Abstract
Major advances have been made in coronary artery stent technology over the last decades. Drug-eluting stents reduced in-stent restenosis and have shown better outcomes compared with bare metal stents, yet some limitations still exist to their use. Because they delay healing of the vessel wall, longer dual antiplatelet therapy is mandatory to mitigate against stent thrombosis and this limitation is most concerning in subjects at high risk for bleeding. The COBRA PzF nanocoated coronary stent has been associated with accelerated endothelialization relative to drug-eluting stents, reduced inflammation and thromboresistance in preclinical studies, suggesting more flexible dual antiplatelet therapy requirement with potential benefits especially in those at high bleeding risk. Here, we discuss the significance of COBRA PzF in light of recent experimental and clinical studies.
Keywords: : COBRA PzF stent, healing, high bleeding risk, oral anticoagulation, Polyzene F, stent
Lay abstract
Coronary artery disease occurs when the inner walls of the coronary arteries thicken due to plaque build-up. As the plaque develops, the inside of the artery narrows, and blood flow to the heart muscle is restricted. Coronary stents act as a miniature circular scaffolding that flexes to fit the shape of the artery. In the COBRA PzF nanocoated coronary stent, Polyzene F nanocoating (PzF) has been applied to the stent’s surface. Experimental studies have shown that PzF nanocoatings promote healing after stent implantation and reduce the attachment of platelets and inflammatory cells. The principal safety and effectiveness for the COBRA PzF stent has been proven by clinical studies, showing that the COBRA PzF stent was as safe and effective as other approved bare metal coronary stents. Here, we summarize the findings of experimental and clinical studies with the COBRA PzF stent and discuss potential future directions of anti-platelet and anti-coagulant medications following COBRA PzF stent implantation.
Evolution of stent devices
Percutaneous coronary intervention (PCI), combined with stent implantation, remains the gold standard for treatment of highly stenosed coronary arteries. Drug-eluting stents (DES) have reduced the risk of in-stent restenosis (ISR) compared with bare metal stents (BMS), however, they have been associated with a prolonged arterial healing process [1]. Ever since their introduction in the early 2000s, three generations of DES have followed each other, with improvements in biocompatibility, stent platform, antiproliferative agent and polymer coatings.
Strut thickness is known to be an important determinant of neointimal proliferation and thrombogenicity [2,3]. The ISAR-STEREO trial including 651 patients with symptomatic coronary artery disease who were randomly assigned to receive stents with similar designs, but differences in strut thickness (ACS Multi-Link RX Duet, strut thickness 140 μm, or ACS RX Multi-Link, strut thickness 50 μm), showed a significantly lower incidence of 6-month angiographic ISR in the thin-strut group versus the thick-strut group (15.0 vs 25.8%; relative risk, 0.58 [95% CI: 0.39–0.87], p = 0.003) [3]. Similar results were reported when comparing angiographic ISR after implantation of thin-strut stents (ACS RX Multi-Link, strut thickness 50 μm) to thick-strut stents of a different design (BX Velocity, strut thickness 140 μm) (relative risk of ISR, 0.57 [95% CI: 0.39–0.84], p < 0.05) [4]. Preclinical studies reported an increased thrombogenicity of thick-strut stents [5,6]. In addition, thicker strut dimensions impair maneuvering and crossing of the device. A recent study including 506 patients with small vessel lesions (i.e., vessel diameters ≤2.5 mm) who were retrospectively evaluated and divided into two groups according to stent strut thickness (74 vs 81 μm) reported a lower incidence of target lesion failure in the 74 μm-group (4.3 vs 9.8%, p = 0.042) at 16 months, suggesting that even small differences in strut thickness can have an important impact on neointimal formation [7]. Therefore, second- and third-generation DES have been designed with thinner strut stent platforms, most commonly using a cobalt-chromium (CoCr) alloy, which offers improved flexibility and deliverability.
DES are covered with cytostatic drugs (e.g., paclitaxel, sirolimus or everolimus) which are delivered to the vascular wall in order to inhibit proliferation of vascular smooth muscle cells. Although these drugs have evolved over the last two decades, exhibiting enhanced biocompatibility, improved kinetics, and wider therapeutic index, they are equally cytotoxic to vascular smooth muscle cells and vascular endothelial cells. Rapid endothelial regrowth is an important determinant of vascular healing, and stent struts lacking endothelialization have been associated with late and very late stent thrombosis, which occurs even in second-generation DES [8]. Therefore, recent efforts have been made to develop drug-free devices.
Polymer coatings are responsible for controlling drug release in DES. However, durable polymers of first-generation DES, such as poly(styrene-b-isobutylene-b-styrene), polyethylene-co-vinyl acetate and poly-n-butyl methacrylate, have been associated with enduring inflammatory responses at the implantation site, leading to delayed endothelialization, late acquired malapposition and neointimal proliferation [9–11]. Significantly reduced inflammatory reactions have been observed with polymers in second-generation DES. Especially fluoropolymers, as used in Xience® CoCr everolimus-eluting stent (CoCr-EES), have been associated with superior biocompatibility. Fluoropolymer-coated stents had significantly less thrombosis and platelet adhesion compared with their bare metal counterparts in experimental studies of Kolandaivelu et al. [12]. Furthermore, endothelialization of fluoropolymer-coated surfaces was similar to control surfaces in vitro [13]. When evaluating the very late (≥1 year) pathologic responses to stent implantation in 92 autopsy cases, our group observed less inflammation and lower neointimal formation with fluoropolymer-coated CoCr-EES compared with first-generation DES and BMS [14]. These data suggest that long-term vascular responses are favorable in fluoropolymer-coated DES and perhaps even superior to BMS. Recently, efforts have been made to replace permanent polymers with biodegradable materials or even to forgo polymer coating. Numerous clinical studies have demonstrated noninferiority of biodegradable polymer DES versus durable polymer first- and second-generation DES [15–23]. Still, the extraordinary biocompatibility of fluoropolymers challenges the dogma that durable polymers are a major source of thrombotic complications and should be avoided.
The COBRA PzF stent took advantage of all major improvements in stent design, combining a thin-strut CoCr stent platform, coated with a nanothin fluoropolymer in the absence of antiproliferative agents.
