | 1.00 | 80 | 1.00 | 138 | 1.00 | 153 | 1.00 | 38 | 1.00 |
| 2–3 | 452 | 1.30 (1.14–1.49) | 69 | 1.05 (0.74–1.49) | 38 | 1.34 (0.85–2.12) | 113 | 1.62 (1.20–2.19) | 102 | 1.33 (1.02–1.75) | 113 | 1.32 (1.02–1.72) | 17 | 0.69 (0.38–1.28) |
| 4+ | 424 | 1.77 (1.53–2.05) | 71 | 1.34 (0.93–1.93) | 28 | 1.92 (1.13–3.28) | 104 | 1.48 (1.07–2.03) | 82 | 2.13 (1.56–2.89) | 112 | 2.09 (1.58–2.77) | 27 | 2.00 (1.12–1.56) |
| P trend | <0.001 | 0.24 | 0.05 | 0.006 | <0.001 | <0.001 | 0.004 | |||||||
| P (per 1 SARC‐F) b | 1.12 (1.09–1.15) | 1.07 (0.99–1.15) | 1.19 (1.08–1.32) | 1.06 (1.00–1.12) | 1.15 (1.09–1.23) | 1.17 (1.11–1.23) | 1.14 (1.02–1.28) | |||||||
Adjusted for age at Q×5, and race/ethnicity, neighbourhood socio‐economic status, education, and baseline variables including BMI (<25, 25–29.9, ≥30 kg/m2, missing), smoking (non‐smoker, former smoker by pack years <20, 20+, don't know; current smokers by pack years <20, 20+, don't know), alcohol consumption (0 ethanol, 1 < 12, 12–<24, ≥24 g/day), physical activity (hours spent in moderate/vigorous activity), Mediterranean diet score (0–9), and history of chronic conditions, as well as BMI, chronic conditions, and previous cancer at Q×5. In race/ethnic‐specific analyses, race/ethnicity was not included in the model.
As above and also parity, age at menarche, menopausal status, and menopausal hormone therapy.
Finally, we investigated the associations between each of the five components of SARC‐F and risk of all‐cause, CVD‐specific, and cancer‐specific mortality (Table 7). Risks of all‐cause mortality were significantly increased in relation to having difficulty or cannot do these five SARC‐F components; the respective HRs in men and women were strongest for walking (HR = 3.25 and 2.96), intermediate for strength (HR = 2.53 and 2.07) and climbing stairs (HR = 2.52 and 2.15), and weaker for falls (HR = 1.92 and 1.66) and rise from chair (HR = 1.58 and 1.42). Interestingly, when we mutually adjusted for the other SARC‐F components, all the HRs remained statistically significant but the respective HR estimates for walking, strength, and climbing weakened considerably while the HR estimates for falls and rise from chair remained largely unchanged. The mutually adjusted HRs for the five SARC‐F components ranged from 1.55 (rise from chair) to 1.90 (falls) in men and 1.39 (rise from chair) to 1.93 (walking) in women. These risk patterns in association with the individual SARC‐F components were also found separately for CVD‐specific and cancer‐specific mortality (Table 7).
Table 7.
