Abstract
Aim:
This study evaluated the effect of an interactive, web-based educational program on parents’ opioid risk knowledge, risk perceptions, analgesic self efficacy and decision-making.
Patients & methods:
Totally, 64 parents from a tertiary care pediatric healthcare setting were assessed for risk understanding at baseline, immediately and 3 days after receiving the educational program.
Results:
Participants gained increased opioid risk knowledge, enhanced risk perceptions as well as enhanced analgesic self efficacy after program exposure. The program had no effect on parental decisions about when to give or withhold a prescribed opioid.
Conclusion:
The interactive web-based program improved parental knowledge about opioid risks. Program enhancements may be needed to improve pain management decisions about when it is safe to use opioids and when they should be withheld.
Keywords: : opioid risk, pain, patient understanding, pediatrics, web-based patient education
Prescription opioids remain a primary means of treating severe, acute postoperative, dental and trauma-related pain in children. However, these potent analgesics are associated with high rates of opioid-related adverse events (ORAE) in children and adolescents [1]. Indeed, adverse effects occur in up to half of all children taking opioids, and although most are self-limiting, others, which are left unrecognized, pose significant risk for serious morbidity and mortality [1–5]. The annual rates of serious ORAE parallel prescribing rates [6–8] with more than 21,000 emergency room admissions [9] and hundreds of accidental prescription opioid overdose deaths in children and adolescents [8]. Although many opioid-related overdoses may be related to misuse, prescribed use has also been associated with serious events and death in children [1]. Details from reports of accidental opioid-related deaths and neurologic injury in children show a critical lack of parental recognition and attention to early signs of opioid toxicity during medical use [10–12]. As many as 10% of parents have been found to give larger or more frequent doses of prescribed analgesics in an attempt to relieve pain, leaving children vulnerable to potential overdose and toxicity [13,14] and therapeutic errors accounted for nearly three quarters of ORAEs that resulted in emergency room admission, hospitalization or death [1]. Additionally, half of parents save their children’s leftover prescription opioids and adults who do so endorse future use of leftovers for themselves or other family members [15–17]. These findings are particularly important since preventable medication adverse events and misuse are often associated with nonadherence behaviors [18,19].
Together, studies suggest a critical need for parental guidance that addresses effective and safe prescription opioid use for managing their children’s acute pain. Uncertainty and lack of knowledge about ORAE is widespread among parents whose children are prescribed analgesics [20–22] and may lead some parents to withhold analgesics altogether in order to reduce risk or to continue giving a prescribed drug when potentially serious adverse effects are present. Lack of preparation for or misunderstanding of treatment effects has been found to be largely responsible for medication instruction nonadherence [23–29]. Interventions that improve parental recognition of ORAEs and that advise appropriate actions to take when pain and other symptoms occur are imperative for safe and effective use of as needed (i.e., pro re nata [prn]) analgesics.
The purpose of this study was to evaluate a prototype web-based educational program (adaptable for smartphone use) that would help parents gain analgesic and pain management knowledge regarding adherent and safe use. The specific aims of this study were to:
Evaluate the ability of the program to improve parental knowledge about ORAEs, analgesic self efficacy and their ability to make safe and effective opioid decisions.
Evaluate the usability and parental satisfaction with the prototype program.
This study tested the primary hypothesis that ‘parental ORAE knowledge and their perception of potentially serious opioid-related risks would increase significantly from pre-intervention to post-intervention’.
Patients & methods
With institutional IRB approval and verbal consent, we consecutively recruited parents who had at least one child under the age of 18 years living in the home setting. Parents were recruited from the surgical waiting room as their children were undergoing elective surgical procedures. We included parents who had access to a smartphone (for follow-up surveys) and the ability to read and understand English and excluded those whose children had been treated for chronic pain or oncologic conditions.
Intervention
The web-based educational intervention incorporated critical information obtained from focused interviews with stakeholders including parents whose children had used opioids, those who had never used opioids, physician prescribers and pediatric pain specialists. The educational content of the program addressed the identified and prioritized needs of stakeholders which included items measured using Likert scales (Table 1). The content was also derived from evidence-based analgesic information and guidelines [30], multimodal pain management practices [30,31], and expert physician and nurse input. The educational information was meant to be universal or generic in order to have the maximum potential value to inform postprocedural or postinjury pain management when opioids and nonopioids are prescribed for acute, short-term pain management.