Description of the COBRA PzF Stent
The COBRA PzF coronary stent device is characterized by ultrathin (71 μm) cobalt-chromium struts, coated by a nanothin (≤50 nm thickness) layer of Polyzene-F® polymer (PzF; CeloNova BioSciences, Inc., TX, USA) with a modified open cell design (Figure 1; Table 1). It received CE Mark approval in 2012 and was launched in Europe and in the Middle East in 2013. It was approved by the US FDA in 2017. The COBRA PzF stent is premounted on a rapid-exchange balloon delivery catheter and is available in sizes ranging from 2.5 to 4.0 mm in diameter and 8–30 mm in length.
Figure 1. . The COBRA PzF nanocoated stent system.
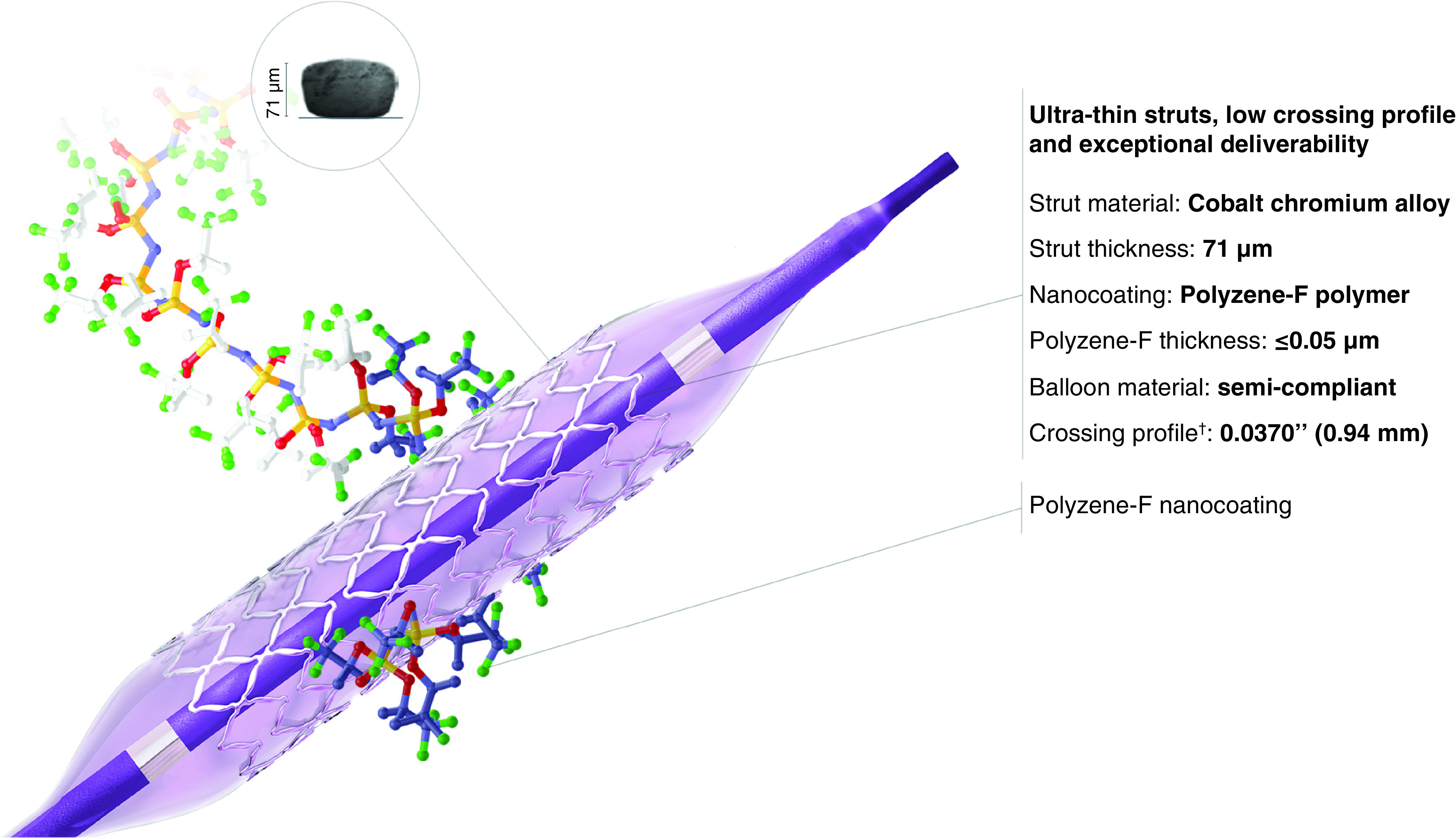
COBRA PzF is a coronary stent device characterized by ultrathin (71 μm) cobalt-chromium struts, coated by a nanothin (≤50 nm thickness) layer of Polyzene-F® with a modified open cell design.
Reproduced with permission from CeloNova (https://celonova.com/cobra-pzf-nanocoated-stent/).
Table 1. . Characteristics of the coated COBRA-PzF stent.
| Manufacturer | CeloNova |
|---|---|
| Stent material | L-605 cobalt-chromium CoCr alloy |
| Polymer coating | Polyzene-F (poly-bis[trifluoroethoxy] phosphazene) |
| Strut thickness | 71 μm |
| Polymer thickness | 0.050 μm |
| Drug component | – |
| Available sizes | Diameter: 2.5–4.0 mm Length: 8–30 mm |
CoCr: Cobalt–chromium.
PzF (poly[bis[trifluoroethoxy]phosphazene) polymer is a soft rubber-like inorganic, high molecular weight fluoropolymer that possesses a backbone of alternating nitrogen and phosphorus atoms and trifluoroethoxy side groups [24]. Early work by Welle et al. showed PzF coating results in hydrophobic surface properties, causing high adsorption of serum albumin, but low adsorption of fibronectin and fibrinogen [25]. PzF polymer is stable in contact with blood and retains its mechanical properties over at least 24 months [24]. In addition, the trifluoroethanol component is known to have anti-inflammatory properties and to stabilize proteins in solution against thermal denaturation [26]. In pilot experiments by Richter et al., PzF polymer surface modification of vascular stents improved thromboresistance and reduced late ISR compared with BMS in a rabbit iliac artery model [26]. Likewise, PzF-nanocoated CoCr stents implanted in the right coronary artery of 30 mini-pigs showed a significantly lower average loss in lumen diameter (2.1 ± 3.05%) compared with bare CoCr stents (9.73 ± 4.93%) and PzF-nanocoated stainless steel stents (9.71 ± 7%; p = 0.04) [27]. Surprisingly, drug-free PzF-nanocoated stents had significantly less late ISR compared with paclitaxel-coated DES (0.3 ± 0.3 mm vs 0.8 ± 0.2 mm in stents coated with an intermediate dose of paclitaxel and 1.5 ± 0.6 mm in high-dose stents; p = 0.04) and showed less inflammation (Kornowski scores of 0.2 ± 0.1 in drug-free stents, 1.7 ± 0.8 in intermediate-dose stents, and 1.3 ± 1.0 in high-dose stents; p = 0.04) [28].