Association between each of the five components of SARC‐F score and all‐cause mortality, in men and women, Multiethnic Cohort (2012–2019)
| Strength (lifting) | Walking | Rise from chair | Climb stairs | Falls | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SARC‐F | #case (C)/noncase (NC) | HR (95% CI) | #C/NC | HR (95% CI) | #C/NC | HR (95% CI) | #C/NC | HR (95% CI) | #C/NC | HR (95% CI) a |
| All men—all‐cause mortality a | ||||||||||
| Score 0 | 2998/20 128 | 1.00 | 4341/23 023 | 1.00 | 2566/16 016 | 1.00 | 2775/19 453 | 1.00 | 3818/21 227 | 1.00 |
| Score 1 + 2 c | 2326/3889 | 2.53 (2.36–2.72) | 1002/1003 | 3.25 (2.93–3.61) | 2764/7935 | 1.58 (1.48–1.69) | 2555/4538 | 2.52 (2.35–2.70) | 1448/2580 | 1.92 (1.77–2.09) |
| Score 1 + 2 d | 1.73 (1.59–1.88) | 1.67 (1.48–1.89) | 1.55 (1.45–1.63) | 1.70 (1.57–1.86) | 1.90 (1.75–2.06) | |||||
| All men—CVD‐specific mortality a | ||||||||||
| Score 0 | 1016/20 128 | 1.00 | 1501/23 023 | 1.00 | 850/16 016 | 1.00 | 926/19 453 | 1.00 | 1312/21 227 | 1.00 |
| Score 1 + 2 c | 854/3889 | 2.57 (2.31–2.86) | 371/1003 | 3.24 (2.81–3.75) | 1021/7935 | 1.64 (1.48–1.82) | 945/4538 | 2.52 (2.27–2.81) | 542/2580 | 1.92 (1.71–2.16) |
| Score 1 + 2 d | 1.79 (1.58–2.03) | 1.92 (1.61–2.28) | 1.61 (1.45–1.78) | 1.71 (1.51–1.94) | 1.91 (1.70–2.15) | |||||
| All men—cancer‐specific mortality a | ||||||||||
| Score 0 | 943/20 128 | 1.00 | 1281/23 023 | 1.00 | 802/16 016 | 1.00 | 907/19 453 | 1.00 | 1128/21 227 | 1.00 |
| Score 1 + 2 c | 479/3889 | 1.76 (1.56–2.00) | 152/1003 | 1.69 (1.39–2.05) | 620/7935 | 1.21 (1.08–1.36) | 513/4538 | 1.60 (1.42–1.82) | 280/2580 | 1.35 (1.17–1.57) |
| Score 1 + 2 d | 1.66 (1.44–1.90) | 1.45 (1.18–1.79) | 1.20 (1.07–1.34) | 1.44 (1.24–1.66) | 1.35 (1.16–1.56) | |||||
| All women—all‐cause mortality b | ||||||||||
| Score 0 | 1613/21 493 | 1.00 | 4026/33 558 | 1.00 | 2161/20 346 | 1.00 | 1943/23 479 | 1.00 | 3496/29 111 | 1.00 |
| Score 1 + 2 c | 3868/14 345 | 2.07 (1.94–2.22) | 1488/2362 | 2.96 (2.73–3.21) | 3313/15 385 | 1.42 (1.33–1.51) | 3519/12 290 | 2.15 (2.01–2.30) | 1907/6204 | 1.66 (1.55–1.78) |
| Score 1 + 2 d | 1.56 (1.45–1.69) | 1.93 (1.76–2.12) | 1.39 (1.31–1.49) | 1.46 (1.35–1.58) | 1.64 (1.53–1.75) | |||||
| All women—CVD‐specific mortality b | ||||||||||
| Score 0 | 511/21 493 | 1.00 | 1422/33 558 | 1.00 | 719/20 346 | 1.00 | 626/23 479 | 1.00 | 1180/29 111 | 1.00 |
| Score 1 + 2 c | 1460/14 345 | 2.23 (2.00–2.49) | 562/2362 | 2.86 (2.54–3.21) | 1251/15 385 | 1.53 (1.38–1.69) | 1335/12 290 | 2.34 (2.10–2.60) | 763/6204 | 1.86 (1.68–2.06) |
| Score 1 + 2 d | 1.75 (1.55–1.97) | 1.87 (1.63–2.14) | 1.50 (1.32–1.66) | 1.53 (1.36–1.73) | 1.83 (1.65–2.03) | |||||
| All women—cancer‐specific mortality b | ||||||||||
| Score 0 | 566/21 493 | 1.00 | 1180/33 558 | 1.00 | 660/20 346 | 1.00 | 668/23 479 | 1.00 | 1022/29 111 | 1.00 |
| Score 1 + 2 c | 828/14 345 | 1.58 (1.40–1.77) | 212/2363 | 1.69 (1.44–1.99) | 724/15 385 | 1.13 (1.01–1.27) | 719/12 290 | 1.43 (1.27–1.60) | 350/6204 | 1.20 (1.05–1.37) |
| Score 1 + 2 d | 1.53 (1.35–1.72) | 1.53 (1.29–1.82) | 1.12 (1.00–1.26) | 1.22 (1.07–1.40) | 1.19 (1.04–1.35) | |||||
Adjusted for age at Q×5, race/ethnicity, neighbourhood socio‐economic status, education, and baseline variables including BMI (<25, 25–29.9, ≥30 kg/m2, missing), smoking (non‐smoker, former smoker by pack years <20, 20+, don't know; current smokers by pack years <20, 20+, don't know), alcohol consumption (0 ethanol, 1 < 12, 12–<24, ≥24 g/day), physical activity (hours spent in moderate/vigorous activity), Mediterranean diet score (0–9), and history of chronic conditions, as well as BMI, chronic conditions, and previous cancer at Q×5, and SARC‐F without the component under investigation.