Table 1. . Self-rated analgesic informational priorities of providers and patients.
| Content | Provider ranking | Patient ranking |
|---|---|---|
| Assess pain intensity | 6 (4–8) | 8 (7–10) |
| Assess level of sedation | 10 (5–10) | 7 (5–9) |
| Assess constipation | 6 (5–9) | 6.5 (3–8) |
| Opioid tapering advice | 8 (7–10) | 9 (6–10) |
| Disposal advice | 8 (5–8) | 8 (4–10) |
| Symptom-guided advice | 8 (8–10) | 9 (8–10) |
Data from focus interviews; ratings could range from 0 to 10, where 10 reflects extremely important. Data presented as median (range).
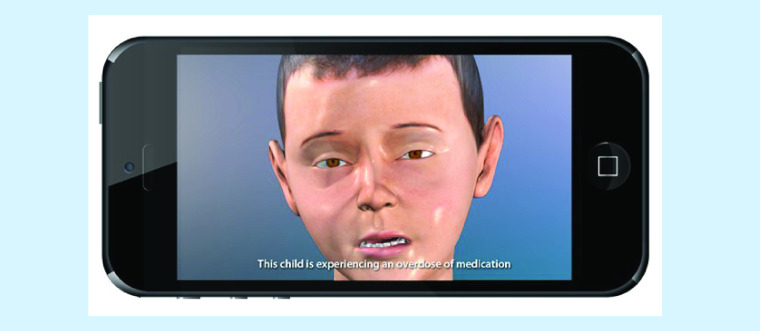
Initially, we created 3D computer models of a virtual pediatric patient designed to simulate various real-life scenarios. The base anatomical and physiologic visualization were modeled with both 2D and 3D computer graphic software and manipulated to create a realistic visual representation of virtual patients and scenarios. The principle software programs utilized in the creation of these models and simulations are Maya, Adobe After Effects, HTML and JAVA.
Initial prototypes and content were reviewed by the investigators to determine authenticity and accuracy of information and these were modified as necessary. The final prototype incorporated guided exercises into the multimedia intervention to assess parents’ understanding of treatment information at the time of information sharing and to provide immediate corrected feedback [32]. Figure 1 depicts screenshots from the program.
Figure 1. . Screenshot from the pain app prototype.
Main measures
ORAE knowledge & risk perceptions
This survey measures knowledge or awareness of several common, nonserious and less common but potentially serious ORAE in a binary manner (yes/no) and the perceived risk or seriousness of each (from 0 to 5, where 0 = not serious and 5 = extremely serious) [33–35]. ORAE risk perception was previously found to have predictive validity for parents’ ability to recognize symptoms and for their decisions to give a prescribed opioid to a child with or without ORAE symptoms [33,34].
Analgesic self efficacy
This tool measured parents’ perceived confidence to safely and effectively give an analgesic to treat their children’s pain. Medication self efficacy mediates symptom management and health behavior and predicts medication adherence behavior [36]. The tool used here included seven items (e.g., “I am sure that I can recognize and reduce the most important risks of pain relievers”) each scored from 0 (not confident) to 5 (extremely confident) to yield a sum score ranging from 0 to 35, where higher scores reflect higher perceived self efficacy.
Opioid decisions
Situational decisional exercises were used to assess parents’ safe and effective opioid decision-making ability. Previously, we demonstrated construct and predictive validity of hypothetical prescription opioid decisions with strong associations between decisions and ORAE risk knowledge, risk perceptions and postoperative analgesic use [33–35]. The first scenario depicts the child with high pain (7 out of 10 on the FACES Pain Scale – the commonest threshold parents use to treat pain with potent analgesics) [35,37] and no signs of ORAE since the last analgesic dose. This exercise was used to measure parents’ willingness to give the prescribed opioid to a child with no evident risk (i.e., safe and effective use). A second scenario depicts a child with the same degree of pain but also with the earliest warning of toxicity, excessive sedation. This exercise was used to assess the parent’s decision to give/withhold the prescribed opioid in a potentially unsafe situation. Immediately after each scenario, parents were asked to choose an analgesic strategy from the list of hypothetically prescribed analgesics. These decisions were coded as binary data (parent would withhold = 0 or would give the prescribed opioid dose = 1).
