Abstract
Low skeletal muscle mass is known to be associated with poor morbidity and mortality outcomes in cancer, but evidence of its impact on health‐related quality of life (HRQOL) is less established. This systematic review and meta‐analysis was performed to investigate the relationship between skeletal muscle mass and HRQOL in adults with cancer. Five databases (Ovid MEDLINE, Embase via Ovid, CINAHL plus, Scopus, and PsycInfo) were systematically searched from 1 January 2007 until 2 September 2020. Studies reporting on the association between measures of skeletal muscle (mass and/or radiodensity) derived from analysis of computed tomography imaging, and a validated measure of HRQOL in adults with cancer, were considered for inclusion. Studies classifying skeletal muscle mass as a categorical variable (low or normal) were combined in a meta‐analysis to investigate cross‐sectional association with HRQOL. Studies reporting skeletal muscle as a continuous variable were qualitatively synthesized. A total of 14 studies involving 2776 participants were eligible for inclusion. Skeletal muscle mass classified as low or normal was used to dichotomize participants in 10 studies (n = 1375). Five different cut points were used for classification across the 10 studies, with low muscle mass attributed to 58% of participants. Low muscle mass was associated with poorer global HRQOL scores [n = 985 from seven studies, standardized mean difference −0.27, 95% confidence interval (CI) −0.40 to −0.14, P < 0.0001], and poorer physical functioning domain HRQOL scores (n = 507 from five studies, standardized mean difference −0.40, 95% CI −0.74 to −0.05, P = 0.02), but not social, role, emotional, or cognitive functioning domain scores (all P > 0.05). Five studies examined the cross‐sectional relationship between HRQOL and skeletal muscle mass as a continuous variable and found little evidence of an association unless non‐linear analysis was used. Two studies investigated the relationship between longitudinal changes in both skeletal muscle and HRQOL, reporting that an association exists across several HRQOL domains. Low muscle mass may be associated with lower global and physical functioning HRQOL scores in adults with cancer. The interpretation of this relationship is limited by the varied classification of low muscle mass between studies. There is a need for prospective, longitudinal studies examining the interplay between skeletal muscle mass and HRQOL over time, and data should be made accessible to enable reanalysis according to different cut points. Further research is needed to elucidate the causal pathways between these outcomes.
Keywords: Oncology, Body composition, Sarcopenia, Computed tomography, EORTC QLQ‐C30, FACT
Introduction
Suboptimal levels of some body composition parameters, particularly skeletal muscle, are predictive of poor cancer outcomes such as decreased overall survival, 1 , 5 shorter disease‐free survival, 6 , 9 increased postoperative complications, 10 , 13 and increased chemotherapy toxicity. 14 , 15 Body composition analysis has therefore become an important method in oncology research. Cross‐sectional areas (CSA) of skeletal muscle measured using analysis of computed tomography (CT) imaging are highly correlated with whole body stores, 16 and the mean radiodensity of this CSA is considered a marker of fatty infiltration, 17 providing a convenient source of body composition data in patients who routinely undergo radiological imaging for cancer diagnosis, staging, and monitoring. 18
Traditional morbidity and mortality endpoints are now being complemented by other outcome measures as enhancements in life‐prolonging treatments bring about improved survival rates in people with cancer. 19 , 21 Consequently, measurement of health‐related quality of life (HRQOL) has become increasingly important in evaluating effectiveness of health interventions. 22 , 23 HRQOL is a subjective and complex outcome and is understood to encompass core domains of physical, psychological, social, and functional wellbeing. 19 , 24 Cancer diagnosis and treatment can affect HRQOL across the spectrum of these interacting domains, 25 and the use of validated, cancer‐specific HRQOL assessment tools allows for an insight into the relationship between a patient's overall wellbeing, life satisfaction, and health status in the context of their disease. 20 , 24 , 26 Understanding HRQOL is key to patient‐centred care, enabling researchers and clinicians to identify a need for supportive interventions, informing treatment decision making, and challenging assumptions about what patients consider important. 24
There is some evidence that low skeletal muscle mass measured using CT imaging analysis is linked to reduced HRQOL in patients with cancer. 27 , 28 It is hypothesized that this relationship between skeletal muscle mass and HRQOL reflects the interplay between reduced strength and impaired physical function, with independence and emotional wellbeing. 27 , 29 In a 2017 cross‐sectional study of 734 newly diagnosed non‐small cell lung cancer patients, low muscle mass was demonstrated to negatively affect physical and role functioning domains of HRQOL in both genders, and overall HRQOL in male patients. 27 Similarly, a 2018 cross‐sectional study of 237 patients with incurable lung and gastrointestinal cancers found that low skeletal muscle was associated with worse overall HRQOL and greater symptoms of depression. 28 The evidence‐base is still emerging, and this association requires further exploration so that effective interventions that improve HRQOL are developed. This systematic review and meta‐analysis was performed to investigate the relationship between CT‐derived measures of skeletal muscle mass, and HRQOL in adults with cancer.
The specific aims of this review were to (i) compare HRQOL scores between adults with either low or normal skeletal muscle mass and (ii) to examine the correlation between skeletal muscle mass and HRQOL. The secondary aims were to examine the relationship between change in skeletal muscle mass and change in HRQOL and to examine the correlation between CT‐derived skeletal muscle radiodensity and measures of HRQOL.
Methods
This systematic review and meta‐analysis was reported according to the Preferred Reporting Items for Systematic review and Meta‐Analysis guidelines. 30 This review was prospectively registered with the PROSPERO international database of systematic reviews on 26 December 2020, prior to data extraction and analysis (CRD42020198972). In a deviation to the published protocol, measures of adipose tissue were excluded as a secondary outcome due to the small number of included studies reporting on its association to HRQOL.
Data sources and search strategy
A systematic search of the Ovid MEDLINE, Embase via Ovid, CINAHL plus, Scopus, and PsycInfo databases was conducted on 2 September 2020. A combination of keywords and subject headings such as ‘sarcopenia’, ‘skeletal muscle’, ‘quality of life’, and ‘cancer’ were used for each database; details of search terms for each database are available in Supporting Information, Appendix S1. The search was run from 1 January 2007 until present, with dates chosen to ensure all relevant studies since the first published use of this technique in the oncology population 31 were captured.
Study selection criteria
Full text studies reporting on the relationship between body composition assessed using CT imaging analysis (Intervention), with HRQOL assessed using a validated tool (Outcome), in adults aged over 18 years with any cancer at any stage of treatment (Population), were eligible for inclusion. Analysis of the association between CT‐derived skeletal muscle index (SMI, cm2/m2) or cross‐sectional skeletal muscle area (cm2), and global HRQOL scores at baseline was considered the primary outcome; the term ‘global’ is hereafter used to refer to ‘global’, ‘overall’, or ‘total’ HRQOL scores, depending on the tool used for assessment. Domain HRQOL scores, longitudinal changes in skeletal muscle mass and/or HRQOL, and skeletal muscle radiodensity measured as mean Hounsfield Units (HU) of the cross‐sectional skeletal muscle area and analysed in relation to HRQOL, were secondary outcomes. Studies not published in the English language, conference abstracts, narrative review articles, and letters to the editor were excluded. Reference lists of all studies meeting inclusion criteria were hand searched. Individual studies included within systematic reviews were also screened for relevance. For studies meeting the above inclusion criteria but not reporting an analysis of the association between body composition and HRQOL, study authors were contacted for further information.
Following database searching, references were exported to Endnote X9 32 for removal of duplicates. Article titles and abstracts were independently screened by two researchers using Covidence systematic review software. 33 Full text articles were then independently reviewed by the same individuals for inclusion against the eligibility criteria. At each stage, consensus was achieved through discussion prior to progression of screening.
Data extraction
A template was created to facilitate extraction of data from included studies, for synthesis and potential meta‐analysis. One researcher extracted data relating to study characteristics: author, year of publication, study design, country of origin, setting, patient demographics (number of participants, cancer type and stage, age, and gender), body composition assessment data (software used, tissue types measured and radiodensity ranges used, anatomical site of analysis, timing/frequency of body composition measurements, and thresholds for determination of optimal vs. suboptimal values), and HRQOL assessment data (validated tool used, timing/frequency of assessment, and HRQOL scores). Two researchers independently extracted primary outcome data related to low muscle mass prevalence and associations between skeletal muscle mass and global scores of HRQOL at baseline. Discrepancies in data extraction were addressed by repeat review of relevant studies, to ensure accuracy. One researcher extracted secondary outcome data relating to associations between skeletal muscle mass and domain HRQOL scores, changes in skeletal muscle and HRQOL over time, and association between skeletal muscle radiodensity and baseline HRQOL scores.