As above and also parity, age at menarche, menopausal status, and menopausal hormone therapy.
Scores of 0, 1, and 2 were assigned, respectively, to responses of no difficulty, some difficulty, and cannot do for strength, walking, rise from chair, and climb stairs. Scores of 0, 1, and 2 were assigned to responses of no falls, falls requiring no treatment, and falls requiring treatment. The other SARC‐F components were not included in these models.
As in Footnote c, and mutually adjusted for the other SARC‐F components.
Discussion
The ability to rapidly screen for sarcopenia for risk stratification and to initiate targeted intervention is critical to slow its progression and lessen the consequences of sarcopenia. 3 This large investigation of SARC‐F in the MEC adds new information on the prevalence of indicators of sarcopenia (SARC‐F ≥ 4) and pre‐sarcopenia (SARC‐F 2–3) and its utility in three minority groups (Native Hawaiians, Japanese Americans, and other Asian Americans including mainly Filipino and Chinese from Hawaii) that have not been included in studies of SARC‐F, as well as in groups (African Americans, Latinos, and Whites) that have been studied, but in relatively small numbers. 12 Using an identical protocol to determine SARC‐F scores and the availability of extensive baseline and follow‐up covariate information, we found that SARC‐F score was significantly associated with all‐cause mortality, as well as CVD‐specific and cancer‐specific mortality in each of the 12 sex–race/ethnic groups in this cohort. Our results also suggest that SARC‐F score was monotonically associated with overall mortality, capturing health information that goes beyond being a surrogate for ALM/HT2. Self‐reported difficulties with performing each of the five SARC‐F components (climbing stairs, lifting, falls, rise from chair and walking across a room) were also associated with overall, CVD‐specific, and cancer‐specific mortality in men and women.
In a sub‐study of 1538 MEC subjects with both SARC‐F and DXA measured ALM/HT2, SARC‐F correlated significantly with ALM/HT2 in women, notably in African American and Latino women; the latter had the highest prevalence of SARC‐F score ≥ 4 (15.4%) in this subgroup of MEC women. The lack of a statistically significant association in men may be due to the low prevalences of SARC‐F ≥ 4 (2.2%) and SARC‐F 2–3 (9.3%) in this subgroup of MEC men. The low sensitivity and high specificity of SARC‐F in our sub‐study as a predictor of ALM/HT2 agrees with previous studies. 4 , 16 , 27 In a 2018 meta‐analysis, SARC‐F had low sensitivity (ranged from 7% to 27%) but high specificity (ranged from 80% to 97%). 27 As others have reported, 16 when we lowered the SARC‐F cut‐off points from ≥4 to ≥2, sensitivity increased while specificity decreased but the sensitivity still remained low. The recent SDOC finding of no association between DXA‐determined muscle mass and adverse outcomes 9 highlights the importance of identifying screening tools for sarcopenia that are not dependent on muscle mass measurements.