Optimizing the interface with blood proteins and suggesting enhanced biocompatibility in the absence of anti-proliferative drugs, COBRA PzF was designed to reduce thrombogenicity and to foster endothelialization.
Preclinical studies with the COBRA PzF device
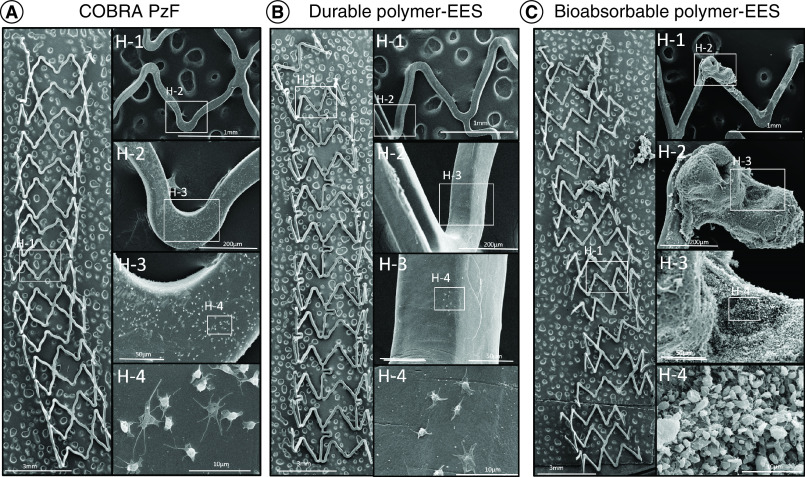
The safety profile of the COBRA PzF stent was investigated in various animal and in vitro studies (Table 2). Experiments in the porcine coronary artery model showed that compared with BMS, the COBRA PzF stent exhibited significantly larger lumen areas, lower neointimal thickness, and %stenosis, both at 28 days and at 90-days follow-up [29]. Furthermore, inflammation was reduced with COBRA PzF devices. These findings were corroborated by reduced monocyte adhesion and thrombus formation compared with BMS in ex vivo swine carotid-jugular arterio-venous shunt models (Figure 2) [29].
Table 2. . Preclinical studies investigating COBRA-PzF.
| Study | Publication year | Models used | Findings | Ref. |
|---|---|---|---|---|
| Koppara et al. | 2016 | Porcine coronary artery model | Similar endothelial coverage but lower neointimal thickness and reduced inflammation in COBRA-PzF compared with BMS | [29] |
| Porcine ex vivo shunt model | Lower stent surface area occupied by platelet aggregates as compared with BMS | |||
| Human monocyte adhesion assay | Lower human monocyte/macrophage and giant cell adherence on COBRA-PzF as compared with BMS | |||
| Cytokine content in monocyte supernatant | Lower levels of IL-4, IL-10 and IL12p40 in the supernatant of monocytes attached to COBRA-PzF | |||
| Jinnouchi et al. | 2019 | Porcine ex vivo shunt model | Less clots in COBRA-PzF compared with durable-polymer DES and bioabsorbable-polymer DES Similar inflammatory cell adhesion in COBRA-PzF and durable polymer DES, but lower inflammatory cell adhesion in COBRA compared with bioabsorbable polymer DES |
[30] |
| Rabbit iliac artery model | Greater endothelial coverage in COBRA-PzF compared with durable-polymer DES and bioabsorbable-polymer DES | |||
| Maillard et al. | 2020 | Rabbit iliac artery model | Nearly complete endothelial coverage of COBRA-PzF at 7 days after stent implantation | [31] |
BMS: Bare metal stent; DES: Drug-eluting stent.
Figure 2. . Representative SEM images in a swine arteriovenous shunt model.
(A–C) The left images of each panel are representative high power (15× magnification) images of COBRA PzF (A), durable polymer EES (B) and bioabsorbable polymer EES (C), the right images are high-power views. COBRA-PzF and durable EES showed minimal platelet aggregation on the strut surfaces, whereas visible clot formation occurred in bioabsorbable polymer EES.
EES: Everolimus-eluting stent; SEM: Scanning electron microscopy.
Reproduced with permission from [30].
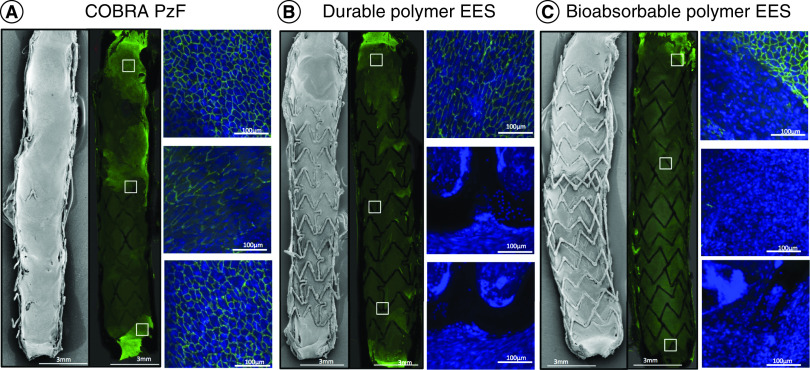
While the same study demonstrated a similar endothelial coverage over luminal surfaces in the COBRA PzF stent and BMS [29], our group recently demonstrated enhanced endothelial coverage with COBRA PzF stents compared with contemporary conventional DES in the rabbit iliac artery model [30]. At 14 days, the COBRA PzF stent showed significantly greater endothelial strut coverage compared with bioabsorbable polymer DES and compared with durable polymer DES (99.0% vs 29.6% vs 17.7%; p < 0.001), as assessed by scanning electron microscopy imaging (Figure 3) [30]. Furthermore, endothelial barrier protein expression, assessed by immunofluorescent staining for the p120/vascular endothelial cadherin complex, was significantly higher in the COBRA PzF stent compared with bioabsorbable polymer and durable polymer DES (82.6% vs 14.4% vs 12.8%; p ≤ 0.001). Likewise, Evans blue uptake, a marker of leaky endothelium/incomplete endothelial barrier function, was least in COBRA PzF stent when compared with durable polymer and bioabsorbable polymer DES (22.0% vs 70.0% vs 66.4%; p = 0.03). We also evaluated the acute inflammatory potential of the COBRA PzF stent in comparison with contemporary DES in a porcine arteriovenous shunt model by immunofluorescent staining against a neutrophil marker (PM-1) and a monocyte marker (CD14). Inflammatory cell adhesion was similar between COBRA PzF stents and durable polymer DES, but significantly lower in COBRA PzF stents compared with bioabsorbable polymer DES [30].