Procedure
Following recruitment and verbal consent, parents completed the pre-intervention survey to assess ORAE knowledge, risk perceptions and analgesic self efficacy. The following data were also collected: demographics, past experience using common analgesics (e.g., have you used a prescribed opioid [examples of common generic names were given] to treat your child’s pain in the past?) and presence of analgesics in the home at the time of the survey.
Immediately following the baseline survey, parents completed the interactive web-based educational program using a dedicated iPad. Immediately after the intervention parents were resurveyed regarding their ORAE knowledge, risk perceptions, analgesic self efficacy. Seven days later, parents were sent a survey link that re-assessed these outcomes and parents’ decision-making abilities. All of the surveys were completed via a computer-based Qualtrics™ platform, and parents themselves entered their assigned ID number to facilitate links between the surveys without revealing any personal identifiers. In this manner, complete parental privacy was maintained as all links to personal contact information were removed immediately following the final survey.
Sample size
The sample size determination was based on recent data [38] showing that use of an interactive program increased risk knowledge by 10%. To detect a similar change in parents’ ORAE risk knowledge, we determined that a sample size of 58 (α 0.05, β 0.20, 2-tailed) would be needed. This sample size was deemed to be adequate for this Phase I pilot study which would also inform the power analysis for a potential Phase II proposal.
Data analyses
Statistical analysis was performed using SPSS © Statistical Software (version 24; IBM Corp., NY, USA). Comparisons between the pre- and post-knowledge tests and self efficacy scores (i.e., baseline to Time 1 (T1) and Time 2 (T2) were made using analyses of variance for repeated measures (to measure change in scores over time) or Friedman’s test for data that were not equally distributed. χ2 test with Fisher’s exact tests (as appropriate) were used to measure the change in percentage of parents who made the decision to give an opioid for each simulated decision between pre- and post-intervention assessments.
Results
A total of 64 parents agreed to take part in the study; however, 6 did not meet eligibility criteria as they reported no children in the home under the age of 18 years. They were therefore excluded from analyses, leaving 58 participants for the analyses. The majority of parent participants were female (78%), non-Hispanic (97%) and white (92%), and the median number of children in the home was 2 (range 1–7). Half of the participants had at least a 4-year college degree, while 18% had completed an associate’s degree or trade school, 20% had some college experience, 9% had a high-school diploma and 2% reported less than a high-school diploma.
Past pain management experience
All parents had previously used acetaminophen and nonsteroidal anti-inflammatories and 24 (38%) had used an opioid to treat their child’s pain. Similarly, most reported having either acetaminophen (97%) or an nonsteroidal anti-inflammatories (98%) in the home at the time of the baseline survey, whereas only a minority (n = 15 [23%]) had an opioid in the home.
Opioid risk knowledge & perceptions
Table 2 shows the changes in knowledge of each of the ORAEs and Table 3 shows parental perceptions of risk seriousness over time. There were significant improvements in knowledge from baseline to the post-intervention assessments for nausea and vomiting, oversedation, slowed-breathing and addiction. There were increases in the perceived seriousness of the risk of oversedation from baseline to Time 1 and Time 2, with no difference between the post-intervention assessments (i.e., Time 1 to Time 2). There were no changes in the perceptions of seriousness of nausea and vomiting, addiction or poor pain relief.
Table 2. . Percentage of parents with opioid-related adverse effect knowledge over time.
| Adverse effect | Baseline | Immediately after intervention | Day 7 after intervention |
|---|---|---|---|
| Nausea/vomiting | 46 (79%) | 57 (98%); 0.002 | 34 (100%); 0.003 |
| Constipation | 46 (79%) | 57 (98%); 0.002 | 33 (97%); 0.027 |
| Oversedation | 46 (79%) | 57 (98%); 0.002 | 33 (97%); 0.027 |
| Slowed breathing | 38 (67%) | 56 (97%); <0.001 | 34 (100%); <0.001 |
| Addiction | 50 (86%) | 57 (98%); 0.032 | 34 (100%); 0.024 |
Data presented as n (%); p-value vs baseline.