Our results based on SARC‐F scores showed a ~2‐fold higher occurrence of sarcopenia in women than in men, and a ~2‐fold difference in the occurrence across race/ethnicity, highest in Latinos and African Americans, intermediate in Native Hawaiians and other Asians, and lowest in Whites and Japanese Americans. It is of interest that the occurrence of sarcopenia tended to be higher in men than in women when sarcopenia was determined based on muscle mass definitions according to AWGS, IWGS, EWGSOP, and other entities, but the occurrence of sarcopenia based on SARC‐F (cut‐off point ≥ 4) was not higher in men than in women (see table 3 of review by Voelker et al. 16 ). Because the occurrence of clinically determined sarcopenia varies depending on the cut‐off points and methods used to measure sarcopenia, 28 we compared our results mainly to studies that used SARC‐F scores. Older White women (n = 141, average age of 83) in Pittsburgh showed SARC‐F score ≥ 4 of 21.3%, 29 similar to our findings (23.3%) among White women (ages 80–84) in the MEC. Malmstrom and colleagues conducted the other US study and presented SARC‐F scores from the African American Health (AAH) study, the National Health and Nutritional Examination Survey (NHANES), and the Baltimore Longitudinal Study of Aging (BLSA). 12 Our finding of a higher SARC‐F score in women than in men was also observed in the AAH and the NHANES studies. For example, in NHANES, SARC‐F ≥ 4 was 15.4% in men and women combined, which we calculated was 19.7% in women (n = 1702) and 11.9% in men (n = 1586). SARC‐F ≥ 4 scores among MEC African American men (14.5%) and women (25.7%) were similar to results reported for African American men (16.4%) and women (26.5%) in the AAH study. 12 However, the NHANES study found comparable SARC‐F ≥ 4 scores among Mexican Americans (14.4%), other Hispanics (18.0%), Whites (15.1%), Blacks (16.5%), and other races (17.1%). 12 The NHANES results on Hispanics were similar to results of 19.5% reported for a community‐based study of older (aged ~73.2) women (80% of 487) residing near Mexico City. 30 Reasons for the higher SARC‐F scores among MEC Latino men and women are not known, but they tended to have a less healthy lifestyle (low physical activity, low Mediterranean diet scores) and more chronic conditions than other racial/ethnic groups (Table 2). We are not aware of published data on SARC‐F scores in Native Hawaiians, Japanese Americans, and other Asian Americans, but some studies in Japan have reported very low SARC‐F ≥ 4 occurrence (~3–4%) among community dwellers, 31 but considerably higher (14.3% for men and 28.4% for women) among diabetics. 32 Differing SARC‐F scores among different racial/ethnic groups may reflect the social gradient during childhood and over the lifecourse. 33 Lifestyle and biomarker determinants of SARC‐F among MEC participants will be investigated in the future.
Although there is substantial literature on the association of mortality with sarcopenia based on various clinical definitions of sarcopenia, 34 , 35 , 36 studies on SARC‐F scores and mortality are sparse, based on relatively short follow‐up periods and adjusted for limited number of covariates. 18 , 19 , 37 In the MEC, covariate adjusted HRs between all‐cause mortality and SARC‐F (as continuous and categorical variables) were statistically significant, in both men and women, and by race/ethnicity; the HRs for SARC‐F ≥ 4 ranged from 2.87 to 5.12 in men, and from 2.20 to 4.17 in women. The HR in each racial/ethnic group was somewhat higher in men than in women except for Native Hawaiians. Our results add to previous longitudinal results on SARC‐F and mortality. 12 With adjustment for sex and age, SARC‐F scores ≥ 4 predicted mortality in both AHH [odds ratio (OR) = 1.87, 95% CI 1.17–2.98] and BLSA (OR = 3.0, 95% CI 1.6–5.7). In BLSA, which also evaluated each component of SARC‐F in association with mortality, strength, rising from a chair, and climbing stairs were each significantly associated with mortality. 12 In the MEC, in both men and women, SARC‐F scores were associated with mortality, showing a significant association for both shorter (<6 years) and longer (≥6 years) follow‐up, which differs from the meta‐analysis results of an increased risk limited to <5 years (HR 4.30, 95% CI 1.82–10.17) but not ≥5 years of follow‐up (HR 1.32, 95% CI 0.86–2.02). 17 We will continue to monitor the impact of SARC‐F on mortality with additional years of follow‐up.