Results synthesis and statistical analysis
Studies classifying skeletal muscle mass as a dichotomous variable (low or normal) using published SMI cut points, or based on a priori classification of clinically relevant muscle mass loss, were combined in a meta‐analysis to investigate cross‐sectional association with HRQOL. Study authors were contacted to request recalculation of data using a published SMI cut point appropriate for their cohort, if this was not reported in their original publication. Meta‐analysis was performed using RevMan software (Version 5.4), 34 using inverse variance analysis with a random effects model due to study heterogeneity. Standardized mean differences with 95% confidence intervals (CI) were calculated as the summary effect measure, as all of the HRQOL tools used in the studies included in the meta‐analysis were scored in the same direction (lower scores reflecting worse HRQOL). 35 A standardized mean difference of 0.2, 0.5, and 0.8 was interpreted to represent small, moderate, and large effect, respectively. 36 Where possible, HRQOL data were in the form of mean ± standard deviation for each category of muscle mass (low or normal). For studies reporting 95% CI, data were converted to standard deviation according to published guidelines. 35 Subgroup separation of studies was used in forest plot presentations to distinguish studies presenting data as mean difference between low and normal muscle mass. This subgroup analysis additionally functioned as a sensitivity analysis, enabling examination of the impact of including studies reporting multivariate data where adjustments were made for important confounding variables, compared with studies reporting univariate data only. Meta‐analyses for global HRQOL and the physical function domain were repeated, with studies alternatively grouped based on the cut point used to stratify participants with low or normal muscle mass, to examine if there is differentiation of pooled data from studies using different cut points. Statistical heterogeneity was assessed using interpretation of the I 2 value, where I 2 values of 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively. 37
Primary and secondary outcome data not suitable for meta‐analysis due to use of incompatible statistical tests or HRQOL domain scores were synthesized qualitatively.
Quality assessment
Each of the included studies was assessed for quality and risk of bias by two reviewers, using the Academy of Nutrition and Dietetics Quality Checklist for Primary Research. 38 Assessments were undertaken independently, and then, a consensus was formed through group discussion with a third reviewer to resolve conflicts. Comparability of study groups was marked as ‘N/A’ when groups were defined by outcome rather than randomization, or if there was only one group (Question 3). For studies in which CT‐derived body composition was a primary outcome, detailed description of methodology required listing the site of analysis, software used, and radiodensity reference ranges for identification of tissue types, as a minimum standard for quality in reporting (Question 6). For studies in which the primary research question differed to that of this review, statistical analysis was deemed to be appropriate if statistical tests used were able to address the study's aims (Question 8).
Results
Study selection
The systematic literature search yielded 6090 studies after removal of duplicates (Figure 1). Following title and abstract screening, 5892 studies were excluded. Full text review of 198 studies was conducted; reasons for exclusion are shown in Figure 1. Authors from 13 studies were contacted via email seeking further data if the paper reported measures of CT‐derived body composition along with HRQOL scores but did not report an analysis of the relationship between these variables. A total of 14 studies met the criteria for inclusion. 27 , 28 , 39 , 50
Figure 1.
Preferred Reporting Items for Systematic review and Meta‐Analysis (PRISMA) flow diagram of study selection.
Study characteristics
Table 1 summarizes the characteristics of the 14 included studies. Analysis of associations between CT‐derived body composition and HRQOL was conducted for 2776 participants in total. Colorectal cancer was the most frequently reported cancer type, 40 , 42 , 43 , 48 , 49 followed by lung, 27 , 28 , 40 , 41 breast, 39 , 40 , 47 gastrointestinal, 28 , 41 , 45 prostate, 40 , 46 head and neck, 44 , 50 and ‘other’ cancers such as gynaecologic, genitourinary, neurologic, haematological, melanoma, and unknown primary. 41 Cancer stage of participants included early/operable disease 39 , 45 , 46 , 49 and advanced disease 27 , 28 , 40 , 42 , 47 , 48 ; three studies involved both staging groups. 43 , 44 , 50 Treatment status at the time of CT imaging used for analysis of body composition was not always clearly described. In one study, CT imaging was conducted prior to any surgery or chemo‐radiation. 50 In the study by Gigic et al., 43 CT imaging used for analysis was conducted prior to surgery for the whole cohort, but 38% of participants had already commenced chemotherapy, and in the study by Blauwhoff‐Buskermolen et al., 40 all participants were chemotherapy naïve, but 15% of participants had required surgery in the previous 6 months. In all other included studies, participant history of chemotherapy, radiotherapy, and/or surgery at the point of CT imaging was unclear.
Table 1.
Characteristics of included studies listed by HRQOL assessment tool
| Author (year) | Country | Study design | Cancer type/s | Sample size | Gender, Female n (%) | Setting | Age, years, mean ± SD | Body composition analysis software | Body composition measure (s) | Site of CT analysis | HRQOL assessment tool |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gigic et al. (2020) 43 | Germany | Prospective cohort | Colorectal | 138 | 39 (28) | Outpatient | 61 ± 11.5 | Syngo Volume tool |
SMI VFA SFA |
L3/4 | EORTC QLQ‐C30 |
| Derksen et al. (2020) 42 | Netherlands | Prospective cohort | Colorectal | 221 | 79 (36) | Outpatient | 63.5 ± 8.4 | SliceOmatic | SMI | L3 | EORTC QLQ‐C30 |
| Daly et al. (2020) 41 | Ireland, Scotland | Cross‐sectional |
Gastrointestinal (40%) Lung (26%) Other (34%) a |
1027 | 503 (49) | Inpatient and outpatient |
Median (IQR) 66 (57–74) |
Ireland: OsiriX software version 4.1.1 Scotland: ImageJ software (version 1.47) |
SMI MA |
L3 | EORTC QLQ‐C30 |
| Blauwhoff‐Buskermolen et al. (2017) 40 | Netherlands | Cross‐sectional |
Colorectal Lung Breast Prostate |
241 | 111 (46) | Outpatient | 64 ± 10 | SliceOmatic | SMI | L3 or T4 | EORTC QLQ‐C30 |
| Bye et al. (2017) 27 | Norway | Cross‐sectional | Lung | 734 | 314 (43) | Outpatient | 65.4 ± 9.4 | SliceOmatic |
SMI SMD |
L3 |
EORTC QLQ‐C30 EORTC QLQ‐LC13 |
| Huang et al. (2017) 45 | China | Before–after | Gastric | 110 | 29 (26) | Outpatient | 63.2 ± 10.4 | INFINITT Healthcare Version 3.0.11.3 | SMI | L3 |
EORTC QLQ‐C30 EORTC QLQ‐STO22 |
| van Roekel et al. (2017) 49 | Netherlands | Cross‐sectional | Colorectal | 104 | 42 (40) | Outpatient | 64.3 ± 9 | SliceOmatic |
SMI VAT IMAT MA |
L3 | EORTC QLQ‐C30 |
| Thoresen et al. (2012) 48 | Norway | Prospective cohort | Colorectal | 50 | 24 (48) | Outpatient |
Median (IQR) 64 (41–85) |
SliceOmatic | SMI | L3 | EORTC QLQ‐C30 |
| Aleixo et al. (2020) 39 | USA | Cross‐sectional | Breast | 99 | 99 (100) | Outpatient | 56.4 ± 13.1 | SliceOmatic |
SMI SMD SMG |
L3 | FACT‐G |
| Sheean et al. (2019) 47 | USA | Cross‐sectional | Breast | 41 | 41 (100) | Outpatient | 59.6 ± 11.9 | SliceOmatic |
SMI SMD VAT SAT TAT |
L3 |
FACT‐B FACT‐ES |
| Nipp et al. (2018) 28 | USA | Cross‐sectional |
Lung (56.5%) Gastrointestinal (43.5%) |
237 | 109 (46) | Outpatient | 64.4 ± 10.9 | OsiriX | SMI | L3 | FACT‐G |
| Hua et al. (2020) 44 | China | Before–after | Nasopharyngeal | 56 | 9 (16) | Outpatient | 44.2 ± 10.93 | Monarco TPS | SMI | C3 (converted to L3) b | WHOQOL‐100 |
| Mitsui et al. (2020) 46 | Japan | Retrospective cohort | Prostate | 301 | 301 male (100) | Outpatient |
Median (IQR) 68 (63–71) |
Synapse Vincent V4 |
SMI VATI SATI |
L3 | EPIC |
| Wang et al. (2016) 50 | USA | Before–after | Oropharyngeal | 50 | 2 (4) | Outpatient | 57 ± 7 | MATLAB version 13.0 |
Total psoas area Lean psoas area SMD |
L4 |
UWQOL HNQOL |
Breast, gynaecologic, genitourinary, neurologic, haematological, melanoma, unknown primary, and others.
Skeletal muscle area at L3 estimated using published formula. 51
EORTC QLQ‐C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – Core 30 20 ; EORTC QLQ‐STO22, Gastric cancer module 52 ; EPIC, Expanded Prostate Cancer Index Composite instrument 53 ; FACT‐G, Functional Assessment of Cancer Therapy – General 54 ; FACT‐B, Breast 55 ; FACT‐ES, Endocrine Symptoms 56 ; HNQOL, Head and Neck Quality of Life Instrument 57 ; HRQOL, health‐related quality of life; HU, Hounsfield Units; IMAT, intermuscular adipose tissue; IQR, interquartile range; MA, mean muscle attenuation [mean radiodensity (in HU) of cross‐sectional muscle area]; PD1, first progression of disease; SAT, subcutaneous adipose tissue (interchangeable with SFA); SATI, subcutaneous adipose tissue index; SD, standard deviation; SFA, subcutaneous fat area (interchangeable with SAT); SMD, skeletal muscle radiodensity (a measurement of MA); SMG, skeletal muscle gauge (SMI × SMD); SMI, skeletal muscle index; SMM, skeletal muscle mass; TAT, total adipose tissue (SAT + VAT); TPA, total psoas area; UWQOL, University of Washington Quality of Life Instrument 58 ; VAT, visceral adipose tissue (interchangeable with VFA); VATI, visceral adipose tissue index; VFA, visceral fat area (interchangeable with VAT); WHOQOL‐100, The World Health Organization Quality of Life assessment. 59
Body composition analyses
Body composition analysis was conducted on CT images of the lumbar spine in all but one study, 44 most frequently at the level of the third lumbar vertebra (L3) (Table 1). Most studies used the same radiodensity range of −29 to +150 HU for identification of skeletal muscle during CT imaging analysis; Gigic et al. 43 used a more narrow range of 40 to 100 HU based on plausibility testing of their cohort in a previous publication, 60 and Aleixo et al. 39 did not report the specific radiodensity range used. Wang et al. 50 alternatively used tracing of psoas muscle borders to determine cross‐sectional skeletal muscle area.