Table 3. . Perceived seriousness of opioid-related adverse events and parental analgesic self efficacy.
| Adverse effect | Baseline | Immediately after intervention | Day 7 after intervention | ANOVA: F statistic; p-value |
|---|---|---|---|---|
| Perceived seriousness of opioid-related risks (mean ± SD; possible range: 0–5) | ||||
| Nausea/vomiting | 2.38 ± 1.45 | 2.98 ± 1.46 | 3.06 ± 0.98 | 3.86; 0.023 |
| Oversedation | 3.0 ± 1.53 | 4.48 ± 0.99 | 4.29 ± 0.91 | 24.26; <0.001 |
| Addiction | 4.40 ± 1.27 | 4.66 ± 0.81 | 4.71 ± 0.72 | 1.41; 0.249 |
| Poor pain relief | 2.97 ± 1.40 | 3.47 ± 1.37 | 3.38 ± 1.23 | 2.19; 0.116 |
| Analgesic self efficacy (mean ± SD; possible range: 0–35) | ||||
| Perceived self efficacy | 28.34 ± 5.7 | 31.48 ± 4.1 | 32.32 ± 3.73 | 9.85; <0.001 |
ANOVA: Analyses of variance.
Analgesic self efficacy
Table 3 describes parental perceptions of analgesic self efficacy at baseline and post- intervention times. One-way analyses of variance demonstrated that the change in self efficacy ratings increased significantly from Baseline to Time 1 and Time 2 follow-up assessments, but no change from Time 1 to Time 2.
Opioid decision-making
There was no difference from baseline to follow-up in participant decisions to give an opioid to the hypothetical child with oversedation (27 vs 30.3%, respectively; OR 1.2 [95% CI: 0.48, 3.04]) or with no adverse drug effect (63 vs 73%; OR: 1.6 [0.64, 4.01]). Notably, 79% of parents who had treated a child with an opioid previously chose to give the prescribed opioid to the hypothetical child with no ORAE compared with only 53% of those who had never treated a child with an opioid (OR: 3.44 [1.07–11.01]; p = 0.033). Similarly, a majority (87%) of those who reported having an opioid in the home at the time of the baseline survey made the hypothetical decision to give an opioid for this scenario compared with only 55% who did not have an opioid at home (OR: 5.3 [1.08–26.01]; p = 0.034). There were no relationships between past opioid use, opioid presence in the home, perceptions of ORAE seriousness, self efficacy or ORAE risk perceptions on either hypothetical decision to give or withhold an opioid in this small sample. Parental ratings of the qualities of the educational program were excellent (Table 4).
Table 4. . Parental ratings of the qualities of the program.
| Qualities | Agree or strongly agree |
|---|---|
| The program was easy to use | 57 (98%) |
| The program improved my skill in assessing my child’s pain | 51 (89%) |
| The program helped me to recognize important symptoms | 56 (97%) |
| The program improved my skill in making safe and effective decisions about pain medicines | 55 (95%) |
| The program features supported my learning | 53 (91%) |
Discussion
This study demonstrated that ORAE information given via an interactive educational web-based platform effectively increased parents’ perceived ability to manage their children safely with analgesics (i.e., increased self efficacy) and their awareness of possible ORAEs. Furthermore, the intervention increased parents’ risk perceptions for the targeted and potentially serious ORAE, oversedation, without impacting risk perceptions of other less-serious ORAEs (e.g., nausea). Despite these outcomes, there was no effect of the intervention on parental decisions to give or withhold an opioid for the hypothetical high-risk and low-risk scenarios. These findings suggest that the interactive web-based application has the potential to improve parental knowledge of opioid risks. Yet, modifications may be needed to improve safe and effective decision-making among parents whose children are prescribed opioids for a painful condition.
We previously demonstrated that factual knowledge or awareness of ORAEs alone (i.e., the fact that oversedation is a possible opioid effect) was insufficient for many parents to recognize important symptoms of oversedation and to stop giving a prescribed opioid when this first sign of toxicity was present [34]. Notably, we also previously found that increased perception of the seriousness of oversedation was associated with the appropriate decision to withhold opioids when this serious ORAE was present. Although our prototype intervention improved parental knowledge and the targeted serious risk perception (i.e., oversedation), the information did not significantly improve the decision about when to withhold an opioid. Recent data suggest that specific, interactive and scenario-guided instruction about what to do when pain and potentially serious ORAEs manifest may improve both risk perception and decision-making [39]. Information provided by the prototype here did offer suggestions regarding safe use and handling of opioids, so differences in outcomes may be related to the small sample size in the present study, or, perhaps, the nature of the information and interactive exercises. For instance, in the previous study, interactivity included analgesic decision-making, whereas in this prototype the interactivity was not related to decision-making. Enhancing the program with guided decisional instruction for each type of risk situation (i.e., oversedation and high pain) such as that shown to be recently effective toward decisional outcomes, may better help to improve decision-making.