Our findings on SARC‐F with CVD‐specific and cancer‐specific mortality add new information on the utility of the SARC‐F scores. We found strong significant associations between SARC‐F ≥ 4 and CVD mortality by sex–race/ethnicity; the HRs ranged from 3.06 in Latino to 4.73 in White men, and from 2.33 in African American to 5.13 in Native Hawaiian women. Our results differ from NHANES results on sarcopenia, determined using bioimpedance analysis, which showed an association with CVD mortality in women (HR 1.61) but not in men (HR 1.07). 38 Also unlike the null results on SARC‐F and cancer‐specific mortality in NHANES, 38 SARC‐F scores in the MEC were associated with cancer‐specific mortality in men and women across race/ethnicity; results were statistically significant in 8 of 12 sex–racial/ethnic subgroups. The stronger findings on cancer in the MEC may be due, in part, to the three times larger number of cancer deaths. However, similar to the NHANES results, 38 we observed equally strong associations between SARC‐F and all‐cause mortality irrespective of BMI.
Finally, our results suggest that each of the five SARC‐F components is associated with risk of overall, CVD‐specific, and cancer‐specific mortality in men and women, providing further evidence of the utility of these easy to implement questions, particularly in population‐based observational studies. The editorial comments of Cesari and Kuchel in response to the recent SDOC findings on the definition of sarcopenia 39 emphasize the role of complementary methods to screen for sarcopenia and that this is a continually evolving field that will require further development, with careful consideration of the age, race/ethnicity, comorbid conditions, and other characteristics of the population under study. 40
Study strengths and limitation
To our knowledge, this is the largest single study to date on SARC‐F in a community setting, covering major racial/ethnic groups in the USA, and a substantial number of early old age (<70), as well as later old age (>90), men and women. The five questions we used on SARC‐F were essentially identical to the questions used in the original SARC‐F study. 12 Despite having poor sensitivity but high specificity, SARC‐F appeared to be very useful to screen for individuals at high risk of adverse outcomes including all‐cause mortality as well as CVD‐specific and cancer‐specific mortality. A main study limitation is the lack of measurements of clinical tests, such as gait speed, grip strength, or chair stands. In addition, our sub‐study on DXA‐assessed sarcopenia was based on a modest sample size in each ethnic group. Our mortality analysis was also based on a relatively short period of follow‐up of Q×5 respondents.
Conclusions
SARC‐F is a simple tool consisting of five questions that can be incorporated easily as a screening tool in the clinic, and it can be used as a continuous or as a categorical variable. SARC‐F has great potential as a first step to screen for sarcopenia for risk stratification and targeted intervention.
Conflict of interest
The authors confirm that they have no personal or financial conflicts of interest.
Funding
This work was supported by pilot funds from the USC Norris Comprehensive Cancer Center NCI P30 Cancer Center Support Grant Developmental Funds. The Multiethnic Cohort Study is funded in part by grant U01 CA164973 and the Adiposity Phenotype Study by grant P01 CA168530.