Figure 3. . Superior healing with COBRA PzF.
Representative SEM and confocal microscopy images with immunofluorescent staining against CD31 (green), showing the inner surface of stented rabbit iliac arteries, stented with (A) COBRA PzF, (B) durable polymer EES and (C) bioabsorbable polymer EES. The boxed areas of the low-power images indicate areas of high-power (20×) views. CD31-positive area, indicating endothelialization, is greater in COBRA PzF compared with durable polymer EES and bioabsorbable polymer EES.
EES: Everolimus-eluting stent; SEM: Scanning electron microscopy.
Reproduced with permission from [30].
A recent study evaluated the time course of re-endothelialization with the COBRA PzF stent and reported nearly complete coverage of the stented area at 7 days after stent implantation (99.54 ± 0.25%) in the rabbit iliac artery model, which is the fastest re-endothelialization ever reported in the literature of coronary stents [31].
Clinical studies with the COBRA PzF device
Assessment of The Latest Non-Thrombogenic Angioplasty Stent Trial (ATLANTA) and ATLANTA 2 were first-in-man single-arm studies investigating safety and efficacy of a PzF-nanocoated stent, using the Catania™ stent system (CeloNova BioSciences), a predecessor of the COBRA PzF stent [32]. Clinically driven target lesion revascularization at 12 months was 3.6% (two of 55 patients with symptomatic ischemic heart disease), with no deaths, strokes or myocardial infarctions in ATLANTA [32]. Target lesion revascularization rate at 12 months was 6.5% and no late stent thrombosis was recorded up to 12 months, even though dual antiplatelet therapy (DAPT) was prescribed for only 1 month post procedure (unless patients had a myocardial infarction) in the 300 patients recruited for ATLANTA 2 [33]. Adding to these promising results, a substudy in 15 patients from ATLANTA reported 99.5% covered struts, assessed by optical coherence tomography, at 6 months follow-up [34].
The first clinical study reporting procedural and 1-year clinical outcomes following COBRA PzF coronary stent implantation enrolled 100 real-world patients (71% men, mean age 71.4 ± 11.0 years), 38% of whom had acute coronary syndromes and 26% of whom had multivessel disease (Table 3) [35]. Target vessel failure rate as a composite of all-cause mortality, myocardial infarction or target vessel revascularization was 12%, including 2% mortality, 5% periprocedural myocardial infarction (according to the definition proposed by the Academic Research Consortium [36]), and 5% target lesion revascularization. These rates compared favorably to those seen with other commercially available BMS in the USA [37–39]. Of note, there were no cases of definite stent thrombosis.
Table 3. . Clinical studies with COBRA-PzF.
| Study | Publication year | Study participants | Follow-up duration | Study design | End point findings | Ref. |
|---|---|---|---|---|---|---|
| Maillard et al. | 2017 | 100 patients (38% with ACS); 151 lesions | 1 year | Single-center study | Primary end point: target vessel failure 12%, including 2% mortality, 5% periprocedural MI and 5% target lesion revascularization |
[35] |
| Cutlip et al. | 2017 | 296 patients (31% with ACS); 300 lesions | 9 months | Multi-center study | Primary end point: target vessel failure 11.5%, including 0.3% cardiac death, 7.0% MI (1% spontaneous MI), 5.9% target vessel revascularization Secondary end point: angiographic in-stent late lumen loss 0.84 ± 0.48 mm |
[40] |
| Maillard et al. | 2020 | 940 patients (47% with ACS; 62% with high bleeding risk); 1229 lesions | 1 year | Multi-center study | Primary end point: Major adverse cardiac event rate of 9.0%, including 3.7% cardiac death, 4.8% MI, 4.3% target lesion revascularization Secondary end point: definite stent thrombosis in 0.7% |
[41] |
| Maillard et al. | 2020 | 77 patients with high bleeding risk (18.2% with ACS); 120 lesions | 1 year | Single-center study | Primary end point: treatment with clopidogrel for 1 month, followed by aspirin monotherapy did not result in stent thrombosis Secondary end point: major adverse cardiac events rate of 3.8%, including 0% cardiac death, 0% MI, 3.8% target lesion revascularization |
[42] |
ACS: Acute coronary syndrome; MI: Myocardial infarction.
Likewise, the multicenter, prospective, single-arm, nonrandomized PzF Shield (COBRA PzF Stent in Native Coronary Arteries for Early Healing, Thrombus Inihibition, Endothelialization and Avoiding Long-Term Dual Anti-Platelet Therapy) trial, performed in the USA and Europe with 296 patients (70% men, mean age 66 ± 10 years) reported target vessel failure rates (defined as a composite of cardiac death, myocardial infarction, or clinically driven target vessel revascularization) of 11.5% at 9 months, and a mean late lumen loss of 0.84 ± 0.48 mm [40]. In addition, clinically driven TLR was 4.6% and no case of stent thrombosis was reported.
Considering the antithrombotic properties of the COBRA PzF stent reported from preclinical studies with enhanced endothelialization and low adhesion of platelets and inflammatory cells, and in light of only two subacute stent thrombosis events and no late stent thrombosis among 650 patients receiving either the Catania or COBRA PzF stent [32,33,35,40], the COBRA PzF stent has been suggested specifically for the treatment of patients with high bleeding risk.
Significance of COBRA PzF in patients with high bleeding risk?
Following stent implantation, DAPT, consisting of aspirin in combination with an ADP receptor P2Y12 inhibitor, is required until endothelialization of the inner stent surface is complete to mitigate against stent thrombosis and ischemic events. While endothelialization of BMS in humans is complete at 1 month after implantation, DES are not fully covered with endothelial cells until 6–12 months [1]. Thus, following PCI for stable coronary artery disease, a minimum duration of DAPT of 1 month is recommended for patients treated with BMS, whereas the default guideline recommendation for DAPT duration after DES implantation is 6–12 months [43,44]. For many years, patients who could not receive DAPT for more than 1 month because of active bleeding, nonadherence to medical therapy, or planned surgery, have preferably been treated with BMS. However, second-generation DES have proved to be safer than BMS even with respect to ST [45], and recent studies suggest shorter durations (as little as 1 month) of DAPT for newer-generation DES [46–48].