Safe and effective treatment of pain with opioid analgesics entails appropriate utilization of risk knowledge and perceptions (i.e., knowing which ORAEs are most serious) and decision-making skill (i.e., knowing when it is okay to give a prescribed opioid and when these drugs should be withheld). Effective pain management, thus, involves a complex decision-making process that may benefit from targeted risk information and advice. Interestingly, parents in this study gained a sense of confidence in their ability to manage their children’s pain safely and effectively (i.e., improved self efficacy). However, this confidence was not reflected in better decision-making following the intervention. This discordant finding may have been influenced in part by past or present experience and comfort with prescribed opioids, since we also found that parents who had treated their children with opioids and who had an opioid in the house prior to study participation were significantly more likely to give an opioid when presented with hypothetical scenarios. Additionally, we did not record the ages of children in the household, and age could have a potential effect on parental decision-making. Importantly, simplistic ORAE information may improve risk awareness but may leave parents with uncertainty about what actions to take to avoid important risk while effectively managing pain. Incorporating guided advice into the next version of the app prototype may better enhance parental decision-making.
Although this preliminary study found that the prototype improved parental knowledge, these findings have several limitations. First, a selection bias is quite possible given the nature of convenience sampling self-selection. Parents who chose to take part may have had a high interest in managing pain as evident by high rates of analgesics in the home at baseline. Furthermore, this study used a pre- and post-intervention design to assess the outcomes, and these may have been influenced by other interim confounders. That we found an immediate effect prior to any subsequent patient teaching lessens the potential for confounding information. However, a subsequent randomized controlled trial is necessary to reduce the potential impact of selection bias and confounders. Next, the sample size was large enough to detect significant effects of the intervention on risk perception and efficacy, but was insufficient to show an effect on decision-making. Consideration of sample size in future studies will need to consider all important outcomes to better examine main and secondary effects. Further, our sample was primarily comprised of white, highly educated mothers and may therefore not reflect broader populations of parents who manage pain in their children. Testing the prototype among older adolescents who begin to make pain management decisions for themselves will also be needed to examine whether this app could translate beyond parents. Finally, although hypothetical decisions have been found to correlate with real-life decisions in multiple studies, it will be important to examine the effects of a refined pain management app on actual analgesic decisions to determine the clinical impact on safety and effectiveness.
Conclusion
In summary, the web-based pain program used in this study was found to effectively improve parent analgesic self efficacy and ORAE knowledge and risk perception. However, our preliminary findings suggest a need for program enhancements and study in a larger, heterogeneous group of parents in order to determine effectiveness on pain and symptom management decision-making.
Future perspective
In future, most preventive health education will be provided to patients via smartphone apps or enhanced web-based platforms. The use of the internet and phone apps has become ubiquitous in our society. Giving patient education material via these platforms better engages patients and facilitates easy updates and symptom guided instruction. Pain management can be complex where patients have to balance risks and benefits of treatments as they navigate pain and related symptom management. Web-based platforms thus can be used to enhance complex decision-making by addressing multiple related symptoms at the touch of an icon.
Summary points.
Interactive web-based analgesic information can enhance parental knowledge of important risks of prescribed analgesics.
In this study, web-based information selectively enhanced the perception of risk of the potentially serious opioid-related adverse effect (i.e., oversedation).
Completing the web-based educational program enhanced parents’ perceived self efficacy about managing pain in their children with analgesics.
Findings support the use of the interactive web-based program to enhance parents’ knowledge and perceptions of important prescription opioid risks; however, enhancements are needed to guide safe analgesic decisions.
Acknowledgments
The authors would like to recognize the assistance of M Weber, who helped with subject identification and training of research assistants.
Footnotes
Author contributions
T Voepel-Lewis and AR Tait were responsible for the study design, methods, analyses, data interpretation, manuscript preparation and editing. A Belcher was responsible for subject recruitment, data entry and manuscript review/editing. R Levine assisted with study design, beta testing and manuscript editing.
Financial & competing interests disclosure
R Levine is the President and Chief Medical Officer and President of ArchieMD, Inc. but was funded independently for this project by a grant from the National Institute on Drug Addiction (NIDA; R43 DA042645-01). R Levine was responsible for the development of the interactive program but had no involvement in subject recruitment, data collection, analysis or interpretation of the data. None of the other investigators have any financial, commercial or other interests in ArchieMD, Inc. T Voepel-Lewis is also funded by a grant from NIDA (R01 DA044245). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical disclosure
This study was reviewed and approved by the Institutional Review Board at the University of Michigan Medical School (IRBMED, HUM#00119290) with verbal consent from parents.