Analysis of skeletal muscle
Ten studies categorized a total of 1375 participants according to baseline skeletal muscle mass using a range of cut points, presented in Table 2. In nine studies, 28 , 40 , 42 , 44 , 46 , 49 widely used cut points based on association with mortality in cancer patients, 1 , 3 or consensus guidelines for diagnosis of cancer cachexia, 61 were used to dichotomize participants as having either low muscle mass or not. The study by Huang et al. 45 used a percentage change in SMI pre‐surgery to post‐surgery (≥10% vs. <10%) to dichotomize low and normal muscle mass, as this extent of muscle loss was deemed clinically relevant. 62 Across the 10 studies, 795 participants (58%) were classified as having low muscle mass.
Table 2.
Studies with participants grouped according to baseline skeletal muscle mass (low or normal)
| Author (year) | Sample size a (% of whole study) |
Cancer type/s Stage |
Treatment status at point of baseline CT image | Cut point for low muscle mass | Source of cut point | Low muscle mass prevalence, n (%) | Timing of HRQOL assessments | Analysis type | |
|---|---|---|---|---|---|---|---|---|---|
| Women | Men | ||||||||
| Daly et al. (2020) 41 | 428 (41.7) |
Gastrointestinal Lung Other Stages III–IV Incurable |
81% receiving active palliative chemotherapy Unclear treatment and surgical status |
<41 cm2/m2 |
<43 cm2/m2 if BMI < 25 kg/m2 <53 cm2/m2 if BMI ≥ 25 kg/m2 |
Martin et al. 2013 1 | 192 (45) | Baseline only (within 12 weeks of CT) |
Multivariate Adjusted for weight loss, ECOG‐PS, mGPS, and low MA |
| Derksen et al. (2020) 42 | 221 (100) |
Colorectal Stage IV Unresectable |
Post‐chemotherapy treatment Prior surgical resection in unspecified number of participants |
<41 cm2/m2 |
<43 cm2/m2 if BMI < 25 kg/m2 <53 cm2/m2 if BMI ≥ 25 kg/m2 |
Martin et al. 2013 1 , b | 117 (53) |
Baseline (enrolment) Every 9 weeks until PD1 |
Univariate |
| Mitsui et al. (2020) 46 , c | 301 (100) |
Prostate Stages I–III Resectable |
Chemo/radiotherapy status not reported Pre‐surgery |
— |
<43 cm2/m2 if BMI < 25 kg/m2 <53 cm2/m2 if BMI ≥ 25 kg/m2 |
Martin et al. 2013 1 | 91 (30) |
Baseline (pre‐surgery) 2 weeks, 1 month, 3 months, 6 months, 12 months post‐surgery |
Multivariate Adjusted for significant variables on univariate analysis: HT and VAT/SAT ≥ 1.35 |
| Hua et al. (2020) 44 | 56 (100) |
Nasopharyngeal Stages II–IV |
Pre‐CCRT Unclear surgical status |
<41 cm2/m2 |
<43 cm2/m2 if BMI < 25 kg/m2 <53 cm2/m2 if BMI ≥ 25 kg/m2 |
Martin et al. 2013 1 | 34 (61) |
Baseline (mid‐point of CCRT, at 15F) 3 weeks post baseline |
Univariate |
| Sheean et al. (2019) 47 , d | 41 (100) |
Breast Stage IV |
Mixed/unclear treatment status Prior surgery in 30 participants (73%) |
<41 cm2/m2 | — | Martin et al. 2013 1 | 14 (34) | Baseline only (during treatment) | Univariate |
| Nipp et al. (2018) 28 | 237 (100) |
Lung (56.5%) Gastrointestinal (43.5%) Unresectable |
Mixed/unclear treatment status |
<39 cm2/m2 | <55 cm2/m2 | Fearon et al. 2011 61 | 131 (55) | Baseline (within 30 days before/after CT) |
Multivariate Adjusted for gender, age, marital status, education, and cancer type |
| Blauwhoff‐Buskermolen et al. (2017) 40 , e | 241 (100) |
Colorectal III–IV Lung, Breast, Prostate Stage IV |
Pre‐palliative chemotherapy Prior surgery in past 6 months in 37 participants (15%) |
Colorectal, breast, prostate cancer |
Fearon et al. 2011 61 Unpublished cut point for CT imaging at T4 f |
142 (59) | Baseline (pre‐palliative chemotherapy) | Univariate | |
| <39 cm2/m2 | <55 cm2/m2 | ||||||||
| Lung cancer | |||||||||
| <51.9 cm2/m2 | <66.0 cm2/m2 | ||||||||
| Huang et al. (2017) 45 | 110 (100) |
Gastric Stages I–III |
Chemo/radiotherapy status not reported Pre‐surgery (<1 month) |
Muscle mass loss ≤10% from baseline to 1 week post‐surgery | Puthucheary et al. 2013 53 | 35 (32) |
1, 3, and 6 months post‐surgery |
Univariate | |
| van Roekel et al. (2017) 49 | 92 (88) |
Colorectal Stages I–III |
Pre‐chemo/radiotherapy treatment in 93% of participants, post‐commencement of treatment (4–36 days) in 7% of participants Surgical status not reported |
<41 cm2/m2 |
<43 cm2/m2 if BMI < 25 kg/m2 <53 cm2/m2 if BMI ≥ 25 kg/m2 |
Martin et al. 2013 1 | 29 (32) | Once only, 2–10 years post‐diagnosis (5.2 ± 1.7 years) |
Multivariate Adjusted for gender, age at diagnosis, BMI at HRQOL assessment, number of comorbidities, tumour stage, and chemotherapy treatment |
| Thoresen et al. (2012) 48 | 28 (56) |
Colorectal Stage IV |
Mixed/unclear chemo/radiotherapy treatment and surgical status for subgroup of participants in current analysis | ≤38.5 cm2/m2 | ≤52.5 cm2/m2 | Prado et al. 2008 3 | 10 (36) | Once only (within 30 days of CT) |
Univariate Multivariate Adjusted for age and gender |
Sample size: number of participants included in analysis of relationship between skeletal muscle mass and HRQOL.
In the original publication, skeletal muscle index was categorized as loss (>2% loss), stable (≤2% loss to ≤2% gain), or gain (>2% gain). Upon our request, the authors repeated the analysis using their choice of preferred cut point for categorization of participants into two groups.
Not included in meta‐analysis due to use of a prostate cancer‐specific tool, which does not generate a global score.
Not included in meta‐analysis due to incompatible data [presented as median (interquartile range)].
Groups defined using diagnosis of cachexia, using cut point in addition to weight loss >2% in previous 6 months.
According to study authors, values based on Fearon et al. 61 cut off for lumbar (L3) CT imaging analysis, in an unpublished study of patients with SMI data for both L3 and T4.
CCRT, concurrent chemoradiotherapy; ECOG‐PS, Eastern Cooperative Oncology Group Performance Status 63 ; HT, hypertension; MA, mean muscle attenuation; mGPS, modified Glasgow Prognostic Score (a measure of systemic inflammation) 64 ; SAT, subcutaneous adipose tissue; PD1, first progression of disease; VAT, visceral adipose tissue.
Six studies assessed the relationship between skeletal muscle mass and quality of life using continuous linear associations of correlation or linear regression. 27 , 39 , 41 , 43 , 50 There was heterogeneity in the assessment of the relationship and data reported; thus, these results were synthesized qualitatively.