The funders had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supporting information
Figure S1. MEC cohort participants: from baseline and each of the follow‐up
Acknowledgements
We gratefully acknowledge Dr Eileen Crimmins for her very helpful advice on geriatric assessment of MEC participants in the fifth follow‐up questionnaire. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 40
Wu A. H., Setiawan V. W., Lim U., Tseng C.‐C., White K. K., Shepherd J., Lenz H. J., Cheng I., Stram D. O., Haiman C., Wilkens L. R., and Le Marchand L. (2022) Prognostic utility of self‐reported sarcopenia (SARC‐F) in the Multiethnic Cohort, Journal of Cachexia, Sarcopenia and Muscle, 13, 987–1002, 10.1002/jcsm.12916
References
- 1. Cruz‐Jentoft AJ, Landi F, Topinkova E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care 2010;13:1–7. [DOI] [PubMed] [Google Scholar]
- 2. Biolo G, Cederholm T, Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr 2014;33:737–748. [DOI] [PubMed] [Google Scholar]
- 3. Bauer J, Morley JE, Schols A, Ferrucci L, Cruz‐Jentoft AJ, Dent E, et al. Sarcopenia: a time for action. An SCWD Position Paper. J Cachexia Sarcopenia Muscle 2019;10:956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dodds RM, Granic A, Robinson SM, Sayer AA. Sarcopenia, long‐term conditions, and multimorbidity: findings from UK Biobank participants. J Cachexia Sarcopenia Muscle 2020;11:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 9. Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, et al. Sarcopenia definition: the position statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc 2020;68:1410–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cawthon PM, Manini T, Patel SM, Newman A, Travison T, Kiel DP, et al. Putative cut‐points in sarcopenia components and incident adverse health outcomes: an SDOC analysis. J Am Geriatr Soc 2020;68:1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grosicki GJ, Travison TG, Zhu H, Magaziner J, Binder EF, Pahor M, et al. Application of cut‐points for low muscle strength and lean mass in mobility‐limited older adults. J Am Geriatr Soc 2020;68:1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC‐F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016;7:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malmstrom TK, Morley JE. SARC‐F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc 2013;14:531–532. [DOI] [PubMed] [Google Scholar]
- 14. Malmstrom TK, Morley JE. Sarcopenia: the target population. J Frailty Aging 2013;2:55–56. [DOI] [PubMed] [Google Scholar]
- 15. Dent E, Morley JE, Cruz‐Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging 2018;22:1148–1161. [DOI] [PubMed] [Google Scholar]
- 16. Voelker SN, Michalopoulos N, Maier AB, Reijnierse EM. Reliability and concurrent validity of the SARC‐F and its modified versions: a systematic review and meta‐analysis. J Am Med Dir Assoc 2021;22:1864–1876.e16. [DOI] [PubMed] [Google Scholar]
- 17. Ida S, Kaneko R, Imataka K, Okubo K, Shirakura Y, Azuma K, et al. Verification of the predictive validity for mortality of the SARC‐F questionnaire based on a meta‐analysis. Aging Clin Exp Res 2021;33:835–842. [DOI] [PubMed] [Google Scholar]
- 18. Requena Calleja MA, Arenas Miquélez A, Díez‐Manglano J, Gullón A, Pose A, Formiga F, et al. Sarcopenia, frailty, cognitive impairment and mortality in elderly patients with non‐valvular atrial fibrillation. Rev Clin Esp (Barc) 2019;219:424–432. [DOI] [PubMed] [Google Scholar]
- 19. Yang M, Jiang J, Zeng Y, Tang H. Sarcopenia for predicting mortality among elderly nursing home residents: SARC‐F versus SARC‐CalF. Medicine (Baltimore) 2019;98:e14546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lim U, Monroe KR, Buchthal S, Fan B, Cheng I, Kristal BS, et al. Propensity for intra‐abdominal and hepatic adiposity varies among ethnic groups. Gastroenterology 2019;156:966–975.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kolonel LN, Henderson BE, Hankin JH, Nomura AMY, Wilkens LR, Pike MC, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;151:346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist 1970;10:20–30. [DOI] [PubMed] [Google Scholar]