Recently, the multicenter prospective e-COBRA study evaluated safety and efficacy of the COBRA PzF stent in routine clinical practice in patients deemed appropriate for shorter duration of DAPT [41]. The primary end point was MACE (cardiac death, myocardial infarction and target lesion revascularization) at 12 months, the secondary end point was definite stent thrombosis at 12 months. Among 940 patients (72% men, mean age 72.8 ± 13.4 years), 62.5% of whom were considered to have a high bleeding risk and 47% had high ischemic risk (21% STEMI and 26% NSTEMI). DAPT duration was at the principal investigator’s discretion. A total of 6% of patients were on single antiplatelet therapy post PCI. A total of 25 and 70% of patients on oral anticoagulant therapy (OAC) stopped DAPT by 1 and 3 months, respectively. Among those without OAC, 16 and 43% stopped DAPT by 1 and 3 months, respectively. MACE occurred in 9.0% of patients, and definite stent thrombosis was reported in 0.7%, suggesting COBRA PzF may be an alternative when short DAPT or even mono antiplatelet therapy is needed.
A recent prospective, consecutive, observational study investigated the safety of single antiplatelet therapy following COBRA PzF implantation in 77 patients (58.5% men, mean age 78.7 ± 8.89 years) at high bleeding risk [42]. A total of 38 patients (49.3%) were discharged with a combination of clopidogrel and OAC (i.e., coumadin or nonvitamin K oral antagonist [novel oral anticoagulant [NOAC]]), while the other 39 patients (50.7%) were discharged with clopidogrel alone. No patient reached the primary end point of definite stent thrombosis at 1 month (stent thrombosis rate 0.0%). In addition, MACE at 12 months (cardiac death, myocardial infarction and target lesion revascularization) was reported in 3.8%, and no severe bleeding events, strokes, or late stent thromboses were noted. These results compared favorably to those reported with other current devices in older populations [49] or patients with high bleeding risk [47].
Recently, preliminary results of the COBRA-REDUCE trial (Randomized Trial of COBRA PzF Stenting to REDUCE Duration of Triple Therapy; Clinical Trial Registration NCT02594501) were presented at TCT Connect (17 October 2020). This multicenter, prospective, randomized, parallel-group, open-label, assessor-blinded clinical trial, conducted at 59 sites in the USA and Europe, was designed to investigate whether coronary stenting with the COBRA PzF stent followed by 14 days of clopidogrel reduced bleeding without increasing thrombo-embolic events compared with FDA-approved DES followed by 3 or 6 months of clopidogrel in patients taking OAC and aspirin [50].
From a total of 996 patients enrolled (73% male, mean age 75 years), 495 patients were randomized to the COBRA stent group, and 501 patients to DES. 36% of patients were diabetics. All patients had at least one, and 42% had at least two major criteria of high bleeding risk as defined by the Academic Research Consortium for High Bleeding Risk [51]. Preliminary data on 483 patients in the COBRA stent group and 484 patients in the DES control group at 6 months follow-up showed the primary end point, bleeding complications (BARC class ≥2) beyond 14 days until 6 months postrandomization, occurred at 7.5% in the COBRA stent group and 8.9% in the DES group without reaching statistical significance (p = 0.48). While DAPT duration was mandated in the study, novel oral anticoagulant (NOAC) dosing was at the PI’s discretion. Subsequently, significantly more patients in the DES/3–6 months DAPT group received a reduced dose of NOAC while on DAPT (56 vs 46%; p = 0.006). This may have contributed to less bleeding in the DES group. The investigators noted that further exploration and analysis to assess the impact of antithrombotic therapy duration, dosing, and the role of aspirin are underway and will be reported in the final results. Nevertheless, when comparing all bleeding events (BARC 1–5) after randomization, fewer events were observed in the COBRA stent group than in the DES group (13.0 vs 18.3%, p = 0.026).
The co-primary end point, a composite of death, myocardial infarction, stroke and stent thrombosis at 6 months was reported in 7.7% of patients treated with COBRA PzF stents and 14 days of DAPT, compared with 5.2% in the standard DES/3–6 months DAPT group. According to these preliminary data, the COBRA PzF stent did not meet the criteria for noninferiority (p for noninferiority = 0.061). However, aside from seven patients who withdrew consent and seven patients who were lost to follow-up, the 6 months follow-up was still incomplete for 15 patients (nine patients in the COBRA PzF group and six patients in the DES group). Since the p-value was only very narrowly missed, the results of these patients might potentially turn the scales, and the final study results are still to be awaited [52]. In addition, 20% of lesions treated with the COBRA PzF stent were at bifurcations, while it was 15% in the DES control group (p = 0.034). Bifurcation lesion intervention is associated with a higher complication rate compared with nonbifurcation lesions [53], which might have impacted the study results. Indeed, ischemia-driven target lesion revascularization at 6 months follow-up was more frequent in the COBRA PzF group compared with the standard DES control group (3.7 vs 0.9%, p = 0.04).
The final results of COBRA-REDUCE will reveal if in patients on oral anticoagulation 14 days DAPT following implantation of the COBRA PzF stent is non-inferior to 3–6 months DAPT treatment in patients receiving a standard DES. Nevertheless, preliminary data suggest 14 days DAPT following implantation of the COBRA PzF stent is safe, particularly with respect to stent thrombosis which was reported in only 0.6% of patients, corroborating the results of previous clinical studies [35,40,42]. Of note, for both groups the preliminary results of COBRA-REDUCE compare favorably with other trials conducted in patients at high bleeding risk, such as LEADERS FREE [16] and Onyx One [54] where DAPT duration was prescribed for 30 days post PCI. The awaited 1-year results of COBRA-REDUCE will shed light on this and clarify particularly in the DES group if 3 months of DAPT should be applied instead of 30 days for this high bleeding risk population.