Two studies investigated the association between HRQOL and SMI as both a categorical and continuous variable. 41 , 42
HRQOL assessment tools
The most frequently utilized HRQOL assessment tool was the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 30 (EORTC QLQ‐C30), 20 used in eight studies. 27 , 40 , 43 , 45 , 48 , 49 The Functional Assessment of Cancer Therapy (FACT) scale 54 was used in three studies: one study used the 27 item general scale (FACT‐G), 28 and two studies 39 , 47 used the breast cancer‐specific version of this tool (FACT‐B). 55 Other tools used for quality of life assessment are reported in Table 1. 52 , 56 , 57 , 58 , 59 , 65 Global or overall HRQOL was measured in 12 studies (n = 2425); in a majority of studies (nine studies, n = 2048, 84%), 27 , 40 , 45 , 48 , 49 this measure was derived through dedicated items in the questionnaire using the EORTC QLQ‐C30 or WHOQOL‐100. 20 , 59 In three studies (n = 377, 16%), 28 , 39 , 47 overall HRQOL was determined as a sum of individual domain scores using the FACT scale. 54
Timing of baseline HRQOL assessments
Baseline HRQOL assessment was conducted within 1 month of CT imaging in four studies, 27 , 28 , 45 , 48 and within 3 months in two studies. 41 , 47 In seven studies, 39 , 40 , 42 , 44 , 46 , 50 timing of HRQOL in relation to CT imaging was not specified. Rather, the two assessments were reported as occurring at around the same period of time in the context of participants' treatment; prior to surgery, 43 , 46 chemo and/or radiotherapy, 39 , 40 , 44 , 50 or enrolment in a research trial. 42 One study 49 conducted HRQOL assessments between 2 and 10 years following body composition analysis and was included in meta‐analysis as it was the first and only time point of HRQOL assessment.
Follow‐up assessments
Two studies used repeat CT imaging analysis to assess longitudinal changes in body composition for analysis against changes in HRQOL. 42 , 50 Meta‐analysis of follow‐up HRQOL data was not possible, due to the heterogeneity in timing and assessment measures used.
Quality assessment of included studies
A summary of the quality criteria checklist assessing the relevance and validity of included studies is presented in Table 3. In all studies, the process of participant selection was subject to bias, as only patients for whom a routinely conducted CT image was available for baseline assessment were eligible for inclusion. Bias in participant selection was also demonstrated in other criteria such as inclusion of participants with a particular language background or treatment plan. Because of this bias, all studies included in this review were assigned a ‘no’ for the second validity criteria question at a minimum, and therefore received a ‘neutral’ quality rating. All studies received a rating of N/A for the question of study group comparability, as participants were either not grouped, or they were grouped according to body composition variable outcomes. One study provided only a brief description of CT‐derived body composition methodology, 39 all other studies reported sufficient detail of this methodology relevant to their primary outcomes. In four studies, investigators conducting body composition analysis were blinded to participant details 27 , 41 , 42 , 47 ; in the remaining 10 studies, it was unclear whether blinding occurred.
Table 3.
Quality assessment of included studies
| Academy of Nutrition and Dietetics Quality Criteria Checklist for Primary Research | Aleixo et al. (2020) 39 | Blauwhoff‐Buskermolen et al. (2017) 40 | Bye et al. (2017) 27 | Daly et al. (2020) 41 | Derksen et al. (2020) 42 | Gigic et al. (2020) 43 | Hua et al. (2020) 44 | Huang et al. (2017) 45 | Mitsui et al. (2020) 46 | Nipp et al. (2018) 28 | Sheean et al. (2019) 47 | Thoresen et al. (2012) 48 | van Roekel et al. (2017) 49 | Wang et al. (2016) 50 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall quality rating | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø | Ø |
| Relevance questions | ||||||||||||||
| Would implementing the studied intervention or procedure (if found successful) result in improved outcomes for the patients/clients/population group? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Did the authors study an outcome (dependent variable) or topic that the patients/clients/population group would care about? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Is the focus of the intervention or procedure (independent variable) or topic of study a common issue of concern to dietetics practice? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Is the intervention or procedure feasible? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Validity questions | ||||||||||||||
| 1. Was the research question clearly stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Was the selection of study subjects/patients free from bias? | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| 3. Were study groups comparable? | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 4. Was method of handling withdrawals described? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 5. Was blinding used to prevent introduction of bias? | Unclear | Unclear | Yes | Yes | Yes | Unclear | Unclear | Unclear | Unclear | Unclear | Yes | Unclear | Unclear | Unclear |
| 6. Were intervention/therapeutic regimes, exposure factor or procedure and any comparisons(s) described in detail? Were intervening factors described? | No a | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 7. Were outcomes clearly defined and the measurements valid and reliable? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 8. Was the statistical analysis appropriate for the study design and type of outcome indicators? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Are conclusions supported by results with biases and limitations taken into consideration? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10. Is bias due to study's finding or sponsorship unlikely? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
The Academy of Nutrition and Dietetics Quality Checklist for Primary Research contains four relevance questions, and 10 validity questions assessing the means by which the study has addressed issues of bias and generalisability, and quality in reporting of methods and statistical analysis. 37 Studies are assigned a positive (+) rating if these factors are adequately addressed with a ‘yes’ assigned to most questions, a negative (−) rating if they are not, and a neutral (Ø) rating if the answers to particular validity questions (2, 3, 6, and 7) are ‘no’, indicating a lack of strength in quality.
Specific radiodensity range used to identify skeletal muscle through CT imaging analysis not reported in methods.
Primary outcome: Association of muscle mass with global HRQOL scores at baseline
Seven of the studies outlined in Table 2 used HRQOL assessment tools that produced a global health score. 28 , 40 , 42 , 44 , 45 , 48 , 49 Meta‐analysis of pooled HRQOL global score data from a total of 985 participants is presented in Figure 2. The first forest plot subgroup contains five studies in which data were univariate, without adjustment for confounding factors. 40 , 42 , 44 , 45 , 48 Data from two studies were multivariate (second subgroup). 28 , 49 The summary effect measure from all seven studies showed that skeletal muscle mass below an optimum threshold was associated with poorer global HRQOL scores (standardized mean difference −0.27, 95% CI −0.40, −0.14, small effect size). Inclusion of both univariate and multivariate data did not affect the significance of the pooled result. There was very low statistical heterogeneity within these data (I 2 = 2%). The inclusion or exclusion of van Roekel et al. 49 (where follow‐up HRQOL was 2–10 years post‐CT analysis) did not affect the findings of the meta‐analysis (data not shown). As five different cut points were applied across the seven studies to classify participants with low or normal muscle mass, the meta‐analysis was repeated with studies grouped according to cut point used (Figure 2A, presented in Appendix S2 ). Statistical analysis of the subgroups was not possible due to the small number of studies 35 ; however, the visual assessment of the forest plots did not indicate there was evidence of systematic bias (Figure 2A).
Figure 2.
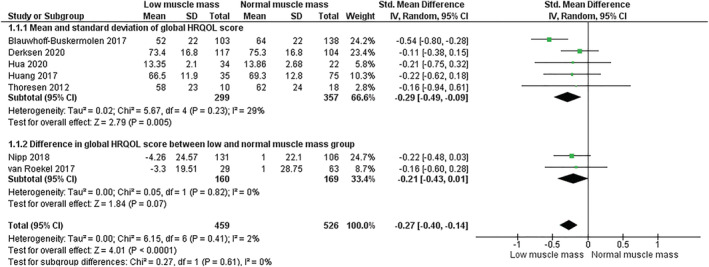
Meta‐analysis of baseline global HRQOL scores, with participants grouped according to low or normal skeletal muscle mass stores. The five studies in first subgroup reported only univariate data without adjustment for confounding factors. The two studies in the second subgroup reported multivariate data. Cross‐sectional area of skeletal muscle was measured at the third lumbar vertebra (L3) in all but two studies, where L3 measurements were imputed from alternate sites of analysis: third cervical vertebra (C3) in the study by Hua et al. 44 and fourth thoracic vertebra (T4) in 36% of participants in the study by Blauwhoff‐Buskermolen et al. 40 As the choice of cut point used to detect low or normal muscle mass affects the classification of participants, a second forest plot was generated to demonstrate the pooled results of studies grouped by cut point, presented in Appendix S2. ‘Total’ refers to sample size of low or normal skeletal muscle mass groups in each study. CI, confidence interval.
The study by Sheean et al. 47 assessed the relationship between skeletal muscle as a categorical variable and global HRQOL scores but was not included in the meta‐analysis as data were presented as median (interquartile range). This small study (n = 14 low muscle mass, n = 27 normal muscle mass) found no difference in global HRQOL scores between the two groups using both the FACT‐B tool [low muscle mass 108 (93–119) vs. normal muscle mass 100 (87–118) P = 0.29] and the FACT‐ES tool [low muscle mass 174 (151–191) vs. normal muscle mass 158 (142–178) P = 0.10]; for both tools, a higher score represents a higher HRQOL. Subgroup analysis suggested that obesity may be a confounding variable in the analysis, with those who were obese reporting poorer HRQOL. 47
Five studies reported on the relationship between skeletal muscle stores as a continuous variable and global HRQOL scores at baseline, presented in Table 4. 27 , 39 , 41 , 43 Four studies found weak associations, which were not statistically significant. 39 , 41 , 43 In large study of advanced lung cancer patients (n = 734), Bye et al. 27 reported a significant association between SMI and global HRQOL in male patients in both univariate analysis (P = 0.001) and after adjusting for age and tumour stage (P < 0.05); in this non‐linear analysis, global HRQOL scores deteriorated once SMI fell to a breakpoint of 42–45 cm2/m2.
Table 4.