- 23. Spector WD, Fleishman JA. Combining activities of daily living with instrumental activities of daily living to measure functional disability. J Gerontol B Psychol Sci Soc Sci 1998;53:S46–S57. [DOI] [PubMed] [Google Scholar]
- 24. Cornwell EY, Waite LJ. Measuring social isolation among older adults using multiple indicators from the NSHAP study. J Gerontol B Psychol Sci Soc Sci 2009;64:i38–i46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van de Velde S, Levecque K, Bracke P. Flanders versus The Netherlands: focus on differences between depressive symptoms in men and women measured on the basis of CES‐D8. Tijdschr Psychiatr 2011;53:73–82. [PubMed] [Google Scholar]
- 26. Harmon BE, Boushey CJ, Shvetsov YB, Ettienne R, Reedy J, Wilkens LR, et al. Associations of key diet‐quality indexes with mortality in the Multiethnic Cohort: the Dietary Patterns Methods Project. Am J Clin Nutr 2015;101:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. TIda S, Kaneko R, Murata K. SARC‐F for screening of sarcopenia among older adults: a meta‐analysis of screening test accuracy. J Am Med Dir Assoc 2018;19:685–689. [DOI] [PubMed] [Google Scholar]
- 28. Bischoff‐Ferrari HA, Orav JE, Kanis JA, Rizzoli R, Schlögl M, Staehelin HB, et al. Comparative performance of current definitions of sarcopenia against the prospective incidence of falls among community‐dwelling seniors age 65 and older. Osteoporos Int 2015;26:2793–2802. [DOI] [PubMed] [Google Scholar]
- 29. Kotlarczyk MP, Perera S, Nace DA, Resnick NM, Greenspan SL. Identifying sarcopenia in female long‐term care residents: a comparison of current guidelines. J Am Geriatr Soc 2018;66:316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parra‐Rodriguez L, Szlejf C, Garcia‐Gonzalez AI, Malmstrom TK, Cruz‐Arenas E, Rosas‐Carrasco O. Cross‐cultural adaptation and validation of the Spanish‐language version of the SARC‐F to assess sarcopenia in Mexican community‐dwelling older adults. J Am Med Dir Assoc 2016;17:1142–1146. [DOI] [PubMed] [Google Scholar]
- 31. Kera T, Kawai H, Hirano H, Kojima M, Watanabe Y, Motokawa K, et al. Limitations of SARC‐F in the diagnosis of sarcopenia in community‐dwelling older adults. Arch Gerontol Geriatr 2020;87:103959. [DOI] [PubMed] [Google Scholar]
- 32. Ida S, Murata K, Nakadachi D, Ishihara Y, Imataka K, Uchida A, et al. Development of a Japanese version of the SARC‐F for diabetic patients: an examination of reliability and validity. Aging Clin Exp Res 2017;29:935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shaw SC, Dennison EM, Cooper C. Epidemiology of sarcopenia: determinants throughout the lifecourse. Calcif Tissue Int 2017;101:229–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Batsis JA, Mackenzie TA, Barre LK, Lopez‐Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr 2014;68:1001–1007. [DOI] [PubMed] [Google Scholar]
- 35. Sim M, Prince RL, Scott D, Daly RM, Duque G, Inderjeeth CA, et al. Sarcopenia definitions and their associations with mortality in older Australian women. J Am Med Dir Assoc 2019;20:76–82.e2. [DOI] [PubMed] [Google Scholar]
- 36. Yuki A, Ando F, Otsuka R, Shimokata H. Sarcopenia based on the Asian Working Group for Sarcopenia criteria and all‐cause mortality risk in older Japanese adults. Geriatr Gerontol Int 2017;17:1642–1647. [DOI] [PubMed] [Google Scholar]
- 37. Wu TY, Liaw CK, Chen FC, Kuo KL, Chie WC, Yang RS. Sarcopenia screened with SARC‐F questionnaire is associated with quality of life and 4‐year mortality. J Am Med Dir Assoc 2016;17:1129–1135. [DOI] [PubMed] [Google Scholar]
- 38. Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population‐based sample of community‐dwelling older adults. J Cachexia Sarcopenia Muscle 2016;7:290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cesari M, Kuchel GA. Role of sarcopenia definition and diagnosis in clinical care: moving from risk assessment to mechanism‐guided interventions. J Am Geriatr Soc 2020;68:1406–1409. [DOI] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. MEC cohort participants: from baseline and each of the follow‐up