References
Papers of special note have been highlighted as: • of interest
- 1.Chung CP, Callahan ST, Cooper WO et al. Outpatient opioid prescriptions for children and opioid-related adverse events. Pediatrics 142, e20172156 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Highlights the prevalence of opioid-related adverse events and their relationship with therapeutic error.
- 2.Duedahl TH, Hansen EH. A qualitative systematic review of morphine treatment in children with postoperative pain. Paediatr. Anaesth. 17, 756–774 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Sutters KA, Miaskowski C, Holdridge-Zeuner D et al. A randomized clinical trial of the efficacy of scheduled dosing of acetaminophen and hydrocodone for the management of postoperative pain in children after tonsillectomy. Clin. J. Pain 26, 95–103 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregorian RS Jr, Gasik A, Kwong WJ, Voeller S, Kavanagh S. Importance of side effects in opioid treatment: a trade-off analysis with patients and physicians. J. Pain 11, 1095–1108 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Sutters KA, Holdridge-Zeuner D, Waite S et al. A descriptive feasibility study to evaluate scheduled oral analgesic dosing at home for the management of postoperative pain in preschool children following tonsillectomy. Pain Med. 13, 472–483 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bond GR, Woodward RW, Ho M. The growing impact of pediatric pharmaceutical poisoning. J. Pediatr. 160, 265–270, e261 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Burghardt LC, Ayers JW, Brownstein JS et al. Adult prescription drug use and pediatric medication exposures and poisonings. Pediatrics 132, 18–27 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilchrist J, Ballesteros M. Vital signs: unintentional injury deaths among persons aged 0–19 years – United States, 2000–2009. Centers for Disease Control and Prevention, GA, USA: (2012). https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6115a5.htm [Google Scholar]
- 9.Cohen AL, Budnitz DS, Weidenbach KN et al. National surveillance of emergency department visits for outpatient adverse drug events in children and adolescents. J. Pediatr. 152, 416–421 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Kelly LE, Rieder M, van den Anker J et al. More codeine fatalities after tonsillectomy in North American children. Pediatrics 129, e1343–e1347 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Madadi P, Hildebrandt D, Gong IY et al. Fatal hydrocodone overdose in a child: pharmacogenetics and drug interactions. Pediatrics 126, e986–e989 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Coté CJ, Posner KL, Domino KB. Death or neurologic Injury after tonsillectomy in children with a focus on obstructive sleep apnea: Houston, we have a problem! Anesth. Analg. 118, 1276–1283 (2014). [DOI] [PubMed] [Google Scholar]; • Highlights the problem of opioid-related oversedation in high-risk patients and the potential for missed recognition in the home setting.
- 13.Hamers JP, Abu-Saad HH. Children’s pain at home following (adeno) tonsillectomy. Eur. J. Pain 6, 213–219 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Unsworth V, Franck LS, Choonara I. Parental assessment and management of children's postoperative pain: a randomized clinical trial. J. Child Health Care 11, 186–194 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Clark S, Singer D, Matos-Moreno A et al. Narcotics in the medicine cabinet: provider talk is key to lower risk. Report no. 4. C.S. Mott Children's Hospital, University of Michigan, MI, USA: (2016). https://mottpoll.org/reports-surveys/narcotics-medicine-cabinet-provider-talk-key-lower-risk [Google Scholar]
- 16.Kennedy-Hendricks A, Gielen A, McDonald E et al. Medication sharing, storage, and disposal practices for opioid medications among US adults. JAMA Intern. Med. 176, 1027–1029 (2016). [DOI] [PubMed] [Google Scholar]
- 17.McDonald EM, Kennedy-Hendricks A, McGinty EE et al. Safe storage of opioid pain relievers among adults living in households with children. Pediatrics 139 (2017). https://pediatrics.aappublications.org/content/139/3/e20162161.abstract [DOI] [PubMed] [Google Scholar]