Relationship between skeletal muscle mass as a continuous variable, and HRQOL scores (global and domains), at baseline
| Author (year) | Sample size a (% of whole study) |
Cancer type/s Stage |
Treatment status at point of CT imaging | Timing of HRQOL assessments | HRQOL domain |
Coefficient Univariate analysis |
P value | Adjusted for confounders |
Coefficient Multivariate analysis |
P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Aleixo et al. (2020) 39 | 99 (100) |
Breast Stages 0–III |
Pre‐chemotherapy Unclear surgical status |
Baseline only (pre‐chemotherapy) |
Global Physical function Social/family Emotional Functional |
Linear regression −0.12 −0.63 −0.27 −0.006 −0.19 |
0.052 0.002 0.16 0.98 0.18 |
Unadjusted | ||
| Daly et al. (2020) 41 | 428 (41.7) |
Gastrointestinal Lung Other Stages III–IV Incurable |
81% receiving active palliative chemotherapy Unclear treatment and surgical status |
Baseline only (within 12 weeks of CT) |
Global Physical function Role Emotional Cognitive Social |
Spearman −0.052 −0.164 −0.070 −0.078 −0.026 −0.104 |
0.282 0.001 0.149 0.103 0.592 0.034 |
Unadjusted | ||
| Derksen et al. (2020) 42 | 221 (100) |
Colorectal Stage IV Unresectable |
Post‐chemotherapy treatment Prior surgical resection in unspecified number of participants |
Baseline (enrolment) Every 9 weeks until PD1 |
Global Physical domain Role domain Emotional domain Cognitive Social |
Pearson 0.089 0.098 0.002 −0.006 0.091 0.035 |
0.19 0.15 0.97 0.02 0.18 0.61 |
Unadjusted b | ||
| Gigic et al. (2020) 43 | 138 (100) |
Colorectal Stages I–IV Resectable |
Prior chemotherapy in 53 (38%) participants Pre‐surgery |
Pre‐surgery (baseline) 6 and 12 months post‐surgery |
Global Physical domain Role Social |
Spearman 0.03 0.19 0.07 −0.06 |
0.68 0.02 0.42 0.52 |
Age Gender Tumour stage Tumour site Neoadjuvant treatment Baseline HRQOL |
Linear regression −0.19 0.18 −0.27 0.12 |
0.50 0.44 0.47 0.72 |
| Bye et al. (2017) 27 | 734 (100) |
Lung Stages IIIB–IV Incurable |
Pre‐chemotherapy Unclear surgical status |
Baseline only (pre‐chemotherapy) |
Global Males Females Physical domain Males Females Role domain Males Females |
Linear regression: flexible non‐linear modelling with restricted cubic splines |
0.001 0.15 0.016 0.004 0.02 0.012 |
Age Tumour stage |
Linear regression: flexible non‐linear modelling with restricted cubic splines |
All significant associations in univariate analysis remained significant (P < 0.05) Individual P values not reported |
Sample size: number of participants included in analysis of relationship between skeletal muscle mass and HRQOL.
Data in original study were adjusted for multiple cofounders; data supplied for this review are unadjusted.
HRQOL, health‐related quality of life; PD1, first progression of disease.
Secondary outcomes
Association of skeletal muscle mass with domain HRQOL scores at baseline
Meta‐analysis of physical function domain HRQOL scores at baseline included 507 participants across five studies 42 , 44 , 45 , 48 , 49 (Figure 3). The overall summary effect measure indicated that those classified as having low skeletal muscle mass at baseline also had a lower baseline physical functioning score compared with people classified as having normal skeletal muscle mass (standardized mean difference −0.4, 95% CI −0.74, −0.05, small‐moderate effect size). Statistical heterogeneity was assessed as moderate (I 2 = 66%). As three different cut points were applied across the five studies to classify participants with low or normal muscle mass, the meta‐analysis was repeated with studies grouped according to cut point used (Figure 3A, presented in Appendix S2). Statistical analysis of the subgroups was not possible due to the small number of studies 35 ; however, the visual assessment of the forest plots did not indicate there was evidence of systematic bias (Figure 3A). In the study by Daly et al. 41 (n = 428), physical function domain scores were mean dichotomized for logistic regression analysis and were therefore not included in the meta‐analysis. Univariate analysis in that study showed low SMI was associated with poorer physical functioning [odds ratio (OR), 1.72; 95% CI, 1.27–2.33; P < 0.001]. After multivariate assessment [controlling for weight loss, performance status (ECOG‐PS), inflammation (mGPS), and low skeletal muscle radiodensity], low SMI was no longer associated with poorer physical functioning (OR, 1.14; 95% CI, 0.74–1.73; P = 0.555).
Figure 3.
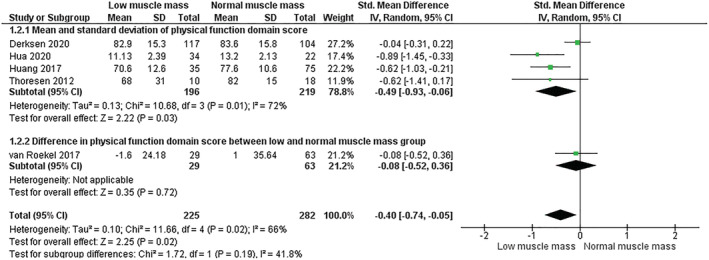
Meta‐analysis of HRQOL physical function domain scores, with participants grouped according to low or normal skeletal muscle mass stores. The four studies in first subgroup reported only univariate data without adjustment for confounding factors. The study in the second subgroup reported multivariate data. Cross‐sectional area of skeletal muscle was measured at the third lumbar vertebra (L3) in all studies excluding Hua et al., 44 where L3 measurements were imputed from analysis of imaging at the third cervical vertebra (C3). As the choice of cut point used to detect low or normal muscle mass affects the classification of participants, a second forest plot was generated to demonstrate the pooled results of studies grouped by cut point, presented in Appendix S2. ‘Total’ refers to sample size of low or normal skeletal muscle mass groups in each study.
A subset of included studies reported on the relationship of other domains of HRQOL with either low or normal skeletal muscle mass. 42 , 44 , 45 , 48 , 49 Meta‐analysis was used to assess the difference in HRQOL for the domains of social, role, emotional, and cognitive functioning, with no significant associations found (summarized in Table 5). Forest plots for these meta‐analyses are presented in Appendix S3 . The study by Mitsui et al. 46 assessed the relationship between low or normal skeletal muscle mass with domains of HRQOL that are specific to prostate cancer treatment 53 and was therefore not included in a meta‐analysis; this study found no significant differences in any of these domains (urinary, bowel, sexual, and hormonal) between low (n = 91) and normal (n = 210) skeletal muscle mass groups at baseline (all P > 0.05).
Table 5.
Meta‐analysis of relationship between SMI and domains of HRQOL: summary of findings
| Domain | Study | Standardized mean difference between low and normal skeletal muscle mass |
|---|---|---|
| Social functioning | Five studies 42 , 44 , 45 , 48 , 49 | n = 507, −0.06, 95% CI −0.24, 0.12, P = 0.53, I 2 0% |
| Role functioning | Four studies 42 , 45 , 48 , 49 | n = 451, −0.25, 95% CI −0.63, 0.13, P = 0.20, I 2 67% |
| Emotional functioning | Three studies 42 , 45 , 48 | n = 359, −0.11, 95% CI −0.33, 0.10, P = 0.29, I 2 0% |
| Cognitive functioning | Three studies 42 , 45 , 48 | n = 359, −0.07, 95% CI −0.28, 0.15, P = 0.54, I 2 0% |
CI, confidence interval; HRQOL, health‐related quality of life; SMI, skeletal muscle index.
Five included studies reported on the relationship between skeletal muscle mass as a continuous variable and different domains of HRQOL at baseline 27 , 39 , 41 , 43 (Table 4). Three studies reported only weak or negligible correlations between SMI and HRQOL scores in all domains of the EORTC QLQ‐C30. 41 , 43 This weak correlation was statistically significant for the physical function domain in two studies; the association was positive in one study and negative in the other. 41 , 43 SMI was reported to be weakly and negatively associated with social function domains scores in the study by Daly et al., 41 and very weakly and negatively associated with emotional function domain scores in the study by Derksen et al. 42 The study by Bye et al. 27 reported a significant association between SMI and the physical and role functioning domains of the EORTC QLQ‐C30 for both genders in both univariate analysis and after adjusting for age and tumour stage; consistent with their global HRQOL findings, scores in these domains began to decline once SMI dropped below a threshold of 42–45 cm2/m2 in male patients, and additionally in female patients at 37–40 cm2/m2. In the study by Aleixo et al., 39 HRQOL in the physical function domain was significantly associated with SMI; however, in contrast with the overall results of the meta‐analysis presented in Figure 3, this study indicated an inverse relationship (univariate analysis β − 0.63, P = 0.02).
Association between changes in skeletal muscle and HRQOL over time
Derksen et al. 42 undertook a secondary analysis to assess the association between changes in skeletal muscle mass [categorized as follows: loss (>2% decrease), stable (≤2% decrease to ≤2% gain), or gain (>2% increase)] from baseline to first progression of disease (PD1), with changes in HRQOL. Compared with the group experiencing muscle mass loss, a clinically relevant increase in global HRQOL scores was associated with the group experiencing stable muscle mass (β 9.9, 95% CI 2.4, 17.5, P < 0.05) and the group experiencing gain in muscle mass (β 14.7, 95% CI 8.0, 21.4, P < 0.05), in a multivariable linear regression analysis adjusted for several important confounding factors such as: age, gender, time to PD1, and previous adjuvant chemotherapy. Clinically relevant association with increased role functioning scores was also found in participants with stable muscle mass (β 12.0, 95% CI 2.2, 21.7, P < 0.05) or gain in muscle mass (β 17.9, 95% CI 9.4, 26.5, P < 0.05). Within group analysis based on cancer treatment protocols indicated that the type of treatment protocol may be a confounding factor.