Conclusion
The duration of DAPT following stent implantation is subject of ongoing debate. The COBRA PzF nanocoated coronary stent has been associated with accelerated endothelialization relative to drug-eluting stents, reduced inflammation, and thromboresistance in preclinical studies, suggesting more flexible DAPT requirements with potential benefits especially in patients at high bleeding risk. Although the safety of shorter duration of DAPT (i.e., 2 weeks) after COBRA PzF stent implantation has been proven, preliminary results of COBRA-REDUCE do not show a significant reduction of bleeding complications (BARC ≥2) in a high bleeding risk cohort. The p-value for noninferiority to DES/3–6 months DAPT treatment regarding the composite of death, MI, stent thrombosis (definite/probable) and ischemic stroke in this high-risk population was missed very narrowly, yet the final results of the trial are to be awaited.
Future perspective
More confirmative studies are needed to determine whether single anti-platelet therapies in subjects at high bleeding risk receiving COBRA PzF are safe. In addition, the minimum duration of DAPT after COBRA PzF stenting appears to be another area in which further data could help to determine its suitability for such patients.
Executive summary.
Performance of COBRA PzF in clinical & preclinical studies
The unique design of the COBRA PzF stent has been associated with enhanced endothelialization and reduced thromboembolic complications in several clinical and preclinical studies.
Antithrombotic strategies with COBRA PzF
Short-term dual antiplatelet therapy (DAPT) following implantation of the COBRA PzF device has shown to be safe. This is especially important in cases where early termination of DAPT is indispensable.
Relevance of COBRA PzF for patients with high bleeding risk
In cases with high bleeding risk, the COBRA PzF stent should be considered as an alternative for bare metal stent which have been associated with higher restenosis rates and worse outcome.
Future perspective
Final results of COBRA-REDUCE will reveal if treatment with the COBRA PzF stent, followed by 2 weeks of DAPT, is non-inferior to standard drug-eluting stents implantation and 3–6 months of DAPT in a high bleeding risk collective.
Footnotes
Financial & competing interests disclosure
R Virmani and AV Finn have received institutional research support from NIH-HL141425, Leducq Foundation Grant, 4C Medical, 4Tech, Abbott Vascular, Ablative Solutions, Absorption Systems, Advanced NanoTherapies, Aerwave Medical, Alivas, Amgen, Asahi Medical, Aurios Medical, Avantec Vascular, BD, Biosensors, Biotronik, Biotyx Medical, Bolt Medical, Boston Scientific, Canon, Cardiac Implants, Cardiawave, CardioMech, Cardionomic, Celonova, Cerus, EndoVascular, Chansu Vascular Technologies, Childrens National, Concept Medical, Cook Medical, Cooper Health, Cormaze, CRL, Croivalve, CSI, Dexcom, Edwards Lifesciences, Elucid Bioimaging, eLum Technologies, Emboline, Endotronix, Envision, Filterlex, Imperative Care, Innovalve, Innovative, Cardiovascular Solutions, Intact Vascular, Interface Biolgics, Intershunt Technologies, Invatin, Lahav, Limflow, L&J Bio, Lutonix, Lyra Therapeutics, Mayo Clinic, Maywell, MDS, MedAlliance, Medanex, Medtronic, Mercator, Microport, Microvention, Neovasc, Nephronyx, Nova Vascular, Nyra Medical, Occultech, Olympus, Ohio Health, OrbusNeich, Ossio, Phenox, Pi-Cardia, Polares Medical, Polyvascular, Profusa, ProKidney, LLC, Protembis, Pulse Biosciences, Qool Therapeutics, Recombinetics, Recor Medical, Regencor, Renata Medical, Restore Medical, Ripple Therapeutics, Rush University, Sanofi, Shockwave, SMT, SoundPipe, Spartan Micro, Spectrawave, Surmodics, Terumo Corporation, The Jacobs Institute, Transmural Systems, Transverse Medical, TruLeaf, UCSF, UPMC, Vascudyne, Vesper, Vetex Medical, Whiteswell, WL Gore, Xeltis. AV Finn has received honoraria from Abbott Vascular; Biosensors; Boston Scientific; Celonova; Cook Medical; CSI; Lutonix Bard; Sinomed; Terumo Corporation; and is a consultant to Amgen; Abbott Vascular; Boston Scientific; Celonova; Cook Medical; Lutonix Bard; Sinomed. R Virmani has received honoraria from Abbott Vascular; Biosensors; Boston Scientific; Cook Medical; Cordis; CSI; Lutonix Bard; Medtronic; OrbusNeich Medical; CeloNova; SINO Medical Technology; ReCore; Terumo Corporation; W. L. Gore; Spectranetics; and is a consultant to Abbott Vascular; Boston Scientific; Celonova; Cook Medical; CSI; Edwards Lifescience; Lutonix Bard; OrbusNeich Medical; ReCore Medical; Sinomedical Sciences Technology; Surmodics; Terumo Corporation; W. L. Gore; Medtronic; Xeltis. M Barakat is an employee of CeloNova BioSciences. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Joner M, Finn AV, Farb A et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J. Am. Coll. Cardiol. 48(1), 193–202 (2006). [DOI] [PubMed] [Google Scholar]; • Shows for the first time a delayed healing in drug-eluting stents (DES) in humans.