- 18.Zandieh SO, Goldmann DA, Keohane CA et al. Risk factors in preventable adverse drug events in pediatric outpatients. J. Pediatr. 152, 225–231 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Zed PJ, Black KJL, Fitzpatrick EA et al. Medication-related emergency department visits in pediatrics: a prospective observational study. Pediatrics 135, 435–443 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Kankkunen P, Vehvilainen-Julkunen K, Pietila A-M, Kokki H, Halonen P. Parents’ perceptions and use of analgesics at home after children’s day surgery. Paediatr. Anaesth. 13, 132–140 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Rony RY, Fortier MA, Chorney JM, Perret D, Kain ZN. Parental postoperative pain management: attitudes, assessment, and management. Pediatrics 125, e1372–e1378 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Tait AR, Voepel-Lewis T, Snyder RM, Malviya S. Parents’ understanding of information regarding their child’s postoperative pain management. Clin. J. Pain 24, 572–577 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donovan JL, Blake DR. Patient non-compliance: deviance or reasoned decision-making? Soc. Sci. Med. 34, 507–513 (1992). [DOI] [PubMed] [Google Scholar]
- 24.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst. Rev. CD000011 (2008). https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000011.pub3/epdf/full [DOI] [PubMed] [Google Scholar]
- 25.Hugtenburg JG, Timmers L, Elders PJ, Vervloet M, van Dijk L. Definitions, variants, and causes of nonadherence with medication: a challenge for tailored interventions. Patient Prefer. Adherence 7, 675–682 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieuwlaat R, Wilczynski N, Navarro T et al. Interventions for enhancing medication adherence. Cochrane Database Syst. Rev. 11, CD000011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnipper JL, Kirwin JL, Cotugno MC et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch. Intern. Med. 166, 565–571 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Wroe A, Thomas M. Intentional and unintentional nonadherence in patients prescribed HAART treatment regimens. Psychol. Health Med. 8, 453–463 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Wroe AL. Intentional and unintentional nonadherence: a study of decision making. J. Behav. Med. 25, 355–372 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Verghese ST, Hannallah RS. Acute pain management in children. J. Pain Res. 3, 105–123 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong I, St John-Green C, Walker SM. Opioid-sparing effects of perioperative paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) in children. Paediatr. Anaesth. 23, 475–495 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tait AR, Voepel-Lewis T, Chetcuti SJ, Brennan-Martinez C, Levine R. Enhancing patient understanding of medical procedures: evaluation of an interactive multimedia program with in-line exercises. Int. J. Med. Inform. 83, 376–384 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voepel-Lewis T, Zikmund-Fisher B, Smith EL, Zyzanski S, Tait AR. Opioid-related adverse drug events: do parents recognize the signals? Clin. J. Pain 31, 198–205 (2015). [DOI] [PubMed] [Google Scholar]; • Highlights the potential lack of recognition of serious opioid-related adverse events among parents whose children are prescribed opioids.
- 34.Voepel-Lewis T, Zikmund-Fisher BJ, Smith EL et al. Parents’ analgesic trade-off dilemmas: how analgesic knowledge influences their decisions to give opioids. Clin. J. Pain 32, 187–195 (2016). [DOI] [PubMed] [Google Scholar]; • Demonstrates that prescription opioid risk perceptions and not awareness, alone are important toward safe and effective opioid dosing decisions.
- 35.Voepel-Lewis T, Zikmund-Fisher BJ, Smith EL, Zyzanski S, Tait AR. Parents’ preferences strongly influence their decisions to withhold prescribed opioids when faced with analgesic trade-off dilemmas for children: a prospective observational study. Int. J. Nurs. Stud. 52, 1343–1353 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Schwarzer R. Self-efficacy: Thought Control of Action. Routledge, Taylor & Francis Group, NY, USA: (1992). [Google Scholar]
- 37.Demyttenaere S, Finley GA, Johnston CC, McGrath PJ. Pain treatment thresholds in children after major surgery. Clin. J. Pain 17, 173–177 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Tait AR, Zikmund-Fisher BJ, Fagerlin A, Voepel-Lewis T. Effect of various risk/benefit trade-offs on parents’ understanding of a pediatric research study. Pediatrics 125, e1475–e1482 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voepel-Lewis T, Zikmund-Fisher BJ, Boyd CJ et al. Effect of a scenario-tailored opioid messaging program on parents’ risk perceptions and opioid decision-making. Clin. J. Pain 34, 497–504 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Shows how risk messages can selectively enhance prescription opioid risk knowledge and perceptions leading to safer dosing decision-making.