Wang et al. 50 found that loss in total psoas muscle CSA from pre‐treatment to 3‐month follow up was correlated with decline in domain scores for activity (r −0.399, P = 0.019), recreation/entertainment (r −0.438, P = 0.0096), and swallowing (r −0.401, P = 0.019) (University of Washington Quality of Life 58 tool) and in the emotion domain of the University of Michigan Head and Neck Quality of Life tool (UM HNQOL 57 ) (r − 0.453, P = 0.007).
Association between skeletal muscle radiodensity and baseline HRQOL
Four studies investigated the relationship between skeletal muscle radiodensity and HRQOL. 27 , 39 , 41 , 49 Two studies found no association. 39 , 49 Conversely, a large study of 734 participants 27 demonstrated that skeletal muscle radiodensity was negatively associated with physical functioning (P male = 0.015, P female < 0.001), and this remained for female patients only, after adjustment for age and stage of disease (P male = 0.053, P female = 0.002); in this non‐linear association, HRQOL scores declined after skeletal muscle radiodensity reached a breakpoint of 32–34 HU in both genders. A study of 428 participants with mixed cancer types also showed that lower skeletal muscle radiodensity was associated with worse physical functioning on both univariate analysis (OR 2.31, 95% CI 1.69, 3.19, P < 0.001) and on multivariate analysis after controlling for weight loss, performance status (ECOG‐PS), inflammation (mGPS), and low SMI (OR 1.67, 95% CI 1.09, 2.56, P = 0.018). 41
Discussion
This systematic review has summarized and synthesized the literature on the relationship between CT‐derived assessment of skeletal muscle mass and HRQOL in adults with cancer. In the majority of studies, this analysis was conducted using dichotomization of participants according to skeletal muscle mass status. Meta‐analysis of these studies showed that adults with low skeletal muscle mass have lower scores of HRQOL (global and physical function domains) compared with those who have normal skeletal muscle mass. A limitation of the evidence‐base is that different cut points were applied between studies for the classification of skeletal muscle status that limits the robustness of these analyses and highlights the importance of making individual level data available for reanalysis of data. A subset of studies examined the correlation between skeletal muscle mass across the continuum of values, and HRQOL scores, and found little evidence of an association unless non‐linear analysis was used. We also found that there is a dearth of prospective longitudinal studies examining the change in skeletal muscle mass and the relationship with HRQOL during cancer treatment.
The results of the current meta‐analyses in which low SMI was associated with worse global and physical functioning HRQOL scores (Figures 2 and 3) may reflect a multifactorial and bidirectional relationship between skeletal muscle status and wellbeing. Reduction in skeletal muscle mass is known to contribute to decline in physical strength, 66 , 67 and there is evidence of a link between strength and HRQOL in cancer patients. 68 , 69 Skeletal muscle radiodensity, considered to be a measure of fatty infiltration of muscle, is also linked to muscle strength and function 17 , 70 ; this was reflected in the studies by Bye et al. 27 and Daly et al., 41 in which reduced skeletal muscle radiodensity was associated with worse physical functioning HRQOL domain scores. Conversely, reduced HRQOL associated with side effects of cancer treatment, recovery from surgery, and/or the emotional burden of having a life‐threatening disease 20 may feasibly reduce an individual's engagement in usual daily routine and result in physical inactivity, which is known to be a key contributing factor to muscle wasting. 67 , 71 Four studies included in this review did report measures of muscle strength 40 , 45 , 47 , 49 and one included measures of physical performance (muscle function) 39 among their outcomes; however, no studies conducted an analysis of the relationship between muscle strength or function and HRQOL scores. This highlights the need for further exploration of the impact of muscle mass in addition to muscle strength or function on HRQOL in cancer, in order to better understand this relationship.
Interpretation of the results of this study is complicated by the inability to determine the cause of muscle mass loss. In addition to occurring as part of the ageing process, 72 skeletal muscle stores are readily depleted by illness, 73 injury, 74 and malnutrition, 75 with tumour type also influencing the degree of wasting 29 ; contributions of each factor to a patient's muscle stores at the point of analysis are difficult to establish. Skeletal muscle mass and quality also deteriorate during chemotherapy treatment through a range of molecular pathways. 76 , 77 Most included studies did not report on participants' status of cancer treatment (medical or surgical) at the point of CT imaging, or statistically adjust for important confounding factors. In the studies where treatment status was clearly described, there was significant variation between studies. 40 , 43 , 50 This review highlights that a limitation of the evidence‐base is the insufficient description of treatment status at the point of body composition assessment, and multivariate adjustment for treatment and other confounding factors. Further studies employing multivariate analysis, controlling for variables known to influence both muscle mass and/or HRQOL, are needed.
The meta‐analysed primary outcome data in this review incorporated five different skeletal muscle cut points across seven studies; the potential for classification error in this sample is a major limitation, and the evidence‐base would benefit from a more consistent approach to classifying low muscle mass. Published thresholds for determination of suboptimal skeletal muscle stores are influenced by the phenotypic profile of participants such as ethnicity, 78 tumour type, 29 and gender, 3 and it is important that as far as possible, authors select appropriate cut points for their own population. The use of different cut points can affect the prevalence of low muscle mass identified in a cohort 79 , 80 which must be taken into consideration when interpreting the results of these meta‐analyses. While it has been demonstrated the association to reduced survival outcomes is consistent regardless of cut point used, 81 it remains possible that for the studies included in this review, findings in relation to HRQOL are influenced by the choice of cut point, as none of the thresholds used for low muscle mass detection were established with HRQOL as the dependant variable. The results of this study also indicate that when skeletal muscle is analysed as a continuous variable rather than categorical, there is a less clear relationship with HRQOL. It is possible that without identifying a threshold for skeletal muscle mass below which health outcomes are known to be worse, 1 , 3 it is difficult to elucidate the impact that incremental variations in skeletal muscle mass have on HRQOL scores using linear correlation or regression models, especially where muscle mass stores are not extremely low or high. The visual depiction of the non‐linear relationship between these variables in the study by Bye et al. 27 reveals the skeletal muscle mass threshold below which HRQOL scores begin to decline; interestingly, these breakpoints are similar to the survival based cut points used to dichotomize participants for one or both genders, in the majority of studies included in this review, which supports the findings of the meta‐analyses. 28 , 40 , 42 , 44 , 46 , 49
There are additional limitations to consider in the synthesis and interpretation of the findings of this review. Seven different HRQOL assessment tools were utilized across the 14 included studies, resulting in the incompatibility of some HRQOL data for inclusion in a meta‐analysis. There was also some heterogeneity in the measurement of skeletal muscle mass from CT imaging analysis. One study 50 measured the longitudinal change in CSA of a single abdominal muscle only (psoas), a convenient but unvalidated measure that is less sensitive to change in skeletal muscle mass than total muscle CSA, 82 and two studies 40 , 44 obtained skeletal muscle CSA from non‐lumbar imaging sites which have not been validated. Hua et al. 44 imputed lumbar (L3) skeletal muscle area from measurements at the third cervical vertebra (C3) using a predictive equation reported to demonstrate strong correlation to L3 measurements, 51 and Blauwhoff‐Buskermolen et al. 40 measured CSA at the fourth thoracic vertebra (T4) in 36% of their participants with lung cancer. Additionally, while data for all but four studies were collected prospectively, 39 , 44 , 46 , 49 the requirement for researchers to obtain routinely conducted CT imaging for assessment at a time point appropriate to answer their research question limited the number of participants eligible for inclusion, and created an element of bias in participant selection in all studies. Four studies attempted to address this by investigating between‐group differences in characteristics of participants who were included, compared with those not included based on availability of CT scans. 27 , 28 , 39 , 49 All four studies found at least one variable difference in the final cohort compared with those excluded; religion, 28 age, 39 disease stage and performance status, 27 and the number of comorbidities, education level, and proportion of individuals receiving chemotherapy treatment. 49 These factors may have varying impacts on the outcomes reported, and demonstrate the way in which collection of body composition data through convenience sampling limits the generalisability of results.