- 2.Lu S, Ng J, Ang H et al. Is there light at the end of the thin-strut tunnel?: in vitro insights on strut thickness impact on thrombogenicity in bioresorbable stents or scaffolds. JACC Cardiovasc. Interv. 11(7), 714–716 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Kastrati A, Mehilli J, Dirschinger J et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation 103(23), 2816–2821 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Pache J, Kastrati A, Mehilli J et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J. Am. Coll. Cardiol. 41(8), 1283–1288 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Koppara T, Cheng Q, Yahagi K et al. Thrombogenicity and early vascular healing response in metallic biodegradable polymer-based and fully bioabsorbable drug-eluting stents. Circ. Cardiovasc. Interv. 8(6), e002427 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Otsuka F, Cheng Q, Yahagi K et al. Acute thrombogenicity of a durable polymer everolimus-eluting stent relative to contemporary drug-eluting stents with biodegradable polymer coatings assessed ex vivo in a swine shunt model. JACC Cardiovasc. Interv. 8(9), 1248–1260 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Franchin L, Piroli F, D'Ascenzo F et al. Impact of stent thickness on clinical outcomes in small vessel and bifurcation lesions: a RAIN-CARDIOGROUP VII sub-study. J. Cardiovasc. Med. (Hagerstown) 22(1), 20–25 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Otsuka F, Vorpahl M, Nakano M et al. Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation 129(2), 211–223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Virmani R, Guagliumi G, Farb A et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 109(6), 701–705 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Nakazawa G, Finn AV, Joner M et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation 118(11), 1138–1145 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Carter AJ, Aggarwal M, Kopia GA et al. Long-term effects of polymer-based, slow-release, sirolimus-eluting stents in a porcine coronary model. Cardiovasc. Res. 63(4), 617–624 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Kolandaivelu K, Swaminathan R, Gibson WJ et al. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation 123(13), 1400–1409 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin-Quee SL, Hsu SH, Nguyen-Ehrenreich KL et al. Endothelial cell recovery, acute thrombogenicity, and monocyte adhesion and activation on fluorinated copolymer and phosphorylcholine polymer stent coatings. Biomaterials 31(4), 648–657 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Mori H, Atmakuri DR, Torii S et al. Very late pathological responses to cobalt-chromium everolimus-eluting, stainless steel sirolimus-eluting, and cobalt-chromium bare metal stents in humans. J. Am. Heart Assoc. 6(11), e007244 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massberg S, Byrne RA, Kastrati A et al. Polymer-free sirolimus- and probucol-eluting versus new generation zotarolimus-eluting stents in coronary artery disease: the intracoronary stenting and angiographic results: test efficacy of sirolimus- and probucol-eluting versus zotarolimus-eluting stents (ISAR-TEST 5) trial. Circulation 124(5), 624–632 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Serruys PW, Farooq V, Kalesan B et al. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. JACC Cardiovasc. Interv. 6(8), 777–789 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Kufner S, Ernst M, Cassese S et al. 10-year outcomes from a randomized trial of polymer-free versus durable polymer drug-eluting coronary stents. J. Am. Coll. Cardiol. 76(2), 146–158 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Serruys PW, Kogame N et al. Final 3-year outcomes of mistent biodegradable polymer crystalline sirolimus-eluting stent versus xience permanent polymer everolimus-eluting stent: insights from the DESSOLVE III all-comers randomized trial. Circ. Cardiovasc. Interv. 13(6), e008737 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Choe JC, Cha KS, Lee JG et al. Long-term outcomes of biodegradable versus second-generation durable polymer drug-eluting stent implantations for myocardial infarction. JACC Cardiovasc. Interv. 13(1), 97–111 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Iglesias JF, Muller O, Heg D et al. Biodegradable polymer sirolimus-eluting stents versus durable polymer everolimus-eluting stents in patients with ST-segment elevation myocardial infarction (BIOSTEMI): a single-blind, prospective, randomised superiority trial. Lancet 394(10205), 1243–1253 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Paradies V, Vlachojannis GJ, Royaards KJ, Wassing J, van der Ent M, Smits PC. Abluminal biodegradable polymer biolimus-eluting versus durable polymer everolimus-eluting stent in patients with diabetes mellitus: 5 years follow-up from the COMPARE II trial. Int. J. Cardiol. 290, 40–44 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Natsuaki M, Kozuma K, Morimoto T et al. Five-year outcome of a randomised trial comparing second-generation drug-eluting stents using either biodegradable polymer or durable polymer: the NOBORI biolimus-eluting versus XIENCE/PROMUS everolimus-eluting stent trial (NEXT). EuroIntervention 14(7), 815–818 (2018). [DOI] [PubMed] [Google Scholar]
- 23.El-Hayek G, Bangalore S, Casso Dominguez A et al. Meta-analysis of randomized clinical trials comparing biodegradable polymer drug-eluting stent to second-generation durable polymer drug-eluting stents. JACC Cardiovasc. Interv. 10(5), 462–473 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Tur D, Koršak V, Vinogradova S et al. Investigation of the thermostability of poly [bis (trifluoroethoxy) phosphazene]. Acta Polymerica 36(11), 627–631 (1985). [Google Scholar]
- 25.Welle A, Grunze M, Tur D. Plasma protein adsorption and platelet adhesion on poly. J. Colloid Interface Sci. 197(2), 263–274 (1998). [DOI] [PubMed] [Google Scholar]
- 26.Richter GM, Stampfl U, Stampfl S et al. A new polymer concept for coating of vascular stents using PTFEP (poly(bis(trifluoroethoxy)phosphazene) to reduce thrombogenicity and late in-stent stenosis. Invest. Radiol. 40(4), 210–218 (2005). [DOI] [PubMed] [Google Scholar]; • Presents pilot experiments on PzF-coated vascular stents, showing improved thromboresistance and reduced late in-stent restenosis compared to bare metal stent (BMS) in a rabbit model.
- 27.Stampfl U, Sommer CM, Thierjung H et al. Reduction of late in-stent stenosis in a porcine coronary artery model by cobalt chromium stents with a nanocoat of polyphosphazene (Polyzene-F). Cardiovasc. Intervent. Radiol. 31(6), 1184–1192 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Stampfl U, Radeleff B, Sommer C et al. Paclitaxel-induced arterial wall toxicity and inflammation: part 2 – long-term tissue response in a minipig model. J. Vasc. Interv. Radiol. 20(12), 1608–1616 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Koppara T, Sakakura K, Pacheco E et al. Preclinical evaluation of a novel polyphosphazene surface modified stent. Int. J. Cardiol. 222, 217–225 (2016). [DOI] [PubMed] [Google Scholar]; •• Preclinical study showing evidence of reduced restenosis, inflammation and thrombus formation in COBRA PzF as compared to BMS using a porcine coronary artery model, human monocyte assays and a porcine ex vivo shunt model.
- 30.Jinnouchi H, Mori H, Cheng Q et al. Thromboresistance and functional healing in the COBRA PzF stent versus competitor DES: implications for dual antiplatelet therapy. EuroIntervention 15(4), e342–e353 (2019). [DOI] [PubMed] [Google Scholar]; •• Preclinical study showing enhanced endothelialization of COBRA PzF compared with bioabsorbable and durable polymer DES in a rabbit iliac artery model, as well as a comparable inflammatory cell adhesion between COBRA PzF and durable polymer DES but lower inflammatory cell adhesion in COBRA PzF compared to the bioabsorbable polymer DES in a porcine shunt model.
- 31.Maillard L, Corseaux D, Altié A et al. Time course of reendothelialization with polyzene-F nanocoated cobra PzF™ coronary stent on rabbit iliac arteries. Cardiovasc. Revasc. Med. 21(2), 195–199 (2020). [DOI] [PubMed] [Google Scholar]; • This preclinical study shows a nearly complete coverage of COBRA PzF at as early as 7 days after stent implantation in a rabbit iliac artery model.