Future research should be directed at studies with a more consistent approach to HRQOL assessment, muscle mass stratification, and multivariate adjustment for important confounding factors. Given the body of literature synthesized in this review, use of the EORTC QLQ‐C30 or FACT‐G tools for HRQOL assessment would enable data comparison with the pre‐existing evidence‐base. Incorporation of muscle strength and function assessment into studies investigating CT‐derived skeletal muscle mass or radiodensity and its impact on HRQOL could allow for more robust exploration of the significant findings in our study. There is also a need for further longitudinal research in which both body composition analysis and HRQOL assessment are undertaken concurrently and repeatedly (pre‐treatment and post‐treatment), with clear description of both treatment and nutrition status, to better understand the interrelationship between body composition and HRQOL in the context of cancer treatment. Attenuation of muscle mass loss through exercise, nutrition, and/or pharmaceuticals is emerging as an exciting prospect warranting further exploration 29 , 83 ; with prevalence of low muscle mass in this review between 30% and 61% at a range of time points including pre‐treatment, it would be of benefit to commence any intervention as early as possible following cancer diagnosis. Future studies will be needed to determine the subsequent impact of these interventions on HRQOL. Furthermore, our understanding of the clinical significance of changes in HRQOL scores will be enhanced by the ongoing work to understand minimally important difference. 84
The results of this systematic review and meta‐analysis suggest that suboptimal skeletal muscle mass may be linked to lower levels of HRQOL in adults with cancer. The interpretation of this relationship is limited by the varied classification of low muscle mass between studies. There is a need for prospective, longitudinal studies examining the interplay between skeletal muscle mass and HRQOL over time, and data should be made accessible to enable reanalysis according to different cut points. This review cannot determine the cause and effect relationship between these outcomes. Further exploration of this relationship through targeted research is required in order to prioritize and develop interventions for optimization of skeletal muscle status, to bring about meaningful HRQOL outcomes for patients. Conversely, interventions targeting other determinants of declining HRQOL might also impact favourably on skeletal muscle mass, indicating that skeletal muscle mass should be assessed as an outcome in such studies.
Conflict of interest
None declared.
Funding
This research is supported by an Australian Government Research Training Program (RTP) Scholarship.
Supporting information
Data S1. Appendix S1: Search strategies for individual databases
Data S2. Appendix S2: Forest plots for global HRQOL and physical function domain scores, with studies grouped according to the cut point used to dichotomise participants with low or normal skeletal muscle mass
Data S3. Appendix S3: Forest plots for meta‐analyses of social, role, emotional and cognitive functioning HRQOL scores
Acknowledgements
L.H., C.E.H., J. P, and K.N. devised the search strategy. L.H. and K.F. conducted title/abstract and full text screening. L. H and K.F. conducted data extraction for primary outcomes, and L.H. extracted secondary outcome data. J.P., K.N., and L.H. completed the risk of bias and quality assessment. L.H. and C.E.H. conducted the meta‐analysis. L.H. drafted the manuscript, and all authors provided feedback. The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle. 85
Hanna L., Nguo K., Furness K., Porter J., and Huggins C. E. (2022) Association between skeletal muscle mass and quality of life in adults with cancer: a systematic review and meta‐analysis, Journal of Cachexia, Sarcopenia and Muscle, 13, 839–857, 10.1002/jcsm.12928
References
- 1. Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 2. Deluche E, Leobon S, Desport JC, Venat‐Bouvet L, Usseglio J, Tubiana‐Mathieu N. Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer 2018;26:861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol 2008;9:629–635. [DOI] [PubMed] [Google Scholar]
- 4. Ninomiya G, Fujii T, Yamada S, Yabusaki N, Suzuki K, Iwata N, et al. Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: a retrospective cohort study. Int J Surg 2017;39:45–51. [DOI] [PubMed] [Google Scholar]
- 5. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta‐analysis and systematic review. Eur J Cancer 2016;57:58–67. [DOI] [PubMed] [Google Scholar]
- 6. Deng H‐Y, Zha P, Peng L, Hou L, Huang K‐L, Li X‐Y. Preoperative sarcopenia is a predictor of poor prognosis of esophageal cancer after esophagectomy: a comprehensive systematic review and meta‐analysis. Dis Esophagus 2018;32:1–10. [DOI] [PubMed] [Google Scholar]
- 7. Chang KV, Chen JD, Wu WT, Huang KC, Hsu CT, Han DS. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta‐analysis. Liver Cancer 2018;7:90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang G, Meng S, Li R, Ye J, Zhao L. Clinical significance of sarcopenia in the treatment of patients with primary hepatic malignancies, a systematic review and meta‐analysis. Oncotarget 2017;8:102474–102485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsukioka T, Izumi N, Kyukwang C, Komatsu H, Toda M, Hara K, et al. Loss of muscle mass is a novel predictor of postoperative early recurrence in N2‐positive non‐small‐cell lung cancer. Ann Torac Surg 2018;24:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elliott AJ, Doyle LS, Murphy FC, King MS, Guinan VE, Beddy VP, et al. Sarcopenia: prevalence, and impact on operative and oncologic outcomes in the multimodal management of locally advanced esophageal cancer. Ann Surg 2017;266:822–830. [DOI] [PubMed] [Google Scholar]
- 11. Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long‐term survival after radical gastrectomy for gastric cancer: analysis from a large‐scale cohort. Medicine 2016;95:e3164, 10.1097/MD.0000000000003164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer 2012;107:931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weerink LBM, van der Hoorn A, van Leeuwen BL, de Bock GH. Low skeletal muscle mass and postoperative morbidity in surgical oncology: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle 2020;11:636–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jung H‐W, Kim JW, Kim J‐Y, Kim S‐W, Yang HK, Lee JW, et al. Effect of muscle mass on toxicity and survival in patients with colon cancer undergoing adjuvant chemotherapy. Support Care Cancer 2015;23:687–694. [DOI] [PubMed] [Google Scholar]
- 15. Aleixo G, Williams G, Nyrop K, Muss H, Shachar S. Muscle composition and outcomes in patients with breast cancer: meta‐analysis and systematic review. Breast Cancer Res Treat 2019;177:569–579. [DOI] [PubMed] [Google Scholar]
- 16. Shen W, Punyanitya M, Wang Z, Gallagher D, St‐Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross‐sectional image. J Appl Physiol 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 17. Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf) 2014;210:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. App Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 19. Cella DF, Tulsky DS. Quality of life in cancer: definition, purpose, and method of measurement. Cancer Invest 1993;11:327–336. [DOI] [PubMed] [Google Scholar]
- 20. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 21. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang X‐S, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population‐based registries in 67 countries (CONCORD‐2). Lancet 2015;385:977–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaplan RM, Bush JW. Health‐related quality of life measurement for evaluation research and policy analysis. Health Psychol 1982;1:61–80. [Google Scholar]
- 23. Calman KC. Quality of life in cancer patients—an hypothesis. J Med Ethics 1984;10:124–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fallowfield L. Quality of life: a new perspective for cancer patients. Nat Rev Cancer 2002;2:873–879. [DOI] [PubMed] [Google Scholar]
- 25. Aaronson NK. Methodologic issues in assessing the quality of life of cancer patients. Cancer 1991;67:844–850. [DOI] [PubMed] [Google Scholar]
- 26. Karimi M, Health BJ. Health‐related quality of life, and quality of life: what is the difference? Pharmacoeconomics 2016;34:645–649. [DOI] [PubMed] [Google Scholar]
- 27. Bye A, Sjøblom B, Wentzel‐Larsen T, Grønberg BH, Baracos VE, Hjermstad MJ, et al. Muscle mass and association to quality of life in non‐small cell lung cancer patients. J Cachexia Sarcopenia Muscle 2017;8:759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nipp RD, Fuchs G, El‐Jawahri A, Mario J, Troschel FM, Greer JA, et al. Sarcopenia is associated with quality of life and depression in patients with advanced cancer. Oncologist 2018;23:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aversa Z, Costelli P, Muscaritoli M. Cancer‐induced muscle wasting: latest findings in prevention and treatment. Ther Adv Med Oncol 2017;9:369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body composition as an independent determinant of 5‐fluorouracil‐based chemotherapy toxicity. Clin Cancer Res 2007;13:3264–3268. [DOI] [PubMed] [Google Scholar]
- 32. The EndNote Team . EndNote. EndNote X9 ed. Philadelphia, PA: Clarivate Analytics; 2013. [Google Scholar]
- 33. Covidence Systematic Review Software. Melbourne, Australia: Veritas Health Innovation. Available at www.covidence.org [Google Scholar]
- 34. Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 35. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (Eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane; 2021. Available from www.training.cochrane.org/handbook [Google Scholar]
- 36. Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed. New York: Routledge; 1988. [Google Scholar]
- 37. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process. Academy of Nutrition and Dietetics; 2016. [Google Scholar]
- 39. Aleixo GFP, Deal AM, Nyrop KA, Muss HB, Damone EM, Williams GR, et al. Association of body composition with function in women with early breast cancer. Breast Cancer Res Treat 2020;181:411–421. [DOI] [PubMed] [Google Scholar]
- 40. Blauwhoff‐Buskermolen S, Langius JAE, Becker A, Verheul HMW, de van der Schueren MAE. The influence of different muscle mass measurements on the diagnosis of cancer cachexia. J Cachexia Sarcopenia Muscle 2017;8:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Daly LE, Dolan RD, Power DG, Ní Bhuachalla É, Sim W, Cushen SJ, et al. Determinants of quality of life in patients with incurable cancer. Cancer 2020;126:2872–2882. [DOI] [PubMed] [Google Scholar]
- 42. Derksen JWG, Kurk SA, Peeters PHM, Dorresteijn B, Jourdan M, van der Velden AMT, et al. The association between changes in muscle mass and quality of life in patients with metastatic colorectal cancer. J Cachexia Sarcopenia Muscle 2020;11:919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gigic B, Nattenmüller J, Schneider M, Kulu Y, Syrjala KL, Böhm J, et al. The role of CT‐quantified body composition on longitudinal health‐related quality of life in colorectal cancer patients: the Colocare study. Nutrients 2020;12:1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hua X, Liao J‐F, Liu S, Zhang J, Huang H‐Y, Wen W, et al. Low skeletal muscle mass impairs quality of life in nasopharyngeal carcinoma patients treated with concurrent chemoradiotherapy. Front Nutr 2020;6:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang D‐D, Ji Y‐B, Zhou D‐L, Li B, Wang S‐L, Chen X‐L, et al. Effect of surgery‐induced acute muscle wasting on postoperative outcomes and quality of life. J Surg Res 2017;218:58–66. [DOI] [PubMed] [Google Scholar]
- 46. Mitsui Y, Sadahira T, Watanabe T, Araki M, Maruyama Y, Sato R, et al. Correlation between lumbar skeletal muscle size and urinary incontinence after radical prostatectomy. Low Urin Tract Symptoms 2020;12:245–252. [DOI] [PubMed] [Google Scholar]
- 47. Sheean P, Gomez‐Perez S, Joyce C, Vasilopoulos V, Bartolotta MB, Robinson P, et al. Body composition, serum biomarkers of inflammation and quality of life in clinically stable women with estrogen receptor positive metastatic breast cancer. Nutr Cancer 2019;71:981–991. [DOI] [PubMed] [Google Scholar]
- 48. Thoresen L, Frykholm G, Lydersen S, Ulveland H, Baracos V, Birdsell L, et al. The association of nutritional assessment criteria with health‐related quality of life in patients with advanced colorectal carcinoma. Eur J Cancer Care (Engl) 2012;21:505–516. [DOI] [PubMed] [Google Scholar]
- 49. van Roekel EH, Bours MJL, Te Molder MEM, Breedveld‐Peters JJL, Olde Damink SWM, Schouten LJ, et al. Associations of adipose and muscle tissue parameters at colorectal cancer diagnosis with long‐term health‐related quality of life. Qual Life Res 2017;26:1745–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang C, Vainshtein JM, Veksler M, Rabban PE, Sullivan JA, Wang SC, et al. Investigating the clinical significance of body composition changes in patients undergoing chemoradiation for oropharyngeal cancer using analytic morphomics. Springerplus 2016;5:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jung AR, Roh JL, Kim JS, Choi SH, Nam SY, Kim SY. Efficacy of head and neck computed tomography for skeletal muscle mass estimation in patients with head and neck cancer. Oral Oncol 2019;95:95–99. [DOI] [PubMed] [Google Scholar]
- 52. Vickery CW, Blazeby JM, Conroy T, Arraras J, Sezer O, Koller M, et al. Development of an EORTC disease‐specific quality of life module for use in patients with gastric cancer. Eur J Cancer 2001;37:966–971. [DOI] [PubMed] [Google Scholar]
- 53. Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health‐related quality of life in men with prostate cancer. Urology 2000;56:899–905. [DOI] [PubMed] [Google Scholar]
- 54. Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570–579. [DOI] [PubMed] [Google Scholar]
- 55. Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the functional assessment of cancer therapy‐breast quality‐of‐life instrument. J Clin Oncol 1997;15:974–986. [DOI] [PubMed] [Google Scholar]
- 56. Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT‐B. Breast Cancer Res Treat 1999;55:189–199. [DOI] [PubMed] [Google Scholar]
- 57. Terrell JE, Nanavati KA, Esclamado RM, Bishop JK, Bradford CR, Wolf GT. Head and neck cancer—specific quality of life: instrument validation. Arch Otolaryngol–Head Neck Surg 1997;123:1125–1132. [DOI] [PubMed] [Google Scholar]
- 58. Hassan SJ, Weymuller EA Jr. Assessment of quality of life in head and neck cancer patients. Head Neck 1993;15:485–496. [DOI] [PubMed] [Google Scholar]
- 59. The World Health Organization Quality of Life Assessment (WHOQOL) . Development and general psychometric properties. Soc Sci Med 1998;46:1569–1585. [DOI] [PubMed] [Google Scholar]
- 60. Nattenmueller J, Hoegenauer H, Boehm J, Scherer D, Paskow M, Gigic B, et al. CT‐based compartmental quantification of adipose tissue versus body metrics in colorectal cancer patients. Eur J Radiol 2016;26:4131–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 62. Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310:1591–1600. [DOI] [PubMed] [Google Scholar]
- 63. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–656. [PubMed] [Google Scholar]
- 64. Proctor MJ, Morrison DS, Talwar D, Balmer SM, O'Reilly DSJ, Foulis AK, et al. An inflammation‐based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer 2011;104:726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bergman B, Aaronson NK, Ahmedzai S, Kaasa S, Sullivan M. The EORTC QLQ‐LC13: a modular supplement to the EORTC core quality of life questionnaire (QLQ‐C30) for use in lung cancer clinical trials. Eur J Cancer 1994;30:635–642. [DOI] [PubMed] [Google Scholar]
- 66. Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 2001;56:B209–B217. [DOI] [PubMed] [Google Scholar]
- 67. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61:1059–1064. [DOI] [PubMed] [Google Scholar]
- 68. Morishita S, Tsubaki A, Fu JB, Mitobe Y, Onishi H, Tsuji T. Cancer survivors exhibit a different relationship between muscle strength and health‐related quality of life/fatigue compared to healthy subjects. Eur J Cancer Care (Engl) 2018;27:e12856, 10.1111/ecc.12856 [DOI] [PubMed] [Google Scholar]
- 69. Cešeiko R, Eglītis J, Srebnijs A, Timofejevs M, Purmalis E, Erts R, et al. The impact of maximal strength training on quality of life among women with breast cancer undergoing treatment. Exp Oncol 2019;41:166–172. [DOI] [PubMed] [Google Scholar]
- 70. McGregor RA, Cameron‐Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan 2014;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Colloca G, Di Capua B, Bellieni A, Cesari M, Marzetti E, Valentini V, et al. Muscoloskeletal aging, sarcopenia and cancer. J Geriatr Oncol 2019;10:504–509. [DOI] [PubMed] [Google Scholar]
- 72. Grimby G, Saltin B. The ageing muscle. Clin Physiol 1983;3:209–218. [DOI] [PubMed] [Google Scholar]
- 73. Argilés JM, Campos N, Lopez‐Pedrosa JM, Rueda R, Rodriguez‐Mañas L. Skeletal muscle regulates metabolism via interorgan crosstalk: roles in health and disease. J Am Med Dir Assoc 2016;17:789–796. [DOI] [PubMed] [Google Scholar]
- 74. Demling RH. Nutrition, anabolism, and the wound healing process: an overview. Eplasty 2009;9: e9‐e. [PMC free article] [PubMed] [Google Scholar]
- 75. Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr 2017;36:49–64. [DOI] [PubMed] [Google Scholar]
- 76. Bozzetti F. Chemotherapy‐induced sarcopenia. Curr Treat Options Oncol 2020;21:21. [DOI] [PubMed] [Google Scholar]
- 77. Daly LE, Ni Bhuachalla EB, Power DG, Cushen SJ, James K, Ryan AM. Loss of skeletal muscle during systemic chemotherapy is prognostic of poor survival in patients with foregut cancer. J Cachexia Sarcopenia Muscle 2018;9:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wells JC. Ethnic variability in adiposity, thrifty phenotypes and cardiometabolic risk: addressing the full range of ethnicity, including those of mixed ethnicity. Obes Rev 2012;13:14–29. [DOI] [PubMed] [Google Scholar]
- 79. Hilmi M, Jouinot A, Burns R, Pigneur F, Mounier R, Gondin J, et al. Body composition and sarcopenia: the next‐generation of personalized oncology and pharmacology? Pharmacol Ther 2019;196:135–159. [DOI] [PubMed] [Google Scholar]
- 80. Bijlsma AY, Meskers CG, Ling CH, Narici M, Kurrle SE, Cameron ID, et al. Defining sarcopenia: the impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age 2013;35:871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hopkins JJ, Skubleny D, Bigam DL, Baracos VE, Eurich DT, Sawyer MB. Barriers to the interpretation of body composition in colorectal cancer: a review of the methodological inconsistency and complexity of the CT‐defined body habitus. Ann Surg Oncol 2018;25:1381–1394. [DOI] [PubMed] [Google Scholar]
- 82. Rutten IJG, Ubachs J, Kruitwagen RFPM, Beets‐Tan RGH, Olde Damink SWM, Van Gorp T. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J Cachexia Sarcopenia Muscle 2017;8:630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Prado CM, Purcell SA, Laviano A. Nutrition interventions to treat low muscle mass in cancer. J Cachexia Sarcopenia Muscle 2020;11:366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Musoro ZJ, Hamel J‐F, Ediebah DE, Cocks K, King MT, Groenvold M, et al. Establishing anchor‐based minimally important differences (MID) with the EORTC quality‐of‐life measures: a meta‐analysis protocol. BMJ Open 2018;8:e019117, 10.1136/bmjopen-2017-019117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Appendix S1: Search strategies for individual databases
Data S2. Appendix S2: Forest plots for global HRQOL and physical function domain scores, with studies grouped according to the cut point used to dichotomise participants with low or normal skeletal muscle mass
Data S3. Appendix S3: Forest plots for meta‐analyses of social, role, emotional and cognitive functioning HRQOL scores