- 32.Tamburino C, La Manna A, Di Salvo ME et al. First-in-man 1-year clinical outcomes of the Catania Coronary Stent System with Nanothin Polyzene-F in de novo native coronary artery lesions: the ATLANTA (Assessment of The LAtest Non-Thrombogenic Angioplasty stent) trial. JACC Cardiovasc. Interv. 2(3), 197–204 (2009). [DOI] [PubMed] [Google Scholar]; • First in man single-arm study showing safety and efficacy of a PzF-nanocoated stent with target lesion revascularization occurring in 3.6% at 12 months.
- 33.Tamburino C, Capodanno D, Di Salvo ME et al. Safety and effectiveness of the Catania Polyzene-F coated stent in real world clinical practice: 12-month results from the ATLANTA 2 registry. EuroIntervention. 7(9), 1062–1068 (2012). [DOI] [PubMed] [Google Scholar]
- 34.La Manna A, Capodanno D, Cera M et al. Optical coherence tomographic results at six-month follow-up evaluation of the CATANIA coronary stent system with nanothin Polyzene-F surface modification (from the Assessment of The LAtest Non-Thrombogenic Angioplasty Stent [ATLANTA] trial). Am. J. Cardiol. 103(11), 1551–1555 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Maillard L, Tavildari A, Barra N et al. Immediate and 1-year follow-up with the novel nanosurface modified COBRA PzF stent. Arch. Cardiovasc. Dis. 110(12), 682–688 (2017). [DOI] [PubMed] [Google Scholar]; •• This is the first clinicial study investigating COBRA PzF, showing favorable rates of target vessel failure in COBRA PzF compared to other commercially available BMS in the USA.
- 36.Pinto Slottow TL, Waksman R. Overview of the 2006 Food and Drug Administration Circulatory System Devices Panel meeting on drug-eluting stent thrombosis. Catheter Cardiovasc. Interv. 69(7), 1064–1074 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Fajadet J, Wijns W, Laarman GJ et al. Randomized, double-blind, multicenter study of the Endeavor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: clinical and angiographic results of the ENDEAVOR II trial. Circulation 114(8), 798–806 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Nef HM, Möllmann H, Weber M et al. Cobalt-chrome MULTI-LINK VISION-stent implantation in diabetics and complex lesions: results from the DaVinci-Registry. Clin. Res. Cardiol. 98(11), 731–737 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Wang JC, Carrié D, Masotti M et al. Primary endpoint results of the OMEGA Study: one-year clinical outcomes after implantation of a novel platinum chromium bare metal stent. Cardiovasc. Revasc. Med. 16(2), 65–69 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Cutlip DE, Garratt KN, Novack V et al. 9-Month clinical and angiographic outcomes of the COBRA Polyzene-F NanoCoated coronary stent system. JACC Cardiovasc. Interv. 10(2), 160–167 (2017). [DOI] [PubMed] [Google Scholar]; •• This clinical study presents the results of the PzF Shield Trial, reporting target vessel failure rates of 11.5% at 9 months.
- 41.Maillard L, de Labriolle A, Brasselet C et al. Evaluation of the safety and efficacy of the Cobra PzF NanoCoated coronary stent in routine, consecutive, prospective, and high-risk patients: the e-Cobra study. Catheter Cardiovasc. Interv. (2020) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]; •• Clinical study in 940 patients reporting a satisfactory major adverse cardiac event rate of 9.0% and stent thrombosis rate of 0.7% at 12months after implantation of COBRA PzF despite shorter duration of dual antiplatelet therapy or mono antiplatelet therapy in most patients.
- 42.Maillard L, Vochelet F, Peycher P et al. MAPT (mono antiplatelet therapy) as regular regimen after COBRA PzF™ NanoCoated Coronary Stent (NCS) implantation. Cardiovasc Revasc Med. 21(6), 785–789 (2020). [DOI] [PubMed] [Google Scholar]; •• Investigates the safety of single antiplatelet therapy after COBRA PzF implantation in patients at high bleeding risk and reported no case of stent thrombosis occurred at 1 month. In addition, major adverse cardiac events occurred in 3.8% at 12 months.
- 43.Valgimigli M, Bueno H, Byrne RA et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 39(3), 213–260 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Levine GN, Bates ER, Bittl JA et al. 2016 ACC/AHA Guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 68(10), 1082–1115 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Palmerini T, Biondi-Zoccai G, Della Riva D et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet 379(9824), 1393–1402 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Valgimigli M, Patialiakas A, Thury A et al. Zotarolimus-eluting versus bare-metal stents in uncertain drug-eluting stent candidates. J. Am. Coll. Cardiol. 65(8), 805–815 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Urban P, Meredith IT, Abizaid A et al. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N. Engl. J. Med. 373(21), 2038–2047 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Natsuaki M, Morimoto T, Yamamoto E et al. One-year outcome of a prospective trial stopping dual antiplatelet therapy at 3 months after everolimus-eluting cobalt-chromium stent implantation: shortT and OPtimal duration of Dual AntiPlatelet Therapy after everolimus-eluting cobalt-chromium stent (STOPDAPT) trial. Cardiovasc. Interv. Ther. 31(3), 196–209 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varenne O, Cook S, Sideris G et al. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single-blind trial. Lancet 391(10115), 41–50 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Colleran R, Joner M, Cutlip D et al. Design and rationale of a randomized trial of COBRA PzF stenting to REDUCE duration of triple therapy (COBRA-REDUCE). Cardiovasc. Revasc. Med. (2021) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 51.Urban P, Mehran R, Colleran R et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur. Heart J. 40(31), 2632–2653 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.COBRA PzF Stenting to REDUCE Duration of Triple Therapy – COBRA-RDUCE. Transcatheter Cardiovascular Therapeutics Virtual Meeting (TCT Connect) Bavry AA (Ed.) (2020). [Google Scholar]
- 53.Cornelissen A, Guo L, Sakamoto A et al. Histopathologic and physiologic effect of bifurcation stenting: current status and future prospects. Expert Rev. Med. Devices 17(3), 189–200 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Windecker S, Latib A, Kedhi E et al. Polymer-based or polymer-free stents in patients at high bleeding risk. N. Engl. J. Med. 382(13), 1208–1218 (2020). [DOI] [PubMed] [Google Scholar